Abstract
Quaternary ammonium compounds (QACs) are a class of antimicrobial disinfectants whose use in cleaning products increased during the COVID-19 pandemic. Chemically, their low vapor pressure indicates a proclivity to persist on surfaces, and their presence suggests a level of protection against microorganisms. The widespread application of QACs in response to the SARS CoV-2 virus created a need to evaluate their longevity on surfaces, for both efficacy and possible health risks. There are however, no standardized analytical methods for QAC surface sampling and analysis, and no published studies quantifying their concentrations on mass transportation vehicles-a high occupancy, close-contact microenvironment documented to facilitate the spread the SARS CoV-2 virus. Here, we describe a robust liquid chromatography mass spectrometry (LC-MS) method for the analysis of QACs and simultaneous development of a direct surface sampling and extraction protocol. We demonstrate the applicability of the method through the analysis of surface samples collected from in-service public transportation buses.
The rapid, sensitive LC-MS method included 8 target QACs quantified on a Q-Exactive HF Hybrid Quadrupole-Orbitrap mass spectrometer using an electrospray ionization source and Dionex UltiMate 3000 UHPLC system for analyte separation. QAC standard mixtures at concentrations between 0.1 ng mL−1 and 2000 ng mL−1 were analyzed, and chromatographic separation of all analytes was achieved in less than 10 min. All correlation coefficients were reported at r > 0.986, and LODs ranged from 0.007 to 2.103 ng mL−1 for all compounds, confirming the method's sensitivity. A previously reported surface sampling and extraction protocol was modified to further simplify the procedure and expand the number of target compounds. The new sampling protocol was optimized from 10 commercially available wipes and 4 solvent types by quantifying recovery from the surface. Band-Aid brand small gauze pads saturated with isopropanol had the highest recovery efficiencies, ranging from 61.5 to 102.9% across all analytes. To test the real-world applicability, wipe samples were collected from 4 in-circulation New Jersey Transit buses on 5 separate days over the course of a month to assess the occurrence and longevity of QACs on sanitized mass transportation vehicles. Concentrations of QACs were detected on every wipe sample taken, and at all sampled time points, confirming their persistence on hard surfaces. QACs have the potential to form polymers, and detection of the polymer might serve as a secondary indication of their effectiveness on surfaces. None of the polymers detected however, were unique to QACs from this study. The polymers detected were already present in the wipe and used as an internal standard to demonstrate the efficacy of extraction and analysis of polymeric QACs.
Keywords: Alkyl dimethyl benzyl ammonium chloride, Alkyl (ethyl benzyl) dimethyl ammonium chloride, Surface sampling, Orbitrap mass spectrometry, Public transportation, COVID-19
1. Introduction
Quaternary ammonium compounds (QACs) are a class of cationic surfactants heavily used as biocides [1]. They have multiple fields of application, including industrial, pharmaceutical, horticulture, and consumer goods production [2]. They make for effective disinfectants against a wide range of microorganisms, including enveloped viruses like the SARS CoV-2 virus [3]. The positively charged head of QACs permeate into the negatively charged cytoplasmic membrane, causing a loss of structural integrity and the progressive leakage of intracellular material, killing bacteria [4,5]. In addition to their antimicrobial properties, their low vapor pressure (e.g. 3.6 × 10−10 mmHg at 25 °C) indicates their tendency to persist on surfaces [6,7], making them favorable additives to surface cleaning products. Two classes of QAC additives commonly used in disinfection products include alkyl (ethyl benzyl) dimethyl ammonium chloride (AEBs) and alkyl dimethyl benzyl ammonium chloride (BACs) (Supplemental Fig. 1). At the height of the COVID-19 pandemic, use of disinfection products containing QAC additives increased due to a decrease in availability of other traditional disinfectants like alcohol-based products [8], as well their ability to come in contact with surfaces that are less tolerant of bleach or alcohol (e.g. human skin). However, excess exposure to QACs can lead to adverse health effects including triggering of asthma, contact dermatitis, developmental delays, and reproductive toxicity [6,9,10].
New Jersey was one of the first transit agencies in the country to incorporate large-scale chemical disinfection of their vehicles and facilities early in the COVID-19 pandemic [11]. Public buses are a crucial microenvironment of concern. In New Jersey alone, the public transportation system has 253 bus routes and provides 270 million passenger trips annually [12], making proper disinfection critical for preventing the spread of SARS-CoV-2. It was required that any disinfection would have to be done with a United States Environmental Protection Agency (EPA) approved chemical from List-N, which includes 216 products containing QACs as their active ingredient [1,13]. In accordance with the Center for Disease Control and Prevention (CDC) guidelines, highly trafficked areas (e.g. vehicles, terminals, stations) were required to undergo disinfection, at a minimum of once per day, though it is to the discretion of each individual transit authority to increase the number of times an area undergoes cleaning [14].
The widespread application of QAC-based disinfectants in response to the SARS CoV-2 virus created a need to evaluate their longevity on surfaces, for both efficacy and potential health risks. While this chemical class is increasingly utilized, there are currently no standardized analytical methods to QAC surface sampling and analysis, and no published studies quantifying their concentrations on mass transportation vehicles. For this study, we first enhanced a previously reported surface sampling and extraction protocol, resulting in sufficient recoveries for all QAC analytes. We then introduce a more robust, sensitive liquid chromatography mass spectrometry (LC-MS) method for the analysis of QACs, allowing for the chromatographic separation of all analytes in 10-min. Finally, we validate the protocol's applicability through the analysis of real-world samples collected from in-circulation buses.
2. Materials and methods
2.1. Media
To quantify QACs from solid surfaces, this solvent-assisted protocol was optimized for material used to wipe the surface, the solvent for extracting QACs from the surface, and the analyte extraction conditions from the wipe. Media compared for the optimized surface sampling protocol included: Medi-First gauze pads (5.1 cm × 5.1 cm) purchased from Fisher Scientific, generic alcohol wipe (polyester wipe pre-saturated with 70% isopropyl alcohol), Band-Aid small gauze pads (5.1 cm × 5.1 cm, Johnson & Johnson), Mr. Clean Magic Eraser, Brillo Estracell Kitchen sponge (blue, Armaly Brands), Brillo Estracell Sponge Cloth (green, Armaly Brands), Eraser Daddy 10x (Scrub Daddy), and foam craft brushes (5.1 cm, generic) purchased locally.
2.2. Chemicals
Water was obtained from a Milli-Q water purification system (Millipore). HPLC-grade acetonitrile (ACN) was purchased from Honeywell. HPLC-grade ammonium acetate, acetone, and ethyl acetate were purchased from Thermo Fisher Scientific. Isopropanol and HPLC-grade formic acid were purchased from VWR. Standard mixtures of C12–C14 alkyl (ethyl benzyl) dimethyl ammonium chloride (61% AEB-12, 25% AEB-14; ≥98.0%) and benzalkonium chloride 10% solution (1.79% BAC-10, 67.83% BAC-12, 24.6% BAC-14, 4.54% BAC-16, 1.24% BAC-18) were purchased from Sigma Aldrich. An individual BAC-10 standard was purchased from Toronto Research Chemicals. An additional proprietary compound (X), best described as a hydroxylated QAC containing a long-chained hydrocarbon and an inorganic component, was obtained from the manufacturer and included in testing.
