Abstract
Neurosteroids are involved in the pathophysiology of many neuroendocrine disorders in women. This article describes recent advancements in pharmacology of neurosteroids and emphasizes the benefits of neurosteroid replacement therapy for the management of neuroendocrine disorders such as catamenial epilepsy, postpartum depression (PPD), and premenstrual brain conditions. Neurosteroids are endogenous modulators of neuronal excitability. A variety of neurosteroids are present in the brain including allopregnanolone (AP), THDOC, and androstanediol. Neurosteroids interact with synaptic and extrasynaptic GABA-A receptors in the brain. AP and related neurosteroids, which are positive allosteric modulators of GABA-A receptors, are powerful anticonvulsants, anxiolytic, antistress, and neuroprotectant agents. In catamenial epilepsy, seizures are most often clustered around a specific menstrual period in women. Neurosteroid withdrawal-linked plasticity in extrasynaptic receptors has been shown to play a key role in catamenial seizures, anxiety, and other mood disorders. Based on our extensive research spanning two decades, we have proposed and championed neurosteroid replacement therapy (NRT) as a rational strategy for treating disorders marked by neurosteroid-deficiency, such as catamenial epilepsy and other related ovarian or menstrual disorders. In 2019, AP (renamed as brexanolone) was approved for treating PPD. A variety of synthetic neurosteroids are in clinical trials for epilepsy, depression, and other brain disorders. Recent advancements in our understanding of neurosteroids have entered a new era of drug discovery, one that offers a high therapeutic potential for treating complex brain disorders.
Keywords: Brexanolone, catamenial seizure, neurosteroid, postpartum depression, tonic inhibition
Short Summary:
Neurosteroids play a critical role in catamenial epilepsy and many neuroendocrine disorders. Neurosteroid deficiency leads to reduced tonic inhibition and enhanced seizures, anxiety, or dysphoric behavior. Changes in the abundance or distribution of GABA-A receptors affect the neurosteroid response. Neurosteroid replacement therapy (NRT) is a unique strategy for CE, PPD, and premenstrual mood disorders. A variety of synthetic neurosteroids are in clinical trials for neuroendocrine conditions.
1 |. INTRODUCTION
Steroid hormones play a vital role in many physiological systems. Neurosteroids are mostly synthesized in the brain, and they are capable of rapidly modulating neuronal excitability due to their direct interactions with ion channels or membrane receptors on principal neurons and interneurons.1 Unlike steroids, neurosteroids do not interact with classical steroid hormone receptors.2,3 Neurosteroids are synthesized from cholesterol and circulating steroid hormones precursors in the nervous systems, especially in many brain structures.4 The term “neurosteroid” is commonly used to refer to steroids that are synthesized de novo in the nervous system.5 The term “neuroactive steroid” refer to both natural and synthetic steroids that rapidly alter the excitability of neurons by virtue of their actions at neuronal membrane receptors. Allopregnanolone (AP), allotetrahydro-deoxycorticosterone (THDOC), and androstanediol (AD) are the most widely studied endogenous neurosteroids (Fig.1). Neurosteroids and neurosteroid-based novel drugs offer a vast therapeutic potential for treating conditions such as epilepsy, anxiety, and psychiatric disorders (Table 1).
Fig. 1. Endogenous neurosteroids as powerful modulators of neuronal excitability.

Three prototype neurosteroids are allopregnanolone (AP, brexanolone), allotetrahydrodeoxycorticosterone (THDOC) and androstanediol (AD). The key structural difference between AP and other neurosteroids is illustrated in green bobble.
Table 1.
List of endogenous neurosteroids with neuronal inhibitory and excitatory properties.
| Inhibitory neurosteroids (anxiolytic, sedative, antistress and anticonvulsants) | Excitatory steroids (anxiogenic, dysphorogenic and proconvulsant) |
|---|---|
| Progesterone | Estradiol |
| Allopregnanolone (AP) or brexanolone | Pregnenolone sulfate |
| Pregnanolone | Dehydroepiandrosterone sulfate |
| Dihydroprogesterone | Cortisol |
| Androstanediol (AD) | 11-Deoxycortisol |
| Etiocholanone | |
| Dihydrotestosterone | |
| Deoxycorticosterone | |
| Dihydrodeoxycorticosterone | |
| Allotetrahydrodeoxycorticosterone (THDOC) |
Based on biochemical and molecular investigations, it is clear that neurosteroids are synthesized in the brain or the periphery. Some authors call peripherally-derived steroids as “neuroactive steroids.” As shown in Fig.2, neurosteroids are synthesized from cholesterol or intermediate steroids via progressive A-ring reductions. Based on their chemical characteristic features, neurosteroids are lipophilic and hence can cross the blood-brain barrier. However, the distribution of neurosteroids appears complex based on the analysis of relationship between peripheral and central levels of neurosteroids.6–9 Electrophysiological and pharmacological investigations show that neurosteroid positive modulation of ionotropic receptors on neuronal membranes alters brain function and behavior.10 Generally, neurosteroids are studied under three classes based on their core steroid structure: (i) pregnane neurosteroids, such as allopregnanolone (recently renamed as brexanolone) and THDOC; (ii) androstane neurosteroids, such as AD and etiocholanone; and (iii) sulfated neurosteroids, such as pregnenolone sulfate and dehydroepiandrosterone sulfate.11,12 Neurosteroids and neurosteroid sulfates are known to interact with specific neurotransmitter receptors such as GABA receptors and glutamate receptors. Pregnane and androstane neurosteroids are positive allosteric modulators of GABA-A receptors - the primary rapid inhibitors in the brain. This article describes recent advancements in pharmacology of neurosteroids, with a special emphasis on the development and validation of neurosteroid replacement therapy for neuroendocrine conditions such as catamenial epilepsy, postpartum depression, and other menstrual neuroendocrine conditions. It also highlights the potential molecular mechanisms underlying the neurosteroid modulation of extrasynaptic GABA-A receptor-mediated tonic inhibition and their clinical implications in neurological disorders in women.
Fig. 2.
Biosynthetic pathways of neurosteroids allopregnanolone and THDOC.
2 |. NEUROSTEROID MODULATION OF SYNAPTIC AND EXTRASYNAPTIC GABA-A RECEPTORS
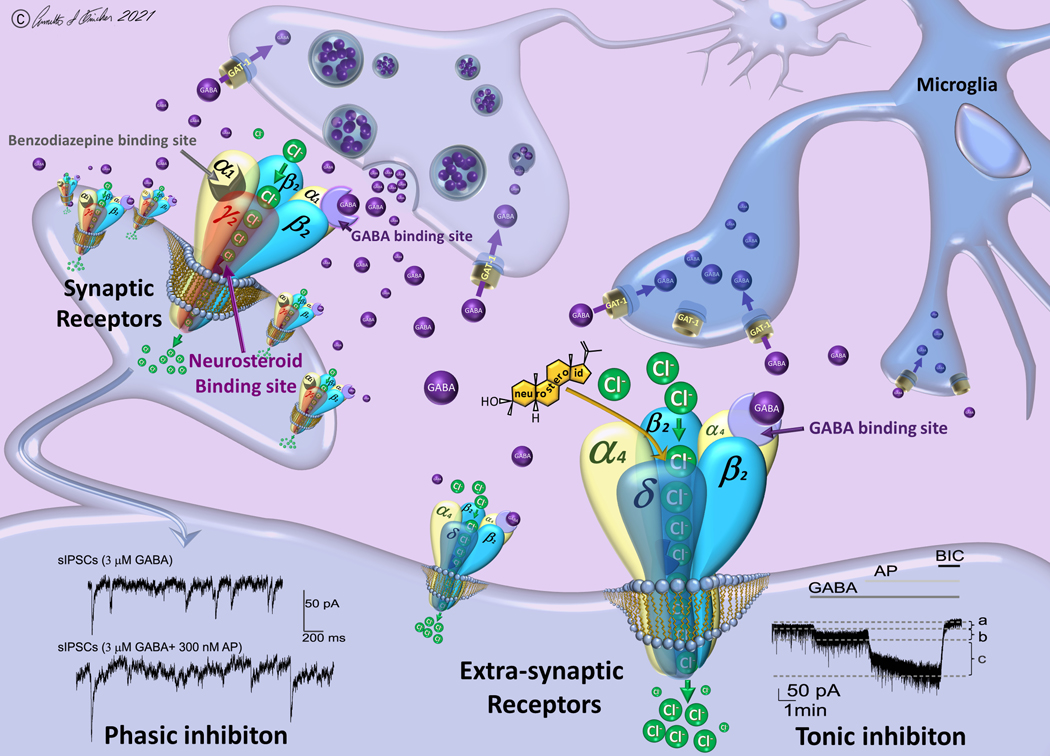
GABA is the principal fast inhibitory neurotransmitter in the brain. GABAergic inhibition involves multiple GABA receptors, including GABA-A, GABA-B and GABA-C receptors. GABA-A receptors, which are ligand-gated chloride ion channels, regulate the neuronal excitability. Biophysical investigations show that GABA-A receptors are composed of five protein subunits (Fig.3) and are further stratified as synaptic (γ-containing) and extrasynaptic (δ-containing) receptors.13,14 Unlike synaptic receptors that produce rapidly-inactivating phasic currents in response to vesicular release of GABA, extrasynaptic receptors produce tonic currents that are continuously activated by ambient GABA present in the extracellular milieu.15 The non-desensitizing tonic current is responsible for setting the baseline inhibitory tone and generating shunting inhibition in brain regions that regulate seizures, anxiety, and mood conditions.
Fig. 3. Neurosteroid modulation of synaptic and extrasynaptic GABA-A receptors.
Neurosteroids such as allopregnanolone (AP) enhance the function of synaptic and extrasynaptic GABA-A receptors by binding to “neurosteroid-binding sites,” which are distinct from the sites for GABA, benzodiazepines, and barbiturates. The left panel shows synaptic receptors composed of 2α2β1γ subunits, which mediate phasic inhibition in response to action potential-dependent release of GABA within the synaptic space. The right panel shows extrasynaptic receptors composed of 2α2β1δ subunits, which primarily contribute to tonic inhibition in response to ambient levels of GABA present in the extracellular fluid. Unlike benzodiazepines that typically bind to synaptic receptors, neurosteroids can bind to both subtypes and thereby enhance the phasic and tonic currents. Traces at the bottom show AP potentiation of phasic current (left) and tonic current (right) in hippocampal neurons. At synaptic sites (phasic inhibition), frequency, amplitude, and decay time of miniature postsynaptic currents (mIPSCs) are potentiated by neurosteroids. At extrasynaptic sites (tonic inhibition), current shift and altered current density is evident with neurosteroid exposure. The tonic inhibition is further affected by the GABA reuptake, which is regulated by neuronal GABA transporter (GAT-1) at the presynaptic terminals and astrocytes/microglia (GAT-2/3). Reuptake into neuronal terminals permits immediate GABA recycling by vesicular uptake, whereas reuptake into astrocytes/microglia leads to metabolism. Overall, neurosteroids can enhance the phasic inhibition and tonic inhibition and thereby promote maximal inhibition in various brain circuits. This network inhibition contributes to their robust actions as anxiolytics, anticonvulsants, and neuroprotectants. AP, allopregnanolone; BIC, bicuculline; GAT-1, GABA transporter-1; GABA, g-aminobutyric acid.
