Abstract
Background:
Understanding the spectrum of COVID-19 in people with HIV (PWH) is critical to provide clinical guidance and risk-reduction strategies.
Setting:
CNICS, a U.S. multisite clinical cohort of PWH in care.
Methods:
We identified COVID-19 cases and severity (hospitalization, intensive care, death) in a large, diverse HIV cohort during March 1-December 31, 2020. We determined predictors and relative risks of hospitalization among PWH with COVID-19, adjusted for disease risk scores.
Results:
Of 16,056 PWH in care, 649 were diagnosed with COVID-19 between March-December 2020. Case fatality was 2%; 106 (16.3%) were hospitalized and 12 died. PWH with current CD4 count<350 cells/mm3 (aRR 2.68; 95%CI 1.93–3.71;P<.001) or lowest recorded CD4 count <200 (aRR 1.67; 95%CI 1.18–2.36;P<.005) had greater risks of hospitalization. HIV viral load and antiretroviral therapy (ART) status were not associated with hospitalization, although the majority of PWH were suppressed (86%). Black PWH were 51% more likely to be hospitalized with COVID-19 compared to other racial/ethnic groups (aRR 1.51; 95%CI 1.04–2.19;P=.03). Chronic kidney disease (CKD), chronic obstructive pulmonary disease, diabetes, hypertension, obesity, and increased cardiovascular and hepatic fibrosis risk scores were associated with higher hospitalization risk. PWH who were older, not on ART, with current CD4<350, diabetes, and CKD were overrepresented amongst PWH who required intubation or died.
Conclusions:
PWH with CD4<350 cells/mm3, and history of CD4<200, have a clear excess risk of severe COVID-19, accounting for comorbidities associated with severe outcomes. PWH with these risk factors should be prioritized for COVID-19 vaccination, early treatment, and monitored closely for worsening illness.
Keywords: COVID-19, HIV, CD4 count, structural determinants of health, immunosuppression
Introduction
The COVID-19 pandemic has had profound direct and structural effects on the health of people with HIV (PWH) in the United States.1 The first observations of COVID-19 in PWH occurred during a period of hospital crowding and rationed testing and treatment2–4. A large population-based study from South Africa was the first to indicate a two-fold higher risk of COVID-19 mortality among PWH5, but lacked data on comorbid conditions. Subsequent global registry data also showed elevated COVID-19 mortality risk in PWH, but had limited ability to examine the contribution of HIV-specific factors and comorbidities to mortality6,7.
Understanding risks for poor COVID-19 outcomes is important since PWH are often marginalized and experience health disparities driven in part by social determinants of health that increase the risk of both exposure to COVID-19 and severity of disease8. PWH experience a disproportionate burden of medical comorbidities9, higher rates of smoking10, drug and alcohol use11–15, and socioeconomic and racial disparities16,17. Not all PWH have overt immunosuppression, but HIV itself, chronic immune activation and exhaustion, and metabolic complications of HIV and antiretroviral therapy (ART) contribute to non-AIDS-defining morbidity, even in PWH with high CD4 counts and suppressed viral load (VL)18–20. Understanding the impact of HIV-associated and other factors on COVID-19 disease progression remains important to enable clinicians to provide appropriate risk assessment and prioritize COVID-19 prevention and treatment efforts, especially in settings where such resources remain limited.
The objective of this study was to identify predictors of COVID-19 severity, including risk for hospitalization, need for mechanical respiratory support, and death, among PWH with COVID-19 in the US.
Methods
The Centers for AIDS Research (CFAR) Network of Integrated Clinic Systems (CNICS) is a prospective observational cohort study of adult PWH in clinical care at academic institutions across the United States21. The study cohort included all PWH in care defined as those who attended one or more in-person or virtual HIV primary care visits between September 1, 2018 and December 31, 2020 at seven CNICS sites: Johns Hopkins University, Case Western Reserve University, Fenway Health, University of Alabama at Birmingham, University of California-San Diego, University of North Carolina at Chapel Hill, and University of Washington. CNICS research has been approved by the institutional review boards at each site.
Methods of data collection in CNICS are previously reported21. Briefly, comprehensive clinical data including diagnoses, laboratory test results, and medications collected through electronic medical records and institutional data systems undergo rigorous quality assessment and are harmonized in a central repository that is updated quarterly. Demographic data including birth sex, patient-reported racial/ethnic identity, and risk factor for HIV acquisition were collected at cohort enrollment. Patient Reported Outcome (PRO) measures of smoking (current vs. former vs. never use of tobacco) and unstable housing/homelessness were collected through tablet-based surveys every 4–6 months during primary care visits22,23, and from medical records.
SARS-CoV-2 infections and Outcomes:
Candidate SARS-CoV-2 infections and COVID-19 cases were identified through laboratory test results and provider documented diagnoses (ICD-10 codes) recorded between March 1 and December 31, 2020 and verified through medical record review. SARS-CoV-2 testing results and hospitalization records electronically imported to CNICS sites from external health systems were reviewed for data completeness. Medical records for all cases identified by diagnosis code without supporting laboratory tests performed within the CNICS system, including externally-reported diagnoses and test results, were reviewed by clinicians at each site using a standardized protocol.
Disease severity indicated by hospitalization for COVID-19, requirement for supplemental oxygen, intensive care admission, and invasive mechanical ventilation were verified by site clinicians and central clinician review of hospitalization discharge summaries. A patient with a positive SARS-CoV-2 test obtained while hospitalized for reasons unrelated to COVID-19 (e.g. pre-operative/admission screening or other incidental diagnosis) was considered a verified case but not as hospitalized due to COVID-19. Deaths occurring within 30 days of COVID-19 diagnosis or discharge from a hospitalization due to COVID-19 were attributed to COVID-19.
