Abstract
Cardiovascular events after primary intracerebral hemorrhage (ICH) have emerged as a leading cause of poor functional outcomes and mortality during the long-term recovery after an ICH. These events encompass arterial ischemic events such as ischemic stroke and myocardial infarction, arterial hemorrhagic events that include recurrent ICH, and venous thrombotic events such as venous thromboembolism. The purpose of this review is to summarize the cardiovascular complications after ICH, epidemiology and associated risk factors, and their impact on ICH outcomes. Additionally, we will highlight possible pathophysiological mechanisms to explain the short and long-term increased risks of ischemic and hemorrhagic events after ICH. Finally, we will highlight potential secondary stroke and venous thrombotic prevention strategies often not considered after ICH, balanced against the risk of ICH recurrence.
Keywords: cerebral hemorrhage, ischemic stroke, myocardial infarction, pulmonary embolism, venous thromboembolism
Introduction
Intracerebral hemorrhage (ICH) is the most devastating form of stroke that affects about 2.9 million people worldwide every year.1 For decades, ICH has remained the least treatable form of stroke with a high associated mortality and morbidity.2 Over a third of patients who survive an ICH can recover in the first year.3 However, nearly one in four ICH survivors with mild to moderate disability initially, experience long-term functional decline, mainly due to acute cerebrovascular and cardiovascular events.4 In fact, incident and recurrent vascular events are a leading cause of readmissions in ICH patients, second only to infections.5,6 Major arterial events, particularly, ischemic stroke and ischemic cardiovascular disease account for nearly 15% of deaths after ICH.7 Despite these emerging data, secondary prevention efforts after ICH focus mainly on blood pressure control and current guidelines equivocate about the use of antithrombotic and statin medications due to concerns about ICH recurrence.8 This review discusses the types of major vascular events after ICH (Figure 1), their epidemiology, purported mechanisms, impact on ICH outcomes, and potential prevention strategies, in an effort to improve overall brain and systemic health.
Figure 1:
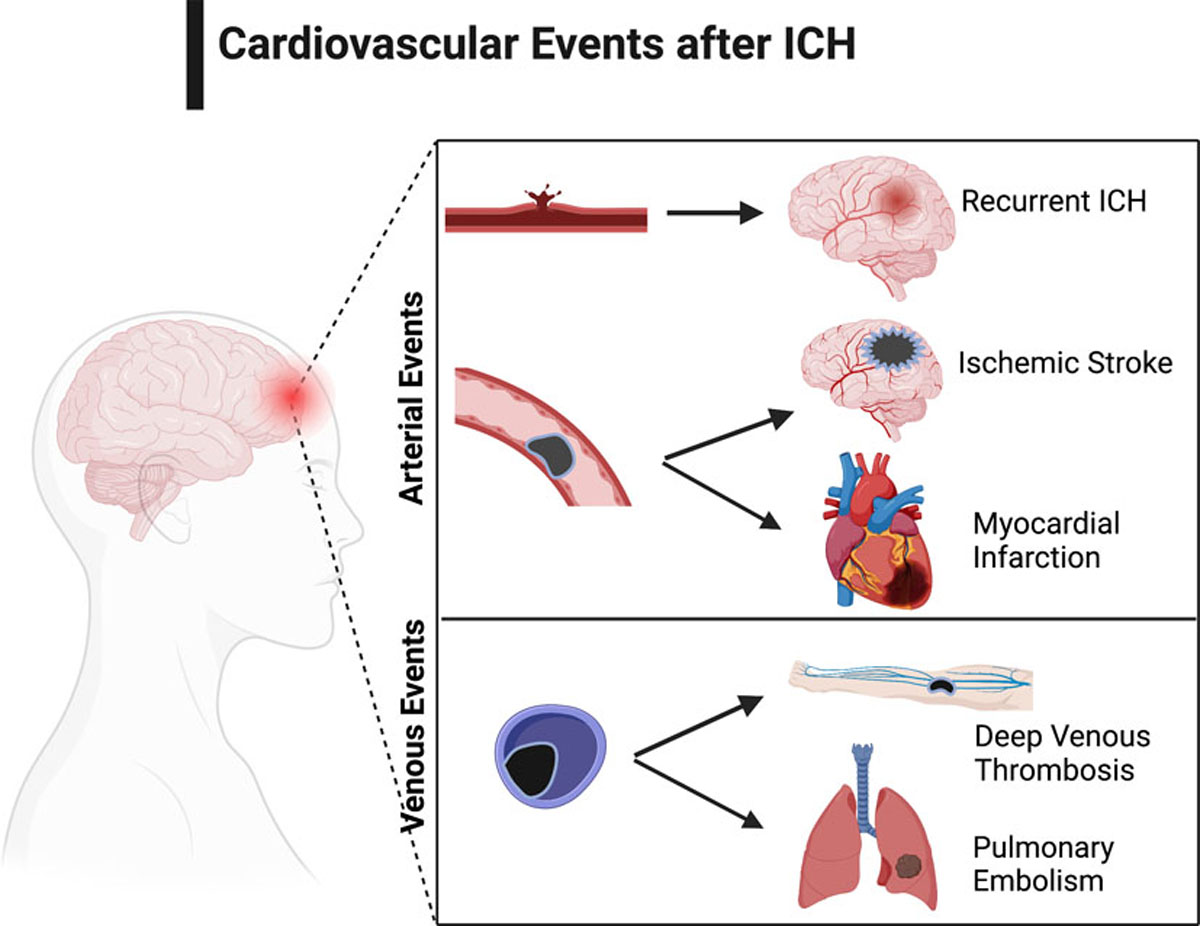
Schematic Diagram Highlighting Cardiovascular and Cerebrovascular Events after Intracerebral Hemorrhage. Created with BioRender.com.
1. ICH and arterial ischemic events
ICH is traditionally believed to mostly increase the risk of ICH recurrence9, but emerging data have shown that ICH survivors may also face a heightened risk of major arterial ischemic events. Large case series have reported rates of arterial ischemic events after ICH, particularly acute ischemic stroke which varies from 3–7% (Table 1), and myocardial infarction which may be as high as 4%.10–38
Table 1.
Annual rates (per 100 patient-years) of Recurrent Intracerebral Hemorrhage (ICH) and Ischemic Stroke (IS) in Patients with Primary Intracerebral Hemorrhage Reported in Previous Studies.
| Study | Country | Study period | Total number of patients | Mean/Median follow-up | Recurrent ICH (No/annual rate) | Recurrent IS (No/annual rate) |
|---|---|---|---|---|---|---|
|
| ||||||
| Population-based | ||||||
| Counsell10,11 | UK | 1981–1986 | 66 | 1.5 years | 4 (4.4%) | 2 (2.1%) |
| Inagawa12 | Japan | 1991–1998 | 279 | 3.1 years | 19 (2.3%) | - |
| Huhtakangas13 | Finland | 1993–2008 | 680 | 3.6 years | 58 (1.7%) | - |
| Pennlert14 | Sweden | 1995–2008 | 815 | 3.1 years | 38 (1.5%) | 63 (2.5%) |
| Flach15 | UK | 1995–2018 | 364 | 3.0 years | 19 (1.7%) | 9 (0.8%) |
| Li16 | UK | 2002–2018 | 255 | 2.5 years | 15 (2.4%) | 7 (1.1%) |
| Poon16 | UK | 2010–2018 | 419 | 1.9 years | 31 (3.9%) | 18 (2.3%) |
| Aked17 | Sweden | 2015–2016 | 60 | 1.0 year | 0 (0) | 1 (1.7%) |
| Hospital-based | ||||||
| Passero18 | Italy | 1978–1982 | 112 | 7.0 years | 27 (3.4%) | 8 (1.0%) |
| Vermeer19 | Netherlands | 1986–1995 | 243 | 5.5 years | 30 (2.1%) | 16 (1.4%) |
| Hill20 | Canada | 1986–1996 | 172 | 3.6 years | 15 (2.4%) | 19 (3.0%) |
| Zia21 | Sweden | 1993–2000 | 353 | 3.0 years | 20 (2.3%) | 24 (2.8%) |
| Viswanathan22 | US | 1994–2004 | 207 | 1.6 years | 39 (11.6%) | 7 (2.1%) |
| Flynn23 | UK | 1994–2005 | 417 | 3.0 years | 14 (0.9%) | 29 (2.3%) |
| Biffi24 | US | 1994–2013 | 1,145 | 3.1 years | 146 (4.2%) | - |
| Yeh25 | Taiwan | 1995–2013 | 3,785 | 3.9 years | 185 (1.3%) | - |
| Hanger26 | New Zealand | 1996–2004 | 768 | 4.0 years | 19 (1.2%) | 17 (1.3%) |
| Chong27 | HK | 1996–2010 | 440 | 5.2 years | 47 (2.1%) | 29 (1.3%) |
| Weimar28 | Germany | 2002–2006 | 496 | 2.0 years | 11 (1.1%) | 21 (2.1%) |
| Asberg29 | Sweden | 2004–2009 | 6,082 | 3.2 years | 234 (1.2%) | 350 (1.9%) |
| Casolla30 | France | 2004–2009 | 310 | 6.0 years | 24 (1.3%) | 33 (1.8%) |
| Skajaa31 | Denmark | 2004–2018 | 13,387 | 1.6 years | 531 (2.5%) | 534 (2.5%) |
| Leasure32 | US | 2005–2011 | 31,355 | 2.9 years | 1330 (1.5%) | - |
| Qiu33 | Singapore | 2006–2013 | 1,708 | 3.7 years | 60 (1.1%) | - |
| Rodriguez-Torres/MGH34 | US | 2006–2013 | 759 | 4.2 years | 118 (3.9%) | - |
| Castello/ERICH35 | US | 2006–2017 | 329 | 1.5 years | 49 (2.9%) | - |
| Kubiszewski36 | US | 2006–2017 | 1,279 | 4.4 years | 128 (4.2%) | - |
| Castello/MGH35 | US | 2010–2017 | 593 | 3.9 years | 62 (4.2%) | - |
| Banerjee37 | UK | 2011–2015 | 1,094 | 3.0 years | 45 (1.9%) | 70 (2.9%) |
| Tsai38 | Taiwan | 2014–2018 | 300 | 1.9 years | 36 (6.3%) | 12 (2.1%) |
Studies were included if they reported annual rates of recurrent intracerebral hemorrhage or ischemic stroke, or if they reported the crude numbers of recurrent stroke and patient-years of follow-up.
