Abstract
BACKGROUND
Uterine natural killer cells (uNK) are the most abundant lymphocytes found in the decidua during implantation and in first trimester pregnancy. They are important for early placental development, especially trophoblast invasion and transformation of the spiral arteries. However, inappropriate uNK function has been implicated in reproductive failure, such as recurrent miscarriage (RM) or recurrent implantation failure (RIF). Previous studies have mainly focussed on peripheral NK cells (pNK), despite the well-documented differences in pNK and uNK phenotype and function. In recent years, there has been an explosion of studies conducted on uNK, providing a more suitable representation of the immune environment at the maternal–foetal interface. Here, we summarize the evidence from studies published on uNK in women with RM/RIF compared with controls.
OBJECTIVE AND RATIONALE
The objectives of this systematic review and meta-analysis are to evaluate: differences in uNK level in women with RM/RIF compared with controls; pregnancy outcome in women with RM/RIF stratified by high and normal uNK levels; correlation between uNK and pNK in women with RM/RIF; and differences in uNK activity in women with RM/RIF compared with controls.
SEARCH METHODS
MEDLINE, EMBASE, Web of Science and Cochrane Trials Registry were searched from inception up to December 2020 and studies were selected in accordance with PRISMA guidelines. Meta-analyses were performed for uNK level, pregnancy outcome and uNK/pNK correlation. Narrative synthesis was conducted for uNK activity. Risk of bias was assessed by ROBINS-I and publication bias by Egger’s test.
OUTCOMES
Our initial search yielded 4636 articles, of which 60 articles were included in our systematic review. Meta-analysis of CD56+ uNK level in women with RM compared with controls showed significantly higher levels in women with RM in subgroup analysis of endometrial samples (standardized mean difference (SMD) 0.49, CI 0.08, 0.90; P = 0.02; I2 88%; 1100 women). Meta-analysis of CD56+ uNK level in endometrium of women with RIF compared with controls showed significantly higher levels in women with RIF (SMD 0.49, CI 0.01, 0.98; P = 0.046; I2 84%; 604 women). There was no difference in pregnancy outcome in women with RM/RIF stratified by uNK level, and no significant correlation between pNK and uNK levels in women with RM/RIF. There was wide variation in studies conducted on uNK activity, which can be broadly divided into regulation and receptors, uNK cytotoxicity, cytokine secretion and effect of uNK on angiogenesis. These studies were largely equivocal in their results on cytokine secretion, but most studies found lower expression of inhibitory receptors and increased expression of angiogenic factors in women with RM.
WIDER IMPLICATIONS
The observation of significantly increased uNK level in endometrium of women with RM and RIF may point to an underlying disturbance of the immune milieu culminating in implantation and/or placentation failure. Further research is warranted to elucidate the underlying pathophysiology. The evidence for measuring pNK as an indicator of uNK behaviour is sparse, and of limited clinical use. Measurement of uNK level/activity may be more useful as a diagnostic tool, however, a standardized reference range must be established before this can be of clinical use.
Keywords: natural killer cells, recurrent miscarriage, recurrent implantation failure, immunology, immunohistochemistry, flow cytometry, reproductive immunology, assisted reproduction, endometrium
Introduction
Recurrent reproductive failure encompassing recurrent miscarriage (RM) and recurrent implantation failure (RIF) affects a small proportion of couples trying to conceive, but up to 50% of these cases remain unexplained. One possible cause for idiopathic cases of RM or RIF is an immunological factor. In the past three decades, significant attention has turned to natural killer (NK) cells, which comprise the highest proportion of immune cells in the placental bed during first trimester pregnancy.
In the non-pregnant endometrium, uterine NK cells (uNK) are mostly inactive but have the ability to undergo differentiation through the menstrual cycle in preparation for pregnancy (Manaster et al., 2008; Strunz et al., 2021). After implantation of the embryo, uNK participate in the process of placentation by facilitating trophoblast invasion and spiral artery remodelling to allow adequate exchange of nutrients and oxygen between mother and baby (Huhn et al., 2021). Maintaining a balance between excessive and insufficient trophoblast invasion is important, as the latter can cause problems ranging from miscarriage to pre-eclampsia and foetal growth restriction, collectively termed the Great Obstetrical Syndromes (Brosens et al., 2011).
Extravillous trophoblasts (EVT) are the only foetal-derived cells in the maternal–foetal interface that express MHC class I antigens. This includes expression of the non-classical class I molecules HLA-E and -G, as well as the classical class I molecule HLA-C, with polymorphic paternal and maternal components that have the potential to evoke a ‘non-self’ response from the maternal immune system (Kovats et al., 1990; King et al., 1996–1997, 2000). The three groups of NK-cell receptors which interact with these antigens are the CD94/NKG2, leucocyte immunoglobulin-like receptor and killer-like immunoglobulin receptor (KIR) families (Parham, 2004), respectively. Activation of uNK promotes cytokine production. For example, HLA-C2 activation of uNK via KIR2DS1 and KIR2DS4 induces granulocyte-macrophage colony-stimulating factor secretion, which promotes migration of trophoblast cells (Xiong et al., 2013; Kennedy et al., 2016). The role of uNK also changes as pregnancy progresses, switching from a predominantly pro-angiogenic growth factor predisposition (e.g. vascular endothelial growth factor-C, angiopetin-1, angiopoietin-2) at 8–10 weeks to a cytokine secretory function (e.g. interferon gamma (IFN-γ), interleukin (IL)-1b, IL-6 and IL-8) at 12–14 weeks (Lash et al., 2010).
There are a number of theories on the origin of uNK that include differentiation from uterine resident haematopoietic stem cells, recruitment from mature peripheral NK cells (pNK) by chemokine signalling or differentiation from immature pNK trafficked from the blood (Díaz-Hernández et al., 2021). One recent study, which examined uNK in a small sample of transplanted uteri, showed that the HLA expression of uNK in transplanted uteri resembled the recipient’s HLA rather than donor’s after transplantation. This suggests that uNK are replenished from circulating rather than tissue resident cells (Strunz et al., 2021), although this does not necessarily show that they originate from circulating NK cells, since the recruitment of circulating progenitors would be equally consistent with the data presented. Indeed, there are stark differences in the phenotype and function of uNK and pNK. uNK are predominantly CD56bright (CD56bright CD16−) (King et al., 1991; Koopman et al., 2003) whereas pNK are predominantly CD56dim (CD56dimCD16+) (Caligiuri, 2008). uNK express the tissue-residence marker CD49a and can be subdivided into three subsets that can be differentiated by their expression of CD39 and CD103, which are not found in pNK (Vento-Tormo et al., 2018). pNK exhibit cytotoxicity and secrete cytokines to effect their function as a first line defence against viruses (Horowitz et al., 2011) and malignant cells (Chiossone et al., 2018). On the other hand, uNK are only weakly cytotoxic against tumour cells and not at all against trophoblast cells (King et al., 1989). This is due to their inability to form activating synapses that trigger perforin release (Kopcow et al., 2005). However, this function can change if decidual or trophoblast cells are subsequently infected by virus (Le Bouteiller and Bensussan, 2017; Shmeleva and Colucci, 2021).
There is still much controversy about the role of NK cells in reproductive failure. The initial notion that uNK, behaving in a similar way to pNK, can ‘kill trophoblasts’ has been dispelled over the years (Moffett and Shreeve, 2015). In place of that, new theories have emerged on the role of NK cells in pathological pregnancies. One theory is that a higher than normal uNK level may cause increased production of angiogenic factors leading to increased peri-implantation blood flow and excessive oxidative stress to trophoblast cells (Quenby et al., 2009; Chen et al., 2016). Other evidence suggests that uNK have a predisposition to secrete pro-inflammatory cytokines akin to Th1-type cytokines while dampening anti-inflammatory Th2-type cytokines that are necessary to maintain healthy pregnancy (Sargent et al., 2006; Makrigiannakis et al., 2011). A recent study exploring the impact of different combinations of parental HLA-C and maternal KIR allotypes on livebirth outcome in women undergoing ART has highlighted that inadequate, rather than excessive, activation of uNK may be the cause of RM and RIF (Alecsandru et al., 2020). There may be a role for intervention based on variation of maternal KIR and foetal HLA-C although this is still at the pre-clinical stage owing to several limitations of present evidence and practical considerations in the clinical setting (Moffett et al., 2016).
In the last meta-analysis of six studies by Seshadri and Sunkara (2014), no difference was found in uNK level when measured as percentage of total stromal cells in women with RM. Since then, there has been an upsurge of studies conducted on uNK in women with RM and RIF. Besides measurement of uNK level, many studies have also been conducted to assess uNK function in these populations of women, which may clarify the pathophysiology underlying reproductive failure.
In light of these new studies, we aim to conduct a systematic review and meta-analysis to evaluate: if there are differences in uNK level in women with RM/RIF vs controls; pregnancy outcome in women with RM/RIF stratified to high and normal uNK level; correlation between uterine and pNK in women with RM/RIF; and if there are any differences in uNK activity in women with RM/RIF vs controls.
Methods
Protocol registration
This review was registered with International Prospective Review of Systematic Reviews (PROSPERO) and the registration number is CRD42020175868.
Study search and screen
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009). A combination of MeSH and keywords for Natural Killer cells, recurrent miscarriage and recurrent implantation failure were used (Supplementary Table SI). Electronic databases that were searched included MEDLINE, EMBASE, Web of Science and Cochrane Central Register of Controlled Trials.
The screening process was conducted independently by two reviewers (E.V.W. and O.G.) according to pre-determined eligibility criteria. Any disagreement about inclusion of a study was resolved by discussion and consensus involving the senior authors (N.S., V.M. and M.J.). Hand searching of references and citations from primary and review papers was conducted to ensure literature saturation.
Study selection
We included all observational studies on humans published as a full article or conference abstract in English from inception until December 2020. Conference abstracts were included if sufficient information was provided for quantitative analysis.
Inclusion criteria were studies with measurement of uNK level or activity in women with RM or RIF. RM was defined as loss of two or more previous pregnancies (Bender Atik et al., 2018) and RIF was defined as inability to achieve clinical pregnancy after two or more fresh or frozen transfers of high-quality embryos (Polanski et al., 2014). The control group included women with no history of reproductive problems, including those undergoing ART because of male factor infertility that resulted in successful pregnancy outcome. Exclusion criteria were usage of immunotherapy, studies on immunogenetics, non-standardized usage of hormonal therapy or no control group (Supplementary Table SII).
Authors were contacted if two studies were found to have the same author, year, institution of publication and study population. If there was no response from the authors, the study that was most recently published was included.
Outcomes measured
The primary outcome was uNK level measured in absolute count, or percentage of stromal cells or lymphocytes in women with RM and RIF.
The secondary outcome was pregnancy outcome, measured in livebirths, or clinical pregnancy rate (CPR), defined as presence of gestational sac and foetal heartbeat. For this outcome, we also evaluated difference in uNK level in women with RM/RIF who achieved successful pregnancy compared with women who did not.
The tertiary outcome waspooled correlation coefficient between pNK and uNK levels in women with RM and RIF.
The final outcome was uNK activity grouped as uNK regulation and receptors, cytotoxicity, effect on uterine vasculature and cytokine production.
Data extraction
The study parameters for data extraction were agreed between the authors and uploaded as a template on Covidence (www.covidence.org, 2021). Data were extracted independently by E.V.W. and O.G. Disagreement was resolved by discussion and consensus with the senior authors. Study information selected included primary author name, year of publication, country of study, study aims and primary outcome, study population, sample size, NK cell measurement including phenotype, timing, method of measurement, reference range, level and activity of uNK, correlation coefficient of pNK and uNK, and pregnancy outcome including clinical pregnancy and livebirth rate. Authors were contacted if there was incomplete information or if data were presented graphically. If there was no response, data were extracted from graphs with an online software WebPlotDigitizer (Rohatgi, 2017). If the data were presented in median and interquartile range, they were converted to mean and standard deviation according to the formula published by Wan et al. (2014).
Quality assessment
The methodological quality of all the included studies was assessed independently by E.V.W. and O.G. using the Risk Of Bias In Non-randomised Studies—of Intervention (ROBINS-I) tool (Sterne et al., 2016). The research question for each outcome was conceptualized based on the patient, intervention, comparator and outcome framework. Potential confounding domains were pre-defined and agreed between authors. Domains that were assessed included pre-intervention, at intervention and post-intervention. Publication bias was evaluated with funnel plot and Egger’s test of statistical significance was performed if the number of studies was more than 10.
Data synthesis
Studies that were homogenous were pooled for meta-analysis using Review Manager (Revman) Version 5.3 The Cochrane Collaboration, 2014. Revman 5.3 (Review Manager (RevMan) [Computer Program], 2014). Standardized mean difference (SMD) of uNK level in women with RM or RIF compared with controls were measured for the primary outcome, and risk ratio of clinical pregnancy and livebirth rate was measured for the secondary outcome. Meta-analysis of correlation coefficient was conducted for studies which assessed phenotype of uNK and pNK the same way. A P-value of <0.05 was considered as significant. Heterogeneity across studies was assessed, and I2 value of >50% was considered as significant. The Mantel–Haenszel fixed effects model was used if there was no significant heterogeneity and Der Simonian–Laird random effects model was used if there was significant heterogeneity.
Subgroup analyses were performed based on study population (including definition of RM/RIF, primary or secondary RM) and method of uNK measurement (including timing, technique, phenotype, unit of measurement). In order to improve reliability of our meta-analysis result, sensitivity analyses were performed for studies with mean values derived from median, graph extraction, critical or serious risk of bias, sampling from menstrual blood, conference abstract, not completely fertile controls, male infertility as controls, or hormonal treatment during time of sampling.
Data synthesis on uNK activity was presented as a narrative synthesis in accordance with reporting guidance of synthesis without meta-analysis (Campbell et al., 2020). Data synthesis was performed by vote counting to determine overall direction of effect where ‘1’ vote was allocated to the study if the direction of effect in outcome measure favoured the case group; ‘0’ was allocated if the direction of effect favours the control group and the study was marked ‘–’ if there was no difference between the groups. The standardized metric was vote counting as per mean difference between case/control groups. Confidence of the effect estimate was determined by application of the binomial test.
Results
Study selection and characteristics
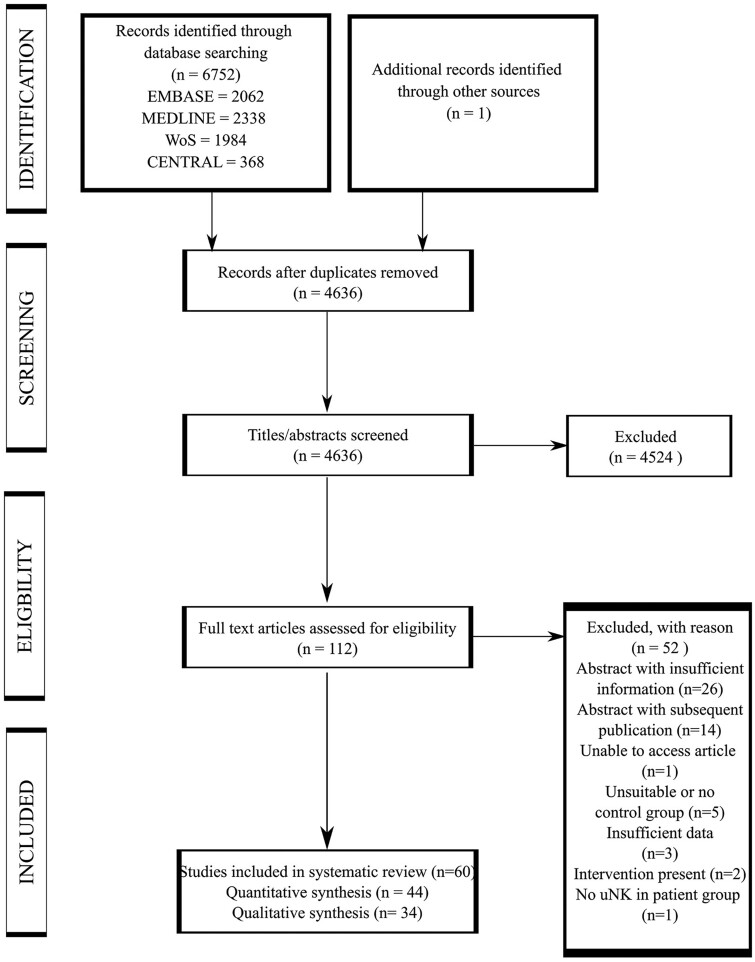
The electronic database search identified 6752 articles including 2117 duplicate articles. After de-duplication, title and abstract screening of 4636 articles yielded 112 articles eligible for full text screening. One further article was identified from snowballing references from full text articles that were included in our systematic review. Ultimately, 60 articles were eligible for inclusion in our systematic review. Forty-four articles were included in the meta-analyses and 34 articles in the qualitative synthesis (Fig. 1). Studies that were excluded from full text screening are presented in Supplementary Table SIII.
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses diagram.
The studies originated from 20 countries including China (16), UK (9), USA (5), Germany (4), Japan (4), France (3), Hong Kong (3), Turkey (3), Ireland (2), Taiwan (1), Spain (1), Belarus (1), Canada (1), Argentina (1), Russia (1), Serbia (1), Saudi Arabia (1), Iran (1), Egypt (1) and the Netherlands (1). All studies that evaluated uNK level, activity and correlation with pNK were case–control studies. For the studies that evaluated pregnancy outcomes, six were prospective cohort and one was a retrospective cohort study design.
Characteristics of the studies included in the meta-analyses are outlined in Table I. There was heterogeneity in the number of previous miscarriages used to define RM, whereby 18 studies used the definition of three or more previous miscarriages, 14 studies used the definition of two or more previous miscarriages, and six studies did not explicitly state number of previous miscarriages in their RM group. Similarly, there was also variation in definition of RIF, with six studies defining RIF as three or more previous failures to achieve clinical pregnancies after transfer of good quality embryos in fresh or frozen cycles, and four studies defining RIF as two or more previous failures.
Table I.
