Abstract
Background:
There are limited data on the trajectory of recovery and long-term functional outcomes after ICH. Most ICH trials have conventionally assessed outcomes at 3 months following the footsteps of ischemic stroke. The ICH Deferoxamine (i-DEF) trial assessed modified Rankin Scale (mRS) longitudinally at pre-specified timepoints from day-7 through the end of the 6-month follow-up period. We evaluated the trajectory of mRS among trial participants and examined the effect of deferoxamine (DFO) on this trajectory.
Methods:
We performed a post-hoc analysis of the i-DEF trial, a multicenter, randomized, placebo-controlled, double-blind, futility-design, phase 2 clinical trial, based on the actual treatment received. Favorable outcome was defined as mRS 0-2. A generalized linear mixed model was used to evaluate the outcome trajectory over time, as well as whether the trajectory was altered by DFO, after adjustments for randomization variables, presence of intraventricular hemorrhage and ICH location.
Results:
A total of 291 subjects were included in analysis (145 placebo and 146 DFO). The proportion of patients with mRS 0-2 continually increased from Day 7 to 180 in both groups (interaction p<0.0001 for time in main effects model), but treatment with DFO favorably altered the trajectory (interaction p=0.0010). Between day 90 and day 180, the DFO group improved (p=0.0001), whereas there was not significant improvement in the placebo arm (p=0.3005).
Conclusions:
A large proportion of patients continue to improve up to 6 months after ICH. Future ICH trials should assess outcomes past 90 days for a minimum of 6 months. In i-DEF, treatment with deferoxamine seemed to accelerate and alter the trajectory of recovery as assessed by mRS.
Registration:
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02175225
Keywords: ICH, Trajectory, Recovery, Outcome, Deferoxamine, i-DEF
Graphical Abstract
Introduction
Intracerebral hemorrhage (ICH) is a devastating condition. Compared to ischemic stroke, ICH tends to be associated with greater morbidity and disability, longer hospitalization, and higher mortality during the acute phase [1-3]. However, sparse data are available regarding the trajectory of recovery and long-term functional outcomes after ICH [3-4], partly because most ICH trials have conventionally assessed outcomes at 3 months in line with ischemic stroke. There is no specific treatment to date for ICH aside from aggressive medical management and supportive care. Better understanding of the course of recovery after ICH and finding new strategies to favorably alter its trajectory could have important public health and care-related cost-saving implications.
The ICH Deferoxamine (i-DEF) trial [5] assessed modified Rankin Scale (mRS) score longitudinally at pre-specified timepoints from day-7 through the end of the 6-month follow-up period. In this study, we evaluated the trajectory of recovery as assessed by mRS among trial participants, and examined the effect of deferoxamine (DFO) on this trajectory.
Secondary brain injury after ICH has been linked to the toxic effects of hemoglobin degradation products and iron accumulation following hemolysis of the red blood cells, oxidative stress, neuroinflammation, and disruption of blood brain barrier and edema formation [6]. DFO is an iron chelator which has diverse neuroprotective properties, including anti-oxidative stress effects by decreasing free iron’s availability for hydroxyl radical formation, inhibiting prolyl 4-hydroxylase, inducing the transcription of heme oxygenase-1, suppressing upregulation of c-Jun N-terminus Kinase (JNK) after ICH, and anti-inflammatory and anti-phagocytic effects [7]. DFO has been shown to exert beneficial effects in various experimental models of ICH [8-9]. It reduces white matter injury (WMI), demyelination, edema formation, neuronal loss, and brain atrophy; all are important determinants of short- and long-term outcomes [9-12].
Methods
This study is performed in agreement with the AHA Journals’ implementation of the Transparency and Openness Promotion Guidelines. The data that support the findings of this study will be provided to researchers upon reasonable request.