A longitudinal analysis from the U.K. that pooled data from two prospective, population-based studies with 674 ICH patients evaluated the incidence of a serious vascular event defined as a composite of recurrent ICH, nonfatal ischemic stroke, non-fatal myocardial infarction, or vascular death. The pooled event rates for a serious vascular event were 15.5% (95% CI, 10.0–24.1%) and 6.8% (95% CI, 3.6–12.5%) among patients with and without atrial fibrillation, respectively.16 In a population-based record linkage study from the Netherlands comprising nearly 2,000 ICH survivors, the 1-year cumulative rate of an arterial ischemic event or vascular death was slightly higher among men compared to women, in the 55–74 years (9.2% vs. 7.4%) and 75–94 year age groups (14.6% vs. 14.1%).39 The 10-year rates reached as high as 40% in the elderly age group, suggesting that ICH survivors experience major arterial ischemic events, both short- and long-term.39 In terms of timing of arterial ischemic events after ICH, prior studies have mostly considered the time after discharge from the ICH or the first 30 days after the index ICH, with the follow-up period extending anywhere between 1 and 10 years (Table 1). Exclusion of the first few weeks after ICH was done due to the high 30-day mortality of ICH, which may yield erroneously high rates of arterial ischemic events.
However, these studies did not include a control group; consequently, the exact nature of the risk of an arterial ischemic event after ICH could not be inferred from these studies. A recent pooled analysis of patient-level data from four U.S. population-based studies with nearly 50,000 participants showed that ICH was associated with an increased risk of an arterial ischemic event (adjusted hazard ratio [aHR], 2.3; 95% CI, 1.7–3.1), compared to the general population, which corresponded to an incidence rate of 3.6% per year versus 1.1% in the general population.40 Similarly, the risks of ischemic stroke (aHR, 3.1; 95% CI, 2.1–4.5) and myocardial infarction (aHR, 1.9; 95% CI, 1.2–2.9) were also high after ICH.40 This risk was consistently elevated both short-term (<1 year after ICH) and long-term (>1 year after ICH), regardless of vascular risk factors including atrial fibrillation and antithrombotic medication use. Although this risk is elevated long-term, there may be a higher risk short-term after the index ICH as observed in an analysis of 1.8 million Medicare beneficiaries in the U.S. where the risk of an arterial ischemic event after ICH was highest in the first 6 months, with the 1-year cumulative incidence being 5.7% (95% CI, 4.8–6.8) in patients with ICH and 1.8% (95% CI, 1.7–1.9) in patients without ICH.41 Further support for the heightened risk of a major arterial ischemic event after ICH comes from a cohort study of 988 subjects from the U.K., where patients with an ICH experienced had a 2.5 times higher risk of an ischemic stroke as compared to the general population (sub HR, 2.49; 95% CI, 1.85–3.34).42 Even among patients without atrial fibrillation, a higher risk of ischemic stroke was observed after ICH (sub HR, 2.28; 95% CI, 1.65–3.16), albeit this risk was nearly half of what was noted in ICH patients with atrial fibrillation (sub HR, 5.47; 95% CI, 2.16–13.83).42
ICH characteristics and risk of arterial ischemic events
The majority of population-based cohort studies assessing the risk of a major arterial ischemic event after ICH lack data on ICH severity characteristics such as hematoma volume, location, and presence of intraventricular hemorrhage. Given the different biological processes implicated in lobar and deep ICHs, hematoma location is of great interest43; however, studies have yielded conflicting results. For instance, a prospective observational study by Casolla and colleagues evaluated 560 ICH patients and found that the 1-year cumulative incidence of major arterial ischemic events was about twice higher after a deep ICH, compared to a lobar ICH (7.3% vs. 3.5%).30 Conversely, ICH recurrence was nearly 3-fold higher in the lobar ICH group (6.1% vs. 2.6%). A deep ICH was independently associated with an increased risk of a major arterial ischemic event (sub HR, 1.85; 95% CI, 1.01–3.40), compared to a lobar ICH.30 Contrary to these findings, the prospective multi-center Clinical Relevance of Microbleeds in Stroke study (CROMIS-2) study, with 1,094 ICH patients reported similar absolute event rates of about 3% for a cerebral ischemic event (ischemic stroke or transient ischemic attack) in lobar and deep ICH.44 Cox regression analysis did not reveal a relationship between hematoma location and a subsequent cerebral ischemic event (aHR, 1.13; 95% CI, 0.66–1.92). Notably, while only 11% of the population had atrial fibrillation in the former study (Casolla et al.)30, the rate of atrial fibrillation at baseline was 37% in the CROMIS-2 study.44 It is therefore possible that a high rate of prevalent atrial fibrillation confounded the relationship between hematoma location and an ischemic arterial event after an index ICH. Interestingly, admission hematoma volume did not influence arterial ischemic events, but a prior history of ischemic stroke or TIA was associated with a higher risk of an arterial ischemic event in both studies.30,44 Lastly, a meta-analysis of population-based and hospital-based studies showed a non-significant trend toward a lower risk of major vascular events in lobar ICH (relative risk [RR], 0.8; 95% CI, 0.5–1.2).16
ICH and covert cerebral infarction
Covert cerebral infarction is nearly 4 times more common than clinically apparent strokes.45 It is therefore not surprising that acute punctate ischemic infarcts occur in about a third of all ICH patients, as seen on the diffusion-weighted imaging (DWI) sequence of a magnetic resonance imaging scan, and can be spatially in the surrounding vicinity or remote from the index hematoma (Figure 2).46,47 Although most studies have reported the incidence of DWI lesions in the first week after ICH, these lesions may appear as late as 30 days after ICH.48,49 Factors implicated in DWI lesions include admission ICH volume, aggressive blood pressure reduction in the acute phase, and pre-existing imaging markers of cerebral small vessel disease- cerebral microbleeds and white matter hyperintensities 48,50, but the exact underlying mechanism has yet to be discerned. Furthermore, underlying stroke mechanisms may influence the pattern of DWI lesions after ICH. For instance, large artery atherosclerosis in conjunction with blood pressure reduction was associated with large DWI lesions after ICH, while cerebral small vessel disease burden was associated with punctate DWI lesions in a prospective study of 305 ICH patients.51 DWI lesions have been shown to adversely affect long-term functional outcomes after ICH. 48,50 Moreover, these lesions may serve as markers for future arterial ischemic risk as observed in a post hoc exploratory analysis of two large ICH trials, where the authors concluded that the presence of a DWI lesion was associated with a 6.9% (95% CI, 2.2–11.6) absolute increase in risk of all stroke that corresponded to a HR of 2.6.52 There was also an increased risk of ischemic stroke (aHR, 3.5; 95% CI, 1.1–11.0). A contemporary study of ICH patients enrolled in the REstart or STop Antithrombotics Randomised Trial (RESTART), found that a DWI lesion was associated with all stroke, (aHR 2.2; 95% CI 1.1 to 4.2) and recurrent ICH (aHR, 4.8; 95% CI 1.8 to 13.2), but not ischemic stroke.53 Both studies, however, were limited by few outcome events that precluded adequate adjustment of covariates. Nevertheless, DWI lesions offer an insight into the microvascular mechanisms associated with recurrent cerebrovascular disease.
