Abstract
The American dog tick, Dermacentor variabilis, is a tick of public and veterinary health importance in North America. Using passive tick surveillance data, we document distribution changes for the American dog tick in Ontario, Canada, from 2010 through 2018. Dermacentor variabilis submissions from the public were geocoded and aggregated—from large to small administrative geographies—by health region, public health unit (PHU) and Forward Sortation Area (FSA). PHU hot spots with high rates of D. variabilis submissions were (1) Brant County, Haldimand-Norfolk and Niagara Regional in the Central West region and (2) Lambton and Winsor-Essex County in the South West region. The number of established D. variabilis populations with ≥ 6 submissions per year increased significantly during the study at regional (PHUs: 22 to 31) and local (FSAs: 27 to 91) scales. The range of D. variabilis increased similarly to the positive control (Ixodes scapularis) during the study and in contrast to the static range of the negative control (Ixodes cookei). Submission hot spots were in warmer, low elevation areas with poorly drained soils, compared to the province’s low submission areas. Dermacentor variabilis is spreading in Ontario and continued research into their vector ecology is required to assess medicoveterinary health risks.
Subject terms: Ecology, Diseases
Introduction
The American dog tick, Dermacentor variabilis, is a temperate and sub-tropical species found throughout central and eastern USA, extending south into Mexico, with an isolated population along the American west coast1. In Canada, the American dog tick occurs in the southern portions of Saskatchewan, Manitoba, Ontario, Quebec and Nova Scotia2. Typically, D. variabilis occurs in deciduous forest ecotones such as fields, forest edges, trails, roadsides and brushy areas along waterways in rural, suburban and urban landscapes1,3. The American dog tick is a three-host tick, with larvae and nymphs feeding on rodents, and adults feeding on medium- to large-sized mammals. Reported hosts of D. variabilis in Ontario are cats (Felis catus), coyotes (Canis latrans), dogs (Canis lupus familiaris), horses (Equus caballus), humans (Homo sapiens), meadow voles (Microtus pennsylvanicus), North American porcupines (Erethizon dorsatum), northern raccoons (Procyon lotor), southern red-backed voles (Myodes gapperi), striped skunks (Mephitis mephitis), Virginia opossums (Didelphis virginiana) and white-footed mice (Peromyscus leucopus)4–9. Given the liberal host range of adult D. variabilis, distribution is limited mostly by the ecological requirements (e.g., appropriate habitat, humidity and temperature) of off-host ticks during diapause, egg development, host seeking, molting and oviposition10.
Dermacentor variabilis transmits several pathogens of public and/or veterinary health concern, including Cytauxzoon felis (cytauxzoonosis), Francisella tularensis (tularemia) and Rickettsia rickettsii (Rocky Mountain spotted fever)2,11. Dermacentor variabilis is a competent vector of Anaplasma marginale (bovine anaplasmosis), but transmission by American dog ticks was not demonstrated during a recent outbreak in Manitoba12. Furthermore, neurotoxins in the American dog tick’s saliva can cause tick paralysis in dogs, horses and humans2,13. Dermacentor variabilis is primarily a biting pest in Ontario, but we know little concerning the vector ecology of the American dog tick in the province.
Despite an apparent low risk of D. variabilis-borne disease in Ontario, climate and land use changes can alter habitat-host-vector-pathogen dynamics14,15. Increases in suitable climate and habitat can amplify disease risks, by increasing tick population numbers and their distribution and lengthening the active season of ticks and their hosts16. To our knowledge, D. variabilis occurrence in Ontario was first reported in 1910, and until at least the 1980s, collection records were sporadic and concentrated south of Toronto (≈ 43.5° N)17–20. Recent work revealed the American dog tick’s range is expanding in Manitoba and Saskatchewan, potentially increasing the risk of disease from D. variabilis-borne pathogens21. The American dog tick is the second most commonly encountered tick in Ontario, behind the blacklegged tick (Ixodes scapularis), yet changes in D. variabilis distribution in the province remain unexplored6. The gold standard for determining a tick’s distribution is active surveillance through host trapping and tick dragging; however, this approach is not feasible given Ontario’s size of approximately 1 million km2. Accordingly, we describe D. variabilis distribution in Ontario using passive tick submissions from the public and determine if there has been a change in American dog tick distribution from 2010 through 2018.
Materials and methods
Study location
Ontario has a population of approximately 14 million and is located in the Great Lakes region of North America. Most of southern Ontario experiences a moderate continental climate and lies within the Mixedwood Plains Ecozone (≈ 83,000 km2); field and agricultural land comprise 59% of land cover in this ecozone (see citation for details of Ontario’s ecozones)22,23. Oaks, maples, yellow birch, ashes, eastern hemlock, American beech, American elm, basswood, wild black cherry, hickories, eastern white pine, firs and spruce dominate forested areas of southern Ontario22,23. In successional habitats, common plants include staghorn sumac, highbush cranberry, clover, red osier dogwood, goldenrod and willow22,23.
Currently, 34 public health units (PHUs) administer public health services in Ontario; however, for this study, we performed analyses with the previous 35-PHU classification that were in existence during the time frame of the study (for PHU acronyms and full names, see Supplementary Table S1). PHUs are further organized into seven health regions: Central East (DUR, HKP, PEL, PTC, SMD, YRK), Central West (BRN, HAL, HAM, HDN, NIA, WAT, WDG), Eastern (EOH, HPE, KFL, LGL, OTT, REN), North East (ALG, NPS, PQP, SUD, TSK), North West (NWR, THB), South West (CHK, GBO, HUR, LAM, MSL, OXE, PDH, WEC) and Toronto (TOR).