Study Design And Participants:
The i-DEF trial was a multicenter, randomized, placebo-controlled, double-blind, futility-design, phase 2 clinical trial in the United States and Canada. The trial was funded by the National Institute of Neurological Disorders and Stroke (U01 NS074425), and approved by the US Food and Drug Administration (IND #77306) and Health Canada (CTA #160713). Details of study design, methods, and results have been previously published [5]. Briefly, patients aged 18–80 years with primary, spontaneous, supratentorial ICH were recruited and randomly assigned to receive DFO (32 mg/kg per day) or placebo (saline) infusions for 3 consecutive days within 24 hours of ICH onset. The mRS score was assessed at day-7 or discharge (whichever is earlier), day-30, day-60, day-90 and day-180. All assessments were done by qualified investigators who were certified in mRS administration and masked to treatment assignment. Good outcome was defined as a dichotomized mRS score of 0-2. The trial was approved by the institutional review board at each participating site, and written informed consent was obtained from each participant or legally authorized representative according to local regulations.
A total of 294 patients were randomized in the i-DEF trial. However, the study infusions were initiated in only 291 participants. Key randomization covariates were clinical site, baseline ICH score (<3 vs. ≥3), ICH-onset-to-treatment time (≤12 h vs. >12 h), warfarin use at ICH onset (yes vs. no), National Institute of Health Scale (NIHSS) score (≤10 vs. >10), and volume of ICH (<10 mL vs. ≥10 mL) at presentation.
Statistical analyses:
Although the primary analysis for the i-DEF trial was based on intent-to-treat population, all analyses in the current study were performed according to treatment actually received (i.e., as treated) in order to assess the true treatment effects. Baseline characteristics were compared among the two treatment groups based on the treatment received,which differs from randomized treatment for 4 subjects. Data are presented as proportion, mean with standard deviation (SD) or median with interquartile range (IQR), as appropriate. Baseline characteristics were compared among DFO- and placebo-treated subjects, using the chi-square test for categorical variables, and analysis of variance or the Kruskal-Wallis test for continuous variables as appropriate. The primary outcome measure was good clinical outcome, defined as a dichotomized mRS score of 0 – 2, and was assessed at Day 7, Day 30, Day 60, Day 90, and Day 180. A longitudinal analysis of the repeated mRS scores was performed, adjusted for the randomization covariates included in the primary analysis (ICH onset-to-treatment time, NIHSS score, and ICH volume) as well as presence of intraventricular hemorrhage (IVH) and ICH location. Two generalized linear mixed models with logit link were constructed: a main effects model and an interaction model. The main effects model was used to evaluate the trajectory of mRS across treatments; the interaction model was used to evaluate a differential effect of time according to treatment received, with focus on the factors of time, treatment received, and the interaction between time and treatment received. The mixed model accommodates the correlation between repeated measures taken within a subject and uses all available data on each subject.
Results
A total of 291 participants received study infusions in i-DEF, with 146 receiving DFO and 145 receiving placebo. Table 1 summarizes the baseline characteristics of the two treatment groups. Patients treated with DFO had a higher prevalence of deep non-thalamic ICH, while patients treated with placebo had a higher prevalence of hypertension, deep thalamic ICH, and IVH. A total of 31/291 (10.65%) participants had missing mRS assessments at various timepoints (Supplemental Table S1). Supplemental Table S2 summarizes the characteristics of these patients, overall and by treatment group. Overall, patients with missing outcome data were older, had a lower prevalence of lobar ICH, lower mean ICH volume, and were less likely to be taking a statin. Among patients treated with placebo, 14 (9.52%) had one or more missing outcome assessment; 17 (11.80%) DFO-treated patients had a missing outcome assessment. Among patients with missing outcome assessments, DFO-treated patients were younger, and had a higher median NIHSS score and blood glucose at presentation, lower prevalence of pulmonary disease, larger median ICH volume, and lower prevalence of IVH and mean IVH volume.
