Abstract
The theory of constructed emotion is a systems neuroscience approach to understanding the nature of emotion. It is also a general theoretical framework to guide hypothesis generation for how actions and experiences are constructed as the brain continually anticipates metabolic needs and attempts to meet those needs before they arise (termed allostasis). In this review, we introduce this framework and hypothesize that allostatic dysregulation is a trans-disorder vulnerability for mental and physical illness. We then review published findings consistent with the hypothesis that several symptoms in major depressive disorder (MDD), such as fatigue, distress, context insensitivity, reward insensitivity, and motor retardation, are associated with persistent problems in energy regulation. Our approach transforms the current understanding of MDD as resulting from enhanced emotional reactivity combined with reduced cognitive control and, in doing so, offers novel hypotheses regarding the development, progression, treatment, and prevention of MDD.
Keywords: metabolism, affect, emotion, interoception, predictive processing, allostasis, depression
1. INTRODUCTION
If you were raised in a Western cultural context, such as a community in North America or Western Europe, then you likely experience a clear distinction between physical events, like a muscle ache, and mental events, like worry. You may believe that these events have different underlying biological causes; for example,if you feel pain in your chest,you may believe that a physician will be able to find the biological source of that pain using an X-ray or some other biological measure, whereas if you have problems with your mood, you might assume the problem lurks in the brain’s neurochemistry. Your mental life is likely composed of mental events such as perceptions, thoughts, beliefs, and emotions, and you might assume that each type of mental event issues from its own assembly of dedicated neurons in your brain, with its own distinctive mental features (e.g., affective feelings of pleasure and discomfort are assumed to be features of emotion, but not cognition). You might assume that everyone on the planet with a neurotypical brain performs cognitive control and experiences emotions as you do, because these are basic functions that are supposedly built into your brain, inherited via specific sets of genes. And because you have likely been taught from a young age that the muscle movements of your face, voice, and body broadcast instances of sadness, fear, and other emotion categories in consistent and specific ways, you might feel confident that you can recognize or “read” emotions in other people’s faces, in the prosody of their voices, and in their body movements (referred to as facial expressions and body language).
Such beliefs are common to many people who were socialized in a Western cultural context and have developed a Western theory of mind, including many research scientists. Correspondingly, these beliefs serve as bedrock assumptions that have given rise to the set of categories (a typology) that are commonly used in psychological science to inspire hypotheses and experimental design, guide inference, and organize knowledge (e.g., sadness is a type of emotion). The many instances of each type are assumed to share a prototypical set of features (see the sidebar titled Category Features),such as a frowning facial expression in instances of sadness,that is supposedly consistent and specific enough to identify the instance (e.g.,diagnose the presence of sadness) and distinguish onetypefromanother(e.g.,distinguishsadnessfromanger;Cowen&Keltner2017,Russell1991).
The typology includes the assumption that there is a meaningful parallelism between a category, the psychological processes that cause the prototypic features of that category, and the dedicated set of biological causes that implement instances of the category. Taken together, these assumptionssuggestthatthecurrenttypologyofmentalandphysicalcategoriesusedinthescience of mind, brain, and body is suitable for integrating empirical findings and building robust, replicable scientific knowledge. Scientists attempt to understand behavior and mental life in terms of these categories and their biological underpinnings and explain illness as a set of problems within them, where problems in categories of mental versus physical events dictate diagnosis and treatment. Major depressive disorder (MDD), for example, is commonly described as having deficits in enhanced emotional reactivity (understood as a problem with the processes that cause emotions, referred to as emotion processing) combined with impaired emotion regulation (understood as a problem with cognitive control), resulting in fatigue, distress, and other symptoms (e.g.,DeRubeis et al. 2008, Disner et al. 2011).
Evidence from psychology, biology, and neuroscience calls these assumptions into question, however. If many of the assumptions of the current scientific typology are called into question, thentheinterpretationsofempiricalresultsmaybesuspectandtheexperimentsthemselves,whose design is also guided by this typology, may be limiting. Assumptions about the typological structure of mental categories have been called into question by empirical evidence, for example. There is a lack of consistency (i.e.,tremendous variation) in the prototypical features of instances within a category as well as a lack of specificity (i.e.,overlapping features) in instances of different categories (for discussion and evidence, see Table 1). Similarly, illnesses that have been designated as “mental” and defined by a specific set of mood disturbances, such as MDD, are heterogeneous within one person over time and across individuals. MDD and other psychiatric disorders can present as a population of highly variable features that are both mental and physical, rather than as a consistent set of disordered categories, neatly dividing into subtypes based on domains of symptoms (Fried 2017, Goldberg 2011). Consequently, there is a lack of clear and consistent criteria for diagnosing depressive disorders as well as substantial variation in treatment response among patients (e.g., Penninx et al. 2018). A new approach would begin with the biological processes that are disrupted, contextualized by evolution and developmental frameworks, and emphasize variation of features within a category and similarity across categories, to search for structure in the variation.
Table 1.
Examples of scientific evidence that disconfirms the assumptions embedded in the Western typology of psychological categories
| Assumption | Examples of conflicting evidence |
|---|---|
| There are firm natural boundaries between mental and physical phenomena in terms of their features and their causes. | Release of cortisol, a key glucocorticoid hormone with a key role in metabolism (e.g., oscillates according to a circadian rhythm) (Oakley & Cidlowski 2013), also increases in preparation for exercise, during stress, and during some instances of emotion. The same brain networks and brain hubs that are important for regulating the autonomic nervous system, endocrine system, and immune system are also implicated in instances of perception, memory, cognitive control, emotion, and other psychological categories (e.g., Crossley et al. 2014, Kleckner et al. 2017 and references therein, Koren et al. 2021, Kragel et al. 2019). |
| Instances of a psychological category issue from an innate, dedicated assembly of neurons in the brain. | In both human and nonhuman animals, there is a many-to-many mapping between emotion categories and individual neurons as well as between emotion categories and populations of neurons within a single brain region, in an intrinsic network, and in a distributed pattern of activity (see tables 1 and 2 of Barrett 2017b and references therein). Networks that are implicated in instances of emotion are also implicated in instances of perception, memory, decision-making, emotion, and other psychological categories (e.g., Barrett & Satpute 2013 and references therein, Kleckner et al. 2017 and references therein). |
| Emotion categories, referred to with words such as “anger,” “sadness,” “fear,” and so on, have distinctive prototypic profiles of mental and physical features. | Patterns of autonomic activity show considerable variability within a single emotion category as well as considerable similarity across categories, demonstrating that autonomic nervous system patterns for emotion categories are neither consistent nor specific, even within a single person across situations (Hoemann et al. 2020, Siegel et al. 2018). The same is true for facial expressions (Barrett et al. 2019, Le Mau et al. 2021). The facial configurations that are presumed to be prototypic also frequently occur during nonemotional events (see Barrett et al. 2019 and references therein). Even affective features vary within a single emotion category (see Barrett 2017b and references therein). |
| Genes are the means of transmitting biological and psychological information across generations, such that certain categories of psychological phenomena (e.g., certain categories of cognition and emotion) are innate and part of a universal human nature. | Growing evidence from molecular genetics and evolutionary developmental biology indicates that there are multiple inheritance systems, including cultural inheritance, that wire a human brain during development and across the life span (e.g., Gendron et al. 2020, Müller 2017). Via these systems, a brain continually hardwires itself to its physical and social worlds (including the physical characteristics of a person’s body). As a consequence, the human species contains many human natures. |
Evidence suggests that there is a many-to-many mapping between a mental phenomenon and its underlying neurobiology. More than one neural assembly has been observed to implement a given psychological phenomenon in the same context (i.e., there is degeneracy, where multiple elements can perform the same function in the same situation; Edelman & Gally 2001), and the same neurobiological processes have been observed in association with different categories of phenomena (i.e., the processes may be domain general with respect to their psychological function; see Table 1 for further discussion). Thus, there is a lack of parallelism among categories that describe mental phenomena and behavior, the psychological processes that are thought to implement those phenomena, and the neurobiological implementation of those processes (for further discussion and evidence, see Table 1). Rather than using Western categories of mental events to organize biological phenomena, a new approach would begin with what is known about the architecture, evolution, and development of the human nervous system to hypothesize how a brain, in constant conversation with the body and the outside world, creates a mind and controls behavior. Furthermore, this approach should aim to identify causal processes that are domain general and consistent with evolution and development (and therefore anatomy), which can then be used to explain both individual differences and general phenomena.
