Abstract
Background:
Endoscopic eradication therapy with radiofrequency ablation and endoscopic mucosal resection is a safe and effective treatment for Barrett’s esophagus (BE). While the outcomes of surveillance endoscopy following successful endoscopic eradication therapy have been described, no prior studies have modeled the natural history after successful ablation or the effect of surveillance endoscopy after successful ablation to prevent progression to invasive esophageal adenocarcinoma.
Methods:
The United States Radiofrequency Ablation Registry is a multi-center registry consisting of patients treated with radiofrequency ablation for BE at 148 institutions (113 community-based, 35 academic-affiliated). The authors fit models to impute the natural history of recurrence and neoplastic progression after any recurrence or retreatment. Natural history estimates of invasive adenocarcinoma after ablation therapy were compared to as-treated estimates at five years to derive the preventive risk difference for surveillance.
Results:
Natural history estimates for the post-ablation progression of high-grade dysplasia (HGD) or intramucosal adenocarcinoma to invasive adenocarcinoma after treatment were 6.3% at five years compared to 1.3% for low-grade dysplasia (LGD). The natural history model found a much higher preventative risk difference for surveillance for HGD/intramucosal adenocarcinoma (−4.8%), compared to LGD (−1.1%). The numbers-needed-to-surveil at five years were 21 and 90 for these groups, respectively, to prevent one case of invasive esophageal adenocarcinoma, making surveillance after successful ablation of baseline HGD more than four times as effective at preventing invasive cancer than after successful ablation of baseline LGD.
Discussion:
Endoscopic surveillance after successful ablation of baseline HGD or intramucosal cancer is much more effective than surveillance after successful treatment of baseline LGD in averting invasive adenocarcinoma. While the modest benefits of surveillance for treated LGD may be greater than the risks for patients at average risk for adverse effects of endoscopy, clinicians should concentrate on retaining baseline HGD or cancer patients in endoscopic surveillance programs.
Keywords: Barrett’s esophagus, surveillance, high-grade dysplasia, intramucosal cancer
Background
Radiofrequency ablation (RFA) is a safe and effective treatment for dysplastic Barrett’s esophagus (BE).(1, 2) After complete eradication of intestinal metaplasia (CEIM), recurrence of BE is common, occurring in 8-10% of treated patients per year, while progression to dysplasia or cancer is less common.(3–7) In order to protect patients from recurrent neoplasia, surveillance endoscopy with retreatment of any recurrent intestinal metaplasia and neoplasia is recommended,(8) and is associated with a low rate of progression to invasive esophageal adenocarcinoma.(7) Surveillance endoscopy also imposes a burden of risk of adverse effects and of cost from the endoscopic procedure.(9, 10) To balance the benefits and risks of surveillance endoscopy, clinicians must perform this exam frequently enough to forestall progression to invasive cancer, but not more frequently as to incur unnecessary cost, patient inconvenience and risk.(11)
While outcomes in surveillance after endoscopic eradication therapy are consistently good in observational studies, (3, 6, 12–14) there are no randomized clinical trials of the effect of surveillance. Previous observational studies have important limitations to their clinical applicability. While we ideally would compare the risks and benefits of post-treatment surveillance to subjects not undergoing such exams, these real-world studies report the observed outcomes under a regimen of surveillance and retreatment. In the absence of randomized data, prior modeling studies seeking to understand the benefit of endoscopic surveillance following endoscopic eradication therapy have either modeled one outcome at a time, which limits the study of the rare but important outcomes like progression to unresectable carcinoma, or they have studied a composite outcome.(3–7)
To overcome these limitations and better inform clinical surveillance decisions, we constructed multi-state survival models to impute the natural history of recurrence and progression after CEIM. We compared these models to the observed outcomes under surveillance and retreatment to estimate the effect of surveillance to prevent progression to invasive esophageal adenocarcinoma. We compared the effect of surveillance after treatment of low-grade dysplasia to that after treatment of high-grade dysplasia or intramucosal adenocarcinoma.
Methods
The United States Radiofrequency Ablation Registry
The United States Radiofrequency Ablation (US RFA) Patient Registry is a multi-center registry consisting of patients treated with RFA for BE at 148 institutions (113 community-based, 35 academic-affiliated). This observational study prospectively collected standardized details of endoscopy, histopathology, surgery, and other events in the routine clinical management starting with RFA for BE. The methods of the US RFA Registry have been described in detail in previous studies. (15) All physicians participating in this registry either elected to use Western institutional review board (IRB) approval or obtained IRB approval through their respective institutions.
Cohort Inclusion and Definition of Outcomes
Participants were included for analysis if they achieved CEIM and had at least one further surveillance visit with histopathology results. CEIM was defined as the first upper endoscopy after RFA treatment with histopathology results with no intestinal metaplasia or associated neoplasia in the tubular esophagus, no dysplasia or adenocarcinoma in the cardia, and no treatments given. Participants that met the definition of CEIM but had a prior histologic grade of invasive adenocarcinoma, a prior esophagectomy, or had no history of histologic grade more advanced than non-dysplastic BE were excluded. Surveillance after CEIM for non-dysplastic BE was not modeled because guidelines recommend against treatment of non-dysplastic BE.(8) Non-dysplastic intestinal metaplasia in the cardia was not considered recurrence.