2.3. Standard solution preparation
Individual analyte stock standards (BAC and X) were made by diluting the purchased standard in ACN to concentrations of 1000 and 5000 μg mL−1, respectively. AEB stock standard was made by dissolving 100 mg in 5 mL of ACN, and diluting to a concentration of 2000 μg mL−1. A working calibration standard solution was prepared by mixing 250 μL of the AEB stock solution, 500 μL of the BAC stock solution, and 100 μL of the X stock solution with 4.15 mL of ACN to get a 100 μg mL−1 QAC mixture. Solutions were made once at the start of experimentation, as a number of QAC analytes were previously reported to be stable for several months with both room temperature and refrigerated storage [15]. Calibration standards were prepared by spiking different volumes of the QAC mixture into 5 mL of extraction solvent and carrying it through the extraction protocol detailed below to make ten standards at the following concentrations: 2000 ng mL−1, 1500 ng mL−1, 1000 ng mL−1, 800 ng mL−1, 500 ng mL−1, 100 ng mL−1, 50 ng mL−1, 10 ng mL−1, 1 ng mL−1, and 0.1 ng mL−1. Concentration levels were selected based off estimated surface application concentrations used in the field (see Section 2.5 Field Sampling). BAC-10 was used as the internal standard for the analysis of all field samples. To create the spiking solution, 100 mg of BAC-10 was dissolved in 2 mL of ACN to get a 50000 μg mL−1 solution. This was further diluted to a final concentration of 5000 μg mL−1.
2.4. Surface sampling, preparation and extraction optimization
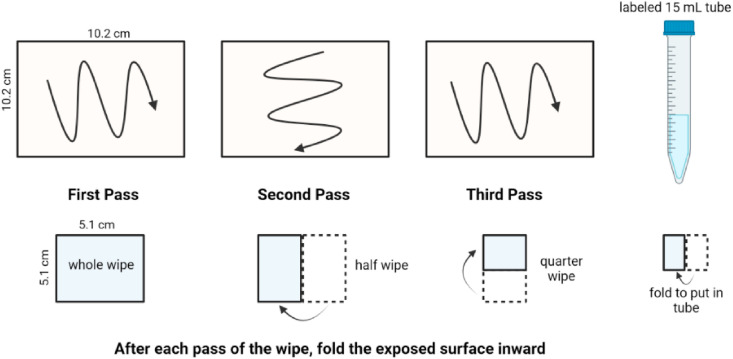
The surface sampling and extraction protocol was adapted from LeBouf et al. [15]. Prior to sampling, wipes were cut into 5.1 cm X 5.1 cm squares, or a rectangle of the same surface area. Additionally, the Brillo Estracell Kitchen Sponge and Eraser Daddy 10x were cut horizontally to separate the rough and smooth textured halves. Sheets of heavy-duty aluminum foil were used as a test surface to carry out experiments. 10.2 cm × 10.2 cm grids were drawn onto the foil with permanent marker to create consistent surface areas (Fig. 1 ). 100 μL of the 100 μg mL−1 QAC mixture was applied to each individual grid and left to dry for 30 min, 5 h, or 24 h. Replicate grids were drawn for each time interval so that a new section was wiped over with each test, rather than re-wiping the same grid at 3 different sampling times. Each wipe was then prepared with 300 μL of solvent, and wiped across the surface in accordance to Fig. 2 [15]. Wipe preparation solvents tested during the optimization process included isopropanol, ACN, acetone, and ethyl acetate; no discoloration was observed on the wipe or tested surface after sample collection. Wipes were placed at the bottom of a 15 mL polypropylene tube. Samples were then extracted in 5 mL of 60:40 ACN: H2O + 0.1% formic acid for 2 h on an orbital shaker at 200 rpm. A 1 mL aliquot of extract was placed into a vial for analysis via liquid chromatography-mass spectrometry (LC-MS).
Fig. 1.
Laboratory setup for preliminary experiments for surface wipe sampling optimization.
Fig. 2.
Wiping protocol used as the guide for sampling of hard surfaces, adapted from LeBouf et al. [15].
2.5. Field sampling
To field test the applicability of this method, the New Jersey Transit Authority (NJT) allowed us to sample from multiple bus surfaces at different time points proceeding the application of various QAC containing cleaning solutions. Wipe samples were taken at the NJT bus depot in Maplewood, NJ from May–June 2021 as part of a pilot study assessing the occurrence and longevity of QACs on high contact surfaces of sanitized mass transportation vehicles. Four in-circulation buses (labeled 1, 2, 3 and 4) were selected for surface sampling—all buses underwent NJT's standard cleaning regiment and were treated 2–3 times throughout the 30-day study time with Maquat 10 (10–10.5% active QACs, EPA Reg. # 10324-63) containing a mixture of BACs and AEBs. Two of the buses (buses 1 and 2) received an additional, one-time treatment with an alternative disinfectant containing compound X. An illustrative representation of the implemented cleaning regiment can be found in Supplemental Fig. 2. Applied surface concentrations of QACs were approximated using percent active ingredient information, found on the SDS of each utilized disinfection product (∼100000 μg mL−1 for NJT disinfectant). On the first day of the time course, all buses were disinfected with the QAC disinfection products before returning to their usual bus routes. Buses selected for sampling were in service throughout the study and maintained their usual daily schedules.
