Abstract
Extracellular vesicles (EVs) are nano-sized membrane-bound particles containing biologically active cargo molecules. The production and molecular composition of EVs reflect the physiological state of parent cells, and once released into the circulation, they exert pleiotropic functions via transferring cargo contents. Thus, circulating EVs not only serve as biomarkers, but also mediators in disease processes or injury responses. In the present study, we performed a comprehensive analysis of plasma EVs from burn patients and healthy subjects, characterizing their size distribution, concentration, temporal changes, cell origins, and cargo protein contents. Our results indicated that burn injury induced a significant increase in circulating EVs, the response peaked at the time of admission and declined over the course of recovery. Importantly, EV production correlated with injury severity, as indicated by the total body surface area and depth of burn, requirement for critical care/ICU stay, hospitalization length, wound infection, and concurrence of sepsis. Burn patients with inhalation injury showed a higher level of EVs than those without inhalation injury. We also evaluated patient demographics (age and sex) and pre-existing conditions (hypertension, obesity, and smoking) and found no significant correlation between these conditions and overall EV production. At the molecular level, flow cytometric analysis showed that the burn-induced EVs were largely derived from leukocytes and endothelial cells (ECs), which are known to be activated postburn. Additionally, a high level of zona-occludens-1 (ZO-1), a major constituent of tight junctions, was identified in burn EV cargos, indicative of injury in tissues that form barriers via tight junctions. Moreover, when applied to endothelial cell monolayers, burn EVs caused significant barrier dysfunction, characterized by decreased transcellular barrier resistance and disrupted cell-cell junction continuity. Taken together, these data suggest that burn injury promotes the production of EVs containing unique cargo proteins in a time-dependent manner; the response correlates with injury severity and worsened clinical outcomes. Functionally, burn EVs serve as a potent mediator capable of reducing endothelial barrier resistance and impairing junction integrity, a pathophysiological process underlying burn-associated tissue dysfunction. Thus, further in-depth characterization of circulating EVs will contribute to the development of new prognostic tools or therapeutic targets for advanced burn care.
Keywords: extracellular vesicles, burn, inhalation injury, inflammation
Introduction
EVs are phospholipid bilayer-encapsulated particles released from various cell types via membrane budding and fission.1 These nano-sized vesicles contain abundant signaling molecules of proteins, lipids, or nucleic acids, serving as important mediators of intercellular communication.2 The size, concentration, and composition of EVs have been reported to reliably reflect the function and pathological status of their cells of origin.3 For instance, activated leukocytes secrete more EVs that are enriched in cytokines and chemokines.4 Platelet-derived phosphatidylserine-positive EVs are significantly higher in patients with coagulopathy.5 Furthermore, increased levels of junction proteins in EVs from epithelial cells are reported as an indicator of impaired epithelial barrier integrity.6 Since EVs are easily accessible from extracellular biofluids such as blood, urine, and saliva, profiling their number and cargo content could serve as a minimally invasive tool for evaluating cell/tissue function, as well as for dynamic monitoring of disease severity, stage, and treatment response.
In addition to their biomarker role, EVs function as active mediators of biological events that contribute to the progression of various diseases.7,8 The effects of EVs are directly associated with their cargo composition. Upon uptake, EVs transfer their cargo molecules to recipient cells, thereby altering the phenotype, signaling events, and function of target cells.9 Some recent studies demonstrate the involvement of EVs in regulating immune responses, coagulation, and organ/tissue function.10–12 For example, our previous study has shown that inflammation stimulates the production of EVs that are capable of promoting neutrophil adhesion and transmigration.13 Platelet-derived EVs can directly interact with coagulation factors and facilitate thrombosis.12 It is worth noting that, due to the protection of EV lipid bilayer, molecular contents in EVs are more stable than in free form, rendering their ability to exert long-lasting effects to recipient cells nearby or in distance.4,14 Thus, circulating EVs may serve as effective mediators in disease processes. In-depth analysis of circulating EVs enables a better understating of disease pathological mechanisms and facilitates the identification of potential therapeutic targets.
Burn injury represents a devastating form of trauma associated with high morbidity and mortality.15 There are approximately 1.1 million burn patients each year in the United States according to American Burn Association.16 Patients with severe burn injury often develop systemic inflammatory response syndrome, leading to multiple organ dysfunction and death.17 Evidence is emerging supporting the involvement of EVs in the pathophysiological response to burns. In burn patients, the plasma concentration of leukocyte-derived EVs is thought to be associated with mortality.18 The levels of several EV cargo proteins, such as serum amyloid A1 (SAA1) and C-reactive protein (CRP), are correlated with the length of hospital stay in female burn patients.19 In addition, burn EVs have been reported to cause macrophage activation and cytokine release.20 Given the potential contributions of EVs to injury responses, we performed a comprehensive profiling of circulating EVs in burn patients with different injury severity and complications. Compared to healthy subjects, burn patients displayed substantially increased levels of circulating EVs, correlating with the percentage of total body surface area (%TBSA) and depth of burn. This increase in plasma EVs declined over the duration of hospital stay. We also found that thermal burn accompanied with inhalation injury caused an even higher level of EV production. The levels of circulating EVs positively correlated with the length of ICU and hospital stay, as well as wound infection or concurrence of sepsis. Additionally, burn EVs contained a unique molecular signature that reflects the presence of inflammatory response, endothelial dysfunction, and barrier tissue injury. Functionally, burn EVs induced a significant reduction in endothelial barrier resistance coupled with cell-cell junction disruption.
Method
Reagents
All antibodies used are listed in Supplementary Table 1.
Study subjects and blood collection
All procedures involving human subjects were performed as per the protocol approved by the University of South Florida Institutional Review Board and according to the principles of the Declaration of Helsinki. After obtaining informed consent, blood was collected by venipuncture from 32 burn patients and 17 healthy donors (no pre-existing medical conditions). Burn patients with history of autoimmune disorders, pre-existing inflammatory conditions, immunodeficiency syndrome, AIDS-related complex, hepatitis, anemia, and pregnancy were excluded from the study. Burn patients younger than 18 or over the age of 90 were also excluded. Burn subjects recruited to this study were graded into minor, moderate and major burn injuries based on the criteria recommended by the American Burn Association (Supplementary table 2). For major burn injuries that required a prolonged hospital stay, blood was collected every week or at regular intervals until the patient was discharged from the hospital. Peripheral blood was collected in heparinized BD vacutainer tubes (Cat # 367880, BD Biosciences, San Jose, CA) and quickly transferred to the laboratory with minimal agitation for further processing. Whole blood was first centrifuged at 250g without brake for 30 minutes at room temperature (RT) to obtain platelet rich plasma. Platelet rich plasma was centrifuged again at 2,500g for 30 minutes at room temperature to obtain platelet poor plasma. Platelet poor plasma was centrifuged further at 12,000g for 2 minutes at 4° C to pellet any residual platelets to obtain platelet free plasma (PFP). PFP was aliquoted and stored at −80 °C until further analysis.
EV Isolation and purification
PFP (800 μl) was ultracentrifuged at 100,000g for 2 hours at 4 °C to obtain the crude EV pellet. The crude EV pellet was then reconstituted with 500 μl of 0.1μm filtered phosphate buffered saline (PBS) and passed through size exclusion chromatography columns (qEV original, Izon, Medford, MA) for purification according to manufacturer’s instructions. EV numbers and plasma protein levels of each elution fraction were determined by nanoparticle tracking analysis (NTA) and Bradford assay, respectively. EV rich, plasma protein poor fractions (fraction 8–12) were pooled, centrifuged, and concentrated by using 10 kDa Amicon centrifugal filters (Cat # UFC801024, Merck Millipore Ltd.) to obtain pure EVs. Pure EV suspensions in 0.1 μm filtered PBS were then stored in 100 μL aliquots and stored at −80 °C for future analysis.
Nanoparticle tracking analysis
EV concentration and size distribution was measured using Nanosight NS300 equipped with Nanosight software 3.2 (Malvern Instruments, UK).13 Briefly, 10 μL pure EV suspension was diluted with 490 μL of 0.1 μm filtered PBS (50× dilution) and injected into the sample chamber through 1 mL syringe. Five videos of 60 seconds were recorded at a camera level of 9. The recordings were then analyzed using a detection threshold of 2 and screen gain of 9.
Transmission electron microscopy
Pure EVs isolated from plasma were fixed with 2% paraformaldehyde in 0.1 M sodium cacodylate for 10 minutes. EVs were then washed twice with 0.1 M sodium cacodylate. EV suspension (5 μL) was loaded onto formvar-coated 200 mesh copper EM grids. Next, 1% uranyl acetate was applied to the EM grid to stain EVs. After 30 seconds of staining, the grids were washed, dried, and observed under a transmission electron microscope (JEOL1400, Tokyo, Japan).
Flow cytometry
Ten μL of pure EV suspension in 90 μL of FACS buffer was labelled with anti-human antibodies (dilution 1:100) for 30 minutes at room temperature. Side-scatter based size gate (corresponding to EV size range of 0.1 μm to 1 μm) was done with the assistance of 0.5, 0.24, 0.20, and 0.16 μm sizing beads (Megamix Plus SSC, Cat # 7803 Biocytex Marseille, France) and all events were recorded with a forward scatter threshold of 200 on a BD™ Canto, and analyzed by BD FACS Diva™ software, v6. The size gate excluded vesicles less than 0.1 μm and vesicles or cell debris larger than 1 μm in size. The same volume of counting beads (size 3 μm) (Cat # 7804, Biocytex, Marseille, France) was used as a reference for analyzing EV concentration. The final EV count was calculated using the following formula: EV concentration = [(Number of EVs counted × EV count bead concentration)/Number of EV count beads counted] according to company instructions. The log height signal was used to quantify EVs. To measure EV cell origins, EV cargo molecules were probed: CD41 for platelet-derived EVs; CD235a for erythrocyte EVs; vascular endothelial growth factor receptor 2 (VEGFR2) for endothelial EVs; and CD66b, CD14, and CD3 for leukocyte EVs.