These scientific objectives—an appreciation of variation, degeneracy, evolution, and domain-general processes—are embodied in the theory of constructed emotion. However, the theory of constructed emotion is more than a scientific approach for understanding the nature of emotion: It is a multidisciplinary enterprise that has the potential to transform the study of mind, brain, and body and aid in reinterpreting existing findings to guide the generation of new hypotheses. We refer to this expanded scope as the constructed mind approach. We begin this review by outlining this approach as a coherent, neurobiologically inspired research framework for understanding how mental and behavioral phenomena arise from the brain’s regulation of the body. We hypothesize that energy regulation is a critical factor in mind and behavior. In Section 3, we explain how the theory distinguishes affect and emotion. Specifically, the brain, to achieve efficient energy regulation, continually models the sensory state of its body in the world, which gives rise to, among other things, affective features of experience such as valence, arousal, and effort; affective feelings, therefore, are best thought of as low-dimensional features of consciousness that are not specific to instances of emotion per se. Correspondingly, any illness involving disruption in the brain’s modeling of the body (and therefore in its regulation of bodily systems), whether considered physical or mental, will involve affective symptoms; and any disorder that involves affective symptoms, whether considered mental or physical, will be accompanied by some dysfunction in energy regulation. Specifically, energy dysregulation may serve as a basic underlying causal factor in illnesses characterized by disruptions in affect, such as MDD. Accordingly, in Section 4, we review findings on brain–symptom relationships and peripheral metabolic phenotypes in MDD to determine whether they are consistent with our hypothesis that affective symptoms arise from disrupted energy regulation, rather than from uncontrolled emotional reactivity. Finally, in Section 5, we discuss the implications of these findings for new hypotheses about the development, progression, and treatment of MDD.(Note that some topics covered in this review are associated with large published literatures, and because of space limitations, comprehensive referencing was not possible; to address this issue,on occasion we refer to published papers from our lab that contain relevant references from other labs, also referring the reader to references therein.)
2. THE CONSTRUCTED MIND APPROACH AS A MULTILEVEL ACCOUNT OF BEHAVIOR AND MIND
The constructed mind approach is an extension of the theory of constructed emotion, which itself began as a more modest theoretical proposal, called the conceptual act theory (Barrett 2006, 2013). Built from psychological and social construction approaches, the conceptual act theory proposed that the human mind transforms feelings of affect into instances of emotion by categorizing them with situation-specific, embodied emotion concepts. Following publication of the initial papers outlining the conceptual act theory, however, a deeper understanding of nervous system structure and function suggested that instances of emotions do not arise from categorizing affect. Instead, they emerge in a brain as it continually makes meaning of sense data from its body and the world by categorizing those data with situation-specific concepts, thereby constructing experience and guiding action (discussed in Barrett 2012, 2013). These hypotheses were then integrated with constructionist hypotheses and research findings in other scholarly fields, including (a) neuroscience, such as neuro-construction (e.g., Karmiloff-Smith 2009, Mareschal et al. 2007) and predictive processing approaches to brain function (discussed in Barrett & Simmons 2015, Chanes & Barrett 2016, Hutchinson & Barrett 2019, Kleckner et al. 2017); (b) physiology, such as the predictive regulation of the body (i.e., allostasis; Sennesh et al. 2022, Sterling 2012) and heterarchical control in physiological function (e.g., Bechtel & Bich 2021, Cohen 1992); (c) developmental science, such as ideas and research findings that arise from rational constructivism and infant brain development (discussed in Atzil et al. 2018,Hoemann et al. 2020); and (d) the extended evolutionary synthesis (discussed in Barrett & Finlay 2018), including research on cultural inheritance (discussed in Gendron et al. 2020). The result—the theory of constructed emotion (Barrett 2017a,b)—is a framework for generating novel hypotheses about the causes of experiences and behaviors, as well as for inferring a different set of explanations for the existing empirical record. The theory starts with the structure and function of the nervous system as a framework for how a brain generates action and experience, rather than starting with folk psychology categories and searching for their neural, physiological, or chemical causes. Similarly, when considering what might go awry in illness, the theory starts with deviations in structure and function of the nervous system and asks how they might give rise to symptoms, rather than starting with symptoms and then searching for their physical causes (discussed in Barrett et al. 2016).
In this section, we outline this multilevel constructionist framework as the constructed mind approach, because the scope of inquiry and target of explanation are broader than instances of emotion per se. The approach is centered on a key insight: Brains evolved to coordinate bodily systems in an efficient manner, providing uninterrupted energy regulation in an ever-changing but only partly predictable world. Brains did not evolve to think, feel, see, and so on—they create these events as they perform a dynamic and complex budgeting process that continues from birth until death. According to this logic, disordered cognition, affect, perception, and other symptoms may be linked to problems in energy regulation. Our goal is not to reduce every mental phenomenon to energy regulation but rather to highlight energy regulation as a key element in the state space of a brain: a complex, nonlinear dynamical system that continually interacts with its body and the surrounding world. Using this framework, we propose three interrelated hypotheses for how a brain creates a mind.
2.1. Hypothesis 1: Mental Categories Are Highly Variable Groups of Instances Whose Similarities Are Intrinsically Context Dependent
In the constructed mind approach, a psychological category, such as sadness or cognitive control, is a grouping of mental instances with highly variable features, because each instance is tailored to the specific situation in which it occurs. Consider the many different contexts that might give rise to instances of sadness. In some situations, you might cry; in others, you might withdraw in silence. You might even smile. In each instance, the physiological changes in your body will vary, because your actions or your intended actions will vary. We hypothesize that sadness and other mental categories are a population of instances whose features vary from one to another because each instance is situated, and situations vary (discussed in Barrett 2017b, Barrett et al. 2019, Hoemann et al. 2020, Siegel et al. 2018). This means that psychological categories are not structured as discrete types with defining features (i.e., natural kinds with firm boundaries in nature) or as prototypes with fuzzy boundaries whose instances share representative or typical features across situations and individuals. Instead, we hypothesize that categories of mental events are populations of situated, variable instances, dynamically constructed on the spot as ad hoc categories (Barsalou 1983) to serve context-specific functions. Ad hoc categories are not stored in the brain to be retrieved but rather constructed in situ, as needed, in much the same way as memories and perceptions are constructed. Hypotheses about how a brain constructs these categories are firmly rooted in the brain’s most important task, which is to predictively regulate the energy needs of the body, a topic to which we now turn.
2.2. Hypothesis 2: A Brain’s Most Important Job Is Coordinating and Regulating the Systems of the Body
Every animal must regulate the use and intake of energy. This regulation becomes particularly complicated when an animal’s body contains multiple organ systems that all perform unique processes and have unique energy needs. Consider a simpler animal such as Caenorhabditis elegans; it is continually sensing the concentration of oxygen in the external environment, which indicates the presence or absence of food, and the level of glucose in its gut (i.e., its internal environment), which indicates energy need (Witham et al. 2016). These simple sensory surfaces are coordinated with each other and with the motor system so that the animal either stays and feeds or moves to find new food (Schiffer et al. 2020). Both moving and feeding require an expenditure of energy to secure more energy (e.g., Sterling & Laughlin 2015). This simple example illustrates that the coordination of multiple systems within a body is intimately linked to energy regulation, even in a small animal with fewer than 10 biological systems and no brain.
Now consider a human body, which has nearly 80 separately identified organ systems. Each system has its own energy needs to be tracked, and to maintain energy efficiency, the systems must be coordinated. As organisms get larger and more complex, efficiency is maintained by this coordination,which is effectively achieved by a brain.Evidence from evolutionary history suggests that, in fact, brains may have evolved along with an increase in the number of bodily systems with moving parts that required coordination (e.g., Gee 2018, Striedter & Northcutt 2020).
Invertebrates,thereappearstohavebeenacoevolutionofnumerousbodypartsandabrainthat sits atop a spinal cord. Some 500 million years ago, under selection pressures related to predation, animals are thought to have evolved more sophisticated abilities to sense and move through the external world, abilities aided by larger, more complicated bodies (e.g., Gee 2018). These larger bodies include more motor parts (a definable head and appendages,such as fins) and newly evolved exteroceptive sensory systems (e.g., olfactory, visual, and gustatory systems), as well as more sophisticated somatosensory and auditory systems. Larger bodies increase the distance between the internal and the external environment,meaning that crucial processes such as waste excretion,nutrient uptake, and circulation could not occur passively by osmosis,requiring new organ systems to actively execute these functions—hence the emergence of newly evolved visceral systems containing both sensory and motor aspects, such as a respiratory system, a gastrointestinal system (along with an enteric nervous system), and an adaptable immune system that can learn from experience (Gee 2018, Striedter & Northcutt 2020). This expansion of biological systems was accompanied by a need to actively coordinate and regulate these systems. A brain serves this purpose, efficiently tracking the influx and efflux of energetic resources to balance the needs of all systems and keep them functioning optimally (Sterling & Laughlin 2015). Animals with smaller bodies and fewer systems, by contrast, accomplished energy regulation without a brain, using neurons that were precursors of the hindbrain (i.e., monoamine nuclei; Parent 1984) and midbrain (i.e., posterior hypothalamus; Lemaire et al. 2021). This evolutionary evidence suggests that energy regulation is fundamentally at the core of brain function.
The anatomical structure of the vertebrate brain reinforces the hypothesis that energy regulation is a core brain function (Figure 1a,b). Developmental and functional evidence is also consistent with the hypothesis that energy regulation is at the core of brain activity. For example, the neurons that make up limbic cortical tissue and are important for controlling and coordinating the internal systems of the body can be considered “core” in an embryological way; they begin to develop from the neural crest of an embryo before those that make up sensory and skeletomotor tissue (for research on rodents, see Bayer & Altman 2004). In addition to regulating metabolism, these limbic regions are involved with a range of psychological events, including skeletomotor action [e.g., in humans (Rizzolatti et al. 1996) and primate tract tracing; reviewed in Paus 2001], vision (in primates; Cavada & Goldman-Rakic 1989, Rockland & Pandya 1979), audition (in primates; Morán et al. 1987, Seltzer & Pandya 1994), memory (e.g., in humans and nonhuman primates; Rolls 2015, 2019), emotion (e.g., in humans; Kober et al. 2008, Wager et al. 2015), and consciousness (e.g., Chanes & Barrett 2016). They are also involved in reward (e.g., Cohen et al. 2008, Liu et al. 2011) and value (e.g., Cai & Padoa-Schioppa 2012, Kolling et al. 2016) precisely because these psychological features are centrally concerned with energy regulation.