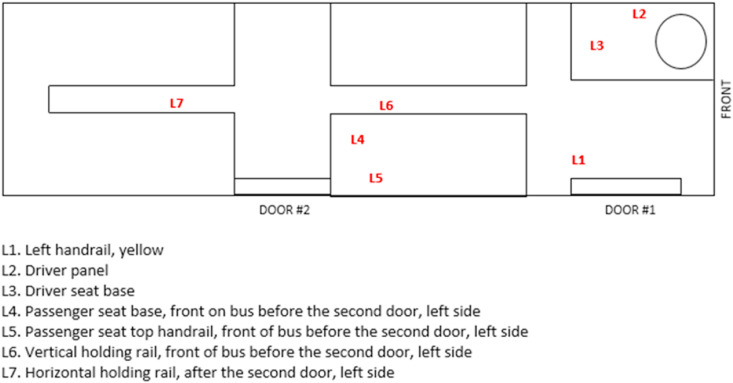
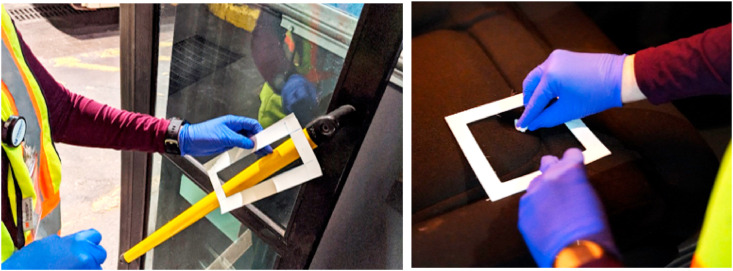
Each bus was sampled at 7 different locations. Sites were selected based on their likelihood for frequent person contact. Location descriptions can be found in Fig. 3 . Various bus surfaces were included amongst the locations wiped for sampling include fiberglass, stainless steel, fabric, and plastic coated. One of two different 103.2 cm2 surface templates was used to mark off each location and keep each sampled area consistent (Fig. 4 ). After a template was positioned on the appropriate surface, a small gauze pad wetted with 300 μL of isopropanol was wiped across the surface using the optimized method as presented in Fig. 2. The wipe was then placed at the bottom of a pre-labeled 15 mLpolypropylene centrifuge tube and sealed. Samples were placed in a cooler packed with ice for transportation back to the lab and stored in a −20 °C freezer until extraction. 5 mL of extraction solvent and 50 μL of BAC-10 internal standard solution was added into each sample tube, followed by an hour bath in an ultrasonic cleaner. Following the extraction protocol, an additional dilution was performed by combining 7 μL of the sample extract with 1493 μL of extraction solvent in an LC vial for analysis to ensure that the final concentration injected would not overload the detector. Samples were collected on 5 different days for the duration of the 30-day time-course at the following time points: days 0, 1, 8, 15, 30. Day 0 was defined as the time immediately after the initial QAC surface cleaner application, before any passengers occupied the bus. Wipes for each successive sample day were taken in areas adjacent to previously sampled sections to avoid accidently re-wiping over already sampled areas. Control samples of compound X were collected after application of the cleaner onto aluminum foil sheets on the same day as the initial treatment to the buses. Sheets were then transported back to the lab to measure decay of compound X in an undisturbed environment. Surface wipes from the foil were taken at the end of each field sampling day.
Fig. 3.
Location descriptions for the surfaces selected for wipe sampling on all buses.
Fig. 4.
Use of templates for surface sampling on the buses, shown at L1 (handrail, yellow) and L3 (driver seat base). The area for both templates used was 103.2 cm2 and ensured consistent surface sampling across locations and days.
2.6. LC-MS analysis
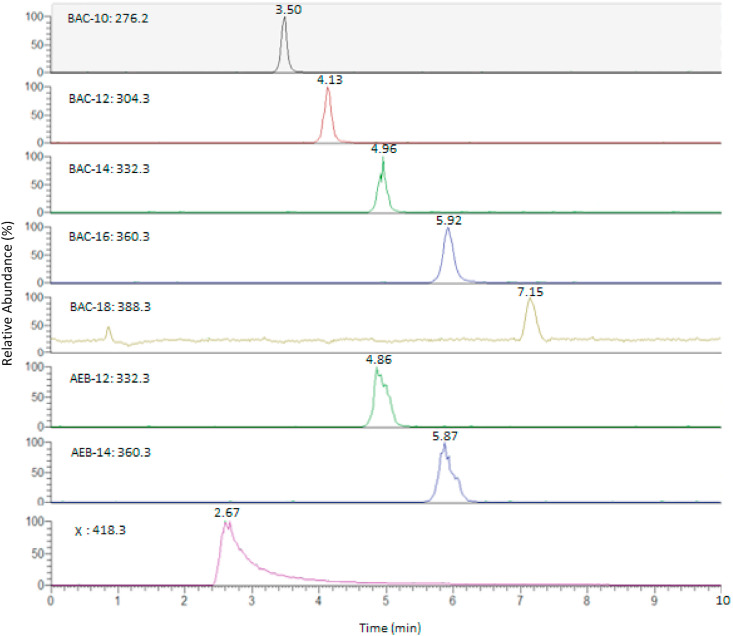
Analysis was performed on a Q Exactive HF Hybrid Quadrupole-Orbitrap mass spectrometer equipped with an electrospray ionization (ESI) source and Dionex UltiMate 3000 UHPLC system (Thermo Fisher Scientific). Daily calibration of the instrument was performed using Pierce LTQ Velos ESI positive calibration solution. Chromatographic separation was carried out on a Supelcosil LC-CN column (7.5 cm × 3 mm, 3 μm) at 25 °C. A mobile phase of H2O, 0.1% formic acid and 100 mM ammonium acetate (solvent A) and ACN (solvent B) was used for a 10-min isocratic elution with a 40:60 solvent A: solvent B composition. The flow rate was 0.4 mL min−1 and the injection volume was 5 μL. The elution order of all analytes, shown in Fig. 5 , was as follows: X < BAC-10 < BAC-12 < AEB-12 < BAC-14 < AEB-14 < BAC-16 < BAC-18. Nitrogen was used for all gas flows. Collision energy was set to 25. The specific precursor and product ions are detailed in Table 1 . Product ions were determined through the direct injection of a 1000–2000 ng mL−1 solution of each commercial standard into the MS and optimizing the collision energy for the recovery of the product ions. Data was collected in positive ionization mode using parallel reaction monitoring (PRM) acquisition mode. Data acquisition and processing was carried out with Thermo Xcalibur (v.4.0.27.19) software.
Fig. 5.
Chromatographic separation of all QACs with their respective precursor ions used for PRM acquisition mode. Standard mixtures were run at 1000–2000 ng mL−1, and their corresponding retention times and relative abundances are shown. Peak tailing was observed in the chromatogram of compound X, and was unable to be completely resolved with the final method parameters.
Table 1.
Precursor and product masses used for identification and quantitation of all QAC compounds.
| Analyte | Precursor Ion | Product Ions |
|---|---|---|
| BAC-10 | 276.2 | 184.2, 91.1 |
| BAC-12 | 304.3 | 212.2, 91.1 |
| BAC-14 | 332.3 | 240.3, 91.1 |
| BAC-16 | 360.3 | 268.3, 91.1 |
| BAC-18 | 388.3 | 297.3, 91.1 |
| AEB-12 | 332.3 | 212.2, 119.1 |
| AEB-14 | 360.3 | 240.3, 119.1 |
| X | 418.3 | n/a |
2.7. Quality assurance
Extraction solvent spiked with the QAC mixture was included in each analytical batch to ensure proper functioning of the instrument and method. Blank mobile phase was included to account for any carryover between injections. Blank extraction solvent, wipes, and foil were analyzed to determine any potential background sources of QACs. All wipes were handled with nitrile gloves and stored in PTFE bags until they were used for sampling. Low measurable concentration of several QAC analytes were detected in the blank wipes. However, background concentration levels were at or near the limit of detection. For this reason, as well as wishing to maintain the integrity of the wipe, we did not pre-clean the wipes prior to taking them into the field. Field control samples, one blank wipe per-bus per-sampling day, were collected. The blank wipe was saturated with 300 μL of isopropanol, not wiped but folded, and placed in a labeled 15 mL tube.