Covariates:
We examined the following chronic comorbid conditions: diabetes defined using a previously validated approach as: hemoglobin A1c (HbA1c) ≥6.5%, a prescription of a diabetes-specific medication, or a diagnosis of diabetes with associated prescription24; treated hypertension as a diagnosis of hypertension and a prescription of an anti-hypertensive medication; obesity as body mass index (BMI) ≥30 kg/m2; hepatitis C virus (HCV) coinfection by the presence of positive HCV antibody or detectable RNA or genotype; and chronic obstructive pulmonary disease (COPD) using a previously validated approach25 as a diagnosis of COPD and ≥90-day continuous supply of long-acting controller medications. We used laboratory test data to compute clinical measures of chronic kidney disease (CKD) defined as last glomerular filtration rate (eGFR) <60 using CKD-EPI without race adjustment26,27, and risk scores for atherosclerotic cardiovascular disease (ASCVD28) and hepatic fibrosis (Fibrosis-4: FIB-429–31). We examined CD4 counts (cells/μL) as both lowest historical value, as a proxy for CD4 nadir, and current CD4 count, as well as HIV VL (copies/mL). All laboratory test results were censored one week prior to COVID-19 diagnosis to avoid confounding.
Statistical analysis
Relative risks for COVID-related hospitalization were calculated using relative risk regression32. We accounted for potential confounding by adjusting models with disease risk scores (DRS)33,34, a prognostic analogue of propensity scores35 useful when studying a limited number of exposed patients and outcomes and a relatively large number of confounders. DRS were constructed independently for each exposure of interest using logistic regression in the full cohort34 for all non-duplicative covariates (e.g., FIB-4 not adjusted for age, as age is a component of FIB-4 calculation) including age, birth sex, race/ethnicity, smoking status, diabetes, hypertension, and CNICS site. We conducted sensitivity analyses adding comorbid conditions to the DRS to further evaluate the specific effects of race/ethnicity and age. All analyses were conducted in Stata version 17 (StataCorp, College Station, TX).
Results
Among 16,056 PWH in care during the study period, 20.9% were female, the median age was 52 years (IQR 40–59), 44.5% were non-Hispanic Black, 37.9% Non-Hispanic White, 12.5% Hispanic, and 5.2% mixed, other, or unreported race/ethnicity. Most (95.5%) were on antiretroviral therapy (ART), and 85.6% had an undetectable HIV VL. Between March and December 2020, 649 PWH had COVID-19 disease, of whom 28% were female, 52% were age 50 or older, 51% were non-Hispanic Black 25% were non-Hispanic White and 20% were Hispanic of any racial identity. The majority of COVID-19 cases (77%) had a documented positive SARS-CoV-2 RT-PCR or antigen test result within the CNICS system. The remaining 23% of cases were verified from a variety of sources including PCR and antigen testing performed in community settings, public health testing, or non-CNICS-affiliated medical sites. As in the overall cohort, the vast majority of PWH with COVID-19 were on ART (95%) and had an undetectable VL (86%). While 45% of PWH with COVID-19 had a lowest CD4 count <200 cells/mm3, more than half (66%) had a current CD4 count ≥500 cells/mm3 (Table 1).
Table 1.
Demographic and clinical characteristics of PWH in the CNICS Cohort with COVID-19 by hospitalization status.
| No./total (%) of patients | ||||
|---|---|---|---|---|
| Total (N = 649) |
P valuea | |||
| Yes (n = 106) |
No (n = 543) |
|||
| Demographics | ||||
| Female | 183/649 (28) | 39/106 (37) | 144/543 (27) | .03 |
| Age in 2020, y | <.001 | |||
| <30 | 51/647 (8) | 5/106 (5) | 46/541 (9) | |
| 30–39 | 123/647 (19) | 10/106 (9) | 113/541 (21) | |
| 40–49 | 140/647 (22) | 18/106 (17) | 122/541 (23) | |
| 50–59 | 200/647 (31) | 34/106 (32) | 166/541 (31) | |
| ≥60 | 133/647 (21) | 39/106 (37) | 94/541 (17) | |
| Race/ethnicity | .001 | |||
| Non-Hispanic Black | 329/649 (51) | 70/106 (66) | 259/543 (48) | |
| Non-Hispanic White | 165/649 (25) | 17/106 (16) | 148/543 (27) | |
| Hispanic | 129/649 (20) | 12/106 (11) | 117/543 (22) | |
| Other | 26/649 (4) | 7/106 (7) | 19/543 (4) | |
| HIV transmission risk factor | .46 | |||
| Non-IDU related | 588 (91) | 94 (89) | 494 (91) | |
| IDU related | 61 (9) | 12 (11) | 49 (9) | |
| Clinical characteristics b | ||||
| Lowest CD4 count, cells/mm3 | .004 | |||
| <200 | 289/645 (45) | 63/105 (60) | 226/540 (42) | |
| 200–349 | 172/645 (27) | 20/105 (19) | 152/540 (28) | |
| 350–499 | 83/645 (13) | 13/105 (12) | 70/540 (13) | |
| ≥500 | 101/645 (16) | 9/105 (9) | 92/540 (17) | |
| Current CD4 count, cells/mm3 | <.001 | |||
| <200 | 42/587 (7) | 18/98 (18) | 24/489 (5) | |
| 200–349 | 70/587 (12) | 20/98 (20) | 50/489 (10) | |
| 350–499 | 86/587 (15) | 11/98 (11) | 75/489 (15) | |
| ≥500 | 389/587 (66) | 49/98 (50) | 340/489 (70) | |
| Currently on ART | 615/649 (95) | 103/106 (97) | 512/543 (94) | .34 |
| Undetectable viral load (<50 copies/mL) | 521/603 (86) | 84/100 (84) | 437/503 (87) | .44 |
| Diabetes | 155/612 (25) | 43/100 (43) | 112/512 (22) | <.001 |
| Hypertension | 256/612 (42) | 60/100 (60) | 196/512 (38) | <.001 |
| CKD (eGFR, mL/min/1.73 m2) | <.001 | |||
| ≥60 | 535/619 (86) | 70/104 (67) | 465/515 (90) | |
| <60 | 84/619 (14) | 34/104 (33) | 50/515 (10) | |
| BMI, kg/m2 | .003 | |||
| <30 | 303/576 (53) | 37/96 (39) | 266/480 (55) | |
| ≥30 | 273/576 (47) | 59/96 (62) | 214/480 (45) | |
| ASCVD risk score, mean (SD) | 9.0% (9.4%) | 11.9% (10.3%) | 8.4% (9.1%) | .006 |
| Cigarette smoker (ever) | 326/649 (50) | 50/106 (47) | 276/543 (51) | .49 |
| COPD | 49/612 (8) | 16/100 (16) | 33/512 (7) | .001 |
| HCV | 99/614 (16) | 20/100 (20) | 79/514 (15) | .25 |
| FIB-4 score | <.001 | |||
| ≤3.25 | 558/570 (98) | 92/99 (93) | 466/471 (99) | |
| >3.25 | 12/570 (2) | 7/99 (7) | 5/471 (1) | |
| FIB-4 score | <.001 | |||
| ≤1.45 | 442/570 (78) | 61/99 (62) | 381/471 (81) | |
| >1.45 | 128/570 (23) | 38/99 (38) | 90/471 (19) | |
Percent may not sum to 100 due to rounding.