Figure 2:
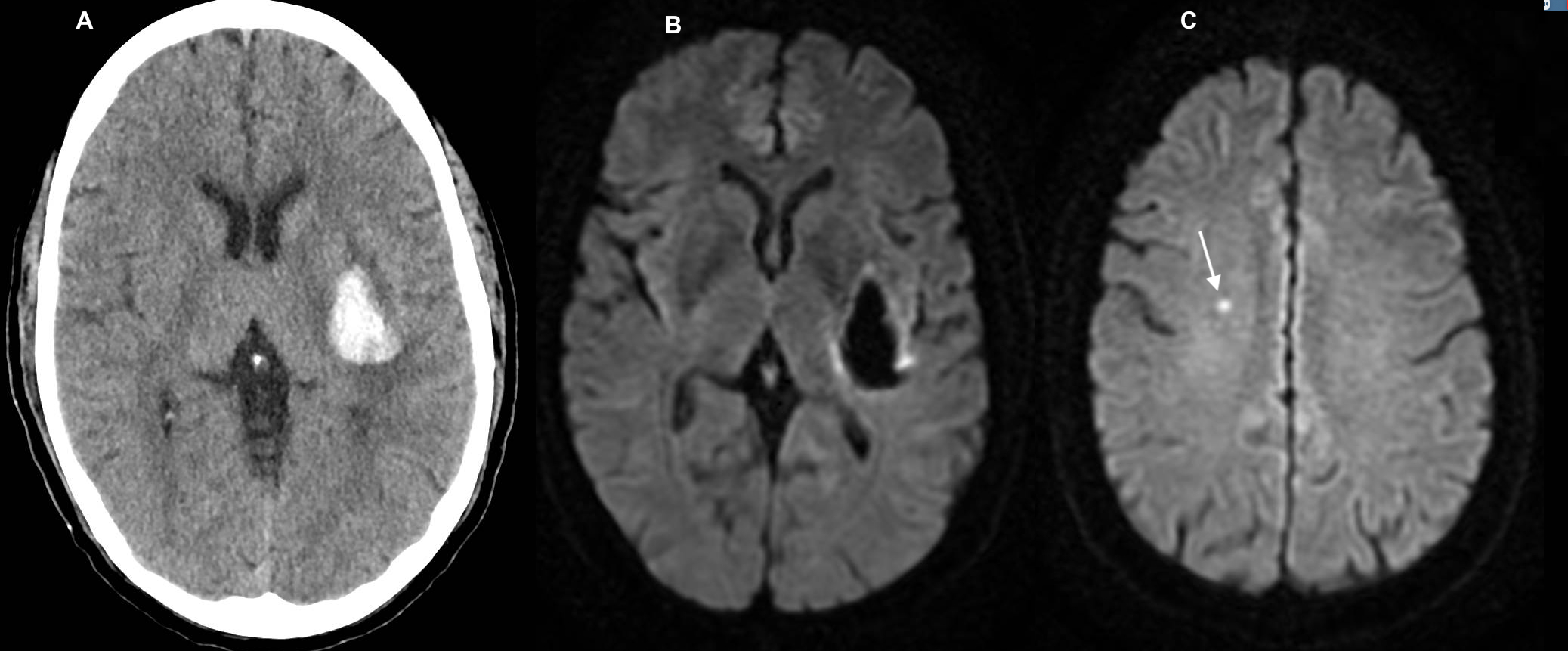
Diffusion-Weighted Imaging (DWI) Lesion after Intracerebral Hemorrhage. Computed Tomography Scan (Panel A) and Magnetic Resonance Imaging Scans of the Hematoma (Panel B) and DWI lesion (Panel C).
Arterial ischemic events and ICH outcomes
Patients who survive the acute phase of ICH often make substantial recovery during the months and years after the event.3 Acute vascular events, mainly stroke, are the second most common reason for readmissions after ICH, after infections.5,6 A longitudinal analysis of prospectively collected claims data in the U.S. showed that cardiac disease and ischemic stroke accounted for nearly 8% and 5% of deaths among ICH survivors with atrial fibrillation, respectively.7 In fact, even among younger ICH patients (18–55 years), recurrent stroke was the cause of death in 15% of patients.54 In addition to being increasingly recognized as a cause of death or readmission after ICH, arterial ischemic events have been shown to adversely affect long-term ICH recovery. For instance, a retrospective cohort study of U.S. Medicare beneficiaries reported a two-fold increased risk of death from an arterial ischemic event among ICH survivors.55 Recurrent stroke, ischemic or hemorrhagic, was associated with a 4-fold heightened risk of functional decline and disability in a single-center, prospective study of 560 ICH patients who survived at least 6 months.4 These data collectively suggest that new arterial ischemic events are not uncommon after ICH, and portend poor prognosis. In a registry study from Finland, patients with ICH who showed good recovery at 3 months went on to have similar outcomes as age- and sex-matched controls.56 In the context of these findings, one may surmise that prevention of incident arterial ischemic events can potentially improve long-term recovery and clinical outcomes after ICH.
Mechanisms of arterial ischemic events after ICH
The elevated risk of ischemic stroke may be attributable to antithrombotic drug cessation after the ICH diagnosis and the lack of an optimal time frame for resumption of these medications. For example, studies have reported that fewer than 50% of patients resume antithrombotic agents in the first year after ICH despite strong indications.57 However, prior studies suggested that only about 20% of patients were on antithrombotic medications prior to ICH58, and rates of observed ischemic strokes among ICH survivors with atrial fibrillation exceed those of expected events for a given CHA2DS2-Vasc score.59 The use of statin medications has also been controversial after ICH. While observational data seem to suggest a 40% risk reduction of major cardiovascular events with the initiation or resumption of statin therapy after ICH60, the presumed increased risk of ICH recurrence, particularly among patients with a lobar ICH, adds to the complexity of clinical decision making.61 Taken together, these factors fail to implicate cessation of antithrombotic medications as the sole mechanism of increased thrombotic risk after ICH.
Another potential explanation is poor risk factor control after ICH. Uncontrolled blood pressure has been demonstrated in one third to half of patients after an ischemic stroke62,63, and these patients have significantly worse blood pressure and risk factor control compared to patients with other cardiovascular conditions such as acute MI.64 This is further supported by the results of a U.S. population-level study, where survivors of ischemic stroke did not experience improvements in cardiovascular health due to secondary prevention efforts that included seven domains -smoking, diet, physical activity, body mass index, blood pressure, total cholesterol, and fasting glucose.65 Given that ICH survivors have more disability than ischemic stroke patients, risk factor control is presumably worse after ICH.
2. Recurrent ICH after primary ICH
Long-term risk of ICH recurrence
The reported annual rates of ICH recurrence range from 0.9% to 11.6% in published literature (30 studies: 8 population-based and 22 hospital-based) as shown in Table 1. Possible reasons for the wide range of recurrent ICH likely include differences in study cohorts with some being enriched with hypertensive ICH while others with a higher proportion of cerebral amyloid angiopathy (CAA), which confers a higher risk of ICH as discussed in the section below. Interestingly, unlike the significant reduction in the recurrence of transient ischemic attack or ischemic stroke over the years,66,67 rates of recurrent ICH after primary ICH did not improve over the past four decades (Table 1).