Table 1:
Baseline Clinical and Demographic Characteristics
| Total n = 291 | DFO (n= 146) | PLACEBO (n=145) | |
|---|---|---|---|
| Age (years) | 59 (51 - 70) | 62 (54 - 70) | |
| Female | 57 (39.0%) | 55 (37.9%) | |
| Race | |||
| White | 83 (56.8%) | 98 (67.6%) | |
| Black | 31 (21.2%) | 33 (22.8%) | |
| Asian | 25 (17.1%) | 12 (8.3%) | |
| Native American or Alaskan | 2 (1.4%) | 0 (0%) | |
| Native Hawaiian/Pacific Islander | 3 (2.1%) | 1 (0.7%) | |
| Unknown/Multiple | 2 (1.4%) | 1 (0.7%) | |
| Hispanic or Latino | 22 (15.1%) | 26 (17.9%) | |
| Glasgow Coma Scale Score) 1 | 14 (12 - 15) | 14 (12 - 15) | |
| NIHSS 2 | 13 (8 - 16) | 13 (8.5 - 19) | |
| Intracerebral hemorrhage score <=2 | 140 (95.9%) | 137 (94.5%) | |
| Medical History | |||
| Hypertension | 113 (77.4%) | 124 (85.5%) | |
| Diabetes mellitus | 32 (21.9%) | 43 (29.7%) | |
| Cardiac disease | 15 (10.3%) | 14 (9.7%) | |
| Pulmonary disease | 30 (20.5%) | 27 (18.6%) | |
| Previous ischemic stroke or TIA | 10 (6.8%) | 16 (11.0%) | |
| Previous ICH | 7 (4.8%) | 3 (2.1%) | |
| Previous drug use | |||
| Antiplatelet agents | 42 (28.8%) | 49 (33.8%) | |
| Warfarin | 1 (0.7%) | 1 (0.7%) | |
| Antihypertensives | 120 (82.2%) | 124 (85.5%) | |
| Statins | 38 (26.0%) | 36 (24.8%) | |
| Modified Rankin Scale score before ICH | |||
| Score of 0 | 132 (90.4%) | 128 (88.3%) | |
| Score of 1 | 14 (9.6%) | 17 (11.7%) | |
| Blood glucose (mg/dL) | 133.5 (114 - 155) | 138 (118 - 164) | |
| Blood pressure (mmHg) | |||
| Systolic | 135 (125 - 148) | 138 (125 - 148) | |
| Diastolic | 71 (63 - 80) | 70 (59 - 80) | |
| Time from ICH to treatment (hrs) | |||
| Median (IQR) | 17.6 (10.8 - 22.5) | 19.5 (11.2 - 22.8) | |
| ≤12 | 44 (30.1%) | 46 (31.7%) | |
| >12 | 102 (69.9%) | 99 (68.3%) | |
| ICH location | |||
| Lobar | 26 (17.8%) | 33 (22.8%) | |
| Deep (thalamic) | 46 (31.5%) | 60 (41.4%) | |
| Deep (non-thalamic) | 74 (50.7%) | 52 (35.9%) | |
| ICH volume (mL) | 12.4 (6.1 – 24.7) | 13.0 (6.7 – 26.7) | |
| Intraventricular hemorrhage 3 | |||
| n | 52 (35.6%) | 68 (46.9%) | |
| Volume (mL) | 3.7 (9.8) | 4.7 (10.0) | |
6 subjects missing GCS (3 DFO, 3 Placebo)
2 subjects missing NIHSS (1 DFO, 1 Placebo)
Based on volumetric measurements by a central reader
Data are median (IQR), n (%), or mean (SD).
In accordance with the CONSORT guidelines on the reporting of randomized clinical trials, statistical tests of baseline characteristics were not conducted; instead, the clinical relevance of the observed imbalances is considered [13]. Table 1 presents baseline characteristics by treatment received,which differs from randomized treatment for 4 subjects.