Passive tick surveillance
We have described Ontario’s passive tick surveillance previously24. Briefly, Public Health Ontario (PHO) morphologically identifies ticks submitted by the public through healthcare providers or PHU offices. This study spanned from 2010 to 2018 and only included ticks submitted from human hosts. Tick submission data include the submitter’s postal code and travel history, along with tick submission date, stage, sex and number of ticks. The Forward Sortation Area (FSA) is the smallest geographic unit used in our analyses and is the first three characters of the six-character postal code of the submitter’s residence. There are 513 FSAs in Ontario with a median area of 24.7 km2 (interquartile range [IQR]: 8.7–151 km2; range: 0.3–408,433 km2), with geographically smaller FSAs in urban centers and larger FSAs in rural areas (see citation for further details on Ontario’s FSAs)25. The median area of the 35 PHUs was 3,806 km2 (interquartile range [IQR]: 2,036–8,988 km2; range: 630–266,291 km2). We used the submitter’s FSA to aggregate data to the PHU level and then to the larger health region level. Ticks potentially acquired outside of Ontario were excluded from analyses (n = 389). EOH, KFL and LGL stopped accepting tick submissions at their PHU offices in 2014 and HDN stopped in 2018; however, healthcare providers could still submit ticks from patients in these PHUs (for provincial summary, we included a subset of PHUs that excludes PHUs that ceased submissions during the study period).
We used existing criteria to determine if D. variabilis was established: a PHU or FSA had an established population if the public submitted at least six ticks in a year from that PHU or FSA. This criterion is based on research focusing on I. scapularis and Ixodes pacificus in the United States of America (USA) and applied at the county-level geography; the criterion has also been applied to D. variabilis and Amblyomma americanum in the USA (lone star tick)26–29. We compared D. variabilis with a relatively newly-established tick (I. scapularis = positive control; a species demonstrated as having an expanding range) and a long-established, nidicolous tick (Ixodes cookei = negative control; a species with a relatively static range)6,30,31. We also use the term occurrence to describe a tick’s distribution; occurrence is when the public submitted at least one tick from a PHU or FSA in a single year.
Mapping and statistical analyses
We calculated provincial, health region and PHU submission rates of D. variabilis per 100,000 population (denominator = 2018 population data) using population data and estimates from Statistics Canada via IntelliHEALTH Ontario (October 19, 2017). We created distribution maps using Esri ArcGIS v10.3 (Esri; Redlands, California, USA; 2014) for tick submission rates for health regions and PHUs. We did not map submissions by FSA, as the high variability in FSA size makes mapping at this level of geography problematic (e.g., most urban FSAs are too small to see on a map and large, rural FSAs would dominate the map). In addition, rate of submission at the FSA-level was out-of-scope for this work since population data for FSAs (including yearly and monthly projections) were not available.
We modelled D. variabilis submission counts using a Poisson regression and the number of submissions as the dependent variable, including year (linear), health region or PHU and their interaction term with year to determine if there are different trends over time by geography (reported as relative risk [RR], 95% confidence interval [CI]). The model also accounted for seasonality with sine and cosine with periods of pi/3 and pi/6 on the 12 months within a year. Population for each month, year and PHU/health region are included as an offset term in the model on the log scale. To examine the geospatial spread of the ticks over time, we modelled the number of FSAs with at least one submission as outcome using Poisson regression, accounting for seasonality (same periods as the model above) and time (in year) without an offset term. R v3.6.2 (R Foundation for Statistical Computing; Vienna, Austria; 2019) was used for Poisson regression analyses (Supplemental Model Outputs). We used Excel v15.0 (Microsoft; Redmond, Washington, USA; 2013) and the Excel add-in Real Statistics Resource Pack v6.8 (Charles Zaiontz; www.real-statistics.com; 2013–2020) for all other analyses. To determine if there were changes in tick submission counts over time for PHUs and FSAs, we used simple linear regressions. We used a two-tailed Mann–Kendall test for changes in tick relative abundance, examining if the proportion of all tick submissions that were D. variabilis, I. scapularis or I. cookei changed over time at the provincial level. We made plots using Excel, except for Supplementary Figures S1, S2 and S5. For all analyses, we used a significance level of α = 0.05.
Results
Provincial summary
There were 17,434 (annual median = 1,901; IQR: 1,020–2,509) D. variabilis submissions made in Ontario (2010–2018), with a provincial submission rate of 124 per 100,000 population (Supplementary Table S1, S2). For a subset of PHUs (subset excludes PHUs that ceased submissions during the study period), 15,298 (annual median = 1704; IQR; 874–2,333) American dog tick submissions were made, with a submission rate of 109/100,000. The mean number of American dog ticks per submission was similar for all PHUs (mean ± SE = 1.08 ± 0.0039; n = 18,809) and the subset (1.08 ± 0.0042; n = 16,528) (t-test: t = 0.2, P = 0.79). For the remainder of the analyses, we will only report “all PHU” dataset results. For I. scapularis, 22,912 (annual median = 2,298; IQR: 1,966–3,245) submissions were made, with a submission rate of 163/100,000. For I. cookei, 2,324 (annual median = 241, IQR: 199–322) submissions were made, with a submission rate of 16.6/100,000.
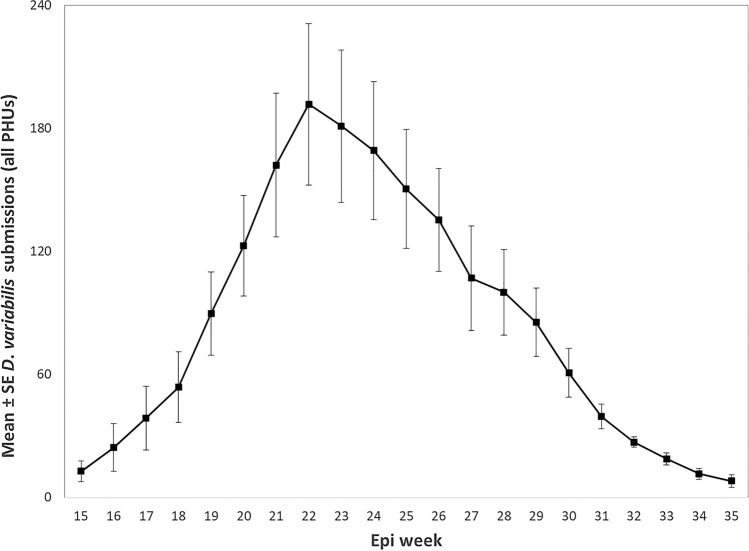
For D. variabilis submissions, 99.9% (17,414/17,428) were adult ticks, < 0.1% (n = 9) were nymphs and < 0.1% (n = 5) were mixed-stage submissions. For adult American dog tick submissions, 61.5% (10,717/17,414) were female, 36.4% (n = 6,334) were male and 1.9% (n = 337) were mixed-sex (i.e., sample contained male and female specimens). Submissions peaked during epidemiological week (epi week) 22 or approximately late May (Fig. 1).
