Abstract
Heme has been shown to have a crucial role in the signal transduction mechanism of the facultative photoheterotrophic bacterium Rhodobacter sphaeroides. It interacts with the transcriptional regulatory complex AppA/PpsR, in which AppA and PpsR function as the antirepressor and repressor, respectively, of photosynthesis gene expression. The mechanism, however, of this interaction remains incompletely understood. In this study, we combined electron paramagnetic resonance (EPR) spectroscopy and Förster resonance energy transfer (FRET) to demonstrate the ligation of heme in PpsR with a proposed cysteine residue. We show that heme binding in AppA affects the fluorescent properties of the dark-adapted state of the protein, suggesting a less constrained flavin environment compared with the absence of heme and the light-adapted state. We performed ultrafast transient absorption measurements in order to reveal potential differences in the dynamic processes in the full-length AppA and its heme-binding domain alone. Comparison of the CO-binding dynamics demonstrates a more open heme pocket in the holo-protein, qualitatively similar to what has been observed in the CO sensor RcoM-2, and suggests a communication path between the blue-light-using flavin (BLUF) and sensing containing heme instead of cobalamin (SCHIC) domains of AppA. We have also examined quantitatively the affinity of PpsR to bind to individual DNA fragments of the puc promoter using fluorescence anisotropy assays. We conclude that oligomerization of PpsR is initially triggered by binding of one of the two DNA fragments and observe a ∼10-fold increase in the dissociation constant Kd for DNA binding upon heme binding to PpsR. Our study provides significant new insight at the molecular level on the regulatory role of heme that modulates the complex transcriptional regulation in R. sphaeroides and supports the two levels of heme signaling, via its binding to AppA and PpsR and via the sensing of gases like oxygen.
Significance
Controlling gene expression is a major challenge in the field of optogenetics underlying the need to explore new systems that can enhance its toolbox. The expression of the photosynthetic genes in Rhodobacter sphaeroides is regulated by two proteins, the light-sensing antirepressor AppA and the transcriptional repressor PpsR, making them attractive candidates in the control of cellular activities with light. However, the mechanism of regulation remains elusive. In this work, we provide mechanistic details on how heme—a well-known biological cofactor able to switch on/off the transcription of important proteins—can modulate the complex transcriptional regulation in R. sphaeroides via a two-level signaling.
Introduction
Heme, a ubiquitous biological cofactor, is well known to switch on/off the transcription of important proteinaceous components (1,2). In the so-called labile heme (LH) pools (3), a term used to refer to the pool of metabolically active cellular heme, the concentration, oxidation state, distribution, speciation, and dynamics of LH remain poorly understood. Heme is insoluble in aqueous solutions and chaperones/transporter proteins (4, 5, 6) have been hypothesized to store and mobilize it within the cell for regulatory control. Such heme-binding/storage proteins like bacterioferritin and heme chaperones like HemW and glyceraldehyde-3-phosphate dehydrogenase have been recently identified to shuttle heme from its site of synthesis to target proteins (6,7). The subcellular distribution of LH is heterogeneous. Estimates for heme concentrations in the cell are in the range up to ∼100 nM and can increase under certain conditions. In particular, reported LH concentrations vary from 20 to 40 nM for the cytosol, to below 2.5 nM for the mitochondria and the nucleus in the unicellular eukaryote Saccharomyces cerevisiae (8). In addition, signaling molecules like nitric oxide have been shown to mobilize LH, resulting in a >10-fold increase in its concentration (8), whereas, in a normal cell, the free heme concentration is considered to be below 10 μΜ (9).
The photosynthetic apparatus of the purple bacterium R. sphaeroides is one of the proteinaceous components whose transcription can be switched on/off by heme. In R. sphaeroides, heme constitutes one of the three major end products of the tetrapyrrole biosynthetic pathway, the other two being bacteriochlorophyll and vitamin B12. The flow of tetrapyrroles in this trifurcated pathway is regulated by several transcriptional and post-transcriptional events that control their synthesis (10). Quantification of heme in R. sphaeroides has shown that there are in total 100,000–200,000 molecules per cell and an overall increase in heme levels per cell in oxygen-limited cultures compared with aerobic cells (11); however, the LH content has not been estimated. R. sphaeroides is a facultative photosynthetic bacterium. It employs the blue-light- and redox-sensitive flavoprotein Activation of Photopigment and PUC A (AppA) to regulate the activity of the aerobic transcriptional repressor PpsR, which functions as a master regulator of photosynthesis development. Heme interacts with the transcriptional regulatory system AppA/PpsR and plays a key role in the formation of the photosynthetic apparatus. AppA/PpsR has been proposed to function as an oxygen-dependent transcriptional rheostat, fine-tuning the transcription of photosynthesis genes as a response to changing oxygen concentration (12). At high-oxygen-level conditions, and at intense light conditions combined with low and intermediate oxygen levels, AppA/PpsR protects the cell from photooxidative damage by preventing the expression of the photosynthetic apparatus (13, 14, 15, 16).
The flavoprotein AppA, which functions as an antirepressor of photosynthesis gene expression, consists of an N-terminal blue-light-using flavin (BLUF) sensor domain (17, 18, 19) and an oxygen-sensing containing heme instead of cobalamin (SCHIC) domain (12,20,21) (Fig.1 A). The light-adapted state of AppA is characterized by a rearrangement of the H bond around the flavin believed to be associated with conformational changes involving the β5 strand (Fig.1 A). We have recently provided insight on the different conformational configurations of W104, a crucial residue for downstream signaling in AppABLUF that resides on the β5 strand (W104 is close to the flavin in the light-adapted state and away from the flavin in the dark-adapted state) (22). AppA is considered to integrate the two stimuli, light and redox, via its BLUF and SCHIC domains, respectively, and to communicate them to PpsR. PpsR is a well-known oxygen- and light-dependent repressor of bacteriochlorophyll, carotenoid, and heme biosynthesis genes and puc operons (23).
Figure 1.
Structure of AppAΔC (PDB: 4hh1) showing the BLUF domain (yellow), the linker region (cyan and light cyan), the 4HB domain (light brown), and the SCHIC domain (green). FAD, His284, and Cys231 are shown. The β5 strand is shown in red. (B) Structure of one monomer of the dimeric PpsR without the HTH domain (PDB: 4hh2). (C) Structure of the AppA-PpsR2 core complex (PDB: 4hh3) showing the N and PAS1 domain of PpsR (monomer 1, light blue, and monomer 2, light brown) and the SCHIC domain of AppA (green) and 4HB (dirty violet). (D) Domain structure in AppA full length, showing the BLUF (yellow), the linker region (cyan and light cyan), the 4HB (light brown), the SCHIC (green), and the Cys-rich (gray) domains. (E) Domain structure in PpsR showing the N (gray), PAS1 (blue), PAS2 (orange), and HTH (ochre) domains. The residue numbers are shown for all constructs used as well as for the residues that ligate to heme iron.
The puc operon of R. sphaeroides comprises the pucBA structural genes, which encode B800-850 light-harvesting beta and alpha polypeptides, respectively (24). The puc promoter contains two PpsR-binding sites (located 8 bp apart) required for repression in vivo. The consensus PpsR-binding sequence is TGTc-N10-gACA (lower case letters indicate lower conservation). PpsR is a multi-domain protein that consists of three PAS domains (N-terminal (N) domain, PAS1 and PAS2) (25,26), a glutamine-rich region (Q linker), and a helix-turn-helix (HTH) motif that binds the palindromic DNA (27) (Fig.1 B). Full-length PpsR exists in an oligomeric state that has already been described as either a dimer (26) or a tetramer (28) with a dynamic dimer-tetramer equilibrium (2PpsR2 PpsR4) and a Kd of 0.9 μΜ (29). The crystal structure of the protein reveals an intricate tetrameric assembly composed of two head-to-tail PpsR dimers. Both the N-terminal and the PAS1 domains form homodimers between which the alpha helical Q linker forms a coiled-coil-like structure that serves as binding site for the PAS2 domains of another PpsR dimer, leading to tetramer formation.
The tetramer architecture alone cannot explain the highly cooperative DNA-binding mode observed for PpsR, because of the large distance between HTH dimers that are separated by a half-turn of the DNA double helix. Active-site titration data and truncated PpsR proteins have supported a 1:8 stoichiometry of puc:PpsR binding (26,28,29). In the absence of a DNA-bound crystal structure for PpsR and based on the active-site titration data and the DNA-bound crystal structure of the FIS protein (PDB: 3jre), a model for DNA binding to an octameric PpsR has been proposed (29). In that model, the alpha helical Q-linkers mediate interaction between symmetry-related tetramers enabling close positioning of two HTH dimers. PpsR binding to a 70-bp (puc I) or 250-bp (puc II) DNA fragment of the R. sphaeroides puc promoter displays cooperative binding (29) in line with previous findings (17). Eight PpsR molecules have been shown to saturate puc II, whereas four PpsR molecules are required for simultaneous binding to two different sites on DNA with an EC50 = 1 μΜ and with looping out of the intervening DNA (29). This is not surprising as, for the majority of helix-turn-helix-containing prokaryotic regulators, the binding to palindromic sequences implies the formation of a tetramer (30).
The SCHIC domain of AppA mediates the interaction with the transcriptional repressor PpsR (Fig.1 C) (12,20), forming an inactive AppA-PpsR2 complex that dissociates upon illumination (17), although recent studies have argued against that assessment (29). An important aspect of AppA regulation is the binding of heme to the SCHIC domain (12,21). Heme has been shown to bind more strongly to the dark-adapted state of AppA compared with the light-adapted state and to significantly reduce the length of the BLUF photocycle (21). Heme binds to the PAS2 and HTH domains of PpsR and induces conformational changes that are propagated to the covalently attached DNA-binding domain, thus fine-tuning the accumulation of free tetrapyrrole. Heme therefore has been hypothesized to provide a mechanism for all characterized photosynthetic purple bacteria to respond to toxic free tetrapyrroles (31).
Although there are crystal structures of the SCHIC domain (21), AppA (29), and PpsR (29), there is no structural information on their heme-bound complexes. The mode of interaction of heme with the transcriptional regulatory system AppA/PpsR and its interplay with DNA binding are poorly understood, and there are no studies on the interaction of PpsR with individual DNA sequences of the puc promoter. In this study, we provide novel information on the interaction of heme with these proteins using absorption, fluorescence, and electron paramagnetic spectroscopies and demonstrate the ligation of heme in PpsR with a previously proposed cysteine residue. We have also probed the heme environment of AppA and PpsR using gaseous ligands, by studying their ligand-binding dynamics. We present evidence for a weak interaction between the BLUF and SCHIC domains in AppA and provide quantitative information on the affinity of PpsR to labeled target DNA fragments and the effect of heme on DNA binding. Our study provides significant insight on important components modulating the complex transcriptional regulation in R. sphaeroides. Using this knowledge, we propose a model for the heme-based regulation.
Materials and methods
Materials
All chemicals were purchased from Sigma-Aldrich. Aqueous stock solutions of hemin for spectroscopic experiments were prepared by dissolving solid hemin in 0.1 M NaOH. Final concentration of stocks was calculated from the optical density at 385 nm (ε385 = 58.4 mM−1 cm−1) (32).