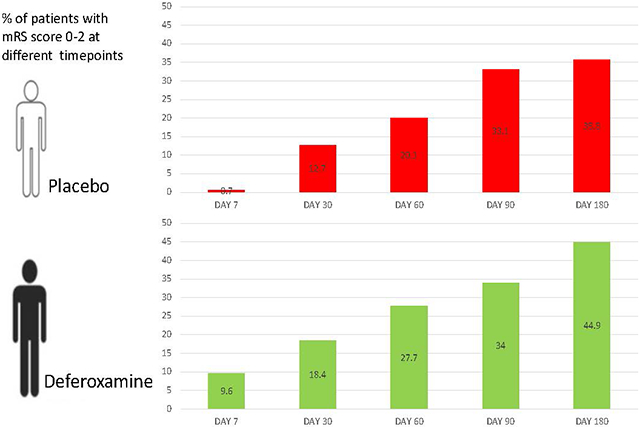
Figure 1 displays the proportions of subjects achieving favorable outcome in the two treatment groups at each assessment timepoint. The proportion of patients with mRS 0-2 continually increased from Day 7 to Day 180 in both groups (p<0.0001 for time in main effects model), but treatment with DFO altered the trajectory (p=0.0010 for interaction). Treatment with DFO accelerated the speed of recovery; a significantly greater proportion of DFO-treated patients achieved favorable outcome by day 7 than placebo (OR 17.2, 95% CI 2.0-148.1, p= 0.0096). Between 90 and 180 days, DFO-treated patients demonstrated a statistically significant increase in the proportion of subjects with good outcomes (OR 1.8, 95% CI 1.3-2.4, p= 0.0001), whereas the placebo group did not (OR 1.2, 95% CI 0.9-1.5, p= 0.3005).
Figure 1:
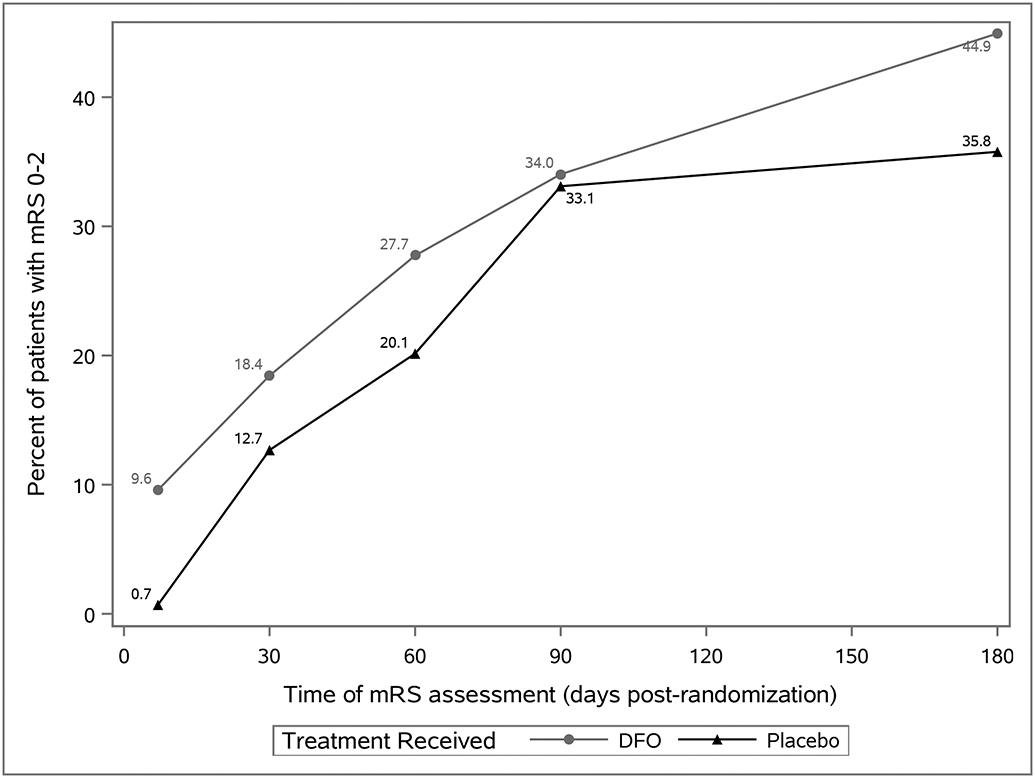
Favorable Outcome (mRS 0-2) Over Time by Treatment Received
Figure 2 displays the proportions of subjects with different ordinal mRS scores at various time points. The proportions of placebo-treated patients with mRS scores 4-6 decreased from 43.5% at 90 days to 32.1% at 180 days (vs. 36.1% to 27.9% in the DFO group) . Between 90 and 180 days, both DFO-treated and placebo groups demonstrated a statistically significant decrease in the proportion of subjects with mortality or disability (DFO-treated OR 0.6, 95% CI 0.5-0.8, p= 0.0015), placebo OR 0.5, 95% CI 0.4-0.7, p< 0.0001).