Both synaptic and extrasynaptic GABA-A receptors have multiple binding sites for many ligands (Fig.3). It is well established that neurosteroids are positive allosteric agonists of synaptic and extrasynaptic GABA-A receptors.10,16,17 At low concentrations (< 0.5μM), they possess strong properties to allosterically potentiate GABA-gated currents. This occurs by binding to “neurosteroid binding sites” on the GABA-A receptor complex.18–21 Multiple lines of evidence indicate the presence of at least two distinct binding sites for neurosteroids. Moreover, such neurosteroid binding sites are quite different from the binding sites for other drugs such as barbiturates and benzodiazepines. Besides allosteric actions, at high concentrations (>1 μM), neurosteroids are known to directly modulate GABA-A receptors.16,22 In fact, pharmacological doses of neurosteroids can reach such concentrations within the brain to affect GABA-A receptor function.23,24 Therefore, it is highly likely that the net inhibition produced by neurosteroids can potentially occur via a combination of allosteric and direct activation mechanisms.
Pharmacological investigations during the past two decades have confirmed that neurosteroids are capable of binding to multiple subtypes of GABA-A receptors and various isoforms, displaying a preferential affinity at extrasynaptic δ-containing receptors.25–29 A defining feature of neurosteroid pharmacology is the broader activity of neurosteroids, as they can bind and activate most isoforms of GABA-A receptors, including those that contain benzodiazepine-insensitive α4 and α6 subunits or those that lack the obligatory γ2 subunit required for benzodiazepine-sensitivity. The low-efficacy δ-containing receptors, which are often present on perisynaptic and extrasynaptic sites in the hippocampus, thalamus, cortex and cerebellum, have received much attention due to their non-sensitizing nature. These extrasynaptic receptors that contribute to tonic inhibition exhibit unique characteristics, especially subunit composition, channel dynamics, and pharmacological sensitivity to various compounds. The extracellular GABA is essential for allosteric actions of neurosteroids. The binding of neurosteroids to extrasynaptic δGABA-A receptors can cause a certain conformational change for greater channel opening and subsequent induction of tonic inhibition.30 This mostly occurs by increasing abundance of δ-containing receptors. Upregulation of δGABA-A receptor expression promotes neurosteroid sensitivity that manifests as increased potentiation of tonic currents.31–33 On the other hand, a relative downregulation of δGABA-A receptors can drastically reduce sensitivity to neurosteroids.29,34–37 These changes are associated with significant alterations in seizure susceptibility and epileptic seizures.38
Recent experiments showed a preferential augmentation of tonic inhibition by neurosteroids. A comparative profile of neurosteroid modulation of tonic current and its correlation with seizure protection are outlined in Table 2. There is greater focus on relative affinity of new compounds at δ-containing receptors. In this connection, a recent pharmacological study has identified a consensus pharmacophore model for neurosteroid interaction at extrasynaptic δGABA-A receptors in the hippocampus.29 Electrophysiological studies show that alterations to the C17 or C20 region of the neurosteroid molecule can profoundly reduce its positive features on receptor function to activate tonic inhibition. As expected, AP and related pregnane analogues exhibited the greatest potency and maximal efficacy in activating tonic current in hippocampal neurons. Furthermore, tonic current potentiation was completely ($95%) diminished in hippocampal granule cells from δ-subunit deficient δ-knockout mice, suggesting that δ-subunit receptors are essential for neurosteroid activity. Therefore, extrasynaptic δGABA-A receptors play a key role in regulating tonic inhibition and neurosteroid sensitivity.32,33,39
Table 2.
Correlation of between neurosteroid activation of tonic inhibition and seizure protection in in mouse 6-Hz model.
| Neurosteroid | Tonic Currents | Seizure Protection ED50 (mg/kg)*** | |
|---|---|---|---|
| E1 μM (pA)* | EF(2-fold GABA) (nM)** | ||
| Brexanolone (AP) | 100.6 | 80 | 4.2 (2.7–5.8) |
| Ganaxolone | 64.0 | 290 | 1.5 (1.3–1.7) |
| Pregnanolone | 44.4 | 780 | 7.7 (6.6–8.8) |
| Isopregnanolone | 15.3 | >10000 | > 100 |
| THDOC | 66.6 | 410 | 5.0 (2.6–7.4) |
| Alfaxolone | 40.9 | 990 | 8.8 (6.1–11.4) |
| ORG-20599 | 86.4 | 120 | 18.6 (16.6–20.6) |
| Androstanediol | 33.2 | 1710 | 44.0 (30.2–58.8) |
E1 μM values represent the mean normalized tonic current responses of drug at 1 μM concentration co-applied with 1 μM GABA. GABA 1 μM tonic current: 0.66 ± 0.22 pA/pF, 19.6 pA.
EF values represent the effective functional concentration of drug (nM) required to double or triple the 1 μM GABA response.
ED50 values represent the dose in milligrams per kilogram that protected 50% of animals in the 6-Hz seizure stimulation test. 95% confidence intervals are listed in parenthesis, according to a normal distribution.
3 |. NEUROSTEROIDS AS ANTICONVULSANTS AND ANXIOLYTIC AGENTS
During the past three decades, neurosteroids have been widely investigated in preclinical models of seizures, anxiety, and excitability disorders. Epilepsy is a chronic neurological condition characterized by repeated seizures, which affects about 65 million people worldwide.40–42 While effective treatments exist for many people with epilepsy, there is no cure for epilepsy. Moreover, antiseizure medications (ASMs) fail to effectively control seizures in one third of patients with intractable seizures.43 Previous studies have taught us that GABAergic drugs possess anticonvulsant properties. As powerful GABAergic agonists, neurosteroids exhibited a broad-spectrum anticonvulsant profile in an array of seizure models.12,44,45 In acute seizure models, neurosteroids are shown to protect against seizures induced by GABA-A receptor antagonists, including pentylenetetrazol and bicuculline, and are effective against pilocarpine-induced limbic seizures as well as seizures in fully-kindled animals exhibiting evoked generalized seizures.45 Based on their structural feature, the pharmacological potencies of neurosteroids may vary in different seizure models.45–47 For example, many neurosteroids are highly active in the 6-Hz model of psychomotor seizures.29,48 Seizures are known to be triggered due to abrupt withdrawal of GABAergic agents including neurosteroids and benzodiazepines, as well as other types of agents such as ethanol and cocaine.49–52 Neurosteroids are shown to be protective against such withdrawal seizures. In general, tolerance develops with repeated administration of benzodiazepines. However, anticonvulsant tolerance is not observed with repeated or chronic administration of neurosteroid.53,54 Despite some limited preclinical reports,55,56 pharmacodynamic tolerance was not evident in clinical trials of neurosteroids.57,58 There are limited studies on the impact of neurosteroids on chronic epilepsy models. There is some evidence to suggest that neurosteroids may play a role in modulating epileptogenesis.38,59–61 Neurosteroid modulation of epileptogenesis is under intense scrutiny. Nevertheless, a deeper understanding of how neurosteroid modulation of phasic and tonic inhibition affects epileptic seizures and epileptogenesis is essential to advancing neurosteroid therapeutics of epilepsy.
Decades of research with steroid hormones and neurosteroids has led to identification of specific compounds for treatment of epilepsy and excitability disorders. During the past two decades, AP and its synthetic analogues have been evaluated in a multitude of clinical trials to assess their utility in epileptic patients.62–64 Ganaxolone, the 3β-methylated analog of AP, and other novel agents are being assessed in clinical trials for epilepsy.65 Natural neurosteroids have low bioavailability and can be reconverted to active 3-ketone-containing progesterone metabolites.2 The synthetic 3β-substituted analogs provide a more promising profile as anticonvulsants. Ganaxolone has a more favorable biopharmaceutical profile than brexanolone due to its higher bioavailability and fewer hormonal side effects.29,66,67 It produces powerful antiseizure activity in a wide range of experimental models and is being evaluated in clinical trials for epilepsy.24,51,54,57,65,68–71 Brexanolone has been investigated as adjunctive therapy in super-refractory status epilepticus.72 In the pilot trial, it exhibited tolerability and was associated with a high rate of successful third-line agent weaning. It was less successful in a subsequent trial. Synthetic neurosteroid analogs such as ganaxolone, zuranolone and other analogs, which activate both synaptic and extrasynaptic GABA-A receptors, are promising compounds for clinical investigations. Systematic randomized clinical trials are required to propel neurosteroid-based therapies from the preclinical realm into routine clinical practice.
4 |. CELL-SPECIFIC AND PHOSPHORYLATION-DEPENDENT ACTIONS OF NEUROSTEROIDS
A better understanding of cellular and molecular actions of neurosteroid on GABAergic inhibition is essential for optimizing the neurosteroid therapeutics. A variety of synthetic compounds around neurosteroid structure are designed to control seizure and excitability conditions. The lead compounds are identified based on in vitro studies in heterologous systems expressing pentameric GABA-A receptors. Therefore, the precise mechanisms of action of such lead compounds in native neurons remain unclear. Recently, we uncovered the mode of action of one synthetic neurosteroid (ganaxolone) as well as those of other analogs in native hippocampal neurons.37 Both phasic and tonic inhibition were profiled using concentration-response relationships in two distinct neuronal cell types: δ-containing dentate gyrus granule cells (DGGCs) and γ2-containing CA1 pyramidal cells (CA1PCs). Since the relative expression of δ-subunits is higher in DGGCs than in CA1 pyramidal neurons, we utilized DG neurons for detailed characterization of 3β-methyl analogs of neurosteroid compounds on phasic and tonic currents. As expected, GX produced three-fold greater potentiation of GABA-gated currents in DGGCs than CA1PCs.37 We identified that, even in the absence of GABA, GX could produce a 2-fold greater inhibitory currents in DGGCs than CA1PCs. The extrasynaptically-mediated tonic currents were investigated in the hippocampal slice preparation. The major findings included GX potentiation and direct activation of tonic currents in DGGCs. The GX-activated current responses are entirely absent in DGGCs isolated from δ-subunit knockout (δKO) mice, confirming its preferential selectivity for extrasynaptic δGABA-A receptors. The kindling model was subsequently utilized to confirm the impact of this mode of action of GX in vivo. In kindled mice, GX produced a dose-dependent protection against evoked seizures; however, this protection was significantly diminished in δKO mice. We identified that certain GX analogs exhibited greater potency and efficacy than GX on tonic inhibition and seizure protection. Together, these findings confirm that GX may control epileptic seizures by two modes of action: potentiation of GABA-A receptor-mediated synaptic and tonic inhibition via allosteric potentiation of GABA response and direct activation of receptors. Therefore, it is highly likely that GX and its analogs are preferential allosteric modulators and direct activators of extrasynaptic δGABA-A receptors, which regulate network inhibition and seizures in a neuronal cell-type specific fashion.14
Protein phosphorylation is a key factor in signal transduction systems, with emerging information on the role of phosphorylation in neurosteroid action. In neurons and neuronal cell types, protein kinases can regulate the function of other proteins by phosphorylating hydroxyl groups on target proteins. Protein kinase can affect GABAergic neurotransmission at multiple levels, including GABA-A receptor surface expression, trafficking, chloride conductance, and sensitivity to neurosteroids. The protein kinase activity levels are possible contributors to these changes. In fact, it is known that many GABA-A receptor subunits contain residues that can be phosphorylated by protein kinases including α4, β, and γ2 subunits.73,74 Phosphorylation of residues within the intracellular loops of the β3 and γ2 subunits maintain the surface expression of GABA-A receptors, whereas dephosphorylation of these subunits may trigger receptor internalization and diminish the abundance of membrane receptors.75,76 PKC activation or inhibition may affect neurosteroid activity at these receptor functions. Application of PKC activator phorbol 12-myristate 13-acetate increases THDOC-potentiated, GABA-gated chloride currents.77 Treatment with the PKC antagonist bisindolylmaleimide diminishes the inhibitory currents by neurosteroids.78 Inhibition of either PKA or PKC reduces neurosteroid-mediated decay of mIPSCs in hippocampal neurons.79 The β- and α4-subunits appear to be involved in these changes. It is demonstrated that phosphorylation of the serine residue of the β-subunits leads to rapid surface expression with enhanced tonic currents in neurons.80 Co-treatment with PKC inhibitor prevents the neurosteroid THDOC-upregulated phosphorylation of the α4 subunits and surface expression of α4GABA-A receptors.81 Moreover, continuous application of AP can affect the phosphorylation and surface expression of the β3GABA-A receptors and tonic currents; these effects are completely prevented by the application of PKC inhibitor.82 A deeper understanding of the functional role of PKC activity on the neurosteroid actions at extrasynaptic GABA-A receptors is essential for defining physiological conditions for better facilitation of tonic inhibition in the brain.