Figure 1.
Dermacentor variabilis submissions per epidemiological week (epi week) for all public health units (PHUs) during the spring and summer: Ontario, Canada (2010–2018). Epi week 22 starts May 25–31, depending on year.
Annual submission counts for D. variabilis (Supplementary Table S2) were correlated with submission counts of all three tick species combined (Pearson correlation: r = 0.96, P = 0.000038), I. scapularis (r = 0.95, P = 0.00010) and I. cookei (r = 0.94, P = 0.00016). The relative abundance of D. variabilis did not change significantly during the study (Mann–Kendall test: Z = 1.1, P = 0.25), similarly there was no change in relative abundance for I. scapularis (Z = -1.0, P = 0.35) and I. cookei (Z = -1.8, P = 0.076) (Table 1).
Table 1.
Mann-Kendal test for changes in proportions (i.e., relative abundance) of all tick submissions that were Dermacentor variabilis, Ixodes scapularis (positive control), Ixodes cookei (negative control) and three species combined: Ontario, Canada (2010–2018).
| Year | Percent of all tick submissions (%) | |||
|---|---|---|---|---|
| Dermacentor variabilis | Ixodes scapularis | Ixodes cookei | Three species combined | |
| 2010 | 37.9 | 48.8 | 7.2 | 93.9 |
| 2011 | 25.5 | 64.4 | 6.4 | 96.3 |
| 2012 | 34.2 | 56.7 | 4.5 | 95.4 |
| 2013 | 34.7 | 50.2 | 4.8 | 89.7 |
| 2014 | 27.6 | 51.7 | 5.9 | 85.2 |
| 2015 | 47.6 | 36.8 | 5.3 | 89.7 |
| 2016 | 41.1 | 44.1 | 4.3 | 89.5 |
| 2017 | 42.3 | 47.1 | 4.1 | 93.5 |
| 2018 | 37.6 | 50.8 | 5.2 | 93.6 |
| Sen’s slope (95% CI) | 1.1 (− 2.6, 3.1) | − 1.3 (− 4.2, 1.5) | − 1.8 (− 4.2, 0.1) | − 0.25 (− 1.7, 1.3) |
| Z-stat | 1.1 | − 1.0 | − 1.8 | − 0.84 |
| P | 0.25 | 0.35 | 0.076 | 0.40 |
Health regions
Dermacentor variabilis submission rates per 100,000 population were highest in the Central West (312) and South West (306) regions (Supplementary Fig. S1). The fastest increases in D. variabilis submissions were from the North East (RR = 1.4, 95% CI: 1.29–1.42) and Central East (RR = 1.3, 95% CI: 1.27–1.32) regions; a RR of 1.4 corresponds to an average annual increase of 40% in submission counts (Supplementary Fig. S2). There was an annual 10% decrease in submission counts from the Eastern region (RR = 0.9, 95% CI: 0.86–0.90).
Public health units
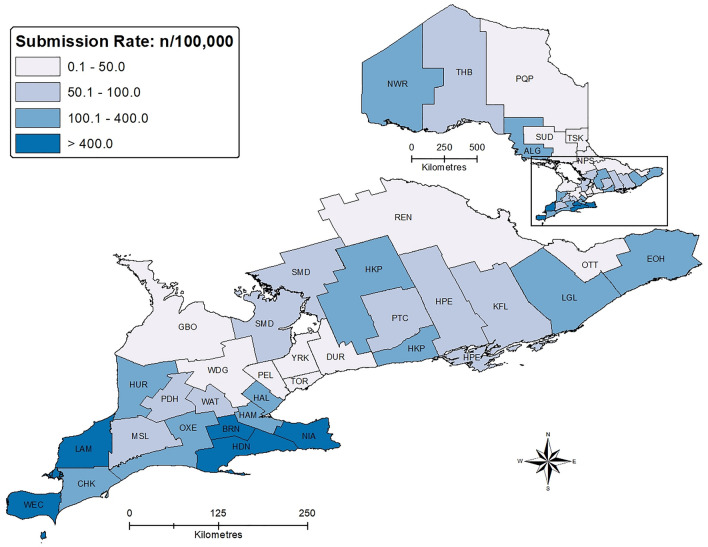
The highest D. variabilis submissions per 100,000 population were from LAM (902), HDN (816), NIA (682), BRN (628) and WEC (586) (Fig. 2, Supplementary Table S1). Multiple D. variabilis (≥ 2 ticks in one submission) submissions per 100,000 population were highest in the same PHUs: LAM (66), HDN (45), BRN (39), NIA (37) and WEC (24).
Figure 2.
Dermacentor variabilis submission rates per 100,000 population, by public health unit: Ontario, Canada (2010–2018).