EV labelling with lipophilic membrane dye Di-8-Anepps
Five μL of plasma were added into 100 μL of FACS buffer. The solution was then stained with 1 μM of a fluorogenic lipophilic probe, Di-8-Anepps (Cat # D3167, Thermofisher Scientific) for 30 minutes at RT. The same volume of counting beads (3 μm) was added into the solution. Membranous vesicles were detected by flow cytometry performed on BD™ Canto and analyzed by BD FACS Diva™ software, v6. The number of Di-8-Anepps+ EVs was calculated based on counting beads using the equation mentioned above.
Immunocapture of EVs with antibody coated magnetic beads
Two hundred μL of PFP was incubated with 20 μL of anti-human CD9 antibody-coated magnetic beads (Cat # 10614D, Thermofisher Scientific) overnight at 4 °C. These magnetic beads were then washed twice with 0.1 μm filtered PBS +1% bovine serum albumin. Washed beads were resuspended in 100 μL FACS buffer and probed with FITC conjugated anti-human CD9 antibody (1:100) for 30 minutes at RT. Intensity of CD9 staining was plotted as flow cytometry histograms indicating immunocapture of CD9+ EVs from plasma. Median fluorescence intensity (MFI) was determined by FACS Diva™ software, v6.
SDS-PAGE
Size exclusion chromatography fractions were lysed with 1× RIPA buffer containing protease inhibitors. The lysates were denatured at 95 °C for 6 minutes. Protein samples were then loaded onto 4–20% Stain Free Tris-Glycine gels (Cat #5678095, BioRad). After electrophoresis, the levels of proteins in SEC fractions were then detected using BioRad ChemiDoc Imaging system.
Transendothelial electrical resistance
Human umbilical vein endothelial cells (HUVECs) were seeded on 0.1% gelatin coated electric cell-substrate impedance sensing (ECIS) electrode arrays. Cells were grown to confluence in Endothelial Cell Growth Medium (C-22010, Promo Cell). Cells were then treated with EVs (~1×107) isolated from healthy or burn subjects. The real-time changes in transendothelial electrical resistance were recorded. Also, the peak resistance drop was normalized to baseline and analyzed.
Immunofluorescence
HUVECs were grown to confluence on 0.1% gelatin-coated coverslips. EVs isolated from plasma were stained with 5 μM carboxyfluorescein succinimidyl ester (#423801, BioLegend) for 30 minutes at RT. After washing two times with PBS, EVs (~1×107) were applied on HUVECs monolayer and incubated for 6 hours. EVs that were not taken up by HUVECs were removed by washing with PBS. Cells were then fixed with 4% PFA for 10 minutes at RT, washed, and permeabilized with PBS+0.05% Triton. After blocking with 10% donkey serum, the coverslips were incubated with rabbit anti-VE-cadherin antibody (#2500S, CST) overnight at 4°C, followed by incubation with donkey anti-rabbit AlexaFluro 568 (A10042, Invitrogen) for 1 hour at RT. ProLong diamond mounting medium with DAPI (#P36962, Life Technologies) was used for nuclei staining and mounting. Images were captured using Leica SP8 Spectral Inverted Laser Scanning Confocal Microscope. The discontinuity of VE-cadherin staining was analyzed using Imaris software (Bitplane).
Statistical analysis
All data are expressed as Mean ± Standard Error Mean (SEM). Normality of the data was assessed by Shapiro-Wilk test. Comparison between more than two groups were performed by the Kruskal Wallis with uncorrected Dunn’s test (GraphPad Prism 7, San Diego, CA). Comparisons between two groups were performed using Mann-Whitney test. Spearman’s correlation analysis was done to compute relation between 2 variables. p < 0.05 was considered significant.
Results
Burn patients display significantly higher levels of circulating EVs, which correlate to burn severity
We first compared the levels of total circulating EVs in burn patients with healthy volunteers. The clinical characteristics, demographics, and co-morbidities for burn subjects are described in Table 1. No burn patients had pre-existing infectious conditions at time of recruitment, ruling out the confounding factors caused by other inflammatory diseases. Plasma EVs were isolated using ultracentrifugation, followed by size exclusion chromatography (SEC) columns. As illustrated in Figure 1A, SEC fractions (8–12) were pooled to collect EVs as these fractions were enriched in EVs and with low plasma protein contamination. SDS PAGE of eluted fractions confirmed the minimal plasma protein contamination in these collected fractions (Fig. 1B). In addition, transmission electron microscopy image showed that the isolated EVs were heterogenous in size (Fig. 1C).
Table 1.
Burn Subject Information
| ID | B01 | B02 | B03 | B04 | B05 | B06 | B07 | B08 | B09 |
|---|---|---|---|---|---|---|---|---|---|
| Age | 28 | 35 | 26 | 38 | 30 | 57 | 71 | 62 | 69 |
| Gender | Male | Female | Male | Male | Male | Female | Male | Male | Female |
| Cause of Burn | Fire | Fire | Fire | Fire | Fire | Grease | Flame | Grease | Grease |
| %TBSA | 29.50% | 24.00% | 93.00% | 28.00% | 30.00% | 10.00% | 16.00% | 5.00% | 6.00% |
| Burn Depth | Second | Second | Mixed | Mixed | Mixed | Mixed | Mixed | Mixed | FT DPT (deep partial thickness) |
| Associated Injuries | Severe inhalation injury, corneal clouding | None | None | Moderate inhalation | None | None | None | None | None |
| BMI | 25.7 | 26.7 | 21.1 | 40.6 | 22.7 | 25.61 | 38.13 | 21.59 | 49.29 |
| Hypertension | No | Yes | No | No | No | No | Yes | No | Yes |
| Smoking | No | Yes | No | Yes | Yes | Yes | No | Yes | No |
| Time of surgery (days postburn) | 4 | 7 | 3 | 2 | 3 | 4 | 4 | 3 | 11 |
| Need for dressing and/or grafts | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes |
| Anabolic treatment | No | No | Yes | Yes | Yes | No | No | No | No |
| Propranolol treatment | Yes | No | No | No | No | No | No | No | No |
| Nutrition | Tube feeding-Impact peptide | Regular diet | Tube feeds | Tube feeding-impact peptide | Regular diet | Regular diet | DM diet | Regular diet | Regular diet |
| Presence of infection | Yes | Yes | Yes | No | No | No | Yes | Yes | No |
| Multiple organ failure | No | No | Yes | No | No | No | No | No | No |
| Presence of sepsis | Yes | No | Yes | No | No | No | No | No | No |
| Length of hospitalization | 40 | 14 | 177 | 10 | 9 | 11 | 16 | 8 | 19 |
| Length of ICU stay | 40 | N/A | 36 | 9 | 1 | N/A | N/A | N/A | N/A |
| Survival | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| ID | B10 | B11 | B12 | B13 | B14 | B15 | B16 | B17 | B18 |
|---|---|---|---|---|---|---|---|---|---|
| Age | 62 | 26 | 39 | 32 | 64 | 67 | 23 | 70 | 72 |
| Gender | Male | Male | Male | Female | Male | Male | Male | Male | Male |
| Cause of Burn | Scald | Flame | Flame | Grease | Flame | Flame | Scald | Flame | Flame |
| %TBSA | 2.00% | 8.75% | 18.50% | 16.00% | 8.50% | 7.00% | 2.00% | 22.50% | 13.50% |
| Burn Depth | Mixed PT | Mixed PT | Mixed PT | DPT/FT | DPT/FT | DPT/FT | FT DPT | FT DPT | Second |
| Associated Injuries | None | None | None | None | None | None | None | None | None |
| BMI | 25.06 | 26.38 | 29.45 | 29.72 | 32.88 | 30.56 | 31.01 | 27.98 | 25.82 |
| Hypertension | Yes | No | Yes | No | Yes | Yes | No | Yes | No |
| Smoking | Yes | No | No | No | Yes | No | No | Yes | Yes |
| Time of surgery (days postburn) | 4 | 2 | 7 | 4 | 4 | 4 | 3 | 3 | 3 |
| Need for dressing and/or grafts | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Anabolic treatment | No | No | No | No | No | No | No | No | No |
| Propranolol treatment | No | No | No | No | No | No | No | No | No |
| Nutrition | Low salt diet | Regular diet | Regular diet | Regular diet | Regular diet | Regular diet | Regular diet | Regular diet | Low salt diet |
| Presence of infection | Yes | Yes | No | No | No | No | No | No | No |
| Multiple organ failure | Yes | No | No | No | No | No | No | No | No |
| Presence of sepsis | Yes | No | No | No | No | No | No | No | No |
| Length of hospitalization | 35 | 3 | 8 | 16 | 11 | 11 | 8 | 27 | 13 |
| Length of ICU stay | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 8 | N/A |
| Survival | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| ID | B19 | B20 | B21 | B22 | B23 | B24 | B25 | B26 |
|---|---|---|---|---|---|---|---|---|
| Age | 29 | 26 | 36 | 63 | 68 | 60 | 44 | 67 |
| Gender | Male | Male | Male | Female | Male | Male | Male | Male |
| Cause of Burn | Flame | Flame | Electrical | Flash flame | Flash flame | Flash flame | Scald grease | Scald |
| %TBSA | 4.00% | 26.50% | 6.00% | 8.00% | 8.00% | 8.50% | 11.00% | 7.10% |
| Burn Depth | Mixed superficial, partial thickness | Mixed superficial, DPT | Mixed superficial to full thickness | Mixed depth | Mixed partial thickness | Mixed superficial, DPT | Mixed depth | Superficial partial thickness |
| Associated Injuries | None | None | None | None | None | None | None | None |
| BMI | 21.7 | 20.54 | 31.21 | 32.12 | 31.87 | 26.5 | 23.24 | 35.28 |
| Hypertension | No | No | No | Yes | Yes | No | No | Yes, |
| Smoking | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Time of surgery (days postburn) | 2 | 7 | 5 | 4 | 5 | 2 | 2 | 3 |
| Need for dressing and/or grafts | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Anabolic treatment | No | No | No | No | No | No | No | No |
| Propranolol treatment | No | No | No | No | No | No | No | No |
| Nutrition | Regular diet + high protein snacks | Regular diet with supplements | Regular diet | Regular diet | Regular diet | Regular diet | Regular diet | Regular diet |
| Presence of infection | No | Yes | No | No | No | No | No | No |
| Multiple organ failure | No | Yes | No | No | No | No | No | No |
| Presence of sepsis | No | Yes | No | No | No | No | No | No |
| Length of hospitalization | 3 | 8 | 19 | 11 | 8 | 11 | 9 | 8 |
| Length of ICU stay | N/A | 7 | 4 | N/A | N/A | N/A | N/A | N/A |
| Survival | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| ID | B27 | B28 | B29 | B30 | B31 | B32 |
|---|---|---|---|---|---|---|
| Age | 34 | 25 | 53 | 63 | 27 | 18 |
| Gender | Male | Male | Male | Male | Male | Female |
| Cause of Burn | Flame | Flame | Flame | Flame | Flame | Flame |
| %TBSA | 20.00% | 21.50% | 14.00% | 5.50% | 5.00% | 6.50% |
| Burn Depth | Partial thickness | Mixed depth | Second | DPT/FT | Superficial partial thickness | Mixed thickness |
| Associated Injuries | None | None | None | low suspicion of inhalation injury | None | None |
| BMI | 27.2 | 29.27 | 28 | 26.01 | 22.91 | 26.62 |
| Hypertension | No | No | No | Yes, | No | No |
| Smoking | Yes | No | Yes | Yes | Yes | Yes |
| Time of surgery (days postburn) | N/A | 2 | N/A | 0 | 2 | 4 |
| Need for dressing and/or grafts | No | Yes | NO | Yes | Yes | Yes |
| Anabolic treatment | No | Yes | No | No | No | No |
| Propranolol treatment | No | No | No | No | No | No |
| Nutrition | Regular diet | High protein diet | Regular diet | High protein, high calorie regular diet | Regular diet | High protein diet |
| Presence of infection | No | No | No | No | No | No |
| Multiple organ failure | No | No | No | No | No | No |
| Presence of sepsis | No | No | No | No | No | No |
| Length of hospitalization | 2 | 9 | 10 | 28 | 7 | 10 |
| Length of ICU stay | N/A | 2 | N/A | 6 | N/A | N/A |
| Survival | Yes | Yes | Yes | Yes | Yes | Yes |
Figure 1. Characterization and quantification of EV number and size.