Abbreviations: ART, antiretroviral therapy; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CKD, chronic kidney disease; CNICS, Center for AIDS Research (CFAR) Network of Integrated Clinical Systems; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; FIB-4, Fibrosis-4 scoring system for liver fibrosis; HCV, Hepatitis C virus; PWH, people with HIV.
Chi-square test of independence for categorical data and independent t-test for continuous data.
Most recent value prior to one-week preceding COVID diagnosis.
One hundred-six (16%) PWH with COVID-19 disease were hospitalized. In univariate analyses, demographic characteristics associated with hospitalization included female sex, Black race, and older age. Compared to non-hospitalized PWH with COVID-19, lowest CD4 count, current CD4 count, elevated FIB-4 and ASCVD risk scores, diabetes, hypertension, CKD (eGFR <60), obesity (BMI ≥30), and COPD, were each associated with risk of COVID-19 hospitalization (all P<.006). Notably, 60% of hospitalized cases had a lowest CD4 count <200 cells/mm3 compared with 42% of non-hospitalized cases, and 50% of hospitalized cases had a current CD4 count ≥500 compared with 70% of non-hospitalized cases. Having a FIB-4 score >3.25 (highly predictive of hepatic fibrosis), was strongly associated with hospitalization (7% vs 1%, P<.001). PWH with a FIB-4 score >1.45 were also more likely to be hospitalized with COVID-19 (38% vs 19%, P<.001). HCV coinfection, smoking, injection drug use (IDU) as the reported risk for HIV, and being unstably housed/homeless were not associated with hospitalization among cases with available data.
In adjusted analysis, low current CD4 count was more strongly predictive of hospitalization than age or any comorbid condition. Both current CD4 count <350 aRR 2.68 (95% CI 1.93–3.71; P<.001) and lowest CD4 count <200 aRR 1.67 (95% CI 1.18–2.36; P<.005) were associated with hospitalization (Figure 1). There was a trend toward protection against hospitalization with higher current CD4/CD8 ratio, i.e., aRR 0.88 for each 1 SD increase in ratio (95% CI 0.75–1.03; P=.08). There was no association between risk of hospitalization and detectable VL. Although there were insufficient PWH not receiving ART to evaluate an adjusted risk, the proportion of hospitalized cases on ART prior to COVID-19 diagnosis (97%) did not differ significantly from non-hospitalized cases (94%).
Figure 1. Relative Risk of hospitalization with COVID-19 among PWH with COVID-19 by key characteristics.
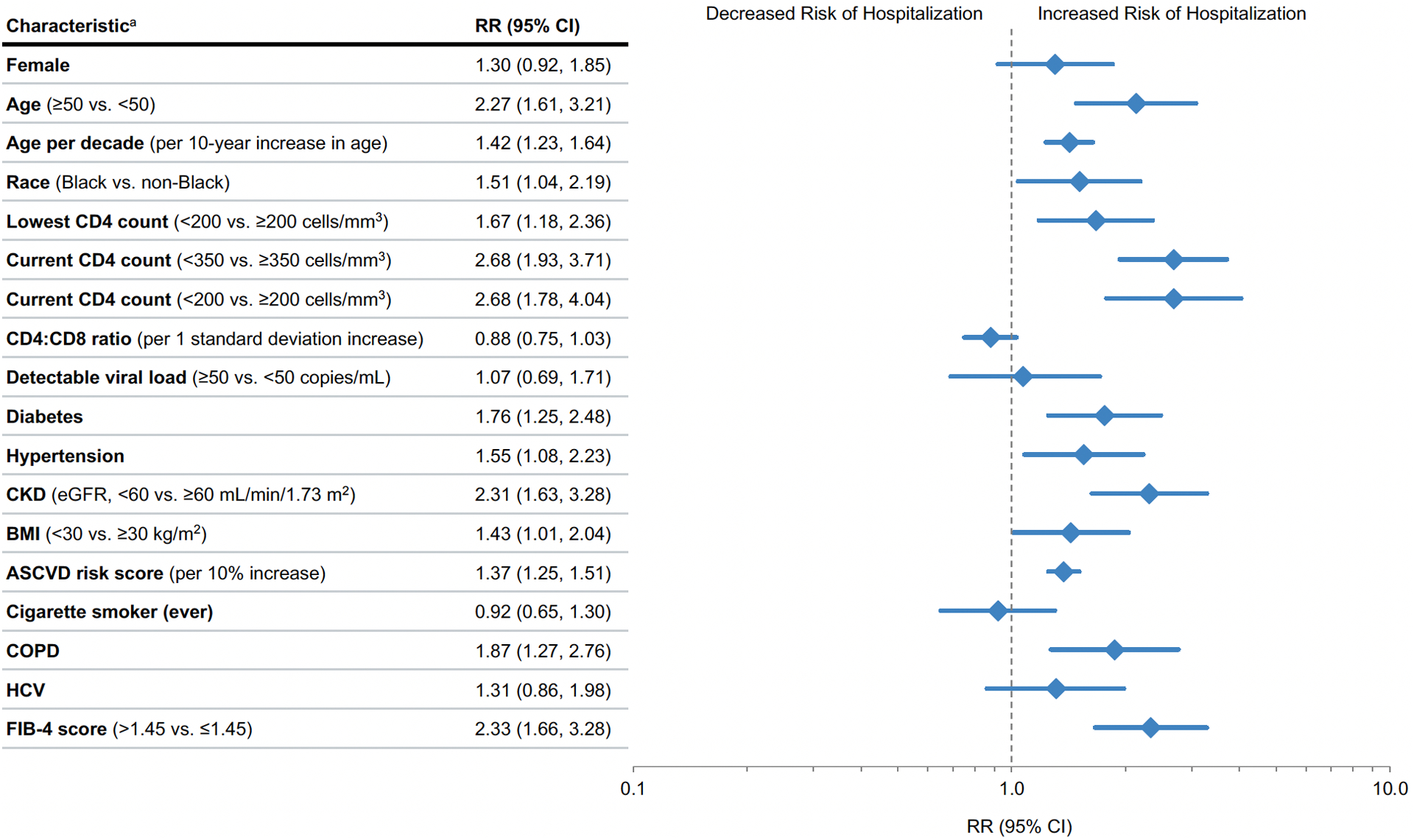
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CKD, chronic kidney disease; CNICS, Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; FIB-4, Fibrosis-4 scoring system for liver fibrosis; HCV, Hepatitis C virus; PWH, people with HIV; RR, relative risk.
