Abstract
Background
Plasma cell mastitis (PCM), also known as mammary duct ectasia, is a chronic nonbacterial breast inflammation characterized by duct expansion and plasma cell infiltration. The severe and intense clinical manifestations profoundly affect the quality of life of female patients. Although the pathological process of PCM is known to include four stages (duct dilatation, inflammation, abscess and fistula), there is still lack of imaging techniques and serum markers with high specificity in clinical practice. Due to recurrent acute attacks and the prolonged healing process of the disease, most patients choose to accept mastectomy.
Summary
We searched for studies, reports and reviews referring to PCM in the past 20 years; more than half of the results were related to animal studies, and little attention has been paid to human beings, which may explain the frequent misdiagnosis of PCM as breast cancer and the limited treatment options. This review focuses on the current diagnostic methods and markers for PCM and hierarchically discusses the typical clinical features, etiological causes and relevant molecular mechanisms of PCM.
Key Messages
We herein highlight the urgent need to develop more specific and sensitive biomarkers in the clinical laboratory. It will help to establish a standardized flowchart for the diagnosis and treatment of PCM in order to improve recovery for female patients.
Keywords: Plasma cell mastitis, Differential diagnosis, Clinical manifestation, Pathogenic mechanism
Introduction
Due to the emphasis on breast-feeding hygiene, the incidence of lactating mastitis has declined. However, the incidence of nonpuerperal mastitis (NPM) is rapidly increasing, among which plasma cell mastitis (PCM) has been highlighted. As the name suggests, PCM is histologically characterized by infiltration of abundant plasma cells into the mammary ducts, which is rare in males [1]. The cause of PCM is still elusive but it is suggested as an autoimmune disease (AID). Some patients have a classic “orange peel” appearance on the skin, which is sometimes mistaken for breast cancer (Table 1).
Table 1.
Summary of PCM in the clinic
| Item | Content |
|---|---|
| Susceptible age | 35–40 years old |
|
| |
| Clinical manifestations | Redness, swelling, heat, pain, lumps with pulsation, nipple retraction, abscess, ulceration, formation of fistula. The course of PCM lasts for several years, and multiple relapses can easily occur |
|
| |
| Pathological characteristics | Mammary duct dilatation, periductal inflammation, plasma cell infiltration |
|
| |
| Diagnostic methods | Ultrasonography, mammography, mammary gland molybdenum target, color Doppler imaging, superb microvascular imaging, MRI, multislice helical CT |
|
| |
| Treatment | Mastectomy or conservative treatment (hormone, antibiotics, etc.) |
Diagnosis of PCM
The current clinical experimental diagnostic criteria for PCM are as follows: (1) nonbreastfeeding and nonpregnant women; (2) with several clinical manifestations, including palpable hard lumps with indistinctive boundaries and poor mobility, or redness, swelling, heat and pain in or around the areola with pulsation, or nipple retraction, nipple discharge and fistula formation; (3) fine-needle puncture biopsy showing abundant infiltrated plasma cells without malignant tumor cells; and (4) consistent results from auxiliary examination methods, such as breast ultrasound, magnetic resonance imaging (MRI) and computed tomography (CT) [2, 3, 4, 5, 6, 7].
Due to the lack of imaging specificity, which is a common characteristic of NPM, PCM can sometimes be misdiagnosed as other breast disorders, such as breast cancer or intraductal papilloma [8, 9, 10] (Fig. 1). Mehta et al. [11] demonstrated that the average resistance index, as determined by color Doppler examination, of malignant lesions is higher than that of benign lesions and that the comprehensive analysis of 3 parameters, that is, the resistance index, pulsatile index and systolic peak velocity, can contribute to the differential diagnosis of PCM.
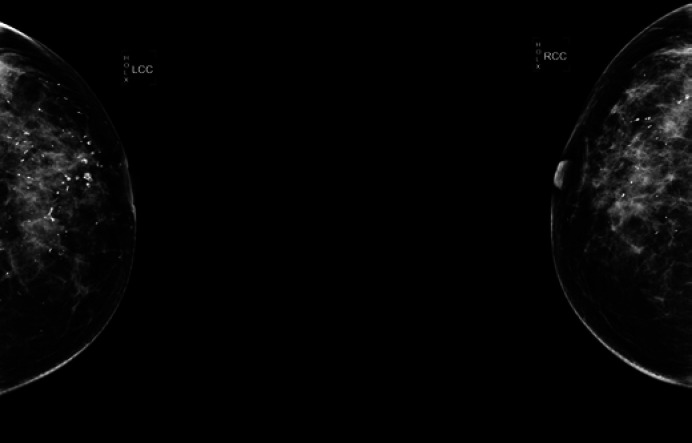
Fig. 1.
Molybdenum target X-ray scanning of a typical PCM patient. The examination shows bilateral nipple retraction, hyperplasia and multiple calcifications and reveals a slightly high-density shadow (3.5 cm in diameter) in the right breast, Breast Imaging Reporting and Data System category 2.