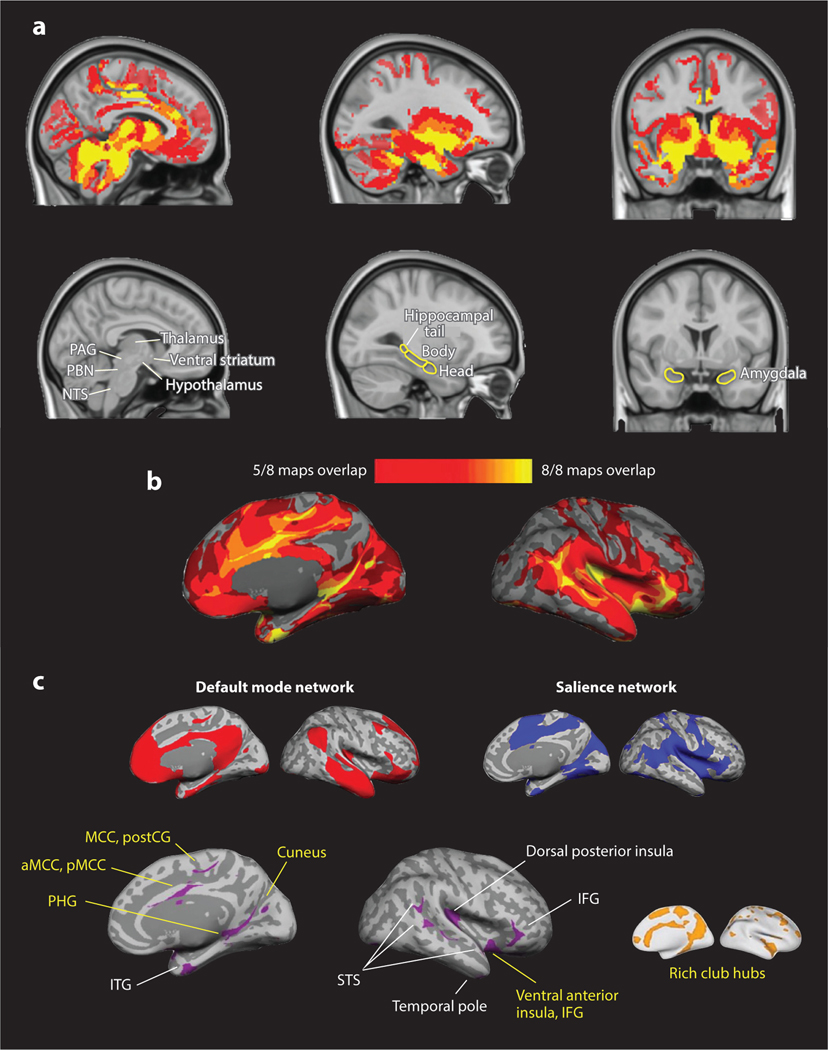
Figure 1.
The anatomical structure of the vertebrate brain supports energy regulation as a core brain function. We consulted anterograde and retrograde tract-tracing studies in macaque monkeys to select eight seed regions in limbic cortices with monosynaptic connections to midbrain and brainstem regions that are known to control the immune, endocrine, and autonomic nervous systems in the service of body regulation. Briefly, brainstem nuclei, such as the nucleus tractus solitarius (NTS), are the first regions of the brain to receive ascending interoceptive sense data about the state of the body and its various systems. Nearby in the brainstem are bed nuclei that are synthesis sites for monoamine neurotransmitters (e.g., locus coeruleus for norepinephrine, dorsal raphe for serotonin, and ventral tegmental area for dopamine) that evolved as metabolic regulators (Beeler et al. 2012, O’Donnell et al. 2012, Yabut et al. 2019) and that project widely throughout the brain to modulate ongoing neural activity (and, therefore, the energetics of the brain). These brainstem regions directly project to the hypothalamus (in rodents; McKellar & Loewy 1981), which contains an array of nuclei that are collectively thought to be a main controller of energy regulation via their sensitivity to metabolic and reproductive hormones, circadian rhythm oscillations, and vascular and immune mediators (reviewed in Blessing 1997, Saper 2002). The hypothalamus projects to a set of regions hypothesized to be crucial for regulating the body. They also project to the amygdala (Pritchard et al. 2000); the hippocampus (e.g., Insausti & Amaral 2012) and the basal ganglia (Haber 2003); the medial aspects of the cerebral cortex (specifically, the cingulate cortices, the posterior orbital frontal cortex, and the abutting ventral anterior insula; see Kleckner et al. 2017 and references therein), all of which are themselves interconnected and are evolutionarily new to the vertebrate brain (as part of the forebrain); and the cerebellum (Cacciola et al. 2019). Ascending interoceptive information continues to midbrain structures such as the parabrachial nucleus (PBN), the periaqueductal gray (PAG), and the deep/intermediate layers of the superior colliculus (SC), which have been implicated in cardiovascular, respiratory, and thermoregulatory control as well as in the control of other visceral and endocrine processes and immune function. These midbrain structures project to the hypothalamus, the amygdala, the hippocampal complex (including entorhinal cortex), and the medial portions of the cerebral cortex mentioned above (Barbas 2015, García-Cabezas et al. 2019). Being located medially in each cerebral hemisphere, the limbic cortical regions, like brainstem and midbrain regions, are topographically at the core of the brain. For each seed region, we computed a discovery map of voxels whose time course correlated with the seed region. (a) A conjunction of all eight maps presented in the volume to display subcortical regions. (b) A conjunction of maps depicted on the cortical surface. These maps highlight the anatomy that supports energy regulation in the body. (c) Cluster analysis of the eight discovery maps revealed that the system for allostasis is composed of two large-scale intrinsic networks (red, blue) that share several hubs (purple). Hubs belonging to the brain’s “rich clubs” are labeled in yellow. Maps were constructed with resting-state blood oxygen level–dependent data from 280 participants binarized at p > 10–5, then replicated on a second sample of 270 participants. Abbreviations: IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; PHG, parahippocampal gyrus; pMCC, posterior midcingulate cortex; postCG, postcentral gyrus; STS, superior temporal sulcus. Figure adapted with permission from Barrett et al. (2016).
It is never possible to claim that the brain evolved “for” any particular function (which would be teleological; Mayr 1974), but the evidence reviewed thus far is consistent with the hypothesis that the brain’s most important job is to efficiently coordinate and regulate the systems of its body as an animal moves and grows within an ever-changing and only partly predictable external world (e.g., Ashby 1960, Sterling & Laughlin 2015). In the next section, we consider a framework for this regulation and its implementation in the brain to suggest that the means by which the brain performs energy regulation are, in fact, domain-general processes of the mind and behavior.
2.3. Hypothesis 3: Predictive Regulation of the Body Is a Domain-General Process That Is Central to Action and Experience
The most efficient way to regulate a system is to model it, that is, to predict and correct those predictions as needed (as in cybernetics; e.g., Conant & Ashby 1970). This strategy allows an animal to prepare its response to the anticipated changes in the world, which is more energy efficient and less error prone, rather than reacting suddenly after the fact (Sterling 2012). Correspondingly, a brain is thought to run an internal model of its body in the world, continually anticipating the needs of the body and attempting to meet those needs before they arise (Sterling 2012, Sterling & Laughlin 2015).This process is called allostasis: the dynamic,predictive coordination of the body’s internal milieu and control of vital biological parameters (e.g., glucose and insulin levels, blood pressure, body temperature) that enable continued life (Sterling 2012) and eventual reproduction (Pontzer 2015). An early example of anticipatory regulation comes from the original studies in classical conditioning, for example, in which Ivan Pavlov famously observed that dogs salivated when presented with an auditory cue that predicted the appearance of food. Salivation represents an early anticipatory visceromotor event that prepares the organism to digest food before the food has been ingested (Pavlov & Thompson 1910). Further research confirmed that this anticipatory response extended to the gut, adrenal glands, and pancreas, such that digestive enzymes, cortisol, and insulin are released into the bloodstream in anticipation of food ingestion (and, consequently, an upcoming increase in blood glucose levels; Brand et al. 1982, Feillet 2010). Visceromotor commands from the brainstem; the midbrain, including the ventromedial hypothalamus; and the limbic cortical regions coordinate multiple organ systems via descending nerve fibers in the vagus and sympathetic nerves; these fibers innervate the viscera and relay the appropriate visceromotor commands (e.g.,Powley 2000). Cortisol levels increase in anticipation of food and drop off rapidly after digestion, and they are tightly coordinated by a neurotypical brain (e.g., Feillet 2010). Cortisol is often called a stress hormone,but it has a more basic metabolic role to play in energy regulation, a role that occurs in stressful conditions but is not limited to them (e.g., Oakley & Cidlowski 2013). Regulation of energy by cortisol is an example of the brain’s continual efforts to coordinate the internal systems of the body via allostasis. Persistent, high levels of cortisol (which many researchers link to chronic stress), such as those observed in depression, may reflect disrupted allostasis.