Characteristics of studies included in meta-analyses
| Study ID | Aim(s) of the study | Study design and cohort | Type and timing of sample | Method of analysis, uNK phenotype and unit of measurement | uNK reference range | Data extracted |
|---|---|---|---|---|---|---|
| Chao et al., 1995, Taiwan | To examine the differences in TCR expression in peripheral blood and intra-decidual T cells and in NK cell activity in normal pregnancy, anembryonic pregnancy and recurrent spontaneous abortion. | Case control study comparing women with ≥ 3 uRM (n=10) and fertile controls (n=21) | Decidual tissue; Between 6-10 weeks GA | FC; CD45+/CD14-/CD56+ or CD16+; Percentage of CD45+CD14- lymphocytes | N/A | uNK level and activity |
| Lachapelle et al., 1995, Canada | To clarify the immunologic role of endometrial leycocytes in uRM by analysis of T, B, NK cells and monocytes. | Case control study comparing women with ≥ 3 uRM (n=20) and fertile controls (n=15) | Endometrial tissue; Day 18 to 25 of menstrual cycle | FC; CD3-CD45+CD56+CD16+ and CD3-CD45+CD56+CD16-; Percentage of CD3-CD45+ lymphocytes | N/A | uNK level, pregnancy outcome (narrative only) |
| Lea et al., (1997), UK | To investigate localisation of bcl-2 at the maternal-fetal interface in first trimester and to determine if this may disturb early stages of pregnancy failure | Case control study comparing women with ≥ 3 uRM (n=23) and fertile controls (n=22) | Decidual tissue; Between 7-14 weeks GA | IHC; CD56; Percentage of stained nucleated cells | N/A | uNK level and activity |
| Yamamoto et al., 1999, Japan | To evaluate maternal pNK and dNK in RM with normal chromosomal content | Case control study comparing women with ≥ 3 uRM (n=9) and controls (n=15) | Decidual tissue; Between 5-11 weeks GA | FC; CD45+/CD14-/CD56+ or CD16+ or CD56+/CD16- or CD56+/CD16-/CD3-; Percentage of CD45+CD14- lymphocytes | N/A | uNK level |
| Clifford et al., 1999, UK | To quantify the endometrial CD56+ NK cell population in women with a history of recurrent miscarriage | Case control study comparing women with ≥ 3 uRM (n=29) and fertile controls (n=10) | Endometrial tissue; Between day 7 to 10 after urine LH surge | IHC; CD56+; Absolute number per 10hpf | N/A | uNK level |
| Quenby et al., 1999, UK | To investigate the immunophenotypic profile of the endometrium of women suffering recurrent pregnancy loss | Case control study comparing women with ≥ 3 uRM (n=22) and fertile controls (n=9) | Endometrial tissue; Mid-luteal on day 21 by LMP confirmed by serum oestrogen and progesterone and histological dating (excluded if not day 19-22) | IHC; CD16, CD56, CD57 and CD69; Percentage of total cells per 10 hpf | N/A | uNK level |
| Quack et al., 2001, USA | To determine whether decidual leukocyte subpopulations and their associated activation markers were different between women having RM of either a trisomy 16 compared with a chromosomally normal male conceptus and compared with women having elective pregnancy termination. | Case control study comparing women with ≥ 3 uRM (n=17) and fertile controls (n=20) | Decidual tissue; Between 6-10 weeks GA | IHC; CD56+ Ratio of CD45+ cells and absolute number per 10 hpf | N/A | uNK level |
| Michimata et al., 2002, Japan | To evaluate the ability of immunophenotypes of endometrial leukocytes from patients with histories of recurrent abortion to predict outcome of subsequent pregnancy. | Case control study comparing women with ≥ 2 uRM (n=17) and controls from male infertility (n=15) | Endometrial tissue; Between day 5 to 8 after urine LH surge | IHC; CD45+, CD56+, CD16+; Percentage of CD45+ cells and absolute number per 10 hpf | N/A | uNK level |
| Lédée-Bataille et al., 2004, France | To investigate the endometrial immunohistochemical staining of interleukin (IL)-12 and IL-18 and to quantify the CD56 bright natural killer (NK) cells in relation to Doppler vascular disorder | Case control study comparing women with ≥ 3 RIF (n=35) and fertile controls (n=12) | Endometrial tissue; On Day 20 based on oestrogen-progestin treatment (in patient group and 6 controls) or urinary LH (in 5 controls) | IHC; CD56+; Absolute number per 3 of 100x field | N/A | uNK level and activity |
| Shimada et al., 2004, Japan | To assess the NK cell and NKT cell populations and cytokine expression of T-helper cells in the endometrium of women who suffered from uR | Case control study comparing women with ≥ 3 uRM (n=20) and fertile controls (n=17) | Endometrial tissue; Between day 5 to 9 post ovulation as determined by basal body temperature | FC; CD56+, CD56+CD16+ and CD56+CD16-; Percentage over total lymphocytes | N/A | uNK level |
| Tuckerman et al., 2007, UK | To investigate whether the number of pre-pregnancy endometrial CD56+ cells in women with uRM is able to predict outcome in a subsequent pregnancy. | Case control comparing women with ≥ 3 uRM (n=87) and controls (n=10) | Endometrial tissue; Between day 7 to 10 after urine LH surge | IHC; CD56+; Absolute number in 10 hpf | >13.8% as abnormally high (90th percentile of control group) | uNK level and pregnancy outcome |
| Qu et al., 2008, China | To understand osteopontin expression and regulation in decidua,osteopontin expression was examined in human first-trimester decidua from RM patients | Case control comparing women with uRM (n=22) and controls (n=25) | Decidual tissue; Between 4-9 weeks GA | IHC; CD56+ Staining intensity and absolute number | N/A | uNK staining intensity and activity |
| Ozcimen et al., 2009, Turkey | To compare CD57+ NK cells in normal pregnancies and different types of early pregnancy failure | Case control study comparing women with ≥ 3 uRM (n=23) and controls (n=23) | Decidual tissue; Between 6-12 weeks GA, obtaned within 1 hour of diagnosis of miscarriage by USS | IHC; CD57+; Absolute number per cm2 | N/A | uNK level |
| Bohlmann et al., 2010, Germany | To address the question of possibly altered endometrial immune-cell concentrations | Case control study comparing women with ≥ 2 uRM (n=25) and controls (n=10) | Endometrial tissue; Between day 8 to 9 after urine LH surge | IHC; CD56+; Staining intensity (0-3) | N/A | uNK staining intensity |
| Tuckerman et al., 2010, UK | To investigate whether or not the number of pre-pregnancy endometrial CD56 cells in women with unexplained RM is able to predict outcome in a subsequent pregnancy. | Case control study comparing women with RIF after ≥ IVF cycles or ≥ 2 IVF plus 2 frozen ET cycles (n=40) and controls (n=15) | Endometrial tissue; Between day 7 to 9 after urine LH surge | IHC; CD56+ and CD16+; Percentage of total stromal cells | >13.8% as abnormally high (90th percentile of control group) | uNK level and pregnancy outcome |
| Laird et al., 2011, UK | To compare numbers of CD56+ cells in peripheral blood and endometrium | Case control study comparing eNK and pNK in women with ≥ 3 uRM (n=25); Separate analysis of IHC vs FC of CD56+ eNK in fertile controls (n=20) | Endometrial tissue; Between day 7 to 9 after urine LH surge | IHC; CD56+; Percentage of total stromal cells | N/A | uNK and pNK correlation |
| Parkin et al., 2011, USA | To determine if there is a functional difference between the uNK in women with UI versus those with uRM, the percentage of NK cells (CD56+) as well as NK cell cytotoxic(CD16) and inhibitory (NKG2a) receptors were compared to controls | Case control study comparing women with uRM (n=24) and fertile controls (n=10) | Endometrial tissue; Mid-secretory phase | IHC; CD56+; Percentage of stromal cells for uNK level | N/A | uNK level only |
| Santillan et al., 2015, Spain | To define the candidates for this test and to find the best methodology and to establish a reasonable cut-off value for normal uNK and to check if pNK correlate with uNK | Case control study comparing women with ≥ 3 RIF (idiopathic, n=32; other causes, n=41) and fertile controls (n=17) | Endometrial tissue; between Day 19 to 23 based on LMP in women with regular periods or Day 5 to 9 after positive urine LH | For uNK: IHC, CD56+, Percentage of total stromal cells and absolute number per 10 hpf For pNK: FC, CD3-CD56+ or CD16+, percentage of total lymphocytes | For uNK: Low (<150 cells/10 hpf), Moderate (150-250 cells/10hpf), Intense (>250 cells/hpf); For pNK: High (>12% of total lymphocyte) | uNK level, correlation pNK and uNK; uNK activity |
| Giuliani et al., 2014, USA | To compare the expres-sion of CD56, CD16, and NKp46 in the eutopic endometrium from women with uRM or UI to fertile patients and correlate this with the presence or the absence of endometriosis | Case control study comparing women with ≥ 2 uRM patients (n=13) and fertile controls (n=10) | Endometrial tissue; between Day 7 to 9 post urine LH surge | IHC; CD56+ and CD16+; Percentage to total endometrial stromal cells | N/A | uNK level |
| Junovich et al., 2013, Argentina | To determine uterine and systemic values of CD16+/- NK cells, IL-6, and VEGF during the implantation window and to establish a correlation between the number and phenotype of endometrial versus peripheral blood NK cells in unexplained infertility patients with RIF compared to fertile women. | Case control study comparing women with unexplained infertility and ≥ 2 failed IVF versus fertile controls | Endometrial tissue; between Day 5 to 9 after ovulation confirmed by USS | FC; CD56+CD9+ and CD56+CD16+CD9+; Percentage over 1x105 endometrial cells | N/A | uNK level, correlation and activity |
| Fu et al., 2013, China | To study function of dNK and successful pregnancy and examine NK cell subsets, distribution and cytokine secretion profiles. | Prospective case control comparing uRM and controls | Decidual tissue; Between 6-12 weeks confirmed by USS | FC, CD3- /CD56+; Percentage NOS | N/A | uNK level and activity |
| Liu et al., 2014, UK | To examine the hypothesis that prognostic value of uNK measurement on pregnancy outcome is improved when combined with histological dating | Retrospective study of women with ≥ 3 uRM and ≥ 3 RIF | Endometrial tissue; between Day 7 to 9 post urine LH surge | IHC; CD56+; Percentage of total stromal cells | >13.9% as abnormally high | Pregnancy outcome |
| Wang et al., 2014, China | To investigate the relationship between expression of KIR on dNK and gestational age in this relationship | Case control study comparing women with ≥ 2 uRM patients (n=30) and fertile controls (n=30) | Decidual tissue; At first trimester, gestational age dated by LMP | FC; CD56+, CD56+/CD16-; CD56+/CD16+, CD56-/CD16+, CD56-CD16-; Percentage of total leucocytes | N/A | uNK level and activity |
| Hosseini et al. (2014), Iran | To compare NK cell subsets in menstrual and peripheral blood of RM patients and fertile women | Case control study comparing women with ≥ 2 uRM patients (n=14) and fertile controls (n=9) | Menstrual blood | FC; CD3-CD56+CD16+, CD3-CD56+CD16+ | N/A | uNK level and activity |
| Sotnikova et al., 2014, Russia | To detail the mechanisms of the interaction of dNK and trophoblast cells during normal and pathological early pregnancy. | Case control study comparing women with ≥ 2 uRM patients (n=26) and controls (n=37) | Decidual tissue; Between 7-11 weeks GA | FC; CD45+/CD14-/CD56+; Percentage of CD45+CD14- lymphocytes | N/A | uNK level and activity |
| Gao et al., 2015, China | To measure the frequency of T and NK cells in uRM patients and normal pregnant women by FC. | Case control study comparing women with uRM patients (n=30) and controls (n=30) | Decidual tissue; In first trimester pregnancy | FC; CD16+CD56+; Percentage of total lymphocytes | N/A | uNK level |
| Almasry et al., 2015, Saudi Arabia | To evaluate the remodelling of decidual spiral arteries in the early human deciduas in women with uRM and their possible relationship with the immunoexpressive behaviour and ultrastructural properties of dNKC | Case control study comparing women with ≥ 3 uRM (n=40) and fertile controls (n=30) | Decidual tissue; Between 6-10 weeks GA | IHC; Absolute number of cells/mm2 per 5 hpf | N/A | uNK level and activity |
| Eskicioğlu et al., 2016, Turkey | To determine role of HLA-G, CD8, CD16, CD56, IFN-g and TNF-a for RM in feto–maternal interface. | Case control study comparing women with ≥ 2 uRM patients (n=10) and controls (n=11) | Decidual tissue; Between 6-11 weeks GA for RM and <10 weeks GA for controls; | Western blot; CD56+ and CD16+ Protein expression normalised to B-Actin band intensities | N/A | uNK level |
| Radović Janošević et al., 2016, Serbia | To identify subpopulations of decidual lymphocytes of RM by IHC study of decidua | Case control study comparing women with ≥ 3 uRM patients (n=30) and controls (n=30) | Decidual tissue; at time of miscarriage or TOP | IHC; CD56+ or CD57+ Absolute number of cells per 10 hpf | N/A | uNK level |
| Chen et al., 2017, Hong Kong | To compare the uNK percentage in women with recurrent reproductive failure and fertile controls | Case control study comparing women with ≥ 3 uRM patients (n=97), RIF patients after ≥ 3 fresh /frozen cycle embryo transfer (n=34) and controls (n=84) | Endometrial tissue; Day 7 post urine LH surge | IHC; CD56+; Percentage of stromal cells per 10 hpf | N/A | uNK level |
| Jiang et al., 2017, China | To define a more precise parameter for a better understanding of NK cells and its relation with Tregs in women with RIF. | Case control study comparing women with ≥ 2 RIF (n=32) and controls with male infertility (n=23) | Endometrial tissue; At Day 7 post urine LH surge | IHC; CD56+ and CD57+; Percentage of total stromal cells | N/A | uNK level and activity |
| Kuon et al., 2017b, Germany | To analyze uNK cell concentration in the endometrium of idiopathic RM patients and fertile controls to establish possible cut-off values. | Case control study comparing women with ≥ 3 uRM (n=58) and fertile controls (n=217) | Endometrial tissue; Day 7-10 post urine LH surge, confirmed by endometrial glands and stroma evaluation | IHC; CD56+; Absolute number cells/mm2 | N/A | uNK level |
| Dong et al., 2017, China | To define the decidual immune cells and to simultaneously detect changes in the Th1/Th2 and decidual NK1 /decidual NK2 ratios of the decidual tissues using FC. | Case control study comparing women with ≥ 2 uRM (n=20) and fertile controls (n=20) | Decidual sample; 1st trimester (6-11 weeks), obtained by ERPC within 3 days of fetal loss in uRM or 7 days in controls | FC; CD3-CD56+/CD56brightCD16- Percentage of parental population (i.e. CD3-CD56+) | N/A | uNK level and activity |
| Guo et al., 2017, China | To confirm that miR-133a negatively regulates HLA-G expression to influence dNK function via KIR2DL4 in RM patients. | Case control study comparing women with R (n=11) and fertile controls (n=12) | Decidual sample; At 7 to 12 weeks GA | FC; CD45+/CD3-/CD56+/CD56brightCD16-; Percentage of CD45+/CD3- lymphocytes | N/A | uNK level and activity |
| El-Azzamy et al., 2018, USA | To investigate a possible role of uNK cells in vascular development and describe characteristics of endometrial vascular patterns in women with RPL | Case control study comparing women with uRM (n=15) and controls (n=7) | Endometrial tissue; Day 7-9 post urine LH surge | IHC; CD56+ and CD16+; Absolute number of positive cells per mm2 | N/A | uNK level and activity |
| Liu et al., 2019, China | To investigate whether Th, Tc, NK and NKT cells population and cytokine expression is associated with msicarriages with abnormal chromosome karyotype | Case control study comparing women with uRM (n=10) and controls (n=21) | Decidual sample; At 6-10 weeks GA | FC; CD3-CD56brightCD16-; Percentage of lymphocytes NOS | N/A | uNK level and activity |
| Marron et al., 2019, Irelanda | To determine whether endometrial immune profiles in adverse reproductive outcomes such as RIF and RM differ from each other and male-factor controls | Case control study comparing women with ≥ 2 RIF (n=181),≥ 2 RM (n=155) and male factor infertility controls (n=35) | Endometrial tissue; Day 21 to 24 menstrual cycle after HRT (5 days of vaginal progesterone) | FC; Total CD56 as percentage over total endometrial cells and CD45+CD3-CD5-CD56brightCD16- as percentage over CD45+ leucocytes | N/A | uNK level only |
| Marron and Harrity, 2019b, Irelanda | To describe a novel technique for calculation of local endometrial lymphocyte concentrations, and to compare results between RIF and RM with male-factor controls. | Case control study comparing women with ≥ 2 RIF (n=149),≥ 2 RM (n=121) and male factor infertility controls (n=29) | Endometrial tissue; Day 21 to 24 menstrual cycle after HRT (5 days of vaginal progesterone) | FC; Median uNK concentration (cells/mg) | >90th centile versus 25th to 75th centile | Pregnancy outcome only |
| Lu et al., 2020, China | To explore expression of CD82 on dNK and role of trophoblast cells, CD29 and CD82 on adhesive ability of dNK to DSC in vitro | Case control study comparing women with ≥ 2 uRM (n=8) and controls (n=45) | Decidual tissue; 1st trimester (6-8 weeks) | FC; CD45+CD3-CD56bright or CD56dim; Percentage of over total CD56 | N/A | uNK level and activity |
| Wei et al., 2020, China | To localize IDO in the endometrium, investigate IDO expression between patients associated with RM andhealthy fertile controls, and undertake a correlation study on IDO and other immune cells. | Case control study comparing women with ≥ 2 uRM (n=58) and male factor infertility controls (n=49) | Endometrial tissue; At mid luteal phase confirmed by H&E staining | IHC; CD56+; Percentage of total endometrial cells | N/A | uNK level and activity |
| Lyzikova et al., 2020, Belarus | To investigate if dysregulation of uNK, FoxP3 cells, PGRMC1 expression and if crosstalk between these factors play a role in RM. | Case control study comparing women with ≥ 2 uRM (n=39) and controls (n=63) | Endometrial tissue; Day 7 to 9 after ovulation confirmed by USS | IHC; CD56+; Absolute number of cells/mm2 per 10hpf | N/A | uNK level and activity |
| Zhao et al., 2020, Hong Kong | To investigate the density and clustering of four different immune cells simultaneously in precisely timed endometrial specimens and compared the results between women with RM and fertile controls subjects | Case control study comparing women with ≥ 3 uRM (n=39) and fertile controls (n=63) | Endometrial tissue; Day 7 after LH surge | IHC; CD56+; Percentage of total stromal cells | N/A | uNK level |
| Babayeva et al. (2020), Turkey | To evaluate whether there was a significant difference in the number of endometrial CD56+ NK between women with RIF and women who had a live birth. | Case control study comparing women with ≥ 3 RIF (n=25) and fertile controls (n=25) | Endometrial tissue; Day 21 to 24 based on LMP | IHC; CD56+; Absolute number of cells/mm2 | N/A | uNK level |
| Chen et al. (2021), Hong Kongb | To investigate if frequency of euploid miscarriage is increased in women with high uterine CD56+ cell density | Prospective cohort study comparing women with ≥ 3 uRM (n=42) and controls (n=12) from previous study | Endometrial tissue; Day 7 after LH surge | IHC; CD56+; Percentage of CD56+ uNK in | > 4.5% as abnormally high | Pregnancy outcome only |
Studies with duplicate data, different data were extracted from each study for separate meta-analyses;
uNK level data not extracted as controls from previous study. uRM, unexplained recurrent miscarriage; RIF, recurrent implantation failure; IVF, in vitro fertilization; GA, gestational age; ERPC, evacuation of retained products of conception; LMP, last menstrual period; USS, ultrasound; LH, luteinizing hormone; HRT, hormone replacement therapy; IHC, immunohistochemistry; FC, flow cytometry; hpf, high power field; H&E, haematoxylin and eosin; NK, Natural Killer; uNK, uterine Natural Killer cells; pNK, peripheral Natural Killer cells; dNK, decidual Natural Killer cells; NKT, Natural Killer-T cells; Th, T helper cells; Treg, T regulatory cells; TCR, T-cell receptor; IL, interleukin; bcl-2, B-cell leukaemia/lymphoma 2; PGRMC1, progesterone receptor membrane component 1; IDO, indoleamine 2,3 dioxygenase
Diversity was detected in the inclusion and exclusion criteria; five studies specifically stated exclusion of abnormal foetal karyotype, nine studies excluded abnormal parental karyotype, whereas seven studies excluded abnormal ‘genetic’, ‘chromosome’ or ‘karyotype’. Not all of the studies specified age as a limiting factor; and some studies controlled for this confounding variable by performing statistical tests of significance between the patient and control groups. There was also variability in the definition of the control group; 16 studies defined controls as having previous successful livebirths, five studies defined controls as having male factor infertility, 10 studies defined controls who had no history of previous miscarriages or failed IVF, 15 studies defined controls as women with healthy pregnancies who opted to undergo elective termination of pregnancy, and six studies did not explicitly state the pregnancy history of their control group.