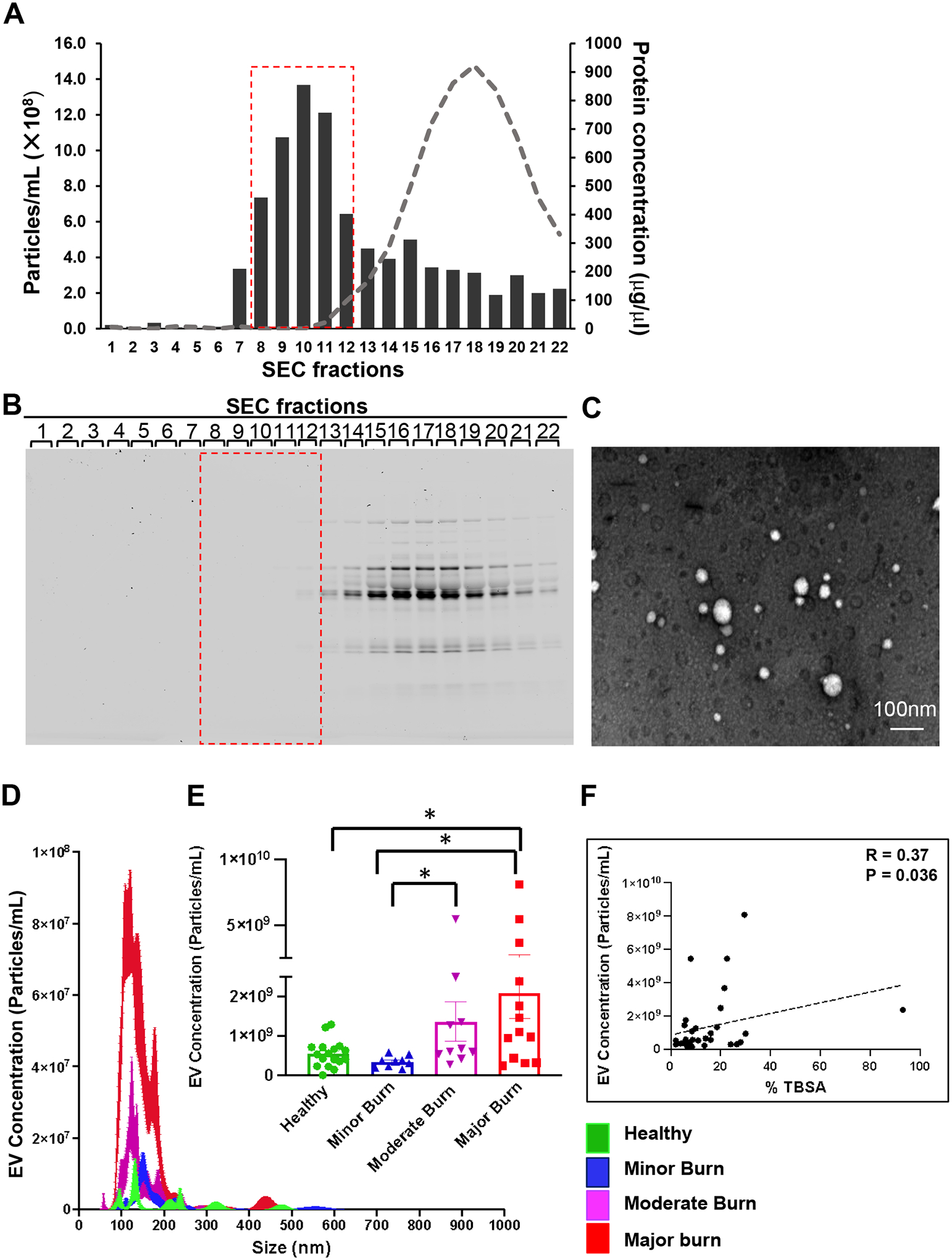
Crude EV pellet was obtained by ultracentrifugation of plasma of burn patients. Pure EVs were collected by size exclusion chromatography (SEC). (A) Particle and protein concentration of different SEC fractions. EV rich fractions (8–12, red box) contain minimal plasma protein contamination (grey curve). (B) Protein staining of gel loaded with SEC fractions verifies that the collected fractions (red box) are rich in EVs and with minimal plasma protein contamination. (C) Transmission electron microscopy image shows isolated EVs. (D) Representative NTA tracing showing concentration and size of EVs in healthy and burn subjects. (E) Quantitative dot plots showing total EV numbers (obtained from NTA) are increased with burn severity. (F) Spearman correlation analysis showing significant positive correlation between EV concentrations and %TBSA of burn injury. All data are shown as mean ± SEM. * p<0.05 by Kruskal Wallis with uncorrected Dunn’s test. N=17 healthy controls, 9 minor burns, 10 moderate burns, and 13 major burns.
The number and size of total circulating EVs in burn subjects were then analyzed via nanoparticle tracking analysis (NTA). Both the representative curve (Fig. 1D) and the quantification data (Fig. 1E) showed significantly increased EV numbers in major burn patients compared with healthy controls. More importantly, there was an increasing trend of circulating EVs with the severity of burn injury. Indeed, Spearman correlation analysis confirmed a significant positive correlation between EV concentrations and %TBSA of burn injury (Fig. 1F).
Next, we validated our NTA data by measuring EVs through probing EV phospholipid membrane and specific EV markers.3 The results of staining EVs with lipophilic dyes Di-8-Anepps also showed a significant increase in EVs in burn patients compared to healthy subjects (Fig. 2 A&B). Consistently, the number of Di-8-Anepps+ vesicles was positively correlated with %TBSA of burn injury (Fig. 2C). Moreover, EVs are enriched in tetraspanin protein CD9, which serves as a reliable marker for EVs.21 As shown in Figure 2D, a higher level of CD9+ EVs was detected in the plasma of burn patients than in healthy controls. Also, patients with full thickness burns displayed more circulating CD9+ EVs than patients with partial thickness burns (Fig. 2E). Consistently, staining for another EV marker, phosphatidylserine (PS), with Annexin-V also showed remarkably higher EV concentrations in burn patients compared to healthy subjects (Fig. 2F). These results together demonstrated that burn injury promoted a significantly increased production of circulating EVs, the levels of which were correlated with burn severity.
Figure 2. Burn injury greatly increases the level of circulating EVs.