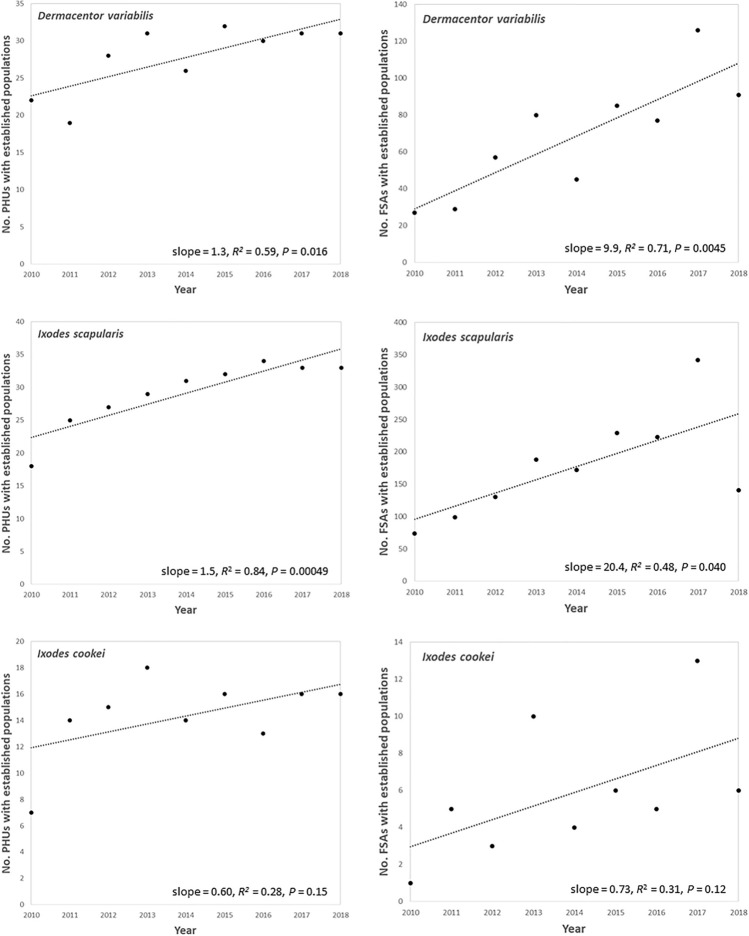
There were significant increases in the number of PHUs with an established D. variabilis population from 2010 through 2018 (R2 = 0.59, P = 0.016) (Fig. 3). The number of PHUs with an established I. scapularis (positive control) population increased significantly during the study (R2 = 0.84, P = 0.00049), while there was no change for I. cookei (negative control, R2 = 0.28, P = 0.15). The fastest increase in American dog tick submissions was from ALG (RR = 1.5, 95% CI: 1.37–1.56), with relatively fast increases in HAL, PTC, REN, HKP and WEC (RR = 1.3–1.4) (Supplementary Fig. S3).
Figure 3.
Relationship between Dermacentor variabilis submissions and time by public health unit (PHU, left panel) and Forward Sortation Area (FSA, right panel), compared with Ixodes scapularis (positive control) and Ixodes cookei (negative control): Ontario, Canada (2010–2018).
Forward sortation areas
FSAs were available for 84.5% (14,729/17,434) of submissions. FSAs with relatively higher D. variabilis counts (≥ 12 submissions per year) were primarily located in the Central West (FSAs for BRN, HAM and NIA: L0R, L0S, L2A, L3C, L3K, L3M, L8E, N0A, N0E, N1A, N3R, N3S, N3T, N3Y) and South West (FSAs for LAM and WEC: N0N, N0R, N7S, N7T, N8H, N8M, N9E, N9J, N9V, N9Y) regions (data not shown).
The number of FSAs with an established D. variabilis population increased significantly during the study (R2 = 0.71, P = 0.0045) (Fig. 3). The number of FSAs with an established I. scapularis (positive control) population increased significantly (R2 = 0.48, P = 0.040), while there was no change for I. cookei (negative control, R2 = 0.31, P = 0.12) (Fig. 3). There were significant increases in the number of D. variabilis-positive FSAs (at least one submission per year = occurrence) during the study (R2 = 0.73, P = 0.0036), higher compared to I. scapularis (positive control, R2 = 0.56, P = 0.20) and I. cookei (negative control, R2 = 0.46, P = 0.045) (Supplementary Fig. S4). There was a 10% annual increase in the number of D. variabilis-positive FSAs (RR = 1.1, 95% CI: 1.09–1.13) (Supplementary Fig. S5).
Discussion
Dermacentor variabilis is a potential vector of several pathogens of public and/or veterinary health concern and, in Ontario, is expanding its range from key hotspots of relatively high abundance. Ontarians submitted D. variabilis from all health regions and PHUs of the province, with the highest submission rates from the Central West (BRN, HDN and NIA) and South West (LAM and WEC) regions. In addition, these hot spots experienced the highest multiple-tick submission rates, suggesting higher densities of ticks that potentially act as sources of expansion. While not a part of our analyses, submission hot spots were in the Mixedwood Plains Ecozone; specifically, in areas with higher temperatures (> 2,200 growing-degree-days above 5 °C) and lower elevation (< 225 m)32. The characterization of these high-submission areas is similar to studies showing that the American dog tick favours conditions associated with low elevation (e.g., higher humidity, higher temperature), favouring expansion and establishment10,33–35. In neighbouring Michigan, USA, American dog ticks were more abundant along large bodies of water in low elevation areas of the state’s southeast region and Northern Peninsula, contiguous with high submission areas of Ontario36. In southern Maine, USA, D. variabilis were more common in warmer areas at low elevation31. High-submission areas in Ontario have a high-percentage of poorly-drained, gleysolic soils (associated with wetlands or where wetlands once existed), consistent with reports of increased D. variabilis abundance in high-humidity habitats with damp soils32,37,38. There is an opportunity to model suitable habitat for D. variabilis in Ontario at a finer geographic scale (i.e., FSA), using passive and active surveillance data under current and future climate projections.