BAC-10 was selected as an internal standard used for quantification of the targeted QACs, as it is chemically representative of the measured analytes and was not used in either tested disinfectant product sprayed on the buses. In addition to the externally spiked BAC-10, a secondary internal standard was monitored throughout final wipe optimization experimentation and all field sample analyses. The selected wipe for field sampling (Band-Aid small gauze pads) contained a measurable polymer peak with a reproducible retention time (RT = 0.99) and molecular weight (m/z = 432.28). Wipes analysis resulted in an average peak area of 3.2 × 108 ± 26.1%, suggesting that this polymer within the wipe acted as a stable marker for method performance. However, our field samples found no measureable surface concentrations of QAC-based polymers; therefore, we could not utilize this internal standard in the same manner as the BAC-10. Nevertheless, we took advantage of this pre-existing polymer to both validate our final surface sampling protocol and QC subsequent extractions of collected field samples.
3. Results and discussion
3.1. Method optimization
The modifications made to the referenced wipe sampling method [15] were meant to simplify the surface sampling protocol. In the first set of optimization experiments, a 100 μg mL−1 QAC mixture was applied to an aluminum foil surface. Following a 30-min drying period, the surface sampling efficiency of 10 different commercially available wipes and sponges, as well as either isopropanol or ACN as wipe preparation solvents was explored. Sampling efficiency was calculated by taking the peak area of a QAC analyte from a wipe sample after extraction and dividing it by the peak area of a QAC analyte from a 5 mL aliquot of extraction solvent spiked with 100 μL of 100 μg mL−1 of QAC mixture and carried through the extraction protocol (Equation (1)).
| (analyte areasample / analyte areaspiked) x 100% | Eq. 1 |
Analyte area sample is the peak area of the individual QAC taken from each tested wipe; analyte area spiked is the peak area of the individual QAC taken from extraction solvent spiked with 100 μg mL−1 QAC mixture.
Preliminary results suggested that the top three performing wipes were Medi-First gauze pads, Band-Aid small gauze pads and Mr. Clean Magic Eraser, ranging in efficiencies of 54.1–92.0%, 46.3–96.0%, and 15.4–38.6%, respectively, across all analytes. Both isopropanol and ACN had similar results when calculating efficiencies, prompting further testing to determine optimal solvent type.
Next, optimization experiments focused on improving efficiency of the compound with the lowest recovery, compound X. 100 μL of a 100 μg mL−1 solution of X was directly applied to a new aluminum foil surface and left to dry for either 30 min, 5 h, or 24 h, and then sampled. These sampling time points of were selected to mimic a realistic timeline between disinfection and human contact. Only the Band-Aid small gauze pads, Medi-First gauze pads, and Mr. Clean Magic Eraser were tested. Additionally, isopropanol, ACN, ethyl acetate and acetone were evaluated as potential wipe solvents. Resulting recovery efficiency calculations are displayed in Table 2 . Mr. Clean Magic Eraser was the most variable across all solvent types and times points, with recoveries ranging from as low as 0.61%to as high as 41.9%. The Medi-first gauze and Band-Aid gauze had comparable results for all 3 time points and required further optimization to determine which would be selected for the final protocol. Isopropanol was selected as the wipe solvent due to its reliably high recovery percentages. From a practical standpoint, alcohol-based cleaners are the most common disinfectants used to kill surface microorganisms in highly trafficked areas (i.e., hospitals, schools, stores, etc.) [16], therefore the smell of isopropanol would be familiar and not alarming to passengers, as the buses remained in service throughout this study.
Table 2.
Percent recovery of compound X at 3 different sampling time points. All samples were extracted using the orbital shaker for 2 h.
| 30 min |
5 h |
24 h |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Medi | Band-Aid | Mr. Clean | Medi | Band-Aid | Mr. Clean | Medi | Band-Aid | Mr. Clean | |
| isopropanol | 48.8% | 42.0% | 30.0% | 41.6% | 56.3% | 41.9% | 48.4% | 74.3% | 37.3% |
| ACN | 19.7% | 13.4% | 11.8% | 22.1% | 35.3% | 8.90% | 42.6% | 23.4% | 23.1% |
| acetone | 43.1% | 19.6% | 0.61% | 22.5% | 47.2% | 1.55% | 50.9% | 36.6% | 0.93% |
| ethyl acetate | 45.6% | 44.1% | 27.9% | 32.4% | 32.8% | 25.3% | 64.3% | 45.6% | 20.9% |
To decrease sample processing time, extraction via orbital shaker (200 rpm, 2 h) vs. an ultrasonic cleaner at 35 °C for 1 h was evaluated. With the exception of the Band-Aid gauze at 24 h post application, results indicated an increase in recovery percentage for both isopropanol-spiked Band-Aid gauze and Medi-first gauze when utilizing the ultrasonic cleaner (Table 3 ). For this reason, as well as the significant decrease in analysis time, the ultrasonic cleaner at elevated temperature was selected for the analysis of all samples going forward.
Table 3.
Percent recovery of compound X at 3 different time points after extraction with either with an orbital shaker (room temperature, 200 rpm, 2 h) or an ultrasonic cleaner (35 °C, 1 h). All results shown are using isopropanol as spiking solvent for the wipes.
| 30 min |
5 h |
24 h |
||||
|---|---|---|---|---|---|---|
| Medi | Band-Aid | Medi | Band-Aid | Medi | Band-Aid | |
| Orbital Shaker | 48.8% | 42.0% | 41.6% | 56.3% | 48.4% | 74.3% |
| Sonicator | 65.9% | 46.6% | 56.6% | 58.4% | 66.8% | 60.0% |
The final set of optimization experiments examined the consistency of the Medi-first gauze pads and the Band-Aid small gauze pads. Due to the low recovery of compound X, we chose the protocol that demonstrated its greatest repeatability. 100 μL of 100 μg mL−1 solution of X was spiked onto a new sheet of aluminum foil. 4 replicate samples were taken with each wipe type at 30 min, 5 h, and 24 h. Wipes were saturated with isopropanol and extracted using the ultrasonic cleaner. Resulting recovery efficiency calculations are shown in Table 4 . Overall, both wipes displayed variable recoveries.
Table 4.
Repeatability of both Medi-first gauze pads and Band-Aid small gauze pads in the recovery of compound X at 3 different sampling time points.
| 30 min |
5 h |
24 h |
||||
|---|---|---|---|---|---|---|
| Medi | Band-Aid | Medi | Band-Aid | Medi | Band-Aid | |
| replicate 1 | 87.0% | 20.7% | 55.5% | 20.7% | 10.8% | 12.6% |
| replicate 2 | 12.6% | 11.0% | 24.7% | 11.0% | 37.0% | 33.5% |
| replicate 3 | 53.2% | 38.8% | 54.4% | 38.8% | 11.7% | 35.7% |
| replicate 4 | 15.1% | 11.7% | 100.2% | 11.7% | 41.5% | 101.5% |
| avg. | 42.0% | 20.5% | 58.7% | 20.5% | 25.3% | 45.8% |
| stnd. dev. | 35.3% | 12.9% | 31.1% | 12.9% | 16.3% | 38.6% |
The Medi-first gauze pads had higher average recoveries but larger deviations. The opposite trend was observed with Band-Aid small gauze pads, which had lower average recoveries and lower relative standard deviations for all time points. While the calculated recoveries were not optimal for this set of experiments, better repeatability led to the selection of the Band-Aid small gauze pads as the final wipe type to be used for the procedure.