Nevertheless, there are serum markers that can help to exclude other pathologies and contribute to the diagnosis of PCM. Immunoglobin (Ig)M is the first antibody synthesized in the primary immune response. As a pentamer, IgM also has the strongest complement activation ability among all antibodies. IgA is a major component of mucosal immunity that shows neutralizing antibody activity to prevent pathogens from adhering to and settling on the surface of mucosal cells. IgG is the main antibody in the re-response process and is related to AID; furthermore, it binds strongly to antigens and mediates both opsonization and antibody-dependent cell-mediated cytotoxicity. Complement is a protein with enzyme activity that mediates immune and inflammatory responses after being activated by antigen-antibody complexes or microorganisms. The fluctuation of serum complement content suggests a role in the diagnosis and treatment of certain diseases. Chinese scholars found that serum IgM, IgA, C3 and C4 levels in patients with nonlactating mastitis were increased in several independent clinical studies that recruited a total of 470 hospitalized patients and healthy controls. These researchers also reported that the IgG and C3 levels were significantly higher in patients with PCM in the acute stage than those with PCM in other stages or in healthy controls [12, 13].
Autoantibodies are antibodies produced in the blood circulation of AID patients against their own tissues, organs, cells and intracellular components. These antibodies react with the antigenic epitopes of tissue components and cause autoimmune damage [14]. The presence of high-potency autoantibodies in the blood of patients is one of the characteristics of AID; they are convenient serological markers of AID, among which antinuclear antibody and antihistone antibody are highly sensitive and specific. Xu et al. [12] found that the expression levels of antinuclear and antihistone antibodies in the peripheral blood of patients with PCM were significantly higher than those in healthy individuals. Therefore, the quantified detection of these markers is an alternative for the clinical diagnosis and treatment evaluation of PCM [15].
Developmental Process of PCM
According to the natural course of PCM, it can be divided into four stages: duct dilatation, inflammation, abscess and fistula [16]. Herein, we elaborate on each stage in terms of clinical manifestations, examination characteristics and etiological causes and discuss the main molecular mechanisms in detail (Table 2).
Table 2.
Main etiological causes and molecular mechanisms of each PCM stage
| Stages | Etiological causes | Molecular mechanisms |
|---|---|---|
| Duct dilatation stage | Autoimmune disorder | Patients have fewer peripheral CD4+CD25+ regulatory T cells with aberrant TGF-β and FOXP3 levels than healthy individuals |
|
|
||
| Hormone fluctuation | PCM is often accompanied by serum PRL fluctuation | |
|
|
||
| Others | Abnormal breast structure, smoking habit, age and drug | |
|
| ||
| Acute inflammation stage | Genetic factors | With the p.R96C mutation of the SOCS2 gene, the mammary gland is susceptible to mastitis due to uncontrollable inflammation |
|
|
||
| Membrane proteins and cytokines | ICAM-1, interleukin and TNF-α attract and regulate more plasma cells to persistently amplify local inflammation and to aggravate PCM | |
|
| ||
| Abscess stage | Bacterium | Pathogenic infection (e.g., Enterococcus, anaerobic Streptococcus, Staphylococcus aureus, Bacteroides or nontuberculous mycobacteria) followed by increased secretion of inflammatory factors and chemokines triggers innate immune response and lymphocyte aggregation which contributes to tissue necrosis and abscess |
|
|
||
| Mammary microenvironment | IL-6/JAK/STAT signaling pathway, PI3K-Akt-mTOR signaling pathway | |
|
| ||
| Fistula stage | Abscess rupture | If the wound does not heal or the ulceration repeatedly occurs, a fistula can form |
TGF, transforming growth factor; FOXP3, forkhead box P3; PRL, prolactin; p.R96C, the C base in the reference sequence (OAR3, 129,722,200 bp) to a T substitution at position 96; SOCS2, suppressor of cytokine signaling 2; ICAM, intercellular adhesion molecule; TNF, tumor necrosis factor; IL, interleukin; JAK/STAT, Janus kinase/signal transducer and activator of transcription; PI3K, phosphoinositide-3-kinase; Akt, protein kinase B; mTOR, mammalian rapamycin target protein.
Duct Dilatation Stage
Duct dilatation is the initial stage of PCM in which covert inflammatory reactions occur. No obvious inflammatory manifestations are observed in this stage. The patient only shows signs of nipple or areola pain and a “cheese”-like excretion on nipple squeezing. On ultrasound examination, the lesion is mostly flat, and the blood flow is sparse, with low velocity and low resistance [17, 18, 19]. Molybdenum target examination of the mammary gland shows an asymmetrical shadow with an increased density, a flame-like appearance, an uneven density accompanied by low-density tubular structures and scattered rod-shaped or hollow, small, round calcified shadows along the long axis of the catheter. Nevertheless, multislice helical CT presents small round nodules around the lesions as well as thickened skin in the areola area and widened soft tissue shadows in the main breast ducts [20].
Autoimmune Disease
The pathological basis of duct dilatation is inflammation stimulated by the destruction and shedding of ductal epithelial cells and blockage of the lumen by keratinized debris and lipid secretion [21]. Inflammatory cells, such as plasma cells, neutrophils, lymphocytes and foam cells, infiltrate the lesion site [16]. Therefore, some scholars suggest that PCM is related to AID. Compared to healthy controls, patients with PCM have fewer peripheral CD4*CD25* regulatory T cells, with lower transforming growth factor (TGF)-β levels and forkhead box P3 (FOXP3) transcription levels. The most significant decrease is often observed in acute PCM [22]. CD4*CD25*FOXP3* regulatory T cells are crucial for maintaining immune homeostasis by preventing autoimmunity and reducing inflammation caused by pathogens and environmental damage. FOXP3 expression on T cells is affected by the synergistic effect of TGF-β and interleukin [23]. Therefore, aberrant TGF-β, FOXP3 and CD4*CD25* T cells in patients with PCM indicate autoimmunity-oriented PCM.