Expression and purification of AppAΔC, 4HB-SCHIC, PpsR, and PAS2-HTH
The encoding R. sphaeroides AppA without the Cys-rich domain, AppAΔC (residues 1–398), 4HB-SCHIC (residues 168–398) (Fig.1 D), full-length PpsR (residues 1–464), and PAS2-HTH (residues 258–464) (Fig.1 E) were inserted into a pET-15b vector (Novagen) in frame with an N-terminal His6-tag under control of a T7 promoter. A TEV site (GAAAACCTGTATTTTCAGGGC) was incorporated between the N-terminal His6-tag and the coding sequences using the NEBuilder HIFI DNA Assembly Cloning Kit (NEB) in order to cleave the His6-tag that could interfere with heme binding. Deletion of the Cys-rich domain in AppA has been shown to result in higher yields of soluble protein, whereas, to construct the SCHIC domain expression vector, the residues 168–398 were chosen in order to include the four-helix bundle (186–273) and a part of the linker region (168–185) (29). Inclusion of the four-helix bundle and a part of the linker region has allowed us to distinguish the effect of the BLUF domain alone in heme binding to the SCHIC domain. Throughout the text, we use the term 4HB-SCHIC for our SCHIC construct that contains the four-helix bundle and a part of the linker region and the term AppAΔC for the construct of AppA without the Cys-rich domain. The full-length PpsR (31) and the truncated PpsR variant PAS2-HTH that is able to bind heme and DNA were chosen for this study. All constructs were verified by DNA sequencing. Protein expression was performed using BL21(DE3) Escherichia coli cells. A single colony for each construct was used to inoculate 10 mL of Luria broth (LB) medium containing 10 μg/mL ampicillin. This culture was incubated at 37°C overnight and was then used to inoculate 1 L of LB/ampicillin medium in a 4-L flask. The 1-L culture was incubated at 30°C until the optical density 600 (OD600) reached 0.4–0.5, after which the temperature was decreased to 18°C for 30 min followed by addition of 0.8 mM (0.2 mM for 4HB-SCHIC) isopropyl-β-D-1-thiogalactopyranoside (IPTG) to induce protein expression. Both the expression and purification of AppAΔC were performed in the dark. The cells were harvested by centrifugation (6000 rcf, 4°C) and stored at −20°C. The cell pellet was then resuspended in 40 mL of lysis buffer with 0.2 mM phenylmethylsulfonyl fluoride (PMSF), DNase, protease inhibitor cocktail, and lysozyme. The cells were lysed by sonication and cell debris was removed by centrifugation (39,000 rcf, 1 h). In the case of AppAΔC, the supernatant was incubated with excess flavin adenine dinucleotide (FAD) for 45 min on ice in the dark. The supernatant was loaded onto a Ni-NTA (Qiagen) column, which was washed with buffer containing 20 mM imidazole, and the protein was eluted using 200 mM imidazole. The fractions containing proteins were pooled together, dialyzed, and treated overnight with TEV protease to cleave the His6-tag. PpsR and PAS2-HTH were also loaded onto a HiTrap heparin column and eluted with a NaCl gradient. All proteins were purified to homogeneity using size-exclusion chromatography (Superdex-200) and the purity of the final preparations was assessed by SDS-PAGE. All proteins were isolated as apo-forms. An SDS-PAGE analysis is presented in Fig. S1. Protein concentrations were estimated as shown in the supporting material. To obtain the absorption spectra of the heme-bound complexes, the apoproteins were incubated for 15 min with substoichiometric hemin (∼5 μΜ) in a ∼sixfold to 10-fold excess of protein to maximize heme binding. That ratio was used for all measurements unless stated otherwise.
The following buffers were used for each protein: for AppAΔC and 4HB-SCHIC, lysis/wash buffer (20 mM Tris, 300 mM NaCl, 20 mM imidazole, 5% glycerol, pH 8.5), elution buffer (20 mM Tris, 300 mM NaCl, 200 mM imidazole, 5% glycerol, pH 8.5); for PpsR and PAS2-HTH, lysis/wash buffer (50 mM Tris, 300 mM NaCl, 20 mM imidazole, 10% glycerol, pH 8.0), elution buffer (50 mM Tris, 300 mM NaCl, 200 mM imidazole, 10% glycerol, pH 8.0).
Steady-state optical and fluorescence spectra
Absorption spectra were measured on a PerkinElmer Lambda XLS+ and a Shimadzu 1700 spectrophotometer. Fluorescence emission spectra were obtained with a Horiba Jobin Yvon Fluorolog spectrophotometer using λexc = 295 nm as the excitation wavelength. The applied slit width was set at 5 nm for both the excitation side and the emission side. All fluorescence spectra were measured in the dark at 22°C in a 10 × 1-mm quartz cuvette.
EPR measurements
Electron paramagnetic resonance (EPR) spectra were recorded on an Elexsys 500 X-band spectrometer (Bruker) equipped with a continuous-flow ESR 900 cryostat and an ITC504 temperature controller (Oxford Instruments, Abingdon, UK). Experimental conditions: microwave frequency 9.48 GHz, microwave power 0.25 mW (T 15 K) or 1 mW (T 6.5 K), field modulation frequency 100 kHz, field modulation amplitude 2 mT, T 15K or 6.5K. Apoproteins (∼120–140 μM) were incubated with substoichiometric hemin (final 100μM) for 15 min before freezing in liquid nitrogen.
Heme-binding assays
Tryptophan fluorescence intensity was recorded from 500 nM AppAΔC, PpsR, PAS2-HTH, and 800nM 4HB-SCHIC in 10 mM HEPES, 100 mM NaCl, 5% glycerol, pH 7.6. Heme that was prepared as described above was titrated to each protein and the Trp emission intensity was monitored as a function of heme concentration. Tryptophan fluorescence was observed using an excitation wavelength of λexc = 295 nm and the emission was measured between 300 and 550 nm. Dilution and inner field effects were taken into account in the data analysis. The wavelength λ = 353nm, which gave the largest intensity changes between the protein and the heme-bound protein, was chosen for calculating the dissociation constant, Kd. Kd was obtained by plotting the corrected fluorescence intensity at each titration step against the concentration of heme and fitted to the Morrison (quadratic or tight-binding) equation (33) (Eq. 1) using the Origin 2020b software.
| (1) |
where Fobs is the observed fluorescence, F0 is the initial fluorescence, Kd is the dissociation constant, C is the concentration of the protein, and x is the total heme concentration. We note that for C << this expression approaches a hyperbolic curve.
Fluorescence anisotropy-based DNA-binding assays
Fluorescence anisotropy-based DNA-binding assays with PpsR and PAS2-HTH were performed using 100 nM labeled DNA. Measurements were conducted at room temperature (RT) in buffer containing 10 mM CHES pH 9, 10 mM NaCl, and 15% glycerol. A 19-mer and an 18-mer of double-stranded DNA, corresponding to fragments of the PpsR promoter (27,31,34) and containing the putative binding site for PpsR, were obtained by mixing equimolar amounts of the two complementary oligonucleotides 5′-TTGTCAGCCAACACTGACA-3′ (DNA1 forward) and 5′-TGTCAGTGTTGGCTGACAA-3′(DNA1 reverse) and 5′-TGTCAGCGCAATGTGACA-3′ (DNA2 forward) and 5′-TGTCACATTGCGCTGACA-3′ (DNA2 reverse) (Eurogentec). The 5′ extremity of the forward oligonucleotide was labeled with Texas Red. The oligonucleotides were heated for 3 min at 80°C followed by slow cooling down to RT to allow complete annealing.
Fluorescence anisotropy measurements can provide useful information on the molecular size and the mobility of the fluorophore (35). Steady-state fluorescence anisotropy measurements were performed with a Fluorolog Jobin Yvon Horiba spectrofluorometer in L-format configuration equipped with a polarization accessory. The measurements were performed at an excitation wavelength of λexc = 595 nm with a vertical polarization filter and by averaging the emission in the 610–630-nm range with the polarization filter both parallel and perpendicular with respect to the excitation light polarization. Fluorescence anisotropies were calculated from the fluorescence intensities detected according to the equation
| (2) |
where r is the fluorescence anisotropy, Iv is the fluorescence emission intensity detected with vertical polarization, Ih is the fluorescence emission intensity detected with horizontal polarization, and G(λ) is the correction factor experimentally determined measuring the ratio Iv/Ih with a horizontally polarized excitation. Data processing was done using Origin 2020b and Kd values were determined by fitting an n = 1 Hill equation.
Fluorescence anisotropy decay measurements
Direct information on the orientational dynamics, which depend on the size and shape of the rotating species and on the fluidity of its microenvironment, can be obtained by analysis of the temporal decay of the anisotropy (35,36). r(t) for an excited species in a single type of isotropic environment is given by a linear combination of exponentially decaying functions
where θi are the rotational correlation times and the sum of the factors βi yields the initial emission anisotropy r0.
Nanosecond time-resolved fluorescence measurements
Time-resolved fluorescence measurements in the nanosecond time range were performed as previously described (22). To measure the fluorescence lifetimes, the excitation wavelength was set at 455 nm (FAD excitation) using a pulsed nanoLED (Horiba) with pulse duration 1.2 ns. The fluorescence emission was collected at 520 nm. The sample concentration was in the 5–10 μM range and 10 × 1-mm quartz cuvettes were used for the measurements. The integrity of the sample was monitored by UV-visible (UV-vis) absorption spectroscopy before and after the fluorescence measurements, using a Thermo Scientific Evolution 600 UV-vis spectrophotometer.
Kinetic studies
The kinetics of heme dissociation from the ferric heme-complexes of AppAΔC, 4HB-SCHIC, PpsR, and PAS2-HTH were measured spectrophotometrically using a Jasco V-550 spectrophotometer. Transfer of hemin to myoglobin on reaction of the ferric heme-complexes (5 μM) with a sixfold excess of apo-myoglobin (30 μM) was followed at 408 nm (experiments were carried out in 50 mM HEPES/50 mM NaCl pH 7.5).
Kinetic studies on PpsR and PAS2-HTH
The dissociation rate constant, k−CO, for loss of CO from the ferrous-heme-protein complexes of PpsR and PAS2-HTH was determined by ligand replacement (37). Typically, a mixture of 10% NO and 90% argon was transferred to the headspace of an anaerobic cuvette containing the heme-protein-CO complex (300 μL, 5 μM). Release of CO was monitored by the rate of disappearance of the absorbance at 419 nm.
Stopped-flow experiments to monitor CO binding to the ferrous PAS2-HTH protein were performed in a Biologic SFM 300 instrument equipped with a J&M Tidas diode detector. The ferrous protein was prepared by addition of 0.5 mM dithionite to the ferric protein to obtain the reduced non-liganded form (deoxy). The optical path length of the measuring cell was 0.8 mm. The instrument was extensively flushed with gaseous nitrogen prior to use. The instrument contains three reservoirs, containing protein solution (20 μM), saturated CO solution ([CO] = 1 mM (37), and anaerobic (oxygen-free) buffer. The mixing ratios for the experiments were chosen such that, after mixing, the protein concentration was 10 μΜ and the CO concentration varied (final concentration: 128, 193, 251, 386, 434 and 515 μM CO). The binding of CO to the protein was followed by recording the full spectra of solutions of the protein at various delay times.