Risk factors for ICH recurrence
Hematoma location has been shown to be one of the strongest risk factors for recurrent ICH. In a meta-analysis combing seven published studies looking at risks of recurrent ICH by hematoma location, Li et al. found that lobar ICH was associated with a 2-fold increased risk of recurrent ICH compared to non-lobar ICH (RR, 2.3; 95% CI 1.5–3.3).16 The annual rate of recurrent ICH was 5.1% after lobar ICH and 1.8% after non-lobar ICH in a pooled analysis of two contemporary prospective population-based studies.16 The higher risk of recurrent ICH after lobar ICH is likely explained by the high prevalence of CAA in the group. CAA is a bleeding-prone vasculopathy resulting from beta-amyloid deposition in cortical blood vessels, and a previous meta-analysis showed that CAA-related ICH was associated with a significantly higher risk of recurrent ICH (7.4%) compared to non-CAA-related ICH (1.1%).68 A recent large cohort study of 194,290 patients with ICH also found that ICH patients with a concomitant diagnosis of CAA were three times more likely to have recurrent ICH compared to patients without CAA even after controlling for potential confounders.69 Another contributing factor for the higher ICH recurrence after lobar ICH is worse blood pressure control compared to non-lobar ICH. In fact, less than half of lobar ICH survivors were on blood pressure lowering treatment at hospital discharge while 71% of patients with non-lobar ICH were treated with antihypertensive medication.16 Furthermore, Biffi et al.24 showed that inadequate control of blood pressure during follow-up was an independent risk factor (lobar ICH: aHR, 3.53; 95% CI 1.67–7.54; non-lobar ICH: aHR, 4.23; 95% CI, 1.02–17.52). Of note, 20–50% of the ICH patients have hematomas involving both lobar and deep areas and tend to share the same risk profile for ICH recurrence as that of hypertension-related deep ICH.38,70,71
The risk of ICH recurrence is also influenced by race. Rodriguez-Torers et al. showed that among 2,291 ICH survivors, Black (aHR, 1.98; 95% CI 1.36–2.86) and Hispanic patients (aHR, 1.51; 95% CI, 1.14–2.00) were at higher risks of recurrent ICH.34 Similarly, in a retrospective cohort analysis of 31,355 patients with ICH, a higher risk of ICH recurrence was observed among Black (aHR, 1.22; 95% CI, 1.01–1.48) and Asian patients (aHR, 1.29; 95% CI, 1.10–1.50) compared to White patients. 32 While the mechanisms underlying these racial/ethnic differences remain unclear, emerging evidence suggest that socioeconomic factors that are more likely to disproportionately affect minorities resulting in higher premorbid blood pressure and consequently, higher burden of cerebral small vessel disease.24,34,35 Other proposed independent risk factors for recurrent ICH include age, prevalence of cerebral small vessel disease (i.e. disseminated cerebral superficial siderosis, enlarged perivascular space in the centrum semiovale, cerebral microbleeds and white matter changes),38,72–75 previous history of ischemic stroke,13,33 and renal impairment.76
ICH Characteristics of recurrent ICH
Recurrent ICH often tends to involve similar regions of the brain as the index ICH. In a study of 464 ICH survivors, most recurrences were “lobar-lobar” type26, and recurrences occurred in the same type of location (lobar vs. non-lobar) as the index ICH, although the exact location of recurrence was only the same in only 33% of the patients.18 However, in populations with a high incidence of hypertension-related deep ICH, the most common location of recurrent ICH was deep ganglionic, with a younger age of onset.51 In addition to the hematoma location, recurrent ICH also shares the underlying pathology as the index ICH, as observed in a prospective study of 185 patients with recurrent ICH, where the pathology between the recurrent and the index events were in agreement in 151 cases (81.6%).25 In two population-based studies in the UK, recurrence after lobar ICH were all lobar whereas up to 50% of the recurrence after non-lobar ICH was also lobar.16 Recurrent ICH is usually severe, resulting in severe disability in about half of patients77, and death in over a third of patients.16,18
Timing of ICH recurrence
Very few studies reported the time-course of recurrent ICH. The risk of recurrent ICH was most marked in the first 90 days, especially for lobar ICH, although the risk was still high beyond the subacute phase.16 Hanger et al. also found that of 464 ICH survivors, recurrence rate for ICH was higher in the first year (2.1%) while overall the risk was 1.2%.26 The front-loading risk of recurrent ICH has potential clinical implications as it identifies a group of most vulnerable patients who might benefit from more effective prevention efforts. Secondary prevention trials are evaluating therapies such as intensive blood pressure control (NCT02699645 and NCT03863665), and resumption of antithrombotic and lipid lowering medications, with an eye on the safety endpoint of ICH recurrence.78–81
3. Venous thromboembolism after ICH
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), are serious complications after ICH and prior studies have focused on the risk of VTE mainly during the acute and subacute phases of ICH. The overall rate of symptomatic VTE after ICH ranges from 2–5% (Supplemental Table S1), with the incidence being higher in patients admitted to the intensive care unit.82–94 With routine screening, the incidence may be as high as 25% by the end of two weeks of hospitalization.94 The rate of new symptomatic VTE is 2–3 fold higher after ICH than after ischemic stroke (Supplemental Table S1).82–87,90–93 Furthermore, the cumulative rate of VTE in a heterogenous U.S. cohort was 4.7% (95% CI, 4.5–4.9) at 7 years after ICH, which was similar to that after ischemic stroke (4.4%, 95% CI, 4.3–4.5).95 The time course for the development of VTE was, however, different between ICH and ischemic stroke, with the VTE risk being higher in the first year after ICH than after ischemic stroke. The occurrence of VTE may be influenced by race and ethnicity as noted in a systemic review where the risks of DVT and PE were higher among Black and Asian patients compared to White patients (DVT: 2.0%, 7.1% and 12.5% at 3 months for White, Asian and Black patients respectively; PE: 0.8%, 1.4% and 4.2%, respectively).96
Timing and Risk Factors for VTE after ICH
Although data on the time course of VTE after ICH are limited, it is commonly believed that the highest risk is in the acute phase of ICH. DVTs may occur as early as day 2, peaking between days 3 and 7, but could very well continue after the acute phase.97 The majority of the studies reported a mean/median time to symptomatic VTE diagnosis of around 5–20 days (Supplemental Table S1). Commonly recognized risk factors for VTE include age, prior history of VTE, hemiplegia, immobility, high NIHSS score at baseline, intubation, presence of IVH and prolonged length of stay.98,99 High D-dimer has also been shown to be associated with venous clots in some studies.94,100 More recently, concurrent infection was also proposed as a strong risk factor for VTE after ICH.88,101 Melmed et al. reported that any infection was associated with increased risk of VTE (adjusted odds ratio [aOR], 4.5; 95% CI, 1.6–12.6), which was driven predominantly by respiratory (aOR, 5.7; 95% CI, 2.8–11.7) and blood stream infections (aOR, 4.0, 95% CI, 1.3–11.0).101
VTE prophylaxis and treatment after ICH
As VTE is independently associated with poor outcome in patients with ICH,102 prophylaxis is important. One early study showed that without prophylaxis, three quarters of the ICH patients with hemiplegia developed a DVT, and PE-related death occurred in about 5% of the patients.97,103 Intermittent pneumatic compression (IPC) devices have been used extensively in the hyper acute phase of ICH for VTE prophylaxis, based on the results of the Clots in Legs Or sTockings after Stroke 3 (CLOTS 3) trial, with international guidelines including those from the American Heart Association/Stroke and European Stroke Organisation advocating their use (Table 2).8,104–107 Among 2876 acute stroke patients including 376 with ICH, use of IPCs was associated with a significant reduction in the risk of DVTs at 30 days (8.5% vs. 12.1%), and a significant reduction in death at 6 months (aHR, 0.86, 95% CI, 0.74–0.99).108 Studies assessing the efficacy of pharmacological prophylaxis after ICH have yielded conflicting results with one network meta-analysis not showing any relationship between prophylaxis and incident VTE (OR, 0.93; 95% CI, 0.19–4.37)109, while an earlier meta-analysis found that anticoagulation chemoprophylaxis initiated between 1 and 6 days after admission did result in a significant reduction in PE (1.7% vs. 2.9%, RR, 0.37; 95% CI, 0.17–0.80).110 Several guidelines including the American Heart Association/Stroke8, recommend the use of low-dose chemoprophylaxis after the demonstration of hematoma stability, but their implementation in the real-world setting has been surprisingly low. In a retrospective cohort study using a large U.S. administrative database, Prabhakaran et al. showed that less than a fifth of ICH patients received anticoagulation for VTE prophylaxis, and among those where anticoagulation was initiated, less than half of the patients had a time to initiation of less than two days.111 Should VTEs be diagnosed after ICH, treatment options include anticoagulation, inferior vena cava filter, and specifically in case of PEs, thrombolysis and mechanical thrombectomy.112 However, as patients with ICH were excluded from randomized trials on anticoagulation therapy for VTE, there is lack of high-quality evidence for the treatment of VTE after ICH, especially during the acute phase when hematoma expansion and recurrence risks may be high.
Table 2.
Recommendations for Venous Thromboembolism Prophylaxis in Recent Guidelines for Patients with Intracerebral Hemorrhage
| Recommendation: | Recommendation: | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Guideline | Mechanical | Timing | Quality of evidence | Pharmacological | Timing | Quality of evidence |
|
| ||||||
| AHA/ASA 20158 | Patients with ICH should have IPC | Day of hospital admission | High | After documentation of cessation of bleeding, low-dose LMWH or UFH may be considered in patients with lack of mobility | After 1 to 4 days from onset | Moderate |
|
| ||||||
| Australia and New Zealand 2022104 | IPC may be used | Not specified | Weak | Pharmacological prophylaxis may be considered once haematoma growth has stabilized | After 48–72 hours | Weak |
|
| ||||||
| ESO 2014105 | Recommend IPC in immobile patients. | Not specified | Moderate | No recommendation | Not specified | Low |
|
| ||||||
| HSFC 2020106 | Patients should be started on IPC devices | Day of hospital admission | High | LMWH can be initiated after documentation of hematoma stabilization on neuroimaging | After 48 hours | Moderate |
|
| ||||||
| NICE 2018107 | Consider IPC for people who are immobile | Within 3 days from onset | Not mentioned | No recommendation | NR | NR |
ESO=European Stroke Organisation; AHA/ASA=American Heart Association/American Stroke Association; NICE=National Institute for Health and Care Excellence; HSFC=Heart and Stroke Foundation of Canada. IPC=Intermittent Pneumatic Compression.
Conclusion
To summarize, 2.9 million patients experience intracerebral hemorrhage each year worldwide,1 and many of these patients survive and can recover.3 Intracerebral hemorrhage may be a risk marker for cardiovascular disease.113,114 Emerging data indicate that patients with ICH are not only at risk for recurrent bleeding, but also at a higher risk of arterial ischemic events than the general population. There is, however, equipoise about the use of established strategies like antithrombotic and lipid-lowering medications after ICH.8. In light of the increased risk of arterial ischemic events, the focus should also be on de novo initiation of secondary stroke prevention and not just reinstatement of previous medications. Further research is needed to aid the careful selection of specific ICH populations (such as deep ICH) and underlying etiologies (like atrial fibrillation), where the benefit of prevention outweighs the risk of recurrent ICH. This review highlights the need for randomized clinical trials to assess the net clinical benefit of antithrombotic therapy and statin medications in this high-risk population.115–117
Supplementary Material
Acknowledgments
Disclosures
Dr. Li is supported by a fellowship award from the Medical Research Foundation (MRF) outside the submitted work. Dr. Murthy is supported by the National Institutes of Health (NIH) (K23NS105948) and reports personal compensation for medicolegal consulting in stroke and neurological disease.