Hormone Fluctuation
Breast remodeling is mainly regulated by 17β-estradiol, progesterone (P4) and serum prolactin (PRL) [24, 25]. Orotic acid is a normal component of biological fluids and plays an important role in promoting cell growth, protein synthesis, tissue regeneration and wound healing. Ghrelin, a peptide hormone that contains 28 amino acids, is found in milk and blood. This hormone promotes wound healing and controls inflammation [26].
In the last century, clinicians observed that NPM was often accompanied by hyperprolactinemia, with PRL levels exceeding the laboratory reference range (high: 400–500 mIU/L) on 2 independent occasions [27]. To date, a few reports have documented peripheral hormone fluctuations during NPM in mammals [28, 29]. We counted the number of mastitis patients who were hospitalized from June 2009 to August 2018 in the Department of Breast Surgery at our hospital. Of 350 cases, there were 120 cases of NPM; among these patients, 79% (95/120) had PCM. Unfortunately, only 9% (31/350) of the patients had a single serum PRL measurement, and none had 2 measurements during the treatment course. Therefore, continuous monitoring of serum PRL in NPM has not drawn much attention to date, and its potential role is likely far underestimated.
There have been no reports describing the exact role of serum PRL in PCM other than that written by Peters and Schuth [27]. These researchers analyzed the data from 108 patients and suggested that NPM (duct ectasia) induces transient hyperprolactinemia with a 3-week duration. Although 7 patients with PCM were enrolled in this study, they showed normal serum PRL levels, with the exception of 1 patient with persistent hyperprolactinemia. Hyperprolactinemia usually occurs under physiological (pregnancy, lactation, exercise and stress), pharmacological (antipsychotic, antiemetic and opioid use) and pathological (hypothalamic/pituitary lesions, hypothyroidism and chronic renal failure) conditions [30]. We recently reported a case of recurrent PCM in a patient with a high level of serum PRL immediately before the clinical manifestation, and we successively documented the dynamic changes in serum PRL for the first time [25]. Our study indicates that breast tissue-derived PRL initiates a cascade of events leading to irreversible PCM, suggesting that serum PRL examination, in some circumstances, may be a practical and promising method for predicting PCM.
PRL is mainly secreted by lactotrophic cells of the anterior pituitary gland. In cows, the milk PRL concentration is significantly increased in cows affected by chronic mastitis and is positively correlated with the neutrophil count in milk samples. This previous study further showed that PRL in cows could promote proinflammatory activity by interacting with bovine mammary epithelial cells. By activating the nuclear factor (NF)-κB pathway, PRL may trigger the increased secretion of cytokines, such as interleukin (IL)-1β, IL-6, IL-8, colony-stimulating factor 2 (GM-CSF), and tumor necrosis factor (TNF)-α, in a dose-dependent manner [29]. Liu et al. [31] suggested that IL-6/Janus kinase (JAK) 2/signal transducers and activators of transcription (STAT) 3 signaling are involved in the pathogenesis of PCM in mice induced by injecting IL-6 into the mouse mammary gland. The roles of PRL in stimulating the proliferation and inflammatory activity of immune cells have also been investigated in humans. Brand et al. [28] demonstrated that PRL, alone or in combination with lipopolysaccharide, promotes the secretion of TNF-α and IL-12 via NF-κB and interferon regulatory factor-1 in the peripheral white blood cells (WBCs) of patients with hyperprolactinemia, which in turn triggers immune responses and plasma cell infiltration in the breast. Friedrich et al. [32] found that serum PRL concentrations were associated with several inflammatory biomarkers, such as the IL-6 level and WBC count, by analyzing 1,839 men and 1,905 women.
Abnormal Breast Structure
Nipple inversion or deformity, catheter stenosis and breast dysplasia can cause milky orifice occlusion, blockage of metabolite excretion or catheter expansion [33, 34]. Moreover, lipid degradation products decompose in milk ducts, which repeatedly stimulate the milk ducts and areola, resulting in the local infiltration of plasma cells, neutrophils and lymphocytes [16].
Smoking
Smoking is a risk factor for PCM [35, 36]. Bundred et al. [37] found that smoking accelerates the accumulation of toxic substances from smoke oil in the breast or produces various harmful metabolites that cause local breast tissue damage, promoting anaerobic bacterial growth, purulent infection and nipple discharge. Smoking damages the breast duct by affecting hormone levels and blood flow, and these injuries are prone to breeding anaerobic bacteria, which could aggravate the course of the disease [38].
Age
PCM often occurs in nonbreastfeeding and nonpregnant women, and thus the age of onset may help in its diagnosis [16]. As age increases, the mammary gland undergoes degeneration. The ductal epithelial cells fall off, and the accumulation of ductal secretions causes blockage. Furthermore, the degrading ovary secretes decreased hormone levels, weakening systemic resistance and increasing the susceptibility of the mammary gland.