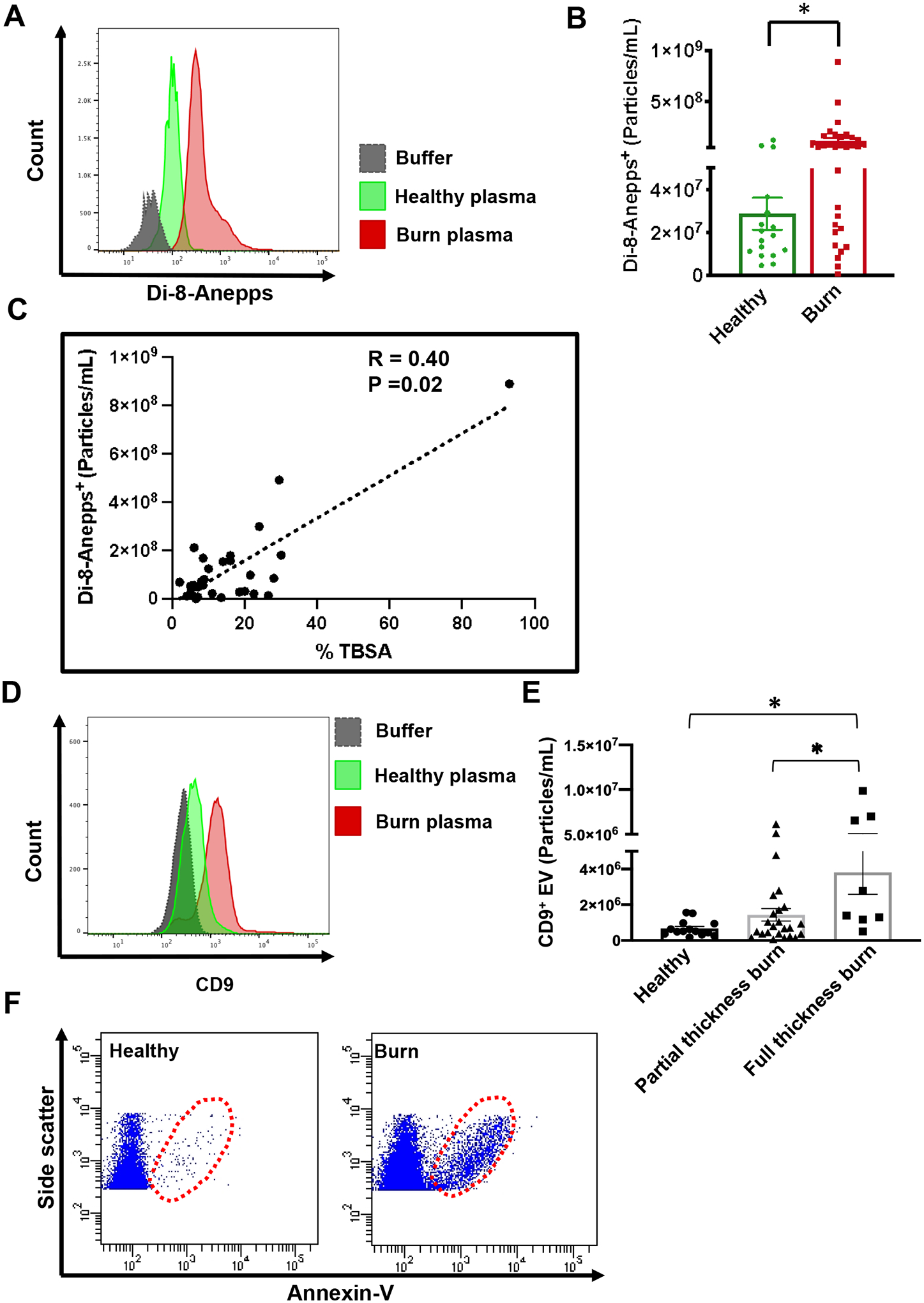
(A) Representative flow cytometry histogram (A) and quantitative dot plot (B) showing increased plasma EV staining with Di-8-Anepps in burn patients compared to healthy subjects. (C) Spearman correlation showing significant positive correlation of Di-8-Anepps staining of plasma EVs with % TBSA of burn injury. (D) Representative flow cytometry histogram showing increased immunocapture of CD9+ EVs from burn plasma compared to healthy controls. (E) Patients with full thickness burns display higher levels of CD9+ EVs than patients with partial thickness burns. (F) Representative flow cytometry dot plots showing EVs detected by Annexin V staining are increased in burn patients compared to healthy subjects. * p<0.05 by Mann-Whitney test. N=17 healthy controls and N=32 burn patients.
Burn-induced EV production peaks at time of admission and subsides over the course of hospitalization
The levels of circulating EVs after burn injury can be very dynamic, which prompted us to investigate the temporal changes of EV concentrations after burn. A major burn patient (26-year-old male, 93% TBSA, mixed thickness burns caused by flame) with no pre-existing comorbidities was hospitalized for in-patient treatment for approximately 2 months. The levels of circulating EVs were monitored throughout the course of hospitalization. Di-8-anepps staining showed the highest EVs concentration at the time of admission; EV levels substantially declined after the acute phase of burn injury (Fig. 3 A&B). Similarly, CD9 fluorescent intensity, an indicator of plasma EV numbers, also peaked at the first blood draw right after admission and subsided over the duration of hospital stay (Fig. 3 C&D).
Figure 3. EV concentrations peak at admission and subside with recovery.
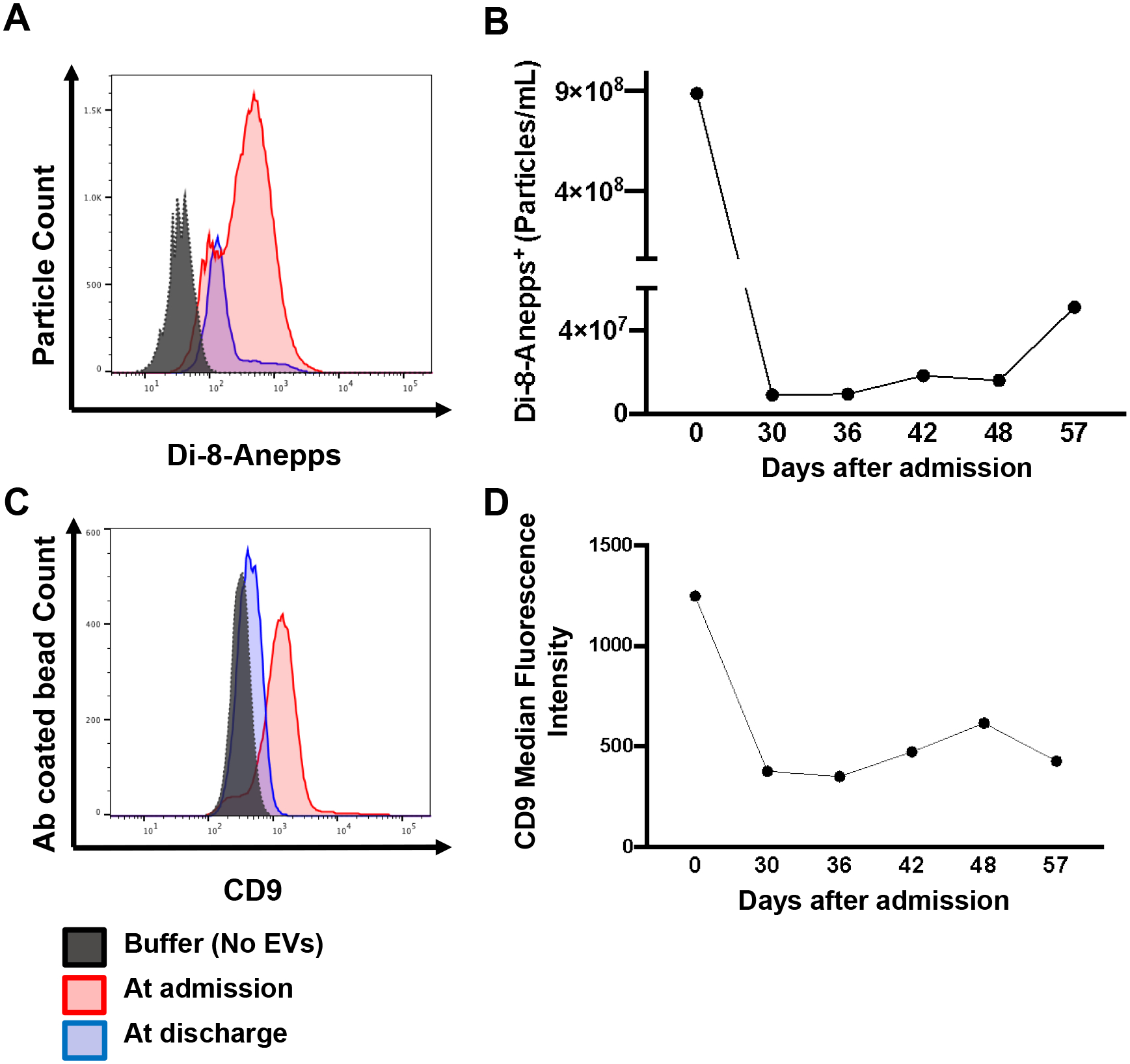
(A) Representative flow cytometry histogram showing a decreased number of EVs stained by Di-8-Anepps in the last blood draw before discharge compared to the first blood draw at admission in a 93%TBSA burn patient. (B) Time-course measurement of the Di-8-Anepps+ EVs over duration of hospital stay in a 93%TBSA burn patient. (C) Representative flow cytometry histogram showing CD9+ EVs in 93%TBSA burn patient plasma. CD9+ EVs are decreased at discharge compared to at admission in a 93%TBSA burn patient. (D) Time-course measurement of the median fluorescence intensity of CD9 indicates decreased CD9+ EVs in plasma over time.
Associated inhalation injury enhances burn-induced increase in EV numbers
Inhalation of smoke or chemical fumes can result in severe damage in respiratory system, which is associated with significantly higher morbidity and mortality in burn patients.22 We compared circulating EV numbers in a major burn patient with severe inhalation injury (29.5% TBSA, second degree burn) to burn patients with similar %TBSA but no inhalation injury. NTA curve in Figure 4A showed that severe inhalation injury led to a substantially higher level of circulating EVs after burn. Consistent with NTA data, remarkably increased EVs in burn patient with inhalation injury were also detected by Di-8-Anepps staining (Fig. 4B) and immunocapture of CD9+ EVs (Fig. 4C).
Figure 4. Circulating EV concentration is increased in burn with inhalation injury as compared to similar burns without inhalation injury.
