Abstract
Background
Stem cell therapy holds promise to improve healing and stimulate tissue regeneration after burn injury. Preclinical evidence has supported this; however, clinical studies are lacking. We examined the application of bone marrow-derived mesenchymal stem cells (BM-MSC) to deep second-degree burn injuries using a two-dose escalation protocol.
Methods
Ten individuals aged 18 years or older with deep second-degree burn wounds were enrolled. The first five patients were administered 2.5 × 10³ BM-MSC/cm2 to their wounds. After safety of the initial dose level was assessed, a second group of five patients was treated with a higher concentration of 5 × 10³ allogeneic BM-MSC/cm2. Safety was assessed clinically and by evaluating cytokine levels in mixed recipient lymphocyte/donor BM-MSC reactions (INFγ, IL-10 and TNFα). At each visit, we performed wound measurements and assessed wounds using a Patient and Observer Scar Assessment Scale (POSAS).
Results
All patients responded well to treatment, with 100% closure of wounds and minimal clinical evidence of fibrosis. No adverse reactions or evidence of rejection were observed for both dose levels. Patients receiving the first dose concentration had a wound closure rate of 3.64 cm2/day. Patients receiving the second dose concentration demonstrated a wound closure rate of 10.47 cm2/day. The difference in healing rates between the two groups was not found to be statistically significant (P = 0.17).
Conclusion
BM-MSC appear beneficial in optimising wound healing in patients with deep second-degree burn wounds. Adverse outcomes were not observed when administering multiple doses of allogeneic BM-MSC.
Lay Summary
Thermal injuries are a significant source of morbidity and mortality, constituting 5%–20% of all injuries and 4% of all deaths. Despite overall improvements in the management of acutely burned patients, morbidities associated with deeper burn injuries remain commonplace. Burn patients are too often left with significant tissue loss, scarring and contractions leading to physical loss of function and long-lasting psychological and emotional impacts.
In previous studies, we have demonstrated the safety and efficacy of administering bone marrow-derived mesenchymal stem cells (BM-MSC) to chronic wounds with substantial improvement in healing and evidence of tissue regeneration. In this report, we have examined the application of BM-MSC to deep second-degree burn injuries in patients.
The aim of the present phase I/II clinical trial was to examine the safety and efficacy of administering allogeneic BM-MSC to deep second-degree burns. We utilised two different dose levels at concentrations 2.5 × 103 and 5 × 103 cells/cm2. Patients with deep second-degree burn wounds up to 20% of the total body surface area were eligible for treatment. Allogeneic BM-MSC were applied to burn wounds topically or by injection under transparent film dressing <7 days after injury. Patients were followed for at least six months after treatment.
Using two dose levels allowed us to gain preliminary information as to whether different amounts of BM-MSC administered to burn wounds will result in significant differences in safety/ clinical response. Once the safety and dose-response analysis were completed, we evaluated the efficacy of allogeneic stem cell therapy in the treatment of deep second-degree burn wounds.
In this study, we examined the role of allogeneic BM-MSC treatment in patients with deep second-degree burn injuries, in a dose-dependent manner. No significant related adverse events were reported. Safety was evaluated both clinically and by laboratory-based methods. Efficacy was assessed clinically through evidence of re-pigmentation, hair follicle restoration and regenerative change. While these findings are encouraging, more studies will be needed to better establish the benefit of BM-MSC in the treatment of burn injuries.
Keywords: Second-degree burn, bone marrow–derived mesenchymal stem cells, cell therapy, wound healing, burn scar, thermal injury
Introduction
Burn wounds have a devastating humanitarian and economic impact, and are responsible for long-term loss of function and disability. Burn patients experience pain, disfigurement, lack of socialisation, depression and permanent disabilities in both the workplace and daily living activities that can last throughout their lifetime.1 The financial costs are significant and widespread, impacting the work force, insurers and government agencies. In 2006, it was estimated that burn injury costs in the United States exceeded 7.5 billion dollars,2 which, adjusting for inflation, would exceed 10 billion dollars today. These costs, however, are likely to be much higher, as determining costs for burn injuries is often difficult given their long-term effects and impact on multiple services. In addition, as acute trauma care has improved significantly, more severely burned patients are surviving to face long-term disabilities.
While there has been progress in supportive therapy, advances in the treatment of burn wounds has not changed appreciably in decades.3 Standard of care methods include escharotomy, debridement, application of simple and complex dressing materials, temporary coverage with cadaver skin, and grafting of autologous skin or cultured skin cells. Despite these therapies, hypertrophic scaring occurs frequently and can lead to the formation of contractures.4 Contractures represent a great source of morbidity for burn patients. Scar contracture rates have not significantly changed with improvements in burn care despite the use of treatments designed to mitigate the effects of hypertrophic scarring including scar massage, topical treatments, steroid injections and compression garments.5 New insights into improving healing and promoting tissue regeneration after burn injury are urgently needed.
Since thermal burns result in a complex array of injury including immediate loss of tissue, ischemia/reperfusion and a destructive inflammatory response, optimal healing of burn wounds requires coordination of multiple cellular processes. Consequent to a severe burn, numerous cytokines, such as tumour necrosis factor alpha (TNF-α), interleukin (IL)-1β and interferon gamma (IFN-γ), IL-6 and IL-12, are released and may become systemically elevated,6 leading to a pro-inflammatory state and altering the normal healing process. The ability to attenuate this pro-inflammatory state, both systemically and locally within the burn wounds, remains elusive. Unfortunately, despite the advancements in the acute management of severe thermal injuries, the prevalence of hypertrophic scarring and scar contractures after thermal injury has not lessened and remains a significant problem for many burn victims.5
Stem cell-based therapy for the treatment of burns offers the promise of accelerating healing as well as initiating tissue regeneration with reduced scarring. Much of the research on the use of stem cells in burn treatment has focused on either the enhancement of burn wound healing and closure, or in the attenuation of the effects of the inflammatory response.7 Stem cell treatments generally show a great deal of promise in both areas. With deeper burn injury, there is comprehensive loss of skin and adnexal structures (including hair, nerves and sweat glands), which are reservoirs rich in local stem cells. The innate capacity for wound healing and skin regeneration is therefore greatly compromised. Other sources of stem cells will be needed to correct this deficit.
We previously investigated the ability of autologous bone marrow stem cells to accelerate healing of chronic wounds.8 A benefit of using autologous cells is the minimal risk of provoking an immune response. However, there are concerns when considering autologous bone marrow cells for burn patients.9 Bone marrow suppression and dysfunction are known to occur after significant burn injury and, therefore, marrow from burned patients is more likely to be adversely affected.10,11 In addition, the expansion of autologous bone marrow stem cells can take more than four weeks, significantly past the critical therapeutic window for burn injuries. Earlier treatment is crucial as prolonged healing increases the risks of infection, hypertrophic scarring with contracture and skin fragility that is prone to reinjury. Therefore, a readily available allogeneic source of stem cells is needed to overcome these obstacles.