Drugs
The pituitary gland secretes catecholamine hormones, also known as prolactin release-inhibiting factor, dopamine and epinephrine, to inhibit PRL release. Drugs for neurological disorders inhibit prolactin release-inhibiting factor receptors or reduce the activity of PRL release factors [39]. This change leads to excessive PRL secretion, which increases intraductal excretion and blocks catheters. The long-term use of contraceptives may also be a risk factor for PCM [40].
Acute Inflammation Stage
The inflammatory stage is also called the “mass stage.” PCM can occur suddenly, and the main clinical symptoms are redness, swelling, heat and pain of the skin with gradually enlarged lesions. Patients complain of pain, lumps and discomfort in the breast. As secretions accumulate in the duct, inflammatory infiltration is aggravated, and fibrous tissue proliferates around the duct wall to destroy it. Then, the intraductal accumulation penetrates the duct into the periductal area and stroma, where it forms a granuloma with a nodular surface, unclear boundary and skin adhesion. Adjacent lymph nodes may swell, and the surrounding breast tissue becomes involved. Commonly, lesions extend along the areolar area to form irregular masses with increased edema.
Genetic Factors
Studies have shown that animal mastitis is related to genetic factors. The somatic cell count is one of the most widely used predictors of mastitis. The bacterial clearance of those with a low somatic cell score is higher than that of individuals with a high somatic cell score [41]. Rupp et al. [42] found that the p.R96C point mutation in the suppressor of the cytokine signaling 2 (SOCS2) gene was related to the somatic cell count. SOCS2 is involved in inflammation, growth and lactation. p.R96C point mutations damage the binding ability of SOCS2 to the growth hormone receptor phosphopeptide and inhibit its interaction with the JAK/STAT signaling pathway. Individuals with SOCS2 gene deficiency fail to control the inflammatory process, resulting in impaired resistance to mastitis.
Membrane Proteins and Cytokines
Intercellular adhesion molecule (ICAM)-1 and 2 are cell surface glycoproteins that regulate the interaction between immune cells and mediate cell recognition, activation, adhesion and metastasis. They attract plasma cells by margination and extravasation to aggregate them at inflammatory sites. ICAM-1 is associated with AID and has a relatively high specificity in PCM because ICAM-2 can also be found in other inflammatory diseases of the breast [43]. Dong et al. [43] examined the expression level of ICAM-1 in PCM, non-PCM mastitis and normal breast tissue samples via immunohistochemistry. The results showed that the expression level of ICAM-1 was the highest in both the ductal epithelium and endothelium in the PCM group. ICAM-1 induced the accumulation and extravasation of a vast number of inflammatory cells, mainly plasma cells, around the breast ducts, which exhibited the unique pathological characteristics of PCM [43].
Among various kinds of inflammatory cells, plasma cells play a variety of roles in regulating the inflammatory response. They not only produce antibodies, but are also elaborately regulated by multiple proinflammatory cytokines. IL-1 and TNF-α are inflammatory factors that are involved in various inflammatory diseases. Both are significantly upregulated in PCM, and patients can benefit from TNF-α antagonists [44].
Abscess Stage
As the disease course is prolonged, patients with PCM are prone to infection by pathogens. The breast mass transforms into an abscess, and nipple discharge becomes yellow or bloody.
Bacteria
There is controversy about whether PCM is associated with bacterial infection. In 1989, Dixon et al. [45] isolated and successfully cultured Enterococcus, anaerobic Streptococcus, Staphylococcus aureus and Bacteroides from the nipple discharge of patients with PCM. Moreover, the bacterial culture rate reached 62%, which was significantly higher than that among patients with nipple discharge caused by other reasons [45]. Another theory is that PCM is related to nontuberculous mycobacterial infection [46]. Skin nontuberculous mycobacterial infections may appear as purulent plaques and ulcers, with similar clinical manifestations as PCM [47].
Mammary epithelial cells (MECs) are the body's natural immune barrier. When MECs are cultured with Escherichia coli, S. aureus or other bacteria, the MECs activate the innate immune response by activating innate immune cells and promoting neutrophil infiltration into breast tissue, causing strong inflammation [48, 49]. Nucleotide-binding oligomerization domain containing 2 (NOD2) is a cytosolic pattern recognition receptor belonging to the NOD-like receptor family. It is involved in the recognition of infectious bacteria by MECs and further triggers the innate immune response [50]. Muramyl dipeptide (MDP) is a specific ligand of NOD2 and is often found in both gram-negative and gram-positive bacteria [51]. When the mammary gland is infected with bacteria, MECs recognize MDP via NOD2 and produce chemokines and proinflammatory factors by activating NF-κB signaling pathways [52], while 17β-estradiol and progesterone reduce the expression of MEC NOD2 induced by MDP [24]. NOD1 is activated by the peptidoglycan fragment γ-D-glutamylmesodiaminopimelic acid of gram-negative bacteria [51].
Mammary Microenvironment
During the abscess stage, abundant plasma cells invade the ductal epithelium and promote IL-6 secretion in PCM patients. Studies have confirmed that IL-6 binding to glycoprotein 130 promotes activation of the JAK/STAT signaling pathway followed by upregulation of the antiapoptotic genes Bcl-2 and ICAM-1 [31, 43, 53]. Bcl-2 prolongs the survival time of plasma cells, while ICAM-1 enhances plasma cell penetration into the ductal epithelium. Activation of this signaling pathway also stimulates B-cell differentiation into plasma cells, which effectively forms a positive feedback loop of IL-6/JAK/STAT signaling [54, 55]. More recently, Wang et al. [33] proved that the PI3K-Akt-mTOR signaling pathway is prominently upregulated in human PCM tissue and that the injection of an exosome release inhibitor in a mouse PCM model was effective in relieving local mammary gland inflammation.