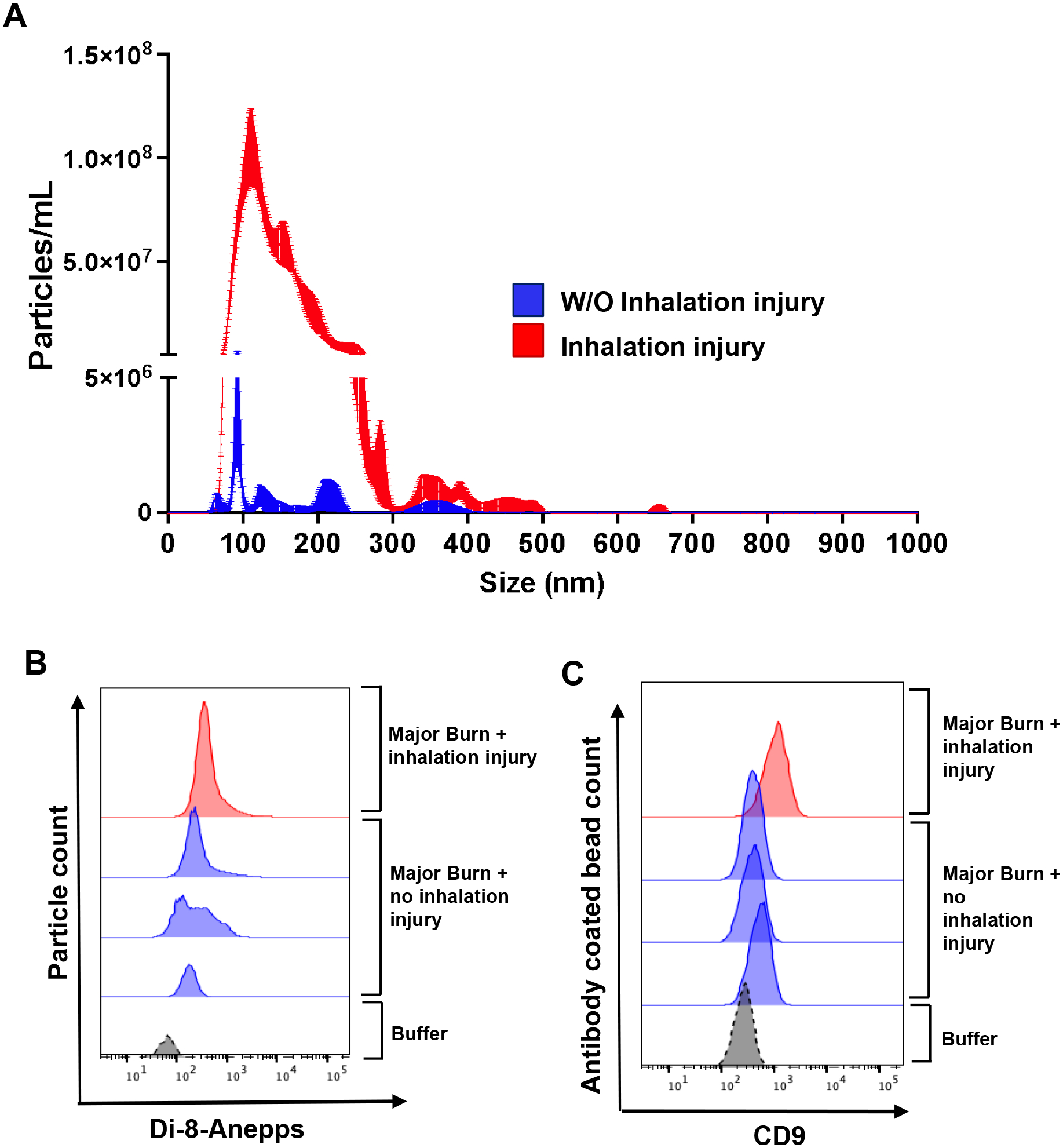
(A) Representative NTA tracing showing total EV numbers are increased in burn with inhalation injury (red) than in burn without inhalation injury (blue). (B-C) EVs measured by Di-8-Anepps+ staining (B) and CD9 immunocapture (B) are increased in burn patient with severe inhalation injury compared to patients with similar %TBSA burns but no inhalation injury. All burn patients examined have similar ~30%TBSA burns. N=3 burns without inhalation injury and n=1 burn with inhalation injury.
Patient demographics and pre-existing conditions
Patient demographics should be considered when evaluating disease severity, treatment strategy, and survival outcomes.16,23 We therefore examined the effects of sex and age on EV concentrations after burn injury. As shown in Figure 5A, there was no significant difference in the levels of EVs between male and female burn patients. Next, burn subjects were categorized into two age groups using the threshold of 65 years. We found that the EV concentrations in Adult and Aged groups were not significantly different (Fig. 5B). Consistently, the spearman r value in Figure 5F also showed a low, non-significant correlation between age and plasma EV concentrations in burn subjects.
Figure 5. The effects of patient demographics and co-morbidities on EV concentration after burn.
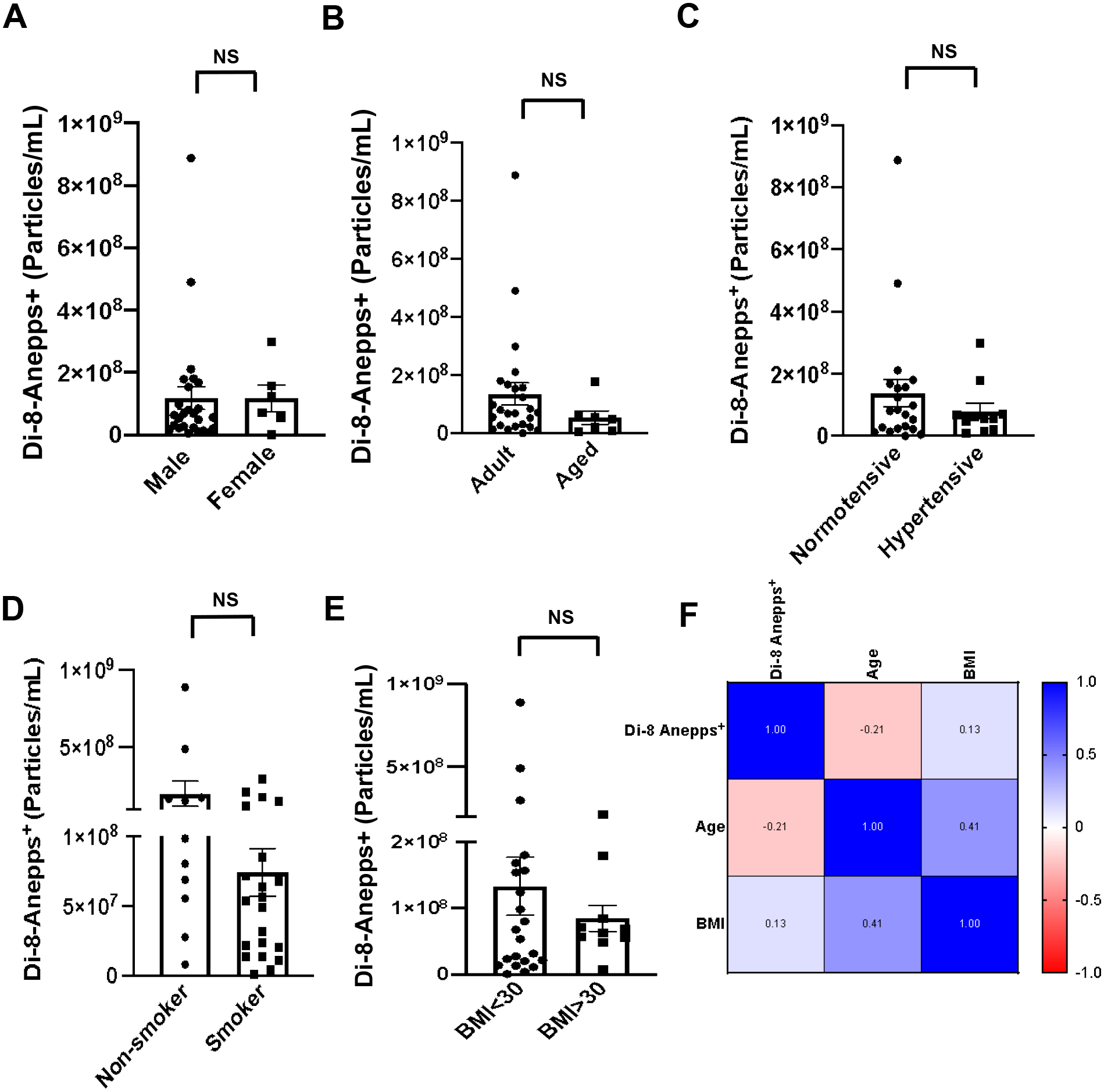
Quantitative analysis of Di-8-Anepps+ vesicles shows no significant change in EV numbers with sex (A), age (B), hypertension (C), smoking status (D), or BMI (E) in burn trauma. (F) Spearman correlation matrix shows no correlation of age or BMI with total EV numbers in burn patients. All data are shown as mean ± SEM. NS= not significant by Mann-Whitney test. Total N=32 burn patients.
We then explored whether pre-existing co-morbidities affect EV production caused by burn injury. Neither hypertension (Fig. 5C), smoking (Fig. 5D), nor obesity (Fig. 5E) significantly affected the numbers of circulating EVs after burn. The spearmen correlation matrix in Figure 5F verified that there was no significant correlation between BMI and EV numbers in burn patients.
Circulating EV levels are associated with clinical outcomes in burn patients
We then investigated whether there is any correlation between the levels of circulating EVs and clinical outcomes in burn patients. Wound infection remains the primary cause of morbidity and death after major burn. Our results in Figure 6A demonstrated that burn patients with wound infection displayed a much higher EV concentration than those without infection. Likewise, significantly increased EV levels were detected in burn patients developing sepsis (Fig. 6B). Moreover, burn patients requiring ICU care exhibited significantly higher levels of circulating EVs than other burn patients (Fig. 6C), and their plasma EV levels positively correlated with the length of ICU stay (Fig. 6D). Also, there was a significant positive association between circulating EV numbers and the length of hospitalization (Fig. 6E), suggesting the predictive value of plasma EVs for clinical outcomes in burn patients.
Figure 6. The levels of circulating EVs correlate with the clinical outcomes in burn patients.