While several types of stem cells are present in adult tissue, mesenchymal stem cells (MSCs), originally derived from the stromal component of bone marrow, are of particular interest to many investigators due to several unique characteristics.12 MSCs are multipotent cells capable of differentiating into bone, adipose, cartilage and fibroblastic lineages. Aside from their potential of being a substrate material for tissue, MSCs appear to be most important in the orchestration of tissue repair.13
MSCs have been studied in a variety of clinical applications to repair and regenerate damaged tissue and have thus far demonstrated an excellent safety profile. Previous work by our group and others have demonstrated the safety and efficacy of delivering bone marrow stem cells, including MSCs, to chronic wounds with significant improvement in healing and scarring.8,14,15
In promoting a healing response, MSCs may augment local reparatory responses by engaging host cells, such as fibroblasts, keratinocytes, macrophages and progenitor cells, at the site of injury.13,16 Furthermore, through the secretion of soluble factors, MSCs may induce angiogenesis, which is a critical step in wound healing.17 In 2005, Rasulov et al. transplanted allogeneic MSCs in a patient with severe burns and reported significant augmentation of haemostasis and epithelisation of the wound bed without significant adverse events.18 Another two patients with severe second- and third-degree burns were treated with allogeneic MSCs combined with an artificial dermal substitute derived from porcine collagen type 1, resulting in decreased scarring and aesthetically favourable findings when compared to areas without allogeneic MSCs with no adverse events.19 More recent work has indicated that membrane-bound particles, extracellular vesicles, released by MSCs and carrying complex mixtures of proteins, transcription factors and nucleic acids may be largely responsible for the healing effects attributed to MSCs.20,21 Regardless of the specific mechanisms involved, however, there is still little known regarding the safety and clinical application of multiple administrations of allogeneic MSCs in the treatment of burn injury.
The preliminary data from this pilot study establish the safety of BM-MSC therapy for burn wounds in two dose escalations while providing both observational and objective data that demonstrate the effectiveness of this therapy for improving the healing of burn injury.
Materials and methods
Clinical trial design
Individuals were enrolled into a phase 1 clinical trial (NCT02104713) to determine the safety of allogeneic stem cell therapy for deep second-degree burn wounds. Patients were evaluated and treated at the Jackson Memorial Hospital-Ryder Trauma Center in Miami, Florida after assessment by an academic burn surgeon. Patients were consented and after meeting inclusion and exclusion criteria were enrolled in the study.
The study design was a dose-escalation trial with two dose concentrations set at 2.5 × 10³ allogeneic BM-MSC/cm2and 5 × 10³ allogeneic BM-MSC/cm2. Ten patients with deep second-degree burns were enrolled with five patients in each dose concentration group. On completion of the first (lower) dose group, safety was assessed before advancing to the higher dose concentration. Each patient group was eligible to receive a maximum of two administrations, at the predetermined dose concentration. A decision to administer a second dose was based on the progression of healing, determined at no more than 10 days after first administration of BM-MSC. A total of 10 patients were enrolled into the two dose concentration groups.
Bone marrow mesenchymal stem cells isolation and expansion
Bone marrow was aspirated from the posterior iliac crest of a young healthy male donor after obtaining informed consent and meeting donor eligibility criteria. BM-MSC isolation and expansion of clinical grade material was performed at the Interdisciplinary Stem Cell Institute cGMP facility at the University of Miami. Briefly, bone marrow mononuclear cells were isolated using lymphocyte separation media (Lonza) and cultured in media consisting of alpha MEM (Gibco) supplemented with 2 mM L-glutamine (Gibco), 20% fetal bovine serum (Hyclone), 100 units/mL penicillin and 100 μg/mL streptomycin (Gibco) for their first passage. Subsequent passages used media consisting of alpha MEM (Gibco) supplemented with 2 mM L-glutamine (Gibco) and 20% fetal bovine serum (Hyclone). Cells were incubated at 37 °C in a 5% CO2 humidified atmosphere. Cultures were expanded (three passages) and cryopreserved for clinical usage.
Allogenic BM-MSC treatment
After debridement and local wound care with standard of care dressings, allogeneic BM-MSC in preservative-free normal saline were applied topically under transparent occlusive film or subcutaneously by injection along the wound edges and wound bed (Figure 1b). Topical application was preferred over subcutaneous injection unless wounds demonstrated excessive weeping, bleeding or with anatomical features not easily sealed with occlusive dressings. Of the 10 patients reported here, only one had BM-MSC delivered by local injection due to the inability to seal the wound area with an occlusive dressing.
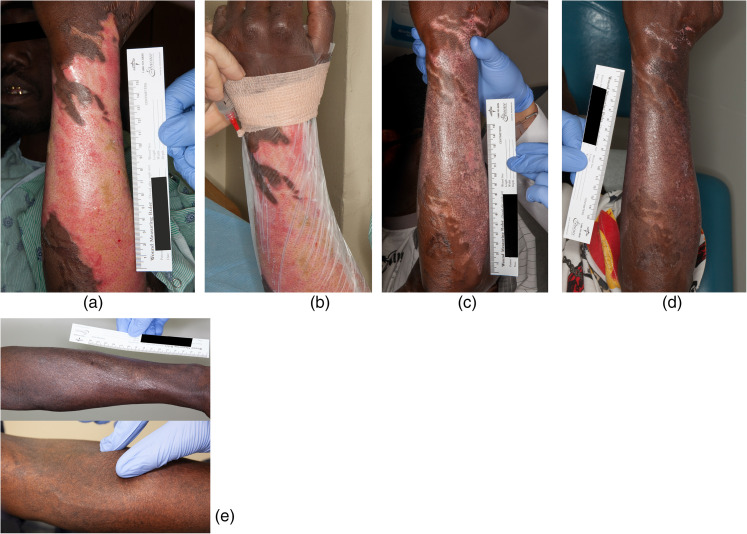
Figure 1.
Case 6 was a 47-year-old African American man who accidentally submerged his arm in hot oil attempting to catch a falling fry basket. (a) Wound before treatment. (b) Transparent film dressing applied to patient’s arm and BM-MSC were injected between the wound and transparent dressing. (c) Ten days after first treatment. (d) Seven days after second treatment (17 days after first treatment). (e) One year after treatment and showing limited to no fibrosis with pinch test.
The patients received a maximum of two administrations of allogeneic BM-MSC followed by standard of care treatment.