Once the recovery conditions were determined for compound X, we verified that the other QAC recovery efficiencies remained essentially unchanged. An additional experiment was performed by spiking 100 μL of the 100 μg mL−1 QAC mixture onto aluminum foil and wiping after 30 min. The resulting surface recovery efficiencies for all QAC analytes of interest ranged from 61.5% to 102.9%. The duration of the ultrasonic extraction was used as reported with a demonstrated recovery of >90% for 7 of the 8 QACs. The original sampling method protocol reported recoveries of 96%, 95%, and 92% for BAC-12, BAC-14, and BAC-16 respectively [15]. Our modified procedure showed an increase in the recovery of the same compounds at 100.1%, 102.0%, and 92.7% for BAC-12, BAC-14, and BAC-16 respectively. Additionally, LeBouf et al. [15] reports recoveries of greater than 90% for 5 total QACs, however this modified approach shows that we were able to include a larger array of compounds with comparable recoveries for 8 QACs.
A highly sensitive and robust LC-MS method was created for rapid analysis of the targeted QACs. The method reported in LeBouf et al. [15] was used as a reference to help build a method that accommodated additional QACs (BAC-10, BAC-18, AEB-12, AEB-14, X). To try and improve peak tailing observed with the elution of compound X, the ratio of aqueous to organic solvent in the mobile phase was optimized. An increase in aqueous content caused a broadening and eventual disappearance of the analyte peak; therefore, a mobile phase composition of 40:60 solvent A: solvent B, the original composition [15], was selected.
Analytical figures of merit calculations used for the evaluation of method performance are listed in Table 5 . To calculate these values, standard mixtures were spiked into extract solvent at different concentrations and carried through the extraction protocol. Linearity can be defined as the method's ability to return values directly proportional to the concentration of the target analyte [17]. The calibration curves for all analytes resulted in correlation coefficients of r > 0.986, confirming strong linear relationships within the range of 0.01 ng mL−1 to 2000 ng mL−1. It is important to note that the standards contained different ratios of QACs; therefore, the actual concentrations within the calibration curve varied for each individual analyte, and were lower than the overall QAC mixture concentration. Precision was calculated by analysis of triplicate injections of a low (10 ng mL−1), medium (0.5 μg mL−1), and high (1.5 μg mL−1) concentration standard and finding the average %CV. All AEB and BAC analytes resulted in %CV values < 4% apart from BAC-14, whose calculated %CV values for low, medium, and high concentration standards were 45%, 60%, and 84% respectively. Compound X also had a higher variation than the other AEBs and BACs, (16%).
Table 5.
Key analytical figures of merit calculations used for evaluation of method performance. Linear dynamic range is defined as the range that a linear relationship was observed in our calibration curves, listed out as the minimum and maximum concentration of standard that was reliably analyzed.
| Analyte | Linear Dynamic Range (ng mL−1) | r | LOD (ng mL−1) | LOQ (ng mL−1) | Precision (%CV) |
|---|---|---|---|---|---|
| BAC-10 | 0.0179–35.8 | 0.999 | 0.025 | 0.080 | 4.01 |
| BAC-12 | 0.0678–1360 | 0.997 | 0.009 | 0.030 | 1.32 |
| BAC-14 | 2.46–369 | 0.986 | 5.53 | 18.4 | 63.1 |
| BAC-16 | 0.0454–90.8 | 0.999 | 0.030 | 0.100 | 2.50 |
| BAC-18 | 0.00124–24.8 | 1.00 | 0.003 | 0.010 | 2.62 |
| AEB-12 | 0.0610–1220 | 0.998 | 0.016 | 0.050 | 2.47 |
| AEB-14 | 0.0248–496 | 0.999 | 0.007 | 0.020 | 1.03 |
| X | 0.100–2000 | 0.993 | 2.10 | 7.01 | 16.1 |
The limit of detection (LOD) was defined as the lowest concentration level that can be determined to be statistically different from a blank and is matrix, method, and analyte specific [18]. Similarly, the limit of quantification (LOQ) was defined as the lowest amount of analyte that can be quantified. Mathematically, the LOD and LOQ were defined as 3 and 10 times the standard deviation of the lowest concentration standard that gives reproducible results, respectively [18]. The LC-MS LODs for all AEB analytes were 0.007–0.016 ng mL−1, BAC analytes were 0.003–0.03 ng mL−1, and X was 2.10 ng mL−1. The LC-MS LOQs for all AEB analytes were 0.02–0.05 ng mL−1, BAC analytes were 0.01–0.1 ng mL−1, and X was 7.01 ng mL−1. BAC-14 had an increased LOD and LOQ at 5.532 ng mL−1 and 18.4 ng mL−1, respectively. No measurable concentration of BAC-14 was detected in the blank samples analyzed; therefore, its poor sensitivity can most likely be attributed to its analysis under compromised conditions. Compound X's elevated LOD and LOQ are most likely related to the difference in its functional groups compared to the other QACs analyzed, similarly leading to its analysis under compromised conditions. Structural differences can result in different ionization efficiencies, which may impact each specific compound's sensitivity.
Recent literature reported studies exploring the change in QAC concentration pre- and post- COVID have reported similar method validation results for BAC compounds analyzed via LC-MS in various matrices. Zheng et al., 2021 looked at 18 various QACs in blood reported LODs between 0.01 and 0.1 ng mL−1 for BAC compounds [19]. The reported LOD for BAC-14 (0.10 ng mL−1) was more sensitive than determined for our study. Another study exploring the concentration of 19 QACs in residential dust calculated LODs of 0.0002–0.0025 μg g−1 [20]. Similarly, they reported more sensitive LODs for BAC-14 (0.0008 μg g−1) than our study. Method LODs for BAC analytes in our current study are better than several additional studies previously published [15,21,22]. This is the first LC-MS method explicitly looking at the identification and quantitation of AEB compounds. Like the BAC analytes, the calculated LODs for AEB compounds in this study are better than previously published values for QACs [[23], [24], [25]].
3.2. Field sampling
These surface sampling and instrument analysis protocols were field tested by the collection of wipe samples from 4 disinfected, highly trafficked mass transit vehicles as part of a pilot study conducted with NJT. Results from 7 sampling sites within each bus are reported. The intent of the study was to compare the surface lifetime of 2 different QAC based disinfection products used for the sanitization of NJ Transit buses. Emphasis was placed on exploration of the differences between single and recurrent applications of cleaning product throughout the 30-day time-course, as a single application of an effective cleaner would provide time and resource conserving benefits to NJT. All buses were repeatedly sanitized with NJT's BAC and AEB cleaner, with initial cleaning occurring at the start of the study, and reapplication of product occurring 2–3 more times throughout the 30-days. Buses 1 and 2 also received additional treatment with the disinfectant spray containing compound X once at the start of the study.