Constructing the post-ablation natural history imputation model
We constructed a model US RFA cohort before any treatment of recurrent intestinal metaplasia or neoplasia for model-based imputation of the natural history without any retreatment. We fit time-to-event, multi-state models that modeled recurrence of BE, progression through stages of dysplasia, intramucosal adenocarcinoma, invasive adenocarcinoma, or death from other causes as discrete states (Supplemental Figure 1). These states were as follows: maintaining CEIM, recurrence with only non-dysplastic intestinal metaplasia, recurrence with indefinite for dysplasia or low-grade dysplasia, recurrence or progression to high-grade dysplasia, recurrence or progression to intramucosal adenocarcinoma, progression to invasive esophageal adenocarcinoma, and death from any other cause. Invasive adenocarcinoma was defined as T1b or higher stage adenocarcinoma. Recurrence or progression to adenocarcinoma and death from any cause without adenocarcinoma were modeled as absorbing states from which participants could not emerge. Passage between states in the model occurs at a constant rate known as the transition intensity, which is estimated based on the observed data. Transitions from an initial recurrence grade to a higher one are sometimes observed, but once treatment occurs after a recurrence, the natural history can no longer observed, which introduces a potential for bias. In particular, those cases where immediate treatment was performed may be a higher risk subset than those treated after biopsies reveal recurrence or after referral to a treatment center, but are not observed in the data defining transition between intermediate states. In order to assess the potential impact of this bias we performed sensitivity analyses where the transition intensities estimated from the data were increased by varying proportions up to three-fold, representing selection bias for cases where immediate treatment is not performed. More frequent surveillance was also modelled in sensitivity analyses assuming it was 50%, 100%, and 200% more effective than what was observed. To simulate the case if the cohort was surveilled at the rate recommended by upcoming guidelines, we modeled an increase in surveillance effectiveness proportional to the increase in surveillance visits under the guidelines compared to the observed visits over five years. The natural history model was stratified by worst prior histologic grade before CEIM and had covariates for time-dependent age and baseline Barrett’s segment length. The fit of the imputation model was assessed by comparing the cumulative incidence of invasive esophageal adenocarcinoma as simulated by the model with that observed in the actual data after CEIM but prior to any re-treatment, i.e., during the “natural history” of the CEIM patients (Supplemental Figures 2 and 3). This calibration was possible because patients in the US RFA Registry returned for endoscopic surveillance at different intervals, depending on patient compliance and provider instructions, leading to a natural experiment describing the outcomes of surveillance endoscopy performed on different intervals. Multi-state survival models were fit in R version 4.0.5 and using the msm package version 1.6.8.
Imputing Events after Re-treatment to Estimate Natural History
Once a cohort member is treated, their natural history can no longer be observed in the data. We used the multi-state natural history model to randomly impute 1,000 natural histories after any re-treatment or recurrence. We applied this model of transitions in the actual surveillance cohort to estimate the rate of transition between recurrence of various histologic grades conditional on each participant’s covariates, the time, and histologic grade of recurrence. We fit Kaplan-Meier estimates of the cumulative incidence of invasive adenocarcinoma and report as the imputations estimates the mean of the imputations by time after CEIM. To estimate the five-year risk difference for surveillance, we compared the imputed natural history estimates to as-treated Kaplan-Meier estimates. We performed 1,000 bootstrap samples to estimate the standard error of the risk difference for both population sampling and the imputation processes. Intramucosal adenocarcinoma was combined with high-grade dysplasia for the imputation analyses due to insufficient sample size at five years. We calculated the number-needed-to-surveil as one divided by the risk difference rounded up to the nearest whole person.
Results
Included Participants and Baseline Characteristics
Among 5,521 participants in the US RFA Registry there were 1,401 who met the criteria for inclusion (Figure 1). The included participants were similar in baseline characteristics to the overall registry population (Table 1). The mean age of included participants was 64.5 years (standard deviation 10.3), and the majority were Caucasian (94.4%) and Male (82.6%). The median number of surveillance visits per included participant was 2 (interquartile range 1–4) and the median time under observation was 26.0 months (interquartile range 16.6–39.2).
Figure 1.