We investigated the role of PKC on allosteric potentiation of tonic currents by AP and ganaxolone in native hippocampal neurons.83 Pretreatment of PKC inhibitor GF109203X for 15- or 30-minutes prior to the application of GABA reduced GABA-evoked tonic current density in the hippocampal slices. The PKC inhibitor attenuated the allosteric potentiation of neurosteroids in a time-dependent manner, with nearly a 92% reduction in tonic current. These results demonstrate that neurosteroid potentiation of tonic current is regulated by the extent of PKC activity in the neurons. The net neurosteroid response is greatly influenced by PKC activity, which may alter the surface expression and functional response of extrasynaptic receptors. Several GABA-A receptor subunits are substrates of PKC, including the α4 and β subunits.81,84 Consequently, PKC inhibition may cause decreased phosphorylation and subsequent internalization of receptors resulting in reduced tonic current potentiation.14
In addition, there is great potential for launching neurosteroid combination therapy with other drugs. The effect of combining neurosteroids with clinical GABAergic drugs was investigated using multidisciplinary approaches.85 Isobolographic analysis, a gold-standard arithmetic technique, enabled us to select the best combination of two drugs to achieve maximal efficacy. The results showed a remarkable positive interaction between two neurosteroids and other GABAergic antiepileptic agents such as tiagabine and midazolam. First, by employing electrophysiological recordings in single neurons, we identified which of these two drugs at specified concentrations can produce optimal inhibitory currents for seizure control. Second, by isobologram plotting, we identified that brexanolone or ganaxolone when applied with tiagabine had the best synergistic effect. These neurosteroids also elicited significant inhibitory current with midazolam. Finally, we have confirmed that such combination regimens can effectively reduce seizures in experimental models.85 These findings provide a strong mechanistic rationale for the use of neurosteroids in the clinical setting, with combination therapies composed of midazolam and tiagabine or neurosteroids as key formulations for the treatment of refractory seizures and other hyperexcitability conditions.
5 |. ZINC BLOCKADE OF NEUROSTEROID ACTIONS
Drug-drug interactions are a significant concern for many antiseizure medications. Zinc (Zn2+) is identified as an endogenous antagonist of neurosteroid actions in the brain. Zn2+ is an essential cofactor in many cells including neurons; it is the most abundant transition metal in the vesicles of hippocampal mossy fibers. During neuronal discharges, vesicular Zn2+ is released into the synaptic space in the hippocampal neuronal circuits. Excessive release of Zn2+ has been shown to affect the threshold of seizures.14 In addition, excess levels of Zn2+ can enter the brain from peripheral sources, especially if there is breach of the blood-brain barrier, such as in, neuronal injury, ischemia, or other acute conditions that may affect the neurovascular endothelial permeability of peripheral zinc ions into the brain. It is well-recognized that Zn2+ blocks GABAergic inhibition by plugging the receptor channel complex. Neurophysiological studies shows that Zn2+ may negatively modulate synaptic GABA-A receptors and modify the excitability of the hippocampal networks.86 These actions are mediated by specific bindings sites on GABA-A receptors. There are three distinct Zn2+ binding sites on GABA-A receptors: one at the internal surface of the channel pore and two at the external amino-terminus of the α-β interfaces. These distinct binding sites may contribute to differential effects of Zn2+ on GABA-A receptor isoforms. The incorporation of the γ-subunit after GABA-A receptor co-assembly disrupts two of the Zn2+ binding sites, which leads to a reduced sensitivity to Zn2+ inhibition.87 Therefore, it is highly likely that the level of Zn2+ inhibition is different at synaptic and extrasynaptic GABA-A receptors.
Recently we demonstrated that Zn2+ selectively blocks extrasynaptic δGABA-A receptors in the dentate gyrus.39,83 In the first study, we tested the interaction between AP and Zn2+ in electrophysiological recordings.39 We found that Zn2+ blocked AP potentiation of tonic currents in a concentration-dependent manner, while synaptic currents were unaffected. Moreover, application of Zn2+ chelator prevented these effects of Zn2+, which further confirm the selective Zn2+ blockade of neurosteroid-sensitive tonic inhibition. In the mouse kindling model of epilepsy, intrahippocampal infusion of Zn2+ resulted in rapid epileptiform activity and prevention of the antiseizure activity of AP. Like neurosteroids, Zn2+ may exhibit high-affinity for extrasynaptic δGABA-A receptors by binding at different allosteric sites. In the second study, we investigated the pharmacodynamic interactions of Zn2+ and ganaxolone at extrasynaptic GABA-A receptors and their pharmacological relevance in the kindling model.83 We demonstrated that zinc could totally block GX-induced potentiation of tonic currents, but not synaptic phasic currents. In the in vivo studies in fully-kindled animals, the antiseizure effects of GX were significantly prevented by intrahippocampal administration of Zn2+ in kindled mice. These finding suggest that Zn2+ diminishes the antiseizure effects of GX by selectively blocking the extrasynaptic δGABA-A receptors in the hippocampus. These findings have clinical implications in neurosteroid therapy for brain conditions associated with zinc fluctuations.
6 |. NEUROSTEROID MECHANISMS OF CATAMENIAL EPILEPSY
Recent investigations have shown that epilepsy is highly circadian in nature.88 Hormones and epilepsy have a complex relationship where seizure occurrence is affected by hormone fluctuations. Hormone changes can trigger epileptic seizures in persons with epilepsy. The ovarian cycle is a very common trigger for enhanced seizures, often referred as catamenial epilepsy (CE). CE is characterized by a cyclical seizure exacerbation near specific phases of the menstrual cycle in epileptic women. CE affects up to 70% of women with epilepsy.89,90 The occurrence of seizures during certain phases of the menstrual cycle is most often due to cyclical fluctuation in steroid hormones and neurosteroid levels.46,89,91,92 Since neurosteroids have protective effects, a decline in their levels at a particular menstrual phase could trigger a seizure in preexisting epilepsies.90 CE is managed with common antiseizure medications (ASMs), but many women still experience catamenial exacerbations that can resemble pharmacoresistant seizures.90,93 Presently, there are no FDA-approved drug therapies for treating CE. The lack of understanding of mechanisms of CE and catamenial exacerbation is a major issue in this field.
People with CE are more likely to experience reproductive issues. Seizure themselves can cause disruptions in hormones and menstrual cycle. The subfield of CE research is slow and there are many gaps in clinical knowledge about this condition. Based on the specific pattern of seizure occurrence in relation to menstrual cycle, three types of catamenial seizures have been identified. They include perimenstrual (C1), periovulatory (C2), and inadequate luteal phase (C3).94,95 The perimenstrual (C1) type is the most common clinical type. In perimenstrual CE, women with epilepsy experience an increase in seizure activity on days 23 to −3 of the cycle.90 In most cases, clinical diagnosis of CE is made by longitudinally assessing menstruation and seizure records from the self-reporting. After counting the number of seizures in each of the 4 phases for at least two menstrual cycles, CE may be identified if there is a two-fold or greater increase in frequency of seizures during a particular phase of the cycle. The seizure diary and onset of menstrual bleeding would serve as critical correlation pointers. CE can be diagnosed in women with both ovulatory and anovulatory cycles; nearly 17% of CE patients had anovulatory cycles.96 These women were found to show inadequate luteal phase (C3) or anovulatory luteal seizures.
The underlying drivers of CE are not well understood and may vary between individuals. The neuroendocrine basis of CE may be understood by general insights of how hormones affect seizures. Ovarian cycle-related fluctuations in estrogens and progesterone play a critical role in the pathogenesis of CE. As discussed in next several paragraphs, the sensitive balance between the excitatory hormone estradiol and the neuronal inhibitory hormone progesterone underlies the changes in seizure susceptibility associated with catamenial exacerbation.90,97 Estradiol levels rise during the follicular and luteal phases, which increases the estrogen:progesterone ratio. This imbalance can affect epileptic women’s likelihood of displaying perimenstrual seizures.92,98–100 The cyclical changes in progesterone levels during the menstrual cycle can drastically affect the seizure susceptibility in women with CE.90 Profound impact is evidently observed around the mid-luteal and pre-menstrual phases. Seizure frequency decreases during the mid-luteal phase, which is associated with high progesterone levels, and seizure exacerbation is more frequent before menstruation when progesterone levels are significantly lower or inactive.90,101 There is a strong premise that perimenstrual catamenial seizures are strongly correlated with the abrupt decrease in progesterone near menstruation.102–105
Progesterone plays a critical role in CE. It is an anticonvulsant steroid hormone. Progesterone can serve as an anticonvulsant agent through at least three distinct mechanisms: (a) negatively impacting glutamatergic (excitatory) transmission, (b) binding to progesterone receptors (PRs), (c) and metabolizing to neurosteroids such AP and pregnanolone.106–110 Progesterone serves as the intermediate precursor for the biosynthesis of the neurosteroid AP. The conversion of progesterone into neurosteroids occurs through sequential A-ring reductions of progesterone molecule.22,60,97,106,111 The elevated risk of seizure susceptibility during the phases of the menstrual cycle associated with low levels of progesterone are attributed to the loss of corresponding anticonvulsant neurosteroids, especially AP.33,104,112,113 An interesting plasticity occurs during the perimenstrual period with marked changes in extrasynaptic GABA-A receptors (Fig.4). During this period, extrasynaptic GABA-A receptors are notably upregulated above normal, with possible molecular mechanisms underlying this phenomenon discussed in the next section.33,114 Such an overexpression of extrasynaptic receptors provides neurosteroids with an abundance of locations to exert their anticonvulsant effects.104 Therefore, perimenstrual CE can be attributed to deficiency or withdrawal of neurosteroids. This mechanistic pathway is providing a framework for designing preclinical models and specific treatments for CE.
