Abstract
Background:
Patients with chronic liver disease (CLD) often present with an elevated International Normalized Ratio (INR). Although elevated INR reflects a higher risk of hemorrhage among warfarin users, its clinical significance in CLD patients is less clear.
Objectives:
We used Veterans Health Administration data to quantify the association between INR and (non-variceal) hemorrhage in patients with CLD as compared to warfarin users.
Methods:
We performed a multivariate competing risk analysis to study the association between INR and hemorrhage in the two cohorts. We used an interaction term between INR and cohort (CLD/warfarin users) to test if INR had different effects on hemorrhage in the two cohorts.
Results:
Data from 80,134 patients (14,412 with CLD and 65,722 taking warfarin) were analyzed. The effect of INR on the risk of hemorrhage differed between CLD patients and warfarin users (interaction p<0.001). As INR increased above 1.5, the adjusted hazards ratio (aHR) for hemorrhage in CLD patients increased to 2.25 but remained fairly constant with further elevation in INR values. In contrast, the risk of hemorrhage in patients taking warfarin remained low with INR in the sub-therapeutic (INR < 2.0) and therapeutic ranges (INR 2.0–3.0), and increased exponentially with INR in the supra-therapeutic range (aHR 1.64 with INR >3.0–3.5, and 4.70 with INR > 3.5).
Conclusions:
The relationship between INR and risk of hemorrhage in CLD patients is different from that in warfarin users. Caution should be exercised extrapolating data from warfarin users to make clinical decisions in CLD patients.
Keywords: International Normalized Ratio, Chronic Liver Disease, Warfarin, Hemostasis, Hemorrhage
A: INTRODUCTION:
In the United States, there are 4.5 million adults with chronic liver disease (CLD), and they frequently present with coagulation abnormalities, such as elevated International Normalized Ratio (INR). While elevated INR values signify protection against venous thromboembolism (VTE) and a higher risk of hemorrhage among patients taking warfarin [2], their clinical significance in patients with CLD is less clear [3, 4].
Preclinical data suggest that patients with CLD have rebalanced hemostasis despite coagulation abnormalities [4, 5]. CLD is associated with reduced synthesis of both pro- and anticoagulant factors. The decrease in procoagulant factors, particularly Factors I, II, V, VII, and X, elevates the INR; however, the concurrent decrease in anticoagulant factors (antithrombin, protein C, protein S) and increase in procoagulant Factor VIII [6, 7] are hypothesized to “rebalance” the hemostasis [5, 8]. This rebalancing does not occur in patients taking warfarin where elevation in INR reflects a predominant reduction in vitamin K-dependent procoagulant factors [2]. A preclinical study demonstrated this difference by finding different degrees of reduction of procoagulant factors in plasma from CLD patients and warfarin users who had similar INR values [9]. Unfortunately, there are no clinical data evaluating if these biological differences translate into different risks of hemorrhage in the two populations.
The American Gastroenterology Association guidelines recommend against using INR values to decide about plasma and coagulation factors replacement before invasive procedures in CLD patients [10]. These guidelines are based on “weak” evidence obtained from case-series and single-arm cohort studies. Clinical practice in prevention of hemorrhage among CLD patients is non-uniform. Many physicians still manage elevated INRs in CLD patients similar to patients taking warfarin. They avoid prophylactic anticoagulation in CLD patients with elevated INR [11, 12], and transfuse plasma and coagulation factors to normalize INR [13]. These practices are likely due to a paucity of data showing different risk of hemorrhage for patients with elevated INR from liver disease rather than warfarin therapy.
To provide further evidence in this area, we conducted a large retrospective cohort study using the national Veterans Health Administration (VHA) database. We analyzed the association between INR and risk of non-variceal hemorrhage among patients with CLD and those on warfarin therapy. We hypothesized that elevated INR would have different effects on the risk of hemorrhage in the two populations.
B: METHODS:
B1: Data source:
We used the VHA database for this study. Within this database, we obtained patient demographics, medical comorbidities, vital signs and laboratory values from Veterans Administration Informatics and Computing Infrastructure (VINCI). We obtained prescription data for antiplatelet and anticoagulant therapy from Pharmacy Benefits Management (PBM) database. Information on date and cause of death was derived from Veterans Administration vital status file [14].
B2: Study Population:
This study was approved by the VHA Institutional Review Board.
Using VINCI, we identified a study cohort of patients 18 years or older, diagnosed with CLD between 10/1/2002 and 9/30/2016 using International Classification of Diseases (ICD) 9/10 codes. To provide accuracy of CLD diagnosis through ICD codes, we followed a previously validated algorithm with a positive predictive value of 83% [15]. According to this algorithm [15], subjects were required to have one ICD9/10 code for CLD (chronic hepatitis or cirrhosis) and one ICD9/10 code for a common clinical presentation of CLD other than ascites (e.g., hepatic encephalopathy) to qualify for the diagnosis (Table A, Supplementary Appendix)[16]. To exclude patients on anticoagulant therapy from this cohort, we excluded those with a history of atrial fibrillation or flutter, prosthetic cardiac valve, diagnosis of VTE within 5 years before the diagnosis of CLD, and those with prescriptions for anticoagulant therapy.