Safety analysis: Evaluation of cytokine profile
While safety analysis was primarily based on clinical assessment, an in vitro assay to establish (even subclinical) reactions to donor cells was also utilised. Peripheral blood was taken from participants before and after treatment with donor BM-MSC. Peripheral blood mononuclear cells (PBMC) were isolated from blood samples using density gradient media, Ficoll Paque Plus (GE Health: 17-5442-02), according to standard methods. Burn patient PBMCs were placed in mixed reaction with donor BM-MSC and levels of INF-γ, IL-10 and TNF-α were measured. Assays were performed with burn patient PBMCs obtained before treatment, 1 h after treatment and again 1–2 weeks after the last administration of allogeneic BM-MSC. INF-γ and TNF-α are presumed markers of rejection and IL-10 is a marker of immunosuppression.
To perform these studies, triplicate cultures of PBMCs were seeded in 96-well plates (Thermo Scientific, Nunc) at 2 × 104 mixed with donor BM-MSC dilutions at 8 × 103, 4 × 103, and 2 × 103. Positive controls were PBMCs stimulated with 3% phytohemagglutinin (PHA) and lipopolysaccharides (LPS) and then plated.22,23 To check for background activity, triplicate cultures of BM-MSC media (alpha MEM supplemented with 2 mM L-glutamine, 20% fetal bovine serum, 100 units/mL penicillin and 100 μg/mL streptomycin) alone, BM-MSC alone, PBMC alone and BM-MSC stimulated with 3% PHA and LPS were also plated. The plate was incubated in 37 °C and 5% CO2 for five days. The supernatant of the culture was collected on day 5 and centrifuged at 1600 rpm for 5 min at room temperature. The supernatant after the centrifugation was collected and placed in −80 °C freezer overnight.
Cytokine (IFN-γ, TNF-α, IL-10) levels in the culture supernatant were measured by ELISA, using a commercially available kit (Ebioscience: 88-7346-88) according to the manufacturer's instructions. The plates were measured at a wavelength of 450 nm using a microplate spectrophotometer (Versamax).
Measures
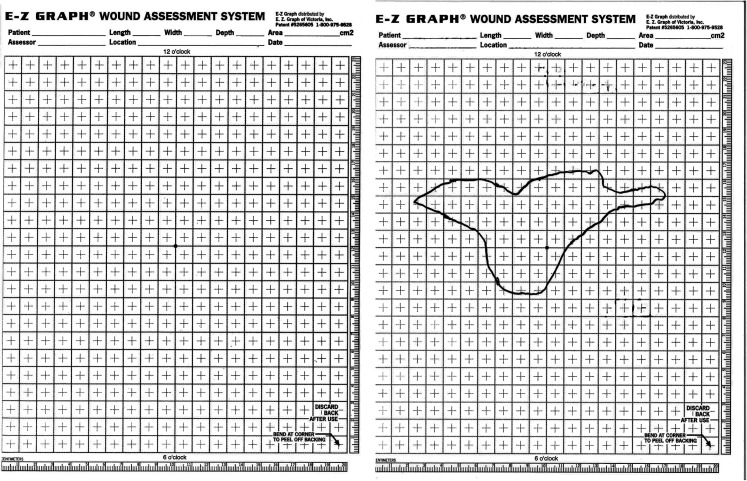
Wound measurements were taken at time of screening, before cell application, every 1–2 weeks after application and then monthly after complete wound closure, as determined by re-epithelisation. Wounds were traced using transparent graph sheets (E-Z graph) and the area (cm2) was calculated by digital analytical software (Figure 5). Wounds were assessed at each visit after initial treatment using a standardised and validated burn scar scale, the Patient and Observer Scar Assessment Scale (POSAS).24 A final score is calculated based on two parts: the observer criteria and the patient criteria. The observer score rated scar vascularity, pigmentation, thickness, relief, pliability and surface area. The patient score rated scar pain, itching, colour, stiffness, thickness and surface area. A score of ‘1’ corresponds to the characteristics of normal skin and goes up to ‘10’, which is the worst outcome. Digital photographs were taken at all time points.
Figure 5.
Screenshot of the E-Z graph® wound assessment system. Left: Empty E-Z Graph used to measure the wound. Right: Tracing graph of the wound healing area. We calculate this traced area using digital analytical software.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) v.25.0 for windows. ANOVA was applied to calculate the differences between the two doses concentrations for the individual POSAS scores. A two-tailed t-test was applied to calculate the differences between the mean wound closure rates of the two dose concentrations.
Isolation of extracellular vesicles
After delivery of BM-MSC to patients, any residual material not administered was brought back to the laboratory on ice for analysis and isolation of extracellular vesicles. Cellular material was removed at low-speed centrifugation and extracellular vesicles were isolated by ultracentrifugation as previously described.17,25 Assessment of particle size and distribution was performed using a Nanosight NS300 (Malvern Panalytical). Preparations were also characterised by western blot analysis for known extracellular markers.
Results
Between October 2014 and May 2018, 13 patients were enrolled (median age = 43 years; age range = 21–63 years). This paper reports on the 10 patients who completed treatment at the first two stem cell dose concentrations (Table 1) with five patients completing each dose group. The various aetiologies of the burns were hot water (n = 2), hot liquid (n = 3) and flame (n = 5). All patients had 100% wound closure with no adverse outcomes or evidence of rejection. There were no changes in the levels of cytokines before and after topical or subcutaneous treatment. The patients’ POSAS scores for each dose group were compared before and after treatment(s) (Table 2).
Table 1.
Patients in clinical trial from the first two doses.
| Patient information | Injury information | Treatment | Healing process | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | Age / Sex | Race | Type of burn* | Burn area | Wound size (cm2) | Doses (cells/cm2) | Type of application | No. of applications | No. of days for closure | Wound closure rate (cm2/day) | |
| Dose 1 | 1 | 63 / M | White | Flame | Upper L arm | 27.90 | 2.5 × 103 | Topical | 1 | 8 | 3.49 |
| 2 | 44 / M | Black | Scald | Upper R chest | 92.50 | 2.5 × 103 | Subcutaneous | 1 | 10 | 9.25 | |
| 3 | 52 / M | Black | Hot liquid | R wrist | 43.50 | 2.5 × 103 | Topical | 2 | 21 | 2.07 | |
| 4 | 56 / M | White | Hot liquid | L forearm | 114.55 | 2.5 × 103 | Topical | 2 | 26 | 3.55 | |
| 5 | 63 / M | Black | Flame | L superior leg | 30.00 | 2.5 × 103 | Topical | 2 | 18 | 1.99 | |
| L inferior leg | 236.40 | Topical | 2 | 45 | 1.50 | ||||||
| Dose 2 | 6 | 47 / M | Black | Hot liquid | L forearm | 821.50 | 5 × 103 | Topical | 2 | 31 | 26.50 |
| 7 | 43 / M | White | Flame | R forearm | 77.40 | 5 × 103 | Topical | 1 | 26 | 5.64 | |
| 8 | 30 / M | White | Flame | L upper leg | 31.50 | 5 × 103 | Topical | 1 | 18 | 1.83 | |
| 9 | 24 / M | White | Flame | L arm | 29.00 | 5 × 103 | Topical | 1 | 17 | 1.52 | |
| R forearm | 78.70 | Topical | 1 | 17 | 5.51 | ||||||
| 10 | 35 / F | Black | Scald | L ankle | 36.10 | 5 × 103 | Topical | 1 | 6 | 5.96 | |
| L leg | 155.43 | Topical | 1 | 6 | 26.34 | ||||||
*The categories of burns are as follows: scald = hot water, flame = due to fire, hot liquid = any other liquid except water.
