Abstract
Treatment of relapsed/refractory or elderly unfit acute myeloid leukemia (AML) is still challenging, and hypomethylating agents in combination with venetoclax, an oral selective BCL2 inhibitor, might be successfully used as salvage therapy. However, clinical trials evaluating the efficacy and safety of this combination in the setting of multiresistant AML treatment also as a bridge to transplant are still ongoing. Here, we reported a 50-year-old male diagnosed with AML with normal cytogenetics and wild type for fms-like tyrosine kinase 3, nucleophosmin 1, and KIT, and treated with decitabine and venetoclax as the fifth line of therapy and after a relapse post-allogeneic transplant. The patient achieved a complete remission and successfully underwent a haploidentical transplant with an overall survival of 48.6 months.
Keywords: Acute myeloid leukemia, Hypomethylating agents, BCL2 inhibitor, Salvage therapy, Hematopoietic stem cell transplantation
Introduction
Acute Myeloid Leukemia (AML) is a heterogeneous group of clonal aggressive hematologic malignancies characterized by a block of differentiation and increased proliferation of myeloid neoplastic cells harboring various cytogenetic and/or molecular abnormalities [1]. The main criterion for AML diagnosis is the presence of at least 20% of leukemic cells with myeloid characteristics in the bone marrow (BM) or peripheral blood, except for AML with specific cytogenetic abnormalities or nucleophosmin 1 (NPM1) mutated forms [1]. AML in the elderly (>60 years old) with recurrent genetic abnormalities is not frequent and has a worse prognosis compared to younger subjects, while more often secondary to myelodysplastic syndromes or therapy-related, accounting for 19% and 7% of cases, respectively [2]. First-line treatments in newly diagnosed AML patients eligible for standard induction and consolidation include cytarabine and daunorubicin-based chemotherapy protocols followed by hematopoietic stem cell transplant (HSCT) in fit patients [3]. Despite 40–90% patients achieving a complete remission (CR), persistent and durable response rates are low, especially in older subjects with 10% of long-term overall survival [3, 4]. This high incidence of relapse or induction failure in older AML subjects is associated with patient-related factors, such as the presence of several comorbidities and a poor performance status, not allowing high-dose standard chemotherapy regimens and HSCT, and with disease-related features because AML in elderly frequently display adverse cytogenetics abnormalities, including complex karyotype [1, 3, 5]. When certain molecular alterations are present, such as somatic mutations in fms-like tyrosine kinase 3 (FLT3) and isocitrate dehydrogenase 1 and 2 (IDH1/2) genes, specific targeted therapies can be used, such as sorafenib, midostaurin, and gilteritinib [6]. Alternatively, hypomethylating agents, including azacytidine and decitabine (5-aza-2′-deoxycytidine), with or without BCL2 inhibitors -venetoclax- can be administered [1, 2]. Hypomethylating agents, nucleoside derivatives, incorporate into DNA and inhibit the activities of DNA methyltransferases (DNMT), leading to transcriptional reinduction of silenced genes [6]. Indeed, in several hematological and solid tumors, hypermethylation of promoter regions of tumor suppressor genes is frequent and causes downregulation of proteins involved in DNA repair, cell cycle regulation, survival, proliferation, and invasion [6]. Therefore, hypomethylating agents can re-establish normal methylation and gene expression; however, response rates are low when these agents are used alone, with a median overall survival of 6–10 months [3, 7], while when antiapoptotic protein inhibitors are added, response rates and overall survival improve by 67%, with a median survival up to 18 months [8]. Despite clinical benefits, this combination can significantly induce hematological toxicities, especially febrile neutropenia that requires pharmacological treatments [9]. Here, we showed a case of a multiresistant frail adult AML patient successfully treated with decitabine and venetoclax followed by allogeneic HSCT.