To understand how a brain might implement allostasis within its electrochemical structure, we turn to the emerging paradigm of predictive processing. Predictive processing refers to a family of theoretical neuroscience approaches (discussed in Hutchinson & Barrett 2019 and references therein) that yield several unintuitive hypotheses about how a brain runs a model of its body in the world in the service of efficient energy regulation, resulting in action and the construction of experience. Such approaches include the Bayesian-brain approach, belief propagation, and active inference (see Hutchinson & Barrett 2019 and references therein), as well as the theory of constructed emotion (e.g., Barrett 2017a,b).
Amid their differences, predictive processing approaches share three key elements (discussed in too many papers to cite here, but for representative examples, see Friston 2010, Hutchinson & Barrett 2019, and references therein): (a) prediction signals (i.e.,the brain’s internal model; Berkes et al. 2011) implemented by reassembling past experiences from memory, sometimes referred to as top-down processing; (b) prediction errors (i.e., the encoded difference between the prediction signals and the sense data arriving from the body’s sensory surfaces), which provide the brain with the opportunity to update its internal model to improve future predictions (and, correspondingly, allostatic efficiency), sometimes referred to as learning (Denève et al. 2017); and (c) precision signals that modulate the probability that prediction and error signals will translate into actual motor (visceromotor and skeletomotor) outcomes, corresponding to attention, executive control, and salience. Prediction, prediction errors, and precision signals all operate simultaneously within the neural hierarchies of the brain. Figure 2 depicts our hypotheses about predictive processing within the cerebral cortex (due to space considerations, we do not discuss predictive processing within the hippocampus and cerebellum; for a brief discussion, see Katsumi et al. 2021 and references therein).
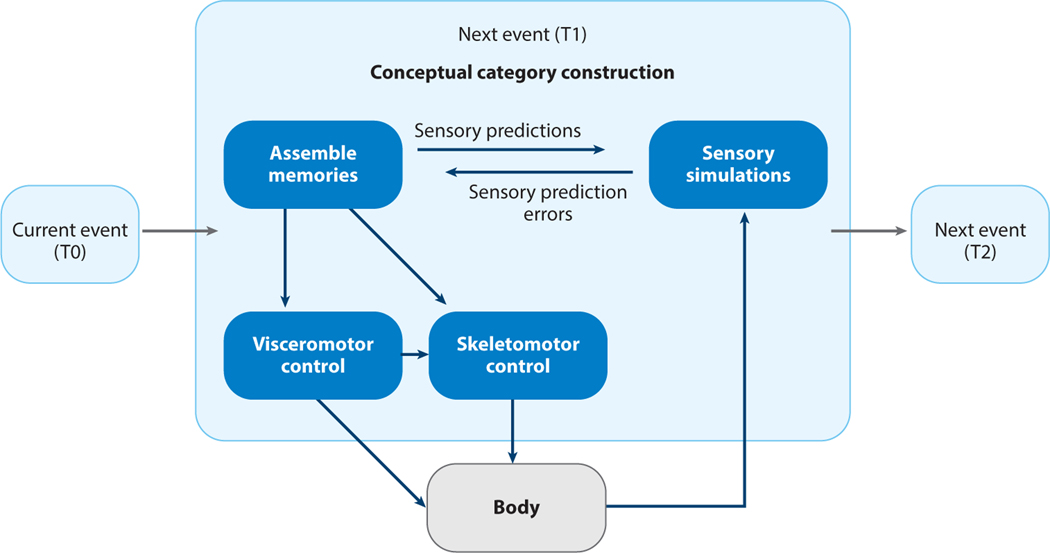
Figure 2.
Toy example of predictive processing within the cerebral cortex. In this example, the present moment is time zero (T0). At T0, a brain’s internal model is representing its confirmed beliefs about the current sensory conditions of the body and the world as it generatively constructs a population of prediction signals by using past experiences of temporal dependencies, based on similarities (or features of equivalence). Figuratively speaking, the brain is asking itself, “In similar past events, what actions did I make next?” Neurons whose cell bodies reside in the deep layers of the cerebral cortex send motor prediction signals (as preparations for both visceromotor and skeletomotor actions) that cascade to subcortical regions and the spinal cord via neurons as they become more detailed and particularized. These neurons in the deep layers of the cerebral cortex have collateral axons that send the same information, called efferent copies of the motor prediction signals, to more granular parts of the cerebral cortex, becoming more detailed and particularized, until they reach primary sensory regions (Barbas 2015) (see Figure 3). These efferent copies can be understood as sensory prediction signals that infer the sensory consequences of the motor predictions (i.e., what a brain expects to see, hear, feel, and so forth if these motor actions are enacted, estimated from the statistical regularities in past experiences). These efferent copies are also called simulations or perceptual inferences because this signal changes the firing of sensory neurons in advance of sense data that will arrive momentarily from the body’s sensory surfaces. Figure adapted with permission from Hutchinson & Barrett (2019).
We hypothesize (following Barrett 2017b) that prediction signals prepare the action plans that are necessary to move your body (including the visceromotor movements that support the energetic requirements of skeletomotor movement) and to begin to construct the mental features that create what you will see, hear, and feel in the next moment, even before the relevant sense data have arrived from the sensory surfaces of the body to the brain. Each prediction signal is computed with a probability of being the best fit to the current circumstances (i.e., a Bayesian prior; Friston 2003, 2010); together, the population of prediction signals are thought to form a probability distribution of possible motor and sensory outcomes that are then tested against incoming sense data from the body and the world. If a prediction signal matches incoming sense data well enough (i.e., the sensory neurons are already firing in a pattern that represents the incoming sense data with some accuracy), then the predictions are confirmed, the motor actions are completed, experience is constructed, and the prediction’s priors are strengthened, increasing the likelihood that it will be constructed again in the future. In such circumstances, prediction signals, which started as visceromotor and skeletomotor reference signals and perceptual inferences, now explain your actions and perceptions. In such cases, your movements and your experience of the world are dominated by your internal model, and the incoming sense data (or what psychologists refer to as stimuli) only serve the function of confirming the model.
Mismatches between the pattern of neural firing that constitutes the prediction signals and the incoming sense data are computed as prediction error signals. These error signals function as teaching signals that are available to modify the internal model to optimize the accuracy of future predictions. This comparison of prediction and prediction error is thought to happen within the brain’s neural hierarchies, perhaps in every neuron (e.g., Denève 2008). When a brain encodes prediction error, it is learning the statistical regularities of the environment, which involves a variety of energetically expensive biological events (e.g., dendritic arborization, protein synthesis, axon terminal modifications) that are managed by the brain in a series of trade-offs.
At the heart of this predictive account of brain function lies a metabolic balancing act (discussed in Barrett 2017b): Learning prediction errors is metabolically costly for a brain, as is the price of failing to learn (e.g., Sterling & Laughlin 2015). On one hand, a brain can incur energetic costs in the present to learn the prediction error, thereby tuning the internal model to predict better and therefore optimize energetic costs in the future. On the other hand, a brain can conserve energy in the present by failing to learn the prediction error, but this might result in future increased costs arising from a miscalibrated and therefore less metabolically efficient internal model (i.e., the priors of the model will match less well the statistical regularities of the environment). To optimize this balancing act, we hypothesize that a brain expends energy to learn prediction errors only when they are predicted to have allostatic implications (i.e., those that are salient, or those with uncertain value). Error signals without allostatic import will be treated as noise and ignored. This modulation by allostatic value is executed by precision signals (Friston 2010, Shipp et al. 2013), a modulatory signaling pattern that either diminishes or strengthens the ease with which prediction error signals continue to propagate according to the predicted allostatic relevance of that error. In psychology, the term attentional control refers to the capacity to selectively focus on specific information in the environment. In the language of the predictive brain, precision signals, which selectively alter the gain on error neurons, can be thought of as applying attention to some error signals over others, thereby altering the rate of learning based on the energetic relevance of those signals (i.e., precision signals apply attentional control over prediction errors).
Precision signals similarly modulate prediction signals to establish their prior probability of matchingincomingsensedata(discussedinBarrett 2017b;e.g.,Kanaietal.2015).Precisionsignals alter the gain on prediction neurons to selectively adjust the ease with which prediction signals reach the brainstem and spinal cord as motor action plans, and reach the cortical sensory systems as perceptual inferences, with the goal of optimizing predictions that are highly likely to be accurate and, correspondingly, to minimize prediction error. It can be said that precision signals apply attention to prediction signals in the service of energetic efficiency, enacting executive control over perceptions and experiences in a goal-directed fashion, where the goal is efficient energy expenditure.
When a brain generatively assembles populations of prediction signals based on computed similarities to the current context, the brain is said to be constructing a category (discussed in Barrett 2017a,b). Many categories constructed by the brain are conceptual because the features of equivalence shared by the instances (the prediction signals) are functional (and therefore abstract; see the sidebar) rather than low-level sensory and motor features. We hypothesize that the brain assembles populations of prediction signals that are similar to one another in their functional features and are particularized into perceptual inferences as they cascade to sensory cortices and into motor reference signals as they cascade down to the spinal cord and effector organs and muscles via the midbrain and brainstem. When prediction signals are confirmed by incoming sensory inputs, those inputs are said to be categorized, influencing priors in the future. Every concept that is constructed by a brain was originally categorized according to its viscerosensory and motor features, consistent with the hypothesis that every concept within a category contains embodied features (e.g., Barsalou 2003, Fernandino et al. 2016).