Non-Standard Abbreviations:
- CAA
cerebral amyloid angiopathy
- DWI
diffusion-weighted imaging
- DVT
deep venous thrombosis
- ICH
intracerebral hemorrhage
- PE
pulmonary embolism
- VTE
venous thromboembolism
Footnotes
Supplemental Material:
References
- 1.Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson LM, Truelsen T, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–281. doi: 10.1016/S2214-109X(13)70089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0 [DOI] [PubMed] [Google Scholar]
- 3.Hemphill JC 3rd, Farrant M, Neill TA Jr. Prospective validation of the ICH Score for 12-month functional outcome. Neurology. 2009;73:1088–1094. doi: 10.1212/WNL.0b013e3181b8b332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasi M, Casolla B, Kyheng M, Boulouis G, Kuchcinski G, Moulin S, Labreuche J, Henon H, Leys D, Cordonnier C. Long-term functional decline of spontaneous intracerebral haemorrhage survivors. J Neurol Neurosurg Psychiatry. 2021;92:249–254. doi: 10.1136/jnnp-2020-324741 [DOI] [PubMed] [Google Scholar]
- 5.Liotta EM, Singh M, Kosteva AR, Beaumont JL, Guth JC, Bauer RM, Prabhakaran S, Rosenberg NF, Maas MB, Naidech AM. Predictors of 30-day readmission after intracerebral hemorrhage: a single-center approach for identifying potentially modifiable associations with readmission. Crit Care Med. 2013;41:2762–2769. doi: 10.1097/CCM.0b013e318298a10f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen T, Liu B, Wan X, Zhang X, Zhang J, Zhou X, Lau AYL, Zhang Y. Risk factors associated with 31-day unplanned readmission in 50,912 discharged patients after stroke in China. BMC Neurol. 2018;18:218. doi: 10.1186/s12883-018-1209-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuohn LR, Leasure AC, Acosta JN, Vanent K, Murthy SB, Kamel H, Matouk CC, Sansing LH, Falcone GJ, Sheth KN. Cause of death in spontaneous intracerebral hemorrhage survivors: Multistate longitudinal study. Neurology. 2020;95:e2736–e2745. doi: 10.1212/WNL.0000000000010736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069 [DOI] [PubMed] [Google Scholar]
- 9.Biffi A, Anderson CD, Battey TW, Ayres AM, Greenberg SM, Viswanathan A, Rosand J. Association Between Blood Pressure Control and Risk of Recurrent Intracerebral Hemorrhage. Jama. 2015;314:904–912. doi: 10.1001/jama.2015.10082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Counsell C, Boonyakarnkul S, Dennis M, Sandercock P, Bamford J, Burn J, Warlow C. Primary Intracerebral Hemorrhage in the Oxfordshire Community Stroke Project .2. Prognosis. Cerebrovascular diseases. 1995;5:26–34. doi: Doi 10.1159/000107814 [DOI] [Google Scholar]
- 11.Li L, Zuurbier SM, Kuker W, Warlow CP, Rothwell PM. Blood Pressure Control and Recurrent Stroke After Intracerebral Hemorrhage in 2002 to 2018 Versus 1981 to 1986: Population-Based Study. Stroke. 2021;52:3243–3248. doi: 10.1161/STROKEAHA.121.034432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inagawa T Recurrent primary intracerebral hemorrhage in Izumo City, Japan. Surgical neurology. 2005;64:28–35; discussion 35–26. doi: 10.1016/j.surneu.2004.09.039 [DOI] [PubMed] [Google Scholar]
- 13.Huhtakangas J, Lopponen P, Tetri S, Juvela S, Saloheimo P, Bode MK, Hillbom M. Predictors for recurrent primary intracerebral hemorrhage: a retrospective population-based study. Stroke. 2013;44:585–590. doi: 10.1161/STROKEAHA.112.671230 [DOI] [PubMed] [Google Scholar]
- 14.Pennlert J, Eriksson M, Carlberg B, Wiklund PG. Long-term risk and predictors of recurrent stroke beyond the acute phase. Stroke. 2014;45:1839–1841. doi: 10.1161/STROKEAHA.114.005060 [DOI] [PubMed] [Google Scholar]
- 15.Flach C, Muruet W, Wolfe CDA, Bhalla A, Douiri A. Risk and Secondary Prevention of Stroke Recurrence: A Population-Base Cohort Study. Stroke. 2020;51:2435–2444. doi: 10.1161/STROKEAHA.120.028992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Poon MTC, Samarasekera NE, Perry LA, Moullaali TJ, Rodrigues MA, Loan JJM, Stephen J, Lerpiniere C, Tuna MA, et al. Risks of recurrent stroke and all serious vascular events after spontaneous intracerebral haemorrhage: pooled analyses of two population-based studies. Lancet Neurol. 2021;20:437–447. doi: 10.1016/S1474-4422(21)00075-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aked J, Delavaran H, Lindgren AG. Survival, causes of death and recurrence up to 3 years after stroke: A population-based study. European journal of neurology. 2021;28:4060–4068. doi: 10.1111/ene.15041 [DOI] [PubMed] [Google Scholar]
- 18.Passero S, Burgalassi L, D'Andrea P, Battistini N. Recurrence of bleeding in patients with primary intracerebral hemorrhage. Stroke. 1995;26:1189–1192. [DOI] [PubMed] [Google Scholar]
- 19.Vermeer SE, Algra A, Franke CL, Koudstaal PJ, Rinkel GJ. Long-term prognosis after recovery from primary intracerebral hemorrhage. Neurology. 2002;59:205–209. [DOI] [PubMed] [Google Scholar]
- 20.Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123–127. doi: 10.1161/01.str.31.1.123 [DOI] [PubMed] [Google Scholar]
- 21.Zia E, Engstrom G, Svensson PJ, Norrving B, Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke. 2009;40:3567–3573. doi: 10.1161/STROKEAHA.109.556324 [DOI] [PubMed] [Google Scholar]
- 22.Viswanathan A, Rakich SM, Engel C, Snider R, Rosand J, Greenberg SM, Smith EE. Antiplatelet use after intracerebral hemorrhage. Neurology. 2006;66:206–209. doi: 10.1212/01.wnl.0000194267.09060.77 [DOI] [PubMed] [Google Scholar]
- 23.Flynn RW, MacDonald TM, Murray GD, MacWalter RS, Doney AS. Prescribing antiplatelet medicine and subsequent events after intracerebral hemorrhage. Stroke. 2010;41:2606–2611. doi: 10.1161/STROKEAHA.110.589143 [DOI] [PubMed] [Google Scholar]
- 24.Biffi A, Anderson CD, Battey TWK, Ayres AM, Greenberg SM, Viswanathan A, Rosand J. Association Between Blood Pressure Control and Risk of Recurrent Intracerebral Hemorrhage. Jama-J Am Med Assoc. 2015;314:904–912. doi: 10.1001/jama.2015.10082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh SJ, Tang SC, Tsai LK, Jeng JS. Pathogenetical subtypes of recurrent intracerebral hemorrhage: designations by SMASH-U classification system. Stroke. 2014;45:2636–2642. doi: 10.1161/STROKEAHA.114.005598 [DOI] [PubMed] [Google Scholar]
- 26.Hanger HC, Wilkinson TJ, Fayez-Iskander N, Sainsbury R. The risk of recurrent stroke after intracerebral haemorrhage. Journal of neurology, neurosurgery, and psychiatry. 2007;78:836–840. doi: 10.1136/jnnp.2006.106500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chong BH, Chan KH, Pong V, Lau KK, Chan YH, Zuo ML, Lui WM, Leung GK, Lau CP, Tse HF, et al. Use of aspirin in Chinese after recovery from primary intracranial haemorrhage. Thrombosis and haemostasis. 2012;107:241–247. doi: 10.1160/TH11-06-0439 [DOI] [PubMed] [Google Scholar]
- 28.Weimar C, Benemann J, Terborg C, Walter U, Weber R, Diener HC, German Stroke Study C. Recurrent stroke after lobar and deep intracerebral hemorrhage: a hospital-based cohort study. Cerebrovascular diseases. 2011;32:283–288. doi: 10.1159/000330643 [DOI] [PubMed] [Google Scholar]
- 29.Asberg S, Farahmand B, Henriksson KM, Appelros P. Statins as secondary preventives in patients with intracerebral hemorrhage. International journal of stroke : official journal of the International Stroke Society. 2018:1747493018816476. doi: 10.1177/1747493018816476 [DOI] [PubMed] [Google Scholar]