Ultrafast spectroscopy
Apoproteins (∼60 μM) were allowed to incubate with substoichiometric heme (∼50 μM) for ∼15 min in 50 mM HEPES/50 mM NaCl buffer, pH 7.5, to yield the heme-bound protein solution. Experiments were performed in 1-mm path length optical cells sealed with a gastight stopper. Oxygen was removed by several cycles of vacuum pumping and exposure to pure argon gas, and the gas phase (Fig. 1)was subsequently replaced by 100% CO. The samples that were reduced with 0.5 mM dithionite were left to equilibrate until formation of the FeII-CO complex was complete, as monitored by the steady-state absorption spectrum. Multicolor femtosecond absorption experiments were performed on a 500-Hz repetition rate setup as described (38), based on a Quantronix Integra-C Ti:sapphire oscillator/amplifier system, with a pump pulse centered at 570 nm and a broad-band continuum probe pulse extending down to ∼350 nm generated in a continuously translated CaF2 window. Both test and reference probe beams pass through the sample, which is rastered with a Lissajous scanner. Data were globally analyzed in terms of multi-exponential decay using the Glotaran package (39).
Results
In order to obtain detailed insight into heme-protein interaction, besides the full-length PpsR protein, we have analyzed truncated constructs of AppA and PpsR. These are AppAΔC, which does not include the C-terminal cysteine-rich domain (29); the heme-binding domain of AppA (SCHIC), which also incorporates the four-helix bundle (4HB-SCHIC) and a part of the linker region (29) (Fig.1 D); and the truncated variant PAS2-HTH of PpsR, which contains the two domains implicated in heme binding (PAS2 and the HTH domain). The optical properties of the heme-bound complexes of all studied constructs and their heme association and dissociation constants are in agreement with the optical properties and dissociation constants of AppA, SCHIC, and PpsR reported in previous studies (12,20,21,31) and are presented in the Supporting material (Figs. S2 and S3). In order to gain structural and functional information on the coordination of heme with each one of the components of the transcriptional regulatory system AppA/PpsR, we employed EPR spectroscopy and Förster resonance energy transfer (FRET) measurements.
Characterization of the heme environment in AppA and PpsR by EPR spectroscopy
Our EPR measurements show heme binding to both AppAΔC and PpsR and in particular reveal the binding interactions with neighboring residues.
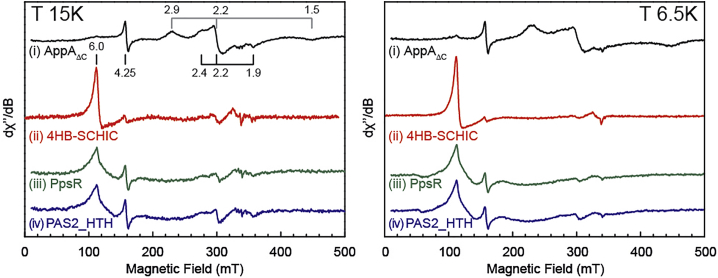
The 15 K spectrum of the AppAΔC-hemin complex consists of two sets of low-spin signals at g = (2.9, 2.2, 1.5) and g = (2.4, 2.2, 1.9) (Fig 2, left panel). The amount of the high-spin signal around g = 6.0 is negligible as seen from experiments under conditions (T = 6.5 K, 1 mW) that enhance high-spin signals (Fig. 2, right panel). In contrast, in the 4HB-SCHIC domain, the spectrum is characterized by a strong signal at g = 6.0 (Fig. 2) that is attributed to loosely bound high-spin ferric heme and a minor low-spin signal at g = (2.4, 2.2, 1.9). Fig. 3 represent a Blumberg-Peisach correlation diagram (40). The rhombicity is plotted against the tetragonal field for a series of low-spin heme proteins and this empirically defines regions of similar axial ligands binding the heme cofactor. The more anisotropic EPR contribution of AppAΔC lies in the region corresponding to a bis-His ligation analogous to bovine liver cytochrome b5. The less anisotropic component has the same g values as 4HB-SCHIC and both lie in the region typical for Cys/His or Cys/OH ligation (41). The His284 residue (Fig. S4 A) has been proposed as the axial ligand for heme binding in the SCHIC domain in AppA (21). Cys231 (Fig. S4 A) is the only cysteine residue present in the helix bundle of the 4HB-SCHIC domain, pointing towards a His284/Cys231 ligation for the ferric form in both proteins. However, the observed differences in heme-binding configuration to the 4HB-SCHIC domain compared with AppAΔC, as deduced from the EPR experiments, suggest that the BLUF domain can modify the conformation of the 4HB-SCHIC domain, affecting the coordination geometry of the heme iron, in line with the proposed weak interaction between the two domains (29). The identity of the His residues forming the bis-His complex is hard to determine. However, given the single koff value for the heme transfer in the AppAΔC complex (see Table S3), we speculate that this is only possible if the exchange between the two species (heme bound to His/Cys and heme bound to His/His) occurs much faster than the heme dissociation. We assume that this exchange can occur fast if His284 is the common ligand between the two species and that the second His ligand is close to His284, which is the case for His308 (Fig. S4 B). In addition, both His284 and His308 are present in flexible loops (Figs.S4 and E). Further studies will provide better insight on the exact stoichiometry in AppAΔC.
Figure 2.
X-band EPR spectra of (i) AppAΔC-hemin complex, (ii) 4HB-SCHIC-hemin complex, (iii) PpsR-hemin complex, and (iv) PAS2-HTH-hemin complex at 15K (left) and at 6.5K (right). The signal at g = 4.3 is due to rhombic iron contamination, minor Mn(II) contamination (six-line signal at g = 2.00) is also visible. [heme] = 100 μM.
Figure 3.
Blumberg-Peisach correlation diagram for low-spin heme species (40). The data corresponding to the proteins studied here are shown in red along data corresponding to a series of previously characterized low-spin heme proteins (see Table S4) shown in black. The empirical regions delimiting a given type of axial heme ligation have been transposed from ref. (40).
The PpsR and PAS2-HTH/hemin complexes are characterized by low-spin signals at g = (2.4, 2.2, 1.9) due to Cys/His or Cys/OH− ligation (Fig. 3) and the presence of a heme-bound high-spin species (g = 6.0) (Fig. 2). These findings identify Cys424 residue in the HTH domain as an axial ligand to the heme under oxidizing conditions, as previously proposed by optical spectroscopy and single-point mutations (31). Under reducing conditions, both His275 (present in the PAS2 domain) (Fig. S4 C) and Cys424 have been proposed to be the axial ligands (31). Table 1 summarizes the g values of the studied heme-bound protein complexes.
Table 1.
Comparison of EPR g values of the studied proteins
| Protein | Ligands | g values |
Crystal field parameters |Δ/λ| |V/Δ| |
References | |||
|---|---|---|---|---|---|---|---|
| gz | gy | gx | |||||
| AppAΔC | His/His | 2.9 | 2.2 | 1.5 | 3.1 | 0.8 | this work |
| Cys/His or Cys/OH- | 2.4 | 2.2 | 1.9 | 6.6 | 0.5 | ||
| PpsR | Cys/His or Cys/OH- | 2.4 | 2.2 | 1.9 | 6.6 | 0.5 | this work |
| PAS2-HTH | Cys/His or Cys/OH- | 2.4 | 2.2 | 1.9 | 6.6 | 0.5 | this work |
FRET measurements
To gain further insight on the location of the heme in both AppAΔC and PpsR, we took advantage of the fluorescent Trp residues in the proximity of the His residues (<30 Å) (Fig. S4), which bind heme as suggested by the absorbance (12,20,21,31) (Fig.S2) and our EPR results (Fig. 2).
A distance (∼25Å) is observed between the only tryptophan residue (W302) present in the heme-binding domain of AppA (PDB: 4heh) and H284 that has been proposed to bind heme (Fig. S4 A). PpsR has one tryptophan in the N domain (W53) and one tryptophan (W361) in the PAS2 domain (Figs.S4 C and S4 G). The latter domain has been proposed to be involved together with the HTH domain in the interaction with heme, based on absorption studies (31). The distance between W361 and the histidine residue H275 that has been proposed to coordinate with the heme iron in reduced conditions is ∼23Å (PDB: 4hh2). Heme can act as a quencher of Trp fluorescence via FRET because the absorption spectrum of the heme overlaps with the emission spectrum of the tryptophan in the 300–420-nm region. Having estimated the Förster distance at which half the energy is transferred (R0), we calculate Trp-heme distances R∼30 Å for the 4HB-SCHIC domain, R∼27 Å for PpsR, and R∼19 Å for the PAS2-HTH domain (see supporting material). These distances are in good agreement with the distances (Trp residue to His residue) obtained from the corresponding crystal structures, mentioned above and summarized in Table S2.
Our FRET measurements corroborate the findings from absorption spectroscopy (12,20,21,31) and our EPR results that implicate His284 and His275 as the heme ligands in AppA and PpsR, respectively. The small deviations between our FRET results and those estimated from the crystal structures are attributed to the fact that we measure distances between Trp and heme and not between Trp and His and the effect that other domains (4HB, N-terminal, PAS1) may have on the conformation of the heme-bound domain in AppA and PpsR.
CO-binding dynamics in AppA and PpsR
Both AppA and PpsR heme-bound complexes have been reported to bind gaseous molecules like CO and NO (12,31). We have further exploited this property using CO as a gaseous ligand in order to probe the heme environment and compare the dynamics of the full-length proteins with the heme-binding domains alone in order to identify important features that control their function. The heme-CO bond can be photolyzed with high quantum yield and CO can escape from the heme pocket and eventually from the protein, or rebind directly to the heme. This dynamic behavior of CO rebinding can be followed by ultrafast transient absorption spectroscopy (42).
AppAΔC and 4HB-SCHIC
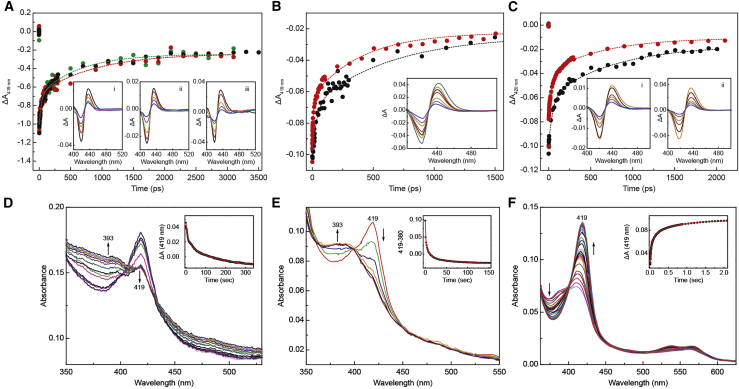
Fig. 4A (inset i, ii and iii) shows the transient absorption spectra in the Soret band region for AppAΔC with the flavin reduced, or oxidized in the dark- and light-adapted states, at selected delay times after CO dissociation in the picosecond and early nanosecond time range. For the AppAΔC protein in all three cases, the heme-CO rebinding kinetics can be described by two phases with time constants of ∼40 ps (30%), ∼700 ps (40%), and the main non-decaying phase (30%). Clearly, the oxidation state of the flavin or the different light conditions do not seem to affect the dynamics of CO rebinding to the heme in the SCHIC domain of the protein (Fig. 4 B).
Figure 4.