Case Report
A 50-year-old male with a history of seminoma diagnosed in 2010 and treated with orchiectomy and chemotherapy (2 cycles of cisplatin, etoposide, and bleomycin) arrived at our observation in March 2018 with a diagnosis of AML wild type for FLT3, NPM1, and KIT made in another institution. The patient received daunorubicin and cytarabine following a 3 + 5 + 10 scheme as first-line induction treatment without any hematological response; therefore, a second-line therapy with FLAG-IDA (fludarabine, cytarabine, idarubicin, and granulocyte-colony stimulating factor) was initiated (Fig. 1). Once achieved a CR, the patient underwent allogeneic HSCT in July 2018 after myeloablative conditioning regimen with busulfan (3.2 mg/kg/day) and fludarabine (40 mg/m2 daily at day −6 and −3) and selecting the HLA-identical 50-year-old sister as donor (serology positive for cytomegalovirus, as well as the recipient). Graft versus Host disease (GvHD) prophylaxis with cyclosporine was started on day 1 [10]. No posttransplant complications or signs of acute GvHD were observed and he achieved a full donor chimerism; however, a grade I cutaneous chronic GvHD was documented. Eight months after transplant, an initial increase in peripheral blood Wilms' tumor (WT1) expression levels and loss of chimerism were detected, and the patient was reevaluated for disease progression confirmed by the reappearance of leukemic cells in the BM. A third-line therapy with MEC (mitoxantrone, etoposide, and cytarabine) was started; however, after two cycles, because of disease persistence (39% of leukemic cells in the BM), the patient switched to a fourth-line treatment with idarubicin and cytarabine without any hematological improvement. Moreover, the patient had recurrent infectious episodes, including an Aspergillosis treated with liposomal amphotericin B and Klebsiella pneumoniae infection. Because of stable disease, decitabine 20 mg/m2 and venetoclax 400 mg were initiated, and the patient achieved a CR in July 2019, 1 year after the first transplant. Few weeks later, the patient was admitted to our Hematology Unit for fever, pancytopenia, and severe asthenia, and an abscess on the left leg positive for Klebsiella pneumoniae was detected, associated with pulmonary findings suggesting a fungal infection. Meropenem and isavuconazole, a second-generation triazole, were started and surgical drainage of the abscess was successfully performed. After infection resolution, the patient was candidate for a second allogeneic HSCT due to multiresistance to chemotherapy and a high risk of relapse; however, a reduced intensity conditioning regimen was performed with busulfan and fludarabine, and hemopoietic stem cells from his haploidentical son were infused in September 2019. GvHD prophylaxis included: cyclosporine and mycophenolate mofetil, started on day 0 and +1, respectively; and cyclophosphamide infused only on days +3 and +4. Antimicrobial and antifungal prophylaxis was performed with meropenem and liposomal amphotericin B, while letermovir 240 mg daily was used for cytomegalovirus prophylaxis during the first 100 days after transplant. No acute GvHD was observed, and at the day +83 evaluation, the patient was in CR and with full donor chimerism. A limited cutaneous chronic GvHD was documented on day +114; however, no immunosuppressive therapies were required. At 9 months after transplant, the patient experienced another fungal infection with bilateral pneumonia on CT scan, thoracic pain, and cough, successfully treated with oral Posaconazole. In October 2020, 1 year after the second HSCT, the patient developed a mild form of ocular and cutaneous chronic GvHD, requiring immunosuppressive therapy with oral cyclosporine and mycophenolate mofetil. Cutaneous manifestations disappeared after 2 months of therapy, while the ocular form persisted, and in February 2021, a mild hepatic chronic GvHD was also detected. At the time of writing, 29 months after the second transplant and with an overall survival of 48.6 months, the patient is alive and still in CR with full donor chimerism while with persistence of ocular and hepatic chronic GvHD, currently treated with mycophenolate mofetil.
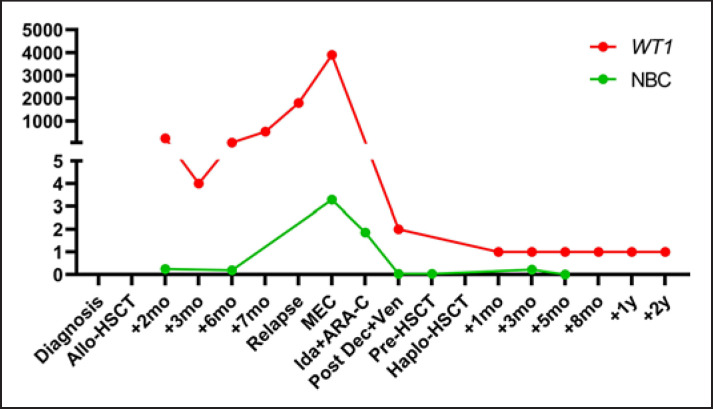
Fig. 1.
