Abstract
Alteration of redox status is one of the molecular pathways commonly associated with pesticide toxicity. Antioxidants, including those obtained from plant phenolics, have been shown to mitigate pesticide-induced cellular injury. The present study was aimed at evaluating the effect of daflon-500®, a flavonoid compound on sub-chronic chlorpyriphos-evoked changes in antioxidant and biochemical parameters in the hypophysis and testes of adult male rats. Twenty-five male albino rats were randomly divided into 5 groups of 5 animals each. Group I (DW) received distilled water (2 ml/kg); group II (SO) was dosed with soya oil (2 ml/kg); Group III (DAF) received daflon-500® at 1000 mg/kg ~ 1/5th of LD50 (≥ 5000 mg/kg); group IV (CP) was administered chlorpyriphos at 7.74 mg/kg ~ 1/10th of LD50 (77.4 mg/kg) while group V (DAF + CP) was previously treated with daflon-500® (1000 mg/kg) and then exposed to CP (7.74 mg/kg), 30 min later. Daily oral regimen administration was done for 60 days after which the animals were sacrificed by cervical venesection after light chloroform anesthesia. The hypophysis and testicular tissues were harvested, and their homogenates were analyzed for malondialdehyde, catalase and superoxide dismutase, and acetylcholinesterase levels. A significant increase in the hypophysis and testicular MDA concentrations, coupled with a decrease in the SOD, CAT, and AChE activities were observed in the CP group. The levels of these oxidative and biochemical parameters were alleviated in the group pretreated with Daflon-500®. Results of this study demonstrated that pre-treatment with Daflon-500® mitigated CP-induced alterations in oxidative and biochemical parameters apparently due to the antioxidant effect of the flavonoid compound.
Keywords: Chlorpyriphos, Oxidative stress, Hypophysis, Testes, Antioxidants, Daflon-500
Introduction
The prevalence of infertility has risen by 50% over the last few years, posing global reproductive health challenges [1]. Exposure to environmental chemicals, including pesticides that adversely affect reproductive organs has been linked to impaired fertility in both humans and animals [2, 3]. Recent studies revealed that pesticides are potentially toxic to non-target organisms, including man [4]. Out of the known insecticides, organophosphates (OPs) have gained more popularity for their use in household and agricultural practices [5]. Chlorpyriphos (CP) is one of the most commonly used OP insecticides for domestic and agricultural pest control [6]. Previous findings have shown that CP causes reproductive dysfunction by altering the endocrine levels and causing damages to the reproductive organs [7, 8]. The toxicodynamics of CP, like other OPs, involves perturbation of acetylcholinesterase (AChE) enzyme, with a resultant build-up of acetylcholine (ACh) in tissues and organs. However, oxidative stress induction, characterized by the high production of reactive oxygen species (ROS) is another established non-AChE mechanism of CP toxicity [9, 10].
Oxidative stress observed sequel to a rise in ROS beyond the normal detoxification and neutralization capacities of the body’s intrinsic antioxidant composition results in cellular injury [11]. Several human and animal studies have revealed that antioxidants including flavonoids obtained from medicinal plants possess great potentials for the mitigation of pesticide-induced toxicity [6, 12]. Daflon-500 mg® is a refined flavonoid sourced from the plant Rutaceae aurantiae and contains 450 mg diosmin and 50 mg hesperidin as its active principles [13]. Diosmin and hesperidin are flavonoid compounds with potent oxidant reducing properties beneficial in the management of oxidative stress-induced subfertility [14]. The present study aimed to assess the effect of Daflon-500® on sub-chronic CP-induced alterations in oxidative changes and AChE activity in male albino rats.
Materials and methods
Experimental animals
Twenty-five male albino rats (120–145 g) obtained from the National Veterinary Research Institute (Vom, Nigeria) were used for this study. They were kept in the animal holding facility of the Department of Veterinary Pharmacology and Toxicology, Ahmadu Bello University, Zaria, Nigeria. Rats were fed pellets made commercially from growers marsh (Vital feeds® Ltd). Water was freely made available. Acclimatization was allowed for two weeks before the commencement of the experiment. The experimental procedures were carried out according to the protocol of the Animal Care and Use Committee of the Ahmadu Bello University, Zaria, Nigeria, and following the guide on Laboratory Animal Care [15].
Chemical procurement and composition
Chlorpyriphos 20% EC (Termikill®, Gujurat, India) was diluted in soya oil (Grand Cereals Oil Mills Ltd., Jos, Nigeria) to a 10% solution ready for use. Daflon-500® (Les LaboratoiresServier, France; 500 mg/tablet) was dissolved in 5 ml of distilled water to make a 100 mg/ml daily preparation before dosing.