(A) Comparison of the transients at 419 nm observed for the heme-CO complexes of flavin-oxidized AppAΔC in the dark-adapted (black) and light-adapted (green) states and for the heme-CO complex of flavin-reduced AppAΔC (red). Inset i: transient absorption spectra of the heme-CO complex of oxidized AppAΔC in the dark-adapted state at various delay times after photodissociation of the CO ligand (2.3, 50, 200, 500, and 1700 ps). Inset ii: transient absorption spectra of the heme-CO complex of oxidized AppAΔC in the light-adapted state at various delay times after photodissociation of the CO ligand (2, 15, 100, 500, 2300 ps). Inset iii: transient absorption spectra of the heme-CO complex of reduced AppAΔC at various delay times after photodissociation of the CO ligand (3, 30, 200, 1300, and 3100 ps). (B) Comparison of the transients at 419 nm observed for the heme-CO complexes of oxidized AppAΔC in the light-adapted (black) states and for the heme-CO complex of the 4HB-SCHIC protein (red). Inset: Transient absorption spectra of the heme-CO complex of the 4HB-SCHIC protein at various delay times after photodissociation of the CO ligand (5, 21, 46, 106, 406, 806, 1906 ps). (C) Comparison of the transient absorption at 420 nm observed for the heme-CO-PpsR (black) and heme-CO-PAS2-HTH complex (red) after photodissociation of the CO ligand. Inset i: transient absorption spectra of the heme-CO-PpsR complex at various delay times after photodissociation of the CO ligand (1.3, 6, 106, 226, 506, and 1106 ps). Inset ii: Transient absorption spectra of the heme-CO-PAS2-HTH complex at various delay times after photodissociation of the CO ligand (1.2, 20, 100, 300, 900, and 2500 ps). (D) Kinetics and spectral changes associated with dissociation of CO from the ferrous-heme-PpsR complex and (E) from the ferrous-heme-PAS2-HTH complex in the presence of NO. The insets show the 419-nm time traces, along with exponential fits. (F) Stopped-flow spectra recorded on binding of CO (515 μM) to the ferrous PAS2-HTH-heme complex (10 μM).
Similar, but not identical, kinetics were observed for the isolated 4HB-SCHIC protein, which is characterized by two rebinding phases with time constants of ∼50 ps (30%), ∼900 ps (40%), and the main non-decaying phase (30%) (Fig. 4 B). The observation that the CO-rebinding dynamics in the 4HB-SCHIC domain are similar to those of the full-length protein is in line with the finding that, in the full-length protein, the redox or light conditions of the BLUF domain do not affect the ligand-binding dynamics in the heme-binding domain. The ligand-binding dynamics in the heme-binding domain are also not significantly affected by the absence or presence of the BLUF domain. Both the BLUF and the SCHIC domain have been shown to use the linker region and the 4HB as binding platforms without strong interactions at their interface (29). The above results are in line with this feature. A comparison of the CO recombination parameters for the proteins of this study and other heme proteins is provided in Table 2.
Table 2.
Parameters of CO recombination in heme proteins
| Protein | Recombination time constants/ps (rebinding fraction) | Non-decaying phase | References |
|---|---|---|---|
| PpsR | 100 (41%), 1400 (48%) | 11% | this work |
| PAS2-HTH | 46 (37%), 660 (48%) | 5% | this work |
| AppAΔC | 40 (30%), 700 (40%) | 30% | this work |
| SCHIC | 50 (30%), 900 (40%) | 30% | this work |
| CooA | 78 (60%), 386 (30%) | 10% | (45,46) |
| DNR | 100 (30%), 900 (35%) | 35% | (47) |
| E. coli DOSH | 1500 (60%) | 40% | (48) |
| E.coli DOS | 1600 (64%) | 36% | (49) |
| FixL | 0 (0%) | 100% | (50) |
| R220H FixL | 280 (25%), 2400 (35%) | 40% | (51) |
| RcoMH-2 | 170 (65%), 500 (35%) | 0% | (52) |
| RcoM-2 | 180 (45.3%), 660 (54.1%) | 0.6% | (44) |
| SUR2A615-933 | 22 (14%), 150 (26%), 2500 (50%) | 10% | (53) |
CooA, CO oxidation transcriptional activator from Rhodospirillum rubrum; DNR, dissimilative nitrate respiration regulator/transcription factor from Pseudomonas aeruginosa; DOS, Escherichia coli direct oxygen sensor (full-length); DOSH, the heme-binding domain of DOS; FixL, transcriptional activator of nitrogen fixation; RcoM-2, regulator of CO metabolism (full-length); RcoMH-2, the heme-binding domain of RcoM-2; SUR2A, sulfonylurea receptor subunit, a member of the ATP-binding cassette transporter superfamily.
PpsR and PAS2-HTH
The insets in Fig. 4 C show the transient absorption spectra in the Soret band region for PpsR and its truncated version PAS2-HTH at selected delay times after CO dissociation, in the picosecond and early nanosecond time range. The transient spectra, with a minimum around 420 nm and a maximum around 440 nm, reflect the formation of a five-coordinate heme upon CO dissociation. Upon CO dissociation, CO located in the heme pocket can either rebind to the heme (geminate rebinding) or migrate out of the protein. CO partially recombines on the picosecond-nanosecond time scale as indicated by the decay of the amplitude of the transient spectra. Their shape also remains unchanged on this time scale, suggesting that the spectral evolution reflects only CO rebinding to the heme and no formation of a six-coordinate complex with two intrinsic residues. The kinetics for all PpsR complexes are characterized by a phase of a few picoseconds that reflects mostly photophysical processes (43) followed by multiphasic CO rebinding. For PpsR, the latter process can be fitted with time constants of 100 ps (41%) and 1.4 ns (48%) and a non-decaying phase (11%) (Fig. 4 C, inset i). In PAS2-HTH, a multiphasic rebinding was also observed with time constants of 46 ps (37%) and 660 ps (48%) and a non-decaying phase (15%) (Fig. 4 C, inset ii). Thus, substantially faster kinetics were observed in the case of the truncated protein PAS2-HTH, suggesting that the N-terminal and PAS1 domain have an effect on the heme environment, in that they exert strain on the heme-binding pocket such that it becomes more open. Interestingly, qualitatively similar effects have been observed when comparing the full-length CO sensor RcoM-2 with its isolated heme domain (slower rebinding in the full-length protein) (44). A comparison of the ligand recombination kinetics for PpsR and PAS2-HTH is given in Fig. 4 C.
CO dissociation and association in PpsR
We have further determined the CO dissociation rates from the ferrous-heme CO complexes of PpsR and PAS2-HTH by measuring the time courses for the replacement of bound CO by NO (37). Addition of 10% gaseous NO results in a gradual decrease of the Soret band of the CO-bound complex (420 nm) with a concomitant increase of an absorbance at ∼390 nm (Figs. 4 D and E). Approximately 27% of a heme-NO-PpsR complex forms with a rate of 0.15 s−1, 27% forms with a rate of 0.04 s−1, and 46% forms with a rate of 0.006 s−1 (Fig. 4 D). In the case of the truncated protein, PAS2-HTH, 43% of the PAS2-HTH-heme NO complex forms with a rate >1 s−1, 39% forms with a rate of 0.15 s−1, and 18% forms with a rate of 0.023 s−1 (Fig. 4 E). These values are in the same range observed for other sensor heme proteins (53). The differences observed between the full-length and the truncated PpsR point toward differences in the heme environment attributed to the presence of the N-terminal and PAS1 domains, as also suggested by the CO-rebinding dynamics. Interestingly, the absorption spectra of the NO-bound species are dominated by a Soret band at ∼393 nm, which is consistent with a five-coordinate NO-bound species, observed in many sensor heme systems (53, 54, 55, 56, 57, 58, 59). The formation of a five-coordinate NO-bound PpsR species has not been reported earlier and it suggests lability of the intrinsic proximal heme ligand.
We employed stopped-flow spectroscopy to study the binding of CO to the ferrous-heme PAS2-HTH domain. In the presence of CO, a heme-CO complex is formed that consequently binds to the protein forming the PAS2-HTH-heme-CO complex (Fig. 4 F). The CO-binding kinetics are highly multiphasic, suggesting considerable heterogeneity.
Heme-induced changes in the flavin fluorescence properties of the BLUF domain
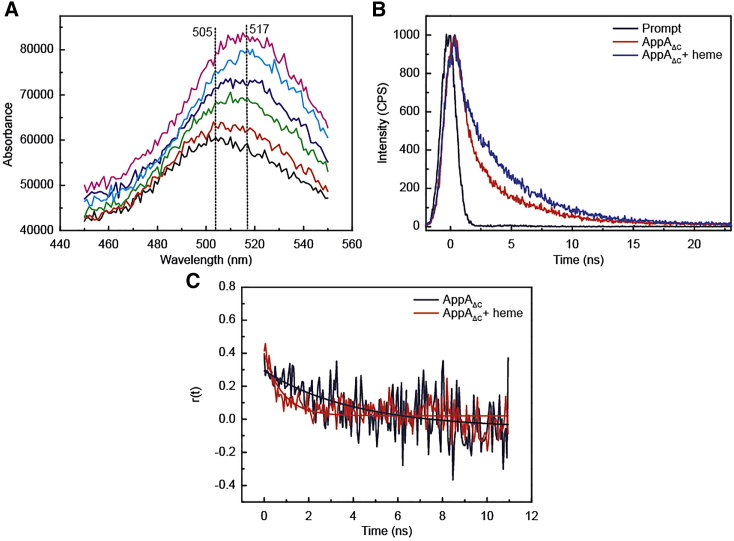
Since blue-light irradiation results in the reorganization of the H-bond network around the flavin, that are transmitted through the C-terminal domain of the protein to partner binding proteins, it is important to know whether, reversely, heme binding in the C-terminal domain has also an effect on the flavin environment. Indeed, heme binding to AppAΔC in the dark-adapted state results in an increase and red shift, from 505 to 517 nm, of the flavin fluorescence (Fig.5 A). Such a red shift is also qualitatively observed upon treatment of AppAΔC with guanidinium chloride (a chemical agent known to denature proteins) (Fig. S5), which is accompanied by the release of the flavin as indicated by the emission maximum at 523 nm. These results suggest that, in heme-bound AppAΔC, flavin is present in an environment with less H bonding, and is hence more flexible, compared with AppAΔC without heme.
Figure 5.
(A) Shift of the fluorescence emission maxima of the dark-adapted AppAΔC at increasing heme concentrations. (B) Fluorescence decay of the dark-adapted AppAΔC in the absence (red) and presence of heme (blue) after excitation at 455 nm (λem = 520 nm). The instrument response function (∼1 ns) is shown in black. (C) Decay of the fluorescence anisotropy of the dark-adapted AppAΔC in the absence of heme (black) and in the presence of heme (red). Fits are shown as solid traces.
To characterize further the flavin environment in the heme-bound AppAΔC, we applied time-correlated single photon counting (TCSPC) and time-resolved fluorescence anisotropy decay measurements. Fig.5 B shows the fluorescence decay of the dark-adapted AppAΔC in the absence and presence of heme. Heme binding to AppAΔC results in a substantial increase of the average fluorescence lifetime of the flavin from ∼2 ns (heme-free AppAΔC) to 4.4 ns (heme-bound AppAΔC) (Table S5). The latter value is in the same range as the fluorescence lifetime of flavin in solution (∼3–5 ns) (60, 61, 62) and suggests that the fluorescence of the flavin in heme-bound AppAΔC is not quenched by adjacent amino acid residues, but that the flavin is exposed to the solvent.
In line with the fluorescence lifetime measurements, the rotational correlation time of the flavin in the dark-adapted AppAΔC drops dramatically in the presence of heme, τh = 0.8 ± 0.1 ns, compared with the absence of heme, τ = 4.1 ± 1.4 ns (Fig.5 C; Table S6). Fast rotational correlation times (∼1 ns) suggest a fast movement of the fluorophore (35) and support the hypothesis that the flavin is exposed to the solvent and hence has some rotational freedom. The value obtained, however, reflects the movement of a flavin chromophore that is still bound, as the rotational correlation time of free flavin is ∼0.24 ns (63). By contrast, longer correlation times (up to tens of nanoseconds) may reflect a slower rotation of the fluorophore or the entire protein complex (35). Hence, overall, the above fluorescence measurements point toward a flavin present in a flexible environment in the dark-adapted state.