Variations in WT1 expression and normalized blast count (NBC). Clinical course of our multiresistant AML patient showing normalized WT1 expression (WT1 copy number/104 ABL copies; red) and NBC by flow cytometry (%CD34+ cells + %immature cells + %blasts/%granulocytes; green line), as previously described [1], are reported from diagnosis to last follow-up (2 years after HSCT, +2 yr). Allo-HSCT, allogeneic HSCT; mo, months; MEC, mitoxantrone, etoposide, and cytarabine; Ida + ARA-C, idarubicin and cytarabine; Dec + Ven, decitabine and venetoclax; haplo-HSCT, haploidentical HSCT; yr, year; WT1, Wilms' tumor 1.
Discussion/Conclusion
The choice of drugs for adult AML treatment is based on the presence of specific molecular alterations, such as FLT3 mutations, and eligibility of patients for standard induction and consolidation chemotherapy followed by HSCT [1, 2]; however, relapse or resistance to induction therapy is common and prognosis varies depending on patient's age, prior allogeneic HSCT, progression-free survival, mutational status, and cytogenetic abnormalities [11]. Indeed, 25–55% of AML patients relapse after allogeneic HSCT, and primary refractory or relapsed subjects after remission benefit of salvage therapy in 25–50% of cases, while only 11% are alive after 5 years with a substantial CR duration of 4.9–9.8 months and a reported overall survival of 6.2–8.7 months [12]. Moreover, no standard chemotherapy strategies are available for treatment of relapse/refractory adult AML patients, especially when a disease-specific molecular alteration is lacking [13]. Our patient was diagnosed with AML wild type for FLT3, NPM1, and KIT and was primary refractory to standard chemotherapy with daunorubicin and cytarabine, and a FLAG-IDA protocol was employed as induction therapy and a bridge to an allogeneic HSCT. Despite an initial hematological response, a durable CR was not maintained as the patient relapsed just after 8 months from the transplant. Mitoxantrone, etoposide and cytarabine first, and idarubicin and cytarabine after were employed as salvage therapy; however, our patient was refractory to both regimens.
Hypomethylating agents are currently the first choice in newly diagnosed unfit AML patients not eligible for standard chemotherapy and subsequent HSCT [1, 2, 7]. These drugs alone have a low CR rate (<20%) while significantly improving overall survival (7.7 vs. 5.0 months, hypomethylating agents vs. supportive care or low-dose cytarabine) [3]. The addition of venetoclax, an oral selective BCL2 inhibitor, to hypomethylating agents has markedly improved the prognosis of AML patients not eligible for standard chemotherapy, with reported median overall survival ranging from 10 to 18 months and with rates of CR or CR with incomplete hematologic recovery of 66–67%, significantly higher compared to those reported in subjects treated with single agents (54% or 28%, venetoclax or azacytidine alone) [3, 8, 9]. Hypomethylating agents in combination with venetoclax are also effective in relapsed/refractory AML with reported overall response rates up to 75% and with a significant increase in overall survival, especially in those subjects treated with azacytidine and venetoclax (up to 25 months) [3, 11, 14]. Moreover, this combination therapy has shown efficacy in minimal residual disease negativization, allowing patients to undergo HSCT; however, clinical trials exploring the efficacy and safety of hypomethylating agents combined with venetoclax as a bridge therapy to HSCT are still under the way, despite good preliminary evidence of favorable outcomes in both newly diagnosed older or relapsed/refractory AML patients [15]. In our case report, we showed the efficacy of venetoclax and decitabine as salvage therapy for the treatment of a multiresistant AML patient and as a bridge to a second haploidentical HSCT, allowing a durable CR with an overall survival of 48.6 months.
In conclusions, AML without specific molecular markers are frequently relapse/refractory diseases requiring different therapeutical strategies based on fitness of patients, presence of comorbidities, or history of myelodysplastic syndromes [1, 2], and allogeneic HSCT is not always feasible, especially in multiresistant subjects [11]. Accumulating evidence is showing efficacy and safety of venetoclax in combination with hypomethylating agents for treatment of newly diagnosed older AML and relapse/refractory subjects, even as a bridge to transplant. Here, we supported the use of venetoclax and decitabine as salvage and bridge therapies in adult AML.