Table 2.
Comparison of scoring of Patient and Observer Scar Assessment Scale among dose groups.
| Treatment group | Initial visit* | P value | Final visit† | P value | |||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||||
| Patient Scar Assessment Scale | |||||||
| Pain | Dose 1 | 5.40 | (2.95–7.85) | 0.603 | 3.20 | (-0.22–6.62) | 0.159 |
| Dose 2 | 6.43 | (3.80–9.06) | 1.00 | (1–1) | |||
| Itchiness | Dose 1 | 5.00 | (1.44–8.56) | 0.182 | 3.80 | (0.22–7.38) | 0.094 |
| Dose 2 | 7.43 | (6.16–8.70) | 1.00 | (1–1) | |||
| Colour | Dose 1 | 9.20 | (8.47–9.93) | 0.255 | 4.40 | (1.59–7.21) | 0.096 |
| Dose 2 | 8.00 | (6.46–9.54) | 2.14 | (1.63–2.65) | |||
| Stiffness | Dose 1 | 5.60 | (3.31–7.89) | 0.616 | 3.40 | (0.03–6.83) | 0.209 |
| Dose 2 | 6.43 | (4.34–8.52) | 1.43 | (1.03–1.82) | |||
| Thickness | Dose 1 | 5.80 | (2.93–8.67) | 0.428 | 4.00 | (0.90–7.10) | 0.082 |
| Dose 2 | 7.29 | (5.12–9.34) | 1.43 | (1.03–1.82) | |||
| Irregularity | Dose 1 | 6.40 | (4.02–8.77) | 0.667 | 4.60 | (1.65–7.55) | 0.085 |
| Dose 2 | 7.14 | (4.94–9.34) | 2.14 | (1.63–2.65) | |||
| Total scar | Dose 1 | 8.00 | (5.60–10.40) | 0.734 | 4.40 | (1.33–7.47) | 0.122 |
| Dose 2 | 7.43 | (5.34–9.52) | 2.14 | (1.63–2.65) | |||
| Observer Scar Assessment Scale | |||||||
| Vascularity | Dose 1 | 7.00 | (6.38–7.62) | 0.034 | 1.80 | (1.07–2.53) | 0.207 |
| Dose 2 | 4.29 | (2.54–6.03) | 1.29 | (0.92–1.65) | |||
| Pigmentation | Dose 1 | 5.80 | (3.21–8.39) | 0.572 | 2.60 | (1.82–3.38) | 0.685 |
| Dose 2 | 5.00 | (3.65–6.35) | 2.43 | (2.03–2.82) | |||
| Thickness | Dose 1 | 4.40 | (2.58–6.22) | 0.287 | 1.60 | (0.82–2.38) | 0.100 |
| Dose 2 | 3.29 | (2.26–4.31) | 1.00 | (1.00–1.00) | |||
| Relief | Dose 1 | 4.20 | (2.30–6.10) | 0.390 | 1.40 | (0.92–1.88) | 0.077 |
| Dose 2 | 3.29 | (2.26–4.31) | 1.00 | (1.00–1.00) | |||
| Pliability | Dose 1 | 4.20 | (2.30–6.10) | 0.390 | 1.40 | (0.92–1.88) | 0.077 |
| Dose 2 | 3.29 | (2.26–4.31) | 1.00 | (1.00–1.00) | |||
| Surface area | Dose 1 | 4.20 | (3.24–5.16) | 0.060 | 1.40 | (0.92–1.88) | 0.077 |
| Dose 2 | 2.86 | (2.07–3.65) | 1.00 | (1.00–1.00) | |||
| Overall opinion | Dose 1 | 5.00 | (2.85–7.25) | 0.306 | 1.80 | (1.41–2.19) | 0.235 |
| Dose 2 | 3.71 | (2.45–4.98) | 1.43 | (1.03–1.82) | |||
*The initial visit is the first follow-up visit after the first BM-MSC treatment.
The final visit is one year after the patient's burn.
Patients receiving the first dose concentration had a wound closure rate of 3.64 cm2/day. Patients receiving the second dose concentration demonstrated a wound closure rate of 10.47 cm2/day. The mean standard deviation in the group receiving the first dose was 2.64 (range = 1.5–9.25 cm2/day) and 10.22 (range = 1.52–26.5 cm2/day) in the group receiving the second dose. The difference in healing rates between the two groups was not found to be statistically significant (P = 0.17).
First dose group
Case 1
A 62-year-old Caucasian man sustained a deep second-degree burn wound caused by a flash flame burn from an electrical box. The designated treatment area was the upper left arm (surface area [SA] = 27.9 cm2). The wound was 100% closed eight days after the first topical application of BM-MSC, deferring the need for a second treatment.
Case 2
A 44-year-old African American man experienced scalded burns from a detached radiator hose while working on his car. The designated treatment area was the upper right chest (SA = 92.5 cm2). The patient received an initial subcutaneous application of BM-MSC in the operating room. The individual was not eligible for a second application of BM-MSC as the wound was completely epithelialised by day 10.
Case 3
A 52-year-old African American man sustained an outdoor patio torch oil burn and the designated treatment area was the right proximal arm (SA = 43.5 cm2). The wound was reassessed seven days after the first topical application of BM-MSC and the open wound measured 14.5 cm2. The second topical application of BM-MSC was performed on day 10. The wound was reassessed on day 21 and was 100% epithelialised.
Case 4
A 56-year-old Hispanic man sustained a burn from hot roofing tar. The designated treatment area was the left forearm (SA = 114.55 cm2). Seven days after the first topical application of BM-MSC, the wound measured 70.03 cm2. The second topical application of BM-MSC was performed on day 12. On day 26, wound closure was 100%.