- 30.Casolla B, Moulin S, Kyheng M, Henon H, Labreuche J, Leys D, Bauters C, Cordonnier C. Five-Year Risk of Major Ischemic and Hemorrhagic Events After Intracerebral Hemorrhage. Stroke. 2019;50:1100–1107. doi: 10.1161/STROKEAHA.118.024449 [DOI] [PubMed] [Google Scholar]
- 31.Skajaa N, Adelborg K, Horvath-Puho E, Rothman KJ, Henderson VW, Thygesen LC, Sorensen HT. Risks of Stroke Recurrence and Mortality After First and Recurrent Strokes in Denmark: A Nationwide Registry Study. Neurology. 2021. doi: 10.1212/WNL.0000000000013118 [DOI] [PubMed] [Google Scholar]
- 32.Leasure AC, King ZA, Torres-Lopez V, Murthy SB, Kamel H, Shoamanesh A, Al-Shahi Salman R, Rosand J, Ziai WC, Hanley DF, et al. Racial/ethnic disparities in the risk of intracerebral hemorrhage recurrence. Neurology. 2020;94:e314–e322. doi: 10.1212/WNL.0000000000008737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu L, Upadhyaya T, See AA, Ng YP, Kon Kam King N. Incidence of Recurrent Intracerebral Hemorrhages in a Multiethnic South Asian Population. J Stroke Cerebrovasc Dis. 2017;26:666–672. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.044 [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Torres A, Murphy M, Kourkoulis C, Schwab K, Ayres AM, Moomaw CJ, Young Kwon S, Berthaud JV, Gurol ME, Greenberg SM, et al. Hypertension and intracerebral hemorrhage recurrence among white, black, and Hispanic individuals. Neurology. 2018;91:e37–e44. doi: 10.1212/WNL.0000000000005729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castello JP, Pasi M, Abramson JR, Rodriguez-Torres A, Marini S, Demel S, Gilkerson L, Kubiszewski P, Charidimou A, Kourkoulis C, et al. Contribution of Racial and Ethnic Differences in Cerebral Small Vessel Disease Subtype and Burden to Risk of Cerebral Hemorrhage Recurrence. Neurology. 2021;96:e2469–e2480. doi: 10.1212/WNL.0000000000011932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubiszewski P, Sugita L, Kourkoulis C, DiPucchio Z, Schwab K, Anderson CD, Gurol ME, Greenberg SM, Viswanathan A, Rosand J, et al. Association of Selective Serotonin Reuptake Inhibitor Use After Intracerebral Hemorrhage With Hemorrhage Recurrence and Depression Severity. JAMA neurology. 2020. doi: 10.1001/jamaneurol.2020.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee G, Wilson D, Ambler G, Hostettler IC, Shakeshaft C, Cohen H, Yousry T, Salman RAS, Lip GYH, Houlden H, et al. Longer term stroke risk in intracerebral haemorrhage survivors. J Neurol Neurosur Ps. 2020;91:840–845. doi: 10.1136/jnnp-2020-323079 [DOI] [PubMed] [Google Scholar]
- 38.Tsai HH, Chen SJ, Tsai LK, Pasi M, Lo YL, Chen YF, Tang SC, Jeng JS. Long-Term Vascular Outcomes in Patients With Mixed Location Intracerebral Hemorrhage and Microbleeds. Neurology. 2021;96:e995–e1004. doi: 10.1212/WNL.0000000000011378 [DOI] [PubMed] [Google Scholar]
- 39.van Nieuwenhuizen KM, Vaartjes I, Verhoeven JI, Rinkel GJ, Kappelle LJ, Schreuder FH, Klijn CJ. Long-term prognosis after intracerebral haemorrhage. Eur Stroke J. 2020;5:336–344. doi: 10.1177/2396987320953394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murthy SB, Zhang C, Diaz I, Levitan EB, Koton S, Bartz TM, DeRosa JT, Strobino K, Colantonio LD, Iadecola C, et al. Association Between Intracerebral Hemorrhage and Subsequent Arterial Ischemic Events in Participants From 4 Population-Based Cohort Studies. JAMA Neurol. 2021;78:809–816. doi: 10.1001/jamaneurol.2021.0925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murthy SB, Diaz I, Wu X, Merkler AE, Iadecola C, Safford MM, Sheth KN, Navi BB, Kamel H. Risk of Arterial Ischemic Events After Intracerebral Hemorrhage. Stroke. 2020;51:137–142. doi: 10.1161/STROKEAHA.119.026207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaist D, Gonzalez-Perez A, Hald SM, Garcia Rodriguez LA. Higher Risk of Ischemic Stroke After an Intracerebral Hemorrhage Than in General Population: A Cohort Study From the United Kingdom. Stroke. 2021:STROKEAHA121037633. doi: 10.1161/STROKEAHA.121.037633 [DOI] [PubMed] [Google Scholar]
- 43.Falcone GJ, Biffi A, Brouwers HB, Anderson CD, Battey TW, Ayres AM, Vashkevich A, Schwab K, Rost NS, Goldstein JN, et al. Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA neurology. 2013;70:988–994. doi: 10.1001/jamaneurol.2013.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banerjee G, Wilson D, Ambler G, Hostettler IC, Shakeshaft C, Cohen H, Yousry T, Al-Shahi Salman R, Lip GYH, Houlden H, et al. Longer term stroke risk in intracerebral haemorrhage survivors. J Neurol Neurosurg Psychiatry. 2020;91:840–845. doi: 10.1136/jnnp-2020-323079 [DOI] [PubMed] [Google Scholar]
- 45.Meinel TR, Kaesmacher J, Roten L, Fischer U. Covert Brain Infarction: Towards Precision Medicine in Research, Diagnosis, and Therapy for a Silent Pandemic. Stroke. 2020;51:2597–2606. doi: 10.1161/STROKEAHA.120.030686 [DOI] [PubMed] [Google Scholar]
- 46.Kimberly WT, Gilson A, Rost NS, Rosand J, Viswanathan A, Smith EE, Greenberg SM. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72:1230–1235. doi: 10.1212/01.wnl.0000345666.83318.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garg RK, Liebling SM, Maas MB, Nemeth AJ, Russell EJ, Naidech AM. Blood pressure reduction, decreased diffusion on MRI, and outcomes after intracerebral hemorrhage. Stroke. 2012;43:67–71. doi: 10.1161/STROKEAHA.111.629493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kidwell CS, Rosand J, Norato G, Dixon S, Worrall BB, James ML, Elkind MS, Flaherty ML, Osborne J, Vashkevich A, et al. Ischemic lesions, blood pressure dysregulation, and poor outcomes in intracerebral hemorrhage. Neurology. 2017;88:782–788. doi: 10.1212/WNL.0000000000003630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menon RS, Burgess RE, Wing JJ, Gibbons MC, Shara NM, Fernandez S, Jayam-Trouth A, German L, Sobotka I, Edwards D, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Annals of neurology. 2012;71:199–205. doi: 10.1002/ana.22668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murthy SB, Cho SM, Gupta A, Shoamanesh A, Navi BB, Avadhani R, Gruber J, Li Y, Greige T, Lioutas VA, et al. A Pooled Analysis of Diffusion-Weighted Imaging Lesions in Patients With Acute Intracerebral Hemorrhage. JAMA Neurol. 2020;77:1390–1397. doi: 10.1001/jamaneurol.2020.2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang A, Ren M, Deng W, Xi M, Tian L, Han Z, Zang W, Hu H, Zhang B, Cui L, et al. Ischemia in intracerebral hemorrhage: A comparative study of small-vessel and large-vessel diseases. Ann Clin Transl Neurol. 2022;9:79–90. doi: 10.1002/acn3.51497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murthy SB, Zhang C, Gupta A, Cho SM, Rivera-Lara L, Avadhani R, Gruber J, Iadecola C, Falcone GJ, Sheth KN, et al. Diffusion-Weighted Imaging Lesions After Intracerebral Hemorrhage and Risk of Stroke: A MISTIE III and ATACH-2 Analysis. Stroke. 2021;52:595–602. doi: 10.1161/STROKEAHA.120.031628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiegertjes K, Dinsmore L, Drever J, Hutchison A, Stephen J, Valdes Hernandez MC, Bhatnagar P, Minks DP, Rodrigues MA, Werring DJ, et al. Diffusion-weighted imaging lesions and risk of recurrent stroke after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 2021;92:950–955. doi: 10.1136/jnnp-2021-326116 [DOI] [PubMed] [Google Scholar]