Statement of Ethics
This study protocol was reviewed and approved by the Institution, Department of Medicine and Surgery, University of Salerno on March 20, 2022. Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Funding Sources
The authors have no funding sources to declare.
Author Contributions
Bianca Serio, Denise Morini, Valentina Giudice, and Roberto Guariglia treated the patient. Rosa Vitolo, Paola Manzo, and Maddalena Langella performed molecular biology analyses. Carmine Selleri overviewed this study.
Data Availability Statement
All data that support the findings of this study are included in this article.
References
- 1.Giudice V, Gorrese M, Vitolo R, Bertolini A, Marcucci R, Serio B, et al. WT1 expression levels combined with flow cytometry blast counts for risk stratification of acute myeloid leukemia and myelodysplastic syndromes. Biomedicines. 2021;9((4)):387. doi: 10.3390/biomedicines9040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granfeldt Østgård LS, Medeiros BC, Sengeløv H, Nørgaard M, Andersen MK, Dufva IH, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015 Nov 1;33((31)):3641–9. doi: 10.1200/JCO.2014.60.0890. [DOI] [PubMed] [Google Scholar]
- 3.Saliba AN, John AJ, Kaufmann SH. Resistance to venetoclax and hypomethylating agents in acute myeloid leukemia. Cancer Drug Resist. 2021;4:125–42. doi: 10.20517/cdr.2020.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farag SS, Archer KJ, Mrózek K, Ruppert AS, Carroll AJ, et al. Cancer and Leukemia Group B 8461 Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood. 2006 Jul 1;108((1)):63–73. doi: 10.1182/blood-2005-11-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krug U, Büchner T, Berdel WE, Müller-Tidow C. The treatment of elderly patients with acute myeloid leukemia. Dtsch Arztebl Int. 2011 Dec;108((51–2)):863–70. doi: 10.3238/arztebl.2011.0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stomper J, Rotondo JC, Greve G, Lübbert M. Hypomethylating agents (HMA) for the treatment of acute myeloid leukemia and myelodysplastic syndromes: mechanisms of resistance and novel HMA-based therapies. Leukemia. 2021 Jul;35((7)):1873–89. doi: 10.1038/s41375-021-01218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015 Jul 16;126((3)):291–9. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richard-Carpentier G, DiNardo CD. Venetoclax for the treatment of newly diagnosed acute myeloid leukemia in patients who are ineligible for intensive chemotherapy. Ther Adv Hematol. 2019 Oct 23;10:2040620719882822. doi: 10.1177/2040620719882822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020 Aug 13;383((7)):617–29. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 10.Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013 Jan;19((1)):117–22. doi: 10.1016/j.bbmt.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Thol F, Heuser M. Treatment for relapsed/refractory acute myeloid leukemia. Hemasphere. 2021 Jun 1;5((6)):e572. doi: 10.1097/HS9.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loke J, Malladi R, Moss P, Craddock C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: a triumph of hope and experience. Br J Haematol. 2020 Jan;188((1)):129–46. doi: 10.1111/bjh.16355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Megías-Vericat JE, Martínez-Cuadrón D, Sanz MÁ, Montesinos P. Salvage regimens using conventional chemotherapy agents for relapsed/refractory adult AML patients: a systematic literature review. Ann Hematol. 2018 Jul;97((7)):1115–53. doi: 10.1007/s00277-018-3304-y. [DOI] [PubMed] [Google Scholar]
- 14.DiNardo CD, Lachowiez CA, Takahashi K, Loghavi S, Xiao L, Kadia T, et al. Venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed and relapsed or refractory acute myeloid leukemia. J Clin Oncol. 2021 Sep 1;39((25)):2768–78. doi: 10.1200/JCO.20.03736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandhu KS, Dadwal S, Yang D, Mei M, Palmer J, Salhotra A, et al. Outcome of allogeneic hematopoietic cell transplantation after venetoclax and hypomethylating agent therapy for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2020 Dec;26((12)):e322–7. doi: 10.1016/j.bbmt.2020.08.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are included in this article.