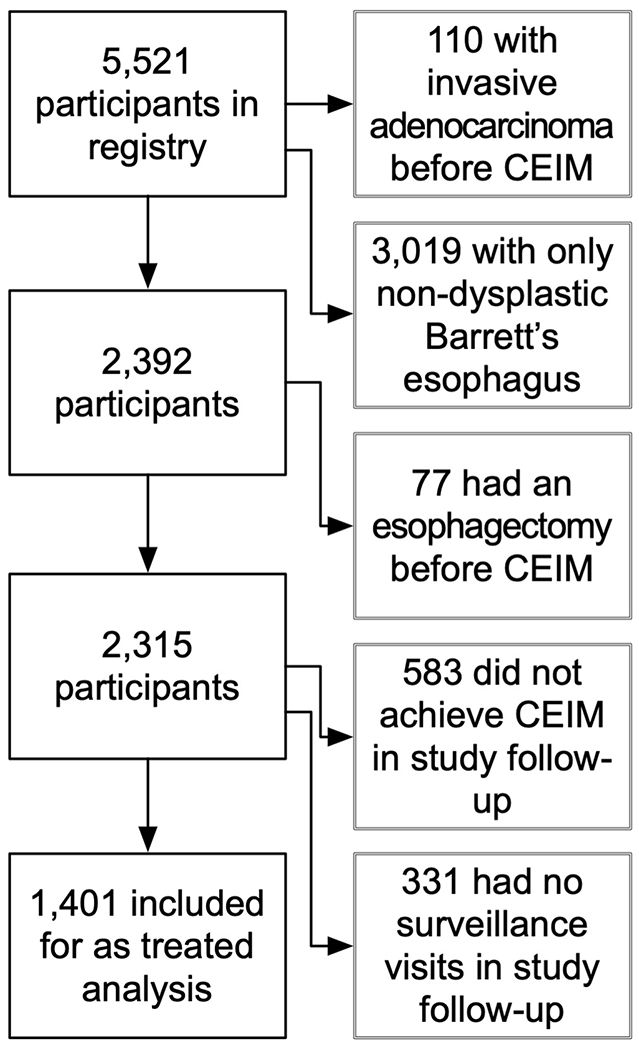
Inclusion and Exclusion of Participants from the Cohort for Analysis. CEIM, complete eradication of intestinal metaplasia.
Table 1.
Baseline characteristics of all United States Radiofrequency Ablation Registry participants, participants achieving complete eradication of intestinal metaplasia, and participants with at least one additional surveillance visit with biopsies.
| All Registry Participants | Participants w/ Dysplastic BE Achieving CEIM | Participants with Dysplastic BE and CEIM having a surveillance visit | |
|---|---|---|---|
| N | 5,521 | 1,732 | 1,401 |
| Enrollment age – mean years (SD) | 61.5 (11.4) | 64.5 (10.3) | 64.5 (10.3) |
| Male gender – N (%) | 4,052 (73.4) | 1,416 (81.8) | 1,157 (82.6) |
| Race/ethnicity – N (%) | |||
| African American | 82 (1.5) | 14 (0.8) | 12 (0.9) |
| Asian | 39 (0.7) | 10 (0.6) | 8 (0.6) |
| Caucasian | 5,126 (92.8) | 1,637 (94.5) | 1,323 (94.4) |
| Hispanic | 137 (2.5) | 23 (1.3) | 18 (1.3) |
| Other | 24 (0.4) | 5 (0.3) | 4 (0.3) |
| Native Hawaiian or Pacific Islander | 1 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown | 112 (2.0) | 43 (2.5) | 36 (2.6) |
|
| |||
| Worst histologic grade prior to CEIM – N (%) | |||
| Non-dysplastic Barrett’s esophagus | 2,607 (47.2) | 0 (0.0) | 0 (0.0) |
| Indefinite for dysplasia | 412 (7.5) | 0 (0.0) | 0 (0.0) |
| Low-grade dysplasia | 1,130 (20.5) | 839 (48.4) | 649 (46.3) |
| High-grade dysplasia | 1,017 (18.4) | 742 (42.8) | 621 (44.3) |
| Intramucosal adenocarcinoma | 245 (4.4) | 151 (8.7) | 131 (9.4) |
| Invasive adenocarcinoma | 110 (2.0) | 0 (0.0) | 0 (0.0) |
| Baseline Barrett’s segment length – mean cm (SD) | 4.1 (3.3) | 4.4 (3.2) | 4.3 (3.2) |
|
| |||
| Nissen fundoplication prior to treatment – N (%) | 295 (5.3) | 86 (5.0) | 63 (4.5) |
| Endoscopic mucosal resection– N (%) | 647 (11.7) | 385 (22.2) | 338 (24.1) |
| Esophagectomy prior to CEIM – N (%) | 125 (2.3) | 0 (0.0) | 0 (0.0) |
N, number; SD, standard deviation; CEIM, complete eradication of intestinal metaplasia; cm, centimeters.
Cumulative Incidence of Invasive Adenocarcinoma in the As-Treated Cohort
Progression to invasive esophageal adenocarcinoma under a regimen of surveillance and retreatment after CEIM was rare (Figure 2), occurring in an estimated 0.2% (95% confidence limits 0.0 – 0.6) of low-grade dysplasia patients, and 1.5% (95% confidence limits 0.3 – 2.7%) of high-grade dysplasia or intramucosal adenocarcinoma patients by five years. The risk was 7.4 times greater for high-grade dysplasia or intramucosal adenocarcinoma compared to low-grade dysplasia.
Figure 2.