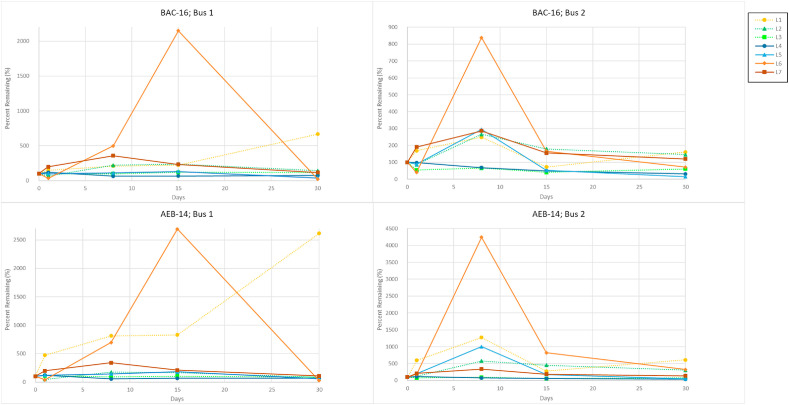
Fig. 6 shows the surface concentration patterns of QACs (BAC-16, AEB-14) that were repeatedly applied to all sampled buses. All 4 buses resulted in similar trends in changes in surface concentrations; only 2 buses, which represent the surface trends observed, are included in this graphic. Both BAC-16 and AEB-14 display elevated relative surface concentration levels detected at all sampled locations, peaking at either day 8 or day 15 before plateauing out to day 30. The observed spikes in QAC concentration are indicative of the cleaning schedule followed for this study, designating when additional sanitization with cleaner was applied to each of the buses. Most locations continued to build on the initial concentration of QAC found on the surface, culminating on day 30 with a similar or larger relative surface amount than seen on day 0. It is clear that repeat application allows for a successful buildup of QACs overtime, which would suggest longer protection of bus surfaces from microorganisms such as the COVID-19 virus.
Fig. 6.
Concentration, expressed as percent of the original concentration seen on day 0 (initial sampling day) remaining vs time for BAC-16 and AEB-14, as measured on 2 buses that underwent repeat disinfection at all 7 sampling locations on days 0–30.
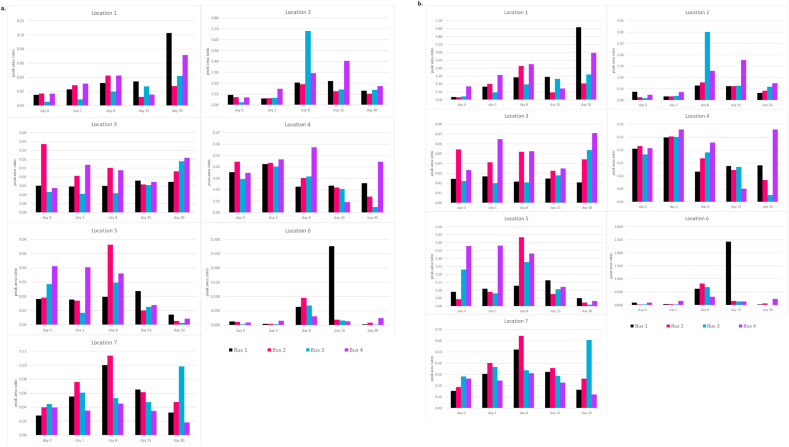
Representative bar graphs displaying the relative concentration patterns for BAC-16 and AEB-14 for all 4 sampled buses are shown in Fig. 7 . All other BAC and AEB analytes (Supplemental Fig. 3) follow a similar pattern with regard to locations on the bus that demonstrate relatively higher concentrations of analytes and the variability in QAC presence throughout the 30-day time course. The similarities in relative surface concentration patterns observed across all QAC compounds per location suggest that the observed variability across the sampling days is dependent on location type rather than QAC identity. This is further supported by the different surface concentration patterns seen in higher frequency contact locations (i.e. L1) vs lower frequency contact areas (i.e. L7). Spikes in concentration recorded in the middle of the sampling period are indicative of the reapplication of disinfectant. QAC compounds were detected at every location for every day sampled even though the buses did not undergo a daily cleaning schedule during the time of the experiment. These results reinforce that BAC and AEB categorized QACs tend to remain on the surface when following a repeat-application schedule, allowing a constant concentration of QACs to build on the surfaces. These findings also speak to the increasing ubiquity of this class of compounds, especially post pandemic when surface disinfection has become a critical part of the COVID-19 protection protocols. In addition to their use in cleaning products, QACs are common additives in many personal care products like soap, hand sanitizer, and floor products [6]. These products are likely to be used by bus riders in alternative settings (i.e. kitchens, home cleaning, personal care product residues, etc.). Their pervasive nature could lead to an unintentional contribution to background levels of QACs, offering further explanation to the continuous detection of the compounds in our samples.
Fig. 7.
Relative surface concentrations for all buses across the 30-day sampling period for each of the 7 sampling locations for individual QAC analytes (a.) BAC-16 (b.) AEB-14. Buses 1 (black)and 2 (red) received both repeat treatment with NJT's AEB and BAC disinfectant, as well as a single application of disinfectant containing compound X at the start of the study.
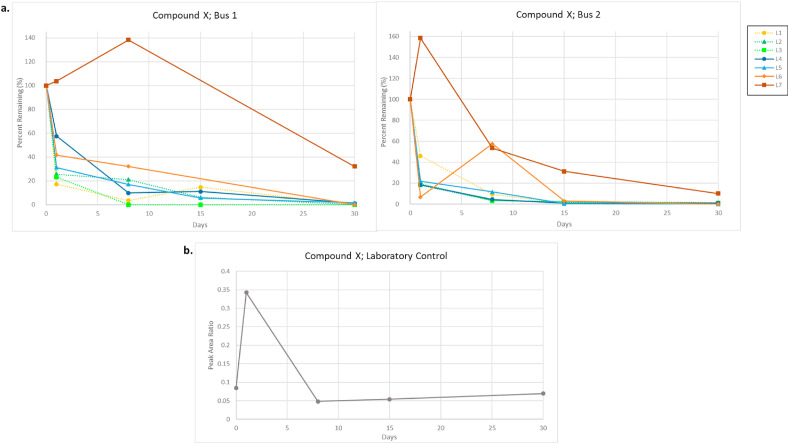
Compound X did not persist on all surfaces tested throughout the 30-day sampling period.