Binding of PpsR and the PpsR-heme complex to DNA
The carboxyl terminus of PpsR, containing the HTH domain that binds to the palindromic motif TGTcN10gACA, is responsible for the repressor activity of the protein (23). As previous studies have not examined the effect of a single palindrome sequence, we have used two different DNA sequences of the puc promoter where only one palindrome is present per sequence in order to examine the effect of each one of the two palindromes that bind to the DNA-binding sites of PpsR. As heme binding affects the interaction between PpsR and its target DNA, we carried out quantitative DNA-binding assays based on fluorescence anisotropy to determine the affinity of the PpsR repressor for the two palindromic sequences of the puc promoter. The two double-stranded DNA fragments that we used, DNA1 and DNA2 (Fig. S4 H), correspond to fragments of the PpsR promoter (27,31,34) and contain the putative binding site for PpsR. Addition of PpsR and PAS2-HTH to labeled DNA increases the fluorescence anisotropy of the Texas Red label because DNA binding to the proteins results in an increase of the volume of the labeled entity and hence slows down its rotational movement.
In the absence of heme, we determined a binding constant for DNA1 (Kd) of 0.9 ± 0.2 μM for PpsR (Fig. 6 A) and 1.25 ± 0.23 μΜ for PAS2-HTH (Fig. 6 B). For both cases, in the presence of the heme, a lower asymptotic anisotropy was observed. This implies that the rotation time for protein-bound DNA1 was shorter, suggesting a weaker interaction with the proteins, which is in line with previous findings (31). Furthermore, the Kd was significantly higher in the presence of heme (8.5 ± 0.7 μM for PpsR-heme and 12.2 ± 1.1 μΜ for PAS2-HTH-heme), indicating weaker binding of DNA1 due to the interaction of the heme molecule with the proteins. Binding of oxidized AppAΔC to PpsR does not seem to affect significantly the binding of DNA1 (Kd = 2.0 ± 0.3 μM), in line with previous studies (20) where only the reduced form of AppA was found to function as an antirepressor of PpsR (Fig. 6 A) (17). As a control, we examined DNA1 binding to oxidized AppAΔC and we observed no binding, as expected (Fig. 6 A). Binding of DNA1 also is not affected by reducing PpsR (1 mM DTT that reduces the disulfide bridge, C424–C251 (28)) (Kd = 1.0 ± 0.3 μΜ) or by heme binding to reduced PpsR (Kd = 1.0 ± 0.3 μΜ) (Fig. 6 C).
Figure 6.
Texas Red-labeled DNA is used in all cases and is referred hereafter as DNA. (A) Binding of PpsR and its complex with AppAΔC and heme to DNA1 determined by fluorescence anisotropy. Binding of DNA1 is also shown for AppAΔC and heme (control). (B) Binding of PAS2-HTH and its complex with heme to DNA1 determined by fluorescence anisotropy. (C) Binding of reduced PpsR and reduced PpsR-heme to DNA1 determined by fluorescence anisotropy. (D) Binding of PpsR and its complex with AppAΔC and heme to DNA2 determined by fluorescence anisotropy. Binding of labeled DNA2 is also shown for AppAΔC and heme (control). (E) Binding of PAS2-HTH and its complex with heme to DNA2 determined by fluorescence anisotropy. (F) Binding of reduced PpsR and reduced PpsR-heme to DNA2 determined by fluorescence anisotropy. Fitting to the binding curves is shown by solid lines. (G) Comparison of the binding of PpsR to DNA1 (DNA2) (100 nM) in the presence of unlabeled DNA2 (DNA1) (500 nM).
Similarly, we determined the affinity of PpsR for the DNA2 sequence at 5.3 ± 2.6 μΜ (Kd), which indicates weaker binding of DNA2 (Fig. 6 D) to the protein compared with DNA1 (Fig. 6 A). A weaker affinity for the DNA2 sequence was also determined for PAS2-HTH (Kd = 16 μΜ). In the presence of heme, for both proteins the anisotropy of DNA2 is significantly lower, suggesting a very weak binding (Figs. 6 D and E). Binding of PpsR to DNA2 is not affected significantly by oxidized AppAΔC binding to PpsR, whereas AppAΔC does not interact with DNA2, as expected (Fig. 6 D).
These findings are similar to those observed for DNA1, as outlined above. A slightly different binding affinity was determined for DNA2 upon reducing PpsR (1mM DTT) (Kd = 1.6 ± 0.9 μM), which was not significantly affected by the presence of heme (Kd = 3.3 ± 2.8 μM) (Fig. 6 F). All results are summarized in Table 3.
Table 3.
Dissociation constants for DNA binding to PpsR
| Protein | Kd for DNA1 (μΜ) | Kd for DNA2 (μΜ) |
|---|---|---|
| PpsR | 0.9 ± 0.2 | 5.3 ± 2.6 |
| PAS2-HTH | 1.25 ± 0.23 | 16 ± 15 |
| PpsR-heme | 8.5 ± 0.7 | |
| PAS2-HTH-heme | 12.2 ± 1.1 | |
| PpsR-AppA | 2.0 ± 0.3 | |
| PpsR/DTT | 1.0 ± 0.3 | 1.6 ± 0.9 |
| PpsR/DTT-heme | 1.0 ± 0.3 | 3.3 ± 2.8 |
Fivefold excess of unlabeled DNA2 or DNA1 in the presence of labeled DNA1 or labeled DNA2, respectively, did not result in significant changes in the affinity compared with the absence of unlabeled DNA (Fig. 6 G). This implies that, in the nanomolar concentration range, no effective competition for the same binding sites occurs. This finding suggests that despite the same consensus sequence TGTcN10gACA of DNA1 and DNA2, protein binding to DNA is rather specific due to the different N10s. However, binding of DNA1 to the binding site 1 of PpsR is stronger (Kd = of 0.9 ± 0.2 μΜ) (Fig. 6 A) compared with binding of DNA2 to the binding site 2 of PpsR (Kd = 5.3 ± 2.6 μΜ) (Fig. 6 D).
Discussion
Weak heme binding to AppA and PpsR is modulated by Cys residues
Previous studies aiming to investigate the heme environment in AppA and PpsR were limited to the application of point mutations and absorption spectroscopy to shed light on the identity of the residues coordinating to the heme iron. In AppA, Cys231 in the 4HB-SCHIC domain and the highly conserved—based on sequence alignments of the SCHIC and B12-binding domains—residue, His284, have been proposed as axial ligands (12,21). A His/Cys ligation is suggested from the EPR spectra of AppAΔC pointing toward a ligation to Cys231, which is the only cysteine residue in the 4HB-SCHIC domain (heme-binding domain). Additional evidence for the involvement of His284 and His275 as the heme ligands in AppAΔC and PpsR, respectively, arise from our FRET measurements in which the estimated distances are in line with the estimated distances from the crystal structures (Table S2). Similarly, a His/Cys ligation also characterizes the heme sensor HRI (heme-regulated eIF2α kinase), whereas, in the truncated protein Δ145-HRI that lacks the His residue, heme iron ligates to a Cys and H2O/OH− ligand (64). These findings favor our assignment to a His/Cys ligation in view of the His residues (His284 and His275) present in the proteins.
In PpsR, Cys424 in the HTH domain has been proposed as the axial ligand under oxidizing conditions, whereas under reduced conditions both His275 and Cys424 have been proposed to be the axial ligands (21). In this work, using EPR spectroscopy and FRET measurements, we provided further insights on the coordination environment of the heme iron. Our EPR measurements on PpsR are in line with a Cys ligation in the ferric state. A cysteine thiolate is a well-known critical group found in many proteins that function as heme sensors. It displays weak coordination to the Fe(III) heme complex and even weaker to the Fe(II) heme complex if it is not supported by another proximal residue. The origin of this redox effect is the stronger electrostatic repulsion due to the largest number of electrons in the Fe(II) heme complex compared with the Fe(III) heme complex (65).
The Kd values calculated from our measurements for AppaΔC and 4HB-SCHIC (Kd = 0.5 ± 0.1 μM for AppAΔC (dark), Kd = 0.15 ± 0.05 μM for AppAΔC (light), and 0.6 ± 0.1 μM for 4HB-SCHIC) are similar to the value (Kd = 1.2 ± 0.4 μΜ) reported for the full-length AppA (12). For PpsR and its truncated version PAS2-HTH, we obtained Kd values of 3.9 ± 1 μM and 4.7 ± 0.7 μΜ, respectively, which are in line with the Kd of 1.9 μΜ that has been reported previously from fluorescence quenching measurements for PpsR (31). These Kd values are all similar and indicative of weak binding of the heme ligand and are in line with a regulatory role of the heme as described in several systems involved in transcription (1). All results are summarized in Table S3. It should be noted that although our tryptophan fluorescence quenching measurements (Fig. S3 B) and absorption measurements (Fig. S2 C) clearly demonstrate heme binding to the light-adapted state of AppA, it has been suggested that this does not occur based on single-wavelength stopped-flow measurements (21).
Heme binding to the 4HB-SCHIC domain of the dark-adapted AppAΔC induces conformational changes in the BLUF domain
A weak interplay between the BLUF and SCHIC domains has been proposed based on the crystal structure of AppAΔC, which lacks a strong interaction at the BLUF-SCHIC interface of AppAΔC. The BLUF and the SCHIC domain are interconnected via a linker region and a 4HB that serve as binding platforms (29). Our EPR and fluorescence spectroscopy studies provide evidence for this type of weak interaction. We propose that, in the dark-adapted state, heme binding has an effect on the flavin environment, as indicated by the changes in the fluorescent properties of the protein. By contrast, in the light-adapted state, the flavin environment is arranged in such a way that changes in the 4HB-SCHIC domain due to heme binding cannot be transmitted. Our findings are in line with the current view that AppA integrates two stimuli (light and redox) (21) and are discussed below:
Dark-adapted AppAΔC
The heme-bound AppAΔC and heme-bound 4ΗΒ-SCHIC domain are characterized by different coordination geometries to the heme iron (Fig. 2), suggesting that the presence of the BLUF domain can modify the conformation of the 4ΗΒ-SCHIC domain. On the other hand, heme binding to the 4ΗΒ-SCHIC domain of the dark-adapted AppAΔC affects the fluorescent properties of the flavin in the BLUF domain. Notably, we observed a substantial change in the environment of the flavin to a more flexible conformation where the flavin is more exposed, as indicated by the red shift of the emission spectrum (Fig. S3 A), the increase of the fluorescence lifetime (Fig. 5 B; Table S5), and the decrease of the rotational correlation time of the flavin in the heme-bound dark-adapted AppAΔC (Fig. 5 C; Table S6). In addition, the photocycle of the heme-bound AppA has been shown to be significantly reduced (decay half-life ∼4 min) compared with AppA without heme (decay half-life ∼15 min) (21). These findings illustrate clearly the weak interconnectivity between the two domains in AppA.
Light-adapted AppAΔC
Interestingly, while heme binding to the dark-adapted AppAΔC has an effect on the flavin environment, as mentioned above and indicated by the changes in the fluorescent properties of the protein, this is not the case for the heme-bound light-adapted AppAΔC (Fig. S3 B). The light-adapted state of AppA is characterized by a rearrangement of the H bond around the flavin believed to be associated with conformational changes involving the β5 strand (Fig.1 A). We have recently observed different conformational configurations in the light-adapted and dark-adapted states of AppAΔC for W104, a crucial residue for downstream signaling in AppABLUF that resides on the β5 strand. We found that W104 is close to the flavin in the light-adapted state and away from the flavin in the dark-adapted state (22).