Fig.4. GABA-A Receptor plasticity model of perimenstrual and ovarian hormone-related brain diseases.
Extrasynaptic GABA-A receptors undergo cyclical plasticity (change in receptor composition and/or abundance) during the ovarian cycle phases due to fluctuations in neurosteroids such as AP. A delta-subunit upregulation may underlie the exacerbation of neuronal excitability and neurosteroid sensitivity, such as those occurring in perimenstrual seizures and post-partum depression. The bottom section shows a model of receptor plasticity around the premenstrual period. Because neurosteroids show enhanced sensitivity, partly due to increase in receptor abundance, during the perimenstrual period that is associated with catamenial-like seizure exacerbation. “Neurosteroid replacement” may provide a rational strategy for treatment of perimenstrual seizures and other brain conditions. A pulse therapy or continuous low-doses of neurosteroid agent may help control catamenial seizures without hormonal side effects. The top panel shows a similar mechanism during pregnancy and post-partum period. In postpartum depression, a rapid drop in neurosteroid levels occurs that could trigger changes in extrasynaptic GABA-A receptors and thereby reduce the net inhibition, which is essential to check the excessive excitability and depression. Neurosteroid replacement therapy, which consists of administering a neurosteroid during the postpartum period to alleviate the mood disorder, is a proven approach for the management of postpartum depression. Intravenous allopregnanolone, renamed as brexanolone (Zulresso®), was approved in 2019 by the FDA for treatment of PPD. Oral neurosteroid analogs are in clinical testing for PPD and related brain conditions (Figure adapted from Reddy, 2016).
There are very few models of CE that show features reminiscent of women with CE. We developed two categories of CE models for pharmacological and molecular studies, utilizing both rat and mice models with the aim of creating a hormonal milieu resembling the perimenstrual period.97,115–117 We used both healthy rats and epileptic animals. The perimenstrual environment was created by a variety of manipulations using the pseudopregnancy state, the exogenous administration of progesterone, and the utilization of a chronic epilepsy model. These studies revolved around the notion that seizure susceptibility and neurosteroid levels are inversely related in females in association with specific changes in GABA-A receptor subunit plasticity. Cyclical protocols with augmentation of endogenous neurosteroid synthesis and withdrawal appear more physiologically relevant than other subacute paradigms.97 This can be accomplished using gonadotropin that increases neurosteroid levels. When gonadotropin is administered, it induces superovulation and the release of progesterone followed by its conversion to allopregnanolone. Concurrently, the increase in allopregnanolone levels can be blocked by finasteride administration to create an artificial neurosteroid withdrawal. During this neurosteroid withdrawal phase the seizure threshold in these animals dropped significantly lower before bouncing back up to normal levels within 72 hours in healthy animals.115 Similarly, we studied this phenomenon in epileptic rats by inducing epilepsy via pilocarpine exposure and then monitoring seizure activity. There was an increase in seizure frequency during the withdrawal period,117 suggesting catamenial seizure exacerbation.
We then replicated this setup in the mouse models, which allow for more mechanistic studies.97,116 We induced NSW in fully kindled animals before subjecting them to the gonadotropin protocol, increasing allopregnanolone levels before blocking its conversion from progesterone after ten days with finasteride. This was associated with significant decreases in after discharge thresholds, increases in seizures, and increases in after discharges. These animals displayed seizures at 50% of their threshold current, which is atypical of mice. We tested neurosteroids during the NSW phase, with withdrawal animals showing much more sensitivity to neurosteroid protection than control animals. This observation indicates the occurrence of an event that coincides with withdrawal-induced neurosteroid sensitivity. We showed that neurosteroid withdrawal triggers upregulation of α4-GABA-A receptors.116 This would promote reduced synaptic inhibition, which could explain increased rates of seizures. Additionally, we demonstrated that increases in neurosteroid sensitivity could be a result of enhanced expression of δ-GABA-A receptors.33 Indeed, tonic current in DGGCs is huge in withdrawal animals in response to AP, but not in the CA1 cells. We found the same occurrence in the δGABA-A receptor knockout mice which were withdrawn from neurosteroids after prolonged exposure. When we tested AP on the phasic and tonic currents, there were no differences between control and knockout withdrawal animals, suggesting that the presence of the δ-subunit is necessary for neurosteroid sensitivity. We used the animals to suppress the increase in the δ-subunit, infusing δ-antisense oligos. In the δ-missense, there was still an increase in sensitivity to anticonvulsant protection to AP. When we infused antisense, the seizure protection was lost, indicating that by diminishing the δ-subunit or its incorporation into the extrasynaptic receptor, we could demonstrate the sensitivity of the neurosteroids.
We used δGABA-A receptor knockout mice to show catamenial-like seizure exacerbation, subjecting them to the gonadotropin induced withdrawal.114 There was a significant increase of afterdischarges in the knockout animals. Generally, wild type fully-kindled animals do not show spontaneous seizures to this extent. At maximum, 20% of the animals that receive a kindling stimulation will experience a spontaneous seizure 20 minutes to 1-hour post stimulation. However, in the knockout animals subjected to withdrawal, close to 50% of the animals showed spontaneous seizures. This indicates hypersensitivity in these animals, which is analogous to catamenial-like exacerbation.
Based on the extensive experimental findings in multiple models, we proposed an extrasynaptic GABA-A receptor plasticity model, which is illustrated in Fig.4.104 During the luteal phase, there is a certain amount of extrasynaptic GABA receptors which respond sensitively to neurosteroids, offering significant seizure protection. The seizure protection is maximal during the luteal phase, and the protection is lost in the menstrual phase due to decreases or withdrawal of neurosteroids. During that time, there is a subsequent change that occurs in the δ-subunit. Essentially, the number of δ-subunit extrasynaptic GABA-A receptors doubles during the perimenstrual phase due to the significant absence of neurosteroid. The brain consequently promotes neuronal tonic inhibition using whatever leftover amounts of neurosteroids are trapped. As a result, we see more tonic current, which then cycles according to the ovarian cycle. In summary, an extrasynaptic δ-linked molecular mechanism is involved in the pathophysiology of catamenial seizures. The perimenstrual decline in neurosteroids triggers the selective overexpression of δGABA-A receptors, but this is not sufficient to suppress the increased seizure susceptibility. This compensatory increase in δGABA-A receptors may act as a “Trojan horse” for the exogenous neurosteroid to inhibit seizures and behavioral dysfunction. Therefore, this extrasynaptic mechanism represents a molecular rationale for neurosteroid therapy of catamenial epilepsy.
7 |. NEUROSTEROID REPLACEMENT THERAPY
CE is classified as pharmacoresistant because of recurrent breakthrough seizures despite treatment with ASMs. Currently, there is no FDA-approved drug therapy for catamenial seizures and related menstrual conditions. The neurosteroid withdrawal (NSW) concept is providing a framework for developing new therapies for perimenstrual CE.118 While many ASMs provide some protection in women with CE, many do not respond to common ASMs. The NSW model has been utilized to confirm that conventional ASMs have a reduced potency in protecting against catamenial seizures. Counterintuitively, our studies have found that neurosteroids and their synthetic analogs may produce enhanced activity in the perimenstrual CE model.51,52,54,97 Unlike benzodiazepines, neurosteroids exhibited enhanced sensitivity in NSW models of CE. Based on these pharmacological studies, we proposed in 2009 that “neurosteroid replacement therapy” (NRT) may be an effective method for controlling catamenial seizure exacerbations.119 We suggested that a neurosteroid or synthetic analog could be administered in a pulse protocol during perimenstrual period or throughout the month, at low doses to avoid side effects, to serve as an innovative CE therapeutic.
Although the molecular mechanisms underlying enhanced neurosteroid sensitivity in CE remain unclear, an upregulation of ɑ4 expression 120 and extrasynaptic δGABA-A receptors 104 may explain this phenomenon This ovarian cycle-regulated, extrasynaptic receptor plasticity mechanism is illustrated in Fig.4. Based on the available experimental and other evidence, it is suggested that the drop in neurosteroids during the perimenstrual period triggers a selective upregulation of ɑ4δ GABA-A receptors in the hippocampus and other brain regions. This alone is not adequate to reduce the increased seizure susceptibility due to the deficiency of neurosteroids, but this compensatory rise in ɑ4δ subunits could possibly act as a vehicle catalyst for exogenously administered neurosteroids to enhance seizure threshold, prevent seizures, and attenuate mood dysfunction. This unique sensitivity to neurosteroids is also displayed by some women with perimenstrual CE, as evident from the results of the NIH progesterone trial, where the best responder group was women with perimenstrual CE.121 This group demonstrated a significant post-treatment surge in AP, an outcome that suggests perimenstrual CE would be a viable target condition for testing the therapeutic benefits of a pulse or cyclical neurosteroid therapy during the perimenstrual period.122 However, there are some limitations in the Herzog study (2012). There was no difference in the primary outcome of ≥50% responder rates between progesterone vs placebo for catamenial or non-catamenial groups. Moreover, post hoc analysis suggests that the level of perimenstrual seizures is a significant predictor of responder rate with progesterone. Thus, neurosteroid may be helpful to some women with CE (C1 level).
Although brexanolone-like natural neurosteroids have great therapeutic potential, there are some obstacles that prevent their widespread clinical use: (i) poor bioavailability; (ii) rapid hepatic inactivation; (iii) short plasma half-life; (iv) limited extrasynaptic receptor selectivity; and (v) poor water-solubility that hinders formulation of an aqueous injection product with optimal stability.10,114 These limitations are surpassed by synthetic water-soluble analogs of brexanolone and ganaxolone (Fig.5). Recently, we synthesized many new water-soluble analogs of brexanolone with improved potency and antiseizure efficacy.37 We achieved a breakthrough in preparing synthetic neurosteroids with excellent water-solubility and preferential extrasynaptic receptor activity. Three major factors governed the creation of new analogs: (i) blood-brain-barrier permeability, which is essential for their action in the brain; (ii) aqueous solubility, which is required for making stable injection products; and (iii) slow metabolism to allow for longer plasma half-lives. The two most promising lead compounds are in advanced development as anticonvulsants and neuroprotectants.
Fig.5. Neurosteroid drug development.
(A) Structure-activity relationship for identification and optimization of novel neurosteroid analogs as modulators of synaptic and extrasynaptic GABA-A receptors. (B) Novel neurosteroid analogs with potential clinical use in neurological disorders. Ganaxolone and SGE-516 are clinical trials for epilepsy and brain conditions. Alfaxolone is available for veterinary clinical use as anesthetic agent.