We identified a comparative cohort of warfarin users by selecting patients 18 years or older, diagnosed with atrial fibrillation or flutter between 10/1/2002 and 9/30/2016 (Table B, Supplementary Appendix) who had a warfarin prescription for at least 30 days. From this cohort, we excluded patients with a history of CLD, prosthetic cardiac valve, diagnosis of VTE within 5 years before the diagnosis of atrial fibrillation/flutter, and those with prescriptions for anticoagulant therapy (other than warfarin).
Patients with history of malignancy and missing data on demographics, vital signs and laboratory values were also excluded from both cohorts (Figure 1). Both cohorts were followed through 09/30/2018.
Figure 1.
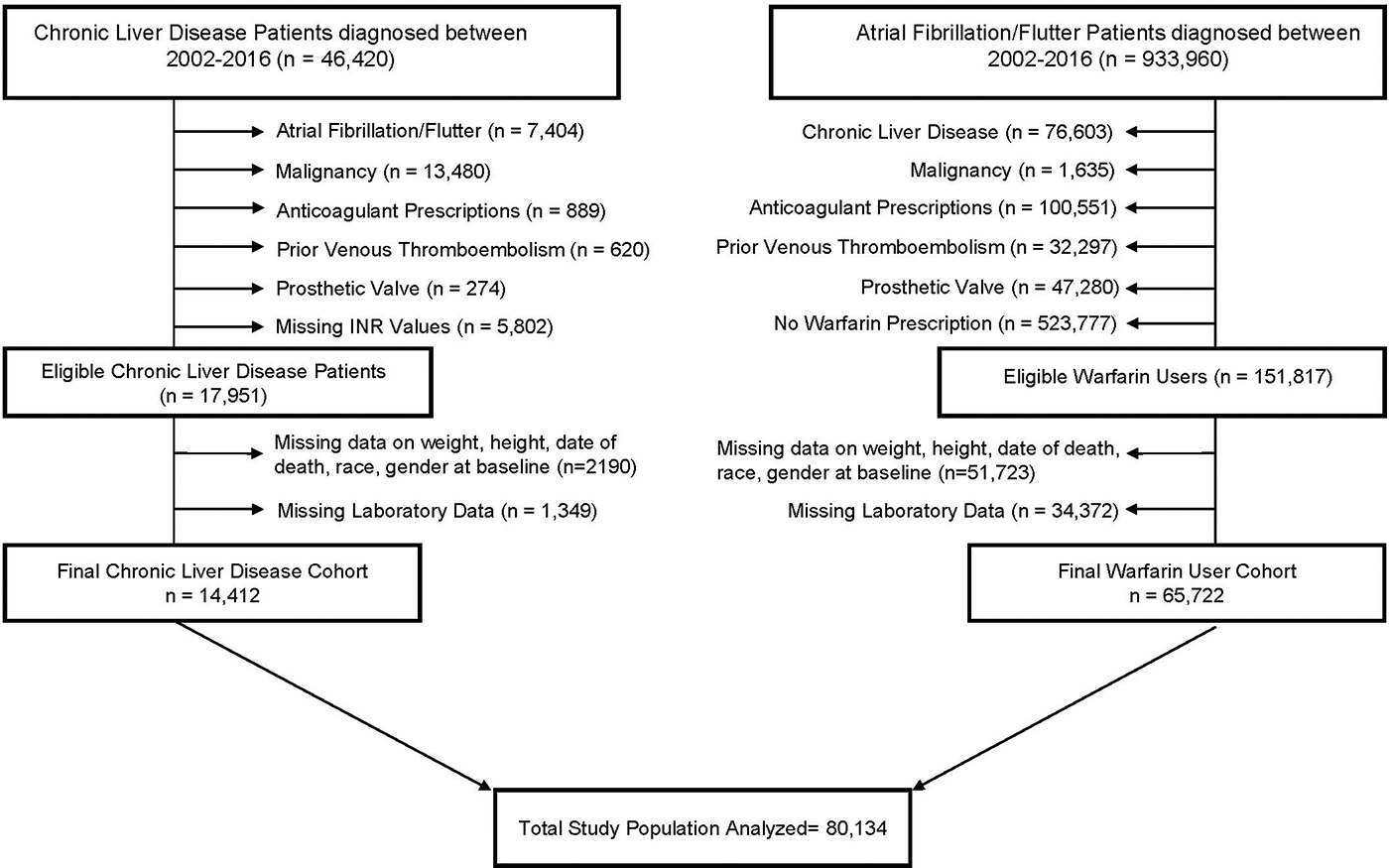
CONOSORT DIAGRAM
B3: Measurement of Covariates:
Demographic characteristics including age, gender, and race were defined as fixed variables, with values closest to time 0 used for analysis. Vital signs (blood pressure, body mass index) and laboratory values [INR, hemoglobin, bilirubin, albumin, estimated glomerular filtration rate (eGFR)] were defined as time-dependent variables with all available values for each patient utilized for analysis. Baseline medical comorbidities (e.g., alcohol abuse and comorbidities in the Charlson Comorbidity Index) were identified using ICD9/10 codes present within one year prior to CLD or atrial fibrillation/flutter diagnosis, and used as fixed variables in the analyses. Prescriptions for antiplatelet and anticoagulant therapy were defined as time-dependent variables (Table D of the Supplementary Appendix).