Reference range for Age per decade is 18–29 years, the the subsequent ordinal categories being 30–39, 40–49, 50–59, and 60+, as in Table 1.
a Relative risk regression models adjusted for demographic and clinical characteristics using disease risk scores, except for the ASCVD risk score analysis which is unadjusted. Disease risk score were constructed independently for each exposure variable of interest using all non-duplicative covariates.
In adjusted analyses, PWH with CKD (eGFR <60) had a more than 2-fold risk of hospitalization (aRR 2.31; 95% CI 1.63–3.28, P<.001), and those with diabetes, hypertension, obesity or COPD also had higher risk of hospitalization (Figure 1). In addition, we found a strong association between clinically validated risk scores for cardiovascular and liver disease (ASCVD, FIB-4) and higher risk of hospitalization. For each 10% increase in ASCVD risk score, the aRR for hospitalization was 1.37 (95% CI 1.25–1.51; P<.001). PWH who had a FIB-4 score above the 1.45 cutoff were 2.33 times more likely to be hospitalized with COVID-19, compared to those below this cutoff (95% CI 1.66–3.28; P<.001).
Although women were over-represented among those hospitalized, after adjusting for other factors, sex was not associated with hospitalization (aRR 1.30; 95% CI 0.92–1.85, P=.14). As seen in the general population, age ≥50 was associated with a more than 2-fold risk of hospitalization (aRR 2.13; 95% CI 1.48, 3.07) P<.001). Adjusting for other covariates, the risk of hospitalization due to COVID-19 increased 42%, on average, for every additional 10-years of age. PWH identifying as non-Hispanic Black were 51% more likely to be hospitalized with COVID-19 compared to other racial/ethnic identities (aRR 1.51; 95% CI 1.04–2.19, P=.03). Hispanic ethnicity was not significantly associated with hospitalization (aRR=0.71 95% CI 0.41–1.24; P=.23).
To further evaluate whether demographic characteristics associated with COVID-19 hospitalization were partially attributable to the differential distribution of comorbidities, we performed sensitivity analyses that also adjusted for comorbidities including diabetes, hypertension, CKD, obesity, COPD and liver disease (FIB-4), and found similar associations between COVID-19 hospitalization and racial/ethnic identity; the associations between age and hospitalization were attenuated but overall similar.
Twelve PWH with COVID-19 (2%) died; three deaths were in persons never hospitalized for COVID-19 and nine died in hospital or within 30 days of discharge. Thirty-one of the 106 hospitalized persons were admitted to the ICU (29%) and 16 (15%) were intubated (Table 2). Due to small numbers who were intubated or died, we report proportions but not adjusted risk estimates for critical illness outcomes. Older PWH, those with CD4 count <350, persons not on ART, and those with diabetes and CKD were overrepresented amongst those who required intubation or died from COVID-19. No one under age 30 was admitted to the ICU or died, whereas 25 (81%) of those admitted to the ICU and nine (75%) of the deaths were among PWH 50 and older. As with most COVID-19 hospitalizations in the US, the majority of PWH (65%, Table 3) received some degree of oxygen or respiratory support, although presentations with gastrointestinal symptoms, sepsis physiology, acute kidney injury, or COVID-19 pneumonia without significant hypoxemia were observed. Thirty-four percent of hospitalized PWH required only nasal canula as the maximal level of support, 10% required High-Flow Nasal Canula, and 15% were intubated; none received extra-corporeal life support (ECLS/ECMO) (Table 3). While the case fatality rate among PWH with COVID-19 was 12 of 649 (2%), 9% of hospitalized COVID-19 cases died, including nearly one-third (31%) of those intubated. Most non-hospitalized COVID-19 cases had no record of receiving any approved or experimental COVID-19 specific treatment (94%), while 64% of hospitalized COVID-19 cases and 77% of critically ill COVID-19 received COVID-19 pharmacotherapy. Consistent with changes in clinical care during 2020, the most common medications administered to hospitalized PWH with COVID-19 were dexamethasone (45%) and remdesivir (42%), with fewer receiving convalescent plasma or hydroxychloroquine, anti-SARS-CoV-2 monoclonal antibodies, or participating in any interventional trial (Supplemental Table 1).
Table 2.
Demographic and clinical characteristics of PWH hospitalized with COVID-19 by severity of COVID-19 disease.