Sub-chronic reproductive toxicity study
Twenty-five rats were grouped into 5 groups of five rats each. Groups I (DW), II (SO), III (DAF), and IV (CP) were administered distilled water (2 ml/kg), Soya oil (2 ml/kg), Daflon-500® (1000 mg/kg [16]) and CP (7.74 mg/kg [16]), respectively. Group V was previously treated with Daflon-500® at 1000 mg/kg and then exposed to CP (7.74 mg/kg), 30 min later [16]. All regimens were given by gavage once daily for 60 days. Thereafter, the animals were sacrificed by cervical venipuncture following light anesthesia with chloroform.
Sample collection and preparation
The hypophysis and testes were removed, blotted dry, and weighed. Thereafter, 0.3 g of each organ was homogenized in 30 ml of cold phosphate-buffered saline using laboratory pestle and mortar and then centrifuged at 3000×g for 10 min [17]. Supernatants obtained from these homogenates were used to assay for the levels of the hypophysis and testicular MDA, SOD, CAT, and AChE.
Assessment of hypophysis and testicular malondialdehyde concentrations
The MDA concentration as an index of lipid peroxidation was determined in the hypophysis and testes based on the method of Draper and Hadley [17]. The principle was based on the measurement of the color developed as a result of the reaction of thiobarbituric acid (TBA) with MDA.
Assessment of hypophysis and testicular superoxide dismutase and catalase activities
The SOD levels in the hypophysis and testes were evaluated as described by Martin et al [18]. The principle of this test was based on monitoring the autooxidation rate of hematoxylin in an aqueous alkaline solution at 560 nm.
The CAT activity in the hypophysis and testes was evaluated as described by Abei [19]. The principle was based on monitoring and measuring the rate of consumption of hydrogen peroxide (H2O2) substrate at 240 nm.
Assessment of hypophysis and testicular tissue acetylcholinesterase activities
Activities of the hypophysis and testicular AChE enzyme were analyzed as described by Ellman et al. [20], using the Abcam Choline/Acetylcholine Colorimetric Assay Kit (Ab65345), following the manufacturer’s instruction.
Effects of treatment on the hypophysis and testicular histopathology
Histopathological slides of the hypophysis and testicular tissues were prepared using the method described by Luna [21]. The tissue samples were fixed in Bouin’s solution for 48 h, embedded in paraffin, passed through graded concentration of alcohol, and cut at 5 μm using a microtome. The sections were then stained with hematoxylin–eosin dye, which was mounted in a neutral deparaffined xylene medium for a light microscope at a magnification of × 250, and lesions observed were recorded.
Data analysis
Data obtained were expressed as mean ± SEM and statistically analyzed with one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc multiple comparison test. All analyses were conducted using Graphpad Prism version 4.0, San Diego, California, USA (www.graphpad.com), and values of p< 0.05 were recorded as significant.
Results
Hypophysis and testicular tissues malondialdehyde concentrations
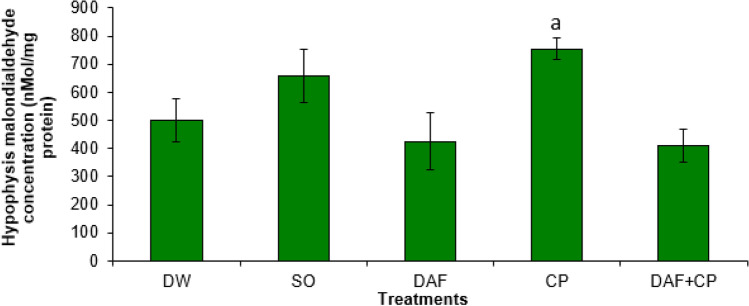
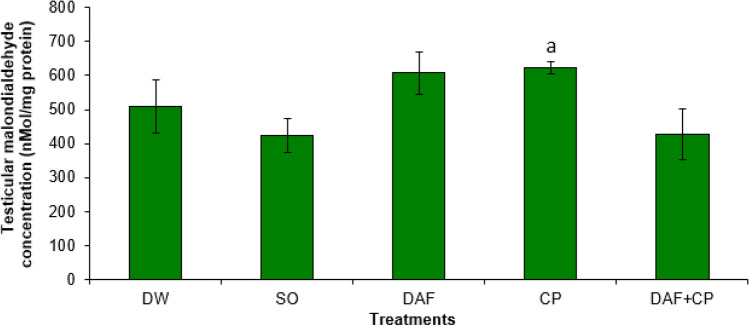
The CP group showed an elevated (p < 0.05) hypophysis MDA concentration compared to that of DAF and DAF + CP groups. However, a non-significant (p > 0.05) change in the hypophysis MDA concentration in the DAF + CP group relative to that of the DW and SO groups was observed (Fig. 1). Figure 2 illustrates an increase (p < 0.05) in testicular MDA level in the CP group compared to that of the DAF + CP group. However, there was no significant (p > 0.05) change in the testicular MDA concentration in the DAF + CP group when compared to that of the DW and SO groups, respectively.