- 54.Verhoeven JI, Pasi M, Casolla B, Henon H, de Leeuw FE, Leys D, Klijn CJ, Cordonnier C. Long-term mortality in young patients with spontaneous intracerebral haemorrhage: Predictors and causes of death. Eur Stroke J. 2021;6:185–193. doi: 10.1177/23969873211017723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parasram M, Parikh NS, Merkler AE, Falcone GJ, Sheth KN, Navi BB, Kamel H, Zhang C, Murthy SB. Risk of Mortality After an Arterial Ischemic Event Among Intracerebral Hemorrhage Survivors. Neurohospitalist. 2022;12:19–23. doi: 10.1177/19418744211026709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saloheimo P, Lapp TM, Juvela S, Hillbom M. The impact of functional status at three months on long-term survival after spontaneous intracerebral hemorrhage. Stroke. 2006;37:487–491. doi: 10.1161/01.STR.0000198868.78546.fc [DOI] [PubMed] [Google Scholar]
- 57.Pennlert J, Asplund K, Carlberg B, Wiklund PG, Wisten A, Asberg S, Eriksson M. Antithrombotic Treatment Following Intracerebral Hemorrhage in Patients With and Without Atrial Fibrillation. Stroke. 2015;46:2094–2099. doi: 10.1161/STROKEAHA.115.009087 [DOI] [PubMed] [Google Scholar]
- 58.Lerario MP, Gialdini G, Lapidus DM, Shaw MM, Navi BB, Merkler AE, Lip GY, Healey JS, Kamel H. Risk of Ischemic Stroke after Intracranial Hemorrhage in Patients with Atrial Fibrillation. PLoS One. 2015;10:e0145579. doi: 10.1371/journal.pone.0145579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuramatsu JB, Gerner ST, Schellinger PD, Glahn J, Endres M, Sobesky J, Flechsenhar J, Neugebauer H, Juttler E, Grau A, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. Jama. 2015;313:824–836. doi: 10.1001/jama.2015.0846 [DOI] [PubMed] [Google Scholar]
- 60.Sprugel MI, Kuramatsu JB, Volbers B, Saam JI, Sembill JA, Gerner ST, Balk S, Hamer HM, Lucking H, Holter P, et al. Impact of Statins on Hematoma, Edema, Seizures, Vascular Events, and Functional Recovery After Intracerebral Hemorrhage. Stroke. 2021;52:975–984. doi: 10.1161/STROKEAHA.120.029345 [DOI] [PubMed] [Google Scholar]
- 61.Endres M, Nolte CH, Scheitz JF. Statin Treatment in Patients With Intracerebral Hemorrhage. Stroke. 2018;49:240–246. doi: 10.1161/STROKEAHA.117.019322 [DOI] [PubMed] [Google Scholar]
- 62.Roumie CL, Zillich AJ, Bravata DM, Jaynes HA, Myers LJ, Yoder J, Cheng EM. Hypertension treatment intensification among stroke survivors with uncontrolled blood pressure. Stroke. 2015;46:465–470. doi: 10.1161/STROKEAHA.114.007566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roumie CL, Ofner S, Ross JS, Arling G, Williams LS, Ordin DL, Bravata DM. Prevalence of inadequate blood pressure control among veterans after acute ischemic stroke hospitalization: a retrospective cohort. Circ Cardiovasc Qual Outcomes. 2011;4:399–407. doi: 10.1161/CIRCOUTCOMES.110.959809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bravata DM, Daggy J, Brosch J, Sico JJ, Baye F, Myers LJ, Roumie CL, Cheng E, Coffing J, Arling G. Comparison of Risk Factor Control in the Year After Discharge for Ischemic Stroke Versus Acute Myocardial Infarction. Stroke. 2018;49:296–303. doi: 10.1161/STROKEAHA.117.017142 [DOI] [PubMed] [Google Scholar]
- 65.Liu C, Roth DL, Gottesman RF, Sheehan OC, Blinka MD, Howard VJ, Judd SE, Cushman M. Change in Life's Simple 7 Measure of Cardiovascular Health After Incident Stroke: The REGARDS Study. Stroke. 2021;52:878–886. doi: 10.1161/STROKEAHA.120.030836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bergstrom L, Irewall AL, Soderstrom L, Ogren J, Laurell K, Mooe T. One-Year Incidence, Time Trends, and Predictors of Recurrent Ischemic Stroke in Sweden From 1998 to 2010: An Observational Study. Stroke. 2017;48:2046–2051. doi: 10.1161/STROKEAHA.117.016815 [DOI] [PubMed] [Google Scholar]
- 67.Santalucia P, Baviera M, Cortesi L, Tettamanti M, Marzona I, Nobili A, Riva E, Fortino I, Bortolotti A, Merlino L, et al. Epidemiologic Trends in Hospitalized Ischemic Stroke from 2002 to 2010: Results from a Large Italian Population-Based Study. J Stroke Cerebrovasc Dis. 2015;24:1917–1923. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 68.Charidimou A, Imaizumi T, Moulin S, Biffi A, Samarasekera N, Yakushiji Y, Peeters A, Vandermeeren Y, Laloux P, Baron JC, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds A meta-analysis. Neurology. 2017;89:820–829. doi: 10.1212/Wnl.0000000000004259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garg A, Ortega-Gutierrez S, Farooqui M, Nagaraja N. Recurrent intracerebral hemorrhage in patients with cerebral amyloid angiopathy: a propensity-matched case-control study. Journal of neurology. 2022;269:2200–2205. doi: 10.1007/s00415-021-10937-4 [DOI] [PubMed] [Google Scholar]
- 70.Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, Goldstein JN, Rosand J, Viswanathan A, Pantoni L, et al. Mixed-location cerebral hemorrhage/microbleeds: Underlying microangiopathy and recurrence risk. Neurology. 2018;90:e119–e126. doi: 10.1212/WNL.0000000000004797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsai HH, Pasi M, Tsai LK, Chen YF, Lee BC, Tang SC, Fotiadis P, Huang CY, Yen RF, Jeng JS, et al. Microangiopathy underlying mixed-location intracerebral hemorrhages/microbleeds: A PiB-PET study. Neurology. 2019;92:e774–e781. doi: 10.1212/WNL.0000000000006953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pinho J, Araujo JM, Costa AS, Silva F, Francisco A, Quintas-Neves M, Soares-Fernandes J, Ferreira C, Oliveira TG. Intracerebral Hemorrhage Recurrence in Patients with and without Cerebral Amyloid Angiopathy. Cerebrovasc Dis Extra. 2021;11:15–21. doi: 10.1159/000513503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Charidimou A, Imaizumi T, Moulin S, Biffi A, Samarasekera N, Yakushiji Y, Peeters A, Vandermeeren Y, Laloux P, Baron JC, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: A meta-analysis. Neurology. 2017;89:820–829. doi: 10.1212/WNL.0000000000004259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castello JP, Pasi M, Kubiszewski P, Abramson JR, Charidimou A, Kourkoulis C, DiPucchio Z, Schwab K, Anderson CD, Gurol ME, et al. Cerebral Small Vessel Disease and Depression Among Intracerebral Hemorrhage Survivors. Stroke. 2022;53:523–531. doi: 10.1161/STROKEAHA.121.035488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu TQ, Lin WZ, Feng YL, Shen FX, Chen J, Wu WW, Zhu XD, Gu L, Fu Y. Leukoaraiosis is associated with clinical symptom severity, poor neurological function prognosis and stroke recurrence in mild intracerebral hemorrhage: a prospective multi-center cohort study. Neural Regen Res. 2022;17:819–823. doi: 10.4103/1673-5374.322469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt LB, Goertz S, Wohlfahrt J, Melbye M, Munch TN. Recurrent Intracerebral Hemorrhage: Associations with Comorbidities and Medicine with Antithrombotic Effects. PLoS ONE. 2016;11:e0166223. doi: 10.1371/journal.pone.0166223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonzalez-Duarte A, Cantu C, Ruiz-Sandoval JL, Barinagarrementeria F. Recurrent primary cerebral hemorrhage: frequency, mechanisms, and prognosis. Stroke. 1998;29:1802–1805. [DOI] [PubMed] [Google Scholar]
- 78.Anticoagulation in ICH Survivors for Stroke Prevention and Recovery (ASPIRE). https://clinicaltrials.gov/ct2/show/NCT03907046. Accessed on March 10, 2022.
- 79.Statins In Intracerbral Hemorrhage (SATURN). https://clinicaltrials.gov/ct2/show/NCT03936361. Accessed on February 15, 2022.
- 80.Triple Therapy Prevention of Recurrent Intracerebral Disease EveNts Trial (TRIDENT). https://clinicaltrials.gov/ct2/show/NCT02699645. Accessed on March 1, 2022. [DOI] [PubMed]
- 81.https://clinicaltrials.gov/ct2/show/NCT03863665. Accessed on March 1, 2022.