B4: Detection of Outcomes:
The primary outcome of interest was incidence of non-variceal hemorrhage within 12 months of INR measurement. We identified non-variceal hemorrhage using previously validated ICD-9/10 codes (Table C, Supplementary Appendix) [17–20] present in the primary or secondary position of inpatient diagnosis or discharge codes. Information on date and cause of death was obtained from Veterans Administration vital status file [21, 22].
B5: Statistical Approach:
We estimated a sample size requirement of at least 722 patients per cohort to show that the rise in risk of hemorrhage among CLD patients is one-half that among warfarin users with similar INR elevation, using β of 0.1 (i.e., a power of 90%), and 2-sided α of 0.05.
Step 1: We assessed the effect of INR on the risk of non-variceal hemorrhage in the two cohorts separately using multivariate time-to-event analyses. We used the method of Fine and Gray [23] to account for the competing risk of non-hemorrhagic death. INR was used as a time-dependent variable with 6 categories in the analyses: INR < 1.5 (referent), INR 1.5–2.0, INR >2.0–2.5, INR >2.5–3.0, INR >3.0–3.5, INR >3.5. For warfarin users, time 0 was defined as 15-days after the 1st warfarin prescription (because INR values immediately after warfarin initiation reflect reduction in factor VII [24]). For patients with CLD, time 0 was the time of first INR measurement, at least 15 days after CLD was first coded. We adjusted the competing risk analysis for known risk factors for hemorrhage including: age, estimated glomerular filtration rate (eGFR), stroke, prior hemorrhage, hypertension, anemia, ethanol abuse, concomitant antiplatelet therapy, platelet count [25–27], and medical comorbidities in Charlson Comorbidity Index [28]. In the cohort of CLD patients, we also adjusted for the variables in the Child–Pugh classification [29] to account for severity of liver disease (Table D of the Supplementary Appendix).
Step 2: We identified covariates that had different effects on the risk of hemorrhage within the two cohorts. We used these covariates as interaction terms (variable*cohort) in the main analysis. In this analysis, we studied the relationship between INR and risk of hemorrhage in the two cohorts adjusting for the same covariates as for step 1 as well as the interaction terms. Using stepwise, backward deletion, we omitted variables (including interaction terms) that had an insignificant relationship (two-sided p-value > 0.05) with the outcome.
Sensitivity Analysis:
We performed a sensitivity analysis for patients with INR values > 2. We generated propensity-score matched pairs of patients with CLD and patients taking warfarin (matched on age, gender, race and BMI). We performed competing risk analysis (adjusted for same covariates as step 1) to study the effect of INR on hemorrhage among the matched pairs. Anticipating a limited number of CLD patients with INR > 2, we used INR as a continuous variable to allow a statistically powered analysis of this sub-group.
All analyses were performed using SAS version 9.2.
C: RESULTS:
A total of 80,134 patients (14,412 patients with CLD, and 65,722 patients taking warfarin) were studied. (Figure 1). CLD patients were younger than patients taking warfarin with median ages of 58 and 74 years, respectively. Most patients in both cohorts (>95%) were male. Among patients with CLD, 25% had at least one elevated INR value (INR > 1.5) whereas among warfarin users, 90% had elevated INR values (Table 1). At diagnosis of CLD, 46% of the patients had MELD score < 10, 51% had MELD scores between 10 and 30, and only 3 % had MELD scores > 30.
Table 1:
Baseline Characteristics of patients with CLD and Warfarin Users
| Demographic and Clinical Characteristics | CLD N=14,412 |
Warfarin Users N=65,722 |
P-Value |
|---|---|---|---|
|
| |||
| Median age (years) | 58.0 | 74.0 | <0.0001 |
|
| |||
| Female Gender (%) | 3.0 | 1.6 | <0.0001 |
|
| |||
| Race (%) | <0.0001 | ||
| White | 85.5 | 89.5 | |
| African American | 11.9 | 8.6 | |
| Other | 2.6 | 1.9 | |
|
| |||
| BMI Kg/m2 (%) | <0.0001 | ||
| <18.5 | 1.7 | 1.2 | |
| 18.5– <25 | 26.6 | 21.8 | |
| 25- <30 | 34.6 | 35.0 | |
| >/= 30 | 37.1 | 42.1 | |
|
| |||
| Charlson Comorbidity Index (median) | 1.0 | 0 | <0.0001 |
|
| |||
| Alcohol Use (%) | 61.7 | 5.0 | <0.0001 |
|
| |||
| Hypertension (%) | 55.4 | 81.7 | <0.0001 |
|
| |||
| Anemia (%) | 45.3 | 23.9 | <0.0001 |
|
| |||
| Antiplatelet use (%) | 13.7 | 38.6 | <0.0001 |
|
| |||
| Platelet count (%) | |||
| <50 k/mL | 4.9 | 0.1 | |
| >/=50 k/mL | 95.1 | 99.9 | |
|
| |||
| INR (% of all INR values) | <0.0001 | ||
| </= 1.5 | 75.0 | 9.6 | |
| >1.5–2.0 | 17.3 | 15.2 | |
| >2.0–2.5 | 4.0 | 47.6 | |
| >2.5–3.0 | 1.6 | 22.5 | |
| >3.0–3.5 | 0.5 | 2.7 | |
| >3.5 | 1.7 | 2.5 | |
Abbreviations: %=percentage of patients, CLD=chronic liver disease, INR=International Normalized Ratio, N=sample size, BMI=body mass index
Within 12 months after INR measurement, 1604 non-variceal hemorrhages occurred: 453 among patients with CLD and 1151 among warfarin users. The absolute incidence of non-variceal hemorrhage was 0.031 per year in patients with CLD and 0.017 per year in patients taking warfarin. The most common location of hemorrhage was upper gastrointestinal tract in patients with CLD and lower gastrointestinal tract in patients taking warfarin (Table 2).