Fig. 1.
Effect of sub-chronic exposure to Daflon-500® and/or chlorpyriphos on hypophysis malondialdehyde concentration in adult male rats. a significantly (p < 0.05) higher when compared to DAF and DAF + CP groups. DW Distilled water, SO Soya oil, DAF Daflon-500®, CP Chlorpyriphos
Fig. 2.
Effect of sub-chronic exposure to Daflon-500® and/or chlorpyriphos on testicular malondialdehyde concentration in adult male rats. a Significantly (p < 0.05) higher when compared to DAF + CP groups. DW Distilled water, SO Soya oil, DAF Daflon 500®, CP Chlorpyriphos
Hypophysis and testicular superoxide dismutase activities
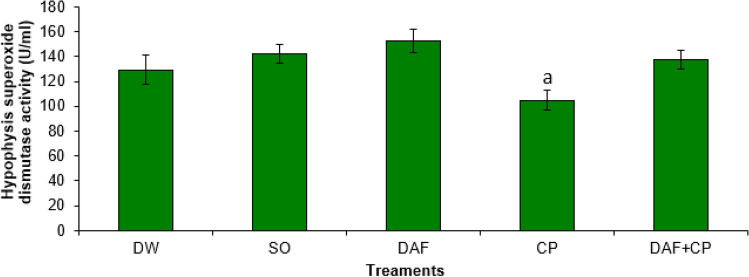
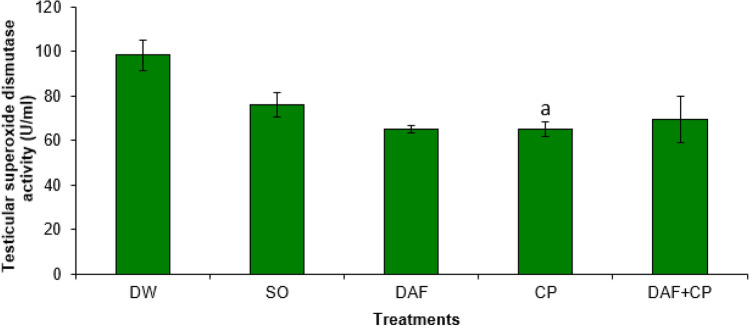
A significantly (p < 0.05) lower SOD activity was observed in the hypophysis of rats in the CP group compared to that of DAF and DAF + CP groups. However, there was no significant (p > 0.05) change in the hypophysis SOD activity in the DAF + CP group compared to that of DW and SO groups (Fig. 3). The testicular SOD activity was significantly (p < 0.05) lower in the CP group relative to that of DW and SO groups. Also, a non-significant change in testicular SOD activity in the DAF + CP group relative to that of the DW, SO, DAF, and CP groups was recorded (Fig. 4).
Fig. 3.
Effect of sub-chronic exposure to Daflon-500® and/or chlorpyriphos on hypophysis SOD activity in adult male rats. a Significantly (p < 0.05) lower when compared to DAF and DAF + CP groups. DW Distilled water, SO Soya oil, DAF Daflon-500®, CP Chlorpyriphos
Fig. 4.
Effect of sub-chronic exposure to Daflon-500® and/or chlorpyriphos on testicular SOD activity in adult male rats. a Significantly (p < 0.05) lower compared to DW and SO groups. DW Distilled water, SO Soya oil, DAF Daflon-500®, CP Chlorpyriphos
Hypophysis and testicular catalase activities
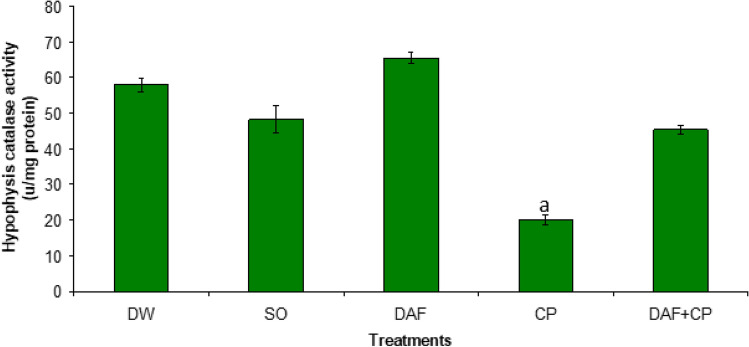
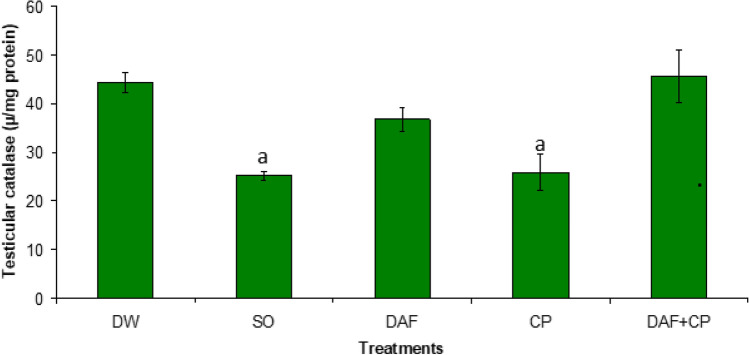
Figure 5 showed a decrease (p < 0.05) in hypophysis CAT activity in the CP group relative to the DW, SO, DAF, and DAF + CP groups. An increase (p < 0.05) in the testicular CAT activity was recorded in the DAF + CP compared to values observed in the CP and SO groups respectively (Fig. 6).