Fistula Stage
The fistula stage is a stable stage but not the last stage of PCM due to recurrent onset. It has the poorest prognosis, and it is associated with the lowest quality of life in patients with PCM. If the lesions in the duct dilatation or the mass stage are not completely resected and recover, they may cause the surgical incision near the nipple to rupture, followed by the formation of a chronic fistula, which is difficult to heal completely [56]. Moreover, due to dilated ducts and impaired drainage ability, abscesses caused by retrograde infection are usually located near the areola and then spread upward to the middle and small ducts away from the areola. Abscess ulceration will produce acne or lipid-like pus within the fistula. Improper treatment protocol selection also contributes to fistula formation. Although radical surgery is preferred and recommended as the first-line method to substantially reduce the risk of recurrence, hormone shock therapy combined with antibiotic therapy has been proven in the clinic to effectively relieve existing symptoms and constrain the progression of PCM [57].
Conclusions
PCM is still a complex disease that affects susceptible nonbreastfeeding and nonpregnant women. Considering that immunological and intracellular inflammatory mechanisms play an important role in the disease process (Fig. 2), it is necessary to enhance the collection and analysis of patients' data to acquire more specific and sensitive biomarkers and promote their use in clinical practice. In addition, research on the cause of PCM should be intensified, and the pathogenesis of PCM should be clarified to provide a standardized scheme for the diagnosis and targeted treatment of PCM. We believe that with the rapid development of science and technology and the continuous improvements in medicine, we can accurately diagnose and cure PCM.
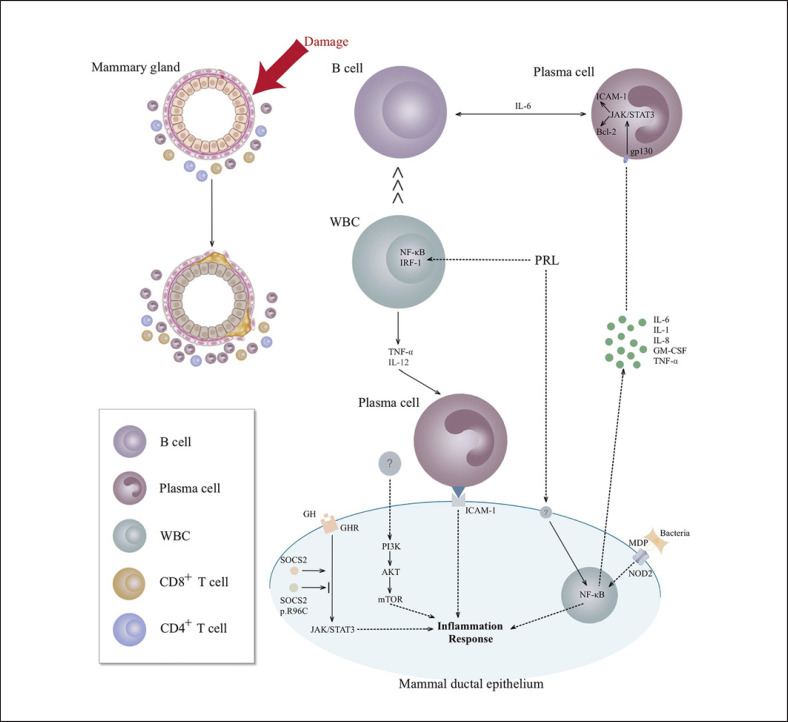
Fig. 2.
Molecular mechanisms of mammary ductal epithelium interaction with the breast microenvironment. PRL promotes proinflammatory activity by activating the NF-κB pathway in mammary epithelial cells to trigger the secretion of cytokines, such as IL-1β, IL-6, IL-8, GM-CSF, and TNF-α, among which IL-6 binds to gp130 in plasma cells to activate the JAK/STAT signaling pathway followed by the upregulation of Bcl-2 (prolonged survival) and ICAM-1 (enhanced penetration and aggregation). Moreover, PRL promotes the secretion of TNF-α and IL-12 via NF-κB and IRF-1 in WBCs. In the presence of IL-6, B cells differentiate into plasma cells, which effectively forms a positive feedback loop of IL-6/JAK/STAT signaling, followed by local accumulation in the breast, while IL-6 from plasma cells promotes B-cell differentiation, and vice versa. In mammary ductal epithelium, SOCS2 binds to the growth hormone receptor phosphopeptide to cause its interaction with the JAK/STAT signaling pathway, which is inhibited by SOCS2 p.R96C point mutations that induce an uncontrolled inflammatory response. The PI3K-Akt-mTOR signaling pathway is prominently upregulated in human PCM tissue with an unknown activator. Bacterial MDP specifically binds to NOD2 on mammary ductal epithelium and activates the NF-κB signaling pathway to produce chemokines and proinflammatory factors. PCM, plasma cell mastitis; PRL, serum prolactin; NF, nuclear factor; IL, interleukin; GM-CSF, colony-stimulating factor 2; TNF, tumor necrosis factor; JAK, Janus kinase; STAT, signal transducers and activators of transcription; IRF-1, interferon regulatory factor 1; WBCs, white blood cells; SOCS2, suppressor of cytokine signaling 2; ICAM, intercellular adhesion molecule; NOD2, nucleotide-binding oligomerization domain containing 2; MDP, muramyl dipeptide; gp130, glycoprotein 130.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by “The Six Top Talent Project” of Jiangsu Province (No. WSW-004), the National Natural Science Foundation of China (No. 81671836) and the Key Laboratory for Laboratory Medicine of Jiangsu Province of China (ZDXKB2016005).