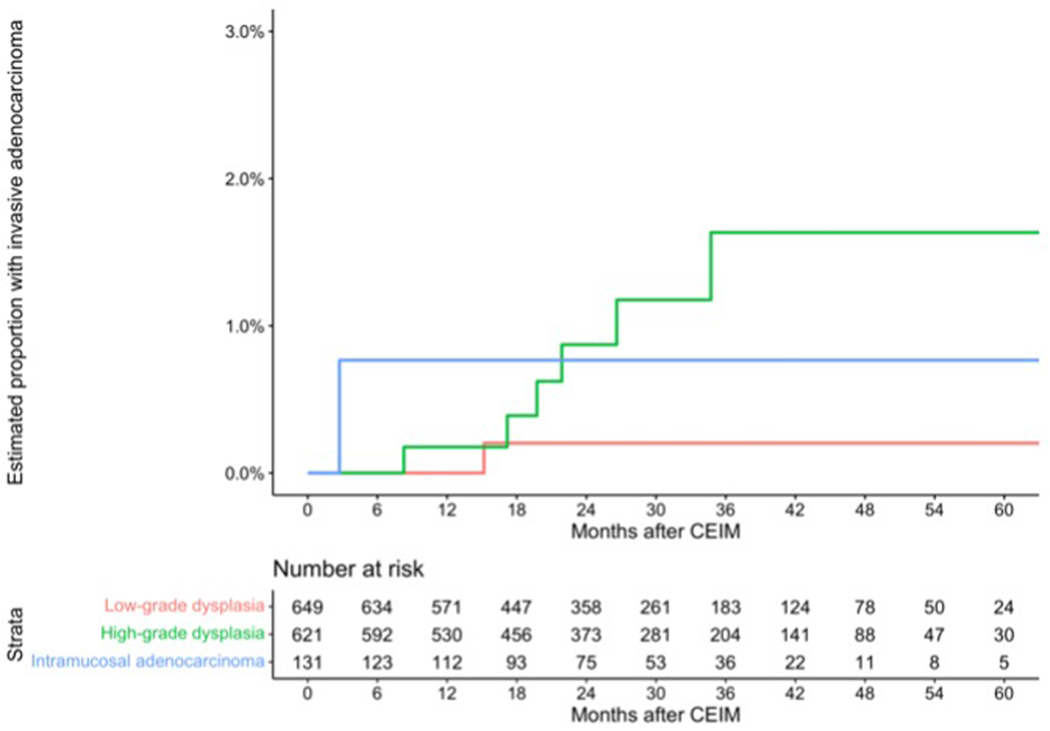
Estimated cumulative incidence of invasive adenocarcinoma and number remaining at risk by months following complete eradication of intestinal metaplasia. These data are “as-treated,” reflecting the impact of endoscopic surveillance.
*, includes indefinite for dysplasia.
Natural History Estimates and Estimated Effects of Surveillance
The estimated cumulative incidence of invasive adenocarcinoma without surveillance and retreatment was much higher than the as-treated cohort for high-grade dysplasia or intramucosal adenocarcinoma, but only slightly higher for low-grade dysplasia (Figure 3). The number-needed-to-surveil to prevent one case of invasive esophageal adenocarcinoma over 5 years were surveillance performed as observed in the registry was 90 for low-grade dysplasia and 21 for high-grade dysplasia or intramucosal adenocarcinoma (Table 2). These effect estimates were moderately sensitive to the varying assumptions of underestimation of risk by our imputation model or more frequent surveillance than was performed in the cohort itself (Table 3).
Figure 3.
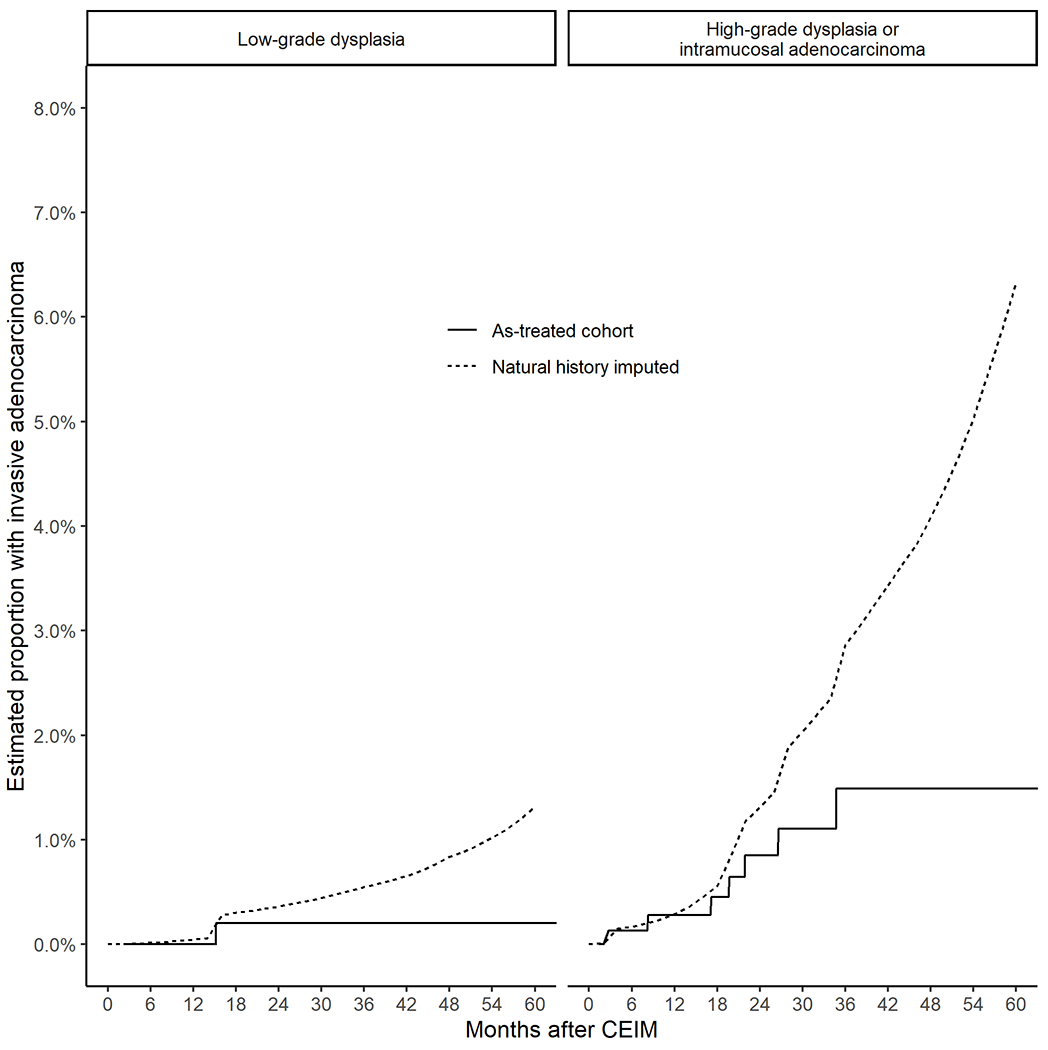
Estimated cumulative incidence of invasive adenocarcinoma in the as-treated populations with low-grade dysplasia and high-grade dysplasia compared to the natural history estimates imputing further progression after any recurrence or retreatment.
Table 2.
Comparing the as-treated cohort to natural history estimates to estimate the risk difference for surveillance and the number needed to surveil to avert one case of invasive adenocarcinoma.
| Five-year cumulative incidence of invasive adenocarcinoma (percent) | Estimated risk difference (95% bootstrap confidence limits) | Number needed to surveil* | ||
|---|---|---|---|---|
| As-treated cohort (95% Greenwood confidence limits) | Natural history estimate (95% imputation limits) | |||
| Low-grade dysplasia | 0.2 (0.0 – 0.6) | 1.3 (0.2 – 4.8) | −1.1 (−2.6 – 0.4) | 90 |