Submission rates and counts for D. variabilis are increasing in Ontario; however, we did not confirm increasing relative abundance at the provincial level during the study. We received approximately 2,400 D. variabilis annually during this study (2013–2018), higher than previous passive surveillance work in Ontario. From 2008 through 2012, there were approximately 1,000 submissions per year in the province, and about 450 submissions per year from 1999 through 2007 (MPN unpublished data)6. A 1975 report noted the collection of eight D. variabilis from humans and dogs in southern Ontario from 1957 through 197539. In southern Ontario from 1967 through 1977, 21 of 65 tick submissions to public health for identification were American dog ticks19. Lindquist et al. (2016) reported that D. variabilis is “locally abundant in southern Québec and Ontario”2. While passive surveillance was not able to detect increased D. variabilis relative abundance at the provincial level, abundance changes at the local level may well be determined in the future using active surveillance (e.g., tick dragging).
Undoubtedly, range expansion of D. variabilis was in progress for years to decades prior to our study, yet we demonstrate expansion in Ontario over a short period of 9 years and provide a baseline for future comparisons. The number of PHUs with established D. variabilis populations increased from 22 to 31 during the study, indicating range expansion at the regional level. We note that the distribution of ticks within an established PHU is likely heterogeneous. At the local level, the number of FSAs with established American dog tick populations increased from 27 to 91 and the number of D. variabilis-positive FSAs (occurrence) increased from 161 to 315. The difference in FSAs between occurrence (at least 1 tick) and establishment (6 or more ticks per year) may represent areas of Ontario where pioneer D. variabilis encounter unfavorable habitat and climate and fail to establish. Furthering our conclusion of range expansion, the number of PHUs and FSAs with established D. variabilis populations increased over time in a manner similar to the positive control (I. scapularis); in contrast, there was no change in the distribution of the negative control (I. cookei). Range expansion of American dog ticks in Ontario agrees with similar observations of expansion in Canada (Manitoba, Saskatchewan) and the USA (Maine, Pennsylvania)21,31,40. Dermacentor variabilis range expansion indicates conditions are favourable for this tick across an area larger than they currently occur in Ontario (continued expansion), potentially increasing threats to public and veterinary health.
Relatively fast increases in D. variabilis submissions identified areas where the ticks are emerging or continuing to proliferate. The greatest increase in D. variabilis submissions over the study period were in the North East region, which was unexpected given the region consisted mostly of PHUs without established tick populations. Submissions increased annually by almost 50% in ALG, the PHU driving the rapid increase in the North East. The recent increase in D. variabilis in ALG (centered in and around Sault Ste. Marie) is likely the result of expansion of D. variabilis populations from the Upper Peninsula of Michigan, or possibly successful introduction and establishment of adventitious ticks from southern Ontario. We presume D. variabilis dispersal in agricultural southern Ontario occurs via host movement among patches of suitable habitat or stepping stones (e.g., isolated woodlots within cultivated land)41. The variability in the rate of increase of submissions by PHU may reflect variability in the size and proximity of these stepping stones (slower where stepping stones are widespread and small), in conjunction with variability in suitable climate and ecological requirements. Geographic expansion of D. variabilis in Ontario does not represent diffusion from a single historical population, rather a mosaic of hot spots expanding at variable rates toward areas with favourable habitat and climate.
While not specifically examined in this study, the range expansion of the American dog tick is likely due to a combination of climate change (e.g., higher annual temperatures and milder winters), changes in land use patterns (e.g., succession of abandoned agricultural land and forest fragmentation), changes in host distribution/abundance (e.g., white-tailed deer, Odocoileus virginianus) and increased human travel (including dog travel). Increasing temperature is an important driver for the range expansion of D. variabilis and other ticks in North America, such as the blacklegged tick and the lone star tick30,42. Worldwide, other tick species are experiencing range expansions, such as I. ricinus in Northern Europe and Dermacentor reticulatus in Central Europe43,44. Dermacentor variabilis is hypothetically expanding along waterways found in high-submission areas of Ontario, similar to American dog tick expansion in Saskatchewan, Canada, along the South Saskatchewan River and Diefenbaker Lake21. For example, hosts travelling along waterways of the Grand River Watershed in Ontario’s Central West region potentially aid the gradual dispersal of adult D. variabilis, especially host animals with relatively larger home ranges such as coyotes, red foxes (Vulpes vulpes) and white-tailed deer. In contrast to other ixodid ticks, American dog ticks rarely feed on birds, limiting long-distance and rapid dispersal2. Rapid and long-distance dispersal of D. variabilis is usually via anthropogenic means, whereby adventitious ticks travel on humans, companion animals or livestock. There is an opportunity to test hypotheses underpinning D. variabilis expansion in Ontario using active and passive surveillance, which can aid in predicting the location of potential emerging populations.
Adult D. variabilis activity in Ontario peaked from late May through early June, similar to elsewhere in the northern portion of the tick’s range; e.g., May (Pennsylvania), late May through early June (Maine, Nova Scotia) and June (Quebec)31,38,45,46. The life cycle of the American dog tick normally takes two years in northern latitudes, longer depending on local host availability and climate2,47. In warmer climates, D. variabilis has a one-year life cycle with longer activity periods and multiple adult cohorts with two (Kentucky, USA) or three (Georgia, USA) activity peaks during the spring and summer48,49. It will be interesting to see if there will be earlier-season shifts in D. variabilis activity in North America due to warming temperatures.