Case 5
A 63-year-old African American man sustained a flame burn while working on his car engine, which caught fire. There were two designated treatment areas on the left leg, superior (SA = 30 cm2) and inferior (SA = 236.4 cm2). Seven days after the first topical application of BM-MSC, the superior wound was 4.8 cm2 and the inferior wound was 32.6 cm2. The second application of BM-MSC was performed on day 11. On day 18, the superior wound was 100% closed. On day 45, the inferior wound was 100% closed.
Second dose group
Case 6
A 47-year-old African American man sustained a burn after accidentally submerging his arm in the hot oil of a deep fryer while working as a chef. The designated treatment area was left forearm (SA = 821.5 cm2) (Figure 1). Ten days after the initial topical application of BM-MSC, the wound area was 16.6 cm2. The second topical application of BM-MSC was performed on day 12. On day 31, the wound was 100% closed.
Case 7
A 43-year-old white Hispanic man sustained a flame burn from an electrical box. The designated treatment area was the right forearm (SA = 77.4 cm2). Seven days after the first topical application of BM-MSC, the wound measured 3.6 cm2. On day 26, the wound was closed 100%.
Case 8
A 30-year-old Hispanic man sustained a flame burn from falling into a bonfire. The designated treatment area was the left upper leg (SA = 31.5 cm2). Eight days after the first topical application of BM-MSC, the wound area was 23 cm2. On day 18, the wound was 100% closed.
Case 9
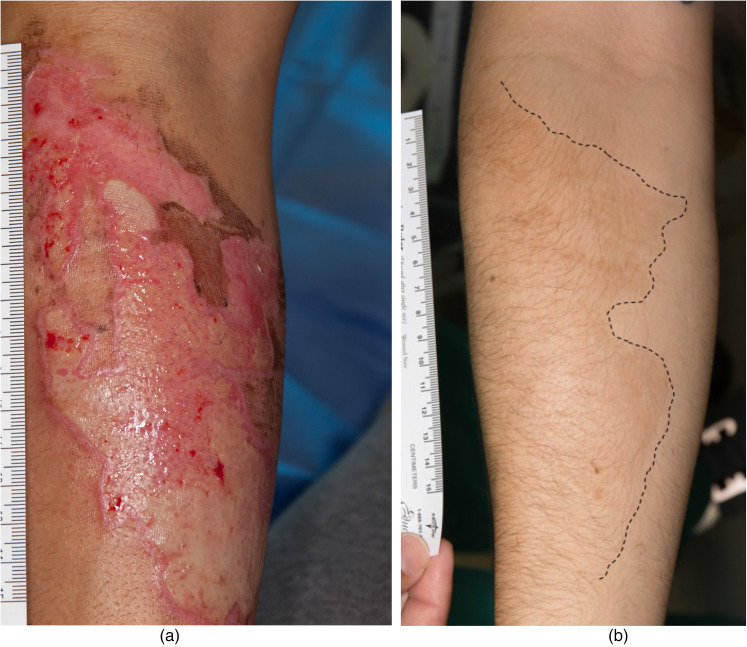
A 24-year-old white Hispanic man sustained a flame burn while pouring a flammable material into a fire. The designated treatment areas were the left arm (SA = 29 cm2) and right forearm (SA = 78.7 cm2) (Figure 4a). Both wounds were 100% closed 17 days after the initial topical application of BM-MSC.
Figure 4.
Case 9 was a 24-year-old white Hispanic man who sustained a flame burn while pouring an accelerant into a fire. The designated treatment area on the left arm was 29 cm2. (a) Before treatment and (b) one year after treatment. The dotted line outlines the treated area of the original wound. More hair development was noted at the site of BM-MSC treatment. The BM-MSC treatment may have created an optimal environment that supplemented hair growth.
Case 10
A 35-year-old African American woman sustained a burn from boiling water. The designated treatment areas were the left ankle (SA = 36.10 cm2) and left lower leg (SA = 155.43 cm2). Both wounds were 100% closed six days after initial topical application of BM-MSC.
Discussion
The standard of care for burn wounds has not changed for many years, with limited choices for treatment. Burn injury and its adverse outcomes can lead to significant scarring and disfigurement, which create a significant physical and psychological burden on many patients and can lead to delays in a patient's re-immersion into society. Prompt wound closure after a burn injury is linked to improved survival and decreased complications such as infection and hypertrophic scarring.26 Therefore, accelerated wound closure is one key to improved healing. Autologous cells can take too long to expand to be of use during the critical acute therapeutic window for burn wounds, whereas allogeneic MSCs represent an option for therapy that can potentially speed recovery in the acute period and improve overall outcomes.
Unlike our clinical trial that utilised BM-MSC for burn injuries, other studies have utilised adipose-derived stem cells (ADSC) as an efficient treatment these types of injuries.27,28 One important aspect is that ADSC also exhibit substantial plasticity to differentiate into multiple cell lineages. A potential advantage for ADSC is that the source material (adipose tissue) may be easier and less expensive to obtain than bone marrow. It has even been proposed that useful ADSC might even be obtainable from discarded burned skin after debridement.29 Isolation procedures for ADSC are however more complex compared to BM-MSC, and detailed biological characteristics are not as well defined to date.30
In addition, it has been reported that adipose tissue offers an abundant source of stromal vascular fraction (SVF) cells for immediate administration and can also give rise to a substantial number of cultured, multipotent adipose-derived stromal cells (ADSC).
Several studies have suggested that SVF treatment exhibited superior statistically significant results compared to ADSC treatment alone, especially in smooth muscle/collagen ratio and in endothelial cell content.31,32 This advantage of SVF over ADSC is supported in the distinctive, heterogeneous cellular composition of SVF, which could be responsible for the better therapeutic result detected in comparative animal studies and SVF is much more easily acquired, without the need for any cell separation or culturing conditions.27,29
Nevertheless, the presence of various cell types with potential to cause immunological rejection in the SVF may restrict use to autologous treatments only whereas ADSCs can be used unmatched in both allogeneic and autologous treatments.
Healthy MSCs appear to be capable of orchestrating the regeneration of lost epidermal and dermal tissues. Thus, use of allogeneic MSCs to accelerate burn wound closure may be able to reduce complications and enhance aesthetic appearance as suggested in the cases presented. In this study, the patients’ ELISAs were non-reactive or minimally reactive for IL-10, TNF-α and IFN-γ, and no adverse outcomes or rejections have been reported thus far. Therefore, our pilot study of two doses demonstrates the safety of the usage of allogeneic BM-MSCs, which may be readily available in a timely manner.
