Abstract
The mechanism of transport of polycyclic aromatic hydrocarbons (PAHs) by Pseudomonas fluorescens LP6a, a PAH-degrading bacterium, was studied by inhibiting membrane transport and measuring the resulting change in cellular uptake. Three cultures were used: wild-type LP6a which carried a plasmid for PAH degradation, a transposon mutant lacking the first enzyme in the pathway for PAH degradation, and a cured strain without the plasmid. Washed cells were mixed with aqueous solutions of radiolabelled PAH; then the cells were removed by centrifugation, and the concentrations of PAH in the supernatant and the cell pellet were measured. The change in the pellet and supernatant concentrations after inhibitors of membrane transport (azide, cyanide, or carbonyl cyanide m-chlorophenyl hydrazone) were added indicated the role of active transport. The data were consistent with the presence of two conflicting transport mechanisms: uptake by passive diffusion and an energy-driven efflux system to transport PAHs out of the cell. The efflux mechanism was chromosomally encoded. Under the test conditions used, neither uptake nor efflux of phenanthrene by P. fluorescens LP6a was saturated. The efflux mechanism showed selectivity since phenanthrene, anthracene, and fluoranthene were transported out of the cell but naphthalene was not.
Extensive research has been carried out on the metabolic pathways and kinetics of biodegradation of polycyclic aromatic hydrocarbons (PAHs) (1, 3) and on the fate of these contaminants in the environment and in bioremediation systems (1). PAHs have high octanol-water partition coefficients (Kow) and partition readily into organic phases (12). The ordering of phospholipids in bacterial membranes creates a hydrophobic region in the interior of the membrane that can act as a reservoir for accumulation of hydrophobic compounds. The partitioning of hexane (22), other alkanes (13), and PAHs (19) into membrane vesicles has been measured, and the partition coefficients correlated with Kow values (8, 19). However, since physiological properties of the membrane vary with environmental conditions and are different in different bacteria, the correlations differ between studies (20).
Little is known, however, about how PAHs travel across bacterial membranes to reach cytoplasmic metabolic enzymes. This question is of particular interest given the high affinity of PAHs for adsorption to soil and their extremely low aqueous solubility. Studies on alkane-degrading organisms have reported microstructural modifications associated with growth on hydrocarbons, including modifications of intracellular membranes and the formation of hydrocarbon inclusions within the cells (17, 18). Active uptake has been proposed for degradation of alkanes such as n-hexadecane in order to explain these observations (6). The limited research on the transport of PAHs, based primarily on work on naphthalene uptake in two different strains of Pseudomonas, suggests that there are two different transport mechanisms. Bateman et al. (2) studied the association between naphthalene and Pseudomonas putida by filtration of cells from naphthalene solutions and flow dialysis. Naphthalene readily partitioned into the cells, where it was degraded, and the rate of degradation was not affected by inhibitors of membrane transport, such as cyanide or azide. These results were consistent with diffusion of the naphthalene across the cell membranes, followed by intracellular metabolism. In contrast, Whitman et al. 23 argued that naphthalene was subject to active uptake in Pseudomonas fluorescens Uper 1. This strain was an active naphthalene degrader, and experiments were conducted with incubation times of up to 4 h. Given the length of the experiments and the active degradation of the substrate by the cells as a growth substrate, the experimental results combined the effects of transport, metabolism, and growth, making conclusions about the role of active transport difficult. In addition, the Kow of naphthalene is sufficiently different from the Kow of the majority of the PAHs that results obtained with naphthalene may not be directly extrapolated to PAHs with much higher Kow.
Efflux pumps provide a means to remove toxic compounds such as toluene from the cell interior. The relative ease with which these compounds permeate cell membranes requires high efflux rates for the target compounds (5). Recently, the active efflux of organic solvents and its relationship to multidrug efflux have received considerable attention. Like multidrug-resistant bacteria, organic solvent-tolerant bacteria are able to transport a broad range of compounds by active efflux mechanisms. When an inhibitor of active membrane processes, such as azide or cyanide, was added to P. putida, the intracellular concentrations of toluene, xylene, benzene, and 1,2,4-trichlorobenzene increased relative to the concentrations in uninhibited cells (5, 16). Another common characteristic of solvent-tolerant bacteria is enhanced resistance upon induction of the gene for the efflux mechanism. Induced P. putida S12 cells accumulated 50% less toluene than noninduced cells accumulated, and the induced cells were also able to survive toluene shock loads (5). The srpABC operon for solvent efflux in P. putida S12 was induced by such solvents as toluene, benzene, xylene, ethylbenzene, aliphatic solvents, and ethanol (10). Similarly, toluene resistance in P. putida DOT T1E was enhanced upon induction of active efflux (16). Results also suggested that there was a low level of constitutively expressed organic solvent efflux in this strain.
A correlation between antibiotic resistance and organic solvent resistance has been observed. Pseudomonas aeruginosa K1112, an antibiotic-resistant bacterium, was also resistant to hexane and xylene, with resistance to toluene developing after 72 h of incubation with toluene (11). P. putida S12 displayed resistance to antibiotics after induction of the efflux pump by organic solvents (10). Studies on multidrug-resistant bacteria have indicated that there are genetic similarities in the efflux pumps of different bacteria (15). The connection between organic solvent resistance and multidrug efflux suggests that there is a generalized efflux mechanism for toxic compounds that may extend to PAHs.