The public and veterinary health importance of American dog ticks in Ontario deserves attention, especially since we have identified population hot spots and expansion. Rocky Mountain spotted fever and tularemia are the primary tick-borne disease threats posed by American dog ticks in Ontario. Rocky Mountain spotted fever is not reportable to public health officials in Ontario; however, researchers in 1978 described a locally acquired case near Ottawa, the only documented case acquired in Ontario50. In a 2006 report, a dog from Ottawa, with no history of travel, was seropositive for R. rickettsii51. Tularemia is endemic throughout Ontario; however, human infection is usually the result of skinning infectious animals (e.g., common muskrats, Ondatra zibethicus; eastern cottontails, Sylvilagus floridanus), rather than tick-borne transmission52. Tularemia is reportable to public health in Ontario, however, it is rare, with only six cases reported in Ontario from 2005 through 201953. Researchers detected F. tularensis and R. rickettsii in American dog ticks in Ontario from the 1960s into the 1980s17,54. In recently published studies (2016–2018), screening of D. variabilis from Ontario revealed the presence of Rickettsia montanensis [spotted fever group rickettsiae (SFGR) with unknown pathogenicity] and Rickettsia peacockii (non-pathogenic, endosymbiotic SFGR)55,56. We infer that the risk of D. variabilis-borne disease in Ontario is low; however, the epidemiological significance of the American dog tick in the province is underexplored.
There are several caveats to our study, which are associated with any surveillance program based on the passive submission of specimens. The location of tick acquisition is not always the same as the submitter’s place of residence; although we excluded ticks where the submitter indicated that they were acquired outside of Ontario. We assumed that most tick exposures occurred near the submitter’s home; therefore, we did not take into account possible human travel within Ontario. Increased awareness of the surveillance program potentially contributed to American dog ticks being submitted from a wider geographic area; however, the extent of this awareness and its impact on submissions is unknown. We also assumed tick submission behaviour did not vary among PHUs; though, the public in areas accustomed to this tick may not have sought identification. In addition, PHUs potentially did not submit all D. variabilis for identification, as they are relatively easy to identify. Our surveillance system did not include submissions from non-human hosts, meaning important hosts were missed (e.g., dogs); future work including collections from companion animals may elucidate further information on the distribution of American dog ticks in Ontario. We acknowledge that the 6-tick criterion for deeming a PHU or FSA as having an established population is a proxy until Ontario- and species-specific benchmarks are developed; this criterion likely overestimates the number of “true established populations” in the absence of confirmatory tick dragging. Further work is needed to determine if the 6-tick criterion is applicable at multiple scales. We should note that additional research is needed to quantify the extent that Ontario population growth (approximately 12.9 million in 2011 and 13.4 million in 2016) and loss of farm land (approximately 64.8 million hectares in 2011 and 64.2 million hectares in 2016) contributes to the spread of the American dog ticks in Ontario, especially at the FSA level57,58. The impact of discontinued passive surveillance meant it was not possible to assess spatiotemporal changes in D. variabilis populations in the Eastern region and we possibly missed areas of high submission rates or areas with rapidly expanding populations.
Conclusions
We have provided a detailed account of D. variabilis distribution and range expansion at multiple spatial scales in Ontario and we suggest continued research into American dog tick populations and their associated pathogens. In addition, further ecological work will add to our understanding of the factors contributing to D. variabilis emergence and expansion.
Supplementary Information
Acknowledgements
We thank Bryna Warshawsky (PHO) and three anonymous reviewers for providing valuable comments to improve the manuscript. We thank PHUs and health care providers for their participation in Ontario's tick surveillance programs.
Author contributions
M.P.N. conceptualized the study and wrote the original manuscript. Y.L. and M.P.N. performed statistical analyses. S.J. performed geospatial analyses. C.B.R., K.C., T.C. and S.N.P. contributed to data acquisition and data curation. All authors read, reviewed and approved the final manuscript.
Data availability
Information about PHO’s data access request process is available on-line at: https://www.publichealthontario.ca/en/Data-and-Analysis/Using-Data/Data-Requests.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-15009-9.
References
- 1.Sonenshine DE. Insects of Virginia No. 13. Ticks of Virginia (Acari: Metastigmata) Res. Div. Bull. 1979;139:1–44. [Google Scholar]
- 2.Lindquist EE, et al. A Handbook to the Ticks of Canada (Ixodida: Ixodidae, Argasidae) Biological Survey of Canada; 2016. [Google Scholar]
- 3.Campbell A, MacKay PR. Distribution of the American dog tick, Dermacentor variabilis (Say), and its small-mammal hosts in relation to vegetation types in a study area in Nova Scotia. Can. J. Zool. 1979;57:1950–1959. doi: 10.1139/z79-258. [DOI] [PubMed] [Google Scholar]
- 4.Barker IK, et al. Distribution of the Lyme disease vector, Ixodes dammini (Acari: Ixodidae) and isolation of Borrelia burgdorferi in Ontario, Canada. J. Med. Entomol. 1992;29:1011–1022. doi: 10.1093/jmedent/29.6.1011. [DOI] [PubMed] [Google Scholar]
- 5.Morshed MG, Scott JD, Fernando K, Mann RB, Durden LA. Lyme disease spirochete, Borrelia burgdorferi endemic at epicenter in Rondeau Provincial Park, Ontario. J. Med. Entomol. 2003;40:91–94. doi: 10.1603/0022-2585-40.1.91. [DOI] [PubMed] [Google Scholar]