Author Contributions
Conceptualization: J.Z., X.Z.; investigation: M.X., J.Z.; data curation: P.V.Z., D.V.V; writing − original draft preparation: M.X., J.Z.; writing − review and editing: J.Z., S.Z.; supervision: J.Z., X.Z.
References
- 1.Palmieri A, D'Orazi V, Martino G, Frusone F, Crocetti D, Amabile MI, et al. Plasma cell mastitis in men: a single-center experience and review of the literature. In Vivo. 2016;30:727–32. doi: 10.21873/invivo.10987. [DOI] [PubMed] [Google Scholar]
- 2.Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol. 1972;58:642–6. doi: 10.1093/ajcp/58.6.642. [DOI] [PubMed] [Google Scholar]
- 3.Thomas WG, Williamson RC, Davies JD, Webb AJ. The clinical syndrome of mammary duct ectasia. Br J Surg. 1982;69:423–5. doi: 10.1002/bjs.1800690724. [DOI] [PubMed] [Google Scholar]
- 4.Tan H, Li R, Peng W, Liu H, Gu Y, Shen X. Radiological and clinical features of adult non-puerperal mastitis. Br J Radiol. 2013;86:20120657. doi: 10.1259/bjr.20120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laas E, Touboul C, Kerdraon O, Catteau-Jonard S. [Inflammatory and infectious breast mastitis outside of pregnancy and lactation: Guidelines] J Gynecol Obstet Biol Reprod (Paris) 2015;44:996–1016. doi: 10.1016/j.jgyn.2015.09.055. French. [DOI] [PubMed] [Google Scholar]
- 6.Cheng L, Reddy V, Solmos G, Watkins L, Cimbaluk D, Bitterman P, et al. Mastitis, a radiographic, clinical, and histopathologic review. Breast J. 2015;21:403–9. doi: 10.1111/tbj.12430. [DOI] [PubMed] [Google Scholar]
- 7.Zervoudis S, Iatrakis G, Economides P, Polyzos D, Navrozoglou I. Nipple discharge screening. Womens Health (Lond) 2010;6:135–51. doi: 10.2217/whe.09.81. [DOI] [PubMed] [Google Scholar]
- 8.Wolfrum A, Kümmel S, Theuerkauf I, Pelz E, Reinisch M. Granulomatous mastitis: a therapeutic and diagnostic challenge. Breast Care (Basel) 2018;13:413–8. doi: 10.1159/000495146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liong YV, Hong GS, Teo JG, Lim GH. Breast ductal carcinoma in situ presenting as recurrent non-puerperal mastitis: case report and literature review. World J Surg Oncol. 2013;11:179. doi: 10.1186/1477-7819-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long Q, Fan Y, Zhang J, Li H, Lv Q. Pruritic breast mass with palpable lymph nodes in a male patient: a case report. Gland Surg. 2021;10((2)):826–31. doi: 10.21037/gs-21-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta TS, Raza S, Baum JK. Use of Doppler ultrasound in the evaluation of breast carcinoma. Semin Ultrasound CT MR. 2000;21:297–307. doi: 10.1016/s0887-2171(00)90024-6. [DOI] [PubMed] [Google Scholar]
- 12.Xu R, Guo QQ, Yang LP, Lai ML, Tong L. [Variations of peripheral blood autoantibody, immunoglobuliln, and complement levels in patients with non-lactational mastitis and their clinical significances] Nan Fang Yi Ke Da Xue Xue Bao. 2016;36:1157–9. Chinese. [PubMed] [Google Scholar]
- 13.Xia YR, Chen HF, Ye MN, Chen LY, Bao YJ. Variations of T lymphocytes, immunoglobulin, and complements levels in peripheral blood of non-lactational mastitis. Chinese Journal of Breast Disease (Electronic Edition) 2012;6:504–14. [Google Scholar]
- 14.Wang D, Lv W, Zhang S, Zhang J. Advances in the Research on Anticardiolipin Antibody. J Immunol Res. 2019;2019:8380214. doi: 10.1155/2019/8380214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis. 2010;69:1420–2. doi: 10.1136/ard.2009.127100. [DOI] [PubMed] [Google Scholar]
- 16.Yu JJ, Bao SL, Yu SL, Zhang DQ, Loo WT, Chow LW, et al. Mouse model of plasma cell mastitis. J Transl Med. 2012;10((Suppl 1)):S11. doi: 10.1186/1479-5876-10-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon S, Lim HS, Ki SY. Ultrasound findings of mammary duct ectasia causing bloody nipple discharge in infancy and childhood. J Ultrasound Med. 2019 doi: 10.1002/jum.14970. [DOI] [PubMed] [Google Scholar]
- 18.Zhu YC, Zhang Y, Deng SH, Jiang Q, Shi XR, Feng LL. Evaluation of plasma cell mastitis with superb microvascular imaging. Clin Hemorheol Microcirc. 2019;72:129–38. doi: 10.3233/CH-180468. [DOI] [PubMed] [Google Scholar]