The objective of this study was to examine the mechanism of uptake of the PAH compounds naphthalene, anthracene, phenanthrene, and fluoranthene by P. fluorescens LP6a. Phenanthrene was selected as a representative PAH for a majority of the studies. P. fluorescens LP6a was selected because of its ability to degrade several PAHs, the genes for which are carried on a plasmid (7). A mutant and a cured strain of LP6a that lacked the ability to degrade PAHs enabled us to study uptake and efflux independent of degradation. Toluene was used to compare the known solvent efflux systems (8–10). The initial PAH concentrations used were below the aqueous solubility limit in order to avoid complications due to kinetics of dissolution from the solid phase or partitioning from an inert solvent.
MATERIALS AND METHODS
Chemicals.
Phenanthrene (99.9% pure) and fluoranthene (98% pure) were obtained from Aldrich (Milwaukee, Wis.). Anthracene (98% pure) and naphthalene (99% pure) were obtained from Sigma Chemical Co. (St. Louis, Mo.). Toluene (99.8% pure) was obtained from Fisher Scientific (Fair Lawn, N.J.). [9-14C]phenanthrene, [side ring U-14C] anthracene, and [methyl-14C]toluene were obtained from Amersham (Arlington Heights, Ill.). [U-14C]naphthalene and [3-14C]fluoranthene were obtained from Sigma Chemical Co. Sodium azide (Fisher Scientific), potassium cyanide (Allied Chemical, New York, N.Y.), and carbonyl cyanide m-chlorophenylhydrazone (CCCP) (Sigma Chemical Co.) were used as inhibitors of transport activities.
Bacterial strains and growth conditions.
P. fluorescens LP6a (wild type), transposon mutant strain 1 (LP6a-1)(7), and a cured strain were used for transport studies. The wild-type strain is capable of metabolizing a range of PAHs. Both LP6a-1, which is blocked in the ability to produce naphthalene dioxygenase enzymes (7), and the cured strain (7) were unable to degrade PAHs. Cultures of the LP6a strains were grown in 100 ml of tryptic soy broth (TSB) (Difco Laboratories, Detroit, Mich.) supplemented with 25 μg of kanamycin (Sigma Chemical Co.) per liter for transposon maintenance in LP6a-1. The cultures were grown at 30°C for 24 h on a rotary shaker at 250 rpm; then the cells were harvested by centrifugation at 8,000 × g for 15 min, washed in 100 ml of 0.1 M phosphate buffer (pH 7), and again collected by centrifugation at 8,000 × g for 15 min. The cells were resuspended in 0.1 M phosphate buffer (pH 7) to give an optical density at 600 nm (OD600) of 1.0.
Azotobacter vinelandii UW (W. Page, University of Alberta) was selected as a representative gram-negative organism lacking PAH metabolic activity and was used as a negative control for PAH efflux. A. vinelandii UW was grown on sterile Burke's buffer with glucose and nitrogen, which contained (per liter of solution) 0.2 g of KH2PO4, 0.8 g of K2HPO4, 0.2 g of MgSO4 · 7H2O, 0.1 g of CaSO4 · 2H2O, 0.005 g of FeSO4 · 7H2O, and 0.00025 g of Na2MoO4 · 2H2O. The cells were grown at 30°C for 24 h on a rotary shaker at 250 rpm, collected, and resuspended in the same manner as the LP6a strains. Since isoleucine is actively transported into Staphylococcus epidermidis ATCC 155, this strain was used as a positive control to confirm that the method of adding an inhibitor and then removing the cell pellet by centrifugation was sensitive enough to demonstrate the presence of active cell transport. S. epidermidis was grown overnight in TSB at 37°C on a rotary shaker at 250 rpm. The overnight culture was diluted to an OD600 of 0.8 in fresh TSB and grown for an additional 3 h at 37°C with shaking. The cell suspension was then washed, resuspended in 33 mM phosphate buffer (pH 7) to an OD600 of 2.1, and shaken at 30°C for 3 h to ensure that all cells were starved for a carbon source.
Glassware treatment.
Due to nonreproducible, high levels of adsorption of PAHs to untreated glassware detected in preliminary experiments, the glassware was treated with chromic acid overnight, rinsed with water, and then treated with concentrated nitric acid overnight. After a final rinse with water, the resulting glassware gave repeatable partitioning of PAH between the glassware and the aqueous phase.
Transport experiments.