The types of samples studied were divided into endometrial tissue obtained from non-pregnant women or decidual tissue obtained after surgical management of miscarriage or termination of pregnancy. For endometrial tissue, nearly all the samples were obtained at mid-luteal phase but method of timing for ovulation varied; 18 studies timed by urine LH, three studies by oestrogen–progesterone therapy, two studies by last menstrual period, two studies by histological dating, and one study used basal body temperature and ultrasound to detect ovulation. Another study used menstrual blood as a source of endometrial tissue (Hosseini et al., 2014). For studies which used decidual tissue obtained at time of surgical procedure, the gestational age ranged from 4 to 12 weeks of pregnancy.
The two main methods of analysis were immunohistochemistry (23 studies) and flow cytometry (14 studies); one study used western blot to quantify CD56 protein expression. In studies which used flow cytometry, there was variation in gating strategy used and the resultant unit of measurement included percentage of total endometrial cells, total leucocytes, CD45+ leucocytes, CD45+CD3− leucocytes, or CD45+CD3−CD56+ lymphocytes. These studies presented their data as total CD56+, CD56+CD16− CD56brightCD16−, CD56+CD16+ or CD57+ uNK.
Studies which used immunohistochemistry expressed uNK level as either percentage of total stromal cells, absolute count or staining intensity. Absolute count was either expressed as number of cells per 10×400 fields, per 3×100 field or per millimetre square. Some studies using immunohistochemistry also identified CD16+ cells as uNK, however, this was considered separately from uNK in our meta-analysis as CD16 is neither a marker of uNK nor an exclusive marker of pNK.
There was no universal reference range used to define normal uNK levels in studies which evaluated pregnancy outcome stratified by normal and high uNK level. Three papers from the same research group (Tuckerman et al., 2007, 2010; Liu et al., 2014) defined high uNK as >13.9% CD56+ cells over total stromal cells assessed by immunohistochemistry. Chen et al. (2021) used the same methodology of uNK count but reported high uNK level as >4.5% based on a value obtained for >95th percentile of 72 fertile controls established in a previous study (Chen et al., 2017). On the other hand, Marron et al. (2019) reported high uNK level based on a cut-off of >90th percentile in a total population of controls, infertile, RIF and RM patients of 455 women. This was expressed as absolute number of cells per mg of endometrial tissue as assessed by flow cytometry.
Quality assessment
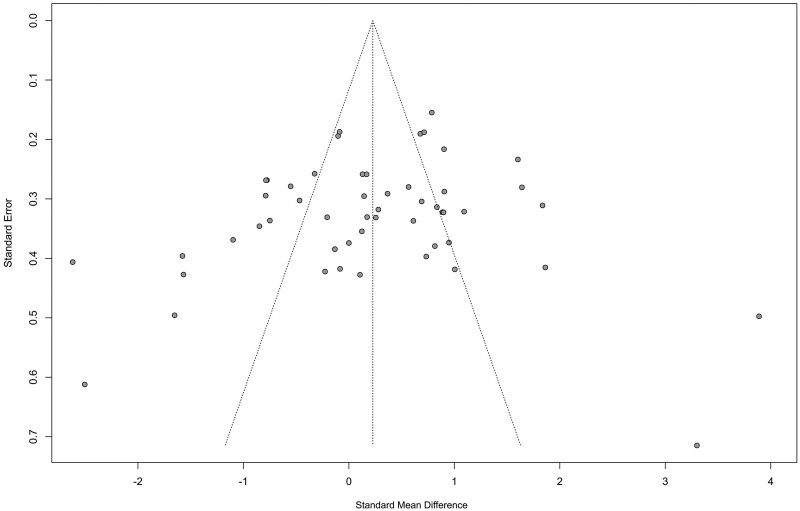
ROBINS-I tool was used to assess the methodological quality of the studies. Studies which did not control for confounding factors, such as maternal age or gestational age, were rated as having serious risk of bias (Table II). In view of inherent changes to immune cells secondary to inflammatory processes, studies that compared decidual samples obtained after miscarriage and termination of an ongoing pregnancy were deemed as having a minimum of moderate risk of bias. Publication bias was not significant for all studies included in the meta-analyses of uNK level (Egger’s test, P = 0.15; Fig. 2).
Table II.
Risk of bias in non-randomized studies of interventions of uNK level/activity in women with RM/RIF compared with healthy controls.
| Study ID | Pre-intervention |
At intervention |
Post-intervention |
Total score | ||||
|---|---|---|---|---|---|---|---|---|
| Confounding bias | Selection bias | Classification bias | Deviation bias | Missing data bias | Measurement of outcome bias | Selective reporting bias | Overall risk of bias judgement | |
| Chao et al. (1995) | Serious | Low | Low | Low | Serious | Moderate | Low | Serious |
| Lachapelle et al. (1996) | Moderate | Low | Low | Low | Serious | Moderate | Moderate | Serious |
| Lea et al. (1997) | Serious | Low | Low | Low | Low | Moderate | Low | Serious |
| Yamamoto et al. (1999) | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Clifford et al. (1999) | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Kwak et al. (1999) | Serious | Moderate | Low | Low | Low | Serious | Low | Serious |
| Quenby et al. (1999) | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Quack et al. (2001) | Serious | Low | Low | Low | Low | Moderate | Low | Serious |
| Emmer et al. (2002) | Serious | Serious | Low | Low | Moderate | Low | Low | Serious |
| Michimata et al. (2002) | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Lédée et al. (2004) | Serious | Low | Low | Low | Moderate | Moderate | Moderate | Serious |
| Shimada et al. (2004) | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Lédée-Bataille et al. (2005) | Serious | Low | Low | Low | Low | Low | Low | Serious |
| Yan et al. (2007) | Serious | Low | Moderate | Low | Serious | Moderate | Low | Serious |
| Tuckerman et al. (2007) | Serious | Low | Low | Low | Low | Low | Low | Serious |
| Qu et al. (2008) | Serious | Low | Moderate | Low | Moderate | Moderate | Low | Serious |
| Lédées et al. (2008) | Serious | Low | Low | Low | Low | Moderate | Serious | Serious |
| Ozcimen et al. (2009) | Moderate | Low | Low | Low | Moderate | Moderate | Low | Moderate |
| Bohlmann et al. (2010) | Serious | Low | Low | Low | Low | Low | Low | Serious |
| Tuckerman et al. (2010) | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Park et al. (2010) | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Laird et al. (2011) | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Parkin et al. (2011) | No info | Low | Low | Low | No info | Low | Low | No info |
| Bao et al. (2012) | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Santillán et al. (2015) | Serious | Low | Low | Low | Low | Moderate | Low | Serious |
| Mariee et al. (2012) | Serious | Low | Low | Low | Critical | Low | Low | Critical |
| Giuliani et al. (2014) | Moderate | Moderate | Low | Low | Low | Low | Serious | Serious |
| Junovich et al. (2013) | Serious | Low | Low | Low | Serious | Low | Low | Serious |
| Fu et al. (2013) | Serious | Low | Low | Low | Serious | Low | Moderate | Serious |
| Wang et al. (2014) | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Sotnikova et al. (2014) | Serious | Low | Low | Low | Low | Moderate | Moderate | Serious |
| Hosseini et al. (2014) | Moderate | Low | Low | Low | Moderate | Moderate | Moderate | Moderate |
| Liu et al. (2014) | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Gao and Wang (2015) | Serious | Low | Moderate | Low | Low | Low | Low | Serious |
| Almasry et al. (2015) | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Eskicioğlu et al. (2016) | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Radović Janošević et al. (2016) | Serious | Low | Low | Low | Low | Moderate | Low | Serious |
| Chen et al. (2017) | Serious | Low | Low | Low | Low | Low | Low | Serious |
| Chen et al. (2018) | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Jiang et al. (2017) | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Kuon et al. (2017b) | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Kuon et al. (2017a) | Serious | Low | Low | Low | Low | Moderate | Moderate | Serious |
| Fukui et al. (2017) | Moderate | Low | Moderate | Low | Moderate | Moderate | Moderate | Moderate |
| Dong et al. (2017) | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Guo et al. (2017) | Serious | Low | Moderate | Low | Low | Moderate | Low | Serious |
| El-Azzamy et al. (2018) | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Liu et al. (2019) | Moderate | Low | Serious | Low | Low | Moderate | Low | Serious |
| Marron and Harrity (2019) | Serious | Low | Low | Low | Low | Low | Moderate | Serious |
| Marron et al. (2019) | Serious | Low | Low | Low | Low | Moderate | Moderate | Serious |
| Li et al. (2019) | Moderate | Low | Low | Low | Serious | Low | Low | Serious |
| Huang et al. (2019) | Serious | No information | Serious | Low | Moderate | Moderate | Low | Serious |
| Toth et al. (2019) | Moderate | Low | Low | Low | Serious | Low | Low | Serious |
| Lu et al. (2020) | Serious | Low | Low | Low | Serious | Moderate | Serious | Serious |
| Wei et al., (2020) | Moderate | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Lyzikova et al. (2020) | Serious | Low | Moderate | Low | Low | Moderate | Low | Serious |
| El-Badawy et al. (2020) | Serious | Low | Low | Low | Low | Moderate | Moderate | Serious |
| Zhao et al. (2020) | Serious | Low | Low | Low | Low | Low | Low | Serious |
| Babayeva et al. (2020) | Moderate | Low | Low | Low | Low | Serious | Serious | Serious |
| Chen et al. (2021) | Moderate | Low | Low | Low | Low | Moderate | Low | Moderate |
| Liu et al. (2020) | Moderate | Moderate | Low | Low | Low | Moderate | Low | Moderate |
RIF, recurrent implantation failure; RM, recurrent miscarriage.
Figure 2.
Funnel plot of all the studies included in the meta-analyses of uterine natural killer level.
Meta-analysis: uNK cell level
Recurrent miscarriage
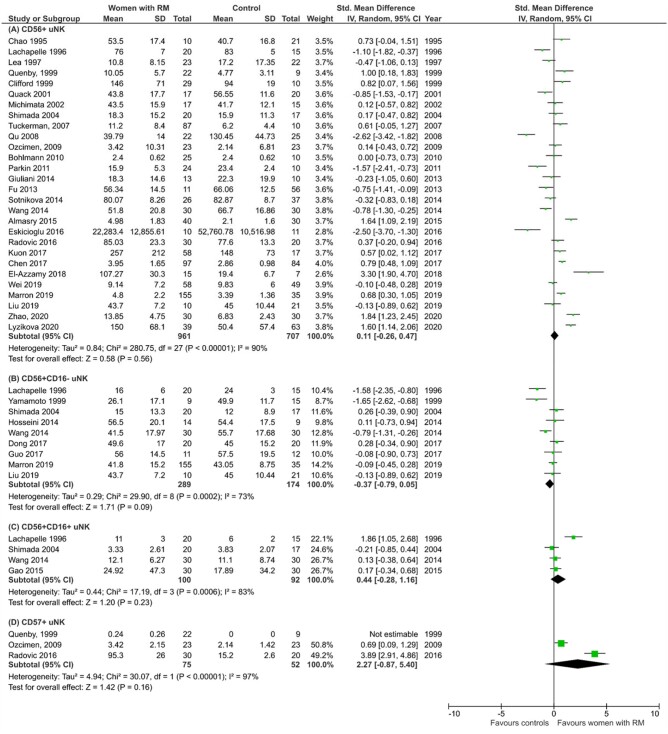
A total of 33 studies reported on uNK level in women with RM compared with controls, including 28 studies on total CD56+ cells (Chao et al. 1995; Lachapelle et al., 1996; Lea et al., 1997; Clifford et al., 1999; Quenby et al., 1999; Quack et al., 2001; Michimata et al., 2002; Shimada et al., 2004; Tuckerman et al., 2007; Qu et al., 2008; Ozcimen et al., 2009; Bohlmann et al., 2010; Parkin et al., 2011; Fu et al., 2013; Giuliani et al., 2014; Sotnikova et al., 2014; Wang et al., 2014; Almasry et al., 2015; Eskicioğlu et al., 2016; Radović Janošević et al., 2016; Chen et al., 2017; Kuon et al., 2017b; El-Azzamy et al., 2018; Liu et al., 2019; Marron and Harrity 2019; Wei et al., 2020; Lyzikova et al. 2020; Zhao et al., 2020), nine studies on CD56+CD16− cells (Lachapelle et al., 1996; Yamamoto et al., 1999; Shimada et al., 2004; Hosseini et al., 2014; Wang et al., 2014; Dong et al., 2017; Guo et al., 2017; Liu et al., 2019; Marron and Harrity 2019), four studies on CD56+CD16+ cells (Lachapelle et al., 1996; Shimada et al., 2004; Wang et al., 2014; Gao and Wang, 2015) and three studies on CD57+ cells (Quenby et al., 1999; Ozcimen et al., 2009; Radović Janošević et al., 2016). As mentioned above, it is important to note that total CD56+ cells represent both uNK and pNK in the uterus, CD56+CD16− cells are predominantly uNK and CD56+CD16+ cells represent pNK in the uterus. CD57 is primarily a marker of mature circulating NK cells (Lopez-Vergès et al., 2010); where decidual NK cells have been examined for this marker, they do not express it (Gamliel et al., 2018).
Meta-analyses of different phenotypes of NK cells found in the uterus are presented in Fig. 3. Overall, there was no significant difference in total CD56+ cells (SMD 0.11, CI −0.26, 0.47; P = 0.56, I2 90%; total 1668 women; Fig. 3A), CD56+CD16− cells (SMD −0.37, CI −0.79, 0.05; P = 0.09; I2 73%; total 463 women; Fig. 3B), CD56+CD16+ cells (SMD 0.44, CI −0.28, 1.16; P = 0.23; I2 83%; total 192 women; Fig. 3C) and CD57+ cells (SMD 2.27, CI −0.87, 5.40; P = 0.16; I2 97%; total 127 women; Fig. 3D) in women with RM compared with controls.
Figure 3.
Level of uNK in women with recurrent miscarriage compared with controls. (A) Total CD56+ uNK. (B) CD56+CD16− uNK. (C) CD56+CD16+ uNK. (D) CD57+ uNK. RM, recurrent miscarriage; uNK, uterine natural killer cells.
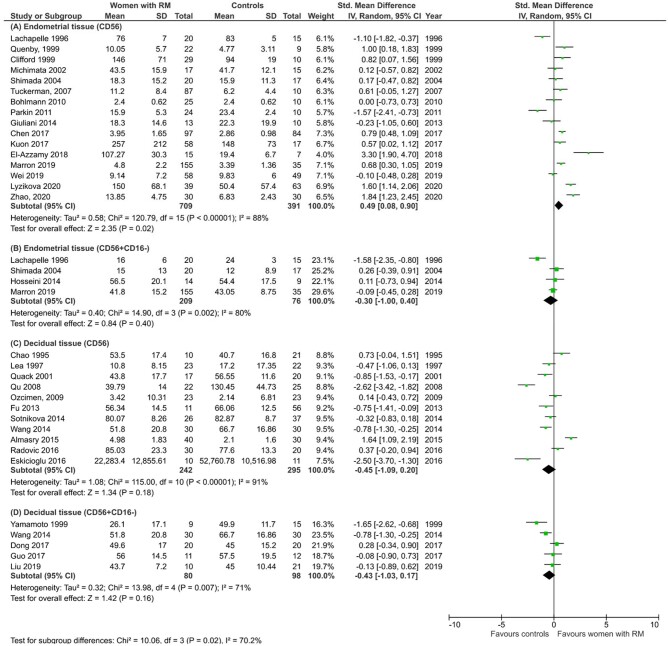
Although each of the subtypes of CD56+ uNK showed no significant difference, subgroup analysis showed significantly higher total CD56+ uNK in women with RM compared with controls in studies using endometrial samples from mid-luteal phase only (SMD 0.49, CI 0.08, 0.90; P = 0.02; I2 88%; total 1100 women; Fig. 4A). This observation was not replicated when decidual tissue in first trimester pregnancy was studied (SMD −0.45, CI −1.09, 0.20; P = 0.18; I2 91%; total 537 women; Fig. 4C). No significant difference was detected by subgroup analysis of CD56+CD16− cells in either endometrial or decidual tissue (Fig. 4B and D). When the patient population was stratified by primary or secondary RM and by two or three previous miscarriages, there was no significant difference in subgroup analysis of CD56+ or CD56+CD16− cells level (Supplementary Fig. S1). Further, no significant difference was found when the studies were categorized by methodology, i.e. immunohistochemistry or flow cytometry and by unit of measurement, i.e. percentage of lymphocytes, percentage of total endometrial cells or absolute cell count (Supplementary Fig. S2). Interestingly, meta-analysis of CD16+ leucocytes (which represent a mixture of pNK, monocytes and macrophages) measured by immunohistochemistry showed a significantly higher level in women with RM compared with controls (SMD 0.55, CI 0.27, 0.83; P = 0.0001; I2 23%; total 223 women; Supplementary Fig. S3).
Figure 4.
Subgroup analyses of of uNK level in women with recurrent miscarriage compared with controls. (A) Endometrium CD56+ uNK. (B) Endometrium CD56+CD16− uNK. (C) Decidua CD56+ uNK. (D) Decidua CD56+CD16− uNK.
Sensitivity analyses did not reveal a change in the main meta-analysis result, including exclusion of 14 studies without fertile controls, one study with abstract only, three studies with male factor infertility controls, one study which used hormonal therapy at time of sampling, 19 studies with serious risk of bias, seven studies in which mean and standard deviation was derived from median, interquartile range or range, and five studies in which data were extracted from the graph (Supplementary Table SIV). However, when sensitivity analysis was performed for the subgroup of CD56+ uNK in endometrium to exclude six studies in which data were extracted from graphs or converted from median, the previously demonstrated significant result became not significant (SMD −0.04, CI −0.45, 0.36; P = 0.83; I2 75%; total 514 women; Supplementary Table SIV).
