Dear Editor
We read with great interest the article in this journal by Tang et al. regarding an impaired immunologic response in solid organ transplant patients after COVID-19 mRNA vaccination.1 Organ transplant patients are vulnerable to COVID-19 infection, progression to severe disease, and mortality given their need for immunosuppressive therapy to prevent transplant rejection. As such, a significantly lower seroconversion rate following vaccination compared to healthy controls prompts the need for effective treatment modalities in this high-risk patient population.1
Organ transplant patients have a markedly low seroconversion rate after 2 doses of COVID-19 mRNA vaccine compared with healthy controls.1 Although COVID-19 vaccines have been effective in mounting an immune response in immunocompetent patients, a more effective alternative is needed for organ transplant recipients. Monoclonal antibodies (mAbs) have been highly studied in the literature as immunotherapy that target specific SARS-CoV-2 domains. As a therapeutic option that does not rely on the body's own immune response, mAbs have value in immunosuppressed patients and considerable potential as a treatment modality for organ transplant patients with COVID-19.2 , 3
To the best of our knowledge, there exists no meta-analysis describing the effect of mAbs on organ transplant recipients with COVID-19. We perform this meta-analysis to evaluate the association between monoclonal antibody therapy and outcomes of organ transplant patient following COVID-19 infection.
An exhaustive electronic search was conducted using PubMed, Embase, Cochrane Library databases, Web of Science, Scopus and medRxiv from December 1st 2019 to May 20th, 2022 without any restrictions on language. The search was performed using the following keywords: “2019-nCoV”, “coronavirus disease 2019″, “COVID-19″, “SARS-CoV-2″, “novel coronavirus”, “transplant recipients”, “transplantation”, “organ-transplant recipients”, “monoclonal antibody”, “neutralizing antibody”, “casirivimab”, “imdevimab”, “sotrovimab”, “bamlanivimab”, “LY-Cov555”. The inclusion criteria were as follows: (1) organ transplant recipients with COVID-19; (2) reports containing original data with available risk estimates and/or with data on the number of clinical outcomes in mAbs and control groups; (3) comparative studies with a control group with no mAbs. The following studies were excluded (1) conference abstracts, editorials, reviews, and case reports; (2) duplicated publications.
Data analysis was conducted using Review Manager, version 5.2 (Cochrane Collaboration, Oxford). Odds ratio (OR) and 95% confidence interval (CI) were calculated. Heterogeneity was assessed with Cochrane's Q-test and quantified with I2 values. A P value of <0.05 was considered statistically significant. This meta-analysis is registered with PROSPERO (International Prospective Register of Systematic Reviews, number CRD42022337101).
A total of 8 studies2, 3, 4, 5, 6, 7, 8, 9 were identified. All studies were retrospective in design. This meta-analysis included 313 patients in the mAbs group and 617 patients in the control group. Demographics and disease characteristics of the 930 patients included in the pooled analysis are summarized in Table 1 . The eight studies were published between 2021 and 2022 with different sample patient sizes that ranged from 40 to 235 patients with COVID-19. Four studies were from USA, three from France and one from Japan. Patients received bamlanivimab or casirivimab-imdevimab or bamlanivimab-etesevimab or sotrovimab. Progression to severe COVID-19 disease included ICU admission and the need for high oxygen support.
Table 1.
Characteristics of included studies.