- 19.Ramalingam K, Vuthaluru S, Srivastava A, Dinda AK, Dhar A. Ultrastructural changes occurring in duct ectasia and periductal mastitis and their significance in etiopathogenesis. PLoS One. 2017;12:e0173216. doi: 10.1371/journal.pone.0173216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song L, Li L, Liu B, Yu D, Sun F, Guo M, et al. Diagnostic evaluations of ultrasound and magnetic resonance imaging in mammary duct ectasia and breast cancer. Oncol Lett. 2018;15:1698–706. doi: 10.3892/ol.2017.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Zhou F, Wang P, Yu L, Ma Z, Li Y, et al. Periductal mastitis: an inflammatory disease related to bacterial infection and consequent immune responses? Mediators Inflamm. 2017;2017:5309081. doi: 10.1155/2017/5309081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grajewski RS, Silver PB, Agarwal RK, Su SB, Chan CC, Liou GI, et al. Endogenous IRBP can be dispensable for generation of natural CD4+CD25+ regulatory T cells that protect from IRBP-induced retinal autoimmunity. J Exp Med. 2006;203:851–6. doi: 10.1084/jem.20050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lourenco EV, La Cava A. Natural regulatory T cells in autoimmunity. Autoimmunity. 2011;44:33–42. doi: 10.3109/08916931003782155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu DD, Wang G, He XJ, Wang JF, Yang B, Sun ZP, et al. 17β-Estradiol and progesterone decrease MDP induced NOD2 expression in bovine mammary epithelial cells. Vet Immunol Immunopathol. 2017;188:59–64. doi: 10.1016/j.vetimm.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Xing MY, Zhang SC, Zha XM, Zhang JX. An unusual cause of high serum prolactin. Clin Chem. 2020;66:499–500. doi: 10.1093/clinchem/hvz031. [DOI] [PubMed] [Google Scholar]
- 26.Karatas F, Aydin S, Kaygusuzoglu E, Yildiz H, Erulas FA, Ozkan Y. Ghrelin and orotic acid increased in subclinical mastitis. Arch Physiol Biochem. 2008;114:178–82. doi: 10.1080/13813450802180940. [DOI] [PubMed] [Google Scholar]
- 27.Peters F, Schuth W. Hyperprolactinemia and nonpuerperal mastitis (duct ectasia) JAMA. 1989;261:1618–20. [PubMed] [Google Scholar]
- 28.Brand JM, Frohn C, Cziupka K, Brockmann C, Kirchner H, Luhm J. Prolactin triggers pro-inflammatory immune responses in peripheral immune cells. Eur Cytokine Netw. 2004;15:99–104.. [PubMed] [Google Scholar]
- 29.Boutet P, Sulon J, Closset R, Detilleux J, Beckers JF, Bureau F, et al. Prolactin-induced activation of nuclear factor kappaB in bovine mammary epithelial cells: role in chronic mastitis. J Dairy Sci. 2007;90:155–64. doi: 10.3168/jds.S0022-0302(07)72617-6. [DOI] [PubMed] [Google Scholar]
- 30.Tritos NA, Klibanski A. Hyperprolactinemia. JAMA. 2015;314((16)):1742–3. doi: 10.1001/jama.2015.7871. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Zhang J, Zhou YH, Zhang HM, Wang K, Ren Y, et al. Activation of the IL-6/JAK2/STAT3 pathway induces plasma cell mastitis in mice. Cytokine. 2018;110:150–8. doi: 10.1016/j.cyto.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich N, Schneider HJ, Spielhagen C, Markus MR, Haring R, Grabe HJ, et al. The association of serum prolactin concentration with inflammatory biomarkers − cross-sectional findings from the population-based Study of Health in Pomerania. Clin Endocrinol (Oxf) 2011;75:561–6. doi: 10.1111/j.1365-2265.2011.04075.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Han Y, Liu J, Zhang Y, Cheng K, Guo J, et al. Exosomes play an important role in the progression of plasma cell mastitis via the PI3K-Akt-mTOR signaling pathway. Mediators Inflamm. 2019;2019:4312016. doi: 10.1155/2019/4312016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ming J, Meng G, Yuan Q, Zhong L, Tang P, Zhang K, et al. Clinical characteristics and surgical modality of plasma cell mastitis: analysis of 91 cases. Am Surg. 2013;79:54–60. [PubMed] [Google Scholar]
- 35.Rahal RM, de Freitas-Júnior R, Paulinelli RR. Risk factors for duct ectasia. Breast J. 2005;11:262–5. doi: 10.1111/j.1075-122X.2005.21684.x. [DOI] [PubMed] [Google Scholar]
- 36.Taffurelli M, Pellegrini A, Santini D, Zanotti S, Di Simone D, Serra M. Recurrent periductal mastitis: surgical treatment. Surgery. 2016;160:1689–92. doi: 10.1016/j.surg.2016.06.048. [DOI] [PubMed] [Google Scholar]
- 37.Bundred NJ, Dover MS, Aluwihare N, Faragher EB, Morrison JM. Smoking and periductal mastitis. BMJ. 1993;307:772–3. doi: 10.1136/bmj.307.6907.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dixon JM, Ravisekar O, Chetty U, Anderson TJ. Periductal mastitis and duct ectasia: different conditions with different aetiologies. Br J Surg. 1996;83:820–2. doi: 10.1002/bjs.1800830630. [DOI] [PubMed] [Google Scholar]