This predictive processing framework supports the hypothesis that perception and action are tightly coupled, which comes as no surprise. What is surprising, however, is that perception and experience are thought to arise from predicted visceromotor and skeletomotor actions, rather than causing those actions. Moreover, a brain is thought to selectively weight different predictions and error signals according to their allostatic relevance, meaning that your perceptions and actions are always constructed with respect to expectations of future energy needs. These hypotheses are further supported by evidence from tract-tracing studies which show that the structure of cortical architecture scientifically predicts the flow of information across the cortex as prediction signals and prediction error signals (referred to as feedback and feedforward signals in that literature). In the next section, we briefly summarize this anatomical evidence, which highlights the centrality of allostasis in a brain that continually constructs conceptual categories to guide movements and create experience.
2.4. Hypothesis 4: Limbic Circuitry Is at the Top of the Predictive Hierarchy
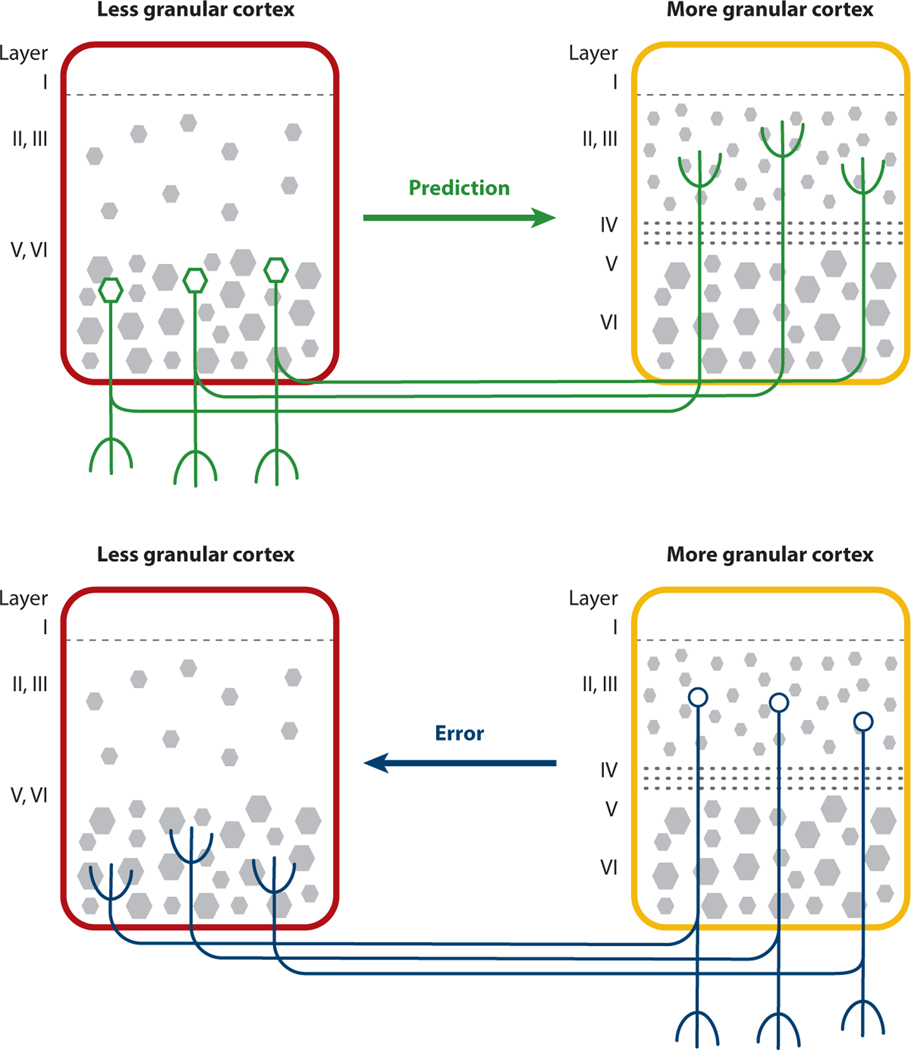
Predictions, prediction errors, and precision weights are hypothesized to be implemented within the brain’s cortical architecture following a loosely hierarchical arrangement. This hypothesis is supported by more than 30 years of empirical research in primates and other mammals, which suggests that the arrangement of neurons within the cerebral cortex describes the flow of predictions and prediction errors across the cortical sheet (for a review of evidence, see Barbas 2015; see also Katsumi et al. 2021 and references therein) (Figure 3). Briefly, the cerebral cortex contains a cytoarchitectural gradient that describes its layered (i.e., laminar) organization. At one end of the gradient, neurons are organized into four layers, representing the lowest degree of laminar differentiation, referred to as agranular tissue; at the other end, neurons are organized into six distinct and well-defined layers, referred to as granular tissue.
Figure 3.
A cytoarchitectural gradient of predictive processing in the cerebral cortex. The cerebral cortex contains a cytoarchitectural gradient that describes its layered (i.e., laminar) organization. At the top of the predictive end of the gradient, neurons are organized into columns of four layers, which is the lowest degree of laminar differentiation, referred to as agranular cortex (e.g., the subgenual anterior cingulate cortex, the posterior orbitofrontal/ventral anterior insular cortex, and the entorhinal cortex). At the other end of the gradient, neurons are organized into columns of six distinct and well-defined layers, referred to as granular cortex; the most differentiated cortical areas (called koniocortices) are primary visual, auditory, and somatosensory cortices. Dysgranular tissue is intermediate in laminar differentiation (e.g., midanterior cingulate cortex). Research shows that the top of the predictive hierarchy in the cerebral cortex begins in agranular cortical regions (Barbas 1986, 2015; also see Chanes & Barrett 2016). Visceromotor and skeletomotor prediction signals begin as highly compressed, low-dimensional (i.e., abstract or conceptual) representations that become more particularized as they descend to the brainstem as motor predictions. Copies of those signals become more particularized as they cascade along the cytoarchitectural gradient in the cerebral cortex to the most granular areas as sensory predictions. Prediction errors are hypothesized to flow in the opposite direction. This hypothesis is consistent with functional evidence that brain activity is organized along a similar gradient (e.g., Katsumi et al. 2021 and references therein, Zhang et al. 2019). Figure adapted with permission from Hutchinson & Barrett (2019).
Agranular regions hypothesized to be the origin of prediction signals within the cortex have another name: They are referred to as limbic (so named by Paul Broca in 1873 because they form the medial border of the cerebral hemispheres). Herein lies the second counterintuitive surprise from a predictive processing perspective: Limbic cortices are primary visceromotor cortices and regulate the autonomic nervous system and the other internal systems of the body (analogous to the primary motor cortex, which controls skeletomotor function; for supporting evidence, see Kleckner et al. 2017 and references therein). For decades, scientists have considered these limbic regions to be the “home” of emotions (e.g., Papez 1937)—the seat of your “inner beast,” highly reactive and in need of control (which was the hypothesized role of the prefrontal cortex; e.g., Ochsner et al. 2009). For example, the finding of impaired limbic and prefrontal connectivity in MDD is described as heightened emotional reactivity combined with impaired cognitive control. If limbic cortices are predictive, rather than reactive, driving perception and action in the brain during all mental events, then this suggests that MDD symptoms may have their roots in altered prediction signals that are insufficient for energetic regulation of the body.
In the next section, we discuss additional evidence that the cortical limbic regions are at the core of the brain’s internal model, regulating the systems of your body via allostasis as they create your experiences, including mood. In this view, any altered functional connectivity between key limbic regions can be understood as a problem in predictive processing or,to put it another way,as a disruption of allostasis and, correspondingly, mood disturbance (discussed in Barrett et al. 2016). In the next section, we also consider evidence that allostasis and the modeled state of the body are integrated within limbic and other key regions, suggesting that depressive symptoms might also arise as disruptions in tuning the brain’s internal model to the external context or the energetic conditions of the body.
3. INTEROCEPTION GIVES RISE TO AFFECT AS A FUNDAMENTAL PROPERTY OF CONSCIOUSNESS
Nearly all limbic regions can be found in two of the brain’s largest intrinsic functional networks, conventionally called the default mode network (DMN; also referred to as the mentalizing network and the semantic network) and the salience network (SN; also referred to as the ventral attention network and the multisensory integration network) (discussed in Barrett 2017b, Chanes & Barrett 2016, Kleckner et al. 2017 and references therein). Given the central role of limbic regions in the maintenance of allostasis within the predictive hierarchy, and the prominent presence of these same limbic regions in both large functional networks, we have hypothesized that the unifying, domain-general function of these networks is allostasis. The DMN is said to run an internal model of the world (Buckner 2012, Hassabis & Maguire 2009), supporting mentalizing and meaning making. We hypothesize that the DMN, together with the hippocampus, generatively constructs the most abstract (i.e., compressed), multimodal features of the brain’s internal model, which are then decompressed into visceromotor, skeletomotor, and sensory details as prediction signals (discussed in Barrett 2017b,Barrett et al. 2016; for supporting evidence on embodied representations of concepts, see Barsalou 2008, Barsalou et al. 2003, Fernandino et al. 2016), consistent with the observation that the DMN constructs perceptual simulations of the world (and simulations of the internal state of the body; Barrett & Simmons 2015). We hypothesize that the SN functions to predict the allostatic relevance of prediction errors, effectively modulating which errors to learn and which to ignore as noise (discussed in Barrett 2017b,Barrett et al. 2016),consistent with its role in multimodal integration, attention regulation, and executive control. Together, these two functional networks are hypothesized to play crucial roles in maintaining allostatic regulation.