Fig. 8a shows the resulting degradation patterns of compound X after a single application on Day 0 (also see Supplemental Fig. 3e.). With the exception of L7, all locations resulted in measurable quantities 18–58% (bus 1) and 7–46% (bus 2) of compound X on the surface after 24-h post-treatment. After one week, sampled locations had ≤32% (bus 1) and ≤12% (bus 2) of the originally applied product remaining. After two weeks post application, locations had ≤15% (bus 1) and ≤3% (bus 2) of applied compound X remaining on the surface. After 30 days, both buses had ≤1% compound X observed, with most sites having no measurable amounts detected. This may be due in part to contact with passengers, causing the applied disinfectant to be removed from high-frequency contact surfaces. L7, the horizontal holding rail at the back of the bus, showed an increase in the concentration of compound X observed after 24 h, as well as measurable concentrations on the surface out to day 30. While it was close to being within the measurement error, this observation may be due to our sampling approach or an uneven distribution of the compound when it was applied. Special care was taken to not wipe over formerly sampled areas and the uneven application of the disinfecting agent or migration of the active from its initial application site, may account for an increase in a measured concentration after Day 0. It is also likely that areas sampled for L7, a location that required standing to access, had low contact frequency with passengers, which in turn would result in an undisturbed settling of the sanitation solution containing compound X.
Fig. 8.
(a.) Observed degradation pattern of compound X after a single application to buses 1 and 2. (b.)Laboratory control of compound X-based disinfectant sprayed onto aluminum foil on Day 0 of field testing. Compound X decreases by nearly 86% between day 1 and day 8 before plateauing for the remainder of the time-course.
The observed reduction pattern can also be attributed to natural decay from surfaces, which was observed through the analysis of laboratory control samples of compound X (refer to Materials and Methods- Field Sampling). These controls follow a similar degradation pattern to that observed in the field. As seen in Fig. 8b, compound X naturally decays by approximately 86% between days 1 and 8, reaching an equilibrium that remained to day 30. Compound X decreases rapidly in an undisturbed lab setting, and even faster in real-world bus samples, so we would not expect the applied amount of compound X to remain on surfaces, even without the inclusion of people commuting on the buses. It is worth noting that an increase in compound X was observed between days 0 and 1. Similar to the field samples, this is most likely due to uneven application (or migration) of the disinfectant spray on the aluminum foil sheets. The poor adhesion of X to surfaces could be due to its physio-chemical properties; however more research would have to be done on that compound to further understand its surface mechanisms.
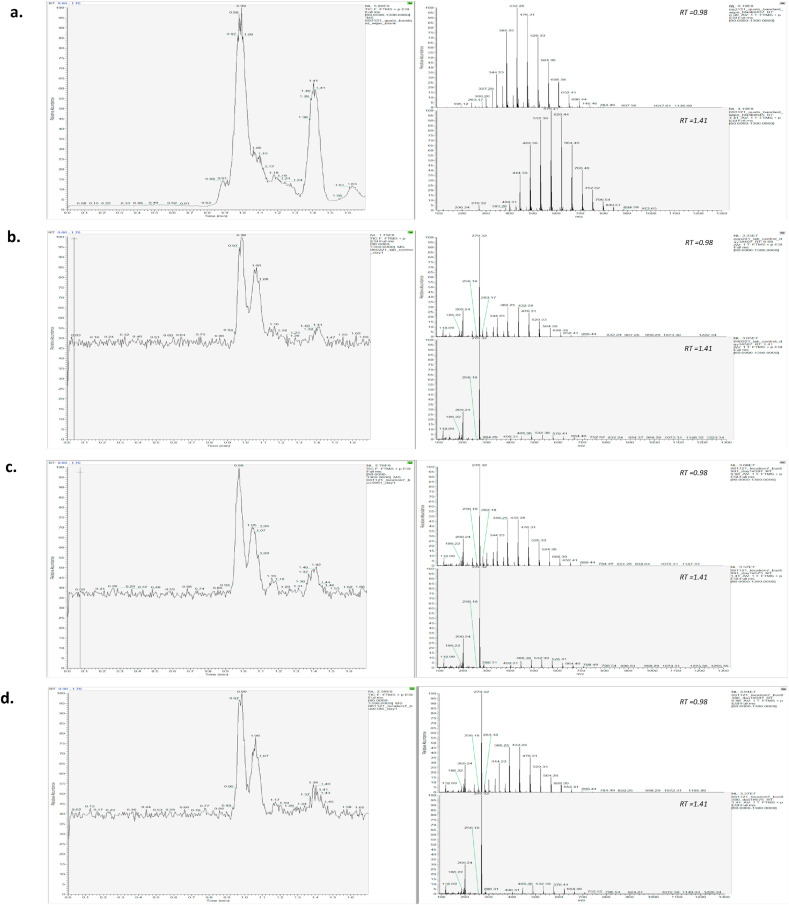
It had been suggested that QACs have the potential to form polymers [26], and their detection would serve as a secondary indication that the disinfectant was effectively remaining on the bus surfaces. Analysis of a blank Band-Aid gauze pad saturated with isopropanol and carried out through the extraction protocol indicated the presence of polymers at retention times of 0.98 and 1.41 min (Fig. 9 a). The background level of QAC polymers allowed us to verify the sampling method's ability to extract and analyze polymers. Polymerization was also observed in the lab control samples, demonstrating its origin from the wipe. The mass spectral signal had a 10–100 fold reduction in intensity compared to a blank Band-Aid gauze pad extraction but their mass-to-charge ratios were the identical, confirming its origin, perhaps demonstrating a loss of this polymer during the wipe procedure. No additional peaks were identified as polymers and we concluded that there were no unique QAC-based polymers sampled from the bus surfaces. Further research is required to explore the extent of QACs ability to polymerize to better understand their role in surface longevity and microorganism protection.
Fig. 9.
Total Ion Chromatogram (TIC) and respective mass spectral patterns for polymer formation observed at RT 0.98 and 1.41 for (a.) a blank Band-Aid wipe spiked with isopropanol (b.) compound X sprayed onto aluminum foil (c.) bus 1 (received all QAC disinfectants), sampled at L7 (d.) bus 4 (received repeat disinfectant with NJT cleaner only), sampled at L7.
4. Conclusion
Modifications were made to a wipe sampling protocol to simplify the collection and extraction of QAC analytes from surfaces, broadening the number of measurable analytes while maintaining proficient recoveries of all compounds (61.5% to102.9%). Additionally, a highly sensitive LC-MS method capable of chromatographic separation of 8 compounds in under 10-min was accomplished. Method LODs were sensitive enough to confidently analyze surface concentrations in the low-ppt range. Field application of our sampling protocol resulted in measurable concentrations of all AEB and BAC analytes after repeat application to the surface of 4 in-circulation buses, confirming that consistent application is necessary to maintain a consistent level of active ingredient on high contact surfaces. These results highlight the importance of monitoring QACs in highly trafficked microenvironments so that we can better understand the extent with which they mitigate the risk of virulent transmission. Moreover, further studies are needed to quantify the levels of QACs found on surfaces so that implications for dermal exposure can be explored.
Declaration of competing interest
We declare that none of the authors of this manuscript have any known conflicts of interest.