- 82.Skaf E, Stein PD, Beemath A, Sanchez J, Bustamante MA, Olson RE. Venous thromboembolism in patients with ischemic and hemorrhagic stroke. Am J Cardiol. 2005;96:1731–1733. doi: 10.1016/j.amjcard.2005.07.097 [DOI] [PubMed] [Google Scholar]
- 83.Goldstein JN, Fazen LE, Wendell L, Chang Y, Rost NS, Snider R, Schwab K, Chanderraj R, Kabrhel C, Kinnecom C, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care. 2009;10:28–34. doi: 10.1007/s12028-008-9134-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gregory PC, Kuhlemeier KV. Prevalence of venous thromboembolism in acute hemorrhagic and thromboembolic stroke. Am J Phys Med Rehabil. 2003;82:364–369. doi: 10.1097/01.PHM.0000064725.62897.A5 [DOI] [PubMed] [Google Scholar]
- 85.Stecker M, Michel K, Antaky K, Cherian S, Koyfmann F. Risk Factors for DVT/PE in Patients with Stroke and Intracranial Hemorrhage. Open Neurol J. 2014;8:1–6. doi: 10.2174/1874205X01408010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ding D, Sekar P, Moomaw CJ, Comeau ME, James ML, Testai F, Flaherty ML, Vashkevich A, Worrall BB, Woo D, et al. Venous Thromboembolism in Patients With Spontaneous Intracerebral Hemorrhage: A Multicenter Study. Neurosurgery. 2019;84:E304–E310. doi: 10.1093/neuros/nyy333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ji R, Li G, Zhang R, Hou H, Zhao X, Wang Y. Higher risk of deep vein thrombosis after hemorrhagic stroke than after acute ischemic stroke. J Vasc Nurs. 2019;37:18–27. doi: 10.1016/j.jvn.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 88.Kim KS, Brophy GM. Symptomatic venous thromboembolism: incidence and risk factors in patients with spontaneous or traumatic intracranial hemorrhage. Neurocrit Care. 2009;11:28–33. doi: 10.1007/s12028-009-9201-4 [DOI] [PubMed] [Google Scholar]
- 89.Chu Q, Liao L, Wei W, Ye Z, Zeng L, Qin C, Tang Y. Venous Thromboembolism in ICU Patients with Intracerebral Hemorrhage: Risk Factors and the Prognosis After Anticoagulation Therapy. Int J Gen Med. 2021;14:5397–5404. doi: 10.2147/IJGM.S327676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yablon SA, Rock WA Jr., Nick TG, Sherer M, McGrath CM, Goodson KH. Deep vein thrombosis: prevalence and risk factors in rehabilitation admissions with brain injury. Neurology. 2004;63:485–491. doi: 10.1212/01.wnl.0000133009.24727.9f [DOI] [PubMed] [Google Scholar]
- 91.Lacut K, Bressollette L, Le Gal G, Etienne E, De Tinteniac A, Renault A, Rouhart F, Besson G, Garcia JF, Mottier D, et al. Prevention of venous thrombosis in patients with acute intracerebral hemorrhage. Neurology. 2005;65:865–869. doi: 10.1212/01.wnl.0000176073.80532.a2 [DOI] [PubMed] [Google Scholar]
- 92.Kawase K, Okazaki S, Toyoda K, Toratani N, Yoshimura S, Kawano H, Nagatsuka K, Matsuo H, Naritomi H, Minematsu K. Sex difference in the prevalence of deep-vein thrombosis in Japanese patients with acute intracerebral hemorrhage. Cerebrovascular diseases. 2009;27:313–319. doi: 10.1159/000202006 [DOI] [PubMed] [Google Scholar]
- 93.Ogata T, Yasaka M, Wakugawa Y, Inoue T, Ibayashi S, Okada Y. Deep venous thrombosis after acute intracerebral hemorrhage. Journal of the neurological sciences. 2008;272:83–86. doi: 10.1016/j.jns.2008.04.032 [DOI] [PubMed] [Google Scholar]
- 94.Cheng X, Zhang L, Xie NC, Ma YQ, Lian YJ. High Plasma Levels of D-Dimer Are Independently Associated with a Heightened Risk of Deep Vein Thrombosis in Patients with Intracerebral Hemorrhage. Mol Neurobiol. 2016;53:5671–5678. doi: 10.1007/s12035-015-9487-5 [DOI] [PubMed] [Google Scholar]
- 95.Murthy SB, Merkler AE, Gialdini G, Chatterjee A, Iadecola C, Navi BB, Kamel H. Long-term Risk of Venous Thromboembolism After Stroke. Stroke. 2017;48. [Google Scholar]
- 96.Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther. 2008;25:831–841. doi: 10.1007/s12325-008-0092-0 [DOI] [PubMed] [Google Scholar]
- 97.Brandstater ME. Venous thromboembolism in stroke. West J Med. 1992;157:666–667. [PMC free article] [PubMed] [Google Scholar]
- 98.Cai Q, Zhang X, Chen H. Patients with venous thromboembolism after spontaneous intracerebral hemorrhage: a review. Thromb J. 2021;19:93. doi: 10.1186/s12959-021-00345-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee SH JW, Choi SK, Kwun BD. Deep vein thrombosis and pulmonary embolism following hemorrhagic stroke. Journal of Neurointensive Care. 2018;1:20–24. [Google Scholar]
- 100.Ogata T, Yasaka M, Wakugawa Y, Kitazono T, Okada Y. [Association of location of deep venous thrombosis and d-dimer value in acute intracerebral hemorrhage]. Nihon Ronen Igakkai Zasshi. 2011;48:686–690. doi: 10.3143/geriatrics.48.686 [DOI] [PubMed] [Google Scholar]
- 101.Melmed KR, Boehme A, Ironside N, Murthy S, Park S, Agarwal S, Connolly ES, Claassen J, Elkind MSV, Roh D. Respiratory and Blood Stream Infections are Associated with Subsequent Venous Thromboembolism After Primary Intracerebral Hemorrhage. Neurocritical Care. 2021;34:85–91. doi: 10.1007/s12028-020-00974-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li J, Wang D, Wang W, Jia J, Kang K, Zhang J, Zhao X. In-hospital venous thromboembolism is associated with poor outcome in patients with spontaneous intracerebral hemorrhage: A multicenter, prospective study. J Stroke Cerebrovasc Dis. 2020;29:104958. doi: 10.1016/j.jstrokecerebrovasdis.2020.104958 [DOI] [PubMed] [Google Scholar]
- 103.Brandstater ME, Roth EJ, Siebens HC. Venous thromboembolism in stroke: literature review and implications for clinical practice. Arch Phys Med Rehabil. 1992;73:S379–391. [PubMed] [Google Scholar]
- 104.The Australian and New Zealand Clinical Guidelines for Stroke. https://appmagicapporg/#/guideline/WE8wOn/section/EKyKVL. 2022;Last checked 30/03/2022.
- 105.Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJ, Krieger D, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9:840–855. doi: 10.1111/ijs.12309 [DOI] [PubMed] [Google Scholar]
- 106.Shoamanesh A, Patrice Lindsay M, Castellucci LA, Cayley A, Crowther M, de Wit K, English SW, Hoosein S, Huynh T, Kelly M, et al. Canadian stroke best practice recommendations: Management of Spontaneous Intracerebral Hemorrhage, 7th Edition Update 2020. International journal of stroke : official journal of the International Stroke Society. 2021;16:321–341. doi: 10.1177/1747493020968424 [DOI] [PubMed] [Google Scholar]
- 107.Dawoud D LS, Glen J, Sharpin C, Committee NG. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. NICE guidelines [NG89]. 2018. [PubMed] [Google Scholar]
- 108.Collaboration CT, Dennis M, Sandercock P, Reid J, Graham C, Forbes J, Murray G. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382:516–524. doi: 10.1016/S0140-6736(13)61050-8 [DOI] [PubMed] [Google Scholar]
- 109.Yogendrakumar V, Lun R, Khan F, Salottolo K, Lacut K, Graham C, Dennis M, Hutton B, Wells PS, Fergusson D, et al. Venous thromboembolism prevention in intracerebral hemorrhage: A systematic review and network meta-analysis. PLoS ONE. 2020;15:e0234957. doi: 10.1371/journal.pone.0234957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paciaroni M, Agnelli G, Venti M, Alberti A, Acciarresi M, Caso V. Efficacy and safety of anticoagulants in the prevention of venous thromboembolism in patients with acute cerebral hemorrhage: a meta-analysis of controlled studies. J Thromb Haemost. 2011;9:893–898. doi: 10.1111/j.1538-7836.2011.04241.x [DOI] [PubMed] [Google Scholar]
- 111.Prabhakaran S, Herbers P, Khoury J, Adeoye O, Khatri P, Ferioli S, Kleindorfer DO. Is prophylactic anticoagulation for deep venous thrombosis common practice after intracerebral hemorrhage? Stroke. 2015;46:369–375. doi: 10.1161/STROKEAHA.114.008006 [DOI] [PubMed] [Google Scholar]
- 112.Key NS, Kasthuri RS. Current treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2010;30:372–375. doi: 10.1161/ATVBAHA.109.197145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lackland DT, Elkind MS, D'Agostino R Sr., Dhamoon MS, Goff DC Jr., Higashida RT, McClure LA, Mitchell PH, Sacco RL, Sila CA, et al. Inclusion of stroke in cardiovascular risk prediction instruments: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1998–2027. doi: 10.1161/STR.0b013e31825bcdac [DOI] [PubMed] [Google Scholar]
- 114.Dhamoon MS, Elkind MS. Inclusion of stroke as an outcome and risk equivalent in risk scores for primary and secondary prevention of vascular disease. Circulation. 2010;121:2071–2078. doi: 10.1161/CIRCULATIONAHA.109.921072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murthy SB, Biffi A, Falcone GJ, Sansing LH, Torres Lopez V, Navi BB, Roh DJ, Mandava P, Hanley DF, Ziai WC, et al. Antiplatelet Therapy After Spontaneous Intracerebral Hemorrhage and Functional Outcomes. Stroke. 2019;50:3057–3063. doi: 10.1161/STROKEAHA.119.025972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Shahi Salman R, Minks DP, Mitra D, Rodrigues MA, Bhatnagar P, du Plessis JC, Joshi Y, Dennis MS, Murray GD, Newby DE, et al. Effects of antiplatelet therapy on stroke risk by brain imaging features of intracerebral haemorrhage and cerebral small vessel diseases: subgroup analyses of the RESTART randomised, open-label trial. Lancet Neurol. 2019;18:643–652. doi: 10.1016/S1474-4422(19)30184-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Bejot Y, Cabrejo L, Cha JK, Ducrocq G, Giroud M, et al. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N Engl J Med. 2020;382:9. doi: 10.1056/NEJMoa1910355 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