Recently, we further investigated the molecular mechanisms of action of brexanolone and related neurosteroid analogs at extrasynaptic δGABA-A receptors in native neurons. Potential interactions between neurosteroids and other combinations were tested in experimental model systems. We tested concentration-response profiles of neurosteroids in multiple cell types.37 Our results demonstrate that brexanolone and analogs are preferential allosteric modulators and direct activators of extrasynaptic δGABA-A receptors in the dentate gyrus. The potentiation of tonic inhibition is both δ-subunit-dependent and protein kinase C activity-mediated. Zinc concentration-dependently blocks AP-potentiated GABA-gated currents in the hippocampus.39,83
8 |. NEUROSTEROIDS IN POSTPARTUM DEPRESSION AND MOOD DISORDERS
Major progress on neurosteroid research during the past two decades has helped develop a neurosteroid therapy for postpartum depression (PPD). In March 2019, the FDA approved brexanolone (Zulresso) as the first drug for treating PPD, a condition that affects about 10–20% of post-partum women. PPD is characterized by clinically-significant depressive symptoms that are often accompanied by anxiety. These are referred to as a “major depressive episode,” and the onset of symptoms occurs after childbirth, especially within the first 4 weeks following delivery.123 PPD significantly impacts patient maternal health-related quality of life. Such a condition hinders a mother’s ability to care for and bond with their babies, and traditional antidepressants take weeks to work—crucial weeks that women with suicidal thoughts or fears of harming their children may not be able to afford. Because these symptoms have a significant negative impact on both mother and infant, immediate clinical intervention is necessary. However, until the evaluation of neurosteroid therapy in 2018, there was no effective treatment for PPD. Antidepressants like fluoxetine had been given but offered limited clinical benefit or delayed benefit due to slow onset (4–6 weeks) of beneficial effects. Such timing was inconsistent with the needs of mothers with baby blues, which happen rapidly within the first several days after childbirth and need to be controlled immediately due to the risk of suicide or harm to the infant. Preclinical research on AP and related neurosteroids has changed this situation.1,10,11,16,33,39,51,52,54,83,106,114,115,124–133 Extensive research on neurosteroid exposure and withdrawal models and their underlying pharmacological mechanisms have paved the way to identify and develop neurosteroid therapy for PPD. Brexanolone therapy for PPD is based on the core neurosteroid replacement strategy, as highlighted in dozens of papers, including identifying a unique “extrasynaptic mechanism” during the perimenstrual and postpartum period.14,104,115,132,134,135 Consequently, neurosteroid therapy of PPD is based on the concept of providing postpartum mothers with doses of AP equivalent to endogenous third-trimester concentrations for a few days.
Brexanolone has shown significant beneficial effects in clinical trials in PPD.136–138 In clinical investigations, a total of 3 multicenter, randomized, double-blind, placebo-controlled trials were conducted in women with PPD. A significant reduction in the degree of depressive behavior signs, as measured by using the Hamilton Rating Scale, was reported at both the end of the 60-h infusion and at 30 days. In contrast to traditional antidepressants, brexanolone was found to be efficacious within 24 hours of administration. However, it causes sedation and alters consciousness in a few patients, requiring a Risk Evaluation and Mitigation Strategy program. Overall, brexanolone therapy produces an effective and ultra-rapid antidepressant effect in women with PPD. Novel oral neurosteroids are in advanced development for PPD. An extensive discussion and meta-analysis have been covered elsewhere.139–142
Major depression affects over 22 million individuals in the U.S. It is typified by harrowing feelings of sadness, hopelessness, pessimism, loss of interest in life, and reduced emotional well-being. It also comes with disturbances of sleep and appetite, decreased energy, and often cognitive disturbances, such as difficulty concentrating and remembering. These persons are at high risk for suicide. While genes and the environment both play a role in an individual’s risk for major depression, chronic stress also can trigger a depressive episode. There is no clear evidence on the functional role of endogenous neurosteroids in depression,143,144 although changes in the levels of certain neuroactive steroids have been reported in some patients.145,146 In contrast, other studies have reported that there are no significant differences in the levels of neurosteroids in patients with depression.147 There are limited preclinical reports that describe the effect of AP and related neurosteroids in rodent models of depression.148,149 Recently, therapeutic drugs have been developed to mimic neurosteroids and tested in clinical trials for amelioration of depression symptoms.58,150 The neurosteroid antidepressant program is based on the GABAergic deficit hypothesis of depression, which states that a deficiency of GABAergic transmission in certain neural circuits is associated with depression. Unlike the well-accepted serotonergic hypothesis of depression, the GABAergic hypothesis needs further experimental and clinical validation. The phase 3 trial of the synthetic neurosteroid zuranolone in 543 patients was announced by the sponsor. According to the sponsor release on June 15, 2021, the WATERFALL trial of oral zuranolone therapy for 15 days showed a quick onset of effect within 3 days in the major depressive disorder, but its effect wanted over time such that the drug did not show any significant superiority to a placebo on key indicators. Common side effects noted include drowsiness, dizziness, and headache. Therefore, the role of neurosteroids in clinical depression warrants further investigation using clinically-relevant experimental models and clinical conditions.
9 |. NEUROSTEROIDS IN PREMENSTRUAL AND ANXIETY DISORDERS
The role of neurosteroids in anxiety disorders has received much attention.124–131,151 Premenstrual dysphoric disorder (PMDD) is a severe form of premenstrual syndrome (PMS) affecting 9% of women of childbearing age. PMDD is a common condition and affects over 3 million women each year in the U.S. PMDD is associated with marked changes in behavior as well as intense emotional lability later in the luteal phase of the menstrual cycle. PMDD is associated with both physical and behavioral symptoms. Behavioral symptoms include extreme sadness, hopelessness, irritability, mood swings, sleep issues, and reduced attention. Physical signs include abdominal bloating, body aches, breast tenderness, cramps, fatigue, headaches, swelling of extremities, and weight gain. It affects the women’s daily functioning and relationships. Presently, there some therapeutic options for PMDD.152,153 There are two main options for the management of PMDD: (i) drugs influencing brain activity, especially the modulation of serotonin transmission, and (ii) drugs that suppress ovulation. Antidepressants, such as selective serotonin reuptake inhibitors, have been shown to be effective in many patients. Serotonergic antidepressants are given continuously or in the luteal phase only. Oral contraceptives, gonadotropin-releasing hormone agonists, danazol, and estradiol all most likely function by ovulation suppression.154,155 The benzodiazepine alprazolam has modest efficacy in some patients, while others have found it to be ineffective.156
Neurosteroids have been implicated in the etiology of PMDD.12,157,158 Although no consistent ovarian hormone level dysfunction has been conclusively linked to PMDD symptoms, it has been shown that, while GnRH-agonist ovarian suppression can mitigate symptoms, progesterone administration can precipitate them.159–161 Progesterone thus plays a significant role in premenstrual symptoms. Fluctuating levels of AP, a progesterone-derived neurosteroid, have been shown to produce anxiety-like behaviors 127 and are believed to trigger affective dysregulation in women with mood disorders.162 Serum concentrations of AP during the luteal phase are lower in women with PMS,163,164 and neurosteroid withdrawal increases anxiety and excitability in animal models.97,165 Therefore, alterations in anxiolytic neurosteroids or neurosteroid withdrawal that occurs during perimenstrual period could be a factor in the pathophysiology of PMS and PMDD. However, progesterone supplementation in women with PMS has no clear beneficial effect.166,167 This could be due to limited neurosteroid biosynthesis, progesterone actions at intracellular receptors, or conversion of progesterone to other steroids with negative properties. Consistent with this notion, progesterone receptors have been linked to enhanced excitability, including seizure susceptibility.61,168 Moreover, in preclinical models, neurosteroid administration is associated with reduced excitability, anxiolytic response, antistress activity, and seizure suppression.16,124,125,132,169,170 Administration of the neurosteroid isomer isoallopregnanolone, also known as sepranolone, to women with PMDD had significant reduction in symptoms compared to placebo.171
10 |. CONCLUSIONS AND FUTURE DIRECTIONS
Dysfunction of GABAergic inhibition in the brain plays a key role in the pathophysiology of epilepsy, PPD, and menstrual mood disorders. Neurosteroids are positive allosteric agonists of synaptic GABA-A receptors and are more efficacious at extrasynaptic receptors that mediate tonic inhibition. Neurosteroids such as AP (brexanolone) have broad-spectrum protective activity and possess therapeutic potential for epilepsy, PPD, and other neurosteroid-deficiency conditions. In 2009, we proposed and investigated the NRT for catamenial seizures and related conditions in women. In 2019, the FDA approved the use of brexanolone for PPD. This is a major milestone in neurosteroid research. There are many synthetic neurosteroids in other clinical trials for epilepsy, mood, and anxiety disorders. Recent advancements in our understanding of extrasynaptic GABA-A receptor-mediated tonic inhibition are providing new therapeutic approaches for epilepsy, status epilepticus, and seizure disorders. The δGABA-A receptors, which are expressed within the dentate gyrus, are involved in elevating tonic inhibition, promoting network shunting and reduction of seizure susceptibility. We identified an SAR for neurosteroid modulation of extrasynaptic GABA-A receptor-mediated tonic inhibition.29 A consensus neurosteroid pharmacophore model at extrasynaptic GABA-A receptors has emerged for activation of tonic current and seizure protection. The discovery of an extrasynaptic molecular mechanism represents a critical milestone for developing novel therapies such as NRT for CE and PPD, which are associated with the rapid decline in the levels of neurosteroids.
Catamenial epilepsy, which is attributed to withdrawal or loss of endogenous neurosteroids around the perimenstrual period, is a unique pharmacoresistant epilepsy in women. Neurosteroids play a dominant pathophysiological role in catamenial seizures, via plasticity of extrasynaptic GABA-A receptor expression and function and other mechanisms. According to this cyclical mechanism, the perimenstrual decline in neurosteroids triggers a selective increase in expression of δGABA-A receptors in the hippocampus. This compensatory increase in δGABA-A receptors may act as a “Trojan horse” for the exogenous neurosteroids to inhibit excessive excitation, seizures, and behavior dysfunction. Therefore, extrasynaptic targeting presents a molecular rationale for neurosteroid therapy of CE. NRT is a viable approach to better control catamenial seizures and menstrual mood disorders. This therapy has a significant impact in reducing the burden of catamenial seizures, PPD, and premenstrual mood conditions such as PMDD in women. Benzodiazepines do not enhance tonic inhibition and thereby offer limited protection against persistent seizures and dysphoric episodes. Neurosteroids exhibit sex-differences in their potency and efficacy on activation of tonic inhibition and seizure protection with greater efficacy in females than males.118,172,173 Additional research is warranted to identify novel neurosteroid analogs for epilepsy, PPD, and related mood conditions.