Figure 2:
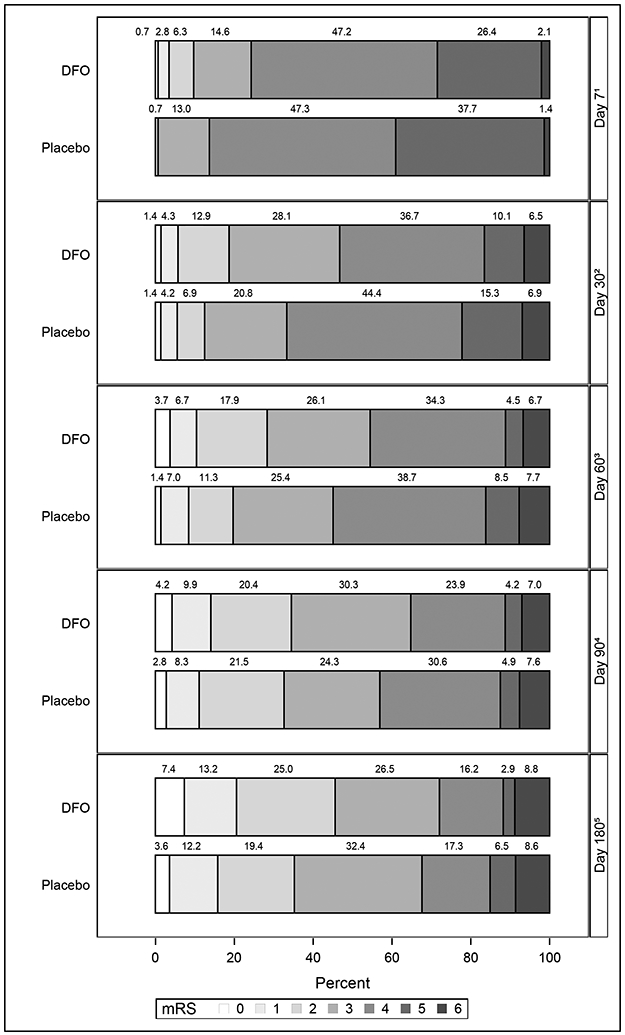
Distribution of mRS Scores Over Time
Discussion
In this post-hoc analysis of the i-DEF trial, we found that: 1) the proportions of patients achieving mRS 0-2 continually increased from day-7 to day-180 in both DFO and placebo groups, 2) the proportion of patients with favorable outcome (mRS 0-2) was higher among DFO-treated subjects than placebo at 7 days; and 3) treatment with DFO altered the trajectory for mRS 0-2, with the DFO group demonstrating additional improvement between day-90 and day-180.
These findings have potential important implications. Most clinical trials in ICH traditionally assess functional outcomes after 90 days. However, accumulating reports over the last few years have indicated that recovery after hemorrhagic stroke may take longer than 90 days, and that many survivors of hemorrhagic stroke continue to improve past 3 months and up to one year [14-17]. There are plausible pathophysiological explanations for these reports. The secondary effects of the mass effect from ICH, associated edema and increased intracranial pressure, intraventricular extension, and hydrocephalus often require several months to resolve and to translate into improvement in resulting disability. Our findings that a large proportion of i-DEF participants continued to improve up to 6 months after ICH add to this growing body of evidence, and suggest that future trials in ICH should assess outcomes at 6 months at a minimum, and preferably longer if feasible, to capture the full extent of recovery [18].