We observed an improvement in the POSAS scores in both dose groups over time. There was a significant difference in the observer scar assessment score for vascularity at the initial visit between the two doses, which is an important factor for wound healing. In some of the variables assessed by POSAS, there was a weak significant difference (P < 0.05) between the two doses (Table 2). A second dose allowed for a sense of relief of certain adverse symptoms that follow a thermal injury, notably itchiness, colour, thickness and irregularity. Looking at the raw means, the second dose group have lower initial observer scores, showing rapid wound bed improvement after the first BM-MSC treatment, in addition to lower final patient scores, showing the improvement of long-term symptoms associated with burn scars as well. A larger sample size may be able to demonstrate a statistically significant difference in the wound bed characteristics initially and the patient symptom relief at the final visit.
All patients had 100% wound closure. Patients receiving the first dose (2.5 × 103 cells/cm2) had a wound closure rate of 3.64 cm2/day. The patients receiving the second dose (5.0 × 103 cells/cm2) demonstrated a wound closure rate of 10.47 cm2/day. The difference in healing rates between the two groups was not found to be statistically significant (P = 0.17). Failure to reach statistical significance may be due to the small sample size as well as differences in wound size and severity. While representing an even smaller sample size (two wounds per group), some indication may come from comparing the healing rates between the two groups for wounds >100 cm2 where the level of significance was <0.05 (P = 0.002). In these wounds, the mean healing rate for the lower dose was 2.5 ± 1.44 cm2/day and 26.42 ± 0.11 cm2/day. Future studies with a larger sample size are needed to further examine these potential effects.
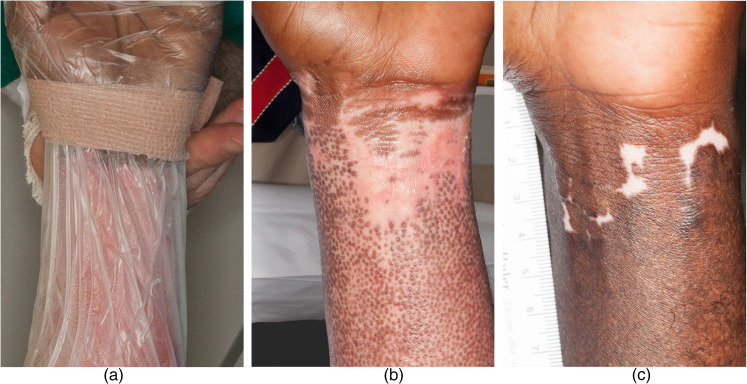
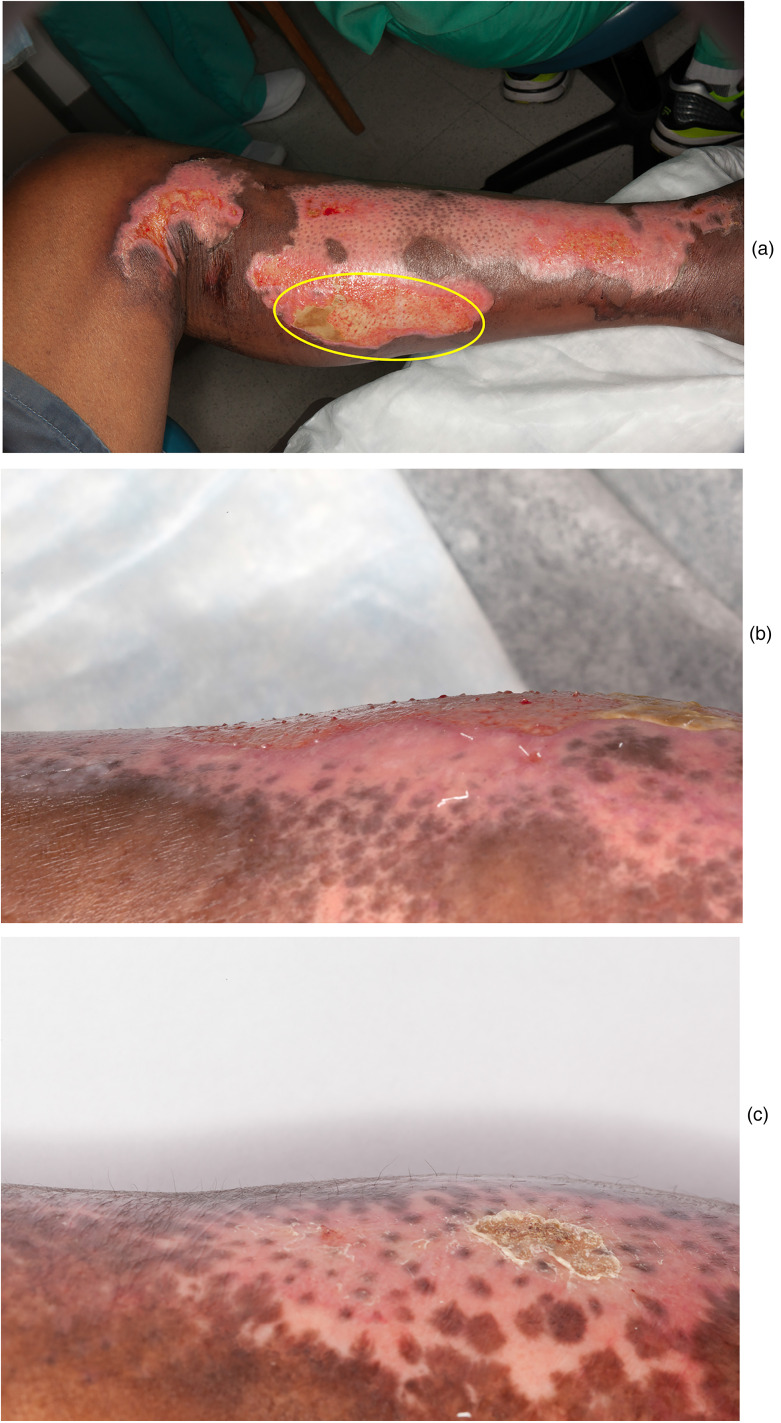
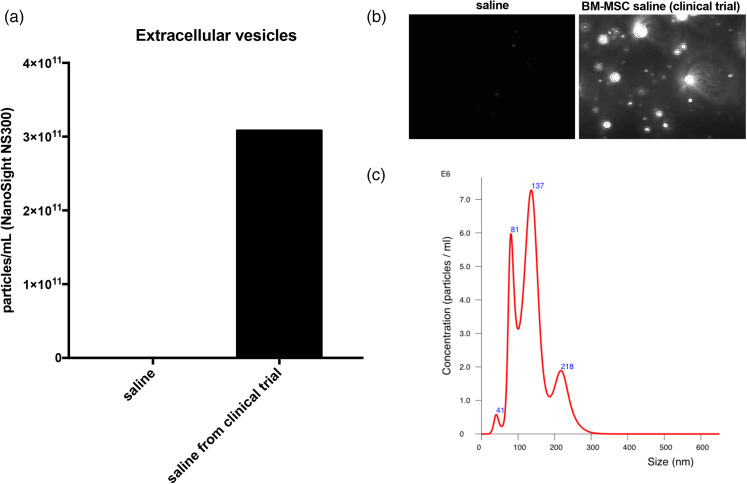
The observational findings in certain cases point to benefits of this treatment. In case 6, the patient is a cook and reported subsequent burn injuries due to hot oil that resulted in significant hypertrophic scarring yet healed exceptionally well with no scarring when treated with BM-MSC during this trial (Figure 1e), thus serving as his own negative control. In case 6, we also noticed delayed re-pigmentation where the bandage was applied during treatment, representing an area that may not have received adequate treatment (Figure 2a–c). Persistent post-burn leukoderma occurs due to the destruction of melanocytes (Figure 1d)33 and can be disfiguring, particularly in dark-skinned patients, where BM-MSCs may prevent this change. Case 5 is notable for hair growth only at the area that was burned deeper when compared to the surrounding area (Figure 3). We hypothesise the area might have been burned deep enough for the treatment to access germinative regions of adnexal structures and stimulate hair regrowth, but not to have eradicated the hair follicle. In case 9, we found more hair development at the site of BM-MSC treatment (Figure 4a–c). In this patient, the BM-MSC treatment may have created an optimal environment that supplemented hair growth. We hypothesised that extracellular vesicles may have aided in orchestrating cutaneous regeneration as there is accruing evidence that several signalling pathways are promoted through vesicles secreted by MSCs.20 We then examined the material delivered to patients (i.e. saline vehicle containing cells) for the presence of extracellular vesicles. After removing all cellular material by centrifugation, we determined that >1011 vesicles per mL were present in the vehicle given to patients (Figure 6). Extracellular vesicles were released from the time cells were harvested and placed in saline for delivery to patients. These findings strongly support that trials administering MSCs to patients are also delivering MSC-derived extracellular vesicles. This may account for some of the rapid clinical findings noted as extracellular vesicles, unlike cells, do not require engraftment and are released as biologically active materials. MSCs may then modulate the burn environment through several unique pathways (Figures 5 and 6).
Figure 2.
Case 6 wrist where the bandage prevented the treatment from reaching shows areas of no pigmentation. (a) The team placing the wrap around the wrist. (b) Ten days after first BM-MSC treatment. There is prominent stimulation of (follicular) re-pigmentation in the area treated. The area less accessible to BM-MSC due to the Coban pressure dressing is not well re-pigmentated. (c) One year after burn injury. Most of the burn injury has re-pigmentated. There is however persistent leukoderma in the area less accessible to BM-MSC due to the Coban pressure dressing.