Ligand-binding dynamics reveal allosteric interactions in both AppA and PpsR
Our findings are in general agreement with allosteric interactions that need to occur in both proteins in order for a light-signaling pathway to take place and point to the proposed cooperativity given the ternary AppA-PpsR-DNA complex that is formed (17). Addition of oxygen to ferrous-heme-bound AppA has been shown to discoordinate heme instead of forming a long-lived oxy complex (12). However, both AppA and PpsR in their heme-bound complexes have been reported to bind gaseous molecules like CO and NO (12, 31), as also demonstrated by our measurements (Figs. 4 and S2). We have further exploited this property using CO as a gaseous ligand in order to probe their heme environment. Photodissociation of the heme-CO bond and subsequent monitoring of the spectroscopic changes occurring upon heme-CO re-ligation provide an exquisitely sensitive probe of the heme-iron environment and allow characterizing the intraprotein migration of that diatomic gaseous molecule. Analysis of the dynamics of CO binding and escape provides insight into how the pathway of the dissociated ligand is controlled by the protein environment (66). We observed multiphasic CO recombination in both proteins, suggesting considerable heterogeneity on the timescale up to a few nanoseconds.
In AppAΔC, there is a substantially higher escape of the CO ligand from the heme pocket in the nanosecond timescale than in PpsR, suggesting a more flexible environment and/or ligand access pathway. Considering that AppA has been suggested to act as an oxygen sensor (12), such a pathway would be necessary for the entry and exit of the oxygen molecule. The presence or absence of the BLUF domain does not seem to affect the CO rebinding in the 4ΗΒ-SCHIC domain, under conditions where heme is reduced. On the contrary, as suggested by our EPR data (Fig. 2), the presence or absence of the BLUF domain seems to affect the binding of ferric heme iron to the 4ΗΒ-SCHIC domain. The reverse is apparently possible as the ferric heme binding to the 4HB-SCHIC domain seems to influence the flavin environment in the dark state (Fig. S3 A). Together, these findings suggest that the redox state of the heme iron may play a role in the transduction mechanism between the BLUF and the SCHIC domain.
Slightly different CO recombination kinetics are observed between the holo-protein and its truncated version in PpsR, suggesting an effect of the N-terminal domain and the PAS1 domain on the local heme environment. Qualitatively, such effects have been observed previously in a bacterial CO-sensor heme protein (44). Whether these differences between the full-length protein and the truncated version of PpsR are due to the absence of the putative disulfide bridge (Cys251-Cys424) (28) in the PAS2-HTH or another conformational change that affects the heme environment remains to be investigated.
Binding of PpsR to DNA
Gas-controlled heme-binding transcription factors have been shown to display high affinities (in the nanomolar range) to DNA (44 nM for dissimilative nitrate respiration regulator [DNR], <2 nM for RcoM-2) and 8nM for CooA (CO oxidation transcription activator from Rhodospirillum rubrum) (44,47,67). In our studies, only one PpsR molecule is implicated in binding each palindrome (Hill coefficient n = 1). We found that DNA1 binds with higher affinity to PpsR (Kd = 0.9 ± 0.2 μΜ) compared with DNA2 binding to PpsR (Kd = 5.3 ± 2.6 μΜ). In the presence of fivefold excess of unlabeled DNA, we did not observe changes in the binding affinity, which suggests that the two DNA sequences are specific for their binding sites (Fig. 6 G). The estimated affinities of DNA to PpsR are in the same order of magnitude as those of previous PAGE studies on PpsR with the puc II sequence encompassing both binding motifs (17), but significantly lower than those determined by gel mobility shift assays for PpsR and a 262 bp puc promoter containing both binding sites (nanomolar range) (17). That may be attributed to the ability of PpsR to interact with other components (e.g., AppA, heme). In order for these multiple interactions to take place, it may be essential that tight binding of any of these components is prohibited. Indeed, the dissociation constants for binding both AppA and heme are in the micromolar range: Kd of 1.3 μΜ for reduced AppA (17) and Kd of 2–4 μΜ for heme binding (Fig. S3 D and (31)).
The affinity we observed for DNA1 (Kd = 0.9 ± 0.2 μΜ) is identical to that observed for full puc II by Winkler et al. (29) but higher than that of DNA2 (Kd = 5.3 ± 2.6 μΜ). Also, we did not observe evidence for mutual influence between DNA1 and DNA2 binding. Together, this suggests that the presence of the connecting sequence may be required to allow higher-affinity DNA2 binding. Under our experimental conditions, we did not observe cooperativity effects in binding of PpsR to either DNA1 or DNA2. This is in contrast to the puc II experiments of Winkler et al. (29) and the puc experiments of Masuda and Bauer (17), suggesting that the separate motifs might not require octomerization of PpsR to bind. Altogether, given that DNA1 affinity is higher than DNA2 and similar to the full sequence affinity that requires an octamerization to bind, these results suggest that, in the full puc II (or puc) sequence, oligomerization is initially triggered by binding of the DNA1 motif.
The effect of heme on DNA binding to PpsR
DNA binding is significantly affected by the presence of heme. As discussed above, heme is able to form a complex with PpsR in the Fe(III) oxidation state, with His275 and Cys424 as the critical residues present in the PAS2 and HTH domains, respectively, that are able to bind to the heme iron. Gel mobility shift assays using the puc promoter, which contains two PpsR-binding sites located 8 bp apart, have shown that heme inhibits the ability of PpsR to form a higher-ordered PpsR-DNA complex. The same effect was also observed with different promoters, but the formation of the PpsR-puc complex was more sensitive to disruption in the presence of heme (31). Footprint assays have confirmed the different conformations of the PpsR-puc complexes with and without bound heme (31). In line with these observations, our fluorescence studies have shown weaker binding of DNA both to PpsR and its truncated version, PAS2-HTH, in the presence of heme. In particular, we observed a ∼10-fold increase in the dissociation constant Kd for DNA1 binding in both heme-bound proteins. Heme is proposed to be accommodated in a cleft near Cys424, and a heme-induced conformational change in the protein may be responsible for this weaker DNA binding (28).
Potential mechanisms that heme uses to control DNA binding to PpsR
One of the proposed mechanisms that control the strength of the DNA binding involves the oxidation-reduction of the disulfide bond between Cys424 in the HTH domain and Cys251 in the PAS1 domain of the protein. PpsR in the oxidized form (disulfide bridge present) has been shown to bind the puc DNA sequence approximately two times stronger in vitro compared with PpsR in the reduced form (free thiols) (17). Therefore, heme binding to Cys424 is expected to disrupt the disulfide bridge, justifying the proposed conformational change and the decreased DNA affinity. However, the observed ∼10-fold increase of the Kd of DNA binding in the presence of heme is significantly higher than the reported ∼twofold increase with reduced PpsR (and the lack of effect observed in our fluorescence anisotropy measurements with short DNA sequences; Figs. 6 C and F), which suggests that other factors, like occupation of the cleft where DNA binds nearby the heme, may also be responsible. Whether the oxidation-reduction mechanism also has an effect on DNA binding in vivo remains to be investigated as in vitro, a trend of increasing DNA-binding activity was observed by reduction of the disulfide bond (68). It should be noted that in vivo studies have shown that PpsR does not contain a disulfide bond either in aerobically or in anaerobically grown cells (68), at variance with an in vitro study that has shown that the disulfide bond of the oxidized PpsR can be formed in the presence of oxygen (17).
Disruption of the disulfide bridge upon heme binding to Cys424 could be considered analogous to the second mechanism reported to control the strength of DNA binding, and that is the binding of AppA to PpsR. Reduced AppA has been shown to form a stable AppA-PpsR2 complex (Kd of 1.3 μM) and to facilitate reduction of the disulfide bond in oxidized PpsR (17). In that complex, the apparent affinity of PpsR for puc I was slightly reduced with respect to that of PpsR in the absence of AppA (29). Our fluorescence anisotropy experiments on DNA binding to the AppA-PpsR complex are in general agreement with DNA-binding data reported by Winkler et al. (29), only small effects on the presence of AppA were found on DNA binding (Figs. 6 A and D).
Physiological implications and the role of heme in transcriptional regulation
Heme binding to PpsR
PpsR, like a number of other transcriptional regulators, is a modular dimeric protein containing PAS domains and the effector domain HTH, which binds DNA. The heme molecule acts as the stimulus that binds to the PAS2 and HTH domains and induces conformational changes that are propagated via the amphipathic α-helical and coiled-coil linkers at the C termini of the core of PAS2 to the covalently attached DNA-binding domain. We propose that, at high oxygen levels, heme iron in its ferric form coordinates weakly, partly via Cys424 (present in the HTH) and partly via His275 to PpsR, thus perturbing DNA binding, but without being able to dissociate DNA as indicated by our affinity measurements. As a result, the formation of the photosynthetic apparatus is inhibited, avoiding photooxidative stress (Fig. 7). However, we anticipate that, at low oxygen levels, heme iron in its Fe(II) oxidation state displays a weak or no coordination with Cys424, resulting in the formation of a five-coordinated species and a significant conformational change in the protein. That conformational change can affect the binding of DNA, which is able to dissociate and activate the expression of genes that encode components of the photosynthetic apparatus (Fig. 7). It should be noted that large conformational changes that take place upon DNA binding are a common characteristic of heme-binding, gas-sensing transcriptional regulators like CooA (69) and DNR (70). Ferrous-heme binding to AppA may also induce conformational changes in the protein due to the weak binding to Cys231 affecting the interaction with PpsR. Future studies, under low oxygen levels in the presence of reducing agents able to reduce the heme iron, are expected to shed more light in that direction.
Figure 7.
Schematic diagram of the interaction of heme with AppA and PpsR in Rhodobacter sphaeroides. In the dark and under aerobic conditions, heme interacts with AppA and induces conformational changes in the BLUF domain. On the contrary, under light conditions, changes in the SCHIC domain due to heme binding cannot be transmitted to the BLUF domain. Heme binding to PpsR decreases the DNA-binding affinity to PpsR but, at high oxygen concentration, inhibits the gene expression of photosynthetic genes. At lower oxygen concentration, binding of ferrous heme is anticipated to result in conformational changes that release DNA and hence promote the expression of photosynthetic genes.
Heme binding to AppA
It is quite surprising that heme is able to interact not only with PpsR but also with AppA. This interaction is prone to provide another level of regulation for the formation of the photosynthetic apparatus. However, the role of heme in AppA remains controversial. Earlier studies have concluded that the AppA-PpsR regulatory system functions as an oxygen-dependent transcriptional rheostat. It was shown that oxygen can dissociate heme from AppA in a concentration-dependent manner (12). Subsequent studies favor a role of heme in controlling the amount of photosynthetic apparatus synthesized. It was shown that AppA can bind heme under both anaerobic and aerobic conditions. Based on the reduced length of the AppA photocycle upon heme binding, it was concluded that, when heme was bound to the oxidized protein, the population of AppA was higher in the dark state (21). Considering that only AppA in the dark state can bind to PpsR (21)—a finding that has been challenged (29)—heme binding favors the formation of the AppA-PpsR complex and hence promotes the biosynthesis of the photosynthetic apparatus. In this way, it is ensured that there is a sufficient amount of heme available to handle the essential function of electron transfer (21). Based on these findings, one could speculate that, under blue-light exposure and regardless of the oxygen levels, heme-bound AppA will favor the formation of the AppA-PpsR complex and hence the biosynthesis of the photosynthetic apparatus. By contrast, in the simultaneous presence of high oxygen levels and light, PpsR will remain bound to DNA and inhibit the formation of the photosynthetic apparatus and the associated photooxidative damage.