The i-DEF trial was a futility design study. Although the primary futility analysis found treatment with DFO futile for improving good clinical outcomes at day-90, DFO was not declared futile in a pre-specified secondary futility analysis at day-180, with an adjusted absolute risk difference of 8.6% in favor of DFO [5]. The current longitudinal analysis of mRS showing that treatment with DFO favorably alters the trajectory of recovery and that the observed improvements in mRS 0-2 with DFO were not attributed to increased mortality or disability in the placebo group during this time period provides additional encouraging results. There are several potential putative mechanisms by which DFO can influence late recovery. DFO has been shown to reduce brain atrophy, chronic hydrocephalus, and to ameliorate cognitive function in animal models of ICH and subarachnoid hemorrhage [10-12, 19]. Furthermore, iron and neuroinflammation play important roles in WMI after ICH by activating JNK signaling pathway which leads to the production of pro-inflammatory cytokines and can induce apoptosis in oligodendrocytes and demyelination in the white matter. DFO reduces JNK positive cells in white matter and reduces white matter necrosis factor alpha and receptor-interacting protein kinase-1 levels and attenuates white matter edema and chronic WMI after experimental ICH [11, 19]. In a prospective study of 167 ICH patients, 37% of patients experienced cognitive decline during a median follow-up period of 4 years, and the severity of WMI and cortical atrophy were associated with cognitive decline [20]. Cognitive decline may alter long-term functional outcomes. We also speculate that treatment with DFO may afford early protection against the cascade of events involved in secondary brain injury, but the severity of injury after ICH may mask some of the early benefits of therapy, and that the benefit does not become fully apparent until adverse consequences of mass effect, intraventricular hemorrhage, and hydrocephalus are resolved over time.
In addition, our finding that a greater proportion of DFO-treated patients than placebo achieved mRS 0-2 at day 7 is relevant. Shorter time to achieve functional independence after ICH is likely to result in shorter hospitalization, lesser need for inpatient rehabilitation, decreased burden on caregivers, and increased probability of rapid return of ICH survivors to their pre-ICH activities. These factors can have important positive cost-savings, lost wages, and societal implications. Time to reach favorable outcome is rarely employed as an outcome measure in ICH trials. This requires more scrutiny in future studies.
Our study has limitations which must be acknowledged. Our analyses are post-hoc in nature, and therefore, are only hypothesis-generating and require further investigations. We also performed multiple tests; given the nature of this post-hoc exploratory analysis, a multiplicity correction was not applied. Lastly, some patients in both groups had missing outcome assessments at various timepoints. However, we performed a sensitivity analysis to assess the impact of missing data by adding an indicator variable for subjects with any missing outcome assessments and relevant interactions to the interaction model [21], and the interaction terms that included the missing data indicator were not significant, indicating that the relationship of outcome to treatment, time and the treatment by time interaction do not differ for subjects with missing outcome data.
Summary/Conclusion
We found that a large proportion of patients with ICH continue to improve up to 6 months after ICH and that treatment with deferoxamine seemed to accelerate and favorably alter the trajectory of recovery. These findings have important implications for future studies of therapeutic interventions targeting the secondary injury and iron-mediated toxicity in ICH. Future ICH trials should assess outcomes past 90 days for a minimum of 6 months, if not longer.
Supplementary Material
Sources of Funding
The iDEF trial was funded by the NIH/NINDS (U01 NS074425). Dr. Selim receives funding from the NIH/NINDS/NIA (U01 NS102289) and (U01 NS 120871).
Non-standard Abbreviations and Acronyms
- DFO
Deferoxamine
- ICH
Intracerebral hemorrhage
- i-DEF
The intracerebral hemorrhage deferoxamine trial
- IVH
Intraventricular hemorrhage
- mRS
Modified Rankin Scale
Footnotes
Disclosures:
Dr. Yeatts receives grant funding from the NINDS (related to and outside of the current submission) and NHLBI (outside of the current submission); compensation for editorial position with Stroke; and from Bard and Emory for DSMB service. Dr. Lioutas receives compensation as a consultant from Qmetis. Dr. Selim receives grant funding from the NINDS (related to and outside of the current work), royalties from Up to Date and Cambridge University Press. He serves on the Advisory Board of MedRhythms Inc.