Neurosteroids offer several advantages over other GABAergic drugs. (i) Neurosteroids can be effective even in benzodiazepine-refractory conditions because they can activate most GABA-A receptor isoforms; (ii) Unlike benzodiazepines, neurosteroids lack tolerance upon repeated use; (iii) Neurosteroids show a rapid onset of action & intermediate duration; (iv) Maximal efficacy is expected even in resistant seizures, due to their direct, non-allosteric actions; (v) They promote tonic inhibition that does not rely on interneurons for producing beneficial actions; (vi) They are readily available and brexanolone is FDA-approved for clinical use; (vii) They are neuroprotectants in many neuronal injury conditions; and (viii) new orally active and water-soluble analogs would allow oral and injectable products. Treatment with neurosteroid products is reported to be generally safe and well-tolerated. The most common side effect of neurosteroids is transient sedation, which is an extension of their therapeutic effects at GABA-A receptors. Adverse events reported by some patients include dizziness, fatigue, and somnolence that are reversible upon discontinuation of therapy. There are potential drug interactions with other GABAergic drugs and zinc, which can prevent the protective effects of neurosteroids by blocking extrasynaptic receptors. In summary, development of a NRT for certain brain conditions with novel neurosteroid products will be highly efficient approach for rapid translation of neurosteroid therapeutics to clinic.
HIGHLIGHTS.
Extrasynaptic GABA-A receptors regulates neuronal network excitability and behavior.
Neurosteroids play a critical role in catamenial epilepsy and many neuroendocrine disorders.
Neurosteroid deficiency leads to reduced tonic inhibition and enhanced seizures, anxiety, or dysphoric behavior.
Changes in the abundance or distribution of GABA-A receptors affect the neurosteroid response.
Neurosteroid replacement therapy is a unique strategy for catamenial epilepsy, post-partum depression, and premenstrual mood disorders.
A variety of synthetic neurosteroids are in clinical trials for neuroendocrine conditions.
ACKNOWLEDGEMENTS
This work was supported by NIH grants NS052158 and NS051398 (to DSR). Dr. Reddy’s research work was supported by the CounterACT Program, National Institutes of Health, Office of the Director and the National Institute of Neurologic Disorders and Stroke [Grant R21-NS076426, U01-NS083460, R21-NS099009, U01-NS117209, and U01-NS117278]. This work was partly supported by Texas A&M Presidential X-Grant (to DSR.). This article is inspired by 11th International Meeting on “STEROIDS AND NERVOUS SYSTEM” (FEB 21-24, 2021), Torino, Italy, and its Satellite Symposium on “ALLOPREGNANOLONE AND ITS SYNTHETIC ANALOGUES: FROM BENCH TO CLINICAL STRATEGIES FOR NEUROPATHOLOGY“. We greatly appreciate the organizers Drs. Roberto Melcangi and Giancarlo Panzica and participants.
ABBREVIATIONS:
- AP
allopregnanolone (brexanolone)
- ASM
antiseizure medications
- AD
androstanediol
- CA1PC
CA1 pyramidal cells
- CE
catamenial epilepsy
- CNS
central nervous system
- DGGC
dentate gyrus granule cell
- δKO
δ-subunit knockout mice
- DKO
GABA-A receptor δ subunit knockout
- GABA
γ-aminobutyric acid
- GX
ganaxolone
- mIPSC
miniature inhibitory postsynaptic current
- NRT
neurosteroid replacement therapy
- NSW
neurosteroid withdrawal
- PKA
protein kinase A
- PKC
protein kinase C
- PMS
premenstrual syndrome
- PMDD
premenstrual dysphoric disorder
- PPD
post-partum depression
- THDOC
allotetrahydro-deoxycorticosterone
Footnotes
Declaration of Competing Interest
Nothing to declare.
REFERENCES
- 1.Kulkarni SK, & Reddy DS Neurosteroids: a new class of neuromodulators. Drugs of today 31, 433–456 (1995). [Google Scholar]
- 2.Rupprecht R. et al. Progesterone receptor-mediated effects of neuroactive steroids. Neuron 11, 523–530 (1993). [DOI] [PubMed] [Google Scholar]
- 3.Rupprecht R, Berning B, Hauser CA, Holsboer F. & Reul JM Steroid receptor-mediated effects of neuroactive steroids: characterization of structure-activity relationship. European journal of pharmacology 303, 227–234 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Baulieu E-E & Robel P. Neurosteroids: a new brain function? The Journal of steroid biochemistry and molecular biology 37, 395–403 (1990). [DOI] [PubMed] [Google Scholar]
- 5.Baulieu E. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology 23, 963–987 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Caruso D. et al. Age-related changes in neuroactive steroid levels in 3xTg-AD mice. Neurobiology of aging 34, 1080–1089 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melcangi RC et al. Levels and actions of progesterone and its metabolites in the nervous system during physiological and pathological conditions. Progress in neurobiology 113, 56–69 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Melcangi RC et al. Neuroactive steroid levels and psychiatric and andrological features in post-finasteride patients. The Journal of steroid biochemistry and molecular biology 171, 229–235 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Meletti S. et al. Decreased allopregnanolone levels in cerebrospinal fluid obtained during status epilepticus. Epilepsia 58, e16–e20 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Reddy DS & Estes WA Clinical potential of neurosteroids for CNS disorders. Trends in pharmacological sciences 37, 543–561 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy DS Pharmacology of endogenous neuroactive steroids. Critical Reviews™ in Neurobiology 15 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Reddy DS Neurosteroids: endogenous role in the human brain and therapeutic potentials. Progress in brain research 186, 113–137 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glykys J, Mann EO & Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? Journal of Neuroscience 28, 1421–1426 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuang S-H & Reddy DS Genetic and molecular regulation of extrasynaptic GABA-A receptors in the brain: therapeutic insights for epilepsy. Journal of pharmacology and experimental therapeutics 364, 180–197 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whissell PD, Lecker I, Wang D-S, Yu J. & Orser BA Altered expression of δGABAA receptors in health and disease. Neuropharmacology 88, 24–35 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Reddy DS & Rogawski MA Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. Journal of Neuroscience 22, 3795–3805 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carver CM & Reddy DS Neurosteroid interactions with synaptic and extrasynaptic GABA A receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology 230, 151–188 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison NL & Simmonds MA Modulation of the GABA receptor complex by a steroid anaesthetic. Brain research 323, 287–292 (1984). [DOI] [PubMed] [Google Scholar]
- 19.Akk G. et al. Mechanisms of neurosteroid interactions with GABAA receptors. Pharmacology & therapeutics 116, 35–57 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosie AM, Clarke L, da Silva H. & Smart TG Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology 56, 149–154 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Hosie AM, Wilkins ME, da Silva HM & Smart TG Endogenous neurosteroids regulate GABA A receptors through two discrete transmembrane sites. Nature 444, 486–489 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Kokate TG, Svensson B. & Rogawski MA Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation. Journal of Pharmacology and Experimental Therapeutics 270, 1223–1229 (1994). [PubMed] [Google Scholar]
- 23.Reddy DS & Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. Journal of Pharmacology and Experimental Therapeutics 334, 1031–1041 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy DS & Rogawski MA Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy research 89, 254–260 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown N, Kerby J, Bonnert T, Whiting PJ & Wafford K. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. British journal of pharmacology 136, 965–974 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stell BM, Brickley SG, Tang C, Farrant M. & Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proceedings of the National Academy of Sciences 100, 14439–14444 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohlfarth KM, Bianchi MT & Macdonald RL Enhanced neurosteroid potentiation of ternary gabaareceptors containing the δ subunit. Journal of Neuroscience 22, 1541–1549 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meera P, Wallner M. & Otis TS Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. Journal of neurophysiology 106, 2057–2064 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carver CM & Reddy DS Neurosteroid structure-activity relationships for functional activation of extrasynaptic δGABAA receptors. Journal of Pharmacology and Experimental Therapeutics 357, 188–204 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianchi MT & Macdonald RL Neurosteroids shift partial agonist activation of GABAA receptor channels from low-to high-efficacy gating patterns. Journal of Neuroscience 23, 10934–10943 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanna E. et al. Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. Journal of Neuroscience 29, 1755–1765 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, Gangisetty O, Carver CM & Reddy DS Estrous cycle regulation of extrasynaptic δ-containing GABAA receptor-mediated tonic inhibition and limbic epileptogenesis. Journal of Pharmacology and Experimental Therapeutics 346, 146–160 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carver CM, Wu X, Gangisetty O. & Reddy DS Perimenstrual-like hormonal regulation of extrasynaptic δ-containing GABAA receptors mediating tonic inhibition and neurosteroid sensitivity. Journal of Neuroscience 34, 14181–14197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mihalek RM et al. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proceedings of the National Academy of Sciences 96, 12905–12910 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng Z. et al. GABAA receptor changes in δ subunit-deficient mice: Altered expression of α4 and γ2 subunits in the forebrain. Journal of Comparative Neurology 446, 179–197 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Spigelman I. et al. Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia 43, 3–8 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Chuang S-H & Reddy DS 3β-Methyl-neurosteroid analogs are preferential positive allosteric modulators and direct activators of extrasynaptic δ-subunit γ-aminobutyric acid type A receptors in the hippocampus dentate gyrus subfield. Journal of Pharmacology and Experimental Therapeutics 365, 583–601 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi S, Rajasekaran K, Williamson J. & Kapur J. Neurosteroid-sensitive δ-GABAA receptors: A role in epileptogenesis? Epilepsia 58, 494–504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carver CM, Chuang S-H & Reddy DS Zinc selectively blocks neurosteroid-sensitive extrasynaptic δGABAA receptors in the hippocampus. Journal of Neuroscience 36, 8070–8077 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs MP et al. Curing epilepsy: progress and future directions. Epilepsy & Behavior 14, 438–445 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hesdorffer DC et al. Research implications of the Institute of Medicine Report, epilepsy across the spectrum: promoting health and understanding. Epilepsia 54, 207–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Younus I. & Reddy DS A resurging boom in new drugs for epilepsy and brain disorders. Expert review of clinical pharmacology 11, 27–45 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Reddy DS Clinical Pharmacology and Therapeutics of Antiepileptic Drugs for Treatment of Epilepsy and Seizure Disorders. International Journal of Pharmaceutical Sciences and Nanotechnology 13, 5165–5180 (2020). [Google Scholar]