The role of the cysteine residues
It should be noted that AppAΔC serves as a very good model for the full-length protein, but caution is needed in extrapolating results from AppAΔC to AppA. The Cys-rich domain, although dispensable for oxygen sensing (12), has been reported to have a significant role in the interaction with PpsR. The redox-active cysteines in the Cys-rich domain of AppA have not yet been structurally solved but are believed to reduce the redox-active Cys in PpsR under anaerobic conditions and lower the DNA-binding affinity of PpsR (17,71). The midpoint potential (Em) of the redox-active pair of cysteines in AppA is reported to be Em = −325 mV (71) and to be very similar to the Em = −320 mV potential of the disulfide pair in PpsR (17,71). The DNA-binding affinity to PpsR seems to be affected slightly by the redox state of the two cysteines present in PAS1 and the HTH domain of PpsR, which has not yet been structurally solved (17). Similarly, the disulfide bridge (Cys399-Cys406, present in the Cys-rich domain) in AppA has been reported to affect the heme-binding properties of the SCHIC domain in the light-adapted state (21). In addition, in the presence of heme, the BLUF photocycle is significantly reduced in oxidized conditions and not affected in reduced conditions, suggesting that the redox state of this disulfide bridge affects signal communication between the BLUF domain and the SCHIC domain (21).
Conclusion
Our study has provided further insight at the molecular level on the interaction of heme with the transcriptional regulatory system AppA/PpsR. It has revealed the involvement of cysteine residues in heme binding, a characteristic of heme-regulated proteins using heme as a signaling molecule and conformational changes in the BLUF domain induced by heme binding to the 4HB-SCHIC domain of AppA. Besides this first level of signaling via its binding, heme can also provide a second level of signaling via the sensing of gases like oxygen. Study of the dynamics have revealed that allosteric interactions need to occur in both proteins in order for the light-signaling pathway to take place. Our study has also provided quantitative information on the individual DNA fragments of the puc promoter that bind to PpsR and has suggested that oligomerization of PpsR is initially triggered by binding of one of the two DNA fragments. Although the affinity values obtained in this study correspond to in vitro results, we anticipate that the competition between the various components involved (heme, DNA, proteins) and the observed behavior can be extrapolated in vivo. Understanding the molecular mechanism of the interaction of heme with PpsR and AppA will allow us to obtain a better understanding of an important component involved in the transcriptional regulation in R. sphaeroides. This knowledge may help us gain insight into this type of regulation in other biological systems, with the ultimate goal to control gene expression, which is a major challenge in the field of optogenetics.
Author contributions
S.M.K., U.L., M.H.V., and A.L. designed research. S.M.K., Z.F., P.D., M.H.V., and A.L. performed experiments. S.M.K., P.D., M.H.V., and A.L. analyzed data. P.D., M.H.V., and A.L. obtained funding. S.M.K. wrote the manuscript with contributions from all authors. All authors read and approved the manuscript.
Acknowledgments
We thank Jinnette Tolentino Collado and Peter Tonge from Stony Brook University for the kind gift of the PpsR plasmid, Éva Hoffmanné Simon for excellent technical assistance, and Jonatan Pasitka for helping with editing the figures. We thank the reviewers for their constructive comments. A.L. acknowledges funding from EFOP-3.6.2-16-2017-00005. A.L. and M.H.V. are grateful to the Balaton project (NKFIH 2017-2.2.5-TÉT-FR-2017-00005 and PHC Balaton 40173VE) for funding the travel exchanges. For EPR experiments, financial support from the IR INFRANALYTICS FR2054 for conducting the research is gratefully acknowledged.
Declaration of interest
The authors declare no competing interests.
Editor: Elizabeth Rhoades.
Footnotes
Sofia M. Kapetanaki's present address is CEA–Institut de Biologie Structurale, Grenoble, France
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2022.04.031.
Contributor Information
Sofia M. Kapetanaki, Email: sofia.kapetanaki@ibs.fr.
Andras Lukacs, Email: andras.lukacs@aok.pte.hu.
Supporting material
References
- 1.Shimizu T., Lengalova A., et al. Martinkova M. Heme: emergent roles of heme in signal transduction, functional regulation and as catalytic centres. Chem. Soc. Rev. 2019;48:5624–5657. doi: 10.1039/C9CS00268E. [DOI] [PubMed] [Google Scholar]
- 2.Gonzaga de França Lopes L., Gouveia Júnior F.S., et al. Henrique Silva Sousa E. Bioinorganic systems responsive to the diatomic gases O2, NO, and CO: from biological sensors to therapy. Coord. Chem. Rev. 2021;445:214096. doi: 10.1016/j.ccr.2021.214096. [DOI] [Google Scholar]
- 3.Gallio A.E., Fung S.S.-P., et al. Raven E.L. Understanding the Logistics for the distribution of heme in cells. J. Am. Chem. Soc. Au. 2021;1:1541–1555. doi: 10.1021/jacsau.1c00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White C., Yuan X., et al. Hamza I. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab. 2013;17:261–270. doi: 10.1016/j.cmet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleischhacker A.S., Ragsdale S.W. An unlikely heme chaperone confirmed at last. J. Biol. Chem. 2018;293:14569–14570. doi: 10.1074/jbc.h118.005247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haskamp V., Karrie S., et al. Jahn D. The radical SAM protein HemW is a heme chaperone. J. Biol. Chem. 2018;293:2558–2572. doi: 10.1074/jbc.ra117.000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweeny E.A., Singh A.B., et al. Stuehr D.J. Glyceraldehyde-3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells. J. Biol. Chem. 2018;293:14557–14568. doi: 10.1074/jbc.ra118.004169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanna D.A., Harvey R.M., et al. Reddi A.R. Heme dynamics and trafficking factors revealed by genetically encoded fluorescent heme sensors. Proc. Natl. Acad. Sci. U. S. A. 2016;113:7539–7544. doi: 10.1073/pnas.1523802113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sassa S. Why heme needs to Be Degraded to iron, Biliverdin IXα, and carbon monoxide? Antioxid. Redox Signal. 2004;6:819–824. doi: 10.1089/ars.2004.6.819. [DOI] [PubMed] [Google Scholar]
- 10.Zappa S., Li K., Bauer C.E. The tetrapyrrole biosynthetic pathway and its regulation in Rhodobacter capsulatus. Adv. Exp. Med. Biol. 2010;675:229–250. doi: 10.1007/978-1-4419-1528-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chidgey J.W., Jackson P.J., et al. Hunter C.N. PufQ regulates porphyrin flux at the haem/bacteriochlorophyll branchpoint of tetrapyrrole biosynthesis via interactions with ferrochelatase. Mol. Microbiol. 2017;106:961–975. doi: 10.1111/mmi.13861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moskvin O.V., Kaplan S., et al. Gomelsky M. Novel heme-based oxygen sensor with a revealing evolutionary history. J. Biol. Chem. 2007;282:28740–28748. doi: 10.1074/jbc.m703261200. [DOI] [PubMed] [Google Scholar]
- 13.Gomelsky M., Kaplan S. appA, a novel gene encoding a trans-acting factor involved in the regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. Rhodobacter Sphaeroides J. Bacteriol. 1995;177:4609–4618. doi: 10.1128/jb.177.16.4609-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomelsky M., Kaplan S. Molecular genetic analysis suggesting interactions between AppA and PpsR in regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 1997;179:128–134. doi: 10.1128/jb.179.1.128-134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braatsch S., Moskvin O.V., Klug G. Responses of the of the Rhodobacter sphaeroides transcriptome to blue light under semiaerobic conditions. J. Bacteriol. 2004;186:7726–7735. doi: 10.1128/jb.186.22.7726-7735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimada H., Iba K., Takamiya K. Blue-light irradiation reduces the expression of puf and puc operons of Rhodobacter sphaeroides under semi-aerobic conditions. Plant Cell Physiol. 1992;33:471–475. doi: 10.1093/oxfordjournals.pcp.a078276. [DOI] [Google Scholar]
- 17.Masuda S., Bauer C.E. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell. 2002;110:613–623. doi: 10.1016/s0092-8674(02)00876-0. [DOI] [PubMed] [Google Scholar]
- 18.Braatsch S., Gomelsky M., et al. Klug G. A single flavoprotein, AppA, integrates both redox and light signals in Rhodobacter sphaeroides. Mol. Microbiol. 2002;45:827–836. doi: 10.1046/j.1365-2958.2002.03058.x. [DOI] [PubMed] [Google Scholar]
- 19.Gomelsky M., Klug G. BLUF: a novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem. Sci. 2002;27:497–500. doi: 10.1016/s0968-0004(02)02181-3. [DOI] [PubMed] [Google Scholar]
- 20.Han Y., Meyer M.H.F., et al. Klug G. A haem cofactor is required for redox and light signalling by the AppA protein of Rhodobacter sphaeroides. Mol. Microbiol. 2007;64:1090–1104. doi: 10.1111/j.1365-2958.2007.05724.x. [DOI] [PubMed] [Google Scholar]
- 21.Yin L., Dragnea V., et al. Bauer C.E. Redox and light control the heme-sensing activity of AppA. MBio. 2013;4:1–9. doi: 10.1128/mbio.00563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karadi K., Kapetanaki S.M., et al. Lukacs A. Functional dynamics of a single tryptophan residue in a BLUF protein revealed by fluorescence spectroscopy. Sci. Rep. 2020;10:2061. doi: 10.1038/s41598-020-59073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomelsky M., Kaplan S. Genetic evidence that PpsR from Rhodobacter Sphaeroides 2.4.1 functions as a repressor of puc and bchf expression. J. Bacteriol. 1995;177:1634–1637. doi: 10.1128/jb.177.6.1634-1637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J.K., Kiley P.J., Kaplan S. Posttranscriptional control of puc operon expression of B800-850 light-harvesting complex formation in Rhodobacter sphaeroides. Rhodobacter Sphaeroides J. Bacteriol. 1989;171:3391–3405. doi: 10.1128/jb.171.6.3391-3405.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elsen S., Jaubert M., et al. Giraud E. PpsR: a multifaceted regulator of photosynthesis gene expression in purple bacteria. Mol. Microbiol. 2005;57:17–26. doi: 10.1111/j.1365-2958.2005.04655.x. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki Y., Fukusumi H., et al. Kataoka M. Role of the N-terminal region in the function of the photosynthetic bacterium transcription regulator PpsR. Photochem. Photobiol. 2008;84:839–844. doi: 10.1111/j.1751-1097.2008.00306.x. [DOI] [PubMed] [Google Scholar]