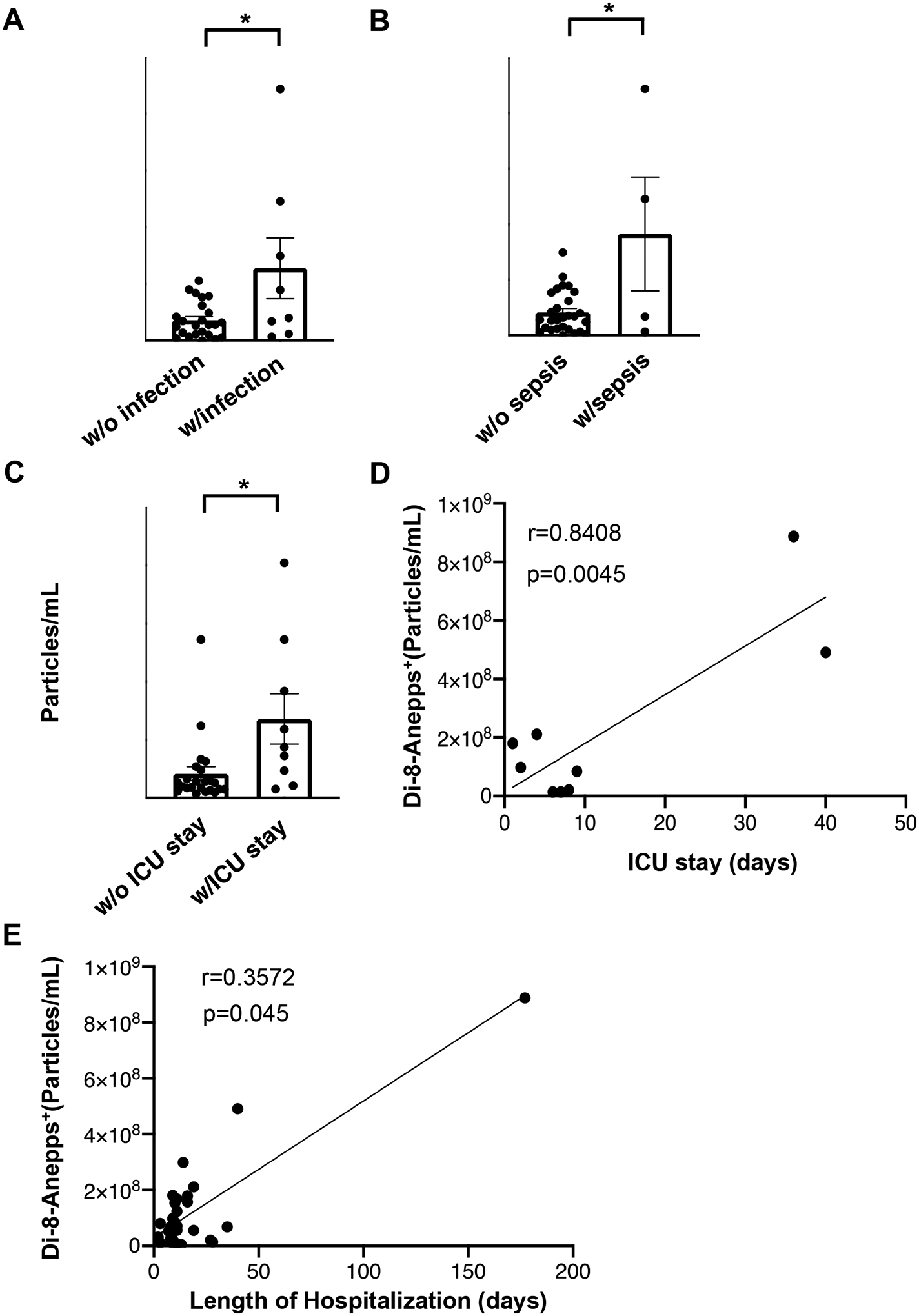
(A) Burn patients with wound infection (n=8) show higher EV concentrations compared to those without infection (n=24). (B) The presence of sepsis is associated with increased EV concentrations in the circulation of burn patients. Burn patients with sepsis n=4; burn patients without sepsis n=28. (C) Burn patients requiring ICU care (n=9) have higher EV concentration than other burn patients (n=23). (D) The levels of burn EVs are positively correlated with the length of ICU stay. (E) EV concentrations in burn patients are positively correlated with the length of hospital stay. * p<0.05
Leukocyte- and endothelium-derived EVs significantly increase after burn injury
The composition and cargo content of EVs mirror the status of their parent cells, thereby serving as reliable indicators of cellular responses and tissue injuries.7 We then investigated the cell origin of EVs produced in response to burn injury. As shown in Figure 7A, burn injury resulted in a higher level of platelet-derived EVs trending towards significance. Furthermore, there was a significant increase in EV numbers released by leukocytes after burn, suggesting the presence of inflammatory responses during the acute phase of burn injury. Moreover, ECs in burn patients produced significantly more EVs than in healthy controls (Fig. 7A), indicating endothelial dysfunction after burn. However, we did not detect any significant changes in the number of red blood cell-derived EVs between healthy and burn patients.
Figure 7. Profiling of cellular origins and cargo molecules of EVs.
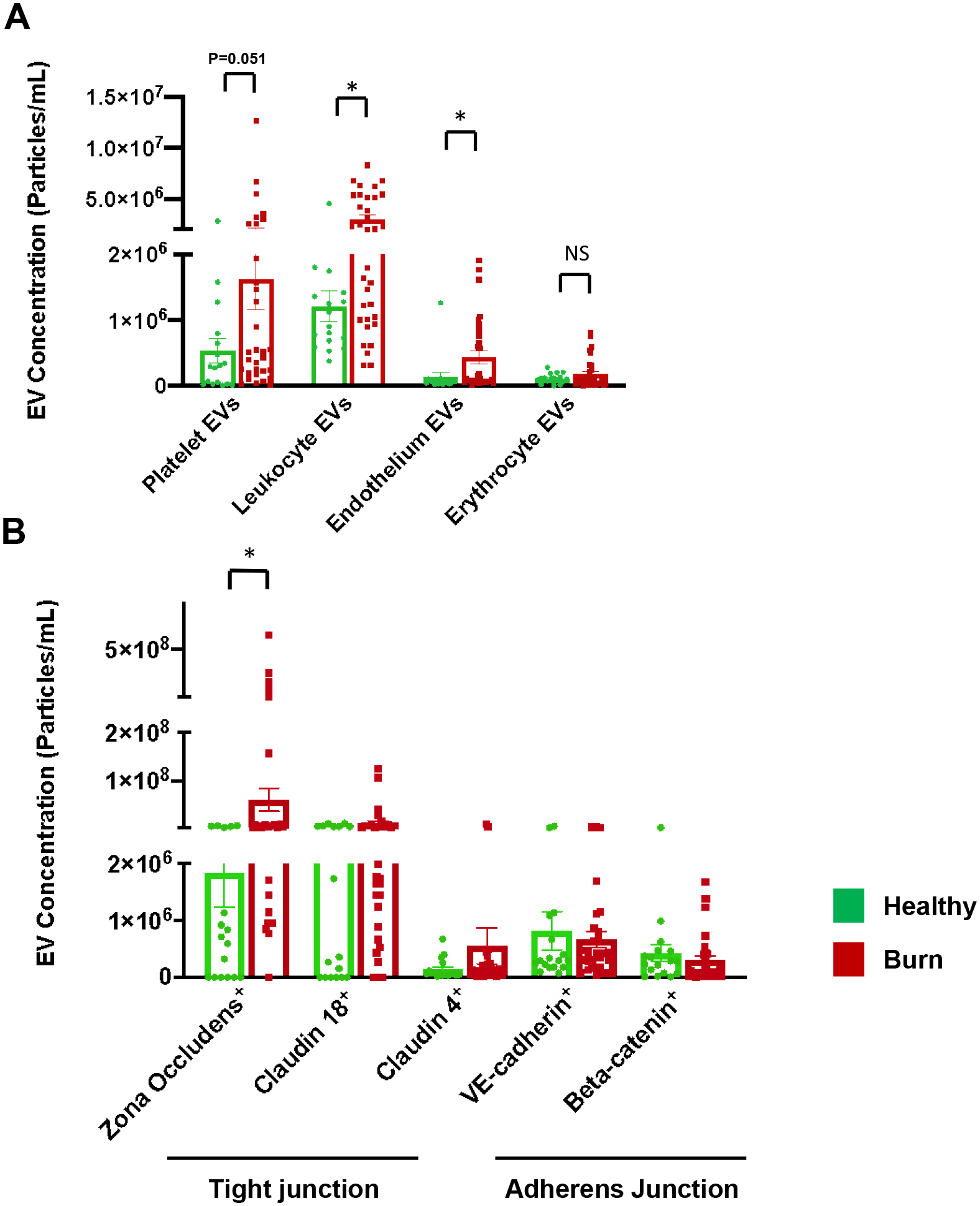
(A) Leukocyte- and endothelium-derived EVs are significantly increased in burn patients compared to healthy controls. Burn injury causes an increase in platelet-derived EVs trending towards significance. (B) The number of ZO-1 bearing EVs is significantly increased in burn patients than in healthy subjects. All data are shown as mean ± SEM. * p<0.05 by Mann-Whitney test. N=17 healthy, 32 burn for all markers.
Junction protein-laden EVs are produced upon burn injury
Burn injury is accompanied by increased permeability as a result of epithelial and endothelial barrier disruption.22,24 We studied the presence of major barrier components tight junction (TJ) and adherens junction (AJ) proteins in EVs in healthy and burn subjects. Our results in Figure 7B showed that burn injury caused a significantly elevated level of EVs containing zona-occludens-1 (ZO-1), a crucial adaptor protein of TJs,25 indicating tight junction injury. Yet, burn injury did not cause significant changes in EVs carrying claudin-18 and claudin-4, which are TJ proteins enriched in lung alveolar epithelial cells.26 We also examined AJ proteins VE-cadherin and beta-catenin in EVs. The concentration of EVs bearing these proteins showed no significant changes between healthy control and burn patients.
Plasma EVs from burn patients cause endothelial barrier dysfunction
Next, we investigated the functional impact of EVs released after burns. The effects of EVs on vascular endothelial barrier function were determined by measuring transendothelial electrical resistance (TER), an indicator of cell-cell adhesive barrier function. TER tracing in Figure 8A showed that burn EVs led to impaired endothelial barrier function. Quantification of peak TER drop indicated that the barrier disruptive effects of burn EVs increased as the burn severity increased (Fig. 8B). Furthermore, we detected the morphological changes of endothelial adherens junction after treatment with burn EVs. Immunofluorescence images in Figure 8C showed intense and continuous staining of VE-cadherin at cell-cell contact of ECs treated with healthy EVs; however, VE-cadherin became disrupted after burn EV treatment. Consistently, quantitative analysis of VE-cadherin staining confirmed that burn EVs significantly increased adherens junction discontinuity (Fig. 8D).
Figure 8. The effects of burn EVs on endothelial barrier function.