| No./total (%) of patients | ||||
|---|---|---|---|---|
| Hospitalized (n = 106) |
Admitted to ICU (n = 31) |
Intubated (n = 16) |
Died (n = 12) |
|
| Demographic characteristics | ||||
| Female | 39/106 (37) | 12/31 (39) | 5 (31) | 4 (33) |
| Age in 2020, y | ||||
| <30 | 5/106 (5) | 0/31 (0) | 0/16 (0) | 0/12 (0) |
| 30–39 | 10/106 (9) | 4/31 (13) | 2/16 (13) | 1/12 (8) |
| 40–49 | 18/106 (17) | 2/31 (7) | 0/16 (0) | 2/12 (17) |
| 50–59 | 34/106 (32) | 9/31 (29) | 4/16 (25) | 2/12 (17) |
| ≥60 | 39/106 (37) | 16/31 (52) | 10/16 (63) | 7/12 (58) |
| Race/ethnicity | ||||
| Non-Hispanic Black | 70/106 (66) | 7/31 (23) | 4/16 (25) | 2/12 (17) |
| Non-Hispanic White | 17/106 (16) | 19/31 (61) | 10/16 (63) | 7/12 (58) |
| Hispanic | 12/106 (11) | 3/31 (10) | 1/16 (6) | 3/12 (25) |
| Other | 7/106 (7) | 2/31 (7) | 1/16 (6) | 0/12 (0) |
| Clinical characteristics a | ||||
| Lowest CD4 count, cells/mm3 | ||||
| <200 | 63/105 (60) | 18/31 (58) | 9/16 (56) | 8/12 (67) |
| 200–349 | 20/105 (19) | 4/31 (13) | 2/16 (13) | 1/12 (8) |
| 350–499 | 13/105 (12) | 6/31 (19) | 2/16 (13) | 1/12 (8) |
| ≥500 | 9/105 (9) | 3/31 (10) | 3/16 (19) | 2/12 (17) |
| Current CD4 count, cells/mm3 | ||||
| <200 | 18/98 (18) | 6/26 (23) | 4/15 (27) | 3/11 (27) |
| 200–349 | 20/98 (20) | 5/26 (19) | 2/15 (13) | 3/11 (27) |
| 350–499 | 11/98 (11) | 3/26 (12) | 2/15 (13) | 1/11 (9) |
| ≥500 | 49/98 (50) | 12/26 (46) | 7/15 (47) | 4/11 (36) |
| Currently on ART | 103/106 (97) | 29/31 (94) | 14/16 (88) | 10/12 (83) |
| Undetectable viral load (<50 copies/mL) | 84/100 (84) | 25/29 (86) | 13/15 (87) | 10/12 (83) |
| Diabetes | 43/100 (43) | 15/29 (52) | 9/16 (56) | 6/11 (55) |
| Hypertension | 60/100 (60) | 19/29 (66) | 11/16 (69) | 8/11 (73) |
| CKD (eGFR), mL/min/1.73 m2 | ||||
| ≥60 | 70/104 (67) | 15/30 (50) | 6/16 (38) | 5/12 (42) |
| <60 | 34/104 (33) | 15/30 (50) | 10/16 (63) | 7/12 (58) |
| BMI, kg/m2 | ||||
| <30 | 37/96 (39) | 9/26 (35) | 3/13 (23) | 2/11 (18) |
| ≥30 | 59/96 (62) | 17/26 (65) | 10/13 (77) | 9/11 (82) |
| ASCVD risk score, mean (SD) | 11.9% (10.3%) | 11.7% (10.7%) | 17.2% (13.4%) | 19.6% (12.9%) |
| Cigarette smoker (ever) | 50/106 (47) | 13/31 (50) | 5/16 (31) | 6/12 (50) |
| COPD | 16/100 (16) | 4/29 (14) | 1/16 (6) | 1/11 (9) |
| HCV | 20/100 (20) | 8/29 (28) | 4/16 (25) | 3/11 (27) |
| FIB-4 score | ||||
| ≤3.25 | 92/99 (93) | 25/28 (89) | 14/16 (88) | 11/11 (100) |
| >3.25 | 7/99 (7) | 3/28 (11) | 2/16 (13) | 0/11 (0) |
| FIB-4 score | ||||
| ≤1.45 | 61/99 (62) | 20/28 (71) | 11/16 (69) | 8/11 (73) |
| >1.45 | 38/99 (38) | 8/28 (29) | 5/16 (31) | 3/11 (27) |
Percent may not sum to 100 due to rounding.
Abbreviations: ART, antiretroviral therapy; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CKD, chronic kidney disease; CNICS, Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; FIB-4, Fibrosis-4 scoring system for liver fibrosis; HCV, Hepatitis C virus; PWH, people with HIV.
Most recent value prior to one-week preceding COVID-19 diagnosis.
Table 3.
Oxygen requirements among PWH hospitalized with COVID-19 by severity of COVID-19 disease.
| No./total (%) of patients | |||
|---|---|---|---|
| Maximum oxygen support type | Hospitalized (n = 106) |
Admitted to ICU (n = 31) |
Died (n = 9) |
| No oxygen required | 35/106 (33) | 1/31 (3) | 0/9 (0) |
| Nasal canula | 36/106 (34) | 2/31 (7) | 1/9 (11) |
| Mask | 3/106 (3) | 1/31 (3) | 1/9 (11) |
| CPAP/BiPAP | 3/106 (3) | 1/31 (3) | 0/9 (0) |
| HFNC | 11/106 (10) | 10/31 (32) | 2/9 (22) |
| Intubation | 16/106 (15) | 16/31 (52) | 5/9 (56) |
| ECMO | 0/106 (0) | 0/31 (0) | 0/9 (0) |
| Unknown | 2/106 (2) | 0/31 (0) | 0/9 (0) |
Percent may not sum to 100 due to rounding.
Abbreviations: CNICS, Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems; CPAP/BiPAP, continuous positive airway pressure/bilevel positive airway pressure; ECMO, extracorporeal membrane oxygenation; HFNC, high-flow nasal cannula; ICU, intensive care unit; PWH, people with HIV
Discussion
We conducted this study in one of the largest and best-characterized multi-center cohorts of PWH and identified factors independently associated with severity of COVID-19 disease in PWH in the US in the first calendar year of the pandemic. Our results demonstrate that both current CD4 count <350 and lowest CD4 <200 cells/mm3 are important predictors of hospitalization among PWH with COVID-19. Though we did not find a statistically significant association between HIV VL suppression and disease severity, our ability to detect an association is limited by the very low numbers of PWH who were not receiving ART or who were virally unsuppressed. PWH with CKD and liver dysfunction had over 2-fold higher risk of more severe COVID-19 disease. In addition, PWH with COPD, diabetes, hypertension, and obesity were also at higher risk of greater COVID-19 severity. Using clinically validated and easily measurable risk scores (ASCVD and FIB-4), we found that cardiovascular as well as liver comorbidity were highly predictive indicators of hospitalization for PWH with COVID-19. Our results confirm previously noted associations between comorbidities and COVID-19 severity, but provide more specificity, especially in characterizing the contribution of chronic comorbid conditions and HIV immunologic history to the risk of hospitalization and severity of COVID-19 disease. Although the majority of PWH in our cohort have reconstituted CD4 counts, prior immune destruction or persistent immune activation, as evidenced by low CD4/CD8 ratio, are also important predictors of COVID-19 severity.