Recurrent implantation failure
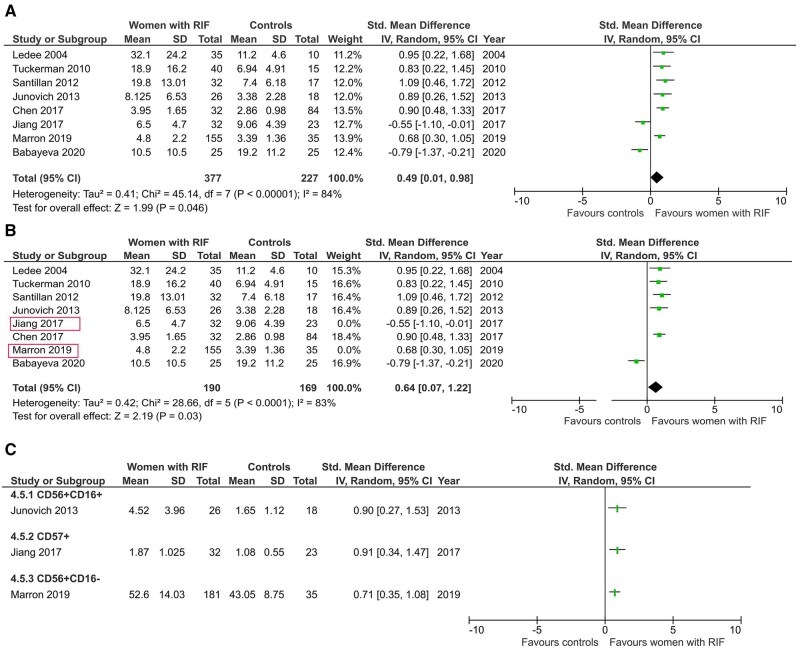
All eight studies on women with RIF measured total CD56+ cells level using endometrial samples obtained during mid-luteal phase (Lédée-Bataille et al., 2004; Tuckerman et al., 2010; Santillán et al., 2015; Junovich et al., 2013; Chen et al., 2017; Jiang et al., 2017; Marron and Harrity 2019; Babayeva et al., 2020). Out of these, three studies also measured CD56+CD16− (Marron and Harrity 2019), CD56+CD16+ (Junovich et al., 2013) and CD57+ cells (Jiang et al., 2017). Meta-analysis showed a significant difference in total CD56+ cells in women with RIF compared with controls (SMD 0.49, CI −0.01, 0.98; P = 0.046; I2 84%; total 604 women; Fig. 5A).
Figure 5.
uNK level in women with recurrent implantation failure compared with controls. (A) Total CD56+ uNK in endometrium. (B) Sensitivity analysis of CD56+ uNK level excluding male factor infertility. (C) Individual study comparison of CD56+CD16+, CD57+ and CD56+CD16−. RIF, recurrent implantation failure.
Sensitivity analysis performed after excluding two studies that used male factor infertility controls (Jiang et al., 2017; Marron and Harrity, 2019) also showed a significantly higher uNK level in women with RIF compared with controls (SMD 0.64, CI 0.07, 1.22; P = 0.03; I2 79%; total 359 women; Fig. 5B). However, this difference lost statistical significance following sensitivity analyses by exclusion of two studies which did not exclusively use fertile controls, two studies which included hormonal intervention, six studies with serious risk of bias, four studies where mean and standard deviation were converted from median and interquartile range and/or range, and two studies from which information was extracted from the graph (Supplementary Table SV).
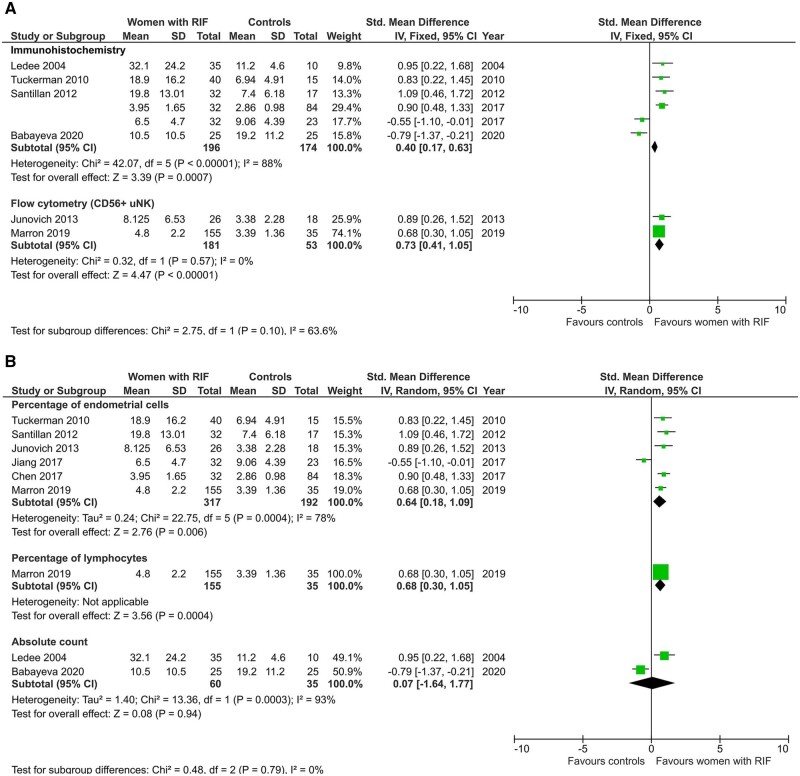
Studies that were stratified by method of analysis showed a significant difference of CD56+ cells level when measured by either immunohistochemistry or flow cytometry (SMD 0.40, CI 0.17, 0.63; P < 0.01; I2 88%; total 370 women and SMD 0.73, CI 0.41, 1.05; P ≤ 0.01; I2 0%; total 234 women; Fig. 6A). Subgroup analysis by unit of measurement showed that CD56+ cells are significantly higher in women with RIF when expressed as percentage of endometrial/stromal cells (SMD 0.64, CI 0.18, 1.09; P = 0.0004; I2 78%; total 509 women; Fig. 6B) but not as absolute count of cells (SMD 0.07, CI −1.64, 1.77; P = 0.94; I2 93%; total 95 women; Fig. 6B).
Figure 6.
Subgroup analyses of uNK level in women with RIF compared with controls. (A) By method of analysis. (B) By unit of measurement.
Meta-analysis: pregnancy outcome
Pregnancy rate
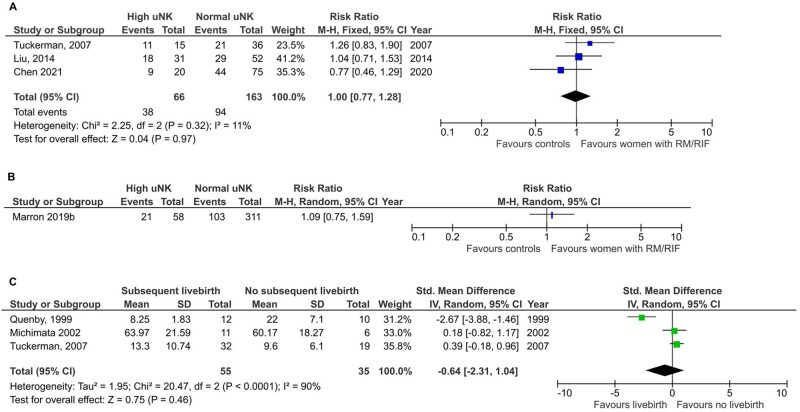
We found seven studies that followed up women with RM until the next pregnancy (Lachapelle et al., 1996; Quenby et al., 1999; Michimata et al., 2002; Tuckerman et al., 2007; Liu et al., 2014; Marron et al., 2019; Chen et al., 2021), three of which reported livebirth rate (Tuckerman et al., 2007; Liu et al., 2014; Chen et al., 2021) and one (Marron et al., 2019) that reported CPR in women with RM stratified by high vs normal uNK level.
Meta-analysis of livebirth rate showed no significant difference in women with RM and RIF with high uNK compared with normal uNK (RR 1.00, CI 0.77, 1.28; P = 0.97; I2 11%; total 229 women; Fig. 7A). The CPR reported by Marron et al. (2019) was also not significantly different (RR 1.09; CI 0.75, 1.59; total 369 women; P = 0.29; Fig. 7B).
Figure 7.
Meta-analysis by pregnancy outcome. (A) Livebirth outcome in women with RM/RIF and normal compared with high uNK level. (B) Individual study comparison of clinical pregnancy rate in women with RM/RIF and normal compared with high uNK level. (C) uNK level in women with RM/RIF with subsequent livebirth compared with miscarriage. Marron 2019b refers to Marron and Harrity, 2019
uNK levels
A meta-analysis of three studies (Quenby et al., 1999; Michimata et al., 2002; Tuckerman et al., 2007) that retrospectively reported on uNK level in women with RM/RIF who had livebirth compared with miscarriage showed no significant difference (SMD −0.64, CI −2.31, 1.04; P = 0.46; I2 90%; total 90 women; Fig. 7C). Lachapelle et al. (1996) also did not find any significant difference in livebirth rate or uNK level but did not report on the values.
Meta-analysis: correlation between peripheral and uNK cells
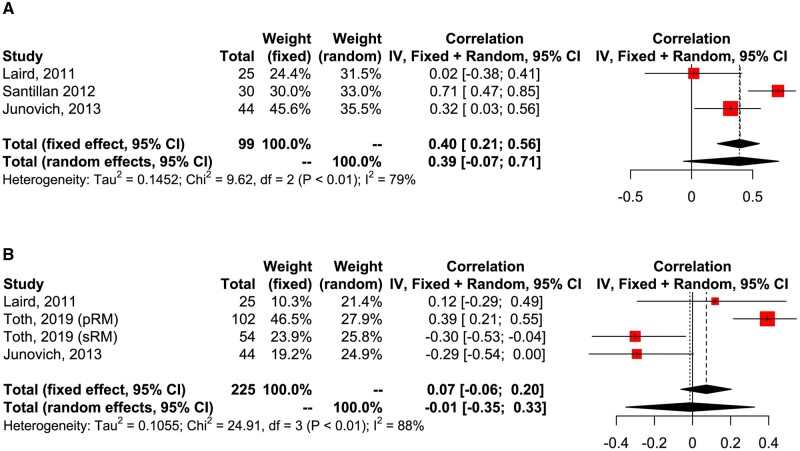
Seven studies reported on correlation between peripheral and uNK cells. Five studies were on women with RM (Park et al., 2010; Laird et al., 2011; Kuon et al., 2017a; Toth et al., 2019; El-Badawy et al., 2020) and two studies on women with RIF (Santillán et al., 2015; Junovich et al., 2013). One study further stratified women with RM into primary RM and secondary RM (Toth et al., 2019). The phenotypes of pNK studied by flow cytometry were variable depending on gating strategy and included total CD56 (Park et al., 2010; Laird et al., 2011; Santillán et al., 2015; Junovich et al., 2013), CD56+CD16+ (Park et al., 2010; Laird et al., 2011; Junovich et al., 2013; Toth et al., 2019), CD56+CD16− (Laird et al., 2011), CD56 or CD16 (Santillán et al., 2015) and CD56dim pNK (El-Badawy et al., 2020).
Meta-analysis of coefficient correlation (CC) was performed only in studies which assessed phenotype of pNK and uNK with the same method. This revealed no significant positive correlation in either total CD56+ pNK and CD56+ cells in the endometrium (pooled CC 0.40; CI 0.21, 0.56; P = 0.10; I2 = 79%; total 99 women; Fig. 8A) or CD56+CD16+ pNK and CD56+ cells in the endometrium (pooled CC 0.07; CI −0.06, 0.20; P = 0.08; I2 = 88%; total 225 women; Fig. 8B). Kuon et al. (2017a) also reported no correlation between CD56+ pNK and CD56+ cells in the endometrium, but this was not included in meta-analysis as r value was not reported.
Figure 8.
Correlation between peripheral NK and uNK. (A) Meta-analysis of coefficient correlation of CD56+CD16− pNK and CD56 uNK. (B) Meta-analysis of coefficient correlation of CD56+CD16+ pNK and CD56+ uNK. pNK, peripheral natural killer cells.
Sensitivity analysis by eliminating studies which combined both fertile women and women with RIF, women with primary RM or secondary RM did not significantly change the result of the meta-analysis (Supplementary Table SVI).
A single study reported a significant correlation between CD56+ cells in decidua of first trimester pregnancy and peripheral blood but this study was not included in the meta-analysis, which focussed on studies on the endometrium (Park et al., 2010). Of note, another study by El-Badawy et al. (2020) reported significant correlation between CD56dim pNK and all of CD56 dim, CD56 bright and CD16dim cells in the menstrual blood.
Narrative synthesis: uNK cells activity
The studies on uNK activity were not amenable to meta-analysis owing to significant heterogeneity, therefore, we have chosen to summarize the findings by narrative synthesis. They were grouped according to outcome measure into four broad functional categories: regulation and receptors; cytotoxicity; expression of cytokines; and effect on uterine vasculature, and the corresponding findings from these studies are found in Tables III–VI, respectively.
Table III.
Uterine NK cell activity, by NK cell regulation and receptors.
| Author/Year | Study design | Study groups and sample number | Tissue analysed | Method of analysis | Outcome measure, e.g. receptor expression | Direction of effect in RM/RIF patients |
|---|---|---|---|---|---|---|
| Kwak et al. (1999) | Prospective case control |
|
Decidual samples at ERPC (uRM patients) and STOP for healthy controls (all <12/40) | IHC/histology |
|
Qualitative analysis |
|
| ||||||
| Emmer et al. (2002) | Prospective Case Control | RM (n = 9); 2 controls (n = 11) | Decidual tissue at time of ERPC or TOP; 2 hysterectomy specimens | IHC | Expression of | |
| • CD56 | ↑ | |||||
| • CD16 | ↑ | |||||
|
| ||||||
| Yan et al. (2007) | Prospective case control |
|
FC; semi-quantitative RT-PCR | uNK expression of | ||
| • KIR2DL4 | ↓ | |||||
|
| ||||||
| Qu et al. (2008) | Prospective case control |
|
Decidual tissue at time of miscarriage or TOP | RT-PCR; IHC | Expression of | |
| • Osteopontin | ↓ | |||||
|
| ||||||
| Bao et al. (2012) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP | PCR; western blot | TLR3 expression | |
| • mRNA (median) | ↑* | |||||
| • Protein (ratio:β-actin) | ↑* | |||||
|
| ||||||
| Fu et al. (2013) | Prospective observational |
|
Decidual samples at time of miscarriage/TOP | FC; ELISA | • CD27+ NK cells: Th17 cell ratio | ↓* |
|
| ||||||
| Hosseini et al. (2014) | Prospective case control |
|
Menstrual blood sample on Day 2 of menstruation | FC | Expression of | |
| • CCR7 | ↑ | |||||
| on | ||||||
| CD56+CD16+ and CD56+CD16− | ||||||
| • CD45RO | ↓* | |||||
| on CD56+CD3− | ||||||
|
| ||||||
| Sotnikova et al. (2014) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP | FC; PCR | Expression of: | |
| • CD56+CD161+ | ↑* | |||||
| • CD56+NKG2A+ | ↓* | |||||
|
| ||||||
| Wang et al. (2014) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP | FC | CD56+/CD16−/CD158a+ cells | |
| • KIR2DL1/S1 | ↓* | |||||
|
| ||||||
| Guo et al. (2017) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP | FC, PCR, Matrigel invasion assay, western blot, ELISA | Expression of: | |
| • KIR2DL4 | ↓* | |||||
| • NKG2A | ↓* | |||||
| Jiang et al. (2017) | Prospective case control |
|
Endometrial tissue taken on Day 7–9 post-LH surge | IHC | Ratio of cells in endometrium: | |
| • CD57+: CD56+ ratio | ↑* | |||||
| Correlation between ratio of | ||||||
| • CD57+ to CD56+ and percentage of | NEGATIVE | |||||
| FoxP3+ in endometrium | RM and controls | |||||
|
| ||||||
| Huang et al. (2019) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP | qRT-PCR; western blot; ELISA | Expression of: | |
| • miR30e | ↓* | |||||
|
| ||||||
| Li et al. (2019) | Prospective case control | 15 RM 15 controls | Endometrial tissue (RM); decidual tissue at TOP for (controls) | FC | Expression of | |
| • CD49a | ↓ | |||||
|
| ||||||
| Lu et al. (2020) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP | FC | Expression of: | |
| • CD82 | ↓* | |||||
| • CD29 | ↑* | |||||
| on CD56dim | ||||||
|
| ||||||
| Wei et al., (2020) | Prospective case control |
|
Endometrial tissue taken on in mid-luteal phase | IHC | Correlation of NK cells with: | |
| IDO | POSITIVE in | |||||
| RM and controls | ||||||
|
| ||||||
| Lyzikova et al. (2020) | Prospective case control |
|
Endometrial samples | IHC | uNK correlation with: | |
| • FoxP3 Tregs | POSITIVE RM and controls* | |||||
| • PGRMC1 | NEGATIVE RM* | |||||
| POSITIVE controls* | ||||||
|
| ||||||
| Zhao et al. (2020) | Prospective case control |
|
Endometrial biopsy on day LH surge +7 | Multiplex IHC staining | Correlation of uNk cell density with: | |
| • CD3+ cell density | POSITIVE* | |||||
Effect direction in relation to RM/RIF group: ↑increase; ↓decrease; ↔no difference between the groups.
P < 0.05.
ERPC, evacuation of retained products of conception; FC, flow cytometry; IHC, immunohistochemistry; RIF, recurrent implantation failure; RM, recurrent miscarriage; STOP, surgical termination of pregnancy; TOP, termination of pregnancy; uRM, unexplained recurrent miscarriage.
Table VI.