For transport experiments, 20 ml of a cell suspension having an OD600 of 1 was mixed with nonlabeled and radiolabeled PAHs in a 250-ml Erlenmeyer flask. Nonlabeled PAHs were prepared as concentrated solutions in ethanol, and then a radiolabeled PAH was added to give 100,000 dpm per 20 ml of final cell suspension. A solution of PAH in ethanol was added (<30 μl) to obtain the desired concentration that was less than the aqueous solubility of the PAH. The experiment started when a PAH was added to the cells. At timed intervals, 1.0-ml samples of cell suspension were pipetted into sterile 1.5-ml microcentrifuge tubes and clarified in a microcentrifuge by centrifugation at 16,000 × g for 20 s. A 0.5-ml sample of each supernatant was pipetted into 10 ml of aqueous scintillation fluor (ACS; Amersham). The remaining supernatant was decanted. The cell pellet was resuspended in 1.0 ml of 0.1 M phosphate buffer (pH 7), and 0.5 ml was counted in 10 ml of ACS by using a Beckman LS3801 liquid scintillation counter. All transport experiments were conducted in triplicate flasks at room temperature.
Two sets of transport experiments were conducted: one in which an inhibitor was added at either 8.5 or 9.5 min and one in which an inhibitor was not added. The time used for addition of the inhibitor allowed the concentrations to stabilize and was between the times used for removing samples for analysis. In preliminary trials, sodium azide concentrations ranging from 10 to 120 mM were tested to determine the minimal effective concentration for inhibition of efflux. Thereafter, azide was typically added at a concentration of 30 mM, which was sufficient to inhibit active transport (4, 21) but was unlikely to affect short-term enzyme activity (14). Each experiment was performed in triplicate with a single batch of cell suspension adjusted to an OD600 of 1.0.
Phenanthrene was used at a final concentration of 6.4 μM, which is less than the aqueous solubility of this compound (7.2 μM) (12). Partitioning was studied by using lower concentrations (2.1, 3.5, and 5.0 μM). Fluoranthene and anthracene were used at concentrations of 1.2 and 0.2 μM, respectively (90% of the aqueous solubility limits). Naphthalene and toluene were used at concentrations of 5.7 and 640 μM, respectively, concentrations which were well below their aqueous solubilities in order to avoid toxicity and (in the case of naphthalene) to ensure complete and rapid dissolution. Abiotic control experiments were performed with 20 ml of sterile 0.1 M potassium phosphate buffer (pH 7) in 250-ml Erlenmeyer flasks. Due to the volatility of naphthalene and toluene, transport studies with these substrates were conducted in 35-ml serum vials sealed with Teflon stoppers. Sampling was accomplished with syringes and 18-gauge needles.
Competitive transport studies.
Competition between transport of phenanthrene and transport of other PAHs was studied by the pulse-chase method. [14C]phenanthrene was added at time zero, and then at 9.5 min a second, unlabeled PAH was added after the phenanthrene had been taken up. Experiments were performed with and without addition of 120 mM azide to cell suspensions 30 min prior to measurement of transport in order to inhibit efflux. Naphthalene was used at a concentration of 5.7 μM, anthracene was used at a concentration of 0.27 μM, and fluoranthene was used at a concentration of 1.2 μM. When naphthalene was used, experiments were conducted in closed serum vials to prevent volatilization.
Analysis of spent medium.
The approximate total concentrations of aromatic compounds (phenanthrene and metabolites) were determined by UV-visible spectroscopy at 254 nm with a Philips PU 8740 UV-visible spectrophotometer. The concentration of phenanthrene was determined with a high-performance liquid chromatograph (HPLC) by using a Waters M-45 HPLC pump, a Waters 75 WISP autosampler, and a Waters 486 tunable absorbance detector set at 254 nm. A Spherisorb 10 RP-18, 10-μm-mesh, reverse-phase column (100 by 4.6 mm) was used for component separation. The mobile phase contained 60% acetonitrile and 40% water, and the flow rate was 2 ml/min. Comparison with authentic standards allowed us to determine concentrations.
RESULTS
Verification of method.
Due to its hydrophobicity and low aqueous solubility, phenanthrene adsorbed to the surfaces of glassware, pipettes, and centrifuge tubes even at concentrations well below its solubility in water. Recovery of [14C]phenanthrene from abiotic controls containing 6.36 μM was independent of time during a 25-min experiment, with 68.3% ± 0.9% recovery in the supernatant without centrifugation. Recovery dropped to 40.6% ± 1.3% (95% confidence interval) when the additional centrifugation step was included. The abiotic losses due to sorption were very rapid, as partitioning was complete within 1 min, the first sampling time. Rinsing the flasks, pipette tips, and centrifuge tubes with methylene chloride after sampling resulted in approximately 100% recovery of phenanthrene, indicating that the losses were reversible and likely due to sorption to surfaces. Losses due to volatilization of phenanthrene were insignificant.
Centrifugation of a suspension of wild-type strain LP6a at an initial OD600 of 1 for 20 s at 16,000 × g resulted in recovery of 95% of the cells in the pellet, based on measuring the OD600 of the supernatant. The short centrifugation time allowed rapid recovery and analysis of the supernatant and the cells, but it did result in a loose cell pellet with associated interstitial liquid. Since hydrophobic compounds such as phenanthrene partition into cell membranes much more than water does, this interstitial liquid contributed negligible error.
