Abstract
Peptides play important roles in the diagnosis, prognostic predictors, and treatment of various kinds of cancer. Peptides (p.C, p.L and p.14), derived from the phage display peptide libraries, specifically binds to colorectal cancer (CRC) cells in vitro. To allow tumor specificity and selectivity for in vivo diagnosis of CRC, biotinylated p.C, p.L and p.14 were conjugated to AuNPs (14 nm) via the biotin-streptavidin interaction. Male Wistar rats were intravenously injected with a single dose (100 µg/kg body weight) of AuNPs (citrate-AuNPs, PEG-AuNPs, p.C-PEG-, p.L-PEG- and p.14-PEG-AuNPs). Animals were monitored for behavioral changes, and sacrificed either 14 days or 84 days post-injection. Biochemical changes, oxidative stress, and histology of the liver and colon were assessed. No significant changes were noted in the rats injected with all the AuNPs, except p.L-PEG-AuNPs that caused significant toxicity (p < 0.05) 14 days post-exposure when compared to control group, as evidenced by increased relative liver weight, increased malondialdehyde levels and histological changes in the liver. These changes, however, returned to normalcy 84 days post-injection. It can be concluded, based on these findings, that p.L induced a transient toxicity in rats after a single intravenous injection, and can therefore be considered non-toxic long-term after a single exposure.
Keywords: Colorectal cancer, Diagnosis, Gold nanoparticles, Peptides, Polyethylene glycol
Introduction
Colorectal cancer (CRC), the third most-common cancer worldwide, is among the leading causes of cancer-related deaths globally [1, 2]. Colonoscopy surgery is the most-sensitive diagnostic method and the most-effective treatment option for CRC to date. This procedure has several limitations compared to other methods of screening. These include expensive procedure and side effects such as use of sedation which often affects patients, need for complete bowel cleansing, damage to nearby organs, and high risk of bowel tears and bleeding, most especially during removal of polyps [3, 4]. Chemotherapeutic agents that often follow surgery lack tissue selectivity. The major challenge to CRC treatment is early detection, thereby reducing the incidence and mortality rate [5]. Therefore, there is a need for the development of more-effective, cost-efficient and readily-available tools for early detection of CRC.
The use of nanoparticles has recently expanded into biomedical research. Gold nanoparticles (AuNPs) possess several physicochemical properties that make them well-suited for biomedical applications. These properties include ease of synthesis [6, 7], biocompatibility, and the ability to be conjugated by biomolecules or ligands such as peptides and polyethylene glycol (PEG), for improved stability, tissue targeting and selectivity [8–10]. Conjugation of AuNPs with a targeting molecule (e.g. antibody or peptide) is directed against cancer receptors in tumors [11].
Peptides have been involved in clinical practice, such as in the diagnosis and treatment of cancers, including breast cancer [12], colorectal cancer [13, 14], lung cancer [15, 16], pancreatic cancer [17] and prostate cancer [18]. The use of peptides in nanomedicine is possible due to their ease of synthesis and modification, small size and low molecular weight, good compatibility, specificity, tumor-penetrating and tumor-targeting abilities, high receptor affinity, and low toxic effects on normal tissues [19–23]. Peptides (p.C, p.L and p.14), with amino acid sequences of CVFSSSYSSSG, LTVSPWY, and PDHERPM, respectively, have been shown to specifically bind to cancer cells that overexpress human epidermal growth factor receptor 2 (HER2). These include breast [24], colorectal, ovarian, prostate, stomach and uterine cancers [19]. It has also been reported that p.C and p.L bind to CRC cell lines, HT-29 cells [25] and p.14 to LoVo cells [26], in vitro, which can theoretically be useful in diagnosing CRC in vivo.
In this study, Citrate-capped AuNPs (Cit-AuNPs) was functionalized with biotinylated p.C, p.L and p.14, and stabilized by PEG, to enhance easy penetration and tumor-targeting efficacy. Before developing this tool for CRC diagnosis, it is pertinent to investigate the possible immediate, delayed or continuous toxic effects of the peptide conjugated to AuNPs, which is necessary because of the various conflicting toxicological studies reported [27–30]. Although bulk gold is considered to be bio-inert, various chemicals involved in the synthesis and functionalization of AuNPs could compromise the safe use in medicine. In this study, the effect of AuNPs on the liver and colon specifically was considered. The liver is the major site of metabolism of many xenobiotics. It is the preferred organ of accumulation of nanoparticles, while accumulation in other organs is dependent on the physicochemical characteristics of the nanoparticles, which include the size, shape and type of the nanoparticles [31]. Non-targeted AuNPs was also reported to accumulate mainly in the liver [32]. The AuNPs used is this study is aimed to be used in CRC diagnosis, and it would be necessary to investigate any toxicity towards healthy colon tissue.
A diagnostic procedure requires a single exposure. This study was thus aimed at investigating the short-term (14 days) and long-term (84 days) toxicity of peptide-PEG-AuNPs after a single exposure.
Materials and methods
Materials
Gold (III) chloride trihydrate (HAuCl4.3H2O; 393.83 g/mol), trisodium citrate (Na3C6H5O7·2H2O; 258.06 g/mol), thiobarbituric acid (TBA), trichloroacetic acid (TCA), and diethyl ether were purchased from Sigma (St. Louis, MO, USA). The PEG molecules, 2-(2-[2-(2-{2-[2-(1-mercaptoundec-11-yloxy)-ethoxy]-ethoxy-ethoxy)-ethoxy]-ethoxy)-ethanol (PEG-OH, C23H48O7S, 468.69 g/mol) and N-(2-{2-[2-(2-{2-[2-(1-mercaptoundec-11-yloxy)-ethoxy]-ethoxy)-ethoxy)-ethoxy]-ethoxy)-ethyl) biotinamide (PEG-biotin, C33H63N3O8S2, 694.00 g/mol) were purchased from Prochimia Surfaces (Poland). Alanine transaminase (ALT), aspartate transaminase (AST), albumin, total and direct bilirubin kits were purchased from Randox Laboratories (Crumlin, UK), protein carbonyl assay kit was obtained from Sigma-Aldrich (USA), and total protein assay kit from Thermo Fisher Scientific (USA). All the reagents used were of analytical grade, and Milli-Q water was used in all the experiments.
Synthesis and functionalization of citrate-capped AuNPs
The synthesis of Cit-AuNPs was performed by the citrate reduction (Turkevich-Frens) method [33, 34], and the functionalization steps were completed according to the method of Sosibo et al. [35] with some modifications (Fig. 1).
Fig. 1.