Figure 3.
Case 5 was a 63-year-old African American man who sustained a flame burn while working on his car engine, which caught fire. (a) Day 0 of treatment with circle around a treated area that represented the deepest region of burn injury. (b) Eleven days after first BM-MSC treatment. There are prominent follicular buds in the deep portion of the treated wound. (c) A total of 34 days after two BM-MSC treatments. There is hair growth in the deeper burned area of the treated wound. This was not seen elsewhere. Perhaps BM-MSC and their paracrine effects of had greater access to the deeper dermis in this area of injury.
Figure 6.
After administration of BM-MSC to a burn patient, the remainder of the unused material was examined for the presence of extracelllular vesicles (EVs) in the sample. The unused material was handled under identical conditions to those used before administration. (a) After removal of cells, the vehicle was found to contain more than 1011 EVs/mL. (b, c) The isolated material also demonstrated particle morphology, size and distribution consistent with EVs as well as expressing characteristic EV markers.
Subsequent to a severe burn, numerous cytokines, such as TNF-α, IL-1β, IFN-γ, IL-6 and IL-12, are released and become systemically elevated.6 MSCs can significantly diminish this excess level of inflammation by attenuating the proliferation of immune effector cells and alter their cytokine profile production.34 This is due, in part, to the release of immunosuppressive and anti-inflammatory factors, such as transforming growth factor (TGF)-β1, IL-4 and indoleamine 2,3-dioxygenase that modulate the inflammatory secretome, and by direct cell-to-cell contact.9 The exact gene mechanism is still unfamiliar, but our group has suggested that extracellular vesicles derived from BM-MSC circulate and deliver mRNA and miRNA that promote the cellular antioxidant system,20 possibly limiting the scarring in our cases (Figure 1d and e). It is possible after our observational findings that these extracellular vesicles derived from BM-MSC may also stimulate quicker pigmentation (Figure 2) and hair growth (Figures 3 and 4). Since case 6 developed hypertrophic scarring in a subsequent burn after being treated, this suggests that topically applied BM-MSC do not permanently change the phenotype of skin at the site of application.
Animal models in previous studies have shown the use of transplanted bone marrow stem cells in severe third-degree burns demonstrated appreciable enhancement of epithelisation of the burn wound without significant adverse events.35 Our limited clinical trial has displayed no adverse outcomes or clinical signs of rejection in humans. Of course, generalisation of our findings to others with deep second-degree burns is limited by the small number of patients. While treated patients did not demonstrate an immune response to allogenic BM-MSC, their long-term effect remains unknown. Therefore, looking at the impact of topically and subcutaneously applied BM-MSC to the patient's systemic immune profile will be beneficial and will give us better insight on the mechanisms associated.
We emphasise that the purpose of this clinical trial is to evaluate the safety of topical and subcutaneous application of BM-MSCs to burn wounds and thus far have had no associated adverse events. The observational results seen during our safety evaluation with rapid wound closure and significant aesthetic appearance, suggest the potential of MSCs to be utilised as part of the standard of care for burn patients to achieve optimal results in the future.
Conclusion
Our initial trial proves safety of topically and subcutaneous application of BM-MSC in deep second-degree burn patients. Treated patients demonstrated improved healing in a dose-dependent manner with evidence of re-pigmentation and regenerative change; however, further studies would be needed to establish efficacy. Application of BM-MSC therapy to deep second-degree burn wounds therefore represents an opportunity for improved outcomes where alternate therapies are limited.
Acknowledgements
The authors thank Olga Orozco and Ronald Maning for their valuable contributions in helping to coordinate this study.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the U.S. Department of Defense (grant number W81XWH-13-2-0024).