- 44.Reddy DS Mass spectrometric assay and physiological–pharmacological activity of androgenic neurosteroids. Neurochemistry international 52, 541–553 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy D. Role of anticonvulsant and antiepileptogenic neurosteroids in the pathophysiology and treatment of epilepsy. Frontiers in endocrinology 2, 38 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy DS Role of neurosteroids in catamenial epilepsy. Epilepsy research 62, 99–118 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Kaminski RM, Marini H, Kim WJ & Rogawski MA Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia 46, 819–827 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaminski RM, Livingood MR & Rogawski MA Allopregnanolone analogs that positively modulate GABAA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia 45, 864–867 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Devaud LL, Purdy RH, Finn DA & Morrow AL Sensitization of gamma-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. Journal of Pharmacology and Experimental Therapeutics 278, 510–517 (1996). [PubMed] [Google Scholar]
- 50.Tsuda M, Suzuki T. & Misawa M. Modulation of the decrease in the seizure threshold of pentylenetetrazole in diazepam withdrawn mice by the neurosteroid 5αpregnan-3α, 21-diol-20-one (alloTHDOC). Addiction biology 2, 455–460 (1997). [DOI] [PubMed] [Google Scholar]
- 51.Reddy DS & Rogawski MA Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. Journal of Pharmacology and Experimental Therapeutics 295, 1241–1248 (2000). [PubMed] [Google Scholar]
- 52.Reddy DS & Rogawski MA Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia 42, 337–344 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Kokate TG et al. Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. Journal of Pharmacology and Experimental Therapeutics 287, 553–558 (1998). [PubMed] [Google Scholar]
- 54.Reddy DS & Rogawski MA Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. Journal of Pharmacology and Experimental Therapeutics 294, 909–915 (2000). [PubMed] [Google Scholar]
- 55.Członkowska AI et al. Tolerance to the anticonvulsant activity of midazolam and allopregnanolone in a model of picrotoxin seizures. European journal of pharmacology 425, 121–127 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Türkmen Ş, Löfgren M, Birzniece V, Bäckström T. & Johansson I-M Tolerance development to Morris water maze test impairments induced by acute allopregnanolone. Neuroscience 139, 651–659 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Sperling MR, Klein P. & Tsai J. Randomized, double-blind, placebo-controlled phase 2 study of ganaxolone as add-on therapy in adults with uncontrolled partial-onset seizures. Epilepsia 58, 558–564 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Gunduz-Bruce H. et al. Trial of SAGE-217 in patients with major depressive disorder. New England Journal of Medicine 381, 903–911 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Biagini G. et al. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Experimental neurology 201, 519–524 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Reddy DS, Gangisetty O. & Briyal S. Disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Neuropharmacology 59, 573–581 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reddy DS & Mohan A. Development and persistence of limbic epileptogenesis are impaired in mice lacking progesterone receptors. Journal of Neuroscience 31, 650–658 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monaghan EP, McAuley JW & Data JL Ganaxolone: a novel positive allosteric modulator of the GABAA receptor complex for the treatment of epilepsy. Expert opinion on investigational drugs 8, 1663–1671 (1999). [DOI] [PubMed] [Google Scholar]
- 63.Nohria V. & Giller E. Ganaxolone. Neurotherapeutics 4, 102–105 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blanco M-J et al. Breakthroughs in neuroactive steroid drug discovery. Bioorganic & medicinal chemistry letters 28, 61–70 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Pieribone VA et al. Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia 48, 1870–1874 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Carter RB et al. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. Journal of Pharmacology and Experimental Therapeutics 280, 1284–1295 (1997). [PubMed] [Google Scholar]
- 67.Reddy DS & Woodward R. Ganaxolone: a prospective overview. Drugs Future 29, 227–242 (2004). [Google Scholar]
- 68.Kerrigan JF et al. Ganaxolone for treating intractable infantile spasms: a multicenter, open-label, add-on trial. Epilepsy research 42, 133–139 (2000). [DOI] [PubMed] [Google Scholar]
- 69.Laxer K. et al. Assessment of ganaxolone’s anticonvulsant activity using a randomized, double-blind, presurgical trial design. Epilepsia 41, 1187–1194 (2000). [DOI] [PubMed] [Google Scholar]
- 70.Bialer M. et al. Progress report on new antiepileptic drugs: a summary of the Twelfth Eilat Conference (EILAT XII). Epilepsy research 111, 85–141 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Reddy DS & Rogawski MA Neurosteroids—endogenous regulators of seizure susceptibility and role in the treatment of epilepsy. Jasper’s Basic Mechanisms of the Epilepsies [Internet]. 4th edition (2012). [Google Scholar]
- 72.Rosenthal ES et al. Brexanolone as adjunctive therapy in super-refractory status epilepticus. Annals of neurology 82, 342–352 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brandon NJ et al. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. Journal of Biological Chemistry 275, 38856–38862 (2000). [DOI] [PubMed] [Google Scholar]
- 74.Moss SJ & Smart TG Modulation Of Amind Acid-Gated Ion Channels By Protein Phoshorlation. International review of neurobiology 39, 1–52 (1996). [DOI] [PubMed] [Google Scholar]
- 75.Kittler JT et al. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proceedings of the National Academy of Sciences 102, 14871–14876 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kittler JT et al. Regulation of synaptic inhibition by phospho-dependent binding of the AP2 complex to a YECL motif in the GABAA receptor γ2 subunit. Proceedings of the National Academy of Sciences 105, 3616–3621 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leidenheimer NJ & Chapell R. Effects of PKC activation and receptor desensitization on neurosteroid modulation of GABAA receptors. Molecular brain research 52, 173–181 (1997). [DOI] [PubMed] [Google Scholar]
- 78.Fáncsik A, Linn DM & Tasker JG Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. Journal of Neuroscience 20, 3067–3075 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harney SC, Frenguelli BG & Lambert JJ Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology 45, 873–883 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Saliba RS, Kretschmannova K. & Moss SJ Activity-dependent phosphorylation of GABAA receptors regulates receptor insertion and tonic current. The EMBO journal 31, 2937–2951 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abramian AM et al. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proceedings of the National Academy of Sciences 111, 7132–7137 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Modgil A. et al. Endogenous and synthetic neuroactive steroids evoke sustained increases in the efficacy of GABAergic inhibition via a protein kinase C-dependent mechanism. Neuropharmacology 113, 314–322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chuang S-H & Reddy DS Zinc reduces antiseizure activity of neurosteroids by selective blockade of extrasynaptic GABA-A receptor-mediated tonic inhibition in the hippocampus. Neuropharmacology 148, 244–256 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adams JM, Thomas P. & Smart TG Modulation of neurosteroid potentiation by protein kinases at synaptic-and extrasynaptic-type GABAA receptors. Neuropharmacology 88, 63–73 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chuang S-H & Reddy DS Isobolographic analysis of antiseizure activity of the GABA type A receptor-modulating synthetic neurosteroids brexanolone and ganaxolone with tiagabine and midazolam. Journal of Pharmacology and Experimental Therapeutics 372, 285–298 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barberis A, Cherubini E. & Mozrzymas JW Zinc inhibits miniature GABAergic currents by allosteric modulation of GABAA receptor gating. Journal of Neuroscience 20, 8618–8627 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hosie AM, Dunne EL, Harvey RJ & Smart TG Zinc-mediated inhibition of GABA A receptors: discrete binding sites underlie subtype specificity. Nature neuroscience 6, 362–369 (2003). [DOI] [PubMed] [Google Scholar]
- 88.Pitsch J. et al. Circadian clustering of spontaneous epileptic seizures emerges after pilocarpine-induced status epilepticus. Epilepsia 58, 1159–1171 (2017). [DOI] [PubMed] [Google Scholar]
- 89.Herzog AG & Fowler KM Sensitivity and specificity of the association between catamenial seizure patterns and ovulation. Neurology 70, 486–487 (2008). [DOI] [PubMed] [Google Scholar]
- 90.Reddy DS The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy research 85, 1–30 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laidlaw J. Catamenial epilepsy. The Lancet 268, 1235–1237 (1956). [DOI] [PubMed] [Google Scholar]
- 92.Bäckström T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurologica Scandinavica 54, 321–347 (1976). [DOI] [PubMed] [Google Scholar]
- 93.Maguire MJ & Nevitt SJ Treatments for seizures in catamenial (menstrual-related) epilepsy. Cochrane Database of Systematic Reviews (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herzog AG, Klein P. & Rand BJ Three patterns of catamenial epilepsy. Epilepsia 38, 1082–1088 (1997). [DOI] [PubMed] [Google Scholar]
- 95.Reddy DS Neurosteroids and their role in sex-specific epilepsies. Neurobiology of disease 72, 198–209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herzog AG et al. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Annals of neurology 56, 431–434 (2004). [DOI] [PubMed] [Google Scholar]
- 97.Reddy DS, Gould J. & Gangisetty O. A mouse kindling model of perimenstrual catamenial epilepsy. Journal of Pharmacology and Experimental Therapeutics 341, 784–793 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Logothetis J, Harner R, Morrell F. & Torres F. The role of estrogens in catamenial exacerbation of epilepsy. Neurology 9, 352–352 (1959). [DOI] [PubMed] [Google Scholar]
- 99.Bäckström T, Zetterlund B, Blom S. & Romano M. Effects of intravenous progesterone infusions on the epileptic discharge frequency in women with partial epilepsy. Acta Neurologica Scandinavica 69, 240–248 (1984). [DOI] [PubMed] [Google Scholar]
- 100.Bonuccellia U. et al. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy research 3, 100–106 (1989). [DOI] [PubMed] [Google Scholar]
- 101.Herzog AG Reproductive endocrine considerations and hormonal therapy for women with epilepsy. Epilepsia 32, S27–S33 (1991). [DOI] [PubMed] [Google Scholar]
- 102.Navis A. & Harden C. A treatment approach to catamenial epilepsy. Current treatment options in neurology 18, 30 (2016). [DOI] [PubMed] [Google Scholar]
- 103.Reddy DS Neuroendocrine aspects of catamenial epilepsy. Hormones and behavior 63, 254–266 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reddy DS Catamenial epilepsy: discovery of an extrasynaptic molecular mechanism for targeted therapy. Frontiers in cellular neuroscience 10, 101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joshi S. et al. A novel therapeutic approach for treatment of catamenial epilepsy. Neurobiology of disease 111, 127–137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reddy DS, Castaneda DC, O’Malley BW & Rogawski MA Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. Journal of Pharmacology and Experimental Therapeutics 310, 230–239 (2004). [DOI] [PubMed] [Google Scholar]
- 107.Reddy DS, Gangisetty O. & Wu X. PR-independent neurosteroid regulation of α2-GABA-A receptors in the hippocampus subfields. Brain research 1659, 142–147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Joshi S. & Kapur J. Neurosteroid regulation of GABAA receptors: a role in catamenial epilepsy. Brain research 1703, 31–40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brinton RD et al. Progesterone receptors: form and function in brain. Frontiers in neuroendocrinology 29, 313–339 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Del Río JP et al. Steroid hormones and their action in women’s brains: the importance of hormonal balance. Frontiers in public health 6, 141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reddy DS & Ramanathan G. Finasteride inhibits the disease-modifying activity of progesterone in the hippocampus kindling model of epileptogenesis. Epilepsy & Behavior 25, 92–97 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Herzog AG Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology 52, 1917–1917-a (1999). [DOI] [PubMed] [Google Scholar]