- 6.Nelder MP, et al. Population-based passive tick surveillance and detection of expanding foci of blacklegged ticks Ixodes scapularis and the Lyme disease agent Borrelia burgdorferi in Ontario, Canada. PLoS ONE. 2014;9:e105358. doi: 10.1371/journal.pone.0105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clow KM, et al. Distribution of ticks and the risk of Lyme disease and other tick-borne pathogens of public health significance in Ontario, Canada. Vector Borne Zoonotic Dis. 2016;16:215–222. doi: 10.1089/vbz.2015.1890. [DOI] [PubMed] [Google Scholar]
- 8.Smith KA, et al. Tick infestations of wildlife and companion animals in Ontario, Canada, with detection of human pathogens in Ixodes scapularis ticks. Ticks Tick Borne Dis. 2019;10:72–76. doi: 10.1016/j.ttbdis.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Scott JD, et al. Extensive distribution of the Lyme disease bacterium, Borrelia burgdorferi sensu lato, in multiple tick species parasitizing avian and mammalian hosts across Canada. Healthcare. 2018;6:131. doi: 10.3390/healthcare6040131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James AM, Burdett C, McCool MJ, Fox A, Riggs P. The geographic distribution and ecological preferences of the American dog tick, Dermacentor variabilis (Say), in the USA. Med. Vet. Entomol. 2015;29:178–188. doi: 10.1111/mve.12099. [DOI] [PubMed] [Google Scholar]
- 11.Blouin EF, Kocan AA, Glenn BL, Kocan KM, Hair JA. Transmission of Cytauxzoon felis Kier, 1979 from bobcats, Felis rufus (Schreber), to domestic cats by Dermacentor variabilis (Say) J. Wildl. Dis. 1984;20:241–242. doi: 10.7589/0090-3558-20.3.241. [DOI] [PubMed] [Google Scholar]
- 12.Yunik ME, Galloway TD, Lindsay LR. Active surveillance of Anaplasma marginale in populations of arthropod vectors (Acari: Ixodidae; Diptera: Tabanidae) during and after an outbreak of bovine anaplasmosis in southern Manitoba, Canada. Can. J. Vet. Res. 2016;80:171–174. [PMC free article] [PubMed] [Google Scholar]
- 13.Trumpp KM, Parsley AL, Lewis MJ, Camp JW, Jr, Taylor SD. Presumptive tick paralysis in 2 American miniature horses in the United States. J. Vet. Intern. Med. 2019;33:1784–1788. doi: 10.1111/jvim.15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Léger E, Vourc'h G, Vial L, Chevillon C, McCoy KD. Changing distributions of ticks: Causes and consequences. Exp. Appl. Acarol. 2013;59:219–244. doi: 10.1007/s10493-012-9615-0. [DOI] [PubMed] [Google Scholar]
- 15.Ogden NH, Mechai S, Margos G. Changing geographic ranges of ticks and tick-borne pathogens: Drivers, mechanisms and consequences for pathogen diversity. Front. Cell. Infect. Microbiol. 2013;3:46. doi: 10.3389/fcimb.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouchard C, et al. Increased risk of tick-borne diseases with climate and environmental changes. Can. Commun. Dis. Rep. 2019;45:83–89. doi: 10.14745/ccdr.v45i04a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artsob H, et al. Isolation of Francisella tularensis and Powassan virus from ticks (Acari: Ixodidae) in Ontario, Canada. J. Med. Entomol. 1984;21:165–168. doi: 10.1093/jmedent/21.2.165. [DOI] [PubMed] [Google Scholar]
- 18.Gregson JD. The Ixodoidea of Canada. Canadian Department of Agriculture Publication 930. Canadian Department of Agriculture; 1956. [Google Scholar]
- 19.Scholten T. Human tick infestations in Ontario: Findings at the Toronto Public Health Laboratory, 1967–1977. Can. J. Public Health. 1977;68:494–496. [PubMed] [Google Scholar]
- 20.Jarvis, D. The Acarina, with a host index to the species found in Ontario. 48thAnn.Rept.Ent.Soc.Ontario190936, 82–109 (1910).
- 21.Dergousoff SJ, Galloway TD, Lindsay LR, Curry PS, Chilton NB. Range expansion of Dermacentor variabilis and Dermacentor andersoni (Acari: Ixodidae) near their northern distributional limits. J. Med. Entomol. 2013;50:510–520. doi: 10.1603/ME12193. [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Natural Resources and Forestry. ForestresourcesofOntario2016 (Ministry of Natural Resources and Forestry, 2018).
- 23.Crins, W. J., Gray, P. A., Uhlig, P. W. C. & Wester, M. C. TheecosystemsofOntario,Part1:Ecozonesandecoregions. (Ministry of Natural Resources and Forestry, 2009).
- 24.Nelder MP, et al. Human pathogens associated with the blacklegged tick Ixodes scapularis: A systematic review. Parasit. Vectors. 2016;9:265. doi: 10.1186/s13071-016-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.University of Toronto. FSA land area file. https://mdl.library.utoronto.ca/collections/numeric-data/census-canada/2016/geo (2018).
- 26.Lehane A, et al. Reported county-level distribution of the American dog tick (Acari: Ixodidae) in the contiguous United States. J. Med. Entomol. 2020;57:131–155. doi: 10.1093/jme/tjz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J. Med. Entomol. 1998;35:629–638. doi: 10.1093/jmedent/35.5.629. [DOI] [PubMed] [Google Scholar]
- 28.Springer YP, Eisen L, Beati L, James AM, Eisen RJ. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae) J. Med. Entomol. 2014;51:342–351. doi: 10.1603/ME13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisen RJ, Eisen L, Beard CB. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 2016;53:349–386. doi: 10.1093/jme/tjv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clow KM, et al. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS ONE. 2017;12:e0189393. doi: 10.1371/journal.pone.0189393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rand PW, et al. Passive surveillance in Maine, an area emergent for tick-borne diseases. J. Med. Entomol. 2007;44:1118–1129. doi: 10.1093/jmedent/44.6.1118. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin, D., Desloges, J. & Band, L. Physical geography of Ontario in Ecologyofamanagedterrestriallandscape:patternsandprocessesofforestlandscapesinOntario (eds. Perera, A. H., Euler, D. L. & Thompson, I. D.) 12–29 (UBC Press, 2000).