Fig. 5.
Effect of sub-chronic exposure to Daflon-500® and/or Chlorpyriphos on hypophysis catalase activity in adult male rats. a Significantly (p < 0.05) lower compared to DW, SO, DAF, and DAF + CP groups. DW Distilled water, SO Soya oil, DAF Daflon-500®, CP Chlorpyriphos
Fig. 6.
Effect of sub-chronic exposure to Daflon-500® and/or Chlorpyriphos on testicular catalase activity in adult male rats. a Significantly (p < 0.05) lower relative to DW and DAF + CP groups. DW Distilled water, SO Soya oil, DAF Daflon-500®, CP Chlorpyriphos
Hypophysis and testicular acetylcholinesterase activities
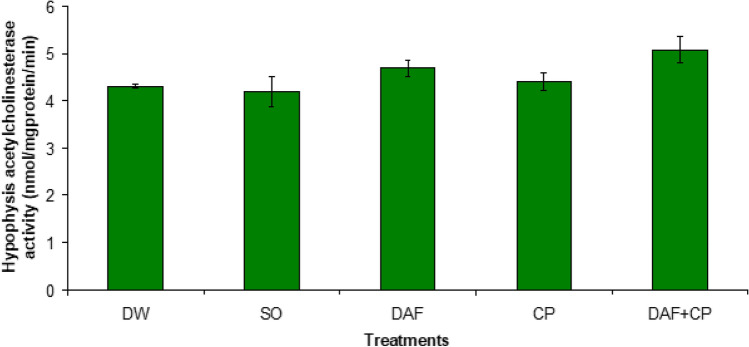
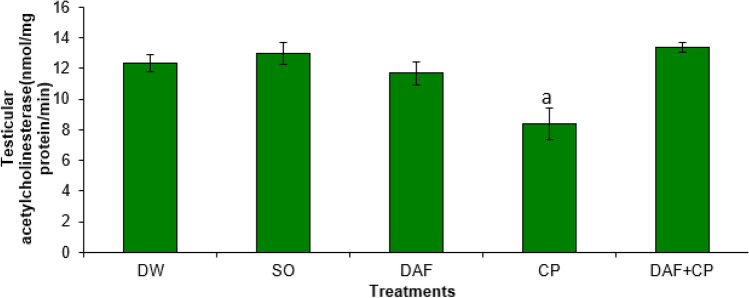
There was no significant difference (p > 0.05) in hypophysis AChE enzyme activities across the groups (Fig. 7). On the other hand, a decreased (p < 0.05) testicular AChE activity was recorded in the CP group compared to the DW, DAF, SO, and DAF + CP groups, respectively (Fig. 8).
Fig. 7.
Effect of sub-chronic exposure to Daflon-500® and/or CP on hypophysis acetylcholinesterase activity in adult male rats. DW Distilled water, SO Soya oil, DAF Daflon-500®, CP Chlorpyriphos
Fig. 8.