Acknowledgments
We would like to acknowledge the support of the Center for Environmental Exposures and Disease (CEED) NIH-NIEHS Grant nos. P30 ES005022 and T32 ES019854 . This research was supported in part by Grant no. 69A3551847102 from the U.S. Department of Transportation, Office of the Assistant Secretary for Research and Technology (OST-R) . We would also like to acknowledge the project contributions of Gedi Mainelis, Nirmala Myers, and Taewon Han and NJ Transit, with special thanks to Chelsea Ramos and the bus depot staff.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.emcon.2022.06.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Hora P.I., Pati S.G., McNamara P.J., Arnold W.A. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ. Sci. Technol. Lett. 2020;7(9):622–631. doi: 10.1021/acs.estlett.0c00437. [DOI] [PubMed] [Google Scholar]
- 2.Buffet-Bataillon S., Tattevin P., Bonnaure-Mallet M., Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds--a critical review. Int. J. Antimicrob. Agents. 2012;39(5):381–389. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 3.McKeen L. Introduction to food irradiation and medical sterilization. Effect Steriliz. Plastics Elastomers. 2012:1–40. doi: 10.1016/B978-1-4557-2598-4.00001-0. [DOI] [Google Scholar]
- 4.Jennings M.C., Minbiole K.P., Wuest W.M. Quaternary ammonium compounds: an antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 2015;1(7):288–303. doi: 10.1021/acsinfecdis.5b00047. [DOI] [PubMed] [Google Scholar]
- 5.Denyer S.P. Mechanisms of action of antibacterial biocides. Int. Biodeterior. Biodegrad. 1995;36(3–4):227–245. doi: 10.1016/0964-8305(96)00015-7. [DOI] [Google Scholar]
- 6.Bellevue and New York University Occupational. Environmental Medicine Clinic Quaternary ammonium compounds in cleaning products: health & safety information for health professionals. Mt. Sinai Selikoff Cent. Occup. Health. 2015 https://www.mountsinai.org/files/MSHealth/Assets/HS/Patient-Care/Service-Areas/Occupational-Medicine/QACsInfoforPhysicians_18.pdf [Google Scholar]
- 7.Pubchem Benzyldimethyltetradecylammonium chloride. https://pubchem.ncbi.nlm.nih.gov/compound/Benzyldimethyltetradecylammonium-chloride 2022; Available from:
- 8.IHS Markit . Stephan Mueller and Stephan Beraud; 2020. The Biocides Market in the Times of Coronavirus.https://ihsmarkit.com/research-analysis/the-biocides-market-in-the-times-of-coronavirus.html [Google Scholar]
- 9.Melin V.E., Melin T.E., Dessify B.J., Nguyen C.T., Shea C.S., Hrubec T.C. Quaternary ammonium disinfectants cause subfertility in mice by targeting both male and female reproductive processes. Reprod. Toxicol. 2016;59:159–166. doi: 10.1016/j.reprotox.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Herron J., Reese R.C., Tallman K.A., Narayanaswamy R., Porter N.A., Xu L.B. Identification of environmental quaternary ammonium compounds as direct inhibitors of cholesterol biosynthesis. Toxicol. Sci. 2016;151(2):261–270. doi: 10.1093/toxsci/kfw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.New Jersey Transit . 2021. Ride to Recovery: NJ Transit's COVID-19 Response & Path Forward. [Google Scholar]
- 12.New Jersey Transit. About Us; 2022. https://www.njtransit.com/about/about-us Available from: [Google Scholar]
- 13.United States Environmental Protection Agency About list N: disinfectants for coronavirus (COVID-19) 2021. https://www.epa.gov/coronavirus/about-list-n-disinfectants-coronavirus-covid-19-0 Available from:
- 14.Centers for Disease Control and Prevention Cleaning and disinfecting your facility- every day and when someone is sick. 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/disinfecting-building-facility.html Available from:
- 15.LeBouf R.F., Virji M.A., Ranpara A., Stefaniak A.B. Air and surface sampling method for assessing exposures to quaternary ammonium compounds using liquid chromatography tandem mass spectrometry. Ann. Work Expo. Health. 2017;61(6):724–736. doi: 10.1093/annweh/wxx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . World Health Organization; 2020. Cleaning and Disinfection of Environmental Surfaces in the Context of COVID-19: Interim Guidance. 15 May 2020. [Google Scholar]
- 17.U.S. Fish & Wildlife Service, Assay validation methods- definitions and terms. Aquat. Anim Health Progr.. p. https://www.fws.gov/aah/PDF/QI-Terms%20and%20Defs.pdf.
- 18.Ripp J. Wisconsin Department of Natural Resources, Laboratory Certification Program; 1996. Analytical Detection Limit Guidance & Laboratory Guide for Determining Method Detection Limits. [Google Scholar]
- 19.Zheng G.M., Webster T.F., Salamova A. Quaternary ammonium compounds: bioaccumulation potentials in humans and levels in blood before and during the covid-19 pandemic. Environ. Sci. Technol. 2021;55(21):14689–14698. doi: 10.1021/acs.est.1c01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng G.M., Filippelli G.M., Salamova A. Increased indoor exposure to commonly used disinfectants during the COVID-19 pandemic. Environ. Sci. Technol. Lett. 2020;7(10):760–765. doi: 10.1021/acs.estlett.0c00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Carballo E., Sitka A., Gonzalez-Barreiro C., Kreuzinger N., Furhacker M., Scharf S., Gans O. Determination of selected quaternary ammonium compounds by liquid chromatography with mass spectrometry. Part I. Application to surface, waste and indirect discharge water samples in Austria. Environ. Pollut. 2007;145(2):489–496. doi: 10.1016/j.envpol.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 22.Amelin V.G., Bol'shakov D.S. Simultaneous identification and determination by LC-MS of quaternary ammonium compounds with other active ingredients in drugs. Pharmaceut. Chem. J. 2020;54(1):85–91. doi: 10.1007/s11094-020-02160-8. [DOI] [Google Scholar]
- 23.Nunez O., Moyano E., Galceran M.T. Determination of quaternary ammonium biocides by liquid chromatography-mass spectrometry. J. Chromatogr. A. 2004;1058(1–2):89–95. doi: 10.1016/j.chroma.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 24.Bassarab P., Williams D., Dean J.R., Ludkin E., Perry J.J. Determination of quaternary ammonium compounds in seawater samples by solid-phase extraction and liquid chromatography-mass spectrometry. J. Chromatogr. A. 2011;1218(5):673–677. doi: 10.1016/j.chroma.2010.11.088. [DOI] [PubMed] [Google Scholar]
- 25.Xian Y., Dong H., Wu Y., Guo X., Hou X., Wang B. QuEChERS-based purification method coupled to ultrahigh performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) to determine six quaternary ammonium compounds (QACs) in dairy products. Food Chem. 2016;212:96–103. doi: 10.1016/j.foodchem.2016.05.151. [DOI] [PubMed] [Google Scholar]
- 26.Elena P., Miri K. Formation of contact active antimicrobial surfaces by covalent grafting of quaternary ammonium compounds. Colloids Surf. B Biointerfaces. 2018;169:195–205. doi: 10.1016/j.colsurfb.2018.04.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.