- 33.Minigan JN, Hager HA, Peregrine AS, Newman JA. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick Borne Dis. 2018;9:354–362. doi: 10.1016/j.ttbdis.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson PR. The distribution of Dermacentor ticks in Canada in relation to bioclimatic zones. Can. J. Zool. 1967;45:517–537. doi: 10.1139/z67-066. [DOI] [PubMed] [Google Scholar]
- 35.Bishopp FC, Trembley TH. Distribution and hosts of certain North American ticks. J. Parasitol. 1945;31:1–54. doi: 10.2307/3273061. [DOI] [Google Scholar]
- 36.Walker ED, et al. Geographic distribution of ticks (Acari: Ixodidae) in Michigan, with emphasis on Ixodes scapularis and Borrelia burgdorferi. J. Med. Entomol. 1998;35:872–882. doi: 10.1093/jmedent/35.5.872. [DOI] [PubMed] [Google Scholar]
- 37.Harlan HJ. Observations of host seeking behaviour in American dog ticks, Dermacentor variabilis (Say) (Acari: Ixodidae) in Ohio. Med. Entomol. 2003;4:23–33. [Google Scholar]
- 38.Dodds DG, Martell AM, Yescott RE. Ecology of the American dog tick, Dermacentor variabilis (Say) Nova Scotia. Can. J. Zool. 1969;47:171–181. doi: 10.1139/z69-039. [DOI] [Google Scholar]
- 39.Judd WW. Recent records of ticks, Ixodes cookei Packard and Dermacentor variabilis (Say) (Acarina: Ixodoidea) in southwestern Ontario. Entomol. News. 1975;86:157–159. [PubMed] [Google Scholar]
- 40.Snetsinger R, Jacobs SB, Kim KC, Tavris D. Extension of the range of Dermacentor variabilis (Acari: Ixodidae) in Pennsylvania. J. Med. Entomol. 1993;30:795–798. doi: 10.1093/jmedent/30.4.795. [DOI] [PubMed] [Google Scholar]
- 41.Saura S, Bodin Ö, Fortin M-J. Stepping stones are crucial for species' long-distance dispersal and range expansion through habitat networks. J. Appl. Ecol. 2014;51:171–182. doi: 10.1111/1365-2664.12179. [DOI] [Google Scholar]
- 42.Sagurova I, et al. Predicted northward expansion of the geographic range of the tick vector Amblyomma americanum in North America under future climate conditions. Environ. Health Perspect. 2019;127:107014. doi: 10.1289/EHP5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mierzejewska EJ, Estrada-Peña A, Alsarraf M, Kowalec M, Bajer A. Mapping of Dermacentor reticulatus expansion in Poland in 2012–2014. Ticks Tick Borne Dis. 2016;7:94–106. doi: 10.1016/j.ttbdis.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Gray JS, Dautel H, Estrada-Peña A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in Europe. Interdiscip. Perspect. Infect. Dis. 2009;2009:593232. doi: 10.1155/2009/593232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gasmi S, et al. Evidence for increasing densities and geographic ranges of tick species of public health significance other than Ixodes scapularis in Quebec, Canada. PLoS ONE. 2018;13:e0201924. doi: 10.1371/journal.pone.0201924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pak D, Jacobs SB, Sakamoto JM. A 117-year retrospective analysis of Pennsylvania tick community dynamics. Parasit. Vectors. 2019;12:189. doi: 10.1186/s13071-019-3451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garvie MB, McKiel JA, Sonenshine DE, Campbell A. Seasonal dynamics of American dog tick, Dermacentor variabilis (Say), populations in southwestern Nova Scotia. Can. J. Zool. 1978;56:28–39. doi: 10.1139/z78-004. [DOI] [PubMed] [Google Scholar]
- 48.Burg JG. Seasonal activity and spatial distribution of host-seeking adults of the tick Dermacentor variabilis. Med. Vet. Entomol. 2001;15:413–421. doi: 10.1046/j.0269-283x.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- 49.Newhouse VF. Variations in population density, movement, and rickettsial infection rates in a local population of Dermacentor variabilis (Acarina: Ixodidae) ticks in the Piedmont of Georgia. Environ. Entomol. 1983;12:1737–1746. doi: 10.1093/ee/12.6.1737. [DOI] [Google Scholar]
- 50.Mackenzie AMR, Rossier E, Polley JR, Corber SJ. Rocky Mountain spotted fever—Ontario. Can. Dis. Wkly. Rep. 1979;5:130–132. [Google Scholar]
- 51.Gary AT, Webb JA, Hegarty BC, Breitschwerdt EB. The low seroprevalence of tick-transmitted agents of disease in dogs from southern Ontario and Quebec. Can. Vet. J. 2006;47:1194–1200. [PMC free article] [PubMed] [Google Scholar]
- 52.Walker WJ, Moore CA. Tularemia: Experience in the Hamilton area. Can. Med. Assoc. J. 1971;105:390–396. [PMC free article] [PubMed] [Google Scholar]
- 53.Ontario Agency for Health Protection and Promotion (Public Health Ontario). 2019 tularemia data at a glance. https://www.publichealthontario.ca/en/diseases-and-conditions/infectious-diseases/vector-borne-zoonotic-diseases/tularemia (2020).
- 54.Wood H, Artsob H. Spotted fever group rickettsiae: a brief review and a Canadian perspective. Zoonoses Public Health. 2012;59(Suppl 2):65–79. doi: 10.1111/j.1863-2378.2012.01472.x. [DOI] [PubMed] [Google Scholar]
- 55.Wood H, Dillon L, Patel SN, Ralevski F. Prevalence of Rickettsia species in Dermacentor variabilis ticks from Ontario, Canada. Ticks Tick Borne Dis. 2016;7:1044–1046. doi: 10.1016/j.ttbdis.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Kaufman EL, et al. Range-wide genetic analysis of Dermacentor variabilis and its Francisella-like endosymbionts demonstrates phylogeographic concordance between both taxa. Parasit. Vectors. 2018;11:306. doi: 10.1186/s13071-018-2886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Statistics Canada. Census profile. 2016 Census. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E (2017).
- 58.Statistics Canada. Land use, census of agriculture historical data. Table: 32–10–0153–01. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E (2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Information about PHO’s data access request process is available on-line at: https://www.publichealthontario.ca/en/Data-and-Analysis/Using-Data/Data-Requests.