Effect of sub-chronic exposure to Daflon-500® and/or CP on testicular acetylcholinesterase activity in adult male rats. a Significantly (p < 0.05) lower when relative to DW, SO, DAF, and DAF + CP groups. DW Distilled water, SO Soya oil, DAF Daflon-500®, CP Chlorpyriphos
Effects of daflon-500® and chlorpyriphos on histo-architecture of organs
Effects of Daflon-500® and chlorpyriphos on histo-architecture of the hypophysis
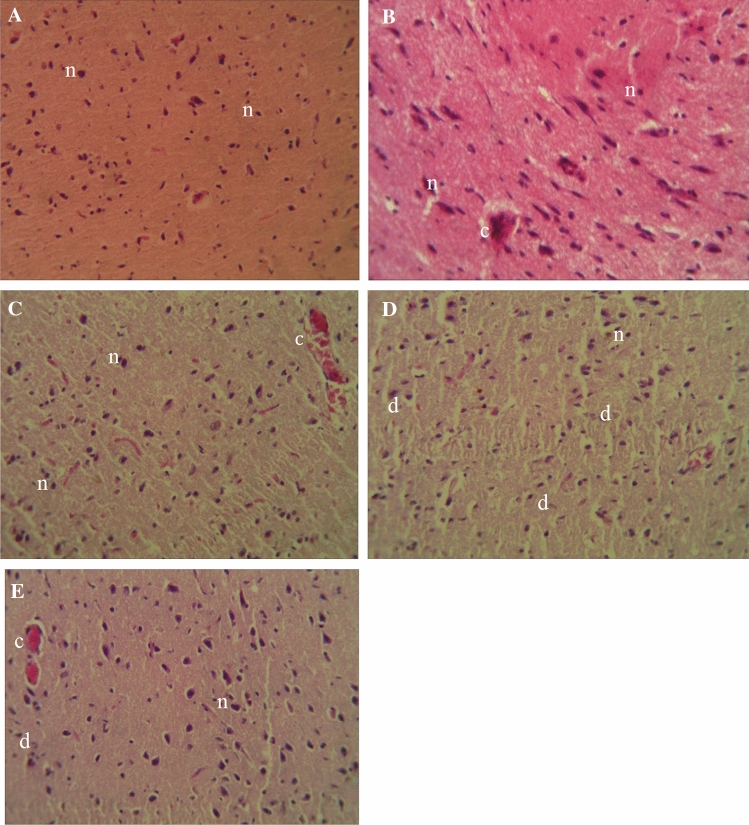
The cytoarchitecture of the hypophysis was normal in the DW, SO and DAF groups (Fig. 9a–c), although mild congestion was observed in the SO and DAF groups. There was a marked increase in the number of degenerated pituicytes within the tissue section of the CP group (Fig. 9d) when compared to the DAF + CP group (Fig. 9e) which had more viable cells.
Fig. 9.
Photomicrograph of sections of the hypophysis of rats exposed sub-chronically to Distilled water (a), Soya oil (b), Daflon-500® (c), Chlorpyriphos (d), and Daflon-500® + chlorpyriphos (e) group showing apparently normal pituicytes (n), congestion (c) and degenerated pituicytes (d); (H and E × 250)
Effects of daflon-500® and chlorpyriphos on histo-architecture of the testes
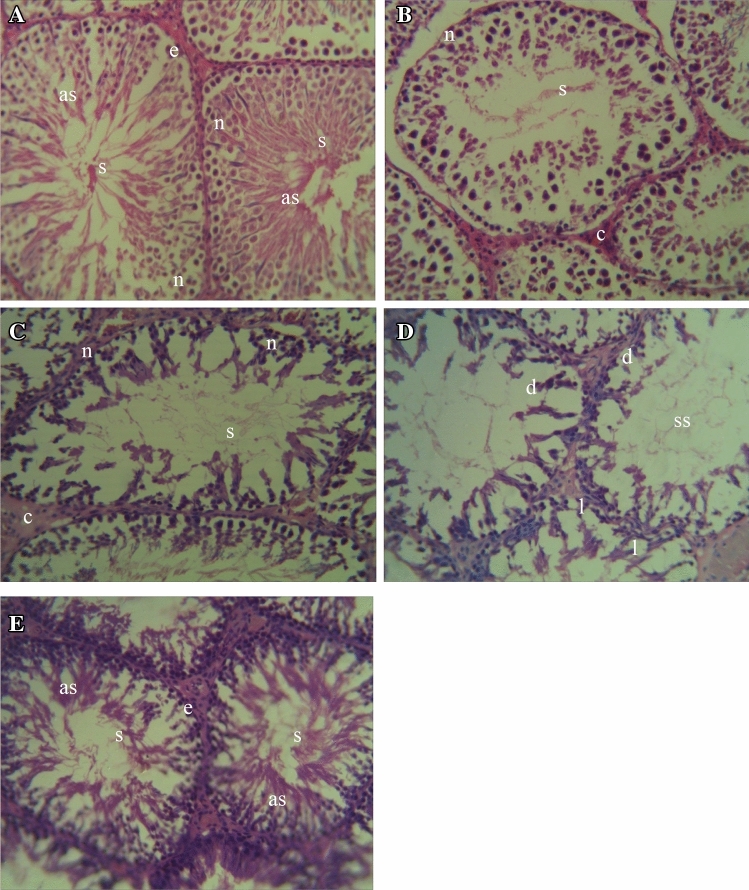
The cytoarchitecture of the testes was apparently normal in the DW, SO and DAF groups (Fig. 10a–c), although congestion was observed within the seminiferous tubules of the SO and DAF groups. The testes of the CP group showed loss of testicular cytoarchitecture, depletion of spermatogenic cells coupled with suboptimal spermatogenic activity (Fig. 10d). There was a relative improvement in the testicular histoarchitecture in the DAF + CP group (Fig. 10e) with relatively intact seminiferous tubules and an apparent increase in the number of normal spermatogenic cells as evidenced by active spermatogenic activity when compared to the CP group.
Fig. 10.
Photomicrograph of sections of the testes of rats exposed sub-chronically to Distilled water (a), Soya oil (b), Daflon-500® (c), Chlorpyriphos (d), and Daflon-500® + chlorpyriphos (e) group showing relatively intact seminiferous tubules (e), spermatozoa (s), active spermatogenic activity (as), normal spermatogenic cells (n), depletion of spermatogenic cells (d), loss of cytoarchitecture (l), suboptimal spermatogenic activity (ss); (H and E × 250)
Discussion
In the present study, the importance of the antioxidant property of daflon-500® following CP exposure is being reported. The increased lipoperoxidative changes recorded in this study as depicted by high MDA levels in the testes and hypophysis of rats exposed to CP reinforced the fact that oxidative stress is an important mechanistic pathway in OP-insecticide poisoning. MDA is an end product of lipid peroxidation produced from an interaction between free radicals and polyunsaturated fatty acid residues within the phospholipid bilayer of cell membranes [6]. The increased MDA concentrations in the hypophysis and testicular tissues observed in the CP group were in agreement with results obtained from previous studies [22, 23]. This reflects a high degree of lipoperoxidative changes, which leads to alterations in the cytoarchitecture, hence functionality of the hypophysis and testis. The reduction in the MDA level in the group pretreated with DAF may be attributed to the antioxidant property of the drug, apparently resulting from its high flavonoid contents, which agree with the preservation of the cytoarchitecture of the hypophysis and testis.
The decreased SOD activity in the hypophysis and testicular tissues observed in the CP group is in agreement with records from earlier studies [22, 24]. SOD is an antioxidant enzyme partly responsible for the elimination of ROS in the body. It does this by speeding the rate of dismutation of superoxide radicals into molecular oxygen and hydrogen peroxide (H2O2) [25]. The lower SOD activity in the hypophysis and testes of rats dosed with CP alone may be attributed to the decrease in the rate of synthesis of the enzyme relative to that of its utilization, or even may be due to its oxidative inactivation after production due to the pro-oxidant effect of the pesticide. However, pretreatment with daflon-500® resulted in increased SOD activity, apparently due to its flavonoid compounds, hesperidin, and diosmin, which possess antioxidant and radical scavenging abilities. These antioxidant flavonoid compounds in Daflon-500® may have assisted in preserving SOD activity since it was not mobilized to counter the CP-evoked elevation of ROS.
The decrease in the hypophysis and testicular CAT activities observed in the CP group agrees with results from previous studies [7, 26]. This may have resulted from excessive H2O2 generated by CP-evoked oxidative stress, which overwhelmed the radical scavenging activities of CAT. It is known that ineffective scavenging of H2O2 results in lower CAT activity [27]. The improvement in CAT activity observed in rats pretreated with DAF may be attributed to the ROS-neutralizing ability of its flavonoid components, leading to a decrease in the extent of H2O2 production.
This present study demonstrated that sub-chronic exposure to CP at the dose given had no significant influence on hypophysis AChE activity. Although inhibition of AChE activity is the main mechanism of OP-induced injuries, this study shows that oxidative injury and toxicity can still occur when an OP compound is given at doses that did not alter AChE activity. However, apart from evoking oxidative stress, studies have shown the ability of CP to provoke cholinergic crisis [9]. This aligns with the result from a previous finding [28], where CP caused injury to organs without significant perturbations in AChE activity. On the other hand, however, testicular AChE enzyme activity significantly decreased in the CP group relative to that of the other groups. The significant decrease in the testicular AChE activity following sub-chronic CP exposure is a demonstration of the widely believed anti-gonadal effect of OP insecticide [6]. Apart from its direct anticholinesterase effect, oxidative stress is also known to play a role in the regulation of AChE activity. The presence of ROS has been observed to disrupt redox processes in the tissues, hence changing the activities of certain enzymes, especially those that are membrane-bound, including AChE [29, 30].
Pretreatment with daflon-500® resulted in the preservation of AChE activity in the testes. This may be linked to the anti-lipoperoxidative activity of its flavonoid components which resulted in the preservation of the structural integrity of the testicular membrane. This is supported by the apparent mitigation of the CP-induced cytotoxic damage to the hypophysis and testes recorded in the DAF pretreated group.
In this study, the potential mechanism of action of daflon-500® is attributed to its flavonoid constituents hesperidin (50 mg) and diosmin (450 mg). Hesperidin has been reported to reduce the generation of ROS in tissues [31] and also stimulates the endogenous antioxidant defense mechanisms [32]. These mechanisms include enhanced activity and production of cellular antioxidant enzymes such as superoxide dismutase, heme oxygenase-1 and catalase coupled with an elevation of the predominant cellular antioxidant called glutathione [33, 34]. Diosmin exerts its antioxidant effect by scavenging the superoxide anions, hence reducing the number of free radicals in circulation [35].
In conclusion, the result from this study demonstrates that sub-chronic exposure to CP causes oxidative injury as demonstrated by increased lipoperoxidation, and reduction in SOD and CAT activities, which was apparently responsible for the cytotoxicity and alterations of the hypophysis and testicular cytoarchitecture in the group exposed to the insecticide only. Similarly, CP exposure caused significantly lower AChE activity in the testes but not in the hypophysis, where there was only an apparent decrease. Pretreatment with Daflon-500® mitigated the oxidative and cellular injury, and AChE inhibition in the hypophysis and testes through its antioxidant and radical scavenging activities. Therefore, the use of daflon-500® may be explored in reducing or mitigating pesticide-induced cytotoxic injury to the reproductive organs in individuals that are constantly exposed to low doses of the insecticide resulting from environmental and occupational exposure.
Acknowledgements
We acknowledged the staff of the Department of Veterinary Pharmacology and Toxicology, Ahmadu Bello University, Zaria especially Mr. D. Otie for his laboratory expertise during the period of this study.
Abbreviations
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- CAT
Catalase
- CP
Chlorpyriphos
- DAF
Daflon-500®
- DW
Distilled water
- H2O2
Hydrogen peroxide
- MDA
Malondialdehyde
- OPs
Organophosphates
- ROS
Reactive oxygen species
- SO
Soya oil
- SOD
Superoxide dismutase
Funding
No funding was received for conducting this study.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
References
- 1.Siegel JS. The demography and epidemiology of human health and aging. Reprod Health. 2012;2:469–531. [Google Scholar]
- 2.Kim J, Shin DH, Lee WJ. Suicidal ideation and occupational pesticide exposure among male farmers. Environ Res. 2014;128:52–56. doi: 10.1016/j.envres.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Neghab M, Momenbella-Fard M, Naziaghdam R, et al. The effects of exposure to pesticides on the fecundity status of farm workers resident in rural region of Fars province, southern Iran. Asian Pac J Trop Bio. 2014;4:324–328. doi: 10.12980/APJTB.4.2014C586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J. Advances in pesticide use in the cocoa belts and perceptions of vegetable farmers. J Hortic. 2015;2:149–152. doi: 10.4172/2376-0354.1000149.. [DOI] [Google Scholar]
- 5.Oates L, Cohen M, Braun L, et al. Reduction in urinary organophosphate pesticides metabolites in adults after a week-long organic diet. Environ Res. 2014;132:105–111. doi: 10.1016/j.envres.2014.03.021.. [DOI] [PubMed] [Google Scholar]
- 6.Ambali SF, Ayo JO, Ojo SA, et al. Chronic chlorpyrifos-induced sensorimotor and cognitive deficit in Wistar rats-reparation by Vitamin C. J Res Environ Sci Toxicol. 2012;1:221–232. [Google Scholar]
- 7.Joshi CS, Mathur R, Gulati N. Testicular toxicity of chlorpyrifos (an organophosphate pesticide) in albino rats. Toxicol Ind Health. 2007;23:439–444. doi: 10.1177/0748233707080908. [DOI] [PubMed] [Google Scholar]
- 8.Shittu M, Olatunji OA, Ambali SF, et al. Ameliorative effect of Hibiscus sabdariffa Linn on subchronic chlorpyrifos-induced alteration in sex and thyroid hormones in male Wistar rats. Am J Pharmacol Toxicol. 2014;9:96–106. doi: 10.3844/ajptsp.2014.96.106. [DOI] [Google Scholar]
- 9.Ambali SF, Ayo JO. Sensorimotor performance deficits induced by chronic chlorpyrifos exposure in Wistar rats: mitigative effect of vitamin C. Toxicol Environ Chem. 2011;93:1212–1226. doi: 10.1080/02772248.2011.585991. [DOI] [Google Scholar]
- 10.Ventura C, Venturino A, Miret N, et al. Chlorpyrifos inhibits cell proliferation through ERK1/2 phosphorylation in breast cancer lines. Chemosphere. 2015;120:343–350. doi: 10.1016/j.chemosphere.2014.07.088. [DOI] [PubMed] [Google Scholar]
- 11.Beam J, Botta A, Barendregt R, et al. Dietary fatty acids, redox signaling, and the heart. In: Laher I, et al., editors. Systems biology of free radicals and antioxidants, part 111. Germany: Springer Verlag; 2014. pp. 1497–1522. [Google Scholar]
- 12.Surai PF, Fisinin VI (2014) Antioxidant systems of the body: From vitamin E to polyphenols and beyond. In: Proceedings of the 35th Western Nutrition Conference. Edmonton, Canada, 24th–25th September 2014, pp 265–277
- 13.Ramelet AA. Clinical benefits of daflon 500 mg in the most severe stages of chronic venous insufficiency. Angiology. 2001;52:49–56. doi: 10.1177/0003319701052001S07. [DOI] [PubMed] [Google Scholar]