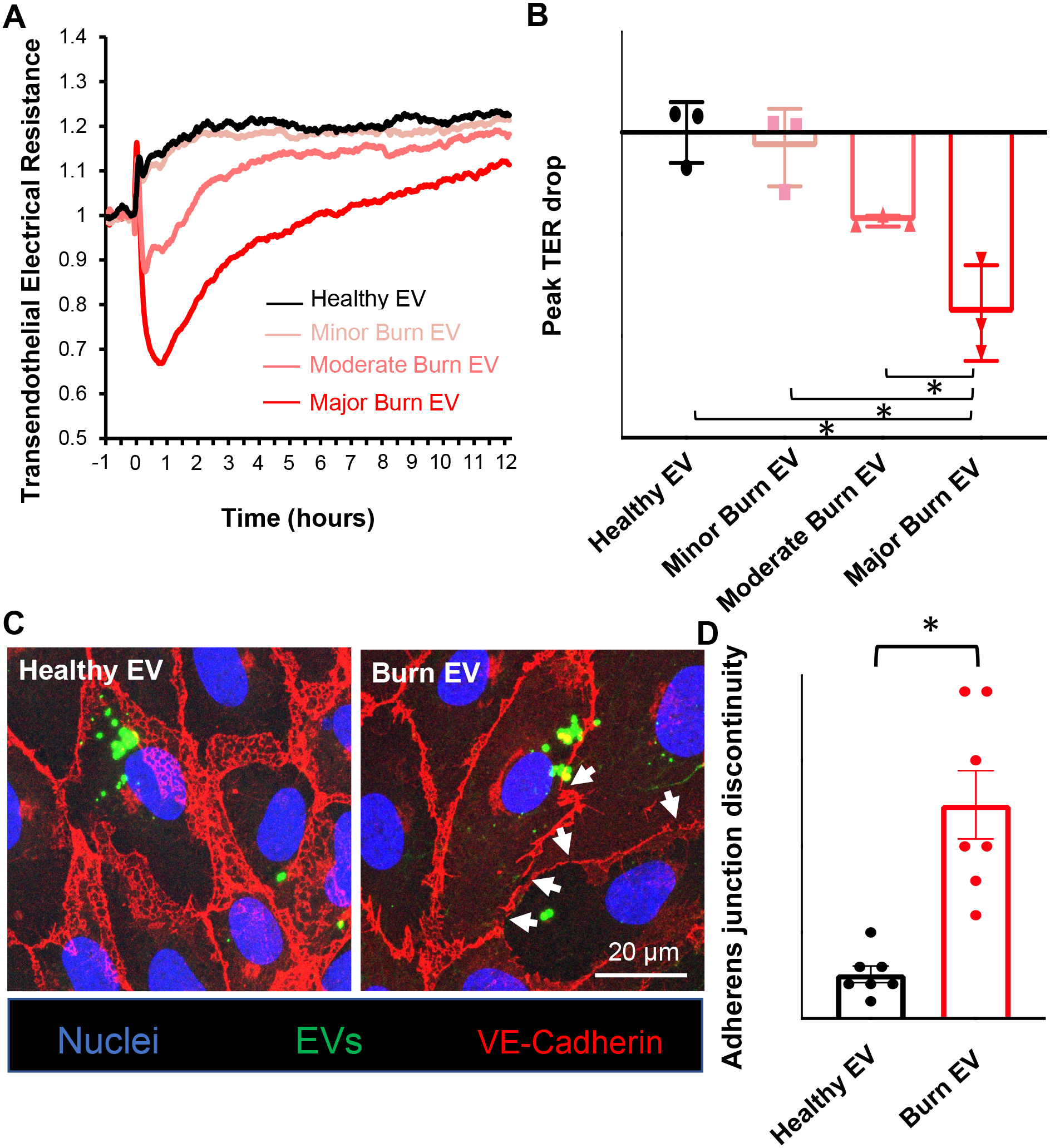
(A) Real-time recording of TER indicates EVs (~1×107) isolated from burn patients cause endothelial barrier dysfunction. (B) Quantification of peak TER drop shows EVs from major burn patients lead to the greatest TER reduction. n=3 (C) Confocal images showing the breakdown of VE-cadherin after treatment with burn EVs (~1×107). White arrows point to disrupted VE-cadherin strands. (D) Quantification of VE-cadherin discontinuity. n=7. Results represent mean ± SEM. *p<0.05
Discussion
The present study provides a comprehensive profile of circulating EVs in burn patients. Our results demonstrate that burn patients display significantly increased plasma levels of EVs, which positively correlate with injury severity and worsened outcome. This increase in EV numbers subsides as patients recover, suggesting a primary role of EVs in the acute phase response to injury. Inhalation injury, a crucial criterion for diagnosis of major burn, remarkably enhances circulating EV concentrations. We also show that age, sex, and co-morbidities (hypertension, smoking, and obesity) exert no significant effects on overall EV release postburn. These findings support the relative specificity of EV response to burn injury. Importantly, increased plasma levels of EVs are associated with worsened clinical outcomes in burn patients, especially those with wound infection or sepsis, and those requiring critical care or lengthened hospital stay. Moreover, we identified leukocytes and ECs as a significant source of EV production postburn, and that burn EVs carry a high level of tight junction molecule ZO-1, indicative of barrier tissue injury. Administration of burn EVs to EC surface induced a significant reduction in cell-cell adhesive barrier resistance and junction integrity, suggesting that increased circulating EVs in burn patients not only serve as a serological marker of aberrant response to burn injury, but also contribute to the pathophysiological mechanisms underlying the injury response.
EVs are categorized into three main subtypes: exosomes, microvesicles, and apoptotic bodies. Exosomes (30–120 nm in diameter) are generated through inward budding of endosomal membranes and released by fusion of multivesicular bodies with plasma membrane; microvesicles (100–1000 nm) are produced via outward budding of plasma membrane, whereas apoptotic bodies are release by apoptotic cells during cell dissembling.21 Studies have suggested EV subtypes may exert distinct functions due to differences in their size and cargo molecules. For instance, compared to small exosomes, microvesicles can deliver more cargo and are potentially more effective to cause functional impacts on recipient cells.27 Also, a functional enrichment study shows exosomes are enriched in signaling proteins, whereas microvesicles are enriched in proteins regulating gene expression and translation.28 Our NTA results show that the majority of EVs released postburn are in 100–200 nm size range, a mixture of exosomes and microvesicles. As the current categorization method is largely based on particle size and biogenesis pathways, tremendous overlap exists in exosome/microvesicles with respect to their size, molecular nature and markers, and it is extremely challenging to completely separate the two subpopulations using the current isolation techniques.27 We, therefore, followed the recommendation of International Society of Extracellular Vesicles (ISEV) and used the general term “extracellular vesicles” for vesicles isolated from burn plasma.27,29
There has been intense debate among researchers on the best approaches in EV purification and analysis. In our study, we used a combination of ultracentrifugation and size exclusion chromatography to isolate EVs with minimal plasma protein contaminants. This combination of two techniques has been shown to yield the best results in terms of EV count and purity.30 Many previous studies have relied on NTA for EV quantification. While NTA is a widely used technique in analyzing small particles, it does not necessarily exclude particles of non-EV nature or cell debris. Hence, additional methods, such as labelling with EV specific markers and transmission electron microscopy, are needed to confirm the presence of EVs. In our study, we used multiple labelling techniques, including the membrane dye Di-8-Anepps and EV specific markers (CD9, Annexin-V), to confirm the level and identity of EVs, which distinguishes our study from other studies.
Circulating EVs possess valuable potential in disease diagnosis, stratification, and prognosis. This is evidenced by a large portion of EV studies focusing on their biomarker roles in several pathological conditions. For instance, increased levels of EVs have been reported in sepsis, correlating with 28-day mortality rate.31 Our data add new knowledge to this area by showing that the increased levels of total EVs in postburn plasma are positively correlated with burn size and depth. In line with this result, there is a recent report about increased production of one type of EVs, microvesicles, in patients with major burn in association with the abbreviated burn severity index (ABSI).18 Compared to that study, ours is unique because we provide a comprehensive, in-depth analysis of EVs covering a wide range of EVs with different subpopulations at different time points after burn injury. To the best of our knowledge, this is the first temporal monitoring of plasma EV concentrations after burn injury for up to two months. The result that EV number peaks at the early onset of injury and declines during the course of recovery suggests a potential utility of measuring plasma EV levels in the dynamic evaluation of patient condition and response to treatment. The application of EVs to assessing treatment efficacy has been reported in other diseases such as cancer.32 The dynamic changes in EV concentrations over the course of burn injury also emphasize the importance of optimizing sampling time and intervals when using burn EVs for clinical assessments in the future.
One major factor that greatly contributes to the severity and worsened outcome of burn injury is inhalation injury. Burn-associated mortality is increased by 17.5% in the presence of inhalation injury.33 Our results show that burn with severe inhalation injury leads to an increased level of circulating EVs, higher than burn alone. This increase in EV concentrations not only reflects the overall severity of burns, but also might be attributable to lung damage. Indeed, we found that EVs of epithelial origins are much higher in the burn patient with severe inhalation injury. Moreover, recent evidence has shown that EVs released from injured lungs contribute to the spread of inflammation systemically.8 These EVs may exert proinflammatory effects and exacerbate organ injuries postburn.
Patient responses to burn injury may vary depending on sex, age, pre-existing conditions, and lifestyle behaviors.16,23 The burn cohort in our study is a mixed population with varied demographics and co-morbidities including smoking, hypertension, and obesity, enabling us to examine the effects of these factors on EV production postburn. To our surprise, however, the results show no significant correlations between the total EV numbers and these parameters. Of note, although burn injury does not seem to induce a difference in the total number of EVs in aged group vs. young group in general, there appears to be a higher level of one subpopulation: neutrophil-derived EVs, in aged patients when compared individually with young adult patients at similar burn sizes. This finding is in line with other studies showing a worsened inflammatory/immune response to injury in the elderly.16 Additionally, the cargo molecules in EVs may be affected by different demographics and clinical conditions of burn patients. For example, certain EV cargo proteins, such as SAA1 and CRP, are thought to be predictive for the length of hospital stay in female, but not male burn patients.19
Prediction of clinical outcomes for burn patients is of great importance in guiding treatment strategies. The prognosis of burn patients is influenced by multiple factors including the size and depth of burns, associated injuries, complications, wound infection, and concurrence of sepsis. Our results show a significant association of EV levels with these factors, supporting the potential application of EVs as prognostic indicator for burns. The translational value of this study is further supported by the findings about unique molecular signature of burn EVs and their functional impact to burn pathophysiology.