- 27.Moskvin O.V., Gomelsky L., Gomelsky M. Transcriptome analysis of the Rhodobacter sphaeroides PpsR regulon: PpsR as a master regulator of photosystem development. J. Bacteriol. 2005;187:2148–2156. doi: 10.1128/jb.187.6.2148-2156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomelsky M., Horne I.M., et al. Kaplan S. Domain structure, oligomeric state, and mutational analysis of PpsR, the Rhodobacter sphaeroides repressor of photosystem gene expression. J. Bacteriol. 2000;182:2253–2261. doi: 10.1128/jb.182.8.2253-2261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkler A., Heintz U., et al. Schlichting I. A ternary AppA-PpsR-DNA complex mediates light regulation of photosynthesis-related gene expression. Nat. Struct. Mol. Biol. 2013;20:859–867. doi: 10.1038/nsmb.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cournac A., Plumbridge J. DNA looping in Prokaryotes: experimental and Theoretical approaches. J. Bacteriol. 2013;195:1109–1119. doi: 10.1128/jb.02038-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin L., Dragnea V., Bauer C.E. PpsR, a regulator of heme and bacteriochlorophyll biosynthesis, is a heme-sensing protein. J. Biol. Chem. 2012;287:13850–13858. doi: 10.1074/jbc.m112.346494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawson R.M.C., Elliot D.C., et al. Jones K.M. Oxford University Press; Oxford: 1975. Data for Biochemical Research; pp. 230–231. [Google Scholar]
- 33.Morrison J.F. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim. Biophys. Acta. 1969;185:269–286. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- 34.Jäger A., Braatsch S., et al. Klug G. The AppA and PpsR proteins from Rhodobacter sphaeroides can Establish a redox-dependent signal Chain but Fail to Transmit blue-light signals in other bacteria. J. Bacteriol. 2007;189:2274–2282. doi: 10.1128/jb.01699-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakowicz J.R. Springer; 2006. Principles of Fluorescence Spectroscopy. [Google Scholar]
- 36.Valeur B. Wiley-VCH Verlag GmbH; 2001. Molecular Fluorescence: Principles and Applications. [Google Scholar]
- 37.Olson J.S., Foley E.W., et al. Paster E.V. Measurement of rate constants for reactions of O2, CO, and NO with hemoglobin. Methods Mol. Med. 2003;82:065–091. doi: 10.1385/1-59259-373-9:065. [DOI] [PubMed] [Google Scholar]
- 38.Nag L., Lukacs A., Vos M.H. Short-lived radical intermediates in the Photochemistry of Glucose oxidase. Chem. Phys. Chem. 2019;20:1793–1798. doi: 10.1002/cphc.201900329. [DOI] [PubMed] [Google Scholar]
- 39.Snellenburg J.J., Laptenok S.P., et al. Stokkum I.H. M.v. Glotaran: a Java-based Graphical user interface for the R package TIMP. J. Stat. Softw. 2012;49:1–22. doi: 10.18637/jss.v049.i03. [DOI] [Google Scholar]
- 40.Blumberg W.E., Peisach J. Low-Spin Compounds of Heme Proteins in Bioinorganic Chemistry. American Chemical Society. 1971;100:271–291. doi: 10.1021/ba-1971-0100.ch013. [DOI] [Google Scholar]
- 41.Smith A.T., Pazicni S., et al. Burstyn J.N. Functional divergence of heme-thiolate proteins: a classification based on spectroscopic attributes. Chem. Rev. 2015;115:2532–2558. doi: 10.1021/cr500056m. [DOI] [PubMed] [Google Scholar]
- 42.Vos M.H. Ultrafast dynamics of ligands within heme proteins. BBA Bioenerg. 2008;1777:15–31. doi: 10.1016/j.bbabio.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Petrich J.W., Poyart C., Martin J.L. Photophysics and reactivity of heme proteins: a femtosecond absorption study of hemoglobin, myoglobin, and protoheme. Biochemistry. 1988;27:4049–4060. doi: 10.1021/bi00411a022. [DOI] [PubMed] [Google Scholar]
- 44.Salman M., Villamil Franco C., et al. Vos M.H. Interaction of the full-length heme-based CO sensor protein RcoM-2 with ligands. Biochemistry. 2019;58:4028–4034. doi: 10.1021/acs.biochem.9b00623. [DOI] [PubMed] [Google Scholar]
- 45.Benabbas A., Karunakaran V., et al. Champion P.M. Effect of DNA binding on geminate CO recombination kinetics in CO-sensing transcription factor CooA. J. Biol. Chem. 2012;287:21729–21740. doi: 10.1074/jbc.m112.345090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumazaki S., Nakajima H., et al. Aono S. Dissociation and recombination between ligands and heme in a CO-sensing transcriptional activator CooA. J. Biol. Chem. 2000;275:38378–38383. doi: 10.1074/jbc.m005533200. [DOI] [PubMed] [Google Scholar]
- 47.Lobato L., Bouzhir-Sima L., et al. Liebl U. Dynamics of the heme-binding bacterial gas-sensing Dissimilative Nitrate Respiration Regulator (DNR) and activation barriers for ligand binding and escape. J. Biol. Chem. 2014;289:26514–26524. doi: 10.1074/jbc.m114.571398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liebl U., Bouzhir-Sima L., et al. Vos M.H. Ligand binding dynamics to the heme domain of the oxygen sensor Dos from Escherichia coli. Biochemistry. 2003;42:6527–6535. doi: 10.1021/bi027359f. [DOI] [PubMed] [Google Scholar]
- 49.Lechauve C., Bouzhir-Sima L., et al. Kiger L. Heme ligand binding properties and intradimer interactions in the full-length sensor protein dos from Escherichia coli and its isolated heme domain. J. Biol. Chem. 2009;284:36146–36159. doi: 10.1074/jbc.m109.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liebl U., Bouzhir-Sima L., et al. Vos M.H. Ultrafast ligand rebinding in the heme domain of the oxygen sensors FixL and Dos: general regulatory implications for heme-based sensors Proc. Natl. Acad. Sci. U.S.A. 2002;99:12771. doi: 10.1073/pnas.192311699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jasaitis A., Hola K., et al. Liebl U. Role of distal arginine in early sensing intermediates in the heme domain of the oxygen sensor FixL. Biochemistry. 2006;45:6018–6026. doi: 10.1021/bi060012i. [DOI] [PubMed] [Google Scholar]
- 52.Bouzhir-Sima L., Motterlini R., et al. Liebl U. Unusual dynamics of ligand binding to the heme domain of the bacterial CO sensor protein RcoM-2. J. Phys. Chem. B. 2016;120:10686–10694. doi: 10.1021/acs.jpcb.6b08160. [DOI] [PubMed] [Google Scholar]
- 53.Kapetanaki S.M., Burton M.J., et al. Raven E.A. A mechanism for CO regulation of ion channels. Nat. Commun. 2018;9:907. doi: 10.1038/s41467-018-03291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshimura T., Suzuki S., et al. Matsubara T. Spectral properties of nitric oxide complexes of cytochrome c' from Alcaligenes sp. NCIB 11015. Biochemistry. 1986;25:2436–2442. doi: 10.1021/bi00357a021. [DOI] [Google Scholar]
- 55.Stone J.R., Marletta M.A. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds M.F., Parks R.B., et al. Spiro T.G. Electronic absorption, EPR, and resonance Raman spectroscopy of CooA, a CO-sensing transcription activator from R. rubrum, reveals a five -coordinate NO- heme. Biochemistry. 2000;39:388–396. doi: 10.1021/bi991378g. [DOI] [PubMed] [Google Scholar]
- 57.Taoka S., Banerjee R. Characterization of NO binding to human cystathionine β-synthase: J. Inorg. Biochem. 2001;87:245–251. doi: 10.1016/s0162-0134(01)00335-x. [DOI] [PubMed] [Google Scholar]
- 58.Silkstone G., Kapetanaki S.M., et al. Wilson M.T. Nitric oxide binds to the proximal heme coordination site of the Ferrocytochrome c/Cardiolipin complex. J. Biol. Chem. 2010;285:19785–19792. doi: 10.1074/jbc.m109.067736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Igarashi J., Sato A., et al. Shimizu T. Activation of heme-regulated eukaryotic Initiation factor 2α kinase by nitric oxide is induced by the formation of a five-coordinate NO-heme complex. J. Biol. Chem. 2004;279:15752–15762. doi: 10.1074/jbc.m310273200. [DOI] [PubMed] [Google Scholar]
- 60.Brazard J., Usman A., et al. Plaza P. New insights into the ultrafast photophysics of oxidized and reduced FAD in solution. J. Phys. Chem. A. 2011;115:3251–3262. doi: 10.1021/jp110741y. [DOI] [PubMed] [Google Scholar]
- 61.Leenders H.R.M., Vervoort J., et al. Visser A.J.W.G. Time-resolved fluorescence studies of flavodoxin. Fluorescence decay and fluorescence anisotropy decay of tryptophan in Desulfovibrio flavodoxins. Eur. Biophys. J. 1990;18:43–55. doi: 10.1007/bf00185419. [DOI] [PubMed] [Google Scholar]
- 62.Leenders R., Van Hoek A., et al. Visser A.J. Flavin dynamics in oxidized Clostridium beijerinckii flavodoxin as assessed by time-resolved polarized fluorescence. Eur. J. Biochem. 1993;218:977–984. doi: 10.1111/j.1432-1033.1993.tb18456.x. [DOI] [PubMed] [Google Scholar]
- 63.Krasnopevtseva M.K., Belik V.P., et al. Vasyutinskii O.S. Decay times and anisotropy in polarized fluorescence of flavin adenine dinucleotide determined with Subnanosecond Resolution. Tech. Phys. Lett. 2020;46:614–616. doi: 10.1134/S1063785020060218. [DOI] [Google Scholar]
- 64.Miksanova M., Igarashi J., et al. Shimizu T. Characterization of heme-regulated eIF2α Kinase: roles of the N-terminal domain in the oligomeric state, heme binding, catalysis, and inhibition. Biochemistry. 2006;45:9894–9905. doi: 10.1021/bi060556k. [DOI] [PubMed] [Google Scholar]
- 65.Shimizu T. Binding of cysteine thiolate to the Fe(III) heme complex is critical for the function of heme sensor proteins. J. Inorg. Biochem. 2012;108:171–177. doi: 10.1016/j.jinorgbio.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 66.Liebl U., Lambry J.C., Vos M.H. Primary processes in heme-based sensor proteins. Biochim. Biophys. Acta - Proteins Proteomics. 2013;1834:1684–1692. doi: 10.1016/j.bbapap.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 67.Youn H., Thorsteinsson M.V., et al. Roberts G.P. Dual roles of an E-helix residue, Glu167, in the transcriptional activator function of CooA. J. Bacteriol. 2005;187:2573–2581. doi: 10.1128/jb.187.8.2573-2581.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cho S.-H., Youn S.-H., et al. Kang S.-O. Redox property and regulation of PpsR, a transcriptional repressor of photosystem gene expression in Rhodobacter sphaeroides. Microbiology. 2004;150:697–706. doi: 10.1099/mic.0.26777-0. [DOI] [PubMed] [Google Scholar]
- 69.Roberts G.P., Kerby R.L., et al. Conrad M. CooA, a paradigm for gas sensing regulatory proteins. J. Inorg. Biochem. 2005;99:280–292. doi: 10.1016/j.jinorgbio.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 70.Giardina G., Rinaldo S., et al. Cutruzzolà F. A dramatic conformational rearrangement is necessary for the activation of DNR from Pseudomonas aeruginosa. Crystal structure of wild-type DNR. Proteins. 2009;77:174–180. doi: 10.1002/prot.22428. [DOI] [PubMed] [Google Scholar]
- 71.Kim S.K., Mason J.T., et al. Setterdahl A.T. Redox properties of the Rhodobacter sphaeroides transcriptional regulatory proteins PpsR and AppA. Photosynth. Res. 2006;89:89–98. doi: 10.1007/s11120-006-9086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.