Recent anatomical and functional evidence provides support for these hypotheses (see Kleckner et al. 2017 and references therein). The two networks, rather than being topographically separate and independent, overlap with one another (Figure 1c). Several interesting anatomical and functional features of this allostatic system bear mentioning. First, as hypothesized, the cortical limbic nodes in both networks have extensive connectivity to subcortical and brainstem structures,as well as to the cerebellum and the hippocampus, all of which contribute to allostasis (Onat & Çavdar 2003, Suarez et al. 2020).
Second, the DMN and SN overlap in the dorsal mid- to posterior insula, which functions as the primary interoceptive cortex. Interoception refers to an integrated representation of the internal state of the body (Craig 2003, Quigley et al. 2021), and the primary interoceptive cortex is thought to be a key site for comparing ascending viscerosensory information from the periphery with interoceptive prediction signals (the sensory simulations, or efferent copies of visceromotor control signals) to confirm or correct the predicted sensory outcomes of allostatic regulation (e.g., Avery et al. 2015). This integrated representation of the body informs subsequent allostatically relevant predictions in the brain’s internal model (as suggested in, e.g., Barrett & Simmons 2015).
Third, the limbic cortices in both networks project to brainstem nuclei that are the synthesis sites for neuromodulators involved in attention and precision (e.g., ventral tegmental area, substantia nigra, dorsal raphe nucleus; Bär et al. 2016, Price & Drevets 2010). This finding suggests that attention may be modulated by allostatic processes that are maintained by the limbic regions in the DMN and SN.
Finally, several of these overlapping regions are “rich club” hubs, defined as regions showing the densest anatomical connections, and are hypothesized to be the brain’s neural backbone due to their role in neural communication and synchrony (van den Heuvel & Sporns 2013) (Figure 1c). Many of these overlapping rich club hubs are also limbic and function as high-level connectors, integrating already highly integrated information across modules or communities of regions (Chanes & Barrett 2016, Zhang et al. 2020).
The myriad psychological events that these networks are implicated in, mentioned above, cover nearly every category of psychological phenomenon, including cognition, emotion, perception, and so on. Integrating such findings with the evidence above, we hypothesize that limbic cortices are part of a highly integrated neural backbone of communication that contributes to various categories of mental events while simultaneously and continuously representing the energetic state of the body in the service of allostasis (see Chanes & Barrett 2016 and references therein).
We have previously hypothesized that interoception is made available to consciousness as low-dimensional features of affect: valence, arousal, and effort (Barrett 2017b, Barrett & Bliss-Moreau 2009,Lindquist et al. 2016). Allostasis and interoception are continuous processes, suggesting that the affective features of experience are also continuously changing, acting as a simple barometer of the brain’s beliefs about the energetic state of the body. Affective features are present even during so-called cognitive events; recent evidence reports that many “neutral”words have affective connotations (Osgood et al. 1975) and even putatively neutral stimuli are experienced with subtle affective features (Lebrecht et al. 2012),suggesting that all elements of our world that are captured by our brain’s conceptual system, created in the context of interoception, have affective qualities. Accordingly, we have proposed that valence, arousal, and other affective features are more general properties of consciousness, rather than specific properties of emotion.
If affect is linked to the energetic state of the body, then intense distress or fatigue might indicate that a brain is running an energetic deficit and/or failing to dynamically update its internal model to support allostasis in the current context (e.g., Bennett et al. 2021). Suppose that a brain’s internal model does not adjust to its current context, perhaps due to an insensitivity to prediction errors or an inaccurate precision weighting of incoming signals. This context insensitivity could be due to a significant metabolic problem that diverts energy resources away from daily functioning and impairs learning. Any failure to update the internal model would disrupt allostasis and introduce inefficiency into visceromotor regulation, which can, in turn, exacerbate current metabolic problems and/or give rise to new ones, disrupting allostasis and producing a downward spiral in efficient energy regulation.In the next section, we explore these possible implications further with a specific clinical example that represents significant affective symptoms and disruptions in energetic efficiency: MDD.
4. EVIDENCE OF METABOLIC DYSFUNCTION IN MAJOR DEPRESSIVE DISORDER THROUGH THE LENS OF THE CONSTRUCTED MIND APPROACH
4.1. Symptoms of Depression Arise from Disrupted Allostasis Rather Than from Hyperactive Emotional Reactivity and Impaired Emotion Regulation
MDD is a prevalent and debilitating condition with a pathophysiology that has historically been described as high emotional reactivity and low cognitive control (DeRubeis et al. 2008, Disner et al. 2011). The neurobiological correlates of MDD are, correspondingly, interpreted using these psychological categories. For example, Increased amygdala activity is usually interpreted as heightened emotional reactivity, and decreased activity in portions of the lateral prefrontal cortex is interpreted as diminished emotion regulation (e.g., cognitive control), both of which give rise to rumination and intense negative mood (e.g., Ottowitz et al. 2002). Other changes, such as increased activity in the cingulate cortices, the hippocampus, and the medial prefrontal cortex, are interpreted as causing an increase in self-referential thought (for a review, see Disner et al. 2011). In addition to focusing on activation magnitude of single regions, other findings of impaired SN and DMN intra- and internetwork connectivity in depression have been interpreted as reflecting cognitive issues in negative attentional biases and rumination, respectively. These findings exist in a larger context of a heterogeneous and often contradictory literature, however. Published neuroimaging studies of depression vary markedly in their findings (Zhuo et al. 2019) such that the “heightened emotional reactivity and reduced cognitive control” hypothesis fails to capture a robust, consistent picture of the depression-related brain correlates.
The constructed mind approach, by contrast, does not treat contradictory findings as disconfirming, but rather as suggestive of degenerate mechanisms related to persistent allostatic dysregulation and inefficient energy regulation that might cause similar symptoms in mood, memory, or attention. Successful allostasis requires coordination between predicted energy needs and the actual movements of the body that dynamically change in response to demands. We consider the possibility that neuroimaging findings from depressed patients represent breakdowns in predictive regulation of these needs, with consequences for movement and energy regulation.
One possibility might be an internal model that has become insensitive to the current context (i.e., prediction signals have reduced precision and weak priors), meaning that the signals are not tuned to current conditions. This imprecision could have several causes. We hypothesize, for example, that DMN regions are important for constructing the most abstract features of prediction signals, modulated by frontoparietal regions that compute the precision of those predictions. Changes in frontoparietal activity or their connectivity to DMN regions, such as the subgenual anterior cingulate cortex (sgACC) (e.g., Li et al. 2018), might be interpreted as a breakdown in this relationship. Additionally, prediction signals might have weak priors in relation to the external world if they are preferentially driven by interoception (the modeled state of the body) at the expense of exteroception (which informs the brain about the state of the world). Altered connectivity of the hippocampus and limbic cortices, such as the cingulate cortices and medial prefrontal cortex, may also be involved; the cerebral cortex is thought to generate predictions based on the sensory statistics of the environment, whereas the hippocampus—which also generates prediction signals—is thought to reweight the cortical prediction signal according to goals (i.e.,the estimated metabolic needs) in the moment (Kumaran et al. 2016, Tingley & Buzsáki 2018). The hippocampus is bathed in ascending viscerosensory inputs from vagal and spinothalamic fibers,via midbrain and brainstem nuclei (Insausti & Amaral 2012), which carry information about the sensory state of the body that informs estimates of upcoming energy requirements.Depression-related changes in activity and/or connectivity in cingulate cortices, the hippocampus, and the medial prefrontal cortex might be understood as possible overweighting of the body (and external context insensitivity) in the formation of prediction signals. Additionally, stronger connectivity between limbic hubs across the DMN and SN, such as the sgACC and anterior midcingulate cortex (aMCC), in concert with weaker connectivity between nodes of the SN and frontoparietal control network might also be reinterpreted as weak precision weighting of predictions, driven by the internal state of the body at the expense of signals from the outside world, resulting in disrupted allostasis. An internal model that is locked into the conditions of the body while ignoring sense data from the external world results in predictions that remain uncorrected by sensory cues in the present, which may give rise to symptoms like context insensitivity and rumination.
Context insensitivity might be further enhanced in a depressed brain by reduced precision for prediction error, such that incoming sense data from the body and/or the world are being improperly weighted, updating the model in ways that are counterproductive to energy efficiency. Changes in amygdala activation in depressed subjects, for example, may reflect either prediction signals with weak priors (discussed above) or the precision estimates which indicate that the incoming prediction errors are not relevant to future allostatic regulation. Such interpretations are consistent with the hypothesis that the amygdala is important for signaling uncertainty, cueing the brain to learn more about present conditions for the purpose of optimizing later predictions (discussed in Barrett 2017b; e.g., Whalen 1998), rather than encoding information that is purely “emotional.”A brain that avoids learning to minimize energy costs perpetuates a positive-feedback cycle such that a failure to encode prediction errors leads to increasingly imprecise predictions, which in turn taxes energetic efficiency and allows allostatic burdens to accumulate. At some point, a brain may attempt to offset the growing energetic cost by failing to update its predictions via the means we have just discussed. Another possible means for cutting energetic costs is to reduce skeletomotor movements and engage in “sickness behaviors” that conserve metabolic resources, in conjunction with feelings of fatigue (Dantzer et al. 2014, Maes et al. 2012). As movements and their associated energetic costs are reduced, the exploratory behavior that involves foraging for prediction errors is also reduced, resulting in symptoms of fatigue and motor retardation that are common in MDD.