Among 16,056 PWH, 106 (0.7%) were hospitalized with COVID-19 between March and December 2020. While identification of non-hospitalized cases was limited by COVID-19 test rationing early in the epidemic, hospitalization with severe disease is less subject to misclassification and bias. Testing for SARS-CoV-2 infection in US hospitals was widely and uniformly available throughout most of the study period, reducing potential for bias in identifying hospitalized patients. The proportions of PWH with COVID-19 requiring hospitalization (16%) and intensive care (5%) and who died (2%) are lower than proportions reported in a Spanish national cohort of PWH with COVID-19 early in the pandemic (64% hospitalized, 6% ICU, 8% died)36, and PWH in New York City with COVID-19 in March-June 2020 (42%, 5%, 13%, respectively)37, which may be explained in part by overwhelmed healthcare capacity during the limited time period of those early cohort studies. In contrast, PWH in a Spanish (PISCIS) registry through December 2020 and US national registry of COVID-19 patients (N3C Cohort) through May 2021, had similar rates of severe outcomes (PISCIS: 13.8%, 0.9%, 1.7%; N3C: 32%, 4%, 2%, respectively) to our cohort38,39.
Cardiovascular, pulmonary and metabolic comorbidities in PWH associated with increased COVID-19 disease severity were consistent with those seen in other cohorts of PWH and in people without HIV.2,40–42 Comorbidities that are caused or exacerbated by smoking, including COPD, hypertension, and diabetes, were all associated with increased risk of hospitalization. Notably, we found that CKD with even modest reductions in eGFR was among the strongest predictors of hospitalization for PWH with COVID-19, consistent with non-HIV cohorts, and not previously well-recognized in PWH, despite the increased prevalence of CKD in PWH.40,41,43
Early studies of PWH with COVID-19 did not detect a strong association between CD4 count and severity of COVID-19 outcomes44,45, and initially it was hypothesized that immune suppression may be protective of cytokine-mediated inflammatory responses in COVID-1946. Our results demonstrate consistent and significant increased risk of hospitalization in PWH with lower CD4 counts, an association that has been confirmed in multiple global settings39,47–49. While most marked in PWH with CD4 <200 cells/mm3, risk of hospitalization was significantly elevated in people with CD4 <350 compared to CD4≥350. In addition to current CD4 count, we also found lowest CD4 count and lower current CD4/CD8 ratio were associated with an increased risk of hospitalization, suggesting an immune exhaustion effect persisting from the time of CD4 nadir despite subsequent CD4 reconstitution. Importantly, in this study, we excluded CD4 counts obtained at or after COVID-19 diagnosis, as SARS-CoV-2 infection can cause profound CD4 and CD8 lymphocyte suppression, which if not carefully parameterized, may have contributed to confounding in other cohort studies. In addition to characterizing HIV-associated risks for hospitalization with greater precision, this study is the first to our knowledge, in PWH or in the general population, to examine ASCVD and FIB-4, which are clinically available, robust indicators of CVD risk and liver comorbidity that were each associated with severity of COVID-19. We recommend these scores be used by clinicians for risk estimation, encouragement of vaccination, and allocation of monoclonal antibodies and other early therapeutics to prevent disease progression.
Our study also confirmed that minoritized racial and ethnic groups had disproportionately severe COVID-19 outcomes, even within a population predominately representing minoritized groups. Black PWH diagnosed with COVID-19 were 50% more likely to be hospitalized than other PWH, a finding that persisted after controlling for multiple medical comorbidities. Racism, anti-LGBTQ bias, and other forms of discrimination and stigmatization also increase allostatic load, in addition to the structural barriers and health inequities faced by many PWH compounded by marginalized gender identities, sexual orientation, and substance use16,50–53.
The greatest strength of this analysis is the extensive and well-characterized multi-site cohort, in which chronic comorbidities and HIV-specific factors have been previously validated. Our analysis has some limitations. The number of COVID-19 cases identified underestimates the true prevalence of SARS-CoV-2 infection and COVID-19 disease, especially for mild cases that may not have presented to clinical care. Thus, the proportion of cases who were hospitalized may be an overestimate, though absolute ascertainment of hospitalization and severe outcomes would not be affected. We were unable to make direct comparisons to the general population; such comparisons have been published, albeit without the rigorous case characterization and clinician hospitalization review as in CNICS.
Conclusions
Given the experience of many long-term survivors living through the first two decades of the AIDS epidemic, the isolation, scientific uncertainty, and lack of actionable medical interventions has revived many parallel concerns for PWH17,54,55. Developing clear understanding of whom amongst those with HIV is at increased risk for severe outcomes with this new pathogen, and which risks are modifiable, can mitigate both the medical and psychological burden to PWH during this pandemic. Results of this large multi-site cohort study demonstrate that older PWH with a CD4 cell count <350 or historic CD4 <200 have higher risk of severe COVID-19 disease. Comorbidities including CKD, liver fibrosis, COPD, diabetes, hypertension, obesity and cardiovascular risk also impart increased risk of severe COVID-19 in PWH. PWH with these risk factors should be strongly encouraged to receive preventive vaccines and boosters, and should be prioritized for allocation of early therapeutics such as monoclonal antibodies.
Supplementary Material
Acknowledgments:
We gratefully acknowledge the CNICS cohort participants and their providers for contributing to the study. Funding for this study and for CNICS came from the National Institute of Allergy and Infectious Diseases (NIAID) [CNICS R24 AI067039; UW CFAR NIAID Grant P30 AI027757; UAB CFAR grant P30 AI027767; UNC CFAR grant P30 AI50410; UCSD CFAR grant P30 AI036214; Case Western Reserve University CFAR grant P30 AI036219; Fenway Health/Harvard CFAR grant P30 AI060354, UCSF CFAR grant P30 AI027763 and JHU CFAR grant P30 AI094189] and the National Institute on Drug Abuse (NIDA) [R01DA047045].
Funding for this study and for CNICS came from the US National Institutes of Health: National Institute of Allergy and Infectious Diseases (NIAID) [CNICS R24 AI067039; UW CFAR NIAID Grant P30 AI027757; UAB CFAR grant P30 AI027767; UNC CFAR grant P30 AI50410; UCSD CFAR grant P30 AI036214; Case Western Reserve University CFAR grant P30 AI036219; Fenway Health/Harvard CFAR grant P30 AI060354, UCSF CFAR grant P30 AI027763 and JHU CFAR grant P30 AI094189] and the National Institute on Drug Abuse (NIDA) [R01DA047045]. The funders had no role in the design, conduct, or reporting of the study.