In negative control experiments we used A. vinelandii UW to ensure that the phenanthrene concentrations measured were not sensitive to inhibitors in the absence of active transport processes. One test was conducted in the absence of azide, and two tests were done in the presence of 120 mM azide added at 8.5 min. Neither the cell pellet nor the supernatant phenanthrene concentrations changed when azide was added. The pellet contained 32% ± 1% of the [14C]phenanthrene radiolabel before and after addition of azide, and the supernatant contained 24 ± 1%. Consequently, addition of azide had no effect on the partitioning of phenanthrene between the cells and the supernatant in this control organism lacking PAH efflux.
Positive control experiments demonstrated the effect of inhibitors on the active uptake of isoleucine by starved cells of S. epidermidis. The control starved cells incubated without glucose or an inhibitor contained a constant level of 10.3% of the total label during the 45-min experiment. When glucose was added as an energy source for uptake, 43.1% of the [14C]isoleucine was found in the cell pellet after 25 min, but when 40 mM azide was added at 8.5 min, the concentration of [14C]isoleucine in the cells decreased to the level in the control and a corresponding increase in the supernatant concentration was observed due to inhibition of active uptake processes. The total recovery of label was 100% ± 2% (n = 3) in all cases. The assay method used, therefore, could detect changes in active cell transport due to addition of inhibitors.
Transport of phenanthrene.
The transport of phenanthrene was studied by using suspensions of LP6a-1 and the cured strain at an OD600 of 1.0 and an initial phenanthrene concentration of 6.36 μM. These strains lacked the ability to metabolize phenanthrene; therefore, partitioning of radiolabel was not affected by the formation of polar metabolites. For suspensions of LP6a-1, an OD600 of 1.0 corresponded to 1.9 × 109 ± 0.4 × 109 CFU ml−1, a dry cell weight of 380 ± 30 μg/ml, and a protein concentration of 186 ± 15 μg/ml. The results for the LP6a wild-type strain were equivalent at an OD600 of 1.0. All gravimetric measurements were calculated on a cell dry weight basis, so that pellet concentrations are presented below in micromoles per gram (dry weight) of cells.
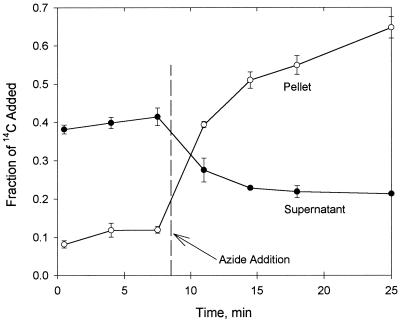
Figure 1 shows the distribution of radiolabel added as [14C]phenanthrene in the LP6a-1 cell pellet and supernatant as a fraction of the total 14C label added. The cell suspensions exhibited no significant change in concentration with time until azide was added at 8.5 min. A separate control without azide showed no significant change with time over the 30-min experiment (data not shown). Steady-state concentrations of phenanthrene were achieved by the time that the first sample was processed and analyzed (approximately 1 to 2 min); therefore, uptake was very rapid. When azide was added, the cell pellet concentration increased immediately to 11.0 ± 0.5 μmol/g, and the supernatant concentration decreased to 1.4 ± 0.1 μM. This response to inhibition of active membrane processes was consistent with interruption of active efflux. The opposite trend would be expected if phenanthrene was transported by active uptake. Equivalent results were obtained with the inhibitors cyanide (10 mM) and CCCP (50 μM) (data not shown).
FIG. 1.
Distribution of radiolabel (added as [9-14C]phenanthrene) in the P. fluorescens LP6a-1 cell pellet and supernatant over time. The data points are means based on three independent experiments. The error bars (where visible) for supernatant and pellet data represent 1 standard deviation. The time when azide was added is indicated.
The transport experiments were repeated with a plasmid-free strain of LP6a in order to determine whether the genes for phenanthrene efflux were plasmid-borne, as the metabolic genes are (7). The results were equivalent to the data shown for LP6a-1 in Fig. 1. After addition of azide, the concentration of phenanthrene in the supernatant decreased from 2.4 ± 0.1 to 1.5 ± 0.1 μM, while the cell pellet concentration increased from 3.1 ± 0.2 to 7.2 ± 0.5 μmol/g. These results suggest that the efflux genes are chromosomally encoded and not linked to the genes for biodegradation of PAHs.
Role of phenanthrene concentration during partitioning.
The data in Fig. 1 were obtained at an initial phenanthrene concentration of 6.36 μM. The same procedure was repeated with initial concentrations of phenanthrene ranging from 2.12 to 6.36 μM in order to study the response of cell transport to the initial solute concentration. In every case, the time course of phenanthrene transport (data not shown) was equivalent to the data in Fig. 1, indicating that efflux was not saturated over the three-fold range of concentrations used. The volume of cells was less than 1% of the total volume of the system, yet when azide was present, 65% of the total phenanthrene was localized in the cell fraction. This partitioning was consistent with the hydrophobicity of phenanthrene and its high affinity for lipid membranes.
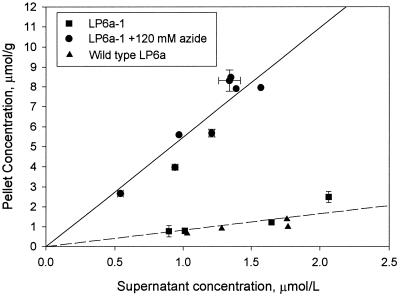
The cell pellet concentrations of phenanthrene in LP6a-1 after 25 min are illustrated in Fig. 2 for initial supernatant concentrations ranging from 2.12 to 6.36 μM. The data show linear partitioning of phenanthrene with and without azide present, and the slopes of the curves give the partition coefficients. The partition coefficient for phenanthrene between the cell pellet and the supernatant increased from 0.78 ± 0.14 to 5.6 ± 0.7 liters/g when azide was added. This seven-fold increase in the partition coefficient was consistent with inhibition of active efflux.
FIG. 2.
Partitioning of phenanthrene between the cell pellet and the supernatant in LP6a-1 and wild-type strains. The error bars (where visible) represent 95% confidence intervals based on three to five independent measurements. Calculations for the wild-type strain are discussed in the text.
The equilibrium partition coefficient (i.e., the coefficient with azide present) was compared to partitioning in phospholipid membranes by assuming that a typical cell is 1 μm in diameter and 3 μm long and the inner and outer cell membranes are 4 nm thick (22). If the phenanthrene partitioned only into the membranes, the gravimetric partition coefficient of 5.6 ± 0.7 liters/g was approximately 1.2 × 104 liters/liter on a membrane volume basis (i.e., micromoles per liter of membrane divided by micromoles per liter of supernantant). In comparison, Sikkema et al.(19) obtained a value of 4,937 ± 86 liters/liter for partitioning of phenanthrene into phospholipids from Escherichia coli. The two values differ by a factor of only 2.5, which is reasonable agreement given the assumptions used to calculate the partition coefficient for the membranes of LP6a.
Transport of phenanthrene with concurrent metabolism.
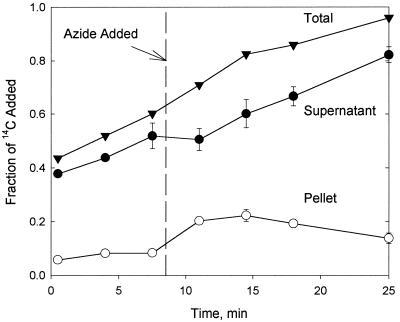
To observe the effects of concurrent metabolism on transport, the uptake experiments described above were repeated with the LP6a wild-type strain (Fig. 3), which is competent for phenanthrene metabolism. In contrast to the results obtained with the mutant, the wild-type supernatant 14C concentration did not reach a steady state before or after azide addition, although there was a significant inflection in the time course as an immediate response to azide addition. The 14C concentration in the pellet reached a steady state prior to azide addition; then it increased significantly (P < 0.01) and then declined slowly. The total recovery of 14C from the azide-treated culture increased throughout the incubation period, reaching 97% by 25 min. These data are consistent with progressive formation of water-soluble phenanthrene metabolites during incubation. The transient increase in pellet-associated 14C and the simultaneous inflection in supernatant 14C can be explained by transient accumulation of parent phenanthrene in the cells due to inhibition of efflux by azide. The subsequent decrease in the pellet concentration and the continued increase in the supernatant 14C level resulted from continued metabolism of membrane-associated phenanthrene (replenished by passive diffusion) and release of polar 14C-labeled metabolites into the supernatant. Transformation of the parent PAH to polar metabolites was reflected in the increasing, high total recovery of radiolabel, as glass-sorbed phenanthrene continued to partition into the aqueous phase and then into the cells and was released as water-soluble metabolites. In the absence of azide (data not shown), there was a continuous increase in the supernatant 14C concentration over 25 min with no inflection point, which represented continuing uptake and metabolism of phenanthrene by the wild type with release of radiolabeled metabolites. Pellet concentrations were constant over 25 min.
FIG. 3.
Distribution of radiolabel (added as [9-14C]phenanthrene) in the P. fluorescens LP6a wild-type cell pellet and supernatant over time. The data points are means based on three independent experiments. The total is the sum of the data for a cell pellet and a supernatant. The error bars (where visible) for supernatant and pellet data represent 1 standard deviation. The time when azide was added is indicated.
The amounts of soluble phenanthrene remaining after incubation with and without azide were quantified by HPLC analysis of methylene chloride extracts of the supernatants and compared with the 14C results (see above). Parallel abiotic control extractions yielded complete recovery of phenanthrene. LP6a-1 supernatants contained 2.56 ± 0.8 μM phenanthrene as determined by HPLC analysis, compared to 2.5 ± 0.2 μM as determined by scintillation counting. Thus, no metabolism occurred, and all of the radiolabel in the supernatant represented parent phenanthrene. In contrast, the wild-type supernatants yielded only 0.39 ± 0.36 μM phenanthrene as quantified by HPLC, compared to 5.6 ± 0.4 μM as determined by scintillation counting, indicating that the majority of the radiolabel in the wild-type supernatant after 25 min was not in phenanthrene but rather was in water-soluble metabolites.
The presence of acidic phenanthrene metabolites was determined by solvent extraction of replicate LP6a wild-type supernatants adjusted to pH 2 or 7. The absorbance values at 254 nm for the acidic extracts were 1.9 ± 0.2 (95% confidence interval; n = 5), compared to 0.80 ± 0.29 (n = 4) for the neutral extracts. Therefore, acidic aromatic compounds were produced by the wild type during incubation. In contrast, the absorbance values for acidic and neutral extracts of LP6a-1 supernatants were equivalent, giving concentrations of 2.7 ± 1.1 and 2.8 ± 0.6 μM, respectively, which is consistent with recovery of parental phenanthrene and the absence of acidic metabolites.
In order to estimate the initial partitioning of phenanthrene between the cells and the supernatant before conversion of radiolabel to metabolites, the concentrations in Fig. 3 were extrapolated to time zero. The same experiment was repeated with lower initial phenanthrene concentrations (as low as 2.12 μM), and the resulting initial cell and supernatant concentrations are shown in Fig. 2. The partition coefficients for phenanthrene in the wild-type and mutant LP6a strains without addition of azide were the same (0.68 ± 0.18 and 0.78 ± 0.14 liter/g [dry weight], respectively). This observation supports the conclusion that the response of the wild-type cells was due to metabolism superimposed on efflux behavior.
Induction of phenanthrene transport.
To determine if induction with the transport substrate could enhance rates of active efflux, an overnight culture of LP6a-1 was suspended at an OD600 of 1.5 in 100 ml of 0.1 M phosphate buffer saturated with phenanthrene and incubated for 2 h at 22°C. The cells were collected, washed, and resuspended to an ODM600 of 1.0 in phosphate buffer, and then they were exposed to 6.36 μM 14C-labeled phenanthrene. The cell concentration was 2.63 ± 0.28 μmol/g without phenanthrene preincubation during a 25-min experiment, compared to 2.45 ± 0.22 μmol/g with preincubation; therefore, phenanthrene was not an inducer of the efflux system.
Transport of other PAHs.
Data from LP6a-1 experiments with anthracene and fluoranthene were equivalent to the data in Fig. 1 in that addition of azide increased the concentration of 14C in the cell pellet (Table 1) and resulted in a corresponding decrease in the supernatant concentration. For example, the supernatant concentration of anthracene was 0.096 ± 0.006 μM prior to azide addition and statistically lower (P < 0.01), 0.062 ± 0.003 μM, after azide addition. The wild-type LP6a strain does not metabolize fluoranthene (7); consequently, with both the wild type and LP6a-1 there was a 2.3-fold increase in the concentration in the cell pellet upon addition of azide.
TABLE 1.
Concentrations of aromatic compounds in P. fluorescens LP6a-1 cell pellets with and without addition of 11mM azide
| Compound | Initial concn (μM) | Concn in cell pellet at 25 min (μmol/g[dry wt])a
|
Significance (P value) | |
|---|---|---|---|---|
| Control (no azide) | Inhibited (azide added at 9.5 min) | |||
| Toluene | 644 | 25 ± 8 | 28 ± 4 | NSb |
| Naphthalene | 5.66 | 0.81 ± 0.08 | 0.81 ± 0.02 | NS |
| Phenanthrene | 6.36 | 2.8 ± 0.3 | 7.2 ± 0.4 | <0.001 |
| Anthracene | 0.20 | 0.11 ± 0.01 | 0.21 ± 0.01 | <0.001 |
| Fluoranthene | 1.16 | 0.65 ± 0.02 | 1.57 ± 0.12 | <0.001 |
Data are means ± 1 standard deviation based on three independent trials.
NS, values are not significantly different.
The transport experiments with naphthalene and toluene were conducted in closed 35-ml serum vials to avoid volatilization losses, and the method was verified by using phenanthrene. The concentrations of naphthalene and toluene in the pellet and supernatant were not changed by the addition of azide at 9.5 min (Table 1). Therefore, naphthalene was not subject to active efflux. The wild-type and LP6a-1 strains are not able to grow on toluene vapor as the sole carbon source; therefore, metabolism of toluene was unlikely during the uptake experiment. Only 1.5% of the total toluene partitioned into the cells; therefore, the scintillation counts for the cell pellets were only 1.5 to 2 times greater than the background level. The accuracy of these results was less than the accuracy of the results for the PAHs; therefore, these data provide an estimate of the partitioning of toluene into LP6a but not a definitive conclusion concerning active efflux of this compound.
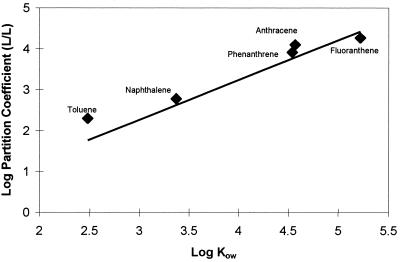
The partition coefficient for each aromatic compound into LP6a-1 when azide was added was calculated by determining the ratio of the pellet concentration to the supernantant concentration. The log partition coefficients were linearly correlated with the Kow for each compound, like the correlation made by Sikkema et al. (19) for PAHs in phospholipids from E. coli (Fig. 4). The agreement between the experimental data and the correlation data suggests that the uptake of aromatic compounds into LP6a was dominated by simple membrane partitioning when azide was present to inhibit active efflux from the cells.
FIG. 4.
Relationship between PAH partition coefficients of LP6a-1, measured in the presence of azide (i.e., in the absence of active efflux), and the corresponding Kow values (12). Partition coefficients were calculated by determining the ratio of pellet concentration to supernatant concentration (see text). The straight line represents the correlation calculated by Sikkema et al. (19).
Competitive transport of aromatic compounds.
The data in Fig. 2 suggest that the partitioning of phenanthrene was linear in uninhibited cells, which suggests that the efflux pump was not saturated over the range of concentrations tested. Competition between aromatic compounds was studied by adding a second PAH to LP6a-1 cells in a steady state with phenanthrene. No significant change in the phenanthrene concentration in the cell pellet or the supernatant was observed after anthracene, fluoranthene, or naphthalene was added (data not shown). The same result was observed in the presence of azide when anthracene was the potential competitor, indicating that equilibrium partitioning in the absence of efflux was noncompetitive at the concentrations tested. The lack of saturation of active efflux indicates either that the transport system has a large capacity relative to the solubility of PAHs or that LP6a has multiple pumps, each of which handles a different substrate(s).
DISCUSSION
The data presented here indicate that P. fluorescens LP6a uses passive transport to take up PAHs such as phenanthrene and a chromosomally encoded active efflux system to regulate the concentration of cell-associated PAHs. While efflux systems for monocyclic aromatic compounds (5) and hydrophobic antibiotics (15) have been reported previously, this is the first report of a system for efflux of tricyclic PAHs. Both phenanthrene and naphthalene are carbon sources for wild-type strain LP6a, yet phenanthrene is subject to active efflux and naphthalene is not. In contrast, fluoranthene, which is not a growth substrate, is actively transported out of the cell. These observations suggest that the efflux system of LP6a may select for compounds with a high Kow (>104). It is not clear whether PAH metabolites are effluxed in the same manner; certainly, they are found in the cell-free supernatant as they are produced, as shown in Fig. 3.
The wild-type, LP6a-1, and cured strains of P. fluorescens LP6a showed the same qualitative response to addition of azide. In every case, the pellet concentration increased and the supernatant concentration decreased compared to cultures without azide. These findings suggest that phenanthrene partitioned into the cell membranes by passive diffusion and was transported back into the medium by an active efflux pump. The efflux pump maintained the intracellular concentration of phenanthrene below its equilibrium level. When this pump was inhibited, the phenanthrene concentrations in the pellet approached equilibrium levels. Similar phenomena have been observed in other cases of active efflux. For example, when active efflux in a multidrug-resistant P. aeruginosa strain was inhibited with CCCP, the concentrations of drugs in the cells increased dramatically (15). The ability of P. putida to export toluene by an efflux mechanism was inhibited by CCCP and cyanide, so that the levels of toluene in the cell pellet rose to the same levels as those in cells without efflux capability (9).
The presence of an active efflux pump in LP6a appears to conflict with the efficient metabolism of PAH compounds, because an efflux pump reduces substrate concentrations within the cell. Unless the metabolic enzymes are saturated, one would expect that higher metabolic rates would result from higher intracellular substrate concentrations. Based on limited data, the supernatant concentrations shown in Fig. 3 suggest that the rates of transformation of [14C]phenanthrene to radiolabeled metabolites were similar before azide was added and during the last 14 min of the experiment. This observation suggests that efflux did not have a significant effect on the rate of phenanthrene transformation.
The fact that the efflux process is chromosomally encoded suggests that it is not coupled to plasmid-encoded PAH biodegradation. The active efflux mechanism could play an important role in resistance to toxicity of hydrophobic compounds by controlling intracellular and membrane concentrations, similar to the control in previously described solvent-resistant strains (5, 16). An important difference between the P. fluorescens LP6a results and the results of previous studies of P. putida (5, 10, 16) is that the efflux mechanism did not require induction in order to be effective. Phenanthrene did not induce efflux, and cells grown on hydrocarbon-free medium were able to efflux PAH immediately (Fig. 1 and 3). These results do not rule out the possibility that other inducers exist.
The lack of saturation of phenanthrene uptake by LP6a-1 in the presence of inhibitory levels of azide (Fig. 2) and the lack of competition in the uptake of aromatic compounds in the presence of azide suggest that uptake into the cell membranes by diffusion dominates the partitioning process. This observation was reinforced by uptake of a series of aromatic compounds, which followed a linear relationship in a log-log plot with the Kow (Fig. 4). The lack of saturation of efflux in this study does not imply that this pathway has an unlimited capacity, but it was not saturated at the concentrations tested, which were below the aqueous solubilities of the PAHs used.
The results obtained for transport of the different PAHs indicate that there is a degree of selectivity in the active efflux pump. The more hydrophobic PAHs like phenanthrene, fluoranthene, and anthracene were transported out of the cells by efflux, whereas the more water-soluble compound naphthalene was not. The preferred growth substrate for LP6a is naphthalene (7), whereas anthracene and phenanthrene were less rapidly degraded and fluoranthene was not metabolized at all. The selectivity for one hydrophobic hydrocarbon instead of another indicates that there is a fundamental difference between PAH efflux in LP6a and the organic solvent and antibiotic efflux in other Pseudomonas spp., which tend to exhibit broad activity for hydrophobic compounds (5, 15). Studies to examine the antibiotic resistance phenotype of LP6a and to generate efflux mutants for genetic analysis are under way.
ACKNOWLEDGMENTS
The financial support of the Natural Sciences and Engineering Research Council is gratefully acknowledged.
Ruth Eckford and Naoyuki Miyata provided substantial assistance in the experimental work and in discussing results.
REFERENCES
- 1.Alexander M. Biodegradation and bioremediation. San Diego, Calif: Academic Press Inc.; 1994. [Google Scholar]
- 2.Bateman J N, Speer B, Feduik L, Harline R A. Naphthalene association and uptake in Pseudomonas putida. J Bacteriol. 1986;166:155–161. doi: 10.1128/jb.166.1.155-161.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerniglia C E. Microbial metabolism of polycyclic aromatic hydrocarbons. Adv Appl Microbiol. 1984;30:31–71. doi: 10.1016/s0065-2164(08)70052-2. [DOI] [PubMed] [Google Scholar]
- 4.Dawson R M C, Elliott D C, Elliot W H, Jones K M. Data for biochemical research. 2nd ed. Oxford, United Kingdom: Oxford University Press; 1969. [Google Scholar]
- 5.de Bont J M. Solvent-tolerant bacteria in biocatalysis. Trends Biotechnol. 1998;16:493–499. [Google Scholar]
- 6.Finnerty W R, Singer M E. Membranes of hydrocarbon-utilizing organisms. In: Ghosh B K, editor. Organization of prokaryotic cell membranes. Vol. 3. Boca Raton, Fla: CRC Press; 1981. pp. 1–44. [Google Scholar]
- 7.Foght J M, Westlake D W S. Transposon and spontaneous deletion mutants of plasmid-borne genes encoding polycyclic aromatic hydrocarbon degradation by a strain of Pseudomonas fluorescens. Biodegradation. 1996;7:353–366. doi: 10.1007/BF00115749. [DOI] [PubMed] [Google Scholar]
- 8.Heipieper H J, Weber F J, Sikkema J, Keweloh H, de Bont J A M. Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994;12:409–415. [Google Scholar]
- 9.Isken S, de Bont J A M. Active efflux of toluene in a solvent-resistant bacterium. J Bacteriol. 1996;178:6056–6058. doi: 10.1128/jb.178.20.6056-6058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kieboom J, Dennis J J, Zylstra G J, de Bont J A M. Active efflux of organic solvents by Pseudomonas putida S12 is induced by solvents. J Bacteriol. 1998;180:6769–6772. doi: 10.1128/jb.180.24.6769-6772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Zhang L, Poole K. Role of the multidrug efflux systems of Pseudomonas aeruginosa in organic solvent tolerance. J Bacteriol. 1998;180:2987–2991. doi: 10.1128/jb.180.11.2987-2991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackay D M, Shiu W Y. Estimating the multimedia partitioning of hydrocarbons: the effective solubility approach. In: Kostecki P T, editor. Hydrocarbon contaminated soils & groundwater: analysis, fate, environmental & public health effects, & remediation. Vol. 2. Boca Raton, Fla: Lewis Publishers Inc.; 1991. pp. 137–154. [Google Scholar]
- 13.McIntosh T J, Simon S A, MacDonald R C. The organization of n-alkanes in lipid bilayers. Biochim Biophys Acta. 1980;597:445–463. doi: 10.1016/0005-2736(80)90219-9. [DOI] [PubMed] [Google Scholar]
- 14.Neidhardt F C, editor. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. [Google Scholar]
- 15.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos J L, Duque E, Godoy P, Segura A. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-TIE. J Bacteriol. 1998;180:3323–3329. doi: 10.1128/jb.180.13.3323-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott C C L, Finnerty W R. A comparative analysis of the ultrastructure of hydrocarbon-oxidizing micro-organisms. J Gen Microbiol. 1976;94:342–350. doi: 10.1099/00221287-94-2-342. [DOI] [PubMed] [Google Scholar]
- 18.Scott C C L, Finnerty W R. Characterization of intracytoplasmic hydrocarbon inclusions from the hydrocarbon-oxidizing Acinetobacter species HO-1N. J Bacteriol. 1976;127:481–489. doi: 10.1128/jb.127.1.481-489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sikkema J, de Bont J A M, Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994;269:8022–8028. [PubMed] [Google Scholar]
- 20.Sikkema J, de Bont J A M, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thayer J R, Wheelis M L. Active transport of benzoate in Pseudomonas putida. J Gen Microbiol. 1981;128:1749–1753. doi: 10.1099/00221287-128-8-1749. [DOI] [PubMed] [Google Scholar]
- 22.White S H, King G I, Cain J E. Location of hexane in lipid bilayers determined by neutron diffraction. Nature. 1981;290:161–163. [Google Scholar]
- 23.Whitman B E, Lueking D R, Mihelcic J R. Naphthalene uptake by a Pseudomonas fluorescens isolate. Can J Microbiol. 1998;44:1086–1093. doi: 10.1139/cjm-44-11-1086. [DOI] [PubMed] [Google Scholar]