| High-grade dysplasia or intramucosal adenocarcinoma | 1.5 (0.3 – 2.7) | 6.3 (2.6 – 11.9) | −4.8 (−5.5 – −4.1) | 21 |
under the base case with endoscopic surveillance as performed in the cohort itself and statistically summarized in Figure 4.
Table 3.
Protective risk difference and number needed to surveil estimates from sensitivity analyses with linear increases in all rates of progression and with linear increases in the rate of surveillance and retreatment by worse prior histologic grade before complete eradication of dysplasia, each with 1,000 replicates.
| Protective risk difference (%) / number needed to surveil* | Rate of surveillance: | ||||
|---|---|---|---|---|---|
| Base case scenario | 50% increase | 100% increase | Per 2022 ACG guidelines† | 200% increase | |
| Rate of progression: | Low-grade dysplasia | ||||
|
|
|||||
| Base case scenario | 1.1 / 90 | 1.6 / 61 | 2.2 / 45 | 2.3 / 42 | 3.3 / 30 |
| 50% increase | 1.6 / 63 | 2.4 / 42 | 3.1 / 31 | 3.4 / 29 | 4.7 / 21 |
| 100% increase | 2.0 / 49 | 3.0 / 32 | 4.1 / 24 | 4.4 / 22 | 6.1 / 16 |
| 150% increase | 2.5 / 39 | 3.8 / 26 | 5.1 / 19 | 5.4 / 18 | 7.6 / 13 |
| 200% increase | 3.0 / 33 | 4.5 / 22 | 6.0 / 16 | 6.5 / 15 | 9.0 / 11 |
|
|
|||||
| Rate of progression: | High-grade dysplasia and intramucosal adenocarcinoma | ||||
|
|
|||||
| Base case scenario | 4.8 / 21 | 7.4 / 13 | 9.8 / 10 | 8.8 / 11 | 14.7 / 6 |
| 50% increase | 8.4 / 11 | 12.6 / 7 | 16.8 / 5 | 15.1 / 6 | 25.2 / 3 |
| 100% increase | 12.3 / 8 | 18.4 / 5 | 24.6 / 4 | 22.1 / 4 | 36.9 / 2 |
| 150% increase | 15.8 / 6 | 23.8 / 4 | 31.7 / 3 | 28.5 / 3 | 47.5 / 2 |
| 200% increase | 19.5 / 5 | 29.3 / 3 | 39.0 / 2 | 35.1 / 2 | 58.5 / 1 |
under the base case scenario endoscopic surveillance is performed as in the cohort itself and described in Figure 4, which is less than recommended,
this was 179% more than observed in the cohort for low-grade dysplasia and 150% more than observed for high-grade dysplasia and intramucosal adenocarcinoma.
ACG, American College of Gastroenterology.