Footnotes
Publication History: A portion of this data was presented at virtualCROI 2021.
Posted history: This manuscript was previously posted to medRxiv: doi: 10.1101/2021.10.15.21265063
Conflicts of Interest and Source of Funding
The authors have no conflicts of interest to disclose.
References
- 1.Armstrong WS, Agwu AL, Barrette EP, et al. Innovations in Human Immunodeficiency Virus (HIV) Care Delivery During the Coronavirus Disease 2019 (COVID-19) Pandemic: Policies to Strengthen the Ending the Epidemic Initiative-A Policy Paper of the Infectious Diseases Society of America and the HIV Medicine Association. Clin Infect Dis. Jan 23 2021;72(1):9–14. doi: 10.1093/cid/ciaa1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vizcarra P, Perez-Elias MJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. Aug 2020;7(8):e554–e564. doi: 10.1016/S2352-3018(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown LB, Spinelli MA, Gandhi M. The interplay between HIV and COVID-19: summary of the data and responses to date. Curr Opin HIV AIDS. Jan 2021;16(1):63–73. doi: 10.1097/COH.0000000000000659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesko CR, Bengtson AM. HIV and COVID-19: Intersecting Epidemics With Many Unknowns. Am J Epidemiol. Jan 4 2021;190(1):10–16. doi: 10.1093/aje/kwaa158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulle A, Davies MA, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. Aug 29 2020;doi: 10.1093/cid/ciaa1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertagnolio S, Thwin SS, Silva R, et al. Clinical characteristics and prognostic factors in people living with HIV hospitalized with COVID-19: findings from the WHO Global Clinical Platform. IAS 2021. 2021;(18-21 July):Abstract 2498. [Google Scholar]
- 7.Wang Y, Feng R, Xu J, Shi L, Feng H, Yang H. An updated meta-analysis on the association between HIV infection and COVID-19 mortality. AIDS. Sep 1 2021;35(11):1875–1878. doi: 10.1097/QAD.0000000000002968 [DOI] [PubMed] [Google Scholar]
- 8.Weiser JK, Tie Y, Beer L, Fanfair RN, Shouse RL. Racial/Ethnic and Income Disparities in the Prevalence of Comorbidities that Are Associated With Risk for Severe COVID-19 Among Adults Receiving HIV Care, United States, 2014–2019. Journal of Acquired Immune Deficiency Syndromes (1999). 2021;86(3):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. Dec 2011;53(11):1120–6. doi: 10.1093/cid/cir627 [DOI] [PubMed] [Google Scholar]
- 10.Frazier EL, Sutton MY, Brooks JT, Shouse RL, Weiser J. Trends in cigarette smoking among adults with HIV compared with the general adult population, United States - 2009–2014. Prev Med. Jun 2018;111:231–234. doi: 10.1016/j.ypmed.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 11.Crane HM, McCaul ME, Chander G, et al. Prevalence and factors associated with hazardous alcohol use among persons living with HIV across the US in the current era of antiretroviral treatment. AIDS Behav. Jul 2017;21(7):1914–1925. doi: 10.1007/s10461-017-1740-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chander G, Josephs J, Fleishman JA, et al. Alcohol use among HIV-infected persons in care: results of a multi-site survey. HIV Med. Apr 2008;9(4):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66(6):769–89. [DOI] [PubMed] [Google Scholar]
- 14.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. Aug 2001;58(8):721–8. [DOI] [PubMed] [Google Scholar]
- 15.Crane HM, Nance RM, Whitney BM, et al. Drug and alcohol use among people living with HIV in care in the United States by geographic region. AIDS Care. Jan 23 2021:1–8. doi: 10.1080/09540121.2021.1874274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. Jul 2020;47:37–44. doi: 10.1016/j.annepidem.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millett GA. New pathogen, same disparities: why COVID-19 and HIV remain prevalent in U.S. communities of colour and implications for ending the HIV epidemic. J Int AIDS Soc. Nov 2020;23(11):e25639. doi: 10.1002/jia2.25639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. May 2014;10(5):e1004078. doi: 10.1371/journal.ppat.1004078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duffau P, Wittkop L, Lazaro E, et al. Association of immune-activation and senescence markers with non-AIDS-defining comorbidities in HIV-suppressed patients. AIDS. Oct 23 2015;29(16):2099–108. doi: 10.1097/QAD.0000000000000807 [DOI] [PubMed] [Google Scholar]
- 20.Dharan NJ, Neuhaus J, Rockstroh JK, et al. Benefit of early versus deferred antiretroviral therapy on progression of liver fibrosis among people with HIV in the START randomized trial. Hepatology. Mar 2019;69(3):1135–1150. doi: 10.1002/hep.30296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. Oct 2008;37(5):948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crane HM, Lober W, Webster E, et al. Routine collection of patient-reported outcomes in an HIV clinic setting: the first 100 patients. Curr HIV Res. Jan 2007;5(1):109–18. doi: 10.2174/157016207779316369 [DOI] [PubMed] [Google Scholar]
- 23.Fredericksen RJ, Crane PK, Tufano J, et al. Integrating a web-based patient assessent into primary care for HIV-infected adults. Journal of AIDS and HIV Research. 2012;4(2):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crane HM, Kadane JB, Crane PK, Kitahata MM. Diabetes case identification methods applied to electronic medical record systems: their use in HIV-infected patients. Curr HIV Res. Jan 2006;4(1):97–106. [DOI] [PubMed] [Google Scholar]
- 25.Crothers K, Rodriguez CV, Nance RM, et al. Accuracy of electronic health record data for the diagnosis of chronic obstructive pulmonary disease in persons living with HIV and uninfected persons. Pharmacoepidemiol Drug Saf. Feb 2019;28(2):140–147. doi: 10.1002/pds.4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Research Support, N.I.H., Extramural Validation Studies. Ann Intern Med. May 5 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA. Jul 9 2019;322(2):113–114. doi: 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]
- 28.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. Jun 24 2014;129(25 Suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 29.Nunes D, Fleming C, Offner G, et al. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol. Jun 2010;105(6):1346–53. doi: 10.1038/ajg.2009.746 [DOI] [PubMed] [Google Scholar]