Yet another possibility is that the brain’s internal model is inaccurate for other reasons. One possibility is that prediction signals do not regulate the body in an energy-efficient manner. For example, some studies, particularly in individuals with unremitting depression (Riva-Posse et al. 2014), show a decrease in connectivity between the sgACC (important for visceromotor regulation) and the aMCC (important for both visceromotor and skeletomotor regulation).This pattern could be interpreted as a failure to coordinate visceromotor and skeletomotor control, resulting in energy-inefficient allostasis.
Finally, energy dysregulation,regardless of whether it stems from an inaccurate internal model, faulty precision weighting of prediction error, or some combination of the two, will be associated with an increasing energetic deficit. The interoceptive consequences of this deficit may give rise to the sustained negative affect and distress that characterize many cases of MDD.
Here, it is crucial to emphasize that there will be multiple mechanisms that give rise to a stagnant internal model, imprecise precision estimates, or a combination of the two, which in turn could result in the exact same symptom, be it fatigue, context insensitivity, or distress. The general trend of heterogeneity in neuroimaging findings related to depression, therefore, may not be a bug in methods or experimental design—it may be a feature of a brain that evolved with degenerate mechanisms. As a result, variable findings might be profitably interrogated to discover multiple trajectories for depression that give rise to similar symptoms. These mechanisms themselves can be thought of as domain general,in that they contribute to many different psychological features, but their neural implementation may be more general and nonpsychological (e.g., limbic tissue and rich club hubs coordinate neural communication at a systemwide level that functions in the service of allostasis). We are describing, in effect, a many (symptom) to many (mechanism) mapping.
Furthermore,the increasing allostatic dysregulation that we hypothesize to be characteristic of MDD could originate from multiple sources. It could begin with an actual, persistent metabolic disorder of the body (e.g., mitochondrial dysfunction) or an internal model that is tuned to realistically expect metabolic costs that cannot be met (e.g.,living in poverty or in a context of persistent aggression), both of which can overextend the body’s energetic budget. Alternatively, it could begin with an internal model that predicts energy needs that are no longer relevant (i.e., incorrect state modeling of the body after illness or exposure to adversity), resulting in inaccurate predictions and/or imprecise precision estimates of the resulting error signal; if the latter is allowed to proceed unchecked,it could result in actual allostatic dysregulation where it did not previously exist.Regardless of the energetic origin,the end result is the same: dysfunctional allostasis associated with altered experience.
4.2. Evidence of Metabolic Dysfunction in Major Depressive Disorder
Several lines of evidence are consistent with the general hypothesis that MDD is associated with impaired energy regulation. Depression has been investigated as a global deficit in efficient energy regulation at a cellular level (Allen et al. 2018); analyses of gene expression in postmortem tissue samples of depressed patients report that 32% of altered protein expression occurred in the dorsolateral prefrontal cortex, specifically in proteins that were involved in metabolic and energy signaling pathways (Martins-de-Souza et al. 2012), and several other studies report impaired cellular metabolism in this same region (Abdallah et al. 2017, Hasler et al. 2007). In depression, deficits in mitochondrial respiratory rate, which indicates the efficiency with which mitochondria utilize glucose to produce biological energy,have been implicated in the pathophysiology of MDD (Bansal & Kuhad 2016, Hroudová et al. 2013). Furthermore, patients with mitochondrial DNA mutations and polymorphisms often present with mood symptoms, such as negative mood and distress (e.g., Fattal et al. 2007, Picard et al. 2015). Depressed individuals who died from suicide show evidence of dysregulation in clock genes that regulate circadian rhythm and are indicative of energetic problems (Li et al. 2013).
Additional evidence for a metabolic basis of depression comes from one of the most popular etiological hypotheses for MDD: alterations in serotonergic neurotransmission. Serotonin has long been conceptualized as the basis for negative mood observed in depression, but it is an evolutionarily ancient molecule that plays a key role in metabolism and energy regulation (for a review, see Yabut et al. 2019). In fact, it was first implicated as a modulator of mood only after reports of significant mood changes from patients who were taking serotonergic modulators to treat various metabolic problems like hypertension and cardiovascular disease (Hillhouse & Porter 2015). Currently, many of the most common pharmacological treatments for depression (selective serotonin reuptake inhibitors) have metabolic side effects, including weight changes and dyslipidemia (e.g., Raeder et al. 2006, Schwartz et al. 2004). Serotonin is thought to exert a variety of metabolic actions in the periphery; serotonin in the gut promotes nutrient storage by increasing gut motility to facilitate nutrient absorption, while serum serotonin enhances insulin secretion from pancreatic islets, indirectly modulating cellular glucose uptake. Serotonin also has receptors on white adipose tissue (WAT) in the periphery and increases adipogenesis (formation of new cells) in WAT, which acts as a storage repository for macromolecule nutrients (for a review and discussion, see Yabut et al. 2019). Centrally, serotonin acts as a crucial mediator of appetite via its synthesis site in the raphe nuclei, integrating information about macromolecule ingestion with circadian rhythms of blood glucose from the suprachiasmatic nucleus of the hypothalamus (e.g., Bezerra de Pontes et al. 2010). We hypothesize that central and peripheral serotonin may work in tandem to optimize nutrient intake and the absorption or metabolism of those nutrients, effectively managing the trade-off between saving (storage in tissue) versus spending (metabolize in blood, uptake into cells) energetic states.In MDD,altered serotonergic transmission may reflect a metabolic problem in the ability to effectively manage that trade-off as coordinated by a predictive brain.
Depression has been linked to insulin resistance and subsequent alterations in brain glucose metabolism (Kan et al. 2013, Lyra e Silva et al. 2019). Insulin resistance involves the insensitivity of insulin receptors and receptor pathways that facilitate glucose uptake into the neurons and neuroglia, which can result in a central loss of glycemic control due to impaired cellular glucose uptake (e.g., Lustman et al. 2000). Both the amygdala and the hippocampus have a high density of insulin receptors in the brain (Unger et al. 1991), suggesting that metabolism in these regions may be specifically impaired in a depressed brain that is insulin resistant, further exacerbating signaling inefficiencies throughout the predictive processing stream (i.e., signaling about uncertainty, prediction errors, and internal model tuning). Insulin resistance and elevated cortisol levels have also been proposed to be an underlying mechanism linking the high comorbidity rates between type 2 diabetes and MDD (Everson-Rose et al. 2004, Leonard & Wegener 2020). Given the tightly coordinated release of insulin and cortisol (e.g., during the anticipatory cephalic phase response described in Section 2.3), this proposal suggests that the unifying link between the two disorders comes from a fundamental deficit in the brain’s ability to anticipate and precisely shift visceromotor control in the direction of the predicted energy demand.
In the last decade, well-established comorbidity rates between MDD and several metabolic disorders such as diabetes, heart disease, obesity, and metabolic syndrome (MetS) have set the stage for reframing MDD as a metabolic disorder (e.g., Gans 2006, McIntyre et al. 2007). Patients with MDD are twice as likely to experience co-occurring cardiovascular disease, diabetes, and stroke (e.g., Kessler et al. 1996), while the severity of symptoms in patients with MetS has been associated with both the severity of depressive-like symptoms in these patients and functional changes in limbic cortices including the hippocampus, anterior cingulate cortex, and hypothalamus (e.g., Cavalieri et al. 2010, Singh et al. 2019). Despite this overlap, the metabolic irregularities observed in depressive disorders are thought to be a consequence of aberrant neurobiology and immune system function, rather than a causal precursor to dysfunction within the brain and immune system.
The inflammatory model of MDD hypothesizes that inflammatory cytokine signaling via the afferent fibers of the vagus nerve is a mechanism of action for aberrant brain energetics; hypothalamus-pituitary-adrenal axis hyperactivation; and disruptions in monoaminergic neurotransmission, synthesis, release, and reuptake (e.g., Dantzer & Capuron 2017, Felger et al. 2007). Within this framework,the metabolic phenotype of MDD is understood as resulting from chronic immune activation, where unchecked inflammatory responses cause a cascade of detrimental effects including increased risk for cardiovascular disorders (i.e., inflammation of the endothelial lining of blood vessels), insulin resistance, and alterations in monoaminergic synthesis. However, metabolic irregularities can precede and actually trigger an inflammatory response in some individuals, as WAT secretes proinflammatory cytokines as signaling molecules (Tilg & Moschen 2006), and insulin resistance has been linked to elevated glucocorticoid secretion (Chan et al. 2005, Werdermann et al. 2021) and altered neurotransmission of monoamines (Kleinridders et al. 2015, Versteeg et al. 2017). Dysfunctional mitochondria can result in depleted energetic availability, which has detrimental effects on signal transduction, and mitochondrial fusion/fission, which could ultimately increase oxidative stress and trigger inflammatory responses (for a review of evidence from rodent literature, see Picard et al. 2015). At the core of these degenerate pathways to MDD lies inefficient allostatic regulation, which itself may take numerous forms that can all give rise to many combinations of the neurobiological and immunological markers of depression that have been well characterized.