Uterine NK cell activity by effect on uterine vasculature.
| Author/year | Study design | Study groups and sample number | Tissue sampled | Method of analysis | Outcome measure | Direction of effect of in RM/RIF patients |
|---|---|---|---|---|---|---|
| Lédée et al. (2004) | Prospective case control |
|
Endometrial tissue at luteal phase, either on Day 20 under oestrogen–progesterone replacement or Day 6 post-urinary LH surge | IHC; USS | No clear correlation between cytokine staining/depletion of IL-12 and IL-18, number of NK cells and ultrasound Doppler in RIF or controls | Qualitative analysis |
|
| ||||||
| Lédée-Bataille et al. (2005) | Prospective case control |
|
Endometrial tissue at luteal phase, either on Day 21 under oestrogen–progesterone replacement | IHC; USS; qRT-PCR | • Mean UA PI | ↑ |
| • Mean number of NK cells | ↑ | |||||
| • Mean ET | ↓ | |||||
| • Endometrial (IL-12; IL-15; IL-18) cytokine mRNA ratio | ↔ | |||||
|
| ||||||
| Lédée et al. (2008) | Prospective case control |
|
Endometrial tissue D7-9 post-LH surge | IHC, rtPCR, USS | • Sub-endometrial flow | |
| • Uterine artery pulsatility | ||||||
| Correlation of cytokine mRNA ratio: | ||||||
| with sub-endometrial flow and CD56+ count | ||||||
| • IL-15 | POSITIVE* | |||||
| With mean UA PI | ||||||
| • IL-15 | ||||||
| • IL-18 | ||||||
| • IL-18BP | NEGATIVE* | |||||
|
| ||||||
| Junovich et al. (2013) | Prospective case control |
|
Endometrial tissue on Day 5–9 after ovulation (by ultrasound) | FC; ELISA | Correlation of CD16+ uNK with endometrial cell culture supernatant protein expression: | |
| • IL-6 (n = 28) |
 NEGATIVE*
NEGATIVE*
|
|||||
| • VEGF (n = 31) | RIF and controls | |||||
|
| ||||||
| Almasry et al. (2015) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP between 6 and 10 weeks gestation | IHC; TEM | Non-remodelling score | POSITIVE* |
| RM and controls | ||||||
|
| ||||||
| El-Azzamy et al. (2018) | Prospective case control |
|
Endometrial tissue taken on in mid-luteal phase Day 7–9 post-ovulation | IHC; western blot; qRT-PCR | Correlation of CD56+ cell count with: | |
| • VSMC expressing SMM | ↔* | |||||
| • CD31+ endothelial cells | ↔* | |||||
|
| ||||||
| Chen et al. (2018) | Prospective case control |
|
Endometrial samples on Day 7 post-LH surge. | Angiogenic array; ELISA | Expression of: | |
| • angiogenin |
 ↑ ↑ |
|||||
| • bFGF | ||||||
| • VEGF-A | ||||||
Effect direction in relation to RM/RIF group: ↑increase;↓decrease; ↔no difference between the groups.
P < 0.05.
bFGF, basic fibroblast growth factor; ET, endometridal thickness; IHC, immunohistochemistry; IL-18BP, Interleukin-18 binding protein; qRT-PCR, real-time quantitative PCR; RIF, current implantation failure; RM, recurrent miscarriage; SMM, smooth muscle myosin; TEM, transmission electron microscopy; TOP, termination of pregnancy; UA PI, uterine artery pulsatility index; USS, ultrasound scan; VSMC, vascular smooth muscle cells.
Table IV.
Uterine NK cell activity by cytotoxicity.
| Author/year | Study design | Study groups and sample number | Tissue sampled | Method of analysis | Outcome measure | Direction of effect of cytotoxicity In RM/RIF patients |
|---|---|---|---|---|---|---|
| Chao et al. (1995) | Prospective case control |
|
Decidual tissue at time of miscarriage/STOP | Cr release assay | Lytic activity20 | ↑ |
|
| ||||||
| Bao et al. (2012) | Prospective case control | RM (n = 32) vs controls (n = 35) | Decidual samples at time of miscarriage/STOP | LDH release assay | % lysed K562 cells | ↑* |
|
| ||||||
| Giuliani et al. (2014) | Prospective case control |
|
Endometrial tissue on Day 7–9 post-LH surge | IHC | Expression of | |
| • % NKp46: endometrial stroma | ↑* | |||||
| • Ratio NKp46: CD56+ | ↑* | |||||
|
| ||||||
| Sotnikova et al. (2014) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP | FC; PCR | Intracellular | |
| • Granzyme | ↑* | |||||
| mRNA expression | ||||||
| • GrB | ↑* | |||||
|
| ||||||
| Fukui et al. (2017) | Prospective case control |
|
Endometrial tissue taken on Day 7–9 post-LH surge | FC: | • NKp44, NKp30 | ↔ RM, RIF or controls |
| • NK p46+ expression | ↓* RM vs controls | |||||
| on CD56; CD56bright and CD56dim | ||||||
|
| ||||||
| Li et al. (2019) | Prospective case control |
|
Decidual samples at time of miscarriage/STOP | Cr release assay | % lysed K562 cells | ↑* |
|
| ||||||
| Li et al. (2019) | Prospective case control |
|
Decidual samples at time of miscarriage/STOP | FC | Protein expression | |
| • Perforin |
 ↑* ↑*
|
|||||
| • GzmB | ||||||
| mRNA expression | ||||||
| • PRF1 |
 ↑ ↑ |
|||||
| • GzmB | ||||||
Effect direction in relation to RM/RIF group: ↑increase; ↓decrease; ↔no difference between the groups.
P < 0.05; confidence of the effect estimate has been determined by application of the binomial test.
Cr, chromium; FC, flow cytometry; GzmB, Granzyme B; IHC, immunohistochemistry; LDH, lactate dehydrogenase; RIF, recurrent implantation failure; RM, recurrent miscarriage; STOP, surgical termination of pregnancy.
Table V.
Uterine NK cell activity by cytokine expression.
| Author/year | Study design | Study groups and sample number | Tissue sampled | Method of analysis | Outcome measure, e.g. cytokine | Direction of effect in RM/RIF patients |
|---|---|---|---|---|---|---|
| Kwak et al. (1999) | Prospective case control |
|
Decidual samples at ERPC (uRM patients) and STOP for healthy controls (all <12/40) | IHC |
|
Qualitative analysis |
|
| ||||||
| Fu et al. (2013) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP | FC; ELISA | • IL-1RA* | ↓* |
| • IL-10 |

|
|||||
| • IFN-γ* | ↓* | |||||
|
| ||||||
| Sotnikova et al. (2014) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP | FC; PCR | Intracellular | |
| • IFN-γ | ↑* | |||||
| mRNA expression | ||||||
| • IFN-γ | ↑* | |||||
|
| ||||||
| Fukui et al. (2017) | Prospective case control |
|
Mid-luteal endometrium | FC | TNF-α and IFN-γ expression | |
| • RM | ↓ | |||||
| • RIF | ↓ | |||||
| on CD56, CD56bright and CD56dim | ||||||
|
| ||||||
| Dong et al. (2017) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP | FC | dNK cytokine ratio expression using FSC/SSC gating strategy | |
| dNK1/dNK2 ratio | ↑ | |||||
| *dNK1 = IFNγ+IL4- and | ||||||
| dNK2 = IFNγ-IL4+ | ||||||
|
| ||||||
| Guo et al. (2017) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP | FC, RT-PCR, Matrigel invasion assay, western blot, ELISA | Expression of cytokines in purified CD94+CD56+CD16− dNK: | |
| IL-8 | ↓ | |||||
| • IP-10 | ↓* | |||||
| • VEGF | ↓* | |||||
| • IFN-γ | ↑ | |||||
|
| ||||||
| Liu et al. (2019), China | Prospective case control |
|
Decidual samples at time of miscarriage/TOP between 6 and 10 weeks gestation | FC | Cytokine expression | |
| • TNF-α (dNK1) | ↑* | |||||
| • IL-4 (dNK2) | ↓* | |||||
| • dNK1: dNK2 ratio | ↑* | |||||
| on CD3−CD56brightCD16− cells | ||||||
|
| ||||||
| Li et al. (2019) | Prospective case control |
|
Decidual tissue at ERPC/TOP between 7 and 10 weeks | FC | mRNA expression • PRF1 • IFN-γ |
 ↑* ↑*
|
| Liu et al. (2020) | Prospective case control |
|
Decidual samples at time of miscarriage/TOP between 6-10 weeks gestation | FC; real-time PCR | Cytokine protein expression | |
| • IFN-γ | ↑* | |||||
| • TNF-α | ↑* | |||||
| • IL-4 | ↓* | |||||
| • IL-10 | ↔ | |||||
| Cytokine mRNA expression | ||||||
| • IFN-γ | ↑* | |||||
| • TNF-α | ↑* | |||||
| • IL-4 | ↓* | |||||
| • IL-10 | ↔ | |||||
Effect direction in relation to RM/RIF group: ↑increase; ↓decrease; ↔no difference between the groups.
P < 0.05.
ERPC, evacuation of retained products of conception; FC, flow cytometry; FSC, forward scatter; IFN-γ, Interferon-γ; IHC, immunohistochemistry; IP-10, interferon gamma-induced protein 10; RIF, recurrent implantation failure; RM, recurrent miscarriage; SC, side scatter; STOP, surgical termination of pregnancy; TNF-α, tumour necrosis factor-α; TOP, termination of pregnancy; VEGF, vascular endothelial growth factor; uRM, unexplained recurrent miscarriage.
Regulation and receptors
There were 17 studies in this category; 16 on women with RM (Kwak et al., 1999; Emmer et al., 2002; Yan et al., 2007; Qu et al., 2008; Bao et al., 2012; Fu et al., 2013; Hosseini et al., 2014; Sotnikova et al., 2014; Wang et al., 2014; Guo et al., 2017; Huang et al., 2019; Li et al., 2019; Wei et al., 2020; Lu et al., 2020; Lyzikova et al., 2020; Zhao et al., 2020) and one on women with RIF (Jiang et al., 2017).
One theory on the origin of uNK is the trafficking of pNK in response to chemokine production from uterine stromal cells (Kitaya et al., 2003; Hanna et al., 2004; Jones et al., 2004). Previous studies found that chemokines (e.g. CXCL10, CXCL12, Chemerin) secreted by decidual cells during pregnancy can support pNK migration through endothelial and stromal cells (Carlino et al., 2008, 2012). From our systematic review, we found three studies that focussed on recruitment of uNK (Qu et al. 2018; Hosseini et al. 2014; Lu et al. 2020). Hosseni et al. (2014) reported preferential recruitment of CD56+CD16+ pNK to the uterus by virtue of higher expression of CCR7 on CD56dim pNK in women with RM, although this finding was not replicated in CD56dim uNK. Concurrently, CD56dim uNK were also found to have increased adhesive ability in response to trophoblast-derived CXCL12 in women with RM (Lu et al., 2020). Nevertheless, our meta-analysis did not show a significantly higher level of CD56dim dNK in women with RM. Another study suggested reduced expression of the chemokine osteopontin in decidua, which was found to be correlated to number of dNK, to be associated with RM (Qu et al., 2008).
The interaction between uNK and trophoblast cells is a key event that determines success of early placentation, but there is no consensus as to whether activation or inhibition of uNK as a result of this interaction contributes to reproductive failure. On the one hand, studies have shown that uNK need to be activated for successful pregnancies (Hiby et al., 2010; Xiong et al., 2013; Long et al., 2015; Kennedy et al., 2016); on the other, studies from our systematic review found significantly lower expression of inhibitory receptors, including KIR2DL4, NKG2A and KIR2DL1 (Yan et al., 2007; Sotnikova et al., 2014; Guo et al., 2017), in women with RM suggesting that overactivation of uNK may cause reproductive problems. In support of this theory, previous immunogenetic studies have also shown a similar repertoire in women with RM (Varla-Leftherioti et al., 2003; Faridi and Agrawal, 2011). One exception may be the interaction between KIR2DL4 and HLA-G. Although HLA-G is known to cause inhibition of immune cells (Carosella et al., 2015), there have been studies which suggest that KIR2DL4 is activated by HLA-G, which in turn supports a role in remodelling of maternal vasculature (Rajagopalan and Long, 2012). Therefore, low expression of KIR2DL4 may, in fact, point to insufficient activation of uNK cells in women with RM. This was supported by Guo et al. (2017) who reported reduced pro-angiogenic and pro-invasion cytokine expression, trophoblast invasive ability, and tube formation, following suppression of KIR2DL4 in vitro.
More recently, a few studies have assessed decidual NK cell (dNK) function by in vitro experiments as an adjunct to ex vivo phenotyping of immune cells. Using villous trophoblast explants to study migration over a collagen surface, Sotnikova et al. (2014) found reduced migration of trophoblast cells after co-incubation with dNK in both women with RM and normal pregnancy groups. This was postulated to be secondary to increased IFN-γ and Granzyme B secretion by CD56+ uNK. Guo et al. (2017) demonstrated the ability of NK cells expressing microRNA 30e (miR30e) to regulate HLA-G expression on a trophoblast cell line, HTR-8/SVneo, and that dampening of HLA-G expression resulted in reduced pro-angiogenic cytokine secretion by dNK, as well as reduced trophoblast invasion and migration. Huang et al. (2019) further expanded on the role of miR30e by showing that upregulation of miR30e can result in suppression of NK cell cytotoxicity against K562 target cells, increased production of pro-angiogenic cytokines (IL-4, IL-10, VEGF, Ang-2) and suppressed expression of pro-inflammatory cytokines (IFN-γ and TNF-α) by uNK. Finally, Lu et al. (2020) found higher CD56dim with low CD82 and high CD29 expression in women with RM, suggesting a role for these in regulation of trophoblast adhesion.
Cross-talk between uNK and other immune cells in the endometrium is crucial to maintain homeostasis in the early pregnancy placental bed. There is emerging evidence on the importance of regulatory T cells (Tregs) in maintaining homeostasis at the maternal–foetal interface (Erlebacher, 2013) and reduced Tregs have been found in the endometrium of women with subfertility (Sauerbrun-Cutler et al., 2021). The relation between NK cells and FOXP3 Tregs, T-helper 17 cells (Th17) and other immune cells were investigated in five studies from our systematic review (Jiang et al., 2017; Lyzikova et al., 2020; Wei et al., 2020; Fu et al., 2013; Zhao et al., 2020). Albeit using a different unit of measurement, two studies were contradictory in their findings; one found no correlation between CD57:CD56 ratio and Treg numbers (Jiang et al., 2017) and another found a positive correlation between CD56+ cells and Treg numbers (Lyzikova et al., 2020). uNK interact with CD14+ macrophages to produce indoleamine 2,3-dioxygenase (IDO) that induces Tregs (Vacca et al., 2010). IDO expression is found to be lower in women with RM (Ban et al., 2013; Wei et al., 2020), but the exact regulatory relation between uNK and IDO remains to be elucidated as there was no apparent correlation between them in contrast to a positive correlation found between IDO and Tregs in controls (Wei et al., 2020).
A study on Th17 showed that in addition to a reduced CD27+ NK to Th17 ratio, supernatants of dNK from women with RM were unable to suppress Th17 expansion under different cytokine conditions (IL-15, IL-12 and IL-18) (Fu et al., 2013). Another study that explored the relation with CD20+ B cells, CD3+ T cells and CD68+ macrophages found a positive correlation between CD56+ uNK and CD68+ macrophages (Zhao et al., 2020).
Cytotoxicity
There is general consensus that uNK do not possess the same cytotoxic ability as pNK (Trundley and Moffett, 2004). In support of this, dNK are unable to form activating synapses that trigger perforin release when interacting with classical cancerous target cells, such as K562 (Koopman et al., 2003). Studies on uNK cytotoxicity are rare because it is technically challenging and resource intensive and, to date, no studies have successfully demonstrated cytotoxicity of endometrial NK cells (Manaster et al., 2008). Therefore, most studies have focussed on pNK cytotoxicity, assuming that it is an indicator of uNK activity.
We found three case control studies on uNK cell cytotoxicity conducted with uNK isolated from first trimester decidua in women with RM compared with controls. (Chao et al., 1995; Bao et al., 2012; Li et al., 2019). All three studies measured lysis of K562 leukaemic cells after co-incubation with uNK at effector:target (E:T) ratios ranging from 5:1 to 40:1. Percentage of target cell lysis was determined by either a Chromium release assay (Chao et al., 1995; Li et al., 2019) or lactate dehydrogenase assay (Bao et al., 2012). Overall, the results demonstrated higher lysis of target cells in women with RM compared with controls. However, consideration needs to be given on the suitability of K562 cell lines as an in vitro model of the placental bed because this only gives information on how uNK react to cancer cells, but not trophoblast cells. A side-by-side comparison of trophoblast cells ex vivo and K562 myeloid leukaemia cells in an earlier study demonstrated that the latter is susceptible to cytotoxicity by dNK, but not the former (King et al., 1989). We postulate two possible explanations for the greater ability of uNK from RM patients to kill K562 myeloid leukaemia cells. Firstly, our findings from this review suggest that there are more pNK in the endometrium of RM patients than the endometrium of controls. pNK have a greater ability to kill K562 cancer cells (King et al., 1989) so this enrichment for pNK in the endometrium of RM patients would lead to an enhanced ability of the total NK cell population to kill K562 cells. Secondly, uNK in RM patients may be more activated, therefore, have enhanced ability to kill K562 cancer cells, although it cannot be concluded that the same effect will also be seen in trophoblast cells.
Two studies in our systematic review examined the expression of granzyme B and perforin, which mediate NK cell cytotoxicity: both molecules were reported to be increased in women with RM (Sotnikova et al., 2014; Li et al., 2019).
pNK expresses three types of NK cytotoxicity receptors (NCR) (NKp46, NKp30 and NKp44), which regulate cytotoxicity and cytokine secretion by NK cells (Hudspeth et al., 2013). Two studies from our systematic review attempted to look at NCR in uNK as a proxy for their cytotoxic ability (Giuliani et al., 2014; Fukui et al., 2017). The expression of NKp46 was found to be significantly lower in uNK of women with RM (Fukui et al., 2017) but higher in women with RIF (Giuliani et al., 2014). Studies on NKp46+ expression on uNK (Giuliani et al., 2014; Fukui et al., 2017) should be interpreted with caution because NKp46+ is universally expressed in all NK cells regardless of activation status (Barrow et al., 2019). Overall, there is little evidence to suggest that expression of NCR on uNK translates to cytotoxicity because uNK can also be controlled via inhibitory co-receptors such as NKp46/NKG2A (El Costa et al., 2009). Moreover, expression of NKp46 in uNK is associated with a different cytokine expression profile compared with pNK (Yokota et al., 2013). No difference in NKp44 and NKp30 receptors were reported (Fukui et al., 2017).
Cytokine expression
Nine studies reported on the expression of cytokines in women with RM. One study also included women with RIF in their samples (Fukui et al., 2017). A majority of these studies sampled first trimester decidua (Fu et al., 2013; Sotnikova et al., 2014; Dong et al., 2017; Guo et al., 2017; Li et al., 2019; Liu et al., 2019, 2020) but two studies used endometrial samples (Fukui et al., 2017; Li et al., 2019). There was significant heterogeneity in phenotype of uNK and type of cytokine expression, which precluded pooling of results. For example, IFN-γ expression has been reported in either CD56+CD27+ uNK (Fu et al., 2013) or just CD56+ uNK (Sotnikova et al., 2014).