- 113.Miziak B, Chrościńska-Krawczyk M. & Czuczwar SJ Neurosteroids and seizure activity. Frontiers in Endocrinology 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clossen BL & Reddy DS Catamenial-like seizure exacerbation in mice with targeted ablation of extrasynaptic δGABA-a receptors in the brain. Journal of neuroscience research 95, 1906–1916 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reddy DS, Kim HY & Rogawski MA Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia 42, 328–336 (2001). [DOI] [PubMed] [Google Scholar]
- 116.Gangisetty O. & Reddy DS Neurosteroid withdrawal regulates GABA-A receptor α4-subunit expression and seizure susceptibility by activation of progesterone receptor-independent early growth response factor-3 pathway. Neuroscience 170, 865–880 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reddy DS & Zeng YC (Wiley Online Library, 2007). [Google Scholar]
- 118.Reddy DS, Thompson W. & Calderara G. Molecular mechanisms of sex differences in epilepsy and seizure susceptibility in chemical, genetic and acquired epileptogenesis. Neuroscience letters 750, 135753 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reddy DS & Rogawski MA Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics 6, 392–401 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith SS, Shen H, Gong QH & Zhou X. Neurosteroid regulation of GABAA receptors: Focus on the α4 and δ subunits. Pharmacology & therapeutics 116, 58–76 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herzog A. et al. Progesterone trial study G. Progesterone vs placebo therapy for women with epilepsy. Neurology 78, 1959–1966 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Herzog AG & Frye CA Allopregnanolone levels and seizure frequency in progesterone-treated women with epilepsy. Neurology 83, 345–348 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nemeroff CB Understanding the pathophysiology of postpartum depression: implications for the development of novel treatments. Neuron 59, 185–186 (2008). [DOI] [PubMed] [Google Scholar]
- 124.Reddy DS & Kulkarni SK Role of GABA-A and mitochondrial diazepam binding inhibitor receptors in the anti-stress activity of neurosteroids in mice. Psychopharmacology 128, 280–292 (1996). [DOI] [PubMed] [Google Scholar]
- 125.Reddy DS & Kulkarni SK Reversal of benzodiazepine inverse agonist FG 7142-induced anxiety syndrome by neurosteroids in mice. Methods and findings in experimental and clinical pharmacology 19, 665–681 (1997). [PubMed] [Google Scholar]
- 126.Reddy DS & Kulkarni SK Neurosteroid coadministration prevents development of tolerance and augments recovery from benzodiazepine withdrawal anxiety and hyperactivity in mice. Methods and findings in experimental and clinical pharmacology 19, 395–405 (1997). [PubMed] [Google Scholar]
- 127.Reddy D. & Kulkarni S. Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain research 752, 61–71 (1997). [DOI] [PubMed] [Google Scholar]
- 128.Reddy DS & Kulkarni SK The role of GABA-A and mitochondrial diazepam-binding inhibitor receptors on the effects of neurosteroids on food intake in mice. Psychopharmacology 137, 391–400 (1998). [DOI] [PubMed] [Google Scholar]
- 129.Reddy DS, Kaur G. & Kulkarni SK Sigma (σ1) receptor mediated antidepressant-like effects of neurosteroids in the Porsolt forced swim test. Neuroreport 9, 3069–3073 (1998). [DOI] [PubMed] [Google Scholar]
- 130.Reddy D. & Kulkarni S. Sex and estrous cycle-dependent changes in neurosteroid and benzodiazepine effects on food consumption and plus-maze learning behaviors in rats. Pharmacology Biochemistry and Behavior 62, 53–60 (1999). [DOI] [PubMed] [Google Scholar]
- 131.Reddy DS & Kulkarni SK Development of neurosteroid-based novel psychotropic drugs. Progress in medicinal chemistry 37, 135–176 (2000). [DOI] [PubMed] [Google Scholar]
- 132.Reddy DS, O’Malley BW & Rogawski MA Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology 48, 14–24 (2005). [DOI] [PubMed] [Google Scholar]
- 133.Reddy D. Pharmacology of catamenial epilepsy. Methods Find Exp Clin Pharmacol 26, 547–561 (2004). [DOI] [PubMed] [Google Scholar]
- 134.Maguire J. & Mody I. GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron 59, 207–213 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maguire JL, Stell BM, Rafizadeh M. & Mody I. Ovarian cycle–linked changes in GABA A receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature neuroscience 8, 797–804 (2005). [DOI] [PubMed] [Google Scholar]
- 136.Kanes S. et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. The Lancet 390, 480–489 (2017). [DOI] [PubMed] [Google Scholar]
- 137.Kanes SJ et al. Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Human Psychopharmacology: Clinical and Experimental 32, e2576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meltzer-Brody S. et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. The Lancet 392, 1058–1070 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Leader LD, O’Connell M. & VandenBerg A. Brexanolone for postpartum depression: clinical evidence and practical considerations. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 39, 1105–1112 (2019). [DOI] [PubMed] [Google Scholar]
- 140.Zheng W. et al. Brexanolone for postpartum depression: a meta-analysis of randomized controlled studies. Psychiatry research 279, 83–89 (2019). [DOI] [PubMed] [Google Scholar]
- 141.Powell JG, Garland S, Preston K. & Piszczatoski C. Brexanolone (Zulresso): Finally, an FDA-approved treatment for postpartum depression. Annals of Pharmacotherapy 54, 157–163 (2020). [DOI] [PubMed] [Google Scholar]
- 142.Hutcherson TC, Cieri-Hutcherson NE & Gosciak MF Brexanolone for postpartum depression. American Journal of Health-System Pharmacy 77, 336–345 (2020). [DOI] [PubMed] [Google Scholar]
- 143.Rupprecht R. & Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends in neurosciences 22, 410–416 (1999). [DOI] [PubMed] [Google Scholar]
- 144.Maguire J. Neuroactive steroids and GABAergic involvement in the neuroendocrine dysfunction associated with major depressive disorder and postpartum depression. Frontiers in cellular neuroscience 13, 83 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Uzunova V. et al. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proceedings of the National Academy of Sciences 95, 3239–3244 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Romeo E. et al. Effects of antidepressant treatment on neuroactive steroids in major depression. American Journal of Psychiatry 155, 910–913 (1998). [DOI] [PubMed] [Google Scholar]
- 147.Agis-Balboa RC, Guidotti A. & Pinna G. 5α-reductase type I expression is downregulated in the prefrontal cortex/Brodmann’s area 9 (BA9) of depressed patients. Psychopharmacology 231, 3569–3580 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Melón L, Hammond R, Lewis M. & Maguire J. A novel, synthetic, neuroactive steroid is effective at decreasing depression-like behaviors and improving maternal care in preclinical models of postpartum depression. Frontiers in endocrinology 9, 703 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zorumski CF, Paul SM, Covey DF & Mennerick S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiology of stress 11, 100196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Clayton A. et al. A Phase 3, Multicenter, Double-Blind, Randomized, Placebo-Controlled Study Evaluating the Efficacy of SAGE-217 in the Treatment of Adult Patients With Major Depressive Disorder. Biological Psychiatry 87, S86 (2020). [Google Scholar]
- 151.Schüle C, Nothdurfter C. & Rupprecht R. The role of allopregnanolone in depression and anxiety. Progress in neurobiology 113, 79–87 (2014). [DOI] [PubMed] [Google Scholar]
- 152.Nevatte T. et al. ISPMD consensus on the management of premenstrual disorders. Archives of women’s mental health 16, 279–291 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Carlini SV & Deligiannidis KM Evidence-based treatment of Premenstrual Dysphoric Disorder: a concise review. The Journal of clinical psychiatry 81, 0–0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maharaj S. & Trevino K. A comprehensive review of treatment options for premenstrual syndrome and premenstrual dysphoric disorder. Journal of Psychiatric Practice® 21, 334–350 (2015). [DOI] [PubMed] [Google Scholar]
- 155.Ismaili E. et al. Fourth consensus of the International Society for Premenstrual Disorders (ISPMD): auditable standards for diagnosis and management of premenstrual disorder. Archives of women’s mental health 19, 953–958 (2016). [DOI] [PubMed] [Google Scholar]
- 156.Schmidt PJ, Grover GN & Rubinow DR Alprazolam in the treatment of premenstrual syndrome: a double-blind, placebo-controlled trial. Archives of general psychiatry 50, 467–473 (1993). [DOI] [PubMed] [Google Scholar]
- 157.Martinez PE et al. 5 α-Reductase Inhibition Prevents the Luteal Phase Increase in Plasma Allopregnanolone Levels and Mitigates Symptoms in Women with Premenstrual Dysphoric Disorder. Neuropsychopharmacology 41, 1093–1102 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schmidt PJ et al. Premenstrual dysphoric disorder symptoms following ovarian suppression: triggered by change in ovarian steroid levels but not continuous stable levels. American Journal of Psychiatry 174, 980–989 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schmidt CL et al. Transfer of cryopreserved-thawed embryos: the natural cycle versus controlled preparation of the endometrium with gonadotropin-releasing hormone agonist and exogenous estradiol and progesterone (GEEP). Fertility and Sterility 52, 609–616 (1989). [DOI] [PubMed] [Google Scholar]
- 160.Björn, I. et al. Increase of estrogen dose deteriorates mood during progestin phase in sequential hormonal therapy. The Journal of Clinical Endocrinology & Metabolism 88, 2026–2030 (2003). [DOI] [PubMed] [Google Scholar]
- 161.Segebladh B, Borgström A, Nyberg S, Bixo M. & Sundström-Poromaa I. Evaluation of different add-back estradiol and progesterone treatments to gonadotropin-releasing hormone agonist treatment in patients with premenstrual dysphoric disorder. American journal of obstetrics and gynecology 201, 139. e131–139. e138 (2009). [DOI] [PubMed] [Google Scholar]
- 162.Schiller CE, Schmidt PJ & Rubinow DR Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology 231, 3557–3567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Girdler SS, Straneva PA, Light KC, Pedersen CA & Morrow AL Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biological psychiatry 49, 788–797 (2001). [DOI] [PubMed] [Google Scholar]
- 164.Monteleone P. et al. Allopregnanolone concentrations and premenstrual syndrome. European journal of endocrinology 142, 269–273 (2000). [DOI] [PubMed] [Google Scholar]
- 165.Smith SS, Gong QH, Hsu F-C, Markowitz RS & Li X. GABA A receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392, 926–929 (1998). [DOI] [PubMed] [Google Scholar]
- 166.Freeman E, Rickels K, Sondheimer SJ & Polansky M. Ineffectiveness of progesterone suppository treatment for premenstrual syndrome. Jama 264, 349–353 (1990). [PubMed] [Google Scholar]
- 167.Freeman EW, Rickels K, Sondheimer SJ & Polansky M. A double-blind trial of oral progesterone, alprazolam, and placebo in treatment of severe premenstrual syndrome. Jama 274, 51–57 (1995). [PubMed] [Google Scholar]
- 168.Shiono S, Williamson J, Kapur J. & Joshi S. Progesterone receptor activation regulates seizure susceptibility. Annals of clinical and translational neurology 6, 1302–1310 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Crawley JN, Glowa JR, Majewska MD & Paul SM Anxiolytic activity of an endogenous adrenal steroid. Brain research 398, 382–385 (1986). [DOI] [PubMed] [Google Scholar]
- 170.Bitran D, Shiekh M. & McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. Journal of neuroendocrinology 7, 171–177 (1995). [DOI] [PubMed] [Google Scholar]
- 171.Bixo M. et al. Treatment of premenstrual dysphoric disorder with the GABAA receptor modulating steroid antagonist Sepranolone (UC1010)—A randomized controlled trial. Psychoneuroendocrinology 80, 46–55 (2017). [DOI] [PubMed] [Google Scholar]
- 172.Christian CA, Reddy DS, Maguire J. & Forcelli PA Sex differences in the epilepsies and associated comorbidities: implications for use and development of pharmacotherapies. Pharmacological Reviews 72, 767–800 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reddy DS, Carver CM, Clossen B. & Wu X. Extrasynaptic GABA-A receptor-mediated sex differences in the antiseizure activity of neurosteroids in status epilepticus and complex partial seizures. Epilepsia 60, 730 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]