Table 2:
Incidence of Non-Variceal Hemorrhage
| Chronic Liver disease (N=14,412) n (%) | Warfarin Users (N=65,722) n (%) | |
|---|---|---|
| Total (Any location) | 453 (3.1% per year) | 1151 (1.7% per year) |
| Intracranial (non-traumatic) | 41 (9.1%) | 198 (17.2%) |
| Orbital | 0 (0%) | 5 (0.4%) |
| Nose & respiratory | 31 (6.8%) | 166 (14.4%) |
| Cardiac | 0 (0%) | 9 (0.8%) |
| Gastrointestinal, Upper | 262 (57.8%) | 271 (23.5%) |
| Gastrointestinal, Lower | 92 (20.3%) | 304 (26.4%) |
| Peritoneal | 10 (2.2%) | 19 (1.7%) |
| Genitourinary (excluded microscopic hematuria) | 10 (2.2%) | 135 (11.7%) |
| Joint/soft tissue | 2 (0.4%) | 22 (1.9%) |
| Hemorrhage, not otherwise specified | 5 (1.1%) | 22 (1.9%) |
Abbreviations: n= number of hemorrhages in each location, % = percentage of hemorrhage in each location
In the separate cohort analyses, age, dementia, gender, HTN, INR and platelet count were found to have different effects on the risk of hemorrhage in the two cohorts (Table E and F of Supplementary Appendix), and thus we included their interaction terms in the combined cohort analysis.
In step 2 (combined cohort analysis), we found a significant difference between the effect of INR on risk of hemorrhage in CLD patients verses warfarin users (interaction p-value < 0.001). As INR increased above the normal range in CLD patients, the adjusted hazards ratio (aHR) of hemorrhage increased to 2.25 but remained relatively stable with further elevation of INR values. In contrast, among patients taking warfarin, the risk of hemorrhage rose exponentially with INR values increasing above 3 (Figure 2).
Figure 2.
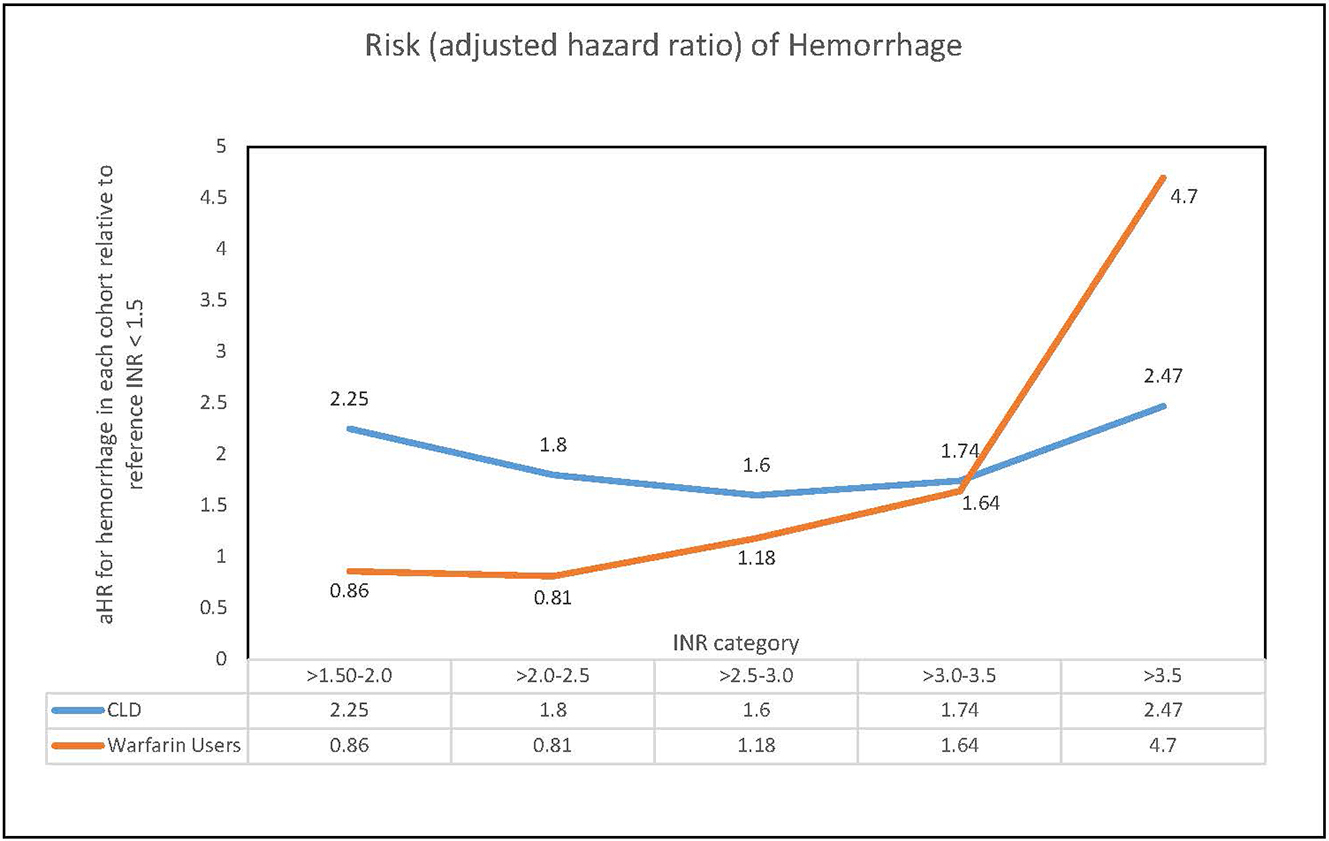
INR specific risk of hemorrhage in chronic liver disease patients and warfarin users
Abbrevations : INR = International Normalized Ratio, CLD = chronic liver disease, Ahr=adjusted harzards ratio
Several factors increased the risk of hemorrhage in both cohorts: alcohol use, anemia, antiplatelet therapy, cardiovascular disease, chronic kidney disease, chronic lung disease, dementia, fracture, hemi- or paraplegia, peptic ulcer disease, surgery and trauma. Thrombocytopenia (platelet count < 50,000/ml) predicted a higher risk of hemorrhage among warfarin users but had no effect on risk of hemorrhage in CLD patients. Similarly, male gender, older age and hypertension predicted a higher risk of hemorrhage among patients taking warfarin. In contrast, among CLD patients, gender and hypertension had no association with the risk of hemorrhage and older age was protective against hemorrhage (Table 3).