EV cargo composition and molecular contents reflect the physiological state and activation status of cells and tissues of their origin, thus providing distinctive signature for cellular functions and tissue injuries in diseases.3,7 Burn injury triggers a biphasic immune response: acute inflammatory phase (first 48–72 hrs) followed by immunosuppression.19 Our results show that burn EV dynamics follows the course of this response. In particular, there is a significant increase in leukocyte-derived EVs in burn patients at the time of admission; we also found neutrophil-derived EVs decline after the acute phase (Supplementary Figure 1). Furthermore, we detected more EVs released from ECs, which are known to be activated during thermal injury.34 The information from EV profiling provides clinically useful information about inflammatory response and endothelial dysfunction in burn patients.
Burns also cause increased permeability and tissue edema by disrupting cell-cell junctions in barrier tissues, such as the endothelium and epithelium.24 We analyzed the presence of different junction proteins in circulating EVs. ZO-1, a scaffold protein connecting tight junctions to intracellular actin fibers in endothelial and epithelial cells,25 was significantly increased in EVs after burn. ZO-1 is known to undergo cellular trafficking from cellular junctions to endosomal pathway during junction disassembly caused by inflammation,35 which might account for increased numbers of ZO-1+ EVs after burn injury. Hence, the increased presence of ZO-1+ EVs might be a biological indicator of tight junction injury. To the best of our knowledge, we are the first to report the increased level of ZO-1+ EVs in human burn plasma. Compared to ZO-1, however, adherens junction proteins, such as VE-cadherin and beta-catenin, showed less abundance in burn EVs, which is not surprising in view of the fact that adherens junctions are mainly expressed in peripheral microvascular endothelium, whereas tight junctions are expressed in not only vascular endothelium but also epithelial tissues of the lung, gut and skin which may undergo massive destruction during major burns.
Circulating EVs not only serve as biomarkers, but also mediators in disease processes and injury responses. Through transferring their cargo content, EVs are capable of directly altering signaling cascades and cellular events of recipient cells. Several lines of evidence show that EVs play essential roles in the progression of various diseases.14 Our data show that burn EVs cause endothelial junction breakdown and barrier disruption, pathological changes known to result in poor organ perfusion and blood-tissue exchange, thereby contributing to multiple organ dysfunction following major burns. In addition, an animal study demonstrates that burn EVs induce macrophage activation and cytokine release.20 These findings on the pathological roles of burn EVs suggest the developmental potential of targeting EVs for burn treatment.
Taken together, this study demonstrates an increased EV production in the circulation of patients with burns, correlating with injury severity and worsened clinical outcome. A significant portion of burn EVs derive from leukocytes and vascular endothelium and are enriched with tight junction molecules. Application of burn EVs to endothelial cell monolayers induces significantly reduced barrier function and disruption of cell-cell junction integrity. Further in-depth analysis of EVs may lead to the development of new diagnostic tools or therapeutic targets for treating burn injury.
Supplementary Material
Supplementary Figure 1. Neutrophil-derived EVs decrease after the acute phase of burn (A)Time-course measurement of neutrophil-derived EVs (CD66b+) over duration of hospital stay in a 93%TBSA burn patient.
Funding support:
This work was supported by the National Institutes of Health grants R35HL150732 and R01GM142110 (to SYY and MHW), the Department of Veterans Affairs grants I01BX000799 and IK6BX004210 (to MHW), Bob & Evelyn Deriso Endowment (to SYY), and Shock Society Faculty Research Award (to XY).
Footnotes
Conflict of Interest: None
References
- 1.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikoloff JM, Saucedo-Espinosa MA, Kling A, Dittrich PS. Identifying extracellular vesicle populations from single cells. Proc Natl Acad Sci U S A 118(38), 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald W, Freeman ML, Lederman MM, Vasilieva E, Romero R, Margolis L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci Rep 8(1):8973, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Meng H, Ma R, He Z, Wu X, Cao M, Yao Z, Zhao L, Li T, Deng R, et al. Circulating Microparticles, Blood Cells, and Endothelium Induce Procoagulant Activity in Sepsis through Phosphatidylserine Exposure. Shock 45(3):299–307, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Schleimer RP. Epithelial-Cell-Derived Extracellular Vesicles in Pathophysiology of Epithelial Injury and Repair in Chronic Rhinosinusitis: Connecting Immunology in Research Lab to Biomarkers in Clinics. Int J Mol Sci 22(21), 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simeone P, Bologna G, Lanuti P, Pierdomenico L, Guagnano MT, Pieragostino D, Del Boccio P, Vergara D, Marchisio M, Miscia S, et al. Extracellular Vesicles as Signaling Mediators and Disease Biomarkers across Biological Barriers. Int J Mol Sci 21(7), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahlund CJE, Eklund A, Grunewald J, Gabrielsson S. Pulmonary Extracellular Vesicles as Mediators of Local and Systemic Inflammation. Front Cell Dev Biol 5:39, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangoda L, Boukouris S, Liem M, Kalra H, Mathivanan S. Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics 15(2–3):260–271, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol 10(6):356–364, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Puhm F, Boilard E, Machlus KR. Platelet Extracellular Vesicles: Beyond the Blood. Arterioscler Thromb Vasc Biol 41(1):87–96, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zifkos K, Dubois C, Schafer K. Extracellular Vesicles and Thrombosis: Update on the Clinical and Experimental Evidence. Int J Mol Sci 22(17), 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee V, Yang X, Ma Y, Cha B, Meegan JE, Wu M, Yuan SY. Endothelial microvesicles carrying Src-rich cargo impair adherens junction integrity and cytoskeleton homeostasis. Cardiovasc Res 116(8):1525–1538, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rontogianni S, Synadaki E, Li B, Liefaard MC, Lips EH, Wesseling J, Wu W, Altelaar M. Proteomic profiling of extracellular vesicles allows for human breast cancer subtyping. Commun Biol 2:325, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bittner EA, Shank E, Woodson L, Martyn JA. Acute and perioperative care of the burn-injured patient. Anesthesiology 122(2):448–464, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primers 6(1):11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang P, Zou B, Liou YC, Huang C. The pathogenesis and diagnosis of sepsis post burn injury. Burns Trauma 9:tkaa047, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Dea KP, Porter JR, Tirlapur N, Katbeh U, Singh S, Handy JM, Takata M. Circulating Microvesicles Are Elevated Acutely following Major Burns Injury and Associated with Clinical Severity. PLoS One 11(12):e0167801, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maile R, Willis ML, Herring LE, Prevatte A, Mahung C, Cairns B, Wallet S, Coleman LG Jr. Burn Injury Induces Proinflammatory Plasma Extracellular Vesicles That Associate with Length of Hospital Stay in Women: CRP and SAA1 as Potential Prognostic Indicators. Int J Mol Sci 22(18), 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willis ML, Mahung C, Wallet SM, Barnett A, Cairns BA, Coleman LG Jr., Maile R. Plasma extracellular vesicles released after severe burn injury modulate macrophage phenotype and function. J Leukoc Biol 111(1):33–49, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol 5:442, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plichta JK, Droho S, Curtis BJ, Patel P, Gamelli RL, Radek KA. Local burn injury impairs epithelial permeability and antimicrobial peptide barrier function in distal unburned skin. Crit Care Med 42(6):e420–431, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolp R, Rehou S, McCann MR, Jeschke MG. Contributors to the length-of-stay trajectory in burn-injured patients. Burns 44(8):2011–2017, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W, Wang Y, Wang P, Wang F. Intestinal barrier dysfunction in severe burn injury. Burns Trauma 7:24, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermette D, Hu P, Canarie MF, Funaro M, Glover J, Pierce RW. Tight junction structure, function, and assessment in the critically ill: a systematic review. Intensive Care Med Exp 6(1):37, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlingmann B, Molina SA, Koval M. Claudins: Gatekeepers of lung epithelial function. Semin Cell Dev Biol 42:47–57, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiteside TL, Boyiadzis M. Response commentary: exosomes vs microvesicles in hematological malignancies. Leukemia 31(10):2277, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willms E, Cabanas C, Mager I, Wood MJA, Vader P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front Immunol 9:738, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3:26913, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh YQ, Almughlliq FB, Vaswani K, Peiris HN, Mitchell MD. Exosome enrichment by ultracentrifugation and size exclusion chromatography. Front Biosci (Landmark Ed) 23:865–874, 2018. [DOI] [PubMed] [Google Scholar]
- 31.Im Y, Yoo H, Lee JY, Park J, Suh GY, Jeon K. Association of plasma exosomes with severity of organ failure and mortality in patients with sepsis. J Cell Mol Med 24(16):9439–9445, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou E, Li Y, Wu F, Guo M, Xu J, Wang S, Tan Q, Ma P, Song S, Jin Y. Circulating extracellular vesicles are effective biomarkers for predicting response to cancer therapy. EBioMedicine 67:103365, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stockly OR, Wolfe AE, Carrougher GJ, Stewart BT, Gibran NS, Wolf SE, McMullen K, Bamer AM, Kowalske K, Cioffi WG, et al. Inhalation injury is associated with long-term employment outcomes in the burn population: Findings from a cross-sectional examination of the Burn Model System National Database. PLoS One 15(9):e0239556, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Huang Q, Guo J, Guo X, Sun Q, Brunk UT, Han D, Zhao K, Zhao M. Local thermal injury induces general endothelial cell contraction through p38 MAP kinase activation. APMIS 122(9):832–841, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Utech M, Mennigen R, Bruewer M. Endocytosis and recycling of tight junction proteins in inflammation. J Biomed Biotechnol 2010:484987, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Neutrophil-derived EVs decrease after the acute phase of burn (A)Time-course measurement of neutrophil-derived EVs (CD66b+) over duration of hospital stay in a 93%TBSA burn patient.