- 14.Rizk SM, Sabri NA. Evaluation of clinical activity and safety of daflon 500mg in type 2 diabetic female patients. Saudi Pharm J. 2009;17:199–207. doi: 10.1016/j.jsps.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canadian Council on Animal Care Guide (CACC) (1993) Canadian Council on Animal Care Guide (CACC), 2nd edn
- 16.Olatunji AO (2017) Ameliorative effects of daflon-500® on sub-chronic chlorpyrifos - induced biochemical and reproductive changes in male albino rats. M.Sc. Dissertation, Ahmadu Bello University, Zaria
- 17.Draper HH, Hardley M. Malondialdehyde deformation as an index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-I. [DOI] [PubMed] [Google Scholar]
- 18.Martin JP, Dailey M, Sugarman E. Negative and positive assays of superoxide dismutase based on haematoxylin autoxidation. Arch Biochem Biophys. 1987;225:329–336. doi: 10.1016/0003-9861(87)90400-0. [DOI] [PubMed] [Google Scholar]
- 19.Abei H. Methods in enzymatic analysis. New York: Academic Press; 1974. Catalase; pp. 673–684. [Google Scholar]
- 20.Ellman GL, Courtney KD, Anders V, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 21.Luna GH. Manual of histologic staining method of armed forces institute of pathology. 35. New York: McGraw-Hill Book Company; 1960. p. 46. [Google Scholar]
- 22.El-bendary HM, Saleh AA, Negm SA, et al. Spermatogenic alterations induced by organophosphorus compounds profenofos, chlorpyrifos and synthetic pyrethroid lambada-cyhalothrin in mice. Annu Res Rev Biol. 2014;4:856–873. doi: 10.9734/ARRB/2014/4925. [DOI] [Google Scholar]
- 23.Heikal TM, Abdel-Tawab H, Mossa AW, et al. Oxidative damage and reproductive toxicity associated with cyromazine and chlorpyrifos in male rats: the protective effects of green tea extract. Res J Environ Toxicol. 2014;8:53–67. doi: 10.3923/rjet.2014.53.67. [DOI] [Google Scholar]
- 24.Gawish AM. The protective role of alpha-lipoic acid against pesticide-induced testicular toxicity-Histopathological and Histochemical Studies. J Aquac Res Development 2010;1:1–7. doi: 10.4172/2155-9546.1000101. [DOI] [Google Scholar]
- 25.Gupta RC. Classification and uses of organophosphates and carbamates. In: Gupta RC, editor. Toxicology of organophosphate and carbamate compounds. 1. San Diego: Academic Press (Elsevier); 2006. pp. 5–24. [Google Scholar]
- 26.Mandal TK, Das NS. Correlation of testicular toxicity and oxidative stress induced by chlorpyrifos in rats. Hum Exp Toxicol. 2011;30:1529–1539. doi: 10.1177/0960327110392400. [DOI] [PubMed] [Google Scholar]
- 27.Salman KA, Ashraf S. Reactive oxygen species: A link between chronic inflammation and cancer. Asia Pac J Mol Biol Biotechnol. 2013;21:42–49. [Google Scholar]
- 28.Slotkin TA. Cholinergic systems in the brain development and disruption by neurotoxins: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001.. [DOI] [PubMed] [Google Scholar]
- 29.Molochkina EM, Zorina OM, Fatkullina LD. H202 modifies membrane structure and activity of acetylcholinesterase. Chem Biol Interact. 2005;57:401–404. doi: 10.1016/j.cbi.2005.10.075. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Fuentes G, Rubio-Escalante FJ, Norena-Barroso E, et al. Impacts of oxidative stress on acetylcholinesterase transcription, and activity in embryos of zebrafish (Danio rerio) following Chlorpyrifos exposure. Comp Biochem Physiol. 2015;173:19–25. doi: 10.1016/j.cbpc.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutri. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 32.Khedr NF. Protective effect of mirtazapine and hesperidin on cyclophosphamide-induced oxidative damage and infertility in rat ovaries. Exp Biol Med (Maywood) 2015;240:1682–1689. doi: 10.1177/1535370215576304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamilselvam K, Nataraj J, Janakiraman U, et al. Antioxidant and anti-inflammatory potential of hesperidin against 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced experimental. Int J Nutr Pharm Neurol Dis. 2013;3:294–302. doi: 10.4103/2231-0738.114875. [DOI] [Google Scholar]
- 34.Roohbakhsh A, Parhiz H, Soltani F, et al. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015;124:64–74. doi: 10.1016/j.lfs.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Silambarasan T, Raja B. Diosmin, a bioflavonoid reverses alterations in blood pressure, nitric oxide, lipid peroxides and antioxidant status in DOCA-salt induced hypertensive rats. Eur J Pharmacol. 2012;679:81–89. doi: 10.1016/j.ejphar.2011.12.040. [DOI] [PubMed] [Google Scholar]