References:
- 1.Krishnamurthi RV, Moran AE, Forouzanfar MH, Bennett DA, Mensah GA, Lawes CM, Barker-Collo S, Conor M, Roth GA, Sacco R, et al.; Global Burden of Diseases, Injuries, and Risk Factors 2010 Study Stroke Expert Group. The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob Heart. 2014; 9:101–106. [DOI] [PubMed] [Google Scholar]
- 2.Lee KB, Lim SH, Kim KH, Kim KJ, Kim YR, Chang WN, Yeom JW, Kim YD, Hwang BY. Six-month functional recovery of stroke patients: a multi-time-point study. Int. J. Rehab. Res 2015;38:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KB, Kim JS, Hong BY, Kim YD, Hwang BY, Lim SH. The motor recovery related with brain lesions in patients with intracranial haemorrhage. Behav Neurol. 2015; 2015: 258161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saulle M, Schambra H. Recovery and rehabilitation after intracerebral hemorrhage. Semin Neurol. 2016;36:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selim M, Foster LD, Moy CS, Xi G, Hill MD, Morgenstern LB, Greenberg SM, James ML, Singh V, Clark WM. et al. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): A multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019;18:428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tschoe C, Bushnell CD, Duncan PW, Alexander-Miller MA, Wolfe SQ. Neuroinflammation after intracerebral helorrhage and potential therapeutic targets. J Stroke. 2020;22:29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selim M Deferoxamine mesylate: a new hope for intracerebral hemorrhage – from bench to clinical trials. Stroke. 2009;40(3 Suppl):S90–1. [DOI] [PubMed] [Google Scholar]
- 8.Gu Y, Hua Y, Keep RF, Morgenstern LB, Xi G. Deferoxamine reduces intracerebral hematoma-induced iron accumulation and neuronal death in piglets. Stroke. 2009;40:2241–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okauchi M, Hua Y, Keep RF, Morgenstern LB, Schallert T, Xi G. Deferoxamine treatment for intracerebral hemorrhage in aged rats: therapeutic time window and optimal duration. Stroke. 2010;41:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni W, Okauchi M, Hatakeyama T, Gu Y, Keep RF, Xi G, Hua Y. Deferoxamine reduces intracerebral hemorrhage-induced white matter damage in aged rats. Exp Neurol. 2015;272:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Q, Gu Y, Hua Y, Liu W, Keep RF, Xi G. Deferoxamine attenuates white matter injury in a piglet intracerebral hemorrhage model. Stroke. 2014;45:290–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin Y, Li G, Sun Z, Xu X, Gu J, Gao F. Comparison of the effects of nimodipine and deferoxamine on brain injury in rat with subarachnoid hemorrhage. Behav Brain Res. 2019;367:194–200. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elboume D, Egger M, Altman DG. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katrak PH, Black D, Peeva V. Do stroke patients with intracerebral hemorrhage have a better functional outcome than patients with cerebral infarction? PMR. 2009; 1:427–433. [DOI] [PubMed] [Google Scholar]
- 15.Schepers VP, Ketelaar M, Visser-Meily AJ, de Groot V, Twisk JW, Lindeman E. Functional recovery differs between ischaemic and haemorrhagic stroke patients. J Rehabil Med. 2008; 40: 487–489. [DOI] [PubMed] [Google Scholar]
- 16.Hanley DF, Thompson RE, Muschelli J, Rosenblum M, McBee N, Lane K, Bistran-Hall A, Mayo SW, Keyl P, Gandhi D, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, openlabel, phase 2 trial. Lancet Neurol. 2016; 15: 1228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sreekrishnan A, Leasure AC, Shi FD, Hwang DY, Schindler JL, Petersen NH, Gilmore EJ, Kamel H, Sansing LH, Greer DM, et al. Functional improvement among intracerebral haemorrhage survivors up to 12 months post Injury. Neurocrit Care. 2017;27: 326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Hemorrhagic Stroke Academia Industry (HEADS) Roundtable Participants. Recommendations for clinical trials in ICH. Stroke. 2020;51:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng H, Li F, Hu R, Yuan Y, Gong G, Hu S, Feng H. Deferoxamine alleviates chronic hydrocephalus after intraventricular hemorrhage through iron chelation and Wnt1/Wnt3a inhibition. Brain Res. 2015;1602:44–52. [DOI] [PubMed] [Google Scholar]
- 20.Benedictus MR, Hochart A, Rossi C, Boulouis G, Henon H, van der Flier WM, Cordonnier C. Prognostic factors for cognitive decline after intracerebral hemorrhage. Stroke. 2015;46:2773–2778. [DOI] [PubMed] [Google Scholar]
- 21.Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychological Methods. 1997;2:64–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