- 30.Jain MK, Seremba E, Bhore R, et al. Change in fibrosis score as a predictor of mortality among HIV-infected patients with viral hepatitis. AIDS Patient Care STDS. Feb 2012;26(2):73–80. doi: 10.1089/apc.2011.0191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H, Nance R, Van Rompaey S, et al. Poorly controlled HIV infection: an independent risk factor for liver fibrosis. J Acquir Immune Defic Syndr. Aug 1 2016;72(4):437–43. doi: 10.1097/QAI.0000000000000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. Apr 1 2004;159(7):702–6. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 33.Hansen BB. The prognostic analogue of the propensity score. Biometrika. 2008;95(2):481–488. [Google Scholar]
- 34.Arbogast PG, Ray WA. Performance of disease risk scores, propensity scores, and traditional multivariable outcome regression in the presence of multiple confounders. Am J Epidemiol. Sep 1 2011;174(5):613–20. doi: 10.1093/aje/kwr143 [DOI] [PubMed] [Google Scholar]
- 35.Tadrous M, Gagne JJ, Sturmer T, Cadarette SM. Disease risk score as a confounder summary method: systematic review and recommendations. Pharmacoepidemiol Drug Saf. Feb 2013;22(2):122–9. doi: 10.1002/pds.3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: A cohort study. Ann Intern Med. Jun 26 2020;doi: 10.7326/M20-3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braunstein SL, Lazar R, Wahnich A, Daskalakis DC, Blackstock OJ. Coronavirus Disease 2019 (COVID-19) infection among people with Human Immunodeficiency Virus in New York city: A population-level analysis of linked surveillance data. Clin Infect Dis. Jun 15 2021;72(12):e1021–e1029. doi: 10.1093/cid/ciaa1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nomah DK, Reyes-Urueña J, Díaz Y, et al. Sociodemographic, clinical, and immunological factors associated with SARS-CoV-2 diagnosis and severe COVID-19 outcomes in people living with HIV: a retrospective cohort study. The Lancet HIV. 2021-November-01 2021;8(11):e701–e710. doi: 10.1016/s2352-3018(21)00240-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, Sun J, Patel RC, et al. Associations between HIV infection and clinical spectrum of COVID-19: a population level analysis based on US National COVID Cohort Collaborative (N3C) data. The Lancet HIV. 2021-November-01 2021;8(11):e690–e700. doi: 10.1016/s2352-3018(21)00239-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko JY, Danielson ML, Town M, et al. Risk Factors for Coronavirus Disease 2019 (COVID-19)–Associated Hospitalization: COVID-19–Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. 2021-June-01 2021;72(11):e695–e703. doi: 10.1093/cid/ciaa1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dandachi D, Geiger G, Montgomery MW, et al. Characteristics, Comorbidities, and Outcomes in a Multicenter Registry of Patients with HIV and Coronavirus Disease-19. Clin Infect Dis. Sep 9 2020;doi: 10.1093/cid/ciaa1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambrosioni J, Blanco JL, Reyes-Urueña JM, et al. Overview of SARS-CoV-2 infection in adults living with HIV. The Lancet HIV. 2021-May-01 2021;8(5):e294–e305. doi: 10.1016/s2352-3018(21)00070-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swanepoel CR, Atta MG, D’Agati VD, et al. Kidney disease in the setting of HIV infection: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney International. 2018-March-01 2018;93(3):545–559. doi: 10.1016/j.kint.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inciarte A, Gonzalez-Cordon A, Rojas J, et al. Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: a single-center, prospective observational study. AIDS. Oct 1 2020;34(12):1775–1780. doi: 10.1097/QAD.0000000000002643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceballos ME, Ross P, Lasso M, et al. Clinical characteristics and outcomes of people living with HIV hospitalized with COVID-19: a nationwide experience. Int J STD AIDS. Apr 2021;32(5):435–443. doi: 10.1177/0956462420973106 [DOI] [PubMed] [Google Scholar]
- 46.Guo W, Ming F, Feng Y, et al. Patterns of HIV and SARS-CoV-2 co-infection in Wuhan, China. J Int AIDS Soc. Jul 2020;23(7):e25568. doi: 10.1002/jia2.25568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffmann C, Casado JL, Harter G, et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. May 2021;22(5):372–378. doi: 10.1111/hiv.13037 [DOI] [PubMed] [Google Scholar]
- 48.Yendewa GA, Perez JA, Schlick K, Tribout H, McComsey GA. Clinical Features and Outcomes of Coronavirus Disease 2019 Among People With Human Immunodeficiency Virus in the United States: A Multicenter Study From a Large Global Health Research Network (TriNetX). Open Forum Infect Dis. Jul 2021;8(7):ofab272. doi: 10.1093/ofid/ofab272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamis KF, Barbera L, Abdo M, et al. Risk Factors for Hospitalization in People With HIV and COVID-19. J Acquir Immune Defic Syndr. 11 January 2021;88(3):e22. doi: 10.1097/QAI.0000000000002780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amirkhan JH. Stress overload in the spread of coronavirus. Anxiety, Stress, & Coping. 2021/March/04 2021;34(2):121–129. doi: 10.1080/10615806.2020.1824271 [DOI] [PubMed] [Google Scholar]
- 51.Serpas DG, García JJ. Allostatic Load and the Wear and Tear of the Body for LGBTQ PoC. Heart, Brain and Mental Health Disparities for LGBTQ People of Color. Springer; 2021:41–52. [Google Scholar]
- 52.Wakeel F, Njoku A. Application of the Weathering Framework: Intersection of Racism, Stigma, and COVID-19 as a Stressful Life Event among African Americans. Multidisciplinary Digital Publishing Institute; 2021:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gil RM, Freeman T, Mathew T, et al. The LGBTQ+ communities and the COVID-19 pandemic: a call to break the cycle of structural barriers. The Journal of infectious diseases. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bassett MT. First AIDS, Now COVID-19: Another Plague Shows Us Who We Are. Social Research: An International Quarterly. 2020;87(2):229–232. [Google Scholar]
- 55.Brown B, Taylor J, Fisher CB. Mitigating Isolation of People Aging With HIV During the COVID-19 Pandemic. Public Health Reports. 2021;136(4):394–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