Three studies from the same research group evaluated dNK1 to dNK2 ratio, whereby dNK category was determined by their expression of either Th1 type cytokines (IFN-γ, TNF-α) or Th2 type cytokines (IL-4, IL-10). Although these studies used different combinations of cytokines to represent dNK1 or dNK2, all three studies found a significantly higher dNK1:dNK2 ratio in women with RM compared with controls (Dong et al., 2017; Liu et al., 2019, 2020). However, these studies were not strictly controlled for gestational age, which could affect the type of cytokine secreted (Lash et al., 2010). For instance, IFN-γ secretion, which can be found physiologically after the first trimester, was thought to help inhibit further invasion of EVT (Lash et al., 2006; Otun et al., 2011). Therefore, studies which did not stratify by gestational age may not be able to detect any true difference between patient and control groups and may explain the conflicting results reported by different studies.
A majority of studies in our systematic review reported increased IFN-γ expression in women with RM (Fu et al., 2013; Sotnikova et al., 2014; Dong et al., 2017; Li et al., 2019) although one study reported a decrease (Fukui et al., 2017). These studies measured the cytokines by different methodologies, including flow cytometry, ELISA and RT-PCR, and in different tissue types thereby precluding synthesis of results. Additionally, Sotnikova et al. (2014) demonstrated elevated IFN-γ mRNA expression in dNK co-cultured with trophoblasts, which was not observed in the RM group, suggesting an alteration in the functional relation between dNK and trophoblast cells. TNF-α expression evaluated by two studies showed conflicting results (Liu 2019; Fukui et al., 2017).
Other cytokines analysed in individual studies included IL-1b, IL-6, IL-22, IL-23, IL-17, IL-10 and IL-1RA (Fu et al., 2013, Guo et al., 2017, Liu et al. 2020).
In summary, the studies reviewed here demonstrated equivocal results with regard to predominant cytokine expression in women with RM/RIF. Moreover, interrogation of any one cytokine in isolation is unlikely to provide useful information as cytokine production by uNK varies with gestational age, method of purification, activation, and may very well differ before and after dNK interact with trophoblasts.
Effect of uNK on uterine vasculature
uNK are thought to be involved in spiral artery remodelling by secretion of angiogenic cytokines such as Ang1, Ang2, VEGF-C and IFN-γ (Robson et al., 2012). Seven of the included studies were grouped in this fourth category of uNK activity; they were found to be too diverse in outcome measure to permit data synthesis. Four studies in total investigated outcomes in patients with RIF (Lédée et al., 2004, 2005, 2008; Junovich et al., 2013) with the remainder investigating RM (Almasry et al., 2015; Chen et al., 2018; El-Azzamy et al., 2018).
All studies in our systematic review supported the, perhaps counterintuitive, hypothesis of increased angiogenesis in women with RM compared with controls (Almasry et al., 2015; Chen et al., 2018; El-Azzamy et al., 2018). This was demonstrated by a higher expression of proangiogenic cytokines including angiogenin, bFGF and VEGF-A in the endometrium (Chen et al., 2018). Spatial assessment by immunohistochemistry revealed impaired vascular remodelling associated with higher uNK (Almasry et al., 2015) and a positive correlation between vascular smooth muscle cells and CD56+ uNK (El-Azzamy et al., 2018). This could support the hypothesis that excessive angiogenic activity may lead to earlier peri-implantation blood flow, which exposes foetal trophoblasts to oxidative stress (Quenby et al., 2009) and subsequent cellular injury. This mechanism may also explain the observation of high uNK levels in women with RM.
In contrast, a study on women with RIF found deficient production of the angiogenic cytokine VEGF in patients compared with controls, which was postulated to have resulted from deficient IL-6 expression leading to an increased cytotoxic response by CD56+CD16+ uNK (Junovich et al., 2013). This may be explained by another possible theory that low production of angiogenic factors can also be associated with insufficient trophoblast invasion (Trundley and Moffett, 2004). This has previously been observed in immunogenetic studies of RM and defective placentation in later pregnancy (Hiby et al., 2008, 2010).
Overall, the direction of effect with respect to uNK involvement in RIF may not be so clear, as evidenced by a series of studies conducted by Lédée et al. (2004, 2005, 2008) on the endometrium of women with RIF. Using a combination of ultrasound assessment of uterine artery blood flow, IL-12, IL-15 and IL-18 cytokine assay by RT-PCR and immunostaining, as well as CD56+ uNK count by immunohistochemistry, they demonstrated that impairment of vascular remodelling may be caused by either insufficient or excessive NK cell recruitment to endometrium, secondary to dysregulated cytokine signalling.
Discussion
Key findings
This systematic review and meta-analysis provides the most comprehensive summary to date of uNK level and activity, and its association with pregnancy outcome, in women with RM or RIF when compared with controls. This is the first meta-analysis investigating the correlation between pNK and uNK, as well as providing an overview on differences in uNK activity in these populations of women. We found significantly higher total CD56+ cells in the uterus in women with RIF compared with controls. Although we did not find a significant difference in overall studies for women with RM compared with controls, the difference became significant when we focussed on studies using endometrial samples from mid-luteal phase only. On the other hand, no difference was found in livebirth rate when women with RM/IF were stratified to normal or high uNK level, and no significant correlation was found between pNK and uNK.
Our review highlights the heterogeneity of studies on uNK activity, which can be broadly divided into uNK regulation and receptors, cytotoxicity, cytokine production and effect on uterine vasculature. There is a general trend suggesting that uNK derived from women with RM/RIF produce more Type 1 cytokines (e.g. IFN-γ and TNF-α) compared with Type 2 cytokines (e.g. IL-4 and IL-10), although some studies showed conflicting results. Most studies reported lower levels of inhibitory receptors and higher levels of angiogenesis under the influence of uNK in women with RM/RIF.
Strengths and limitations
The main strength of our review lies in the meticulous meta-analysis of uNK based on different phenotype followed by robust subgroup and sensitivity analyses to identify sources of heterogeneity. Further, a vigorous search strategy was applied including hand-searching references from all included full text articles. Quality of the studies was assessed individually by two independent reviewers with the ROBINS-I tool, which is a Cochrane tool optimized to assess quality of observational studies. Individual studies were scored with a moderate to serious risk of bias, and we have performed sensitivity analysis to exclude studies with serious risk of bias. Finally, the reliability of all the studies included in the meta-analyses of uNK level was augmented by assessment of publication bias and Egger’s test.
The major limitation of this review lies in the clinical heterogeneity among the studies, including definitions of RM and RIF, and the control subjects used. This is further elaborated below. Other limitations of our meta-analysis included the exclusion of studies not published in English, derivation of mean and standard deviation from median, range and interquartile range and extraction of data from graphs in a subgroup of studies which may have resulted in skewing of data. This was addressed by performing sensitivity analysis for these studies, which did not reveal a change in the main meta-analysis result, i.e. total CD56+ level in women with RM compared with controls. However, when further sensitivity analysis was performed on the subgroup of CD56+ cells in endometrium only, to exclude studies from which data were extracted from graphs or converted from median, the result changed from significantly higher CD56+ uNK in women with RM to no significant difference. For women with RIF, we also found altered significance in the meta-analysis result for total CD56+ cells in endometrium after performing various sensitivity analyses, as reported above. On balance, the inclusion of these studies was deemed important to provide a comprehensive review of all the literature available on the topic. This also emphasizes the need for larger scale, well-designed studies in the future to establish a stronger conclusion. Owing to the diversity of studies on uNK activity, it was not possible to neatly fit all the studies into categories. This highlights the complexity of uNK function and their interaction with surrounding decidual and immune cells. Finally, duplication bias was an issue as some articles were published twice by the same group of authors close to each other. This was overcome by contacting authors for clarification or extracting data from the most recent publication or most complete and larger dataset.
Measurement of uNK level
In the last meta-analysis of six studies on endometrial uNK level in women with RM, Seshadri and Sunkara (2014) found no significant SMD in women with RM compared with controls when uNK number was expressed as percentage of stromal cells. Since the publication of this 2014 review, research on uNK has expanded considerably which allowed us to include 12 and 19 studies that used flow cytometry and immunohistochemistry to identify uNK, respectively. Further, we were able to dissect the population of uNK based on different phenotypes. Although each of the subtypes of CD56+ uNK showed no significant difference, total CD56+ uNK in the endometrium was found to be significantly higher in women with RM compared with controls. The significant difference in total CD56+ cells seen between the groups (when no such difference was detected in CD56+ CD16− cells) could point to the main cell that differs between the groups being circulating, rather than uterine, NK cells.
However, as previously mentioned, there was significant clinical heterogeneity among the studies. First, the variability in definition of RM needs to be considered. Currently, RM is defined as either two (Bender Atik et al., 2018; Practice Committee of the American Society for Reproductive Medicine, 2020) or three (Green Top Guideline, Royal College of Obtetricians and Gynaecologists, 2011) previous consecutive miscarriages, depending on the regulatory body. Despite exclusion of uterine malformations, antiphospholipid syndrome, endocrine dysfunctions, and haematological disorders in most studies, not all excluded parental or foetal chromosomal abnormalities. The clinical use of testing for foetal chromosomal abnormality is also contentious. Although it was previously thought that women with RM and normal foetal karyotype had worse prognoses for future pregnancies compared with sporadic foetal aneuploidy (Carp et al., 2001; Saravelos and Li, 2012), a systematic review found that incidence of chromosomal abnormalities, which accounted for 46% of RM, was not different from sporadic miscarriage (Smits et al., 2020). Recent guidance published in the Lancet recommended that chromosomal testing could be performed for counselling purposes but little evidence is found on its value as a prognostic factor (Coomarasamy et al., 2021).
Moving on to consider RIF, the lack of uniformity in defining this condition was highlighted in a recent review (Polanski et al., 2014). First the most common definition used is failure to achieve clinical pregnancy after ‘minimum of three fresh or frozen cycles’ (Coughlan et al., 2014) or ‘two consecutive cycles’ (Polanski et al., 2014), but some have also defined it based on the previous number of embryos transferred irrespective of the number of cycles (Lédée et al., 2008). RIF and recurrent failure of IVF are terms that may be interchangeably used, but it is important to note that they are not the same because embryo transfer may not have necessarily occurred in the latter.
Second, although most studies were case-controlled observational studies, not all confounding factors will be entirely eliminated. An example was maternal age, which is well known to influence the rate of aneuploidy in embryos (Rubio et al., 2017; Bergh et al., 2019). Moreover, women older than 40 years are 100 times more likely to experience RM (Saravelos and Li, 2012). Another possible confounding factor was the administration of hormonal therapy, which has the potential to influence uNK numbers, although a recent study found no difference in uNK density of IVF patients undergoing natural cycle or hormonal replacement therapy prior to blastocyst transfer (Yang et al., 2019). We have accounted for this by exclusion of studies in which the control group was not treated the same as the patient group and performed sensitivity analysis on studies which administered hormonal therapy.
A third source of heterogeneity was the definition of controls. For this review, we have excluded studies using infertile controls owing to female or embryo factor. Among the included studies, there was no uniformity in the inclusion criteria for controls. Some studies strictly used controls with proven fertility whereas others used controls with no previous history of miscarriage or controls with male factor infertility with successful pregnancy outcome after IVF. Interestingly, our sensitivity analysis showed a lower P-value in RIF patients after exclusion of studies using male factor infertility as controls. As this population of women would have undergone ART to conceive, there may be inherent differences in uNK behaviour in this category of control group compared with fertile women.
Fourth, studies on RM differed in type of tissue analysed, which may include endometrium, decidua from first trimester pregnancy or menstrual blood. As demonstrated by our subgroup analysis, a significant difference in CD56+ level was only detected when mid-luteal endometrial samples were used, but not decidual samples. One caveat of using decidual tissue is the inability to accurately extrapolate uNK frequency and function in vivo. This is because of the time lapse that would have inevitably occurred from foetal demise to surgical removal of pregnancy tissue, initiating a cascade of reactions to eliminate the products of conception and initiate tissue repair that is likely to involve an influx of immune cells. Moreover, uNK level fluctuation at different gestational ages would have skewed results from studies that did not control for this confounding factor.
Studies of uNK in the endometrium are relatively rare but can be useful to assess the immune milieu during the window of implantation. However, this must be timed properly as uNK level is known to fluctuate through the menstrual cycle from 26% during late proliferative up to 83% in late secretory phase (Pace et al., 1989; Flynn et al., 2000; Williams et al., 2009). The majority of studies included in this systematic review timed their samples after the urine LH surge and sampling time can vary from Day 5 to 10 post-ovulation. Russell et al. (2013) demonstrated a 6-to-7-fold increase of CD56+ uNK over a 7-day period from Day 22 of the menstrual cycle, as measured in both absolute count and a percentage by immunohistochemistry. Therefore, the effect of fluctuating uNK within the same group of patients may have nullified the conclusion of these studies. Future studies should focus on accurate timing of their sampling using a unified method, for example, timing it accurately at 7 days post-ovulation by the urine LH surge. Another suggested method to overcome the impracticalities of accurate time of sampling is the utilization of histological dating, as demonstrated by two of the studies included in the systematic review (Quenby et al., 1999; Liu et al., 2014).
Recently, the use of menstrual blood has been proposed as a less invasive surrogate for endometrial sampling and endometrial NK cells obtained from this source displayed a distinct KIR repertoire from pNK (Ivarsson et al., 2017; Feyaerts et al., 2018). Our sensitivity analysis on the study of menstrual blood did not show any significant difference in the meta-analysis (Hosseini et al., 2014).
Fifth is the heterogeneity in techniques used to measure uNK. Immunohistochemistry is widely used to evaluate uNK numbers and can be presented as absolute number/mm3 or percentage of total stromal cells. However, this method is influenced by subjectivity between observers and indeed within a single observer (Mariee et al., 2012). A recent study revealed significant variation in counts between different centres, which was attributed to different techniques of tissue fixation, embedding and sectioning, selection of area for assessment, definition of immune-positive cells and inclusion/exclusion of blood vessels (Lash et al., 2016). In addition, this technique, which only allows the expression of one or two antigens to be evaluated, frequently results in misinterpretation of single-stained CD16+ cells as uNK. Our meta-analysis of immune cells that solely expressed CD16+ by immunohistochemistry showed a significant difference, but this should be interpreted with caution, since a single stain of CD16 does not distinguish uNK from other immune cells, such as monocytes, and more specific markers including CD56, CD49a, CD39 and CD103 are useful. In contrast to immunohistochemistry, flow cytometry introduces a more objective and robust way to selectively assess uNK by the specific antigens CD45+ (leucocyte antigen), CD3 (T-cell antigen), CD56 and CD16. Despite that, a few caveats still exist such as inconsistencies in the order of gating strategy and arbitrary inclusion of cells (Rai et al., 2005). For instance, Marron and Harrity (2019) reported CD56+ uNK level with both total endometrial cell population and CD45+ leucocytes as the denominator. To overcome this source of heterogeneity, we have performed subgroup analysis on unit of measurement as well as method of measurement to assess whether these differences affected the overall result, and the results were not different from the main meta-analysis result.
Sixth, variation in reference range of uNK level can be a source of heterogeneity in the meta-analysis for livebirth outcome. Surprisingly, with an I2 test result on heterogeneity of 11%, the pooled risk ratio on livebirth outcome was found to be no different in women with a high or normal level of uNK regardless of reference range used. This finding indicates uNK level is not useful as a prognostic indicator for subsequent pregnancies and suggests that differences in uNK level observed may be an effect of RM/RIF rather than a cause.
Measurement of uNK activity
As outlined in our narrative review, there is a wide repertoire of uNK activity that can be measured, from recruitment and regulation of uNK to production of cytokines, cytotoxicity and angiogenesis. Overall, most of the studies in each respective category reported conflicting findings and this may be caused by the same confounding factors as discussed for uNK level measurement. Additionally, some of the methodologies employed, such as measuring cytotoxicity against cancer cell lines, is not an accurate representation of in vivo uNK activity. However, future well-designed functional studies may be useful to give an indication of the direction of effect of uNK in women with reproductive problems that in turn may help to direct targeted immunotherapy. Until then, our findings serve to highlight the current poor understanding of uNK function in women with RM and RIF, therefore, it is premature to offer blanket cover immunotherapy, which may have detrimental effects on the physiological function of uNK.
Implications
Future research implications
Although much research has been dedicated to understanding the role of uNK cells in pregnancy, we still do not fully understand their role in pathological pregnancies. One of the issues identified by our systematic review is the heterogeneity among the studies performed on uNK level and activity in women with reproductive failure.
Measurement of uNK level is a good start; however, when investigating these cells researchers should focus on the endometrium and ideally time clinical specimens so that they are obtained during the mid-luteal phase to avoid skewing of results owing to the rapid change in uNK level during the secretory phase of the menstrual cycle. Although flow cytometry is more resource intensive, in our opinion it is a better tool than immunohistochemistry because it is objective and able to discern different subpopulations of uNK using specific cell surface markers. Furthermore, the use of a standardized gating strategy would help to overcome heterogeneity between different studies and indeed between different samples within the same study. Finally, there should be a move away from using CD16 as a sole marker to define uNK, as this does not discern uNK from other immune cells, such as macrophages, thus resulting in inaccurate reports of ‘cytotoxic’ uNK.
Future research should endeavour to understand uNK activity in normal pregnancies to set a baseline before proceeding to evaluate abnormal behaviour in pathological pregnancies. Recent application of single cell RNA sequencing in first trimester pregnancies has revealed three new subpopulations of CD56bright dNK (Vento-Tormo et al., 2018), with dNK1 proposed to play a central role in trophoblast interaction (Huhn et al., 2020). Future studies focussing specifically on dNK1 may be able to detect differences that were not evident before.
It is clear that uNK undergo complex interactions with other cells in the immune milieu of the placental bed as well. The role of other immune cells that are present in the decidua needs to be further investigated, such as innate lymphoid cells, macrophages and T cells, all of which may contribute to the pool of cytokines that were originally thought to be produced by uNK. For instance, CD56+CD94−CD117+CD127+ Type 3 ILC produce IL-22 and IL-8 whereas CD56+CD94− ILC-1 may contribute to IFN-γ production (Vivier et al., 2018).
Additionally, consideration needs to be given to the interaction between uNK and trophoblast cells. Recent immunogenetic studies strongly suggest that certain combinations of parental HLA-C and maternal KIR genotype may result in better pregnancy outcome in women undergoing ART. This may explain the clinical observation of improved pregnancy outcome in some women with RIF when donor eggs are used. Further randomized controlled trials are required to confirm these observations before immunogenetic screening can be utilized in a clinical setting.
From a clinical perspective, there are various risk factors that may also influence pregnancy outcome in women with unexplained RM and RIF that has not been fully explored, such as lifestyle factors, BMI, subclinical chronic endometritis, or low testosterone levels. The interplay between these factors may influence uNK cell function and therefore there is a need for further studies to establish if this relation exists.
Clinical implications
Although measurement of pNK level is an appealing clinical tool owing to convenience, our findings indicate that measurements made on pNK do not allow us to predict uNK level or activity. Therefore, there should be a move away from measuring pNK level, which does not correlate with uNK level. The recent finding that peripheral blood immune cells contribute to uNK (Strunz et al., 2021) suggests that there is a circulating progenitor to uNK. This holds out the potential that measurements of this progenitor in blood could be informative, as well as relatively non-invasive. Identification of the progenitor will be a critical first step before this approach can be tested.