Quantifying Surveillance Visits, Treatments, and their Yield
Surveillance yielded treatment of recurrent disease in an estimated cumulative 65.6% of participants by five years (Figure 4–A). Surveillance endoscopies occurred at an overall average of 1.3 surveillance endoscopies per year. Surveillance endoscopies accumulated at a roughly linear rate and differences in the frequency of surveillance endoscopies by worst prior histologic grade were small (Figure 4–B), meaning that patients treated for baseline low-grade dysplasia had endoscopic surveillance about as often as those treated for baseline high-grade dysplasia. Among surveillance retreatment endoscopies, the worst histologic grade of recurrence was higher among patients with a baseline histology of high-grade dysplasia or intramucosal adenocarcinoma compared to low-grade dysplasia.
Figure 4.
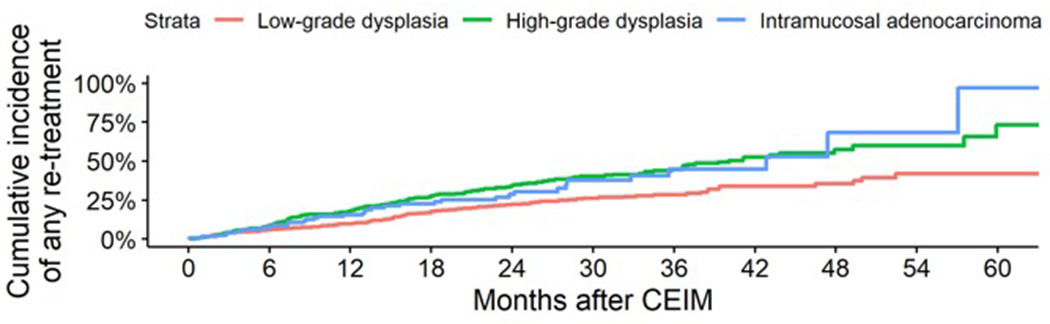
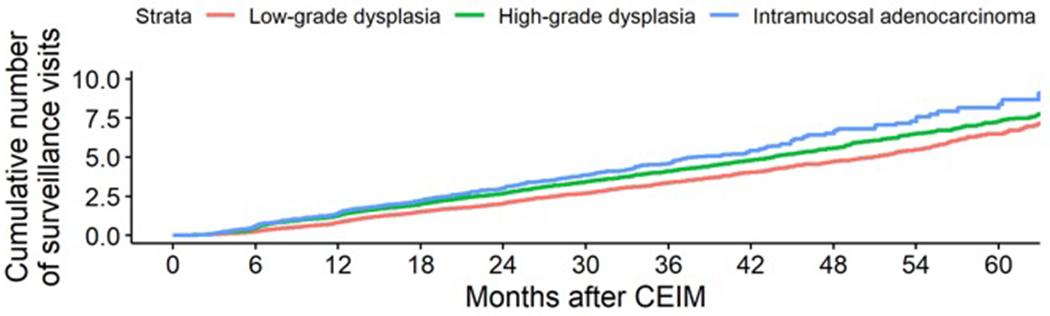
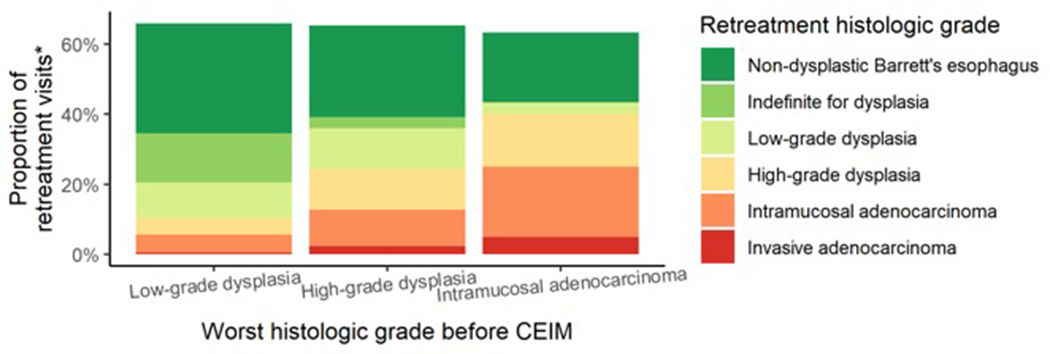
Estimated (A) cumulative incidence of surveillance treatments, (B) cumulative number of surveillance endoscopies by months following complete eradication of intestinal metaplasia, and (C) histologic grade at retreatment by baseline worst prior histologic grade.
* This does not add up to 100% due to retreatment of visible recurrence that occurred with negative histology or without biopsies
Discussion
In this observational analysis of a large, prospective study of surveillance outcomes after endoscopic eradication therapy with RFA among participants with neoplastic BE, we modeled the natural history of recurrence and progression to invasive cancer and estimated the effects of surveillance endoscopy for comparison to the risks. The natural history estimates of the incidence of surveillance invasive adenocarcinoma at five years were 1.3% (95% confidence limits 0.2% - 4.8%) for low-grade dysplasia and 6.3% (2.6 - 11.9%) for high-grade dysplasia or intramucosal adenocarcinoma. The observed rates of invasive adenocarcinoma in the cohort were 0.2% in baseline low-grade dysplasia patients, and 1.5% in baseline high-grade dysplasia or intramucosal adenocarcinoma patients by five years, suggesting a substantial protective effect of surveillance endoscopy. The estimated benefits of post-treatment surveillance in preventing invasive adenocarcinoma were 4.3-fold greater for high-grade dysplasia or intramucosal adenocarcinoma compared to low-grade dysplasia. Because endoscopic surveillance is very safe, the weight of this evidence suggests the benefits of surveillance even in low-grade dysplasia are greater than the low risk of serious adverse effects of surveillance endoscopy in otherwise healthy patients, but the risks may outweigh benefits in some patients at elevated risk for surveillance endoscopy.
While surveillance was more effective for pre-treatment high-grade dysplasia and intramucosal adenocarcinoma and less effective for pre-treatment low-grade dysplasia, the observed frequency of surveillance in the baseline high-grade dysplasia/intramucosal carcinoma cohort was less frequent than guidelines recommend, and similar to guideline-recommended intervals for low-grade dysplasia (Figure 4–B).(8) Despite this pattern, more retreatments were given to patients with pre-treatment high-grade dysplasia and intramucosal adenocarcinoma (Figure 4–B) and the severity of neoplasia retreated was much greater (Figure 4–C). Compared to the initial therapy of dysplastic BE,(1) lesions found during endoscopic surveillance after successful ablation were predominantly of lower grade (Figure 4–C) than the baseline histologic grade, which is why there is a smaller effect for endoscopic surveillance compared to initial treatment of dysplastic BE. The Barrett’s segment length prior to ablation had a detectable effect on the natural history estimates, but the effect was too small to justify varying surveillance intervals (full data not shown).
While this is the first analysis to estimate the natural history after successful ablative therapy, a large body of literature describes the observed outcomes in surveillance. Except for a prior report from this same registry, most studies have not reported the rate of invasive esophageal adenocarcinoma in surveillance due to insufficient sample size.(7) Rates of the combined outcome of high-grade dysplasia, intramucosal adenocarcinoma, and invasive esophageal adenocarcinoma from other post-ablation cohorts are similar to the as-treated estimates of this study.(16) By comparison, the estimated effects of surveillance even for high-grade dysplasia or intramucosal adenocarcinoma were substantially less than the benefit observed from the initial successful ablation of these lesions in randomized clinical trials of endoscopic eradication.(1, 2) Therefore, while endoscopic surveillance following ablation is merited, the majority of the protective effect is already achieved from the initial successful ablation itself.
This analysis has important limitations. While the analysis aims to describe the natural history effects of surveillance, the study design has inherent limitations to describe this. At the point of retreatment of recurrent disease in surveillance, the natural history and treated history diverge and the natural history that would have occurred after treatment is not observed. This missing person-time, which we must impute using a multi-state model, may be systematically different from the observed person-time for progression between intermediate states of recurrence and thus may introduce a bias. This bias may underestimate natural history risk by modeling more cases of microscopic rather than macroscopic recurrence and of low- rather than high-volume centers. Our sensitivity analyses suggest that the stronger the bias of this type, the greater the benefit of surveillance for high-grade dysplasia and intramucosal adenocarcinoma compared to low-grade dysplasia, and the benefit for low-grade dysplasia would remain modest (Table 3). This bias could only be resolved completely with randomization. The base case analysis compares surveillance as it was performed in the registry, which was generally less frequently than is recommended by guidelines, to the natural history estimate. While this approach has the advantage of using real world data instead of theoretical “perfectly-performed” surveillance, it may thus underestimate the benefit of surveillance at the recommended intervals, which may be greater. Estimates extrapolating the effect of more frequent surveillance, such as is recommended in forthcoming guidelines (17), are presented in Table 3.
The strengths of this analysis are the focus on a clinically important but rarely studied outcome, progression to invasive adenocarcinoma, and the estimation of the effects of surveillance. While there are limitations to the analysis’ ability to quantify the effects of surveillance on post-treatment BE compared to a randomized clinical trial, such a study has not been reported. The US RFA Registry is a sample of community and academic ablation centers and has similar rates of recurrence and progression compared to other United States studies.
This analysis of a large, multi-center, United States cohort in surveillance after endoscopic eradication therapy used imputation with multi-state survival models to model the natural history of recurrence and progression, and thus estimate the protective effect of endoscopic surveillance following successful ablation of dysplastic BE. We found the benefit of surveillance after successful initial treatment is concentrated in patients with baseline high-grade dysplasia and intramucosal adenocarcinoma, while benefit was marginal in patients with baseline low-grade dysplasia. Our analysis suggests that substantial efforts are merited to retain those with baseline high-grade dysplasia or intramucosal carcinoma in endoscopic surveillance. Conversely, in patients with low-grade dysplasia and elevated endoscopy risk, the benefits of surveillance should be carefully considered against the risk, and such examinations might provide modest or no survival advantage, especially late in life, when competing causes of mortality rise. These findings reinforce the findings of our prior analysis (11) and support forthcoming surveillance recommendations (17) that will decrease surveillance frequency after successful ablation of baseline low-grade dysplasia and maintain aggressive surveillance after successful ablation of baseline high-grade dysplasia. Further studies should investigate the cost effectiveness of surveillance of low-grade dysplasia.
Supplementary Material
What is known?
Radiofrequency ablation is a safe and effective treatment for dysplastic Barrett’s esophagus.
Surveillance after complete eradication of intestinal metaplasia yields a clinically significant rate of recurrent disease that is usually amenable to further endoscopic treatment.
What is new here?
We used imputation approaches to model the benefit of surveillance rather than the yield.
We found a clinically significant benefit of surveillance and retreatment after endoscopic eradication of high-grade dysplasia, but a more modest benefit in the setting of low-grade dysplasia.
A single surveillance endoscopy performed after successful ablation of high-grade dysplasia prevents four times as much invasive cancer than surveillance exam performed after successful ablation of low-grade dysplasia
Acknowledgements:
We would like to thank the University of North Carolina at Chapel Hill and the Research Computing group for providing computational resources and support that have contributed to these research results.
Financial support:
This work was funded by T32 DK007634
Abbreviations:
- RFA
radiofrequency ablation
- BE
Barrett’s esophagus
- CEIM
complete eradication of intestinal metaplasia
Footnotes
Ethics: All physicians participating in this registry either elected to use Western institutional review board (IRB) approval or obtained IRB approval through their respective institutions.
Potential competing interests: CCC, none declared, NJS, has received research funding from Medtronic, Pentax, Steris, CDx Medical, Lucid, and Interpace Diagnostics and has worked as a consultant for Boston Scientific, Cernostics, Cook Medical, Aqua, Exact Sciences, and Phathom, APT, none declared.
References
- 1.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009;360:2277–88. [DOI] [PubMed] [Google Scholar]
- 2.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. Jama 2014;311:1209–17. [DOI] [PubMed] [Google Scholar]
- 3.Guthikonda A, Cotton CC, Madanick RD, et al. Clinical Outcomes Following Recurrence of Intestinal Metaplasia After Successful Treatment of Barrett’s Esophagus With Radiofrequency Ablation. Am J Gastroenterol 2017;112:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotton CC, Wolf WA, Overholt BF, et al. Late Recurrence of Barrett’s Esophagus After Complete Eradication of Intestinal Metaplasia is Rare: Final Report From Ablation in Intestinal Metaplasia Containing Dysplasia Trial. Gastroenterology 2017;153:681–688 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology 2013;145:79–86 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sami SS, Ravindran A, Kahn A, et al. Timeline and location of recurrence following successful ablation in Barrett’s oesophagus: an international multicentre study. Gut 2019;68:1379–1385. [DOI] [PubMed] [Google Scholar]
- 7.Wolf WA, Pasricha S, Cotton C, et al. Incidence of Esophageal Adenocarcinoma and Causes of Mortality After Radiofrequency Ablation of Barrett’s Esophagus. Gastroenterology 2015;149:1752–1761.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaheen NJ, Falk GW, Iyer PG, et al. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol 2016;111:30–50; quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quine MA, Bell GD, McCloy RF, et al. Prospective audit of upper gastrointestinal endoscopy in two regions of England: safety, staffing, and sedation methods. Gut 1995;36:462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee ASoP, Ben-Menachem T, Decker GA, et al. Adverse events of upper GI endoscopy. Gastrointest Endosc 2012;76:707–18. [DOI] [PubMed] [Google Scholar]
- 11.Cotton CC, Haidry R, Thrift AP, et al. Development of Evidence-Based Surveillance Intervals After Radiofrequency Ablation of Barrett’s Esophagus. Gastroenterology 2018;155:316–326 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotton CC, Wolf WA, Overholt BF, et al. Late Recurrence of Barrett’s Esophagus After Complete Eradication of Intestinal Metaplasia is Rare: Final Report From Ablation in Intestinal Metaplasia Containing Dysplasia Trial. Gastroenterology 2017;153:681–688.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta M, Iyer PG, Lutzke L, et al. Recurrence of esophageal intestinal metaplasia after endoscopic mucosal resection and radiofrequency ablation of Barrett’s esophagus: results from a US Multicenter Consortium. Gastroenterology 2013;145:79–86.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komanduri S, Kahrilas PJ, Krishnan K, et al. Recurrence of Barrett’s Esophagus is Rare Following Endoscopic Eradication Therapy Coupled With Effective Reflux Control. Am J Gastroenterol 2017;112:556–566. [DOI] [PubMed] [Google Scholar]
- 15.Shaheen NJ, Kim HP, Bulsiewicz WJ, et al. Prior fundoplication does not improve safety or efficacy outcomes of radiofrequency ablation: results from the U.S. RFA Registry. J Gastrointest Surg 2013;17:21–8; discussion p 28–9. [DOI] [PubMed] [Google Scholar]
- 16.Krishnamoorthi R, Singh S, Ragunathan K, et al. Risk of recurrence of Barrett’s esophagus after successful endoscopic therapy. Gastrointest Endosc 2016;83:1090–1106.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaheen NJ et al. , Upcoming ACG Clinical Guideline when citable [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