Table 3:
Competing risk analysis for the effect of INR on risk of hemorrhage among patients with chronic liver disease and warfarin users
| Parameter | Adjusted Hazard Ratio of Hemorrhage (95% Confidence Interval) |
|
|---|---|---|
| Chronic Liver Disease | Warfarin users | |
|
| ||
| Age* per decade | 0.88 (0.79–0.98) | 1.12 (1.05–1.19) |
|
| ||
| Gender* (Female versus Male) | 1.45 (0.93–2.28) | 0.53 (0.28–0.98) |
|
| ||
| HTN* | 1.00 (0.83–1.21) | 1.40 (1.16–1.69) |
|
| ||
| International Normalized Ratio* | ||
| ≤1.50 | Referent | Referent |
| 1.50–2.0 | 2.25 (1.77–2.85) | 0.86 (0.71–1.05) |
| >2.0–2.5 | 1.80 (1.11–2.91) | 0.81 (0.67–0.98) |
| >2.5–3.0 | 1.60 (0.74–3.45) | 1.18 (0.97–1.44) |
| >3.0–3.5 | 1.74 (0.421–7.23) | 1.64 (1.29–2.08) |
| >3.5 | 2.47 (0.89–6.82) | 4.70 (3.94–5.60) |
|
| ||
| Platelet count* <50 × 109/L | 1.06 (0.71–1.59) | 3.07 (1.15–8.22) |
|
| ||
| Alcohol Use | 1.40 (1.19–1.65) | |
|
| ||
| Anemia | 1.56 (1.40–1.73) | |
|
| ||
| Anti-platelet therapy | 1.59 (1.41–1.79) | |
|
| ||
| Cardiovascular disease | 1.17 (1.05–1.31) | |
|
| ||
| Chronic Kidney Disease | 2.10 (1.83–2.42) | |
|
| ||
| Chronic Lung disease | 1.25 (1.13–1.39) | |
|
| ||
| Dementia | 1.25 (0.91–1.72) | |
|
| ||
| Fracture | 2.38 (1.33–4.28) | |
|
| ||
| Hemi or paraplegia | 1.57 (1.09–2.26) | |
|
| ||
| Peptic Ulcer disease | 1.37 (1.08–1.74) | |
|
| ||
| Surgery | 5.22 (4.20–6.49) | |
|
| ||
| Trauma | 3.08 (2.50–3.80) | |
|
| ||
indicates interaction term results; Abbreviations: HTN= hypertension, INR= International Normalized Ratio, aHR= adjusted hazards ratio
The association between INR categories and risk of hemorrhage in CLD patients relative to warfarin users is listed in Table 4. With INR values < 3, the risk of hemorrhage in CLD patients was higher than the risk in warfarin users. As INR increased above 3, the risk in warfarin users increased to where there was no significant different between the risk of hemorrhage in CLD patients and warfarin users. (Table 4).