Steps involved in the synthesis of the peptide-PEG-AuNPs
Prior to the synthesis of AuNPs, all glassware used were treated with aqua regia (3:1 HCl:HNO3). The materials were then rinsed with distilled water, followed by Milli-Q water (18.2 MΩ.cm−1 at 25ºC), and oven dried. An aqueous solution of HAuCl4.3H20 (5 mL, 28.95 mM) was brought to boiling in a flask, and stirred continuously with a magnetic stirrer on a hot plate. This was followed by the rapid addition of trisodium citrate (14.5 mL, 38.75 mM) into the flask, resulting in a color change to ruby red within 30 min. This was left at room temperature to cool. The solution was then centrifuged at 12,000×g for 15 min. To remove excess citrate, the resulting pellet was washed with Milli-Q water, and the resulting solution of Cit-AuNPs was stored at 4 °C.
As reported by Sosibo et al. [35] that there was agglomeration when 100% PEG-biotin was used to stabilize AuNPs. In this study, functionalization of the resulting Cit-AuNPs was performed using PEG-OH and PEG-biotin. This was to prevent aggregation of the AuNPs, ensure stability, water solubility, and biological compatibility and reduce non-specific interactions of the nanoparticles with biomolecules [35]. Ethanolic solutions of PEG-OH (18.8 mg/mL, 10.53 µL) and PEG-biotin (8.4 mg/mL, 0.24 µL) were added simultaneously to 20 mL Cit-AuNPs (2 nM,), and the mixture was stirred at 4 °C for 3 h. This was then centrifuged at 12,000×g at 22 °C for 20 min, washed twice with phosphate buffered saline (PBS) to obtain 1% PEG-biotin AuNPs, which contained 99% PEG-OH as a co-stabilizer. The resulting mixture (PEG-AuNPs) was stored at 4 °C in PBS, until further use.
The PEG-AuNPs were further conjugated with biotinylated peptides by first functionalizing it with streptavidin via the streptavidin–biotin chemistry. This was obtained by the addition of 25 µL streptavidin (1 mg/mL) to 800 µL PEG-AuNPs (16 nM), and made up to 2 mL with PBS. The mixture was then incubated overnight at 4 °C after thorough mixing. The mixture was then centrifuged (12,000×g, 22 °C, 20 min) to remove excess streptavidin, and streptavidin-AuNP conjugate was obtained.
An aliquot of each biotinylated peptide (2.94 μL 170 μg/mL p.C, 7.4 μL 70 μg/mL p.L, and 2.54 μL 197 μg/mL p.14) was added to 5 mL streptavidin-AuNP conjugate (12 nM), and incubated overnight at 4 °C, after gentle mixing. The mixture was then centrifuged (12,000×g, 22 °C, 20 min), after making it up to 2 mL with PBS to remove unbound peptide, and the resultant peptide-conjugated AuNPs (p.C-PEG-, p.L-PEG- and p.14-PEG-AuNPs) were stored in PBS at 4 °C until use.
Characterization of AuNPs
Characterization of the synthesized peptide-PEG-AuNPs was performed using the following: UV–Visible spectroscopy (Nanodrop 2000c spectrophotometer) to assess the stability, aggregation levels and concentrations of the nanoparticles; high resolution transmission electron microscopy (HRTEM) (JEOL model 1200 LaB6) to investigate the size and shape of the AuNPs; Zeta potential and the DLS measurements were performed using the Zetasizer Nano ZS (Malvern Instruments) to provide information on the surface charge and polydispersity index (PDI) alongside the hydrodynamic size (Z-average size) of the AuNPs; the Fourier Transform Infrared (FTIR) spectroscopy was performed, using the PerkinElmer FTIR Spectrometer (Spectrum two) to investigate the presence of functional groups on the AuNPs [36].
Animal grouping and treatment
A total of 48 adult male Wistar rats (12 weeks old), weighing between 400 and 420 g, obtained from the animal house of North West University, South Africa, and the SA Vaccine Producers (Animal Unit), South Africa, was used for this study. Animal experimentation was approved by the Nelson Mandela University Research Ethics Committee: Animal, with reference number: A15-SCI-BCM-002, and the animal study conformed to the South African National Standard for the Care and Use of Animals for Scientific Purpose. Animals were divided into eight groups of six rats each, and were acclimatized for 14 days prior to treatment. The rats were fed standard, commercial pelletized rat chow, and given distilled water ad libitum; the animal house was maintained at 22 °C (± 2 °C), with a 12-h light–dark cycle throughout the period of the experiment.
The animal experiment was divided into two phases: phase 1 was designed for short-term acute toxicity study (14 days), while phase 2 was for long-term toxicity study (84 days), after a single exposure, and each phase consisted of four groups. In both phases, group 1 animals received 1 X PBS as the vehicle control while groups 2–6 received a single intravenous injection of 100 µg/kg body weight Cit-, PEG-, p.C-PEG-, p.L-PEG-, p.14-PEG-AuNPs, respectively. The dose used was in the range for low and medium doses (∼0.01 and ∼1.0 mg/kg) in the rat model [37], and fell within the range of the safe doses (3–373 μg/kg) used in the toxicity studies of 14 nm Cit-AuNPs [29]. Equal dosing was ensured, as the dose received was calculated based on the body weight of each rat [38].
During the two-time point studies, assessment of the general health status was done by observing the general behavioral changes, changes in body weights, symptoms of toxicity and mortality throughout the 84 days. Weights of animals were taken 24 h after injection, and then weekly throughout the experiment. Rats were observed for the first 4 h after injection, thereafter over a period of 24 h, and then daily throughout the experiment.
The animals in phases 1 and 2 were sacrificed 14 and 84 days post-treatment, respectively. Animal sacrifice was performed following an overnight fast by quick exposure to diethyl ether. Blood was then withdrawn by cardiac puncture. A portion of blood samples was collected into plain vacutainer tubes, and centrifuged at 3000×g for 10 min for serum collection.
Organs, including the liver, kidneys, brain, heart, spleen, colon, lungs, pancreas, and testes of each of the animals were removed and rinsed in ice-cold physiological saline (0.9% NaCl), blotted with filter paper and weighed. The relative organ weight of each animal was calculated using Eq. 1:
| 1 |
Each organ was divided into separate sections in aluminium foil, snap-frozen in liquid nitrogen, and stored at − 80 °C for further biochemical analyses.
Preparation of liver homogenate
A portion of the liver (1 g) of each rat was homogenized (20% w/v) in ice-cold 1.15% KCl–0.01 M potassium phosphate buffer (pH 7.4) with a Potter–Elvehjem homogenizer (LabSource, USA) Homogenates were centrifuged at 12,000×g at 4 °C for 15 min, and the supernatants were stored at − 80 °C for further biochemical analyses.
Biochemical analyses
Biochemical assays, including serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein, albumin, urea, creatinine, protein carbonyls, and total and direct bilirubin, were determined according to the procedure of commercially-available test kits. Lipid peroxidation was investigated by measuring the formation of malondialdehyde (MDA), according to the method of Varshney and Kale [39].
Statistical analyses
Statistical analyses were performed by one-way analysis of variance (ANOVA), using SPSS software package for Windows (version 16.0, 2007). All results are expressed as Mean ± Standard Deviation (SD). Post-hoc testing was performed for inter-group comparisons using Tukey’s test [40]. In all instances, p values < 0.05 were considered statistically significant.
Results
Characterization of AuNPs
The characteristic resonance peaks of the AuNPs by UV–Vis spectroscopy were in the range of 520–530 nm. The Cit-AuNPs had a wavelength of 520 nm, with slight shifts in the wavelengths of the conjugated AuNPs, as follows: PEG-AuNPs – 522 nm, and p.C-PEG-, p.L-PEG- and p.14-PEG-AuNPs with 524, 524 and 525 nm, respectively (Fig. 2). Spherically-shaped AuNPs, with an average size diameter of 14 ± 1 nm, as determined by HRTEM, were observed (Fig. 3). There was no indication of changes in the shape and sizes of the nanoparticles on conjugation. A thin film (arrow) surrounding the Cit-AuNPs was observed in the peptide-PEG-AuNPs (Fig. 3c–e). The zeta potential measurement showed varied negatively-charged AuNPs, with highest negativity of Cit-AuNPs (Table 1). The polydispersity index of all the AuNPs showed a range of 0.1–0.37, indicating that the AuNPs were being monodispersed, and formed a stable and homogeneous phase. The AuNPs were stable up to 2 months in their respective suspension solutions at 4 °C: Cit-AuNPs – stable in milli-Q water; PEG-AuNPs and p.C-PEG-, p.L-PEG- and p.14-PEG-AuNPs were unstable and aggregated in milli-Q water, but were stable in PBS.
Fig. 2.
UV–Vis spectra of AuNPs showing the surface plasmon resonance shifts
Fig. 3.
Transmission electron micrograph of a Cit-AuNPs (scale bar 10 nm), b PEG-AuNPs (Scale bar 10 nm), c p.C-PEG-AuNPs (Scale bar 10 nm), d p.L-PEG-AuNPs (Scale bar 10 nm), p.14-PEG-AuNPs (Scale bar 20 nm)
Table 1.
Zeta potential and DLS measurements of AuNPs, polydispersity index, and the suspension solution
| AuNPs | Zeta potential (mV) | DLS (nm) | Polydispersity Index | Suspension solution |
|---|---|---|---|---|
| Cit-AuNP | − 31.34 ± 2.37 | 17.94 ± 0.12 | 0.37 ± 0.01 | Milli-Q water |
| PEG-AuNP | − 25.60 ± 1.15 | 19.70 ± 0.20 | 0.19 ± 0.00 | PBS |
| p.C-PEG-AuNP | − 10.90 ± 0.62 | 21.22 ± 0.93 | 0.24 ± 0.01 | PBS |
| p.L-PEG-AuNP | − 9.22 ± 1.22 | 20.93 ± 0.12 | 0.10 ± 0.01 | PBS |
| p.14-PEG-AuNP | − 6.34 ± 1.25 | 22.37 ± 0.29 | 0.25 ± 0.00 | PBS |
Values are reported as Mean ± SD
AuNPs gold nanoparticles, TEM transmission electron microscopy, Cit citrate, PBS phosphate-buffered saline, PEG polyethylene glycol
The FTIR spectra of the AuNPs (Fig. 4) were recorded in the frequency range from 450 to 4000 cm−1 in the % transmittance (%T) mode. Intense peaks were seen at 3390.79, 3370.01, and 3402.77 cm−1, and they correspond to the presence of NH3+ on the amino acid constituent of p.C, p.L, and p.14, respectively. Additional peaks were also seen at 1645, 1649.85 and 1645 cm−1 for p.C-, p.L- and p.14-PEG-AuNPs, respectively, which correspond to the presence of amide bonds in the characteristic vibrational features of proteins. Two peaks at 2985 and 2921 cm−1 on the Cit-AuNPs were noted, corresponding to the presence of alkyl groups (CH2 and CH3). Intense peaks at 1078.01, 1074.06 and 1078.92 cm−1 on p.C-, p.L-, and p.14-PEG-AuNPs were also noted, corresponding to C-O stretching.
Fig. 4.
Fourier transmission infrared spectroscopy of the AuNPs
In vivo acute toxicity
Assessment of general body signs
Rats did not show any signs of adverse reactions or observable toxicity. There were no noted behavioral changes, and no mortalities occurred for the duration of the study.
Effect of AuNPs on food intake and water consumption in rats
During both the 14- and 84-days period (phase 1 and 2, respectively), there were no significant changes in either the food or water intake in the AuNP-treated rats compared to the control group (Data not shown).
Effect of AuNPs on body weights
Figure 5 shows the effect of the AuNPs on the body weights of rats. A single intravenous injection of 100 µg/kg b.w. of the AuNPs did not affect the body weights of rats when compared to the control group, during the 84-days study period (Fig. 5).
Fig. 5.
Body weights (g) of rats during 84 days post-injection of AuNPs
Effects of AuNPs on liver and colon weights
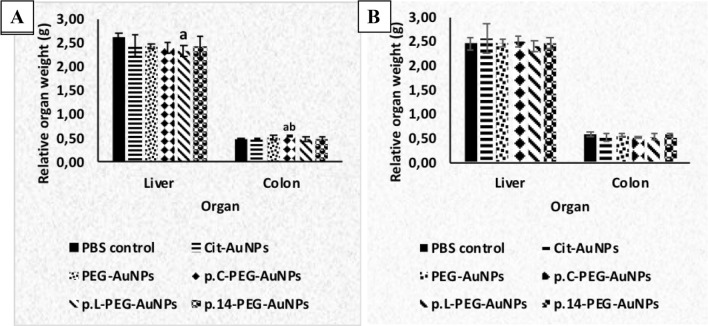
A single intravenous injection of AuNPs did not affect the liver and colon weights of the rats after 14 and 84 days, except the liver, where a significant decrease (p < 0.05) was observed in p.L-PEG-AuNP-treated rats when compared to the control group, at 14 days (Fig. 6a). The weights of the colon of p.C-PEG-AuNP-treated rats significantly increased (p < 0.05) when compared to the control and Cit-AuNP groups at 14 days (Fig. 6a). No significant changes were noted in the organ weights of AuNP-treated rats at 84 days post-exposure.
Fig. 6.
Relative organ weights of rats a 14 days and b 84 days post-injection of AuNPs (per 100 g body weight). Cit citrate, PBS phosphate-buffered saline, AuNPs gold nanoparticles. Values are expressed as Mean ± SD, n = 6. ap < 0.05 when compared to the PBS control group; bp < 0.05 when compared to Cit-AuNP group
Effect of AuNPs on liver marker enzymes
The effect of AuNPs on some liver marker enzymes (ALT, AST and ALP) is presented in Fig. 7. No significant differences (p > 0.05) were noted in the concentrations of serum ALT and AST following a single injection of AuNPs to rats compared to the control at the two-time point studies. However, a significant increase in the level of AST was noted in rats treated with p.L-PEG-AuNPs at 14 days when compared to the p.C-PEG-AuNPs. At 14 days post-treatment, levels of serum alkaline phosphatase were significantly decreased (p < 0.05) in the PEG-, p.C-PEG- and p.L-PEG-AuNPs-treated rats when compared to the control group. No clear trend in the levels of ALP was noted at 84 days. Only p14-PEG-AuNPs presented a significant increase (p < 0.05) in the levels of ALP in rats compared to the p.C-PEG-AuNPs.
Fig. 7.
Effect of a single intravenous injection of AuNPs on serum marker enzymes after a 14 days and b 84 days. Cit citrate, PBS phosphate-buffered saline, AuNPs gold nanoparticles, (/10) actual values divided by 10. Values are expressed as Mean ± SD, n = 6. ap < 0.05, when compared to PBS control group; bp < 0.05, when compared to p.C-PEG-AuNPs group
Hepatic function markers
The effect of a single intravenous injection of AuNPs on albumin and total protein as well as bilirubin is shown in Fig. 8 and Table 2, respectively. No significant (p > 0.05) difference was noted in the levels of albumin and total protein in AuNP-treatment groups when compared to the control group (Fig. 8). At 14 days, a significant (p < 0.05) reduction was noted in the level of total protein in rats treated with p.L-PEG-AuNPs, when compared to Cit-, PEG- and p.14-PEG-AuNPs groups, with a non-significant reduction in comparison to the control group. At day 84, no significant differences were noted in albumin and total protein when compared to the control group. During the two-time point studies, no significant changes in the levels of direct and total bilirubin were noted (Table 2).
Fig. 8.
Effect of AuNPs on the levels of albumin and total protein after a 14 days and b 84 days. Cit citrate, PBS phosphate-buffered saline, AuNPs gold nanoparticles. Values are expressed as Mean ± SD, n = 6. ap < 0.05 when compared to Cit-AuNP group; bp < 0.05 when compared to PEG-AuNPs group; cp < 0.05 when compared to p.L-AuNPs group
Table 2.
Effect of AuNPs on bilirubin levels in rats 14 and 84 days post-exposure
| Treatment | Direct bilirubin (mg/dL) | Total bilirubin (mg/dL) | ||
|---|---|---|---|---|
| 14 days | 84 days | 14 days | 84 days | |
| PBS control | 0.06 ± 0.03 | 0.05 ± 0.02 | 0.19 ± 0.15 | 0.34 ± 0.06 |
| Cit-AuNPs | 0.04 ± 0.02 | 0.08 ± 0.02 | 0.14 ± 0.10 | 0.22 ± 0.08 |
| PEG-AuNPs | 0.04 ± 0.02 | 0.06 ± 0.02 | 0.16 ± 0.09 | 0.30 ± 0.03 |
| p.C-PEG-AuNPs | 0.07 ± 0.03 | 0.06 ± 0.02 | 0.12 ± 0.06 | 0.32 ± 0.04 |
| p.L-PEG-AuNPs | 0.06 ± 0.02 | 0.07 ± 0.02 | 0.12 ± 0.10 | 0.32 ± 0.02 |
| p.14-PEG-AuNPs | 0.06 ± 0.04 | 0.05 ± 0.01 | 0.11 ± 0.08 | 0.28 ± 0.01 |
Values are expressed as Mean ± SD, n = 6
Cit citrate, PBS phosphate-buffered saline, AuNPs gold nanoparticles
Effect of AuNPs on oxidative stress markers
No significant difference was noted in the levels of MDA and protein carbonyls in all the AuNP groups, except a significant (p < 0.05) increase in the level of MDA noted in p.L-PEG-AuNPs-treated rats when compared to the control, Cit-AuNPs and p.14-PEG-AuNP groups at 14 days (Table 3). No significant difference was noted in protein carbonyls in all the AuNPs compared to the control group. However, at day 84, there were no significant differences in the levels of MDA and protein carbonyl in the liver homogenates of AuNP-treated rats compared to the control.
Table 3.
Levels of oxidative stress markers in liver homogenates 14 and 84 days post-exposure
| Treatment | MDA (µmol/mg protein) | Protein carbonyl (nmol/mg protein) | ||
|---|---|---|---|---|
| 14 days | 84 days | 14 days | 84 days | |
| PBS control | 0.16 ± 0.02 | 0.19 ± 0.06 | 2.27 ± 1.01 | 2.31 ± 0.80 |
| Cit-AuNPs | 0.21 ± 0.04 | 0.27 ± 0.10 | 3.15 ± 0.52 | 2.98 ± 1.30 |
| PEG-AuNPs | 0.22 ± 0.04 | 0.18 ± 0.04 | 3.12 ± 1.30 | 2.50 ± 1.36 |
| p.C-PEG-AuNPs | 0.23 ± 0.03 | 0.29 ± 0.08 | 3.92 ± 0.92 | 2.93 ± 0.81 |
| p.L-PEG-AuNPs | 0.30 ± 0.08ab | 0.23 ± 0.06 | 2.96 ± 0.80 | 2.70 ± 0.83 |
| p.14-PEG-AuNPs | 0.20 ± 0.04c | 0.22 ± 0.04 | 2.54 ± 0.70 | 2.75 ± 0.20 |
Values are expressed as Mean ± SD, n = 6. ap < 0.05 when compared to the PBS control group; bp < 0.05 when compared to Cit-AuNPs group; cp < 0.05 when compared to p.L-AuNPs group
Cit citrate, PBS Phosphate-buffered saline, AuNPs Gold nanoparticles
Histology of tissues
Histology of tissues (liver and colon) of all groups of rats in the two-time point study (14 and 84 days post-exposure) was completed (Figs. 9 and 10). In the tissues, no histopathological changes were observed at 14 days post-exposure to the AuNPs, except in p.L-PEG-AuNP-treated rats where some changes were noted in the architectural pattern of the liver (Fig. 9a). In the liver of all the groups, there was congestion of the central vein (CV) and sinusoidal spaces (arrow head), and minimal bile duct (thick arrow) hyperplasia was noted. At day 84, no significant histological changes were observed in both tissues. In rats treated with p.L-PEG-AuNPs (Fig. 9b), there was mild periductal (around bile ducts (BD)), perivascular (around portal vein (PV)) and mononuclear cell infiltration (light arrow) noted in the portal triad configuration, with bile duct hyperplasia (many bile ducts). At day 14, few changes, such as hemosiderin laden macrophages, were noted (Fig. 10a), No histological changes were noted in both liver and colon at 84 days post-exposure (Fig. 10b).
Fig. 9.
Histology of the liver a 14 days, and b 84 days after treatment with AuNPs (Haematoxylin and Eosin, × 400 original magnification, Scale bar = 50 µm). Key: 1 = PBS control; 2 = Citrate-AuNPs; 3 = PEG-AuNPs; 4 = p.C-PEG-AuNPs; 5 = p.L-PEG-AuNPs; 6 = p.14-PEG-AuNPs. CV central vein, thick arrow bile ducts, arrow head sinusoidal spaces, thick arrow chromatin material, BD bile ducts, PV portal vein
Fig. 10.
Histology of the colon a 14 days, and b 84 days post-injection of AuNPs. (Haematoxylin and Eosin, × 400 original magnification, Scale Bar = 50 µm). Key: 1 = PBS control; 2 = Citrate-AuNPs; 3 = PEG-AuNPs; 4 = p.C-PEG-AuNPs; 5 = p.L-PEG-AuNPs; 6 = p.14-PEG-AuNPs. Thick/thin arrow epithelium, C crypts
Discussion
In considering AuNPs for the diagnosis of cancers, which requires a single exposure, acute toxicity studies of the nanoparticles are essential. Acute toxicity studies are, therefore, required to provide first-line information about the short-term effect of these AuNPs on tissues in vivo. Guidelines for the in vivo testing of substances are periodically updated [41]. It should be noted that a single exposure to a substance could result in short-term or long-lasting injury to tissues of an organism, thus, acute toxicity studies are important. Nanoparticles have unique properties including large surface area, small size, easy penetration to cells, the ability to react with biological components, as well as their general in vivo behavior [42]. In this study, a single intravenous injection of conjugated AuNPs to healthy rats was considered to mimic the potential diagnostic application, with various biochemical parameters investigated to provide insight into the possible toxic effect of these AuNPs in the liver and colon of rats 14 and 84 days after exposure.
Characterization studies provide evidence on the physicochemical properties and presence of functional groups on the AuNPs. The characteristic SPR peaks of the AuNPs are in the wavelength range of 520–530 nm (Fig. 2), which is attributed to 14 nm AuNPs [29]. The slight red shift observed in the SPR was caused by the presence of PEG and the peptides on the surface of Cit-AuNPs, with no broadening of peaks, indicating monodispersity of the AuNPs, with no aggregation after conjugation [35]. These results are similar to the study of Sosibo et al. [35] where slight shifts were seen in the SPR peaks of the nanoparticles (520 nm for Cit-AuNPs, 524 nm PEG-AuNPs and 525 nm for TAT-AuNPs).
The tiny film observed in the conjugated AuNPs suggest the presence of additional functional groups and peptides on the surface of the Cit-AuNPs (Fig. 3). Thi Ha Lien et al. [43] reported a similar result, where a thin film was observed surrounding the Cit-AuNPs, indicating the presence of BSA protein on the surface of the nanoparticles. The increased zeta potentials of the conjugated-AuNPs could be linked to the presence of additional functional groups on the Cit-AuNPs [44], such as peptides and PEG.
A slight reduction in the negativity of conjugated AuNPs, compared to Cit-AuNPs, could suggest the presence of polar hydroxyl group on PEG-OH and biotin on PEG-biotin. The presence of NH3+ groups on the peptides could suggest the sharp reduction in the zeta potential values towards neutral. The presence of additional functional groups was also confirmed by increases in the hydrodynamic size of the conjugated-AuNPs with the DLS measurements (Table 1). An increase in the hydrodynamic size of conjugated-AuNPs could result from the presence of PEG and peptides on the surface of Cit-AuNPs. This agrees with studies by Kalmodia et al. [45] and Leopold et al. [46], where significant increases were observed in size distributions on coating of AuNPs with antioxidant peptide (Pep-A) and bovine serum albumin (BSA), respectively. Increased sizes of the AuNPs obtained with the DLS measurement, compared to the size obtained using HRTEM, could be linked to the hydration of the AuNPs during DLS measurements. The polydispersity results showed that the AuNPs were well-dispersed in aqueous media (Table 1), as all the values were less than 0.7 (no agglomeration), which are in the acceptable range described by Jiang et al. [47] and Akrami et al. [48]. Generally, parameters, such as the slight red shifts in wavelength, change in negativity and the presence of additional functional groups revealed the differences between Cit-AuNPs and conjugated-AuNPs [48].
The presence of NH3+ on the amino acid composition of the peptide-AuNPs were in the previously-described range of 3000–3500 cm−1 [45]. Additional characteristic vibrational bands seen on p.C-, p.L- and p.14-PEG-AuNPs (1645.52, 1649.85 and 1645.52 cm−1) correspond to the presence of amide bonds in the characteristic vibrational features of proteins (1500–1700 cm−1) [45, 46]. The two characteristic peaks, corresponding to the alkyl groups (CH2 and CH3) or stretching modes on the AuNPs, was previously described by [45]. These peaks were more pronounced on citrate-AuNPs when compared to PEG-AuNPs and the peptide-AuNPs (Fig. 4). It is suggested that this is due to the loss of the alkyl groups by the washing steps during the synthesis of the peptide-conjugated AuNPs.
The non-significant changes noted in the body weights indicated that the animals were healthy, based on their food and water intake, as well as the injected AuNPs (Fig. 5). This is in agreement with the study of Ghahnavieh et al. [49] in which there was no significant difference in the body weights of mice 14 days after an intraperitoneal injection of 10 nm Cit-AuNPs.
Significant changes in organ weights are regarded as indices of toxicity in animals, as determined by toxicity tests [50]. The unchanged weights of organs in this study is similar to the findings of Zhang et al. [51], which noted no significant changes in organ weights (liver, lungs, kidneys, heart, and spleen) at 28 days post-injection with 5, 10, 30, and 60 nm PEG-AuNPs (4000 µg/kg) in mice. Overall, the unchanged liver and colon weights of rats in the AuNPs groups, compared to the control group, suggest that there was no toxicity to these organs, except the p.L.-PEG-AuNPs with signs of toxicity in the liver at 14 days (Fig. 6).
Alanine aminotransferase and AST are important enzymes used to assess the hepatocellular integrity of the liver (the major metabolic organ). Alanine aminotransferase is predominantly found in the liver while AST is found in equal concentrations in the liver, heart, muscle, kidney and brain [52]. Alkaline phosphatase is a marker of liver or hepatobiliary damage (non-specific) and bone disease, as commonly found on the canalicular membrane of the hepatocytes and in the bone [53]. As these enzymes are present within the hepatocytes, any damage to the hepatocytes would cause leakage of these enzymes from the liver [54, 55].
Elevated levels of ALT, AST, and ALP in the blood thus indicate liver damage, and some other organs. The unchanged levels of these parameters in the serum of rats administered with these AuNPs, when compared to the control group (Fig. 7), could suggest no damage to the liver at both time-points, and is similar to a study by Rambanapasi et al. [29], where no significant changes in the levels of liver damage markers (ALT and ALP) in 14 nm Cit-AuNP-treated rats were noted, even with repeated doses (3–375 µg/kg b.w.) for 7 weeks. However, it must be emphasized that a non-significant and significant increase in the level of AST of rats administered with p.L-PEG-AuNPs) was noted at 14 days post-exposure when compared to the control and Cit-AuNPs, respectively (Fig. 7a).
The predominant isoform of ALP in circulation (in mature rats) originates from the intestine, and are responsive to food intake; that is, decreased total ALP is an indicator of decreased food intake [56, 57]. The observed reduction in ALP levels in all the AuNP-treated groups do not directly correlate with the food intake, and are not linked to food intake in this study. Since neither ALT nor AST were significantly affected by AuNP injections, it is expected that the significant changes of ALP were derived from either bone or hepatobiliary (though not specific) sources, which need further investigation.
Albumin, a major component of total protein, is an indicator of hepatocellular function [53]. Any marked reduction in serum albumin levels indicates an impairment of liver function. The non-significant changes in the levels of albumin and total protein noted in the AuNPs-treated groups in our study suggests normal synthesis of proteins by the liver, as described by Hanafy et al. [54]. P.L-PEG-AuNP was the only AuNP that showed a trend towards reduced protein synthesis (Fig. 8). It was also noted that the liver weights and ALP concentrations were significantly reduced in rats treated with p.L-PEG-AuNP, with a trend towards increased serum AST levels. However, these observed changes were reversed after long-term exposure.
Bilirubin is a product of haemoglobin breakdown, which is normally excreted via the bile and urine. As a marker of hepatocellular function, elevation in the levels of total and direct bilirubin in the serum indicates hepatobiliary and hepatic function disorders [58]. This increase may result from over-production of bilirubin through haemolysis and decreased liver uptake [59, 60]. Elevations in both direct and total bilirubin could also result from decreased conjugation and reduced secretion from the liver, or as a result of blockage of bile ducts [61]. In this study, the unchanged levels of direct and total bilirubin (Table 2) noted suggested no hepatobiliary disorders. These results are similar to a study by Rambanapasi et al. [29], where no significant changes in the level of total bilirubin in 14 nm Cit-AuNPs-treated rats were noted when compared to the control group. The dose (100 µg/kg b.w.) used in the current study falls within the range of the doses (3–375 µg/kg b.w.) of 14 nm Cit-AuNPs administered by Rambanapasi et al. [29].
Peroxidation of lipids, mediated by free radicals, as a result of xenobiotics and environmental pollutants or during pathology, cause cell damage and disrupt the biological membrane, leading to the release of liver marker enzymes into the extracellular fluid [54]. Treatment of rats with p.L-PEG-AuNPs induced the expression of oxidative stress markers (Table 3), suggesting an increased potential of cellular damage. There was, however, no significant increase in the levels of protein carbonyl and, therefore, only minor cellular damage is expected, which was reversed after 84 days. The result of the study differs from that of Abdelhalim et al. [62], where there was noted significant differences in the levels of oxidative stress markers in rats injected intraperitoneally with unconjugated AuNPs when compared to the control group. The disparities could be linked to differences in the size of AuNPs (10 nm), route of administration, duration of exposure (3 or 7 days), as well as surface chemistry.
The central vein congestion and sinusoidal spaces, noted in all the groups, could suggest intrahepatic haemodynamic forces prevalent at necropsy. At 84 days post-exposure, histological assessment of the liver in all AuNP-treated rats were consistent with the biochemical analyses and oxidative stress markers investigated, as no significant changes were noted when compared to the control group. Safe use of these peptides in the diagnosis of CRC is suggested as there were no significant changes in the liver and colon sections of the AuNPs-treated rats at 84 days post-exposure when compared to the control.
In addition to changes in the liver weight, biochemical analyses and histopathological examinations of the liver validated the transient toxic effect of p.L.-PEG-AuNPs. However, there were no histological changes associated with the increased colon weight noted in p.C.-PEG-AuNPs at 14 days.
The toxicity study of 14 nm AuNPs (100 µg/kg b.w.), 14 days after a single intravenous injection to rats, suggests that neither the citrate capping nor the PEG (stabilizing molecule) would contribute towards toxicity of the AuNPs, and should be considered in the synthesis and functionalization of AuNPs. Overall, it can be suggested based on the two-time point toxicity study that p.L-PEG-AuNPs caused an immediate toxic effect without a delayed or continuous effect, with no toxicity in p.C-PEG- and p.14-PEG-AuNPs observed. These could thus be considered a potential agent for use in the diagnosis of CRC in vivo. Further studies on the investigation of various organ toxicities, and the receptors on CRC tumors responsible for the specificity should be performed.
Acknowledgements
This work was supported by the National Research Foundation, South Africa [Grant Number 101132] to Olusola B. Adewale. The authors also acknowledge the technical assistance by Mr. Lawyer Mabulu and Ms. Jabu Madubedube of the Department of Biochemistry and Microbiology, Nelson Mandela University, South Africa, and Prof. M. Meyer of University of the Western Cape, South Africa, for donating the peptides used.
Declarations
Conflict of interests
The authors declare no conflict of interest with this work. This work is contained in the PhD thesis of Olusola B. Adewale at Nelson Mandela University, South Africa. Part of this work has been presented at the 14th International Conference of Nanosciences and Nanotechnologies, 4–7 July 2017, Thessaloniki, Greece, and LAUTECH NANO Conference, 22–24 October 2019, Ogbomoso, Nigeria.
References
- 1.Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–732. doi: 10.1038/s41575-019-0189-8. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society . Colorectal cancer facts and figures. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 4.American Cancer Society . Colorectal cancer facts and figures 2017–2019. Atlanta: American Cancer Society; 2017. [Google Scholar]
- 5.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elia P, Zach R, Hazan S, et al. Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int J Nanomedicine. 2014;9:4007–4021. doi: 10.2147/IJN.S57343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakor AS, Jokerst J, Zavaleta C, et al. Gold nanoparticles: a revival in precious metal administration to patients. Nano Lett. 2011;11(10):4029–4036. doi: 10.1021/nl202559p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkilany AM, Abulateefeh SR, Mills KK, et al. Colloidal stability of citrate and mercaptoacetic acid capped gold nanoparticles upon lyophilization: effect of capping ligand attachment and type of cryoprotectants. Langmuir. 2014;30(46):13799–13808. doi: 10.1021/la504000v. [DOI] [PubMed] [Google Scholar]
- 9.Bohl Kullberg E, Bergstrand N, Carlsson J, et al. Development of EGF-conjugated liposomes for targeted delivery of boronated DNA-binding agents. Bioconjug Chem. 2002;13(4):737–743. doi: 10.1021/bc0100713. [DOI] [PubMed] [Google Scholar]
- 10.Goodman CM, McCusker CD, Yilmaz T, et al. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate Chem. 2004;15(4):897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 11.Arvizo RR, Miranda OR, Moyano DF, et al. Modulating Pharmacokinetics, Tumor uptake and biodistribution by engineered nanoparticles. PLoS ONE. 2011;6(9):e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haggag YA, Matchett KB, Dakir E-H, et al. Nano-encapsulation of a novel anti-Ran-GTPase peptide for blockade of regulator of chromosome condensation 1 (RCC1) function in MDA-MB-231 breast cancer cells. Int J Pharm. 2017;521(1–2):40–53. doi: 10.1016/j.ijpharm.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Comstock SS, Xu D, Hortos K, et al. Association of insulin-related serum factors with colorectal polyp number and type in adult males. Cancer Epidemiol Biomark Prev. 2014;23(9):1843–1851. doi: 10.1158/1055-9965.EPI-14-0249-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabinsky EF, Joshi BP, Pant A, et al. Overexpressed Claudin-1 can be visualized endoscopically in colonic adenomas in vivo. Cell Mol Gastroenterol Hepatol. 2016;2(2):222–237. doi: 10.1016/j.jcmgh.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Guan S, Sun S, et al. Detection of circulating antibodies to linear peptide antigens derived from ANXA1 and DDX53 in lung cancer. Tumor Biol. 2014;35(5):4901–4905. doi: 10.1007/s13277-014-1643-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Li X, Zhang X, et al. Association of serum hemoglobin A1c, C-peptide and insulin-like growth factor-1 levels with the occurrence and development of lung cancer. Mol Clin Oncol. 2014;2(4):506–508. doi: 10.3892/mco.2014.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy R, Zurakowski D, Wischhusen J, et al. Urinary TIMP-1 and MMP-2 levels detect the presence of pancreatic malignancies. Br J Cancer. 2014;111(9):1772–1779. doi: 10.1038/bjc.2014.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han Z, Zhou Z, Shi X, et al. EDB fibronectin specific peptide for prostate cancer targeting. Bioconjugate Chem. 2015;26(5):830–838. doi: 10.1021/acs.bioconjchem.5b00178. [DOI] [PubMed] [Google Scholar]
- 19.Michalska M, Florczak A, Dams-Kozlowska H, et al. Peptide-functionalized ZCIS QDs as fluorescent nanoprobe for targeted HER2-positive breast cancer cells imaging. Acta Biomater. 2016;35:293–304. doi: 10.1016/j.actbio.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Shapira S, Fokra A, Arber N, et al. Peptides for diagnosis and treatment of colorectal cancer. Curr Med Chem. 2014;21(21):2410–2416. doi: 10.2174/0929867321666140205134616. [DOI] [PubMed] [Google Scholar]
- 21.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20(1):122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Thundimadathil J. Cancer treatment using peptides: current therapies and future prospects. J Amino Acids. 2012;2012:13. doi: 10.1155/2012/967347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marqus S, Pirogova E, Piva TJ. Evaluation of the use of therapeutic peptides for cancer treatment. J Biomed Sci. 2017;24(1):21. doi: 10.1186/s12929-017-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shadidi M, Sioud M. Selective targeting of cancer cells using synthetic peptides. Drug Resist Updat. 2003;6(6):363–371. doi: 10.1016/j.drup.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Mazyambe MK. Evaluating the specificity of cancer cell targeting peptides for applications in cancer diagnostics. Cape Town: University of the Western Cape; 2013. [Google Scholar]
- 26.Wang J-J, Liu Y, Zheng Y, et al. Screening peptides binding specifically to colorectal cancer cells from a phage random peptide library. Asian Pac J Cancer Prev. 2012;13(1):377–381. doi: 10.7314/APJCP.2012.13.1.377. [DOI] [PubMed] [Google Scholar]
- 27.Ferchichi S, Trabelsi H, Azzouz I, et al. Evaluation of oxidative response and tissular damage in rat lungs exposed to silica-coated gold nanoparticles under static magnetic fields. Int J Nanomed. 2016;11:2711–2719. doi: 10.2147/IJN.S103140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan HA, Abdelhalim MAK, Alhomida AS, et al. Effects of naked gold nanoparticles on proinflammatory cytokines mRNA expression in rat liver and kidney. BioMed Res Int. 2013;2013:590730. doi: 10.1155/2013/590730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rambanapasi C, Zeevaart JR, Buntting H, et al. Bioaccumulation and subchronic toxicity of 14 nm gold nanoparticles in rats. Molecules. 2016 doi: 10.3390/molecules21060763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uchiyama MK, Deda DK, Rodrigues SF, et al. In vivo and in vitro toxicity and anti-inflammatory properties of gold nanoparticle bioconjugates to the vascular system. Toxicol Sci. 2014;142(2):497–507. doi: 10.1093/toxsci/kfu202. [DOI] [PubMed] [Google Scholar]
- 31.Yahyaei B, Nouri M, Bakherad S, et al. Effects of biologically produced gold nanoparticles: toxicity assessment in different rat organs after intraperitoneal injection. AMB Express. 2019;9(1):38. doi: 10.1186/s13568-019-0762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thovhogi N, Sibuyi N, Meyer M, et al. Targeted delivery using peptide-functionalised gold nanoparticles to white adipose tissues of obese rats. J Nanopart Res. 2015;17(2):112. doi: 10.1007/s11051-015-2904-x. [DOI] [Google Scholar]
- 33.Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Faraday Discuss. 1951;11:55–75. doi: 10.1039/DF9511100055. [DOI] [Google Scholar]
- 34.Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241:20–22. doi: 10.1038/physci241020a0. [DOI] [Google Scholar]
- 35.Sosibo N, Keter F, Skepu A, et al. Facile attachment of TAT peptide on gold monolayer protected clusters: synthesis and characterization. Nanomaterials. 2015;5(3):1211–1222. doi: 10.3390/nano5031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh S, Patil S, Ahire M, et al. Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J Nanobiotechnol. 2012;10(1):1–9. doi: 10.1186/1477-3155-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Z, Monteiro-Riviere NA, Kannan R, et al. A computational framework for interspecies pharmacokinetics, exposure and toxicity assessment of gold nanoparticles. Nanomedicine. 2016;11(2):107–119. doi: 10.2217/nnm.15.177. [DOI] [PubMed] [Google Scholar]
- 38.OECD . Guidance on sample preparation and dosimetry for the safety testing of manufactured nanomaterials. Paris: OECD; 2012. [Google Scholar]
- 39.Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990;58(5):733–743. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 40.Alalaiwe A, Roberts G, Carpinone P, et al. Influence of PEG coating on the oral bioavailability of gold nanoparticles in rats. Drug Deliv. 2017;24(1):591–598. doi: 10.1080/10717544.2017.1282554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.OECD . Test no. 425: acute oral toxicity: up-and-down procedure. Paris: OECD Publishing; 2008. [Google Scholar]
- 42.Schmid G, Kreyling WG, Simon U. Toxic effects and biodistribution of ultrasmall gold nanoparticles. Arch Toxicol. 2017;91(9):3011–3037. doi: 10.1007/s00204-017-2016-8. [DOI] [PubMed] [Google Scholar]
- 43.Thi Ha Lien N, Thi Tuyen N, Emmanuel F, et al. Capping and in vivo toxicity studies of gold nanoparticles. Adv Nat Sci. 2012;3(1):015002. [Google Scholar]
- 44.Verissimo TV, Santos NT, Silva JR, et al. In vitro cytotoxicity and phototoxicity of surface-modified gold nanoparticles associated with neutral red as a potential drug delivery system in phototherapy. Mater Sci Eng C. 2016;65:199–204. doi: 10.1016/j.msec.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 45.Kalmodia S, Vandhana S, Tejaswini Rama BR, et al. Bio-conjugation of antioxidant peptide on surface-modified gold nanoparticles: a novel approach to enhance the radical scavenging property in cancer cell. Cancer Nanotechnol. 2016;7(1):1. doi: 10.1186/s12645-016-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leopold LF, Todor IS, Diaconeasa Z, et al. Assessment of PEG and BSA-PEG gold nanoparticles cellular interaction. Colloid Surface A. 2017;532:70–76. doi: 10.1016/j.colsurfa.2017.06.061. [DOI] [Google Scholar]
- 47.Jiang W, Hibbert DB, Moran G, et al. Characterisation of gold agglomerates: size distribution, shape and optical properties. RSC Adv. 2013;3(20):7367–7374. doi: 10.1039/C3RA22727H. [DOI] [Google Scholar]
- 48.Akrami M, Balalaie S, Hosseinkhani S, et al. Tuning the anticancer activity of a novel pro-apoptotic peptide using gold nanoparticle platforms. Sci Rep. 2016;6:31030. doi: 10.1038/srep31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghahnavieh MZ, Ajdary M, Ghahnavieh MZ, et al. Effects of intraperitoneal injection of gold nanoparticles in male mice. Nanomed J. 2014;1(3):121–127. [Google Scholar]
- 50.Adewale OB, Onasanya A, Anadozie SO, et al. Evaluation of acute and subacute toxicity of aqueous extract of Crassocephalum rubens leaves in rats. J Ethnopharmacol. 2016;188:153–158. doi: 10.1016/j.jep.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X-D, Wu D, Shen X, et al. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int J Nanomed. 2011;6:2071–2081. doi: 10.2147/IJN.S21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dollah MA, Parhizkar S, Latiff LA, et al. Toxicity effect of Nigella sativa on the liver function of rats. Adv Pharm Bull. 2013;3(1):97–102. doi: 10.5681/apb.2013.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18–35. doi: 10.1038/ajg.2016.517. [DOI] [PubMed] [Google Scholar]
- 54.Hanafy A, Aldawsari HM, Badr JM, et al. Evaluation of hepatoprotective activity of Adansonia digitata extract on acetaminophen-induced hepatotoxicity in rats. Evid-Based Complement Altern Med. 2016;2016:4579149. doi: 10.1155/2016/4579149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abou Seif HS. Physiological changes due to hepatotoxicity and the protective role of some medicinal plants. Beni-Suef Univ J Basic Appl Sci. 2016;5(2):134–146. doi: 10.1016/j.bjbas.2016.03.004. [DOI] [Google Scholar]
- 56.Ramaiah SK. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol. 2007;45(9):1551–1557. doi: 10.1016/j.fct.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 57.York MJ. Clinical pathology. In: Faqi AS, editor. A comprehensive guide to toxicology in preclinical drug development. 1. Cambridge: Academic Press; 2013. pp. 168–206. [Google Scholar]
- 58.Okokon JE, Simeon JO, Umoh EE. Hepatoprotective activity of the extract of Homalium letestui stem against paracetamol-induced liver injury. Avicenna J Phytomed. 2017;7(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- 59.Kunjiappan S, Bhattacharjee C, Chowdhury R. Hepatoprotective and antioxidant effects of Azolla microphylla based gold nanoparticles against acetaminophen induced toxicity in a fresh water common carp fish (Cyprinus carpio L.) Nanomed J. 2015;2(2):88–110. doi: 10.7508/nmj.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Sefi M, Ben Amara I, Troudi A, et al. Effect of selenium on methimazole-induced liver damage and oxidative stress in adult rats and their offspring. Toxicol Ind Health. 2014;30(7):653–669. doi: 10.1177/0748233712462445. [DOI] [PubMed] [Google Scholar]
- 61.Hussein RH, Khalifa FK. The protective role of ellagitannins flavonoids pretreatment against N-nitrosodiethylamine induced-hepatocellular carcinoma. Saudi J Biol Sci. 2014;21(6):589–596. doi: 10.1016/j.sjbs.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdelhalim MA, Al-Ayed MS, Moussa SA. The effects of intraperitoneal administration of gold nanoparticles size and exposure duration on oxidative and antioxidants levels in various rat organs. Pak J Pharm Sci. 2015;28(2):705–712. [PubMed] [Google Scholar]