Our systematic review has exposed the limitations of uNK level measurement as a clinical tool. At present, the most obvious issue is the lack of an established reference range, with each centre reporting cut-off values based on their own cohorts. Therefore, a standardized reference range will need to be agreed before clinical utilization of uNK measurement can be realized. In the future, there may be a role for measurement of uNK level to guide clinical diagnosis, although this may only be limited to having an explanatory purpose in women with unexplained RM or RIF: measurement of uNK level may not be useful to guide immunotherapy because the observation of elevated CD56+ uNK in the endometrium of women with RM and RIF could either be a cause or effect of the underlying pathology. Besides that, our review has highlighted the complexity of interaction between NK cells and other cells in the immune milieu of the decidua. Therefore, the practice of suppressing ‘high’ uNK level with immunotherapy need to be considered carefully. The broad-spectrum effect of most immunotherapy may not only target NK cells but also other immune cells, with possible deleterious effects. The lack of high-level evidence and uniformity of immunotherapy regimen in women with RM/RIF was highlighted in recent reviews (Achilli et al., 2018; Woon et al., 2020). Therefore, immunotherapy may be better guided by correcting altered uNK function, rather than uNK number, as is current practice in other fields of medicine such as cancer immunotherapy.
Conclusion
It is evident from our systematic review that, despite extensive research over the past three decades, we are only at the cusp of beginning to understand the role of NK cells in early pregnancy. This is partly attributable to the complexity of their interaction with other cells in the uterine immune milieu and therefore it is not possible to draw conclusions from single cells or molecules. With the application of novel research technologies, such as single cell RNA sequencing, we may be able to further decipher the role of uNK in physiological and pathological pregnancies. Until then, measurement of uNK and administration of immunotherapy should be exclusively performed in research settings.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Data availability
The data underlying this article are available in the article and its online supplementary material.
Authors’ roles
V.M. initiated the review. E.V.W. and O.G. developed the protocol with input from N.S., M.J. and V.M. E.V.W. and O.G. selected the articles, performed data extraction and quality assessment. E.V.W. performed the meta-analysis and O.G. performed the qualitative analysis. E.V.W. wrote the first draft of manuscript with input from O.G. N.S., D.N., M.J. and V.M. provided input on the review and the revisions. All authors contributed to the writing of this article and approved the final version.
Funding
This research was supported by a Borne Foundation PhD studentship awarded to E.V.W.
Conflict of interest
The authors declare that there is no conflict of interest.
Supplementary Material
Contributor Information
Ee Von Woon, Department of Metabolism, Digestion and Reproduction, Institute of Developmental Reproductive and Developmental Biology, Imperial College London, London, UK; The Fertility Centre, Chelsea and Westminster Hospital, London, UK.
Orene Greer, Department of Metabolism, Digestion and Reproduction, Institute of Developmental Reproductive and Developmental Biology, Imperial College London, London, UK.
Nishel Shah, Department of Metabolism, Digestion and Reproduction, Institute of Developmental Reproductive and Developmental Biology, Imperial College London, London, UK.
Dimitrios Nikolaou, The Fertility Centre, Chelsea and Westminster Hospital, London, UK.
Mark Johnson, Department of Metabolism, Digestion and Reproduction, Institute of Developmental Reproductive and Developmental Biology, Imperial College London, London, UK.
Victoria Male, Department of Metabolism, Digestion and Reproduction, Institute of Developmental Reproductive and Developmental Biology, Imperial College London, London, UK.
References
- Achilli C, Duran-Retamal M, Saab W, Serhal P, Seshadri S.. The role of immunotherapy in in vitro fertilization and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril 2018;110:1089–1100. [DOI] [PubMed] [Google Scholar]
- Alecsandru D, Barrio A, Garrido N, Aparicio P, Pellicer A, Moffett A, García-Velasco JA.. Parental human leukocyte antigen-C allotypes are predictive of live birth rate and risk of poor placentation in assisted reproductive treatment. Fertil Steril 2020;114:809–817. [DOI] [PubMed] [Google Scholar]
- Almasry SM, Elmansy RA, Elfayomy AK, Algaidi SA.. Ultrastructure alteration of decidual natural killer cells in women with unexplained recurrent miscarriage: a possible association with impaired decidual vascular remodelling. J Mol Hist 2015;46:67–78. [DOI] [PubMed] [Google Scholar]
- Babayeva G, Purut YE, Giray B, Oltulu P, Alakuş R, Çolakoğlu MC.. Endometrial CD56+ natural killer cells in women with recurrent implantation failure: an immunohistochemical study. tjod 2020;17:236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban Y, Chang Y, Dong B, Kong B, Qu X.. Indoleamine 2,3-dioxygenase levels at the normal and recurrent spontaneous abortion fetal-maternal interface. J Int Med Res 2013;41:1135–1149. [DOI] [PubMed] [Google Scholar]
- Bao SH, Shuai W, Tong J, Wang L, Chen P, Sun J.. Increased expression of Toll-like receptor 3 in decidual natural killer cells of patients with unexplained recurrent spontaneous miscarriage. Eur J Obstet Gynecol Reprod Biol 2012;165:326–330. [DOI] [PubMed] [Google Scholar]
- Barrow AD, Martin CJ, Colonna M.. The natural cytotoxicity receptors in health and disease. Front Immunol 2019;10:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, Nelen W, Peramo B, Quenby S, Vermeulen N. et al. ; ESHRE Guideline Group on RPL. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open 2018;2018:hoy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh C, Pinborg A, Wennerholm UB.. Parental age and child outcomes. Fertil Steril 2019;111:1036–1046. [DOI] [PubMed] [Google Scholar]
- Bohlmann MK, Luedders DW, Strowitzki T, von Wolff M.. Specific secretory phase endometrial leukocytes of women with two and more consecutive idiopathic abortions are not significantly different from healthy controls. Arch Gynecol Obstet 2010;281:983–990. [DOI] [PubMed] [Google Scholar]
- Brosens I, Pijnenborg R, Vercruysse L, Romero R.. The "Great Obstetrical Syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol 2011;204:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MA. Human natural killer cells. Blood 2008;112:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, Hartmann-Boyce J, Ryan R, Shepperd S, Thomas J. et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlino C, Stabile H, Morrone S, Bulla R, Soriani A, Agostinis C, Bossi F, Mocci C, Sarazani F, Tedesco F. et al. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood 2008;111:3108–3115. [DOI] [PubMed] [Google Scholar]
- Carlino C, Trotta E, Stabile H, Morrone S, Bulla R, Soriani A, Iannitto ML, Agostinis C, Mocci C, Minozzi M. et al. Chemerin regulates NK cell accumulation and endothelial cell morphogenesis in the decidua during early pregnancy. J Clin Endocrinol Metab 2012;97:3603–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carosella ED, Rouas-Freiss N, Tronik-Le Roux D, Moreau P, LeMaoult J.. HLA-G: an immune checkpoint molecule. Adv Immunol 2015;127:33–144. [DOI] [PubMed] [Google Scholar]
- Carp H, Toder V, Aviram A, Daniely M, Mashiach S, Barkai G.. Karyotype of the abortus in recurrent miscarriage. Fertil Steril 2001;75:678–682. [DOI] [PubMed] [Google Scholar]
- Chao K H, Yang Y S, Ho H N, Chen S U, Chen H F, Dai H J, Huang S C, Gill T J III. Decidual natural killer cytotoxicity decreased in normal pregnancy but not in anembryonic pregnancy and recurrent spontaneous abortion. Am J Reprod Immunol 1995;34:274–280. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu Y, Cheung WC, Zhao Y, Huang J, Chung JPW, Wang CC, Li TC.. Increased expression of angiogenic cytokines in CD56+ uterine natural killer cells from women with recurrent miscarriage. Cytokine 2018;110:272–276. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu Y, Jiang L, Wang CC, Li TC.. Angiogenic cytokine profiles of human uterine CD56+ natural killer cells in women with recurrent reproductive failure. Am J Reprod Immunol 2016;76:19–28.27813197 [Google Scholar]
- Chen X, Mariee N, Jiang L, Liu Y, Wang CC, Li TC, Laird S.. Measurement of uterine natural killer cell percentage in the periimplantation endometrium from fertile women and women with recurrent reproductive failure: establishment of a reference range. Am J Obstet Gynecol 2017;217:680.e1–680.e6. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang T, Liu Y, Cheung WC, Zhao Y, Wang CC, Laird S, Li TC.. Uterine CD56+ cell density and euploid miscarriage in women with a history of recurrent miscarriage: a clinical descriptive study. Eur J Immunol 2021;51:487–489. [DOI] [PubMed] [Google Scholar]
- Chiossone L, Dumas PY, Vienne M, Vivier E.. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol 2018;18:671–688. [DOI] [PubMed] [Google Scholar]
- Clifford K, Flanagan AM, Regan L.. Endometrial CD56+ natural killer cells in women with recurrent miscarriage: a histomorphometric study. Hum Reprod 1999;14:2727–2730. [DOI] [PubMed] [Google Scholar]
- Coomarasamy A, Dhillon-Smith RK, Papadopoulou A, Al-Memar M, Brewin J, Abrahams VM, Maheshwari A, Christiansen OB, Stephenson MD, Goddijn M.. Recurrent miscarriage: evidence to accelerate action. Lancet 2021;397:1675–1682. [DOI] [PubMed] [Google Scholar]
- Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, Cutting R, Ong K, Sallam H, Li TC.. Recurrent implantation failure: definition and management. Reprod Biomed Online 2014;28:14–38. [DOI] [PubMed] [Google Scholar]
- Covidence Systematic Review Software. 2021. Veritas Health Innovation, Melbourne, Australia. www.covidence.org. (15 June 2021, date last accessed).
- Díaz-Hernández I, Alecsandru D, García-Velasco JA, Domínguez F.. Uterine natural killer cells: from foe to friend in reproduction. Hum Reprod Update 2021;27:720–746. [DOI] [PubMed] [Google Scholar]
- Dong P, Wen X, Liu J, Yan CY, Yuan J, Luo LR, Hu QF, Li J.. Simultaneous detection of decidual Th1/Th2 and NK1/NK2 immunophenotyping in unknown recurrent miscarriage using 8-color flow cytometry with FSC/Vt extended strategy. Biosci Rep 2017;37:BSR20170150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Azzamy H, Dambaeva SV, Katukurundage D, Salazar Garcia MD, Skariah A, Hussein Y, Germain A, Fernandez E, Gilman-Sachs A, Beaman KD. et al. Dysregulated uterine natural killer cells and vascular remodeling in women with recurrent pregnancy losses. Am J Reprod Immunol 2018;80:e13024. [DOI] [PubMed] [Google Scholar]
- El-Badawy O, Helmy AS, Abbas AM, Zahran AM, Afifi NA, Abdel-Rahim MH.. Concordance between peripheral and decidual NK cell subsets and killer immunoglobulin-like receptors in women with recurrent spontaneous miscarriages. J Reprod Immunol 2020;140:103130. [DOI] [PubMed] [Google Scholar]
- El Costa H, Tabiasco J, Berrebi A, Parant O, Aguerre-Girr M, Piccinni MP, Le Bouteiller P.. Effector functions of human decidual NK cells in healthy early pregnancy are dependent on the specific engagement of natural cytotoxicity receptors. J Reprod Immunol 2009;82:142–147. [DOI] [PubMed] [Google Scholar]
- Emmer PM, Steegers EA, Kerstens HM, Bulten J, Nelen WL, Boer K, Joosten I.. Altered phenotype of HLA-G expressing trophoblast and decidual natural killer cells in pathological pregnancies. Hum Reprod 2002;17:1072–1080. [DOI] [PubMed] [Google Scholar]
- Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol 2013;13:23–33. [DOI] [PubMed] [Google Scholar]
- Eskicioğlu F, Özdemir AT, Özdemir RB, Turan GA, Akan Z, Hasdemir SP.. The association of HLA-G and immune markers in recurrent miscarriages. J Matern Fetal Neonatal Med 2016;29:3056–3060. [DOI] [PubMed] [Google Scholar]
- Faridi RM, Agrawal S.. Killer immunoglobulin-like receptors (KIRs) and HLA-C allorecognition patterns implicative of dominant activation of natural killer cells contribute to recurrent miscarriages. Hum Reprod 2011;26:491–497. [DOI] [PubMed] [Google Scholar]
- Feyaerts D, Kuret T, van Cranenbroek B, van der Zeeuw-Hingrez S, van der Heijden OWH, van der Meer A, Joosten I, van der Molen RG.. Endometrial natural killer (NK) cells reveal a tissue-specific receptor repertoire. Hum Reprod 2018;33:441–451. [DOI] [PubMed] [Google Scholar]
- Flynn L, Byrne B, Carton J, Kelehan P, O'Herlihy C, O'Farrelly C.. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am J Reprod Immunol 2000;43:209–217. [DOI] [PubMed] [Google Scholar]
- Fu B, Li X, Sun R, Tong X, Ling B, Tian Z, Wei H.. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci U S A 2013;110:E231–E240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui A, Funamizu A, Fukuhara R, Shibahara H.. expression of natural cytotoxicity receptors on peripheral blood natural killer cells in pregnant women with a history of recurrent pregnancy loss. J Obstet Gynaecol Res 2017;43:1678–1686. [DOI] [PubMed] [Google Scholar]
- Gamliel M, Goldman-Wohl D, Isaacson B, Gur C, Stein N, Yamin R, Berger M, Grunewald M, Keshet E, Rais Y. et al. Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity 2018;48:951–962.e5. [DOI] [PubMed] [Google Scholar]
- Gao Y, Wang PL.. Increased CD56(+) NK cells and enhanced Th1 responses in human unexplained recurrent spontaneous abortion. Genet Mol Res 2015;14:18103–18109. [DOI] [PubMed] [Google Scholar]
- Giuliani E, Parkin KL, Lessey BA, Young SL, Fazleabas AT.. Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am J Reprod Immunol 2014;72:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Fang L, Li B, Xiao X, Chen S, Wang J, Yang F, Chen L, Wang X.. Decreased human leukocyte antigen-G expression by miR-133a contributes to impairment of proinvasion and proangiogenesis functions of decidual NK cells. Front Immunol 2017;8:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Bechtel P, Zhai Y, Youssef F, McLachlan K, Mandelboim O.. Novel insights on human NK cells’ immunological modalities revealed by gene expression profiling. J Immunol 2004;173:6547–6563. Erratum in: J Immunol 2015;194(5): 2447–2448. [DOI] [PubMed] [Google Scholar]
- Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CW, Morgan L. et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest 2010;120:4102–10. Erratum in J Clin Invest 2011;121:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiby SE, Regan L, Lo W, Farrell L, Carrington M, Moffett A.. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum Reprod 2008;23:972–976. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Stegmann KA, Riley EM.. Activation of natural killer cells during microbial infections. Front Immunol 2011;2:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini S, Zarnani AH, Asgarian-Omran H, Vahedian-Dargahi Z, Eshraghian MR, Akbarzadeh-Pasha Z, Arefi S, Jeddi-Tehrani M, Shokri F.. Comparative analysis of NK cell subsets in menstrual and peripheral blood of patients with unexplained recurrent spontaneous abortion and fertile subjects. J Reprod Immunol 2014;103:9–17. [DOI] [PubMed] [Google Scholar]
- Huang Q, Ding J, Gong M, Wei M, Zhao Q, Yang J.. Effect of miR-30e regulating NK cell activities on immune tolerance of maternal-fetal interface by targeting PRF1. Biomed Pharmacother 2019;109:1478–1487. [DOI] [PubMed] [Google Scholar]
- Hudspeth K, Silva-Santos B, Mavilio D.. Natural cytotoxicity receptors: broader expression patterns and functions in innate and adaptive immune cells. Front Immunol 2013;4:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn O, Ivarsson MA, Gardner L, Hollinshead M, Stinchcombe JC, Chen P, Shreeve N, Chazara O, Farrell LE, Theorell J. et al. Distinctive phenotypes and functions of innate lymphoid cells in human decidua during early pregnancy. Nat Commun 2020;11:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn O, Zhao X, Esposito L, Moffett A, Colucci F, Sharkey AM.. How do uterine natural killer and innate lymphoid cells contribute to successful pregnancy? Front Immunol 2021;12:607669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarsson MA, Stiglund N, Marquardt N, Westgren M, Gidlöf S, Björkström NK.. Composition and dynamics of the uterine NK cell KIR repertoire in menstrual blood. Mucosal Immunol 2017;10:322–331. [DOI] [PubMed] [Google Scholar]
- Jiang R, Yan G, Xing J, Wang Z, Liu Y, Wu H, Fan X, Zhou J, Ding L, Sun H.. Abnormal ratio of CD57+ cells to CD56+ cells in women with recurrent implantation failure. Am J Reprod Immunol 2017;78:e12708. [DOI] [PubMed] [Google Scholar]
- Jones RL, Hannan NJ, Kaitu'u TJ, Zhang J, Salamonsen LA.. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab 2004;89:6155–6167. [DOI] [PubMed] [Google Scholar]
- Junovich G, Azpiroz A, Incera E, Ferrer C, Pasqualini A, Gutierrez G.. Endometrial CD16(+) and CD16(-) NK cell count in fertility and unexplained infertility. Am J Reprod Immunol 2013;70:182–189. [DOI] [PubMed] [Google Scholar]
- Kennedy PR, Chazara O, Gardner L, Ivarsson MA, Farrell LE, Xiong S, Hiby SE, Colucci F, Sharkey AM, Moffett A.. Activating KIR2DS4 is expressed by uterine NK cells and contributes to successful pregnancy. J Immunol 2016;197:4292–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Allan DS, Bowen M, Powis SJ, Joseph S, Verma S, Hiby SE, McMichael AJ, Loke YW, Braud VM.. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol 2000;30:1623–1631. [DOI] [PubMed] [Google Scholar]
- King A, Balendran N, Wooding P, Carter NP, Loke YW.. CD3- leukocytes present in the human uterus during early placentation: phenotypic and morphologic characterization of the CD56++ population. Dev Immunol 1991;1:169–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Birkby C, Loke YW.. Early human decidual cells exhibit NK activity against the K562 cell line but not against first trimester trophoblast. Cell Immunol 1989;118:337–344. [DOI] [PubMed] [Google Scholar]