ORCID iDs: Carl I Schulman https://orcid.org/0000-0001-8899-4350
Evangelos V Badiavas https://orcid.org/0000-0002-3664-3409
How to cite this article
Schulman CI, Namias N, Pizano L, Rodriguez-Menocal L, Aickara D, Guzman W, Candanedo A, Maranda E, Beirn A, McBride JD and Badiavas EV. The effect of mesenchymal stem cells improves the healing of burn wounds: A phase 1 dose-escalation clinical trial. Scars, Burns & Healing, Volume 8, 2022. DOI: 10.1177/20595131211070783.
References
- 1.Burd A. Burns: treatment and outcomes. Semin Plast Surg 2010; 24: 262–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelstein E, Corso PS, Miller TR. The incidence and economic burden of injuries in the United States. Oxford; New York: Oxford University Press, 2006. [Google Scholar]
- 3.Lee KC, Joory K, Moiemen NS. History of burns: the past, present and the future. Burns Trauma 2014; 2: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson DA, Renshaw A. An algorithm for the release of burn contractures of the extremities. Burns 2006; 32: 663–668. [DOI] [PubMed] [Google Scholar]
- 5.Richard R, Baryza MJ, Carr JA, et al. Burn rehabilitation and research: proceedings of a consensus summit. J Burn Care Res 2009; 30: 543–573. [DOI] [PubMed] [Google Scholar]
- 6.Gauglitz GG, Song J, Herndon DNet al. et al. Characterization of the inflammatory response during acute and post-acute phases after severe burn. Shock 2008; 30: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler KL, Goverman J, Ma Het al. Stem cells and burns: review and therapeutic implications. J Burn Care Res 2010; 31: 874–881. [DOI] [PubMed] [Google Scholar]
- 8.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol 2003; 139: 510–516. [DOI] [PubMed] [Google Scholar]
- 9.Maranda EL, Rodriguez-Menocal L, Badiavas EV. Role of mesenchymal stem cells in dermal repair in burns and diabetic wounds. Curr Stem Cell Res Ther 2017; 12: 61–70. [DOI] [PubMed] [Google Scholar]
- 10.Burd A, Ahmed K, Lam Set al. et al. Stem cell strategies in burns care. Burns 2007; 33: 282–291. [DOI] [PubMed] [Google Scholar]
- 11.Gamelli RL, Hebert JC, Foster RS Jr. Effect of burn injury on granulocyte and macrophage production. J Trauma 1985; 25: 615–619. [DOI] [PubMed] [Google Scholar]
- 12.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 13.Shabbir A, Zisa D, Lin Het al. et al. Activation of host tissue trophic factors through JAK-STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am J Physiol Heart Circ Physiol 2010; 299: H1428–H1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badiavas EV, Ford D, Liu Pet al. et al. Long-term bone marrow culture and its clinical potential in chronic wound healing. Wound Repair Regen 2007; 15: 856–865. [DOI] [PubMed] [Google Scholar]
- 15.Dash NR, Dash SN, Routray Pet al. et al. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res 2009; 12: 359–366. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Menocal L, Salgado M, Ford Det al. et al. Stimulation of skin and wound fibroblast migration by mesenchymal stem cells derived from normal donors and chronic wound patients. Stem Cells Transl Med 2012; 1: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shabbir A, Cox A, Rodriguez-Menocal Let al. et al. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis In vitro. Stem Cells Dev 2015; 24: 1635–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasulov MF, Vasilchenkov AV, Onishchenko NAet al. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med 2005; 139: 141–144. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa T, Mitsuno H, Nonaka Iet al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg 2008; 121: 860–877. [DOI] [PubMed] [Google Scholar]
- 20.McBride JD, Rodriguez-Menocal L, Badiavas EV. Extracellular vesicles as biomarkers and therapeutics in dermatology: a focus on exosomes. J Invest Dermatol 2017; 137: 1622–1629. [DOI] [PubMed] [Google Scholar]
- 21.Shabbir A, Badiavas EV. Toward an ‘off the shelf’ technology for burn victims: healing wounds with mesenchymal stem cells. Regen Med 2015; 10: 381–384. [DOI] [PubMed] [Google Scholar]
- 22.Oviedo-Orta E, Gasque P, Evans WH. Immunoglobulin and cytokine expression in mixed lymphocyte cultures is reduced by disruption of gap junction intercellular communication. FASEB J 2001; 15: 768–774. [DOI] [PubMed] [Google Scholar]
- 23.Ai W, Li H, Song Net al. et al. Optimal method to stimulate cytokine production and its use in immunotoxicity assessment. Int J Environ Res Public Health 2013; 10: 3834–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Wal MB, Tuinebreijer WE, Bloemen MCet al. et al. Rasch analysis of the patient and observer scar assessment scale (POSAS) in burn scars. Qual Life Res 2012; 21: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBride JD, Rodriguez-Menocal L, Guzman Wet al. et al. Bone marrow mesenchymal stem cell-derived CD63 + exosomes transport Wnt3a exteriorly and enhance dermal fibroblast proliferation, migration, and angiogenesis In vitro. Stem Cells Dev 2017; 26: 1384–1398. [DOI] [PubMed] [Google Scholar]
- 26.Aarabi S, Longaker MT, Gurtner GC. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med 2007; 4: e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng JZ, Farrokhi A, Ghahary Aet al. et al. Therapeutic use of stem cells in treatment of burn injuries. J Burn Care Res 2018; 39: 175–182. [DOI] [PubMed] [Google Scholar]
- 28.Franck CL, Senegaglia AC, Leite LMBet al. et al. Influence of adipose tissue-derived stem cells on the burn wound healing process. Stem Cells Int 2019; 2019: 2340725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan RK, Zamora DO, Wrice NLet al. et al. Development of a vascularized skin construct using adipose-derived stem cells from debrided burned skin. Stem Cells Int 2012; 2012: 841203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mushahary D, Spittler A, Kasper Cet al. et al. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 2018; 93: 19–31. [DOI] [PubMed] [Google Scholar]
- 31.You D, Jang MJ, Kim BHet al. et al. Comparative study of autologous stromal vascular fraction and adipose-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Stem Cells Transl Med 2015; 4: 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 2017; 8: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiwari VK. Burn wound: how it differs from other wounds? Indian J Plast Surg 2012; 45: 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang XX, Zhang Y, Liu Bet al. et al. Mao N: human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005; 105: 4120–4126. [DOI] [PubMed] [Google Scholar]
- 35.Rasulov MF, Vasilenko VT, Zaidenov VAet al. et al. Cell transplantation inhibits inflammatory reaction and stimulates repair processes in burn wound. Bull Exp Biol Med 2006; 142: 112–115. [DOI] [PubMed] [Google Scholar]