- 39.Cong Y, Zou H, Qiao G, Lin J, Wang X, Li X, et al. Bilateral mammary duct ectasia induced by sulpiride-associated hyperprolactinemia: a case report. Oncol Lett. 2015;9:2181–4. doi: 10.3892/ol.2015.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murthy MS. Granulomatous mastitis and lipogranuloma of the breast. Am J Clin Pathol. 1973;60:432–3. doi: 10.1093/ajcp/60.3.432. [DOI] [PubMed] [Google Scholar]
- 41.Bonnefont CM, Toufeer M, Caubet C, Foulon E, Tasca C, Aurel MR, et al. Transcriptomic analysis of milk somatic cells in mastitis resistant and susceptible sheep upon challenge with Staphylococcus epidermidis and Staphylococcus aureus. BMC Genomics. 2011;12:208. doi: 10.1186/1471-2164-12-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rupp R, Senin P, Sarry J, Allain C, Tasca C, Ligat L, et al. A point mutation in suppressor of cytokine signalling 2 (SOCS2) increases the susceptibility to inflammation of the mammary gland while associated with higher body weight and size and higher milk production in a sheep model. PLoS Genet. 2015;11:e1005629. doi: 10.1371/journal.pgen.1005629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong Y, Yu JJ, Shibahara Y, Lu HS, He HY, Liu JD, et al. Intercellular adhesion molecule 1/2 and E-selectin in plasma cell mastitis: immunohistochemical study of 35 cases. Hum Pathol. 2014;45:606–10. doi: 10.1016/j.humpath.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Faccin M, Caillot O, Leveque J, Perdriger A. Plasma cell mastitis in women with rheumatoid arthritis treated with TNFalpha antagonists: report of 2 cases. Joint Bone Spine. 2016;83:593–4. doi: 10.1016/j.jbspin.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Dixon JM. Periductal mastitis/duct ectasia. World J Surg. 1989 Nov-Dec;13((6)):715–20. doi: 10.1007/BF01658420. [DOI] [PubMed] [Google Scholar]
- 46.Ji LY, Xu DL, Yin SP, Liu HC, Li GL, Jiang Y, et al. First Report in China on the Identification and Drug sensitivity of Mycobacterium elephantis isolated from the milk of a cow with mastitis. Biomed Environ Sci. 2017;30:501–7. doi: 10.3967/bes2017.066. [DOI] [PubMed] [Google Scholar]
- 47.Horii KA, Jackson MA. Images in clinical medicine. Piercing-related nontuberculous mycobacterial infection. N Engl J Med. 2010;362:2012. doi: 10.1056/NEJMicm0906854. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert FB, Cunha P, Jensen K, Glass EJ, Foucras G, Robert-Granié C, et al. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet Res. 2013;44:40. doi: 10.1186/1297-9716-44-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brenaut P, Lefèvre L, Rau A, Laloë D, Pisoni G, Moroni P, et al. Contribution of mammary epithelial cells to the immune response during early stages of a bacterial infection to Staphylococcus aureus. Vet Res. 2014;45:16. doi: 10.1186/1297-9716-45-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Travassos LH, Carneiro LA, Girardin S, Philpott DJ. Nod proteins link bacterial sensing and autophagy. Autophagy. 2010;6:409–11. doi: 10.4161/auto.6.3.11305. [DOI] [PubMed] [Google Scholar]
- 51.Chung WO, An JY, Yin L, Hacker BM, Rohani MG, Dommisch H, et al. Interplay of protease-activated receptors and NOD pattern recognition receptors in epithelial innate immune responses to bacteria. Immunol Lett. 2010;131:113–9. doi: 10.1016/j.imlet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kvarnhammar AM, Cardell LO. Pattern-recognition receptors in human eosinophils. Immunology. 2012;136:11–20. doi: 10.1111/j.1365-2567.2012.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y, Zhang J, Zhou YH, Jiang YN, Zhang W, Tang XJ, et al. IL-6/STAT3 signaling pathway is activated in plasma cell mastitis. Int J Clin Exp Pathol. 2015;8:12541–8. [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Sun Y, Zhou Y, Tang X, Wang K, Ren Y, et al. Sinomenine hydrochloride inhibits the progression of plasma cell mastitis by regulating IL-6/JAK2/STAT3 pathway. Int Immunopharmacol. 2019;81:106025. doi: 10.1016/j.intimp.2019.106025. [DOI] [PubMed] [Google Scholar]
- 55.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–56. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 56.Joseph WJ, Jindal R, De La Cruz C. Mammillary fistula revisited: implications for immediate breast reconstruction. Breast J. 2019;25:138–40. doi: 10.1111/tbj.13168. [DOI] [PubMed] [Google Scholar]
- 57.Hanavadi S, Pereira G, Mansel RE. How mammillary fistulas should be managed. Breast J. 2005;11:254–6. doi: 10.1111/j.1075-122X.2005.21641.x. [DOI] [PubMed] [Google Scholar]