| Study | Region | Study design | Sample size | mAbs |
No mAbs |
Patients included | Transplant recipients | Usage of mAbs | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Agea | Male (%) | Agea | Male (%) | |||||||
| Ahearn2 2021 | USA | Retrospective | 181 | 49.3 ± 14.3 | 22(65) | 54 ± 14.5 | 83(56) | Outpatients | 94 kidney, 87 liver | Bamlanivimab or Casirivimab-imdevimab |
| Bello3 2021 | France | Retrospective | 48 | 54 ± 14 | 10 | 59 ± 13 | 20 | Hospitalized, having symptoms for <6 d, and not requiring oxygen | 37 kidney, 2 liver, 5 heart, 2 kidney-pancreas, 1 kidney-liver | Bamlanivimab, bamlanivimab-etesevimab, casirivimab-imdevimab |
| Chavarot4 2022 | France | Retrospective | 125 | 54 (46–62) | 21 (84.0) | 53 (37.8–52) | 54 (54.0) | Outpatient mild-to-moderate Omicron COVID-19 | 125 kidney | Sotrovimab |
| Gueguen5 2022 | France | Retrospective | 235 | 57 (46–64.5) | 45 (56.3) | 57 (44–65) | 90 (58) | Recent symptoms (<5 days) and no need of oxygen | 155 kidney | Bamlanivimab, bamlanivimab-etesevimab, casirivimab-imdevimab |
| Klein6 2021 | USA | Retrospective | 95 | 55.0 (31–79) | 15 (75.0) | 58 (38–78) | 45 (60.0) | (1) were not hospitalized due to COVID-19, (2) did not require oxygen therapy due to COVID-19, and (3) had symptoms for <10 days | 75 kidney | Bamlanivimab, bamlanivimab-etesevimab, casirivimab-imdevimab |
| Sarrell7 2021 | USA | Retrospective | 165 | 55.1 (42.8–63.5) | 56 (60.2) | 52.0 (41.7–66.3) | 39 (54.2) | Outpatient mild to moderate COVID-19 | 50 kidney, 17 liver, 11 lung, 9 heart, 6 dual-organ | Bamlanivimab, casirivimab-imdevimab |
| Tagaya8 2022 | Japan | Retrospective | 41 | 53.72 (26–73) | 13(72) | 48.04 (20–80) | 19 (82.6) | Hospitalized mild-to-moderate COVID-19 | 36 kidney, 3 liver, 2 bone marrow | Casirivimab-imdevimab |
| Wang9 2022 | USA | Retrospective | 40 | 52 (37–61) | 15(56) | 44 (32–54) | 4(31) | Outpatient mild to moderate COVID-19 | 40 kidney | Bamlanivimab, casirivimab-imdevimab |
Age data presented as median (IQR) or mean (SD).
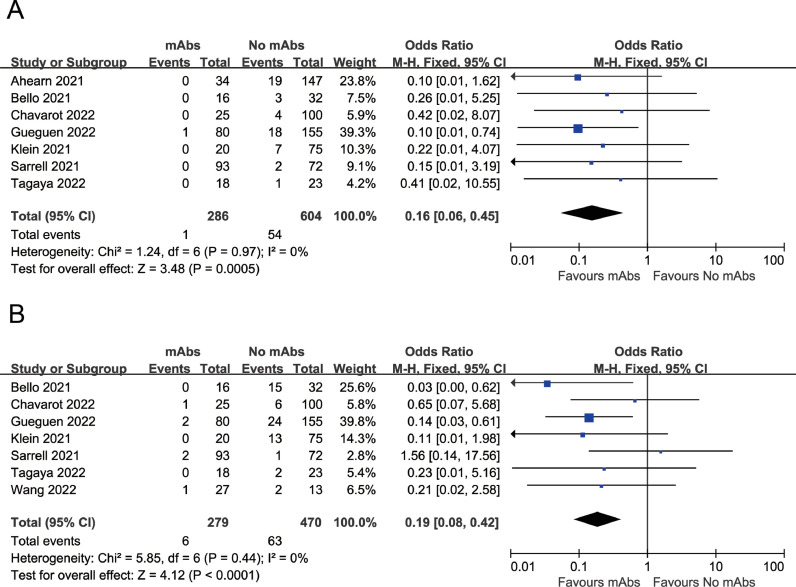
The meta-analysis indicated that the mAbs group had lower mortality compared with the control group (OR=0.16, 95%CI: 0.06 to 0.45, P = 0.0005; I2=0%) (Fig. 1 A). Moreover, mAbs treatment was associated with a reduced risk of developing severe COVID-19 disease as compared to the control group (OR=0.19, 95%CI: 0.08 to 0.42, P < 0.0001; I2=0%) (Fig. 1B).
Fig. 1.
(A) Association between mAbs treatment and mortality (B) Association between mAbs treatment and developing severe COVID-19.
In this study, we find that mAb therapy in organ transplant recipients with COVID-19 is associated with a significant improvement in disease severity as well as overall mortality.
The reduced morbidity and mortality of organ transplant recipients with COVID-19 following mAb treatment is likely related to a decreased progression to severe disease. Neutralizing mAbs target specific domains of the SARS-CoV-2 spike protein, inhibiting viral internalization and reducing viral load and virulence.10 With organ transplant patients having increased baseline risks for disease severity and mortality given pre-existing comorbidities and anti-rejection immunosuppressive therapy, mAbs serve as a protective factor through a lower propensity to progress to severe disease. This reduces the need to discontinue anti-rejection immunosuppressive therapy in patients admitted for severe disease thus reducing risk of transplant rejection. Increased transplant organ survival rates further benefit health, social, and financial factors.