- King A, Burrows T, Loke YW.. Human uterine natural killer cells. Nat Immun 1996. –1997;15:41–52. [PubMed] [Google Scholar]
- Kitaya K, Nakayama T, Okubo T, Kuroboshi H, Fushiki S, Honjo H.. Expression of macrophage inflammatory protein-1beta in human endometrium: its role in endometrial recruitment of natural killer cells. J Clin Endocrinol Metab 2003;88:1809–1814. [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ. et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med 2003;198:1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcow HD, Allan DS, Chen X, Rybalov B, Andzelm MM, Ge B, Strominger JL.. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci U S A 2005;102:15563–15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R.. A class I antigen, HLA-G, expressed in human trophoblasts. Science 1990;248:220–223. [DOI] [PubMed] [Google Scholar]
- Kuon RJ, Vomstein K, Weber M, Müller F, Seitz C, Wallwiener S, Strowitzki T, Schleussner E, Markert UR, Daniel V. et al. The "killer cell story" in recurrent miscarriage: Association between activated peripheral lymphocytes and uterine natural killer cells. J Reprod Immunol 2017a;119:9–14. [DOI] [PubMed] [Google Scholar]
- Kuon RJ, Weber M, Heger J, Santillán I, Vomstein K, Bär C, Strowitzki T, Markert UR, Toth B.. Uterine natural killer cells in patients with idiopathic recurrent miscarriage. Am J Reprod Immunol 2017b;78:e12721. [DOI] [PubMed] [Google Scholar]
- Kwak JY, Beer AE, Kim SH, Mantouvalos HP.. Immunopathology of the implantation site utilizing monoclonal antibodies to natural killer cells in women with recurrent pregnancy losses. Am J Reprod Immunol 1999;41:91–98. [DOI] [PubMed] [Google Scholar]
- Lachapelle M H, Miron P, Hemmings R, Roy D C, . and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol 1996;156:4027–4034. [PubMed] [Google Scholar]
- Laird SM, Mariee N, Wei L, Li TC.. Measurements of CD56+ cells in peripheral blood and endometrium by flow cytometry and immunohistochemical staining in situ. Hum Reprod 2011;26:1331–1337. [DOI] [PubMed] [Google Scholar]
- Lash GE, Bulmer JN, Li TC, Innes BA, Mariee N, Patel G, Sanderson J, Quenby S, Laird SM.. Standardisation of uterine natural killer (uNK) cell measurements in the endometrium of women with recurrent reproductive failure. J Reprod Immunol 2016;116:50–59. [DOI] [PubMed] [Google Scholar]
- Lash GE, Otun HA, Innes BA, Kirkley M, De Oliveira L, Searle RF, Robson SC, Bulmer JN.. Interferon-gamma inhibits extravillous trophoblast cell invasion by a mechanism that involves both changes in apoptosis and protease levels. FASEB J 2006;20:2512–2518. [DOI] [PubMed] [Google Scholar]
- Lash GE, Robson SC, Bulmer JN.. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta 2010;31(Suppl):S87–S92. [DOI] [PubMed] [Google Scholar]
- Le Bouteiller P, Bensussan A.. Up-and-down immunity of pregnancy in humans. F1000Research 2017;6:1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea RG, Al-Sharekh N, Tulppala M, Critchley HO.. The immunolocalization of bcl-2 at the maternal-fetal interface in healthy and failing pregnancies. Hum Reprod 1997;12:153–158. [DOI] [PubMed] [Google Scholar]
- Lédée N, Chaouat G, Serazin V, Lombroso R, Dubanchet S, Oger P, Louafi N, Ville Y.. Endometrial vascularity by three-dimensional power Doppler ultrasound and cytokines: a complementary approach to assess uterine receptivity. J Reprod Immunol 2008;77:57–62. [DOI] [PubMed] [Google Scholar]
- Lédée-Bataille N, Bonnet-Chea K, Hosny G, Dubanchet S, Frydman R, Chaouat G.. Role of the endometrial tripod interleukin-18, -15, and -12 in inadequate uterine receptivity in patients with a history of repeated in vitro fertilization-embryo transfer failure. Fertil Steril 2005;83:598–605. [DOI] [PubMed] [Google Scholar]
- Lédée-Bataille N, Dubanchet S, Coulomb-L'hermine A, Durand-Gasselin I, Frydman R, Chaouat G.. A new role for natural killer cells, interleukin (IL)-12, and IL-18 in repeated implantation failure after in vitro fertilization. Fertil Steril 2004;81:59–65. [DOI] [PubMed] [Google Scholar]
- Li H, Hou Y, Zhang S, Zhou Y, Wang D, Tao S, Ni F.. CD49a regulates the function of human decidual natural killer cells. Am J Reprod Immunol 2019;81:e13101. [DOI] [PubMed] [Google Scholar]
- Liu B, Mariee N, Laird S, Smith J, Li J, Li TC.. The prognostic value of uNK cell count and histological dating in the mid-luteal phase of women with reproductive failure. Eur J Obstet Gynecol Reprod Biol 2014;181:171–175. [DOI] [PubMed] [Google Scholar]
- Liu J, Dong P, Jia N, Wen X, Luo L, Wang S, Li J.. The expression of intracellular cytokines of decidual natural killer cells in unexplained recurrent pregnancy loss. J Matern Fetal Neonatal Med 2020;9:1–7. [DOI] [PubMed] [Google Scholar]
- Liu J, Dong P, Wang S, Li J.. Natural killer, natural killer T, helper and cytotoxic T cells in the decidua from recurrent spontaneous abortion with normal and abnormal chromosome karyotypes. Biochem Biophys Res Commun 2019;508:354–360. [DOI] [PubMed] [Google Scholar]
- Long W, Shi Z, Fan S, Liu L, Lu Y, Guo X, Rong C, Cui X, Ding H.. Association of maternal KIR and fetal HLA-C genes with the risk of preeclampsia in the Chinese Han population. Placenta 2015;36:433–437. [DOI] [PubMed] [Google Scholar]
- Lopez-Vergès S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL.. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010;116:3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Jin LP, Huang HL, Ha SY, Yang HL, Chang RQ, Li DJ, Li MQ.. Trophoblast-derived CXCL12 promotes CD56bright CD82- CD29+ NK cell enrichment in the decidua. Am J Reprod Immunol 2020;83:e13203. [DOI] [PubMed] [Google Scholar]
- Lyzikova YA, Zinovkin DA, Pranjol MZI.. Increase in FoxP3, CD56 immune cells and decrease in glands PGRMC1 expression in the endometrium are associated with recurrent miscarriages. Eur J Obstet Gynecol Reprod Biol 2020;245:121–126. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Petsas G, Toth B, Relakis K, Jeschke U.. Recent advances in understanding immunology of reproductive failure. J Reprod Immunol 2011;90:96–104. [DOI] [PubMed] [Google Scholar]
- Manaster I, Mizrahi S, Goldman-Wohl D, Sela HY, Stern-Ginossar N, Lankry D, Gruda R, Hurwitz A, Bdolah Y, Haimov-Kochman R, Yagel S.. Endometrial NK cells are special immature cells that await pregnancy. J Immunol 2008;181:1869–1876. [DOI] [PubMed] [Google Scholar]
- Mariee N, Tuckerman E, Ali A, Li W, Laird S, Li TC.. The observer and cycle-to-cycle variability in the measurement of uterine natural killer cells by immunohistochemistry. J Reprod Immunol 2012;95:93–100. [DOI] [PubMed] [Google Scholar]
- Marron K, Harrity C.. Endometrial lymphocyte concentrations in adverse reproductive outcome populations. J Assist Reprod Genet 2019;36:837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron K, Walsh D, Harrity C.. Detailed endometrial immune assessment of both normal and adverse reproductive outcome populations. J Assist Reprod Genet 2019;36:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michimata T, Ogasawara MS, Tsuda H, Suzumori K, Aoki K, Sakai M, Fujimura M, Nagata K, Nakamura M, Saito S.. Distributions of endometrial NK cells, B cells, T cells, and Th2/Tc2 cells fail to predict pregnancy outcome following recurrent abortion. Am J Reprod Immunol 2002;47:196–202. [DOI] [PubMed] [Google Scholar]
- Moffett A, Chazara O, Colucci F, Johnson MH.. Variation of maternal KIR and fetal HLA-C genes in reproductive failure: too early for clinical intervention. Reprod Biomed Online 2016;33:763–769. [DOI] [PubMed] [Google Scholar]
- Moffett A, Shreeve N.. First do no harm: uterine natural killer (NK) cells in assisted reproduction. Hum Reprod 2015;30:1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otun HA, Lash GE, Innes BA, Bulmer JN, Naruse K, Hannon T, Searle RF, Robson SC.. Effect of tumour necrosis factor-α in combination with interferon-γ on first trimester extravillous trophoblast invasion. J Reprod Immunol 2011;88:1–11. [DOI] [PubMed] [Google Scholar]
- Ozcimen EE, Kiyici H, Uckuyu A, Yanik FF.. Are CD57+ natural killer cells really important in early pregnancy failure? Arch Gynecol Obstet 2009;279:493–497. [DOI] [PubMed] [Google Scholar]
- Pace D, Morrison L, Bulmer JN.. Proliferative activity in endometrial stromal granulocytes throughout menstrual cycle and early pregnancy. J Clin Pathol 1989;42:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P. NK cells and trophoblasts: partners in pregnancy. J Exp Med 2004;200:951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DW, Lee HJ, Park CW, Hong SR, Kwak-Kim J, Yang KM.. Peripheral blood NK cells reflect changes in decidual NK cells in women with recurrent miscarriages. Am J Reprod Immunol 2010;63:173–180. [DOI] [PubMed] [Google Scholar]
- Parkin KL, Lessey BA, Young SL, Fazleabas AT.. Comparison of NK cell phenotypes in the endometrium of patients with recurrent pregnancy loss versus unexplained infertility: A27. Am J Reprod Immunol 2011;65:1046–7408. [Google Scholar]
- Polanski LT, Baumgarten MN, Quenby S, Brosens J, Campbell BK, Raine-Fenning NJ.. What exactly do we mean by ‘recurrent implantation failure’? A systematic review and opinion. Reprod Biomed Online 2014;28:409–423. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2020;113:533–535. [DOI] [PubMed] [Google Scholar]
- Qu X, Yang M, Zhang W, Liang L, Yang Y, Zhang Y, Deng B, Gao W, Liu J, Yang Q. et al. Osteopontin expression in human decidua is associated with decidual natural killer cells recruitment and regulated by progesterone. In Vivo 2008;22:55–61. [PubMed] [Google Scholar]
- Quack KC, Vassiliadou N, Pudney J, Anderson DJ, Hill JA.. Leukocyte activation in the decidua of chromosomally normal and abnormal fetuses from women with recurrent abortion. Hum Reprod 2001;16:949–955. [DOI] [PubMed] [Google Scholar]
- Quenby S, Bates M, Doig T, Brewster J, Lewis-Jones DI, Johnson PM, Vince G.. Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum Reprod 1999;14:2386–2391. [DOI] [PubMed] [Google Scholar]
- Quenby S, Nik H, Innes B, Lash G, Turner M, Drury J, Bulmer J.. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum Reprod 2009;24:45–54. [DOI] [PubMed] [Google Scholar]
- Radović Janošević D, Popović J, Krstić M, Tubić-Pavlović A, Stefanović M, Pop-Trajković S.. The structure of immunocompetent decidual cells in recurrent missed abortions. Vojnosanit Pregl 2016;73:306–311. [DOI] [PubMed] [Google Scholar]
- Rai R, Sacks G, Trew G.. Natural killer cells and reproductive failure–theory, practice and prejudice. Hum Reprod 2005;20:1123–1126. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Long EO.. KIR2DL4 (CD158d): an activation receptor for HLA-G. Front Immunol 2012;3:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Review Manager (RevMan) [Computer Program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. [Google Scholar]
- Robson A, Harris LK, Innes BA, Lash GE, Aljunaidy MM, Aplin JD, Baker PN, Robson SC, Bulmer JN.. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J 2012;26:4876–4885. [DOI] [PubMed] [Google Scholar]
- Rohatgi A. WebPlotDigitizer. Austin, TX, USA, 2017. [Google Scholar]
- Royal College of Obtetricians and Gynaecologists. Investigation and Treatment of Couples (Green-Top Guideline No. 17). London: RCOG, 2011. [Google Scholar]
- Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, Giles J, Ferrando M, Cabanillas S, Remohí J. et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril 2017;107:1122–1129. [DOI] [PubMed] [Google Scholar]
- Russell P, Sacks G, Tremellen K, Gee A.. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. III: Further observations and reference ranges. Pathology 2013;45:393–401. [DOI] [PubMed] [Google Scholar]
- Santillán I, Lozano I, Illán J, Verdú V, Coca S, Bajo-Arenas JM, Martinez F.. Where and when should natural killer cells be tested in women with repeated implantation failure? J Reprod Immunol 2015;108:142–148. [DOI] [PubMed] [Google Scholar]
- Saravelos SH, Li TC.. Unexplained recurrent miscarriage: how can we explain it? Hum Reprod 2012;27:1882–1886. [DOI] [PubMed] [Google Scholar]
- Sargent IL, Borzychowski AM, Redman CW.. NK cells and human pregnancy–an inflammatory view. Trends Immunol 2006;27:399–404. [DOI] [PubMed] [Google Scholar]
- Sauerbrun-Cutler MT, Huber WJ, Krueger PM, Sung CJ, Has P, Sharma S.. Do endometrial natural killer and regulatory T cells differ in infertile and clinical pregnancy patients? An analysis in patients undergoing frozen embryo transfer cycles. Am J Reprod Immunol 2021;85:e13393. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Sunkara SK.. Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum Reprod Update 2014;20:429–438. [DOI] [PubMed] [Google Scholar]
- Shimada S, Kato EH, Morikawa M, Iwabuchi K, Nishida R, Kishi R, Onoé K, Minakami H, Yamada H.. No difference in natural killer or natural killer T-cell population, but aberrant T-helper cell population in the endometrium of women with repeated miscarriage. Hum Reprod 2004;19:1018–1024. [DOI] [PubMed] [Google Scholar]
- Shmeleva EV, Colucci F.. Maternal natural killer cells at the intersection between reproduction and mucosal immunity. Mucosal Immunol 2021;14:991–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits MAJ, van Maarle M, Hamer G, Mastenbroek S, Goddijn M, van Wely M.. Cytogenetic testing of pregnancy loss tissue: a meta-analysis. Reprod Biomed Online 2020;40:867–879. [DOI] [PubMed] [Google Scholar]
- Sotnikova N, Voronin D, Antsiferova Y, Bukina E.. Interaction of decidual CD56+ NK with trophoblast cells during normal pregnancy and recurrent spontaneous abortion at early term of gestation. Scand J Immunol 2014;80:198–208. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunz B, Bister J, Jönsson H, Filipovic I, Crona-Guterstam Y, Kvedaraite E, Sleiers N, Dumitrescu B, Brännström M, Lentini A. et al. Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy. Sci Immunol 2021;6:eabb7800. [DOI] [PubMed] [Google Scholar]
- Toth B, Vomstein K, Togawa R, Böttcher B, Hudalla H, Strowitzki T, Daniel V, Kuon RJ.. The impact of previous live births on peripheral and uterine natural killer cells in patients with recurrent miscarriage. Reprod Biol Endocrinol 2019;17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trundley A, Moffett A.. Human uterine leukocytes and pregnancy. Tissue Antigens 2004;63:1–12. [DOI] [PubMed] [Google Scholar]
- Tuckerman E, Laird SM, Prakash A, Li TC.. Prognostic value of the measurement of uterine natural killer cells in the endometrium of women with recurrent miscarriage. Hum Reprod 2007;22:2208–2213. [DOI] [PubMed] [Google Scholar]
- Tuckerman E, Mariee N, Prakash A, Li TC, Laird S.. Uterine natural killer cells in peri-implantation endometrium from women with repeated implantation failure after IVF. J Reprod Immunol 2010;87:60–66. [DOI] [PubMed] [Google Scholar]
- Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Fenoglio D, Ragni N, Moretta L, Mingari MC.. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci U S A 2010;107:11918–11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varla-Leftherioti M, Spyropoulou-Vlachou M, Niokou D, Keramitsoglou T, Darlamitsou A, Tsekoura C, Papadimitropoulos M, Lepage V, Balafoutas C, Stavropoulos-Giokas C.. Natural killer (NK) cell receptors' repertoire in couples with recurrent spontaneous abortions. Am J Reprod Immunol 2003;49:183–191. [DOI] [PubMed] [Google Scholar]
- Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, Park JE, Stephenson E, Polański K, Goncalves A. et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018;563:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE. et al. Innate lymphoid cells: 10 years on. Cell 2018;174:1054–1066. [DOI] [PubMed] [Google Scholar]
- Wan X, Wang W, Liu J, Tong T.. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Li YP, Ding B, Zhao YR, Chen ZJ, Xu CY, Fu YB, Wang XT.. Recurrent miscarriage is associated with a decline of decidual natural killer cells expressing killer cell immunoglobulin-like receptors specific for human leukocyte antigen C. J Obstet Gynaecol Res 2014;40:1288–1295. [DOI] [PubMed] [Google Scholar]
- Wei H, Liu S, Lian R, Huang C, Li Y, Chen L, Zeng Y.. Abnormal expression of indoleamine 2,3-dioxygenase in human recurrent miscarriage. Reprod Sci 2020;27:1656–1664. [DOI] [PubMed] [Google Scholar]
- Williams PJ, Searle RF, Robson SC, Innes BA, Bulmer JN.. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol 2009;82:24–31. [DOI] [PubMed] [Google Scholar]
- Woon EV, Day A, Bracewell-Milnes T, Male V, Johnson M.. Immunotherapy to improve pregnancy outcome in women with abnormal natural killer cell levels/activity and recurrent miscarriage or implantation failure: a systematic review and meta-analysis. J Reprod Immunol 2020;142:103189. [DOI] [PubMed] [Google Scholar]
- Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, Bauer J, Hiby SE, Colucci F, Moffett A.. Uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest 2013;123:4264–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Takahashi Y, Kase N, Mori H.. Decidual natural killer cells in recurrent spontaneous abortion with normal chromosomal content. Am J Reprod Immunol 1999;41:337–342. [DOI] [PubMed] [Google Scholar]
- Yan WH, Lin A, Chen BG, Zhou MY, Dai MZ, Chen XJ, Gan LH, Zhu M, Shi WW, Li BL.. Possible roles of KIR2DL4 expression on uNK cells in human pregnancy. Am J Reprod Immunol 2007;57:233–242. [DOI] [PubMed] [Google Scholar]
- Yang WJ, Chen X, Zhao Y, Li TC.. Is uterine natural killer (uNK) cells density related to the number of embryo transfer failure? Hum Reprod 2019;34:305–305. [Google Scholar]
- Yokota M, Fukui A, Funamizu A, Nakamura R, Kamoi M, Fuchinoue K, Sasaki Y, Fukuhara R, Mizunuma H.. Role of NKp46 expression in cytokine production by CD56-positive NK cells in the peripheral blood and the uterine endometrium. Am J Reprod Immunol 2013;69:202–211. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chen X, Zhang T, Chan LKY, Liu Y, Chung JP, Kwong J, Li TC.. The use of multiplex staining to measure the density and clustering of four endometrial immune cells around the implantation period in women with recurrent miscarriage: comparison with fertile controls. J Mol Histol 2020;51:593–603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and its online supplementary material.