In reinterpreting MDD as a metabolic disorder, we are not proposing that the abovementioned immunological and neurobiological frameworks for MDD are incorrect. They may be incomplete, however. Nor are we reducing every feature of depression to metabolic problems. Our goal is to understand depression within a framework for the biology of meaning making, where “meaning” is not an abstract term, but one that is contextualized by the goal of efficient energy expenditure that is at the core of both physiological regulation and psychological experience. By this framework, the heterogeneity in psychiatric biomarker research may not be due to a failure to identify a single, unifying biomarker but rather to the incorrect assumption that a single biomarker exists in the first place, which affects research designs (and, by extension, reported results) by restricting the possible number of biological sources measured in samples of depressed patients, obscuring the full picture at an organism or systemwide level.
Even if the specifics of various hypotheses generated by this framework are incorrect, the constructed mind approach—by virtue of its emphasis on variation and degeneracy, evolution, and domain generality—allows for the flexible interpretation of neurobiological and psychological phenomena within a broader organismic function: allostasis. Accordingly, the mood disruptions observed in both depression and other metabolic disorders can be reinterpreted as arising from disruptions to the predictive processing stream, which affect allostasis and lead to prolonged energetic inefficiencies, as evidenced by the metabolic symptoms of depression and aberrant functional and structural patterns in key limbic cortices that are the neural backbone of allostasis.
5. IMPLICATIONS, HYPOTHESES, AND FUTURE DIRECTIONS
In this review, we have highlighted the utility of the constructed mind approach in generating novel hypotheses about psychiatric disorders such as depression. One of the values of this approach is that it begins with biology and neuroscience and asks questions about the psychological phenomena that arise from those interactions, rather than the other way around. Accordingly, we have proposed that MDD is fundamentally a disorder of allostasis that causes persistent problems in metabolism and energy regulation, supported by evidence that the same psychological symptoms are also present in physical disorders of metabolism and, in turn, that the same metabolic symptomatology is observed in mood disorders like MDD. However, this hypothesis has led us to question the utility of psychological categories, as opposed to processes, for guiding inquiry. We propose that the boundary line that creates categories around mental and physical disorders is a socially constructed one that complicates knowledge accumulation under this category: Metabolic disorders and depression are not comorbidly occurring with one another, nor does one cause another. Instead, they are unified by the same underlying cause, which is a breakdown in the predictive architecture that supports allostatic regulation, and the ensuing consequences of that energetic inefficiency. Future research directions will involve direct experimental manipulations that allow empirical tests of this hypothesis.
CATEGORY FEATURES.
An instance of a category is described according to its features. Features can be physical or psychological in nature. Physical features are those which can be measured objectively (i.e.,are perceiver independent); they include observable changes in a person, such as facial and body movements, voice prosody, changes in nervous system activity, and so forth. Physical features can also describe the surrounding environment, such as wavelengths of light, vibrations in the air, and odorant molecules. Psychological features are a brain’s abstractions that supervene on many different physical features. For example, perceptual features represent neural abstractions of the sensory signals for physical features; wavelengths of photons are perceived as light, vibrations in the air as sound, odorant molecules as smells. Affective features describe the feelings associated with a given instance (Barrett & Bliss-Moreau 2009): Every singular feeling has multiple properties, such as valence, which refers to a spectrum from pleasant to unpleasant, and arousal, which refers to a spectrum from high activation to quiescence. Appraisal features are descriptions of the world as experienced, such as novel, goal conducive, or predictable (Barrett et al. 2007, Clore & Ortony 2008). Functional features are the goals that a person is attempting to meet in each situation, for example, to curry favor, to socially affiliate, or to avoid harm (e.g., Adolphs 2017, Frijda 1986, Lazarus 1993). Temporal features denote the sequence and structure of events that result as the brain segments continuous activity (Zacks & Tversky 2001); the representation of event dynamics drives understanding of intentionality and causality (Kurby & Zacks 2008), and the demarcation of event boundaries is hypothesized to be a key aspect of categorization (Hoemann et al. 2017, Richmond & Zacks 2017).
SUMMARY POINTS.
Major depressive disorder (MDD) has traditionally been understood as a syndrome of symptoms that arise from enhanced emotional reactivity combined with reduced cognitive control, but this hypothesis is rooted in a set of assumptions that have been called into question by recent research in a variety of scientific domains.
The theory of constructed emotion, which is a systems neuroscience approach, offersa different set of hypotheses about the etiology and treatment of depressive symptoms, rooted in the observation that decision making, action planning, and the mental features of experience continually arise in the service of ongoing bodily regulation.
A brain’s most important job is to anticipate the metabolic needs of the body and actto begin to meet those needs before they arise (allostasis), thereby coordinating bodily systems in an energy-efficient manner and providing uninterrupted energy regulation in an ever-changing but only partly predictable world.
The brain achieves allostasis by continually modeling the sensory state of its body (interoception) in the world (exteroception); these high-dimensional features give rise to lower-dimensional, more abstract affective features of consciousness, such as valence, arousal, and effort.
When the brain models the sensory state of the body as having spent metabolic resourcesthat have not yet been replenished, this may be accompanied by momentary distress, fatigue, and a sense of effort.
Any illness involving persistent metabolic disruption resulting from the brain’s predictive modeling of the body and coordination of bodily systems (allostasis), including the coordination between visceromotor and skeletomotor systems, will be accompanied by motor slowing, context insensitivity, and reward insensitivity, as well as affective symptoms such as intense distress, profound fatigue, and lack of motivation (apathy).
Any person diagnosed with a mental illness who is experiencing substantial alteration inmood may be suffering from metabolic dysregulation, stemming from the brain’s predictive modeling of the body.
Any physical illness that involves dysfunction in efficient energy regulation will eventually be accompanied by affective symptoms.
Mental illnesses, such as mood disorders, and physical illnesses, such as heart disease,diabetes, cancer, and even neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease, may share at least one underlying cause (in an interacting web of causes): a breakdown in the anatomical and/or functional predictive architecture that supports efficient allostatic regulation, and the ensuing consequences of the resulting energetic inefficiency.
ACKNOWLEDGMENTS
The authors thank Nate McLaughlin and Francesca Morfini for their assistance in summarizing the neuroimaging and biomarker literature on depression. The writing of this review was supported by funding from the National Cancer Institute (U01CA193632), the National Institute of Mental Health (R01MH113234, R01MH109464), the National Institute on Aging (R56AG058745), the National Science Foundation (BCS1947972), and the Elizabeth R. Koch Foundation (through its Unlikely Collaborators Fund) to L.F.B. and by the US Army Research Institute for the Behavioral and Social Sciences (W911NF-16-1-019) to K.S.Q.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review. The views, opinions, and/or findings contained in this review are those of the authors and shall not be construed as an official Department of the Army position, policy, or decision, unless so designated by other documents; nor do they necessarily reflect the views of the Elizabeth R. Koch Foundation.
LITERATURE CITED
- Abdallah CG, Averill CL, Salas R, Averill LA, Baldwin PR, et al. 2017. Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2(7):566–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. 2017. How should neuroscience study emotions? By distinguishing emotion states, concepts, and experiences. Soc. Cogn. Affect. Neurosci 12(1):24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J, Romay-Tallon R, Brymer KJ, Caruncho HJ, Kalynchuk LE. 2018. Mitochondria and mood: mitochondrial dysfunction as a key player in the manifestation of depression. Front. Neurosci 12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby WR. 1960. Design for a Brain: The Origin of Adaptive Behavior. London: Chapman & Hall [Google Scholar]
- Atzil S, Gao W, Fradkin I, Barrett LF. 2018. Growing a social brain. Nat. Hum. Behav 2(9):624–36 [DOI] [PubMed] [Google Scholar]
- Avery JA, Kerr KL, Ingeholm JE, Burrows K, Bodurka J, Simmons WK. 2015. A common gustatory and interoceptive representation in the human mid-insula. Hum. Brain Mapp 36(8):2996–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal Y, Kuhad A. 2016. Mitochondrial dysfunction in depression. Curr. Neuropharmacol 14(6):610–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär K-J, de la Cruz F, Schumann A, Koehler S, Sauer H, et al. 2016. Functional connectivity and network analysis of midbrain and brainstem nuclei. NeuroImage 134:53–63 [DOI] [PubMed] [Google Scholar]
- Barbas H. 1986. Pattern in the laminar origin of corticocortical connections. J. Comp. Neurol 252(3):415–22 [DOI] [PubMed] [Google Scholar]
- Barbas H. 2015. General cortical and special prefrontal connections: principles from structure to function. Annu. Rev. Neurosci 38:269–89 [DOI] [PubMed] [Google Scholar]
- Barrett LF. 2006. Solving the emotion paradox: categorization and the experience of emotion. Personal. Soc. Psychol. Rev 10(1):20–46 [DOI] [PubMed] [Google Scholar]
- Barrett LF. 2012. Emotions are real. Emotion 12(3):413–29 [DOI] [PubMed] [Google Scholar]
- Barrett LF. 2013. Psychological construction: the Darwinian approach to the science of emotion. Emot. Rev 5(4):379–89 [Google Scholar]
- Barrett LF. 2017a. How Emotions Are Made: The Secret Life of the Brain. London: Palgrave Macmillan [Google Scholar]