While mAbs have considerable potential as a novel therapeutic option, SARS-CoV-2 continues to mutate as increasing variants of concern emerge. Recent studies have shown that newer COVID-19 variants may have developed resistance against neutralizing mAbs developed specifically against SARS-CoV-2 thus decreasing treatment efficacy.10 As such, there is considerable value in having ongoing research and development of mAbs which target the dominant variant strains of COVID-19 in order to keep up with global epidemiological needs.
There are several limitations to our study that should be noted. There was a small sample size of eight included articles for use in the meta-analysis. In addition, all included articles were retrospective in study design, leaving the results vulnerable to selection bias and confounding bias. Furthermore, each study looked at a different number and combination of mAbs without providing individual therapeutic data and as such subgroup analysis restricted to any single mAb was not able to be conducted. Despite these limitations, our study is the first meta-analysis to explore the effect of mAb therapy on organ transplant recipients with COVID-19.
Further research is needed to investigate the impact of mAbs as treatment for organ transplant patients with COVID-19 to provide additional insight into the efficacy of individual mAbs as well as combination therapy on morbidity and mortality. Future studies should aim to provide a large sample size through study designs that are prospective and more robust.
In conclusion, treatment with mAbs in organ transplant patients with COVID-19 is associated with a significant improvement in both severity and mortality. Additional exploration is needed to evaluate the impact of individual mAbs and validate these findings in prospective studies.
Declaration of Competing Interest
The authors declare that they have no competing interest.
Acknowledgments
Funding information
None declared.
Acknowledgements
None.
References
- 1.Tang K., Wu X., Luo Y., Wei Z., Feng L., Wu L. Meta-analysis of immunologic response after COVID-19 mRNA vaccination in solid organ transplant recipients. J Infect. 2022;84(5):e73–e75. doi: 10.1016/j.jinf.2022.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahearn A.J., Thin Maw T., Mehta R., et al. A programmatic response, including bamlanivimab or casirivimab-imdevimab administration, reduces hospitalization and death in COVID-19 positive abdominal transplant recipients. Transplantation. 2022;106(2):e153–e157. doi: 10.1097/TP.0000000000003953. [DOI] [PubMed] [Google Scholar]
- 3.Del Bello A., Marion O., Vellas C., Faguer S., Izopet J., Kamar N. Anti-SARS-CoV-2 monoclonal antibodies in solid-organ transplant patients. Transplantation. 2021;105(10):e146–e147. doi: 10.1097/TP.0000000000003883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavarot N., Melenotte C., Amrouche L., et al. Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with Omicron infection. Kidney Int. 2022;101(6):1290–1293. doi: 10.1016/j.kint.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gueguen J., Colosio C., Del Bello A., et al. Early administration of anti-SARS-CoV-2 monoclonal antibodies prevents severe COVID-19 in kidney transplant patients. Kidney Int Rep. 2022;7(6):1241–1247. doi: 10.1016/j.ekir.2022.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein E.J., Hardesty A., Vieira K., Farmakiotis D. Use of anti-spike monoclonal antibodies in kidney transplant recipients with COVID-19: efficacy, ethnic and racial disparities. Am J Transpl. 2022;22(2):640–645. doi: 10.1111/ajt.16843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarrell B.A., Bloch K., El Chediak A., et al. Monoclonal antibody treatment for COVID-19 in solid organ transplant recipients. Transpl Infect Dis. 2022;24(1):e13759. doi: 10.1111/tid.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tagaya E., Kikuchi K., Mitsuda T., et al. The efficacy of casirivimab/Imdevimab in solid organ transplant recipients with mild-to-moderate COVID-19. Res Square. 2022 doi: 10.21203/rs.3.rs-1361782/v1. [DOI] [Google Scholar]
- 9.Wang A.X., Busque S., Kuo J., et al. SARS-CoV-2 neutralizing monoclonal antibodies for the treatment of COVID-19 in kidney transplant recipients. Kidney. 2021;3(1):133–143. doi: 10.34067/KID.0005732021. 360Published 2021 Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ao G., Li A., Wang Y., Tran C., Qi X. Lack of efficacy for sotrovimab use in patients with COVID-19: a meta-analysis. J Infect. 2022;S0163-4453(22):00210–00219. doi: 10.1016/j.jinf.2022.04.027. [published online ahead of print, 2022 Apr 21] [DOI] [PMC free article] [PubMed] [Google Scholar]