Table 4:
Association between INR and hemorrhage in chronic liver disease patients compared to patients taking warfarin
| INR category | Adjusted Hazard Ratio (CLD patients versus warfarin users) |
95% Confidence Interval |
|---|---|---|
| ≤1.5 | 1.5 | 1.21–1.81 |
| >1.5–2.0 | 4.2 | 3.11–5.68 |
| >2.0–2.5 | 3.7 | 2.24–6.13 |
| >2.5–3.0 | 2.3 | 1.02–4.98 |
| >3.0–3.5 | 1.9 | 0.44–7.81 |
| >3.5 | 0.9 | 0.33–2.48 |
Abbreviations: CLD= Chronic liver disease, INR= International Normalized Ratio
The results of sensitivity analysis were coherent with the main analysis. Among the propensity-score matched pairs of patients with INR values > 2, one-unit increase in INR had no effect on risk of hemorrhage in CLD patients (aHR 0.84, 95% CI: 0.68–1.04) but it increased the risk of hemorrhage by 4% for warfarin users (aHR 1.04, 95%, 95% CI: 1.002–1.07). Some warfarin users had INR values > 20 so we repeated this analysis using the natural logarithm (Ln) to account for outliers. We found a 2-fold increase in risk of hemorrhage per unit increase in Ln(INR) among warfarin users (aHR 2.14, 95% CI 1.83–2.49). This increase is equivalent to a 1.36-fold hemorrhage risk for an INR of 3.0 vs. an INR of 2.0.
D: Discussion:
In this nationwide cohort of more than 80,000 US veterans, elevated INR values had different effects on the risk of hemorrhage among CLD patients and warfarin users.
Our results support preclinical studies suggesting rebalanced hemostasis in patients with CLD. INR is a component of the commonly used indices of severity of liver disease: the Child–Pugh classification and the MELD score [29, 30]. Elevated INR values in CLD reflect severe liver disease with decreased synthetic function. Our analysis shows that CLD patients with INR values above 1.5 are approximately twice as likely to experience hemorrhage as CLD patients with a lower INR (Table 3). The risk of hemorrhage, however, remains consistently elevated in this population with further elevation of INR values. In contrast, the risk of hemorrhage in warfarin users correlates with INR values. As INR increases above 2.5, the risk of hemorrhage increases, and the risk becomes 4.7-fold with INR in the supra-therapeutic range. This difference reflects rebalanced hemostasis in CLD patients with elevated INR values, absent in patients taking warfarin.
Preclinical studies suggest that thrombocytopenia in patients with CLD is balanced by elevation in von Willebrand factor levels [31]. Our findings support this hypothesis by demonstrating no relationship between thrombocytopenia and hemorrhage in patients with CLD. In contrast, warfarin users experienced a higher risk of hemorrhage with platelet counts <50 × 109/L.
This study also supports prior clinical studies [25, 27] showing a higher risk of hemorrhage among warfarin users with older age, hypertension, chronic kidney disease, alcohol use, anemia and other comorbidities (table D, Supplementary Appendix). Older age and hypertension, however, did not predict hemorrhage among CLD patients, which is consistent with available literature [32–34].
The most frequent site for non-variceal hemorrhage in CLD patients was upper gastrointestinal tract (Table 2) likely because patients with advanced liver disease are predisposed to vascular ectasias [35] and peptic ulcer disease [36]. Conversely, warfarin users experienced hemorrhage in a wider variety of locations with most hemorrhages in the lower gastrointestinal tract (Table 2).
The findings from this study inform clinical practice and future research. The increase in the risk of hemorrhage in CLD is likely related to the severity of underlying disease as opposed to INR elevation as it varies minimally between different elevated INR values. The normalization of INR values through infusion of plasma or coagulation factors may therefore be unnecessary and ineffective, as suggested by others [37–39]. Further, these infusions expose CLD patients to several risks: fluid overload [40], allergic reactions [41], transfusion-transmitted infections [42], and thrombosis [43]. Future clinical trials should evaluate the role of normalization of INR values in preventing hemorrhage among patients with CLD.
We used a large, national cohort of veterans to study the relationship between INR and hemorrhage. The VHA is an ideal database for this study as it allows access to laboratory values, vital signs, medications, and patient charts from both inpatient and outpatient encounters. This dataset allowed us to study the dynamic relationship between changing INR values and risk of hemorrhage. We used vital signs and laboratory values as time-dependent variables to account for their temporal association with hemorrhage. Our analysis was further strengthened by use of multiple interaction terms to account for differences in the two populations. Moreover, we used previously validated methods [15, 17–20] for identification of study population and outcomes.
