Abstract
Background:
It is unclear whether programmed death ligand-1 (PD-L1) expression is prognostic or predictive of immunotherapy benefit among stage III non-small-cell lung cancer (NSCLC) patients treated with definitive chemoradiation and adjuvant durvalumab.
Methods:
We determined pre-treatment tumor PD-L1 expression for 312 patients with stage III NSCLC treated with definitive chemoradiation and at least one dose of adjuvant durvalumab between November 2017 and April 2021 across the national Veterans Health Administration. Progression-free survival (PFS) and overall survival (OS) in PD-L1 expression subgroups (<1%, 1–49%, and 50–100%) were compared with 994 patients with stage III NSCLC treated without adjuvant durvalumab from 2015 to 2016.
Results:
PD-L1 expression was <1%, 1–49%, and 50–100% in 109 (34.9%), 96 (30.7%), and 107 (34.3%) patients, respectively. Increasing PD-L1 expression was associated with longer PFS (adjusted hazard ratio [aHR] 0.84 per 25% absolute increase in expression, 95% CI 0.75-0.94, p=0.003) and OS (aHR 0.86 per 25% absolute increase in expression, 95% CI 0.74–0.99, p=0.036). Compared to the no-durvalumab group, PFS was longer for PD-L1 50–100% (aHR 0.44, 95% CI 0.32–0.60, p<0.001) and PD-L1 1–49% (aHR 0.64, 95% CI 0.47–0.86, p=0.003) but not PD-L1 <1% (aHR 0.84, 95% CI 0.64–1.10, p=0.19). Similar results were found for OS, with no significant difference between the no-durvalumab group and PD-L1 <1% (aHR 0.81, 95% CI 0.58–1.13, p=0.22).
Conclusion:
Increasing tumor PD-L1 expression is prognostic for PFS and OS among patients with stage III NSCLC treated with adjuvant durvalumab, and patients with PD-L1 expression <1% may have limited benefit from adjuvant durvalumab.
Introduction
Adjuvant durvalumab, a selective antibody targeting programmed death ligand (PD-L1), has become the standard of care after definitive concurrent chemoradiation for many patients with stage III non-small-cell lung cancer (NSCLC) patients on the basis of the PACIFIC trial which demonstrated a large progression-free survival (PFS) and overall survival (OS) benefit in the intention-to-treat population.1–3 However, an unplanned post-hoc analysis of PACIFIC by tumor PD-L1 expression suggested no OS benefit for patients with PD-L1 expression <1%.4 This result led to the European Medicines Agency (EMA) limiting approval to patients with ≥1% PD-L1 expression. There is significant controversy within the thoracic oncology community as to whether clinical practice should be based on this study given the unplanned and underpowered nature of the analysis.5 In this study, we sought to evaluate the prognostic and predictive value of tumor PD-L1 expression among a cohort of real-world patients treated with definitive chemoradiation and adjuvant durvalumab and compare survival outcomes of PD-L1 groups to patients treated with chemoradiation alone.
Methods and Materials
Data source.
We identified lung cancer patients using the Department of Veterans Affairs Informatics and Computing Infrastructure (VINCI). VINCI is an informatics platform that facilitates access to patient-level electronic health record information and administrative data for all veterans within the VA healthcare system. VINCI also incorporates tumor registry data uploaded from individual VA sites; these data are gathered by trained registrars. This study was approved by the local institutional review board and STROBE reporting guidelines were followed.
Patient selection.
We included patients with histologically confirmed stage III NSCLC (AJCC 8th edition) treated with definitive chemoradiation and who received at least one dose of adjuvant durvalumab between November 2017 to April 2021, as previously described.6,7 Adjuvant durvalumab infusion dates were identified by intravenous infusion records and manually confirmed by chart review. Staging and treatment data were supplemented with tumor registry data where available. For comparison of outcomes to patients treated without durvalumab (“no durvalumab” group), we identified a cohort of stage III NSCLC (AJCC 7th edition) patients treated with definitive chemoradiation alone between January 2015 and December 2016. These patients were identified through tumor registry treatment and staging records.
Outcomes and covariates.
The primary outcome measures were progression-free survival (PFS) and overall survival (OS). Date of radiographic progression was determined by manual review of radiological reports by a licensed physician (MDG and KS). Surveillance scans performed before the date of durvalumab initiation were included in this review, and patients were excluded if they progressed before the date of durvalumab start. Date of death was obtained from the VA Vital Status File (drawn from Medicare, Social Security Administration, and the internal VA death registry) and supplemented with the VA Master Patient Index for more recent deaths.
We obtained tumor PD-L1 expression of the original pre-treatment diagnostic specimen primarily through review of pathology reports (79% of patients) or through manual review of oncologist clinical notes when the original pathology report was unavailable (21%). Of 424 patients initially identified with known PD-L1 status, we excluded 21 patients whose PD-L1 status was determined on tissue samples from metastatic or recurrent lesions. As PD-L1 expression is often ascertained at the time of progression to direct salvage therapy, to mitigate any associated selection bias we excluded an additional 91 patients for whom PD-L1 was reported on the pre-treatment diagnostic specimen greater than 30 days after starting durvalumab. For 34 patients (10.9%) whose PD-L1 results were expressed as a range (e.g. 50–60%), we imputed the midpoint value in the primary analyses (e.g. 55%). In sensitivity analyses this was varied to impute the low or high end of the range.
Demographics including race, sex, and age were obtained through the Master Patient Index. Charlson Comorbidity Index (CCI)8,9 was calculated from inpatient and outpatient ICD-10 diagnosis codes in the year before durvalumab start (or the proxy durvalumab start date described below for no-durvalumab group). Smoking status was obtained through Health Factors data. Concurrent chemotherapy regimen was obtained through intravenous infusion records and supplemented with tumor registry data where available. Use of salvage immunotherapy was determined with intravenous infusion records. The number of durvalumab infusions and reasons for durvalumab discontinuation (classified as completion of planned therapy, progression, immune-related adverse event [irAE], or other) were obtained through manual review of physician notes. Patients were categorized as having durvalumab-related toxicity if the toxicity was possibly, probably, or definitely related to durvalumab in the judgement of the management outpatient oncologist or inpatient physician.
Statistical analysis.
Differences in baseline characteristics were assessed with the chi-square test for categorical variables and the t-test for continuous variables. OS and PFS estimates were generated with the Kaplan-Meier method and were compared between groups with the log-rank test in univariable analyses. Adjusted survival analyses were performed with multivariable Cox regression adjusting for PD-L1 expression (continuous [per 25% absolute increase] or categorical [<1% vs 1–49% vs 50–100%]), age (continuous, per 10 years), sex (male vs. female), race (African American, Caucasian, or other/unknown), smoking status (current, former, never, or unknown), CCI (0–2, 3–5, 6–8, or 9+), AJCC summary stage (IIIA, IIIB, IIIC, or III not otherwise specified), concurrent chemotherapy regimen (carboplatin-paclitaxel vs. other) and histology (adenocarcinoma, squamous cell carcinoma, or other). In the analysis of OS, post-baseline initiation of salvage immunotherapy was incorporated as a time-dependent binary covariate. 5 patients in the PD-L1 groups and 102 patients in the no-durvalumab group had missing progression data and were excluded from the PFS analyses. Statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC) and R v4.1 (R Core Team, Vienna, Austria).
For patients treated with durvalumab, survival time was measured from the first dose of durvalumab to the date of death from any cause (for OS) or to disease progression or death from any cause (for PFS). For the no-durvalumab group, survival time was measured from the date of radiation start plus 84 days, which was the median time from radiation start to durvalumab start among the PD-L1 groups. Patients in the no-durvalumab group who progressed or died prior to this timepoint were excluded (n=48) to reflect the eligibility criteria of PACIFIC, which required no evidence of progression after chemoradiation before starting durvalumab.1
To test the sensitivity of the results to this choice of survival dates, we performed a 90-day landmark analysis in which we measured survival from the date of radiation completion plus 90 days, excluded patients who died or progressed before this date, and included durvalumab patients who started durvalumab within 90 days of radiation completion; this left 215 durvalumab patients and 779 no-durvalumab patients who were analyzable. In all survival analyses, patients were censored at the date of last known follow-up, defined as the most recent encounter with a VA provider. Patients with ongoing follow-up past April 15, 2021 were administratively censored at that time.
Results
Patient characteristics.
Among 985 patients with stage III NSCLC treated with concurrent chemoradiation and at least 1 dose of durvalumab, baseline PD-L1 expression was available in 312 patients (31.6%). Among these, PD-L1 expression was <1%, 1–49%, and 50–100% in 109 (34.9%), 96 (30.7%), and 107 (34.3%) patients, respectively. There were no significant differences among PD-L1 groups in demographics, comorbidity, or cancer stage (Table 1). There were no significant differences in durvalumab treatment tolerance, with similar rates of durvalumab discontinuation due to irAE (18.4% in PD-L1 <1% vs. 18.8% in PD-L1 1–49% vs. 14.0 % in PD-L1 50–100%, p=0.62), median number of durvalumab infusions (10 in PD-L1 <1% vs. 10 in PD-L1 1–49% vs. 13 in PD-L1 50–100%, p=0.09), and rates of completion of intended durvalumab course (23.9% in PD-L1 <1% vs. 25.0% in PD-L1 1–49% vs 33.6% in PD-L1 50–100%, p=0.22).
Table 1.
Characteristics of the sample.
| Variable | PD-L1 <1% | PD-L1 1-49% | PD-L1 50-100% | No durvalum ab | p-value* | p-value† | |
|---|---|---|---|---|---|---|---|
| N | 109 | 96 | 107 | 994 | |||
| Age, median in years (IQR) | 69 (64-73) | 69 (64-73) | 69 (63-72) | 68 (64-71) | 0.98 | 0.82 | |
| Race, n (%) | African American | 29 (26.6) | 18 (18.8) | 26 (24.3) | 163 (16.4) | 0.64 | 0.072 |
| Other/unknown | 5 (4.59) | 5 (5.21) | 3 (2.80) | 61 (6.14) | |||
| Caucasian | 75 (68.8) | 73 (76.0) | 78 (72.9) | 770 (77.5) | |||
| Sex, n (%) | Female | 7 (6.42) | 2 (2.08) | 6 (5.61) | 26 (2.62) | 0.31 | 0.064 |
| Male | 102 (93.6) | 94 (97.9) | 101 (94.4) | 968 (97.4) | |||
| CCI, n (%) | 0-2 | 16 (14.7) | 13 (13.5) | 11 (10.3) | 221 (22.2) | 0.46 | <0.001 |
| 3-5 | 29 (26.6) | 34 (35.4) | 26 (24.3) | 378 (38.0) | |||
| 6-8 | 13 (11.9) | 13 (13.5) | 17 (15.9) | 125 (12.6) | |||
| 9+ | 51 (46.8) | 36 (37.5) | 53 (49.5) | 270 (27.2) | |||
| Smoking, n (%) | Current | 42 (38.5) | 44 (45.8) | 56 (52.3) | 431 (43.4) | 0.79 | 0.03 |
| Former | 52 (47.7) | 29 (30.2) | 37 (34.6) | 338 (34.0) | |||
| Never | 7 (6.42) | 14 (14.6) | 7 (6.54) | 101 (10.2) | |||
| Unknown | 8 (7.34) | 9 (9.38) | 7 (6.54) | 124 (12.5) | |||
| Stage, n (%) | IIIA | 54 (49.5) | 46 (47.9) | 52 (48.6) | 667 (67.1) | 0.79 | <0.001 |
| IIIB | 40 (36.7) | 39 (40.6) | 47 (43.9) | 327 (32.9) | |||
| IIIC | 13 (11.9) | 9 (9.38) | 6 (5.61) | . ( . ) | |||
| III NOS | 2 (1.83) | 2 (2.08) | 2 (1.87) | . ( . ) | |||
| Concurrent chemotherapy, n (%) | Carboplatin/paclitax el | 77 (70.6) | 69 (71.9) | 85 (79.4) | 705 (70.9) | 0.45 | <0.001 |
| Cisplatin/etoposide | 9 (8.26) | 5 (5.21) | 4 (3.74) | 92 (9.26) | |||
| Platinum/pemetrex ed | 9 (8.26) | 13 (13.5) | 10 (9.35) | 6 (0.60) | |||
| Other/unknown | 14 (12.8) | 9 (9.38) | 8 (7.48) | 191 (19.2) | |||
| Histology | Adenocarcinoma | 41 (37.6) | 48 (50.0) | 39 (36.5) | 341 (34.3) | 0.31 | <0.001 |
| Other | 4 (3.67) | 2 (2.08) | 4 (3.74) | 129 (13.0) | |||
| Squamous cell carcinoma | 64 (58.7) | 46 (47.9) | 64 (59.8) | 524 (52.7) | |||
| Reason for durvalumab discontinuation | Completed | 26 (23.9) | 24 (25.0) | 36 (33.6) | . ( . ) | 0.5 | |
| irAE | 20 (18.4) | 18 (18.8) | 15 (14.0) | . ( . ) | |||
| Progression | 25 (22.9) | 23 (24.0) | 19 (17.8) | . ( . ) | |||
| Ongoing | 19 (17.4) | 9 (9.38) | 15 (14.0) | . ( . ) | |||
| Other | 19 (17.4) | 22 (22.9) | 22 (20.6) | . ( . ) | |||
| Completed durvalumab cycles, median (IQR) | 10 (4-17) | 10 (3-20) | 13 (6-24) | . ( . ) | 0.09 |
Comparison of PD-L1 groups only.
Comparison of all groups.
Prognostic value of PD-L1 expression among durvalumab-treated patients.
Median follow-up among censored patients was 19 months for the OS analysis and 18 months for the PFS analysis. In unadjusted analyses, patients with higher tumor PD-L1 expression showed longer PFS (24 month estimates: 29.3% in PD-L1 <1% vs. 43.5% in PD-L1 1–49% vs 57.6% in PD-L1 50–100%, p=0.006 by log-rank; Figure 1A). OS was similar for the <1% and 1–49% groups (24 month estimates: 54.4% in PD-L1 <1% vs. 56.2% in PD-L1 1–49% vs 73.3% in PD-L1 50–100%, p=0.14 by log-rank; Figure 1B). In multivariable Cox regression, increasing PD-L1 expression was associated with longer PFS when analyzed as a continuous variable (adjusted hazard ratio [aHR] 0.84 per 25% absolute increase in expression, 95% CI 0.75–0.94, p=0.003). Compared to the <1% group, the 50–100% group showed longer PFS (aHR 0.51, 95% CI 0.34–0.76, p=0.001), and the 1–49% group trended toward longer PFS (aHR 0.70, 95% CI 0.47–1.03, p=0.07; Table 2). Increasing PD-L1 expression was associated with longer OS as a continuous variable (aHR 0.86 per 25% absolute increase in expression, 95% CI 0.74–0.99, p=0.036). Compared to the <1% group, the 50–100% group showed longer OS (aHR 0.57, 95% CI 0.35–0.94, p=0.028) though the 1–49% group did not (aHR 0.75, 95% CI 0.46-1.22, p=0.24; Table 2). These results were unchanged in sensitivity analyses imputing the low or high end of the PD-L1 expression range.
Figure 1. Progression-free and overall survival by tumor PD-L1 expression.
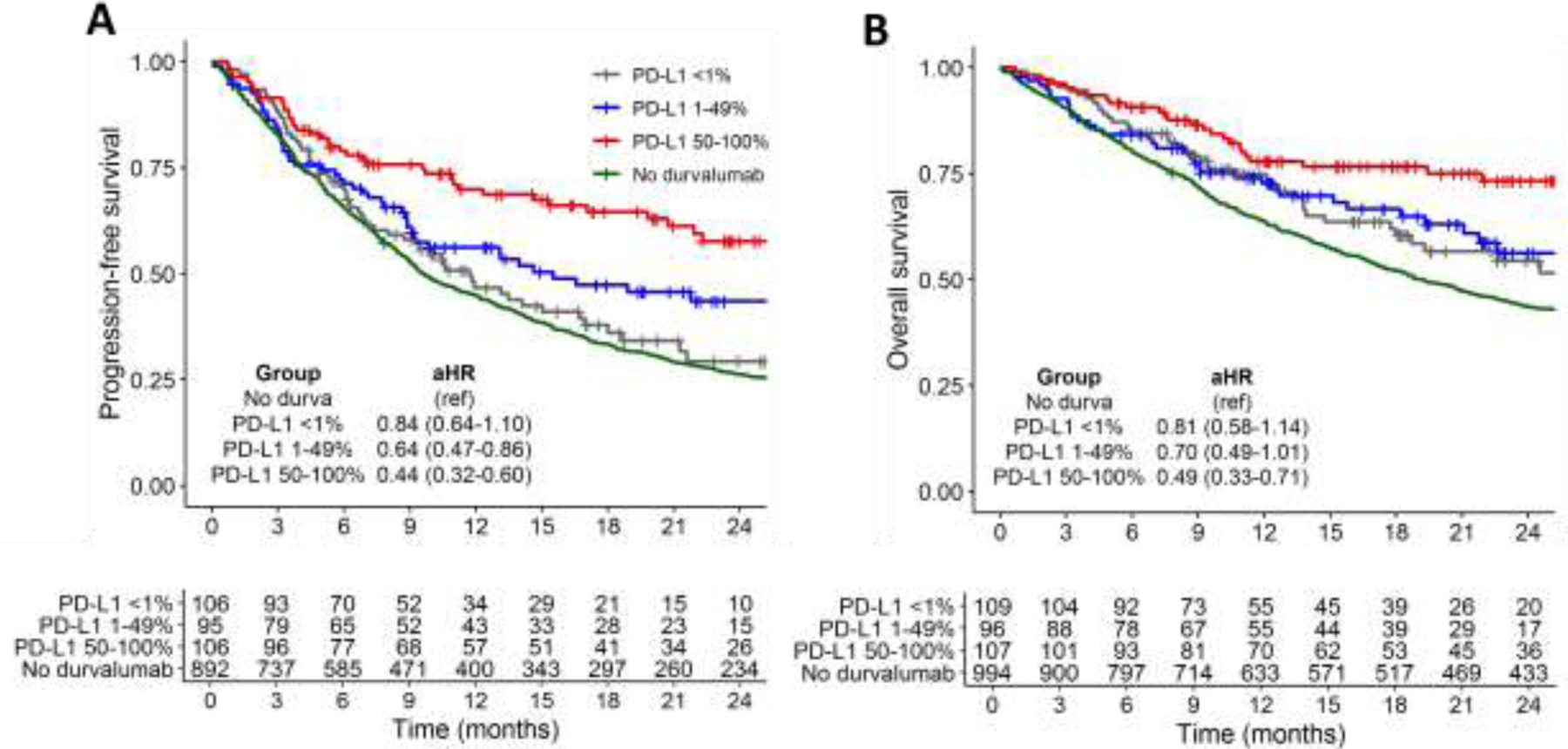
Unadjusted Kaplan-Meier curves for (A) progression-free survival, and (B) overall survival. Adjusted hazard ratio estimates (aHRs) for PD-L1 subgroups relative to the no-durvalumab group are generated from multivariable Cox regression models and shown with 95% confidence intervals.
Table 2.
Multivariable Cox regression for progression-free survival and overall survival among all patients with known PD-L1 status.
| PFS | OS | |||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| PD-L1 expression | <1% | Ref | Ref | Ref | Ref | |
| 1-49% | 0.70 (0.47-1.03) | 0.07 | 0.75 (0.46-1.22) | 0.24 | ||
| 50-100% | 0.51 (0.34-0.76) | 0.001 | 0.57 (0.35-0.94) | 0.028 | ||
| Age (per 10 years) | 1.00 (0.98-1.03) | 0.82 | 1.36 (0.96-1.93) | 0.08 | ||
| Male | 8.16 (1.13-59.1) | 0.04 | 4.21 (0.68-26.2) | 0.12 | ||
| Race | African American | Ref | Ref | Ref | Ref | |
| Caucasian | 1.19 (0.79-1.79) | 0.42 | 1.26 (0.75-2.13) | 0.38 | ||
| Other/unknown | 1.17 (0.54-2.51) | 0.69 | 1.44 (0.60-3.46) | 0.41 | ||
| Smoking | Current | Ref | Ref | Ref | Ref | |
| Former | 1.02 (0.70-1.47) | 0.93 | 0.94 (0.60-1.47) | 0.78 | ||
| Never | 1.04 (0.57-1.90) | 0.90 | 0.84 (0.37-1.92) | 0.67 | ||
| Unknown | 0.93 (0.47-1.80) | 0.82 | 0.53 (0.18-1.52) | 0.24 | ||
| Stage | IIIA | Ref | Ref | Ref | Ref | |
| IIIB | 1.30 (0.92-1.85) | 0.14 | 1.63 (1.05-2.53) | 0.029 | ||
| IIIC | 1.68 (0.98-2.88) | 0.06 | 1.35 (0.66-2.77) | 0.4 | ||
| III NOS | 0.68 (0.12-4.03) | 0.67 | 0.40 (0.05-3.14) | 0.4 | ||
| Chemotherapy | Other/unknown | Ref | Ref | Ref | Ref | |
| Carboplatin / paclitaxel | 0.97 (0.66-1.42) | 0.87 | 0.97 (0.59-1.57) | 0.9 | ||
| Histology | Adenocarcinoma | Ref | Ref | Ref | Ref | |
| Squamous cell carcinoma | 0.68 (0.48-0.95) | 0.03 | 0.73 (0.48-1.11) | 0.15 | ||
| Other | 0.85 (0.23-3.14) | 0.81 | 1.58 (0.38-6.55) | 0.5 | ||
| CCI | 0-2 | Ref | Ref | Ref | Ref | |
| 3-5 | 0.99 (0.59-1.67) | 0.97 | 1.18 (0.61-2.27) | 0.6 | ||
| 6-8 | 1.13 (0.62-2.09) | 0.69 | 1.08 (0.48-2.41) | 0.9 | ||
| 9+ | 0.94 (0.57-1.56) | 0.82 | 0.93 (0.49-1.77) | 0.8 |
PFS: Progression-free survival; OS: overall survival; HR: hazard ratio; CI: Confidence interval; NOS: not otherwise specified; CCI: Charlson Comorbidity Index.
Evaluation of durvalumab benefit in tumor PD-L1 expression groups.
Compared to durvalumab-treated patients with known PD-L1, patients in the no-durvalumab group (n=994) had less severe comorbidity (27.2% with CCI 9+ vs. 44.9%, p<0.001) and were more likely to have stage IIIa disease (67.1% vs 48.7%, p<0.001; Table 1). Relative to the no-durvalumab group, PFS was longer for the PD-L1 50–100% group (aHR 0.44, 95% CI 0.32–0.60, p<0.001) and the PD-L1 1–49% group (aHR 0.64, 95% CI 0.47–0.86, p=0.003) but not the PD-L1 <1% group (aHR 0.84, 95% CI 0.64–1.10, p=0.19) (Figure 1A). Similar results were found for OS, with longer survival for the PD-L1 50–100% group (aHR 0.49, 95% CI 0.33–0.71, p<0.001), a trend toward longer OS for the PD-L1 1–49% group (aHR 0.70, 95% CI 0.48–1.01, p=0.055), but no difference for the PD-L1 <1% group (aHR 0.81, 95% CI 0.58–1.13, p=0.22) (Figure 1B). These results were unchanged in the 90-day landmark sensitivity analysis; relative to the no-durvalumab group, the <1% group showed no difference in PFS (aHR 0.94, 95% CI 0.68–1.29, p=0.69) or OS (aHR 0.81, 95% CI 0.54–1.21, p=0.30), the 1-49% group showed longer PFS (aHR 0.62, 95% CI 0.43–0.89, p=0.009) and a trend toward longer OS (HR 0.64, 95% CI 0.41–1.00, p=0.05), and the 50–100% group showed both longer PFS (aHR 0.45, 95% CI 0.30–0.67, p<0.001) and OS (aHR 0.42, 95% CI 0.25–0.71, p=0.001).
Discussion
In this study of stage III NSCLC patients treated with chemoradiation and adjuvant durvalumab, we demonstrate a prognostic role of tumor PD-L1 expression, with higher levels of expression – especially ≥50% -- associated with longer PFS and OS among patients treated with durvalumab. Further, we found no significant difference in PFS or OS between durvalumabtreated patients with tumor PD-L1 <1% and patients not treated with durvalumab, suggesting that this group may derive minimal benefit from durvalumab. These results indicate that PD-L1 expression should be considered as a stratification factor in future trials of adjuvant immunotherapy and suggest a need for prospective validation of durvalumab efficacy in the PD-L1 <1% subgroup.
Tumor PD-L1 expression has been repeatedly shown to predict response to immunotherapy among patients with metastatic or recurrent NSCLC11–13 and low PD-L1 expression has been an exclusion criteria in multiple landmark immunotherapy trials.14,15 While the prognostic and predictive value of PD-L1 expression is well-established in metastatic NSCLC, it is more controversial in the adjuvant setting, where immunotherapy is intended to eradicate microscopic residual disease. Radiotherapy also affects the tumor microenvironment, modulates the systemic immune response, and induces changes in tumor PD-L1 expression as a mechanism of immune escape that are detectable in circulating tumor cells,16 all of which could affect the prognostic and predictive value of pre-treatment tumor PD-L1 expression.
The largest previous analysis of this question is a post-hoc study of PACIFIC by Paz-Ares et al. that included 451 patients with known PD-L1 status (n=278 treated with durvalumab, 136 treated with placebo) and suggested a lack of OS benefit in the PD-L1 <1% subgroup (durvalumab vs. placebo: HR 1.14, 95% CI 0.71–1.84) and similar objective response rates (durvalumab: 24.7%; placebo: 21.6%), despite a nonsignificant trend toward longer PFS (durvalumab vs. placebo: HR 0.73, 95% CI 0.48–1.11). Our results broadly confirm this finding, with no statistically significant difference in PFS or OS for the PD-L1 <1% group compared to the no-durvalumab group. Similar to the findings of Paz-Ares et al., patients in our study with PD-L1 expression ≥1% showed substantial benefit with durvalumab, and this benefit increased with higher PD-L1 expression. Our findings also echo smaller series that suggested improved prognosis with higher PD-L1 expression.17,18 Overall, our data contributes to a growing literature questioning the efficacy of adjuvant durvalumab in patients with <1% PD-L1 expression and points to a need for further validation of durvalumab benefit in this subgroup.
Our study is subject to several limitations. First, PD-L1 status was not available in the no-durvalumab cohort, precluding an analysis of durvalumab efficacy within PD-L1 strata. While a limitation, PD-L1 expression has not been shown to be strongly prognostic among patients not treated with durvalumab,19,20 and as such we would not expect our results to differ substantially if analyzed within PD-L1 expression strata. It is indeed possible that the benefit of durvalumab in the <1% group would be even smaller that we observed, given that low PD-L1 expression is paradoxically associated with better survival in many series of patients treated before the durvalumab era.19 Second, baseline PD-L1 expression was only ascertainable in a minority of patients treated with durvalumab, and it is possible that this introduced selection bias. Third, PD-L1 ascertainment was performed by multiple FDA approved methodologies, and the optimal staining approach and scoring system has yet to be determined.21,22 The comparison to the no-durvalumab group is further subject to unmeasured confounding including lack of baseline performance status, residual immortal time bias, sample size limitations, and selection biases inherent to retrospective comparisons. Finally, our study period extended across the transition from AJCC 7th edition to AJCC 8th edition staging; this transition included stage migration of a subset of previously stage IIB patients who were reclassified as stage III in the AJCC 8th edition.23 Disproportionate inclusion of these patients in the durvalumab-treated cohort may have produced an optimistic bias in our survival estimates for PD-L1 groups relative to the nodurvalumab group.
In summary, our study supports a prognostic role of tumor PD-L1 expression in a contemporary cohort of stage III NSCLC patients treated with adjuvant durvalumab. Consistent with prior reports, our data further suggest that there may be limited benefit of durvalumab in patients with <1% PD-L1 expression. These results should be validated prospectively.
Funding statement:
This work was supported by the Lung Precision Oncology Program, VA Ann Arbor Healthcare System. The funding source was not involved in data analysis or the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: G.W.S. serves an uncompensated position on the Board of Directors for the Optimal Cancer Alliance. A.K.B, K.S., L.Z., D.E., M.D.G. and N.R. do not have any conflicts of interest.
Data sharing: Patient-level data for these analyses are not available for public use.
References
- 1.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379(24):2342–2350. [DOI] [PubMed] [Google Scholar]
- 2.Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: An update from the PACIFIC trial. J Clin Oncol 2021;39(15_suppl):8511–8511. [Google Scholar]
- 3.Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol 2021;16(5):860–867. [DOI] [PubMed] [Google Scholar]
- 4.Paz-Ares L, Spira A, Raben D, et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann Oncol 2020;31(6):798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters S, Dafni U, Boyer M, et al. Position of a panel of international lung cancer experts on the approval decision for use of durvalumab in stage III non-small-cell lung cancer (NSCLC) by the Committee for Medicinal Products for Human Use (CHMP). Ann Oncol 2019;30(2):161–165. [DOI] [PubMed] [Google Scholar]
- 6.Sankar K, Bryant AK, Strohbehn GW, et al. Real World Outcomes ver-sus Clinical Trial Results of Durvalumab Maintenance in Veterans with Stage III Non-Small Cell Lung Cancer. Cancers 2022;14:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant AK, Sankar K, Strohbehn GW, et al. Timing of Adjuvant Dur-valumab Initiation Is Not Associated With Outcomes in Stage III Non-small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 00, 2022, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 10.Therneau TM, Crowson CS, Atkinson EJ. Adjusted survival curves Published online 2015.ftp://tucows.icm.edu.pl/packages/cran/web/packages/survival/vignettes/adjcurve.pdf [Google Scholar]
- 11.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387(10027):1540–1550. [DOI] [PubMed] [Google Scholar]
- 13.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387(10030):1837–1846. [DOI] [PubMed] [Google Scholar]
- 14.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375(19):1823–1833. [DOI] [PubMed] [Google Scholar]
- 15.Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376(25):2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Kim TH, Fouladdel S, et al. PD-L1 Expression in Circulating Tumor Cells Increases during Radio(chemo)therapy and Indicates Poor Prognosis in Non-small Cell Lung Cancer. Sci Rep 2019;9(1):566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desilets A, Blanc-Durand F, Lau S, et al. Durvalumab therapy following chemoradiation compared with a historical cohort treated with chemoradiation alone in patients with stage III non–small cell lung cancer: A real-world multicentre study. Eur J Cancer 2021;142:83–91. [DOI] [PubMed] [Google Scholar]
- 18.Jazieh K, Gad M, Saad AM, Pennell NA. PD-L1 expression and progression risk of stage IIINSCLC patients on durvalumab consolidation. J Clin Oncol 2020;38(15_suppl):e21070–e21070. [Google Scholar]
- 19.Li H, Xu Y, Wan B, et al. The clinicopathological and prognostic significance of PD-L1 expression assessed by immunohistochemistry in lung cancer: a meta-analysis of 50 studies with 11,383 patients. Transl Lung Cancer Res 2019;8(4):429–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brody R, Zhang Y, Ballas M, et al. PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 2017;112:200–215. [DOI] [PubMed] [Google Scholar]
- 21.O’Malley DP, Yang Y, Boisot S, et al. Immunohistochemical detection of PD-L1 among diverse human neoplasms in a reference laboratory: observations based upon 62,896 cases. Mod Pathol 2019;32(7):929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin H, Wei S, Hurt EM, et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest 2018;128(2):805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11(1):39–51. [DOI] [PubMed] [Google Scholar]