This study also had limitations. Some of the baseline differences between the two populations may have persisted despite statistical adjustments. Since randomization to liver disease and warfarin use is not possible, we performed a propensity-score matched sensitivity analysis to account for some of these differences. Although the most common type of hemorrhage in CLD patients is variceal hemorrhage, we studied non-variceal hemorrhage to allow a fair comparison of INR effects between CLD patients and warfarin users. Information on use of blood products was not available, which might have provided information on utility of plasma and platelet infusions for CLD patients with elevated INR values. Similarly, data on the assay/methodology used for INR measurement was not available. A minor limitation is that VHA data underrepresents females, thus our results should be confirmed in females with CLD. Despite these limitations, this study adds to the knowledge of hemostasis in liver disease by demonstrating a difference in the clinical effects of elevated INR values between patients with CLD and patients taking warfarin.
In summary, the risk of hemorrhage varies little with progressive elevation of INR values in patients with CLD, in contrast to patients taking warfarin. Clinical data from warfarin users should not be extrapolated to prevent and treat hemorrhage in CLD patients as the effect of INR on hemorrhage differs significantly in the two populations.
Supplementary Material
ESSENTIALS:
INR may not affect hemorrhage in chronic liver disease (CLD) patients like in warfarin users.
We retrospectively studied the association of INR with hemorrhage in the two populations.
INR had a different effect on risk of hemorrhage in patients with CLD than in warfarin users.
Caution should be exercised managing elevated INR similarly in the two populations.
F: ACKNOWLEDGEMENTS:
The contents do not represent the views of the U.S Department of Veterans Affairs or the United States Government. This material was produced using resources and facilities at the Saint Louis Veterans Affairs Medical Center, Saint Louis, Missouri. This work was further supported by the National Heart, Lung, and Blood Institute (1K01HL136893–01, 5K12 HL087107–95, and the NIH Loan Repayment Program to Dr Sanfilippo) and the National Center for Advancing Translational Sciences grant (UL1 TR000448).
Footnotes
G: DISCLOSURE of CONFLICTS of INTEREST:
The authors declare no competing financial interests.
References
- 1.CDC: Liver Disease. 2020; Available from: https://www.cdc.gov/nchs/fastats/liver-disease.htm.
- 2.Nassif ME, et al. , Relationship Between Anticoagulation Intensity and Thrombotic or Bleeding Outcomes Among Outpatients With Continuous-Flow Left Ventricular Assist Devices. Circulation: Heart Failure, 2016. 9(5): p. e002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldwell SH, et al. , Coagulation disorders and hemostasis in liver disease: Pathophysiology and critical assessment of current management. Hepatology, 2006. 44(4): p. 1039–1046. [DOI] [PubMed] [Google Scholar]
- 4.Tripodi A and Mannucci PM, The Coagulopathy of Chronic Liver Disease. New England Journal of Medicine, 2011. 365(2): p. 147–156. [DOI] [PubMed] [Google Scholar]
- 5.Tripodi A, et al. , Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology, 2005. 41(3): p. 553–8. [DOI] [PubMed] [Google Scholar]
- 6.Tripodi A, et al. , An Imbalance of Pro- vs Anti-Coagulation Factors in Plasma From Patients With Cirrhosis. Gastroenterology, 2009. 137(6): p. 2105–2111. [DOI] [PubMed] [Google Scholar]
- 7.Tripodi A, et al. , Detection of the imbalance of procoagulant versus anticoagulant factors in cirrhosis by a simple laboratory method. Hepatology, 2010. 52(1): p. 249–55. [DOI] [PubMed] [Google Scholar]
- 8.Tripodi A, et al. , Thrombin generation in patients with cirrhosis: the role of platelets. Hepatology, 2006. 44(2): p. 440–5. [DOI] [PubMed] [Google Scholar]
- 9.Deitcher SR, Interpretation of the international normalised ratio in patients with liver disease. The Lancet, 2002. 359(9300): p. 47–48. [DOI] [PubMed] [Google Scholar]
- 10.O’Shea RS, et al. , AGA Clinical Practice Guideline on the Management of Coagulation Disorders in Patients With Cirrhosis. Gastroenterology, 2021. 161(5): p. 1615–1627.e1. [DOI] [PubMed] [Google Scholar]
- 11.Aldawood A, et al. , The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thrombosis journal, 2011. 9(1): p. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabbagh O, et al. , Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest, 2010. 137(5): p. 1145–9. [DOI] [PubMed] [Google Scholar]
- 13.Shah NL, Northup PG, and Caldwell SH, A clinical survey of bleeding, thrombosis, and blood product use in decompensated cirrhosis patients. Ann Hepatol, 2012. 11(5): p. 686–90. [PubMed] [Google Scholar]
- 14.VA Vital Statistics. 2022. [Google Scholar]
- 15.Nehra MS, et al. , Use of administrative claims data for identifying patients with cirrhosis. Journal of clinical gastroenterology, 2013. 47(5): p. e50–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nehra MS, et al. , Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol, 2013. 47(5): p. e50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shehab N, et al. , Assessment of ICD-10-CM code assignment validity for case finding of outpatient anticoagulant-related bleeding among Medicare beneficiaries. Pharmacoepidemiol Drug Saf, 2019. 28(7): p. 951–964. [DOI] [PubMed] [Google Scholar]
- 18.Delate T, et al. , Assessment of the coding accuracy of warfarin-related bleeding events. Thromb Res, 2017. 159: p. 86–90. [DOI] [PubMed] [Google Scholar]
- 19.Arnason T, et al. , Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res, 2006. 118(2): p. 253–62. [DOI] [PubMed] [Google Scholar]
- 20.Joos C, et al. , Accuracy of ICD-10 codes for identifying hospitalizations for acute anticoagulation therapy-related bleeding events. Thromb Res, 2019. 181: p. 71–76. [DOI] [PubMed] [Google Scholar]
- 21.Savas LS, et al. , Mortality ascertainment of women veterans: a comparison of sources of vital status information, 1979–2002. Med Care, 2009. 47(1): p. 125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sohn MW, et al. , Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr, 2006. 4: p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine JP and Gray RJ, A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association, 1999. 94(446): p. 496–509. [Google Scholar]
- 24.Pitsiu M, et al. , A Bayesian Method Based on Clotting Factor Activity for the Prediction of Maintenance Warfarin Dosage Regimens. Therapeutic drug monitoring, 2003. 25: p. 36–40. [DOI] [PubMed] [Google Scholar]
- 25.Gage BF, et al. , Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J, 2006. 151(3): p. 713–9. [DOI] [PubMed] [Google Scholar]
- 26.Klok FA, et al. , Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. European Respiratory Journal, 2016. 48(5): p. 1369–1376. [DOI] [PubMed] [Google Scholar]
- 27.Pisters R, et al. , A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest, 2010. 138(5): p. 1093–100. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, et al. , A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis, 1987. 40(5): p. 373–83. [DOI] [PubMed] [Google Scholar]
- 29.Child CG and Turcotte JG, Surgery and portal hypertension. Major Probl Clin Surg, 1964. 1: p. 1–85. [PubMed] [Google Scholar]
- 30.Kamath PS, et al. , A model to predict survival in patients with end-stage liver disease. Hepatology, 2001. 33(2): p. 464–70. [DOI] [PubMed] [Google Scholar]
- 31.Lisman T, et al. , Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology, 2006. 44(1): p. 53–61. [DOI] [PubMed] [Google Scholar]
- 32.Beppu K, et al. , Prediction of variceal hemorrhage by esophageal endoscopy. Gastrointestinal Endoscopy, 1981. 27(4): p. 213–218. [DOI] [PubMed] [Google Scholar]
- 33.Ma JL, et al. , New model predicting gastroesophageal varices and variceal hemorrhage in patients with chronic liver disease. Ann Hepatol, 2020. [DOI] [PubMed] [Google Scholar]
- 34.Prediction of the First Variceal Hemorrhage in Patients with Cirrhosis of the Liver and Esophageal Varices. New England Journal of Medicine, 1988. 319(15): p. 983–989. [DOI] [PubMed] [Google Scholar]
- 35.Kalafateli M, et al. , Non-variceal gastrointestinal bleeding in patients with liver cirrhosis: a review. Dig Dis Sci, 2012. 57(11): p. 2743–54. [DOI] [PubMed] [Google Scholar]
- 36.González-González JA, et al. , Nonvariceal upper gastrointestinal bleeding in patients with liver cirrhosis. Clinical features, outcomes and predictors of in-hospital mortality. A prospective study. Annals of Hepatology, 2011. 10(3): p. 287–295. [PubMed] [Google Scholar]
- 37.Stanworth SJ, Hyde CJ, and Murphy MF, Evidence for indications of fresh frozen plasma. Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine, 2007. 14(6): p. 551–556. [DOI] [PubMed] [Google Scholar]
- 38.Tripodi A, et al. , Thrombin generation in plasma from patients with cirrhosis supplemented with normal plasma: considerations on the efficacy of treatment with fresh-frozen plasma. Intern Emerg Med, 2012. 7(2): p. 139–44. [DOI] [PubMed] [Google Scholar]
- 39.Tinmouth A, Evidence for a rationale use of frozen plasma for the treatment and prevention of bleeding. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis, 2012. 46(3): p. 293–298. [DOI] [PubMed] [Google Scholar]
- 40.Demeyere R, et al. , Comparison of fresh frozen plasma and prothrombin complex concentrate for the reversal of oral anticoagulants in patients undergoing cardiopulmonary bypass surgery: a randomized study. Vox Sang, 2010. 99(3): p. 251–60. [DOI] [PubMed] [Google Scholar]
- 41.Reutter JC, et al. , Incidence of allergic reactions with fresh frozen plasma or cryo-supernatant plasma in the treatment of thrombotic thrombocytopenic purpura. J Clin Apher, 2001. 16(3): p. 134–8. [DOI] [PubMed] [Google Scholar]
- 42.Sarani B, et al. , Transfusion of fresh frozen plasma in critically ill surgical patients is associated with an increased risk of infection. Crit Care Med, 2008. 36(4): p. 1114–8. [DOI] [PubMed] [Google Scholar]
- 43.Kujovich JL, Hemostatic defects in end stage liver disease. Crit Care Clin, 2005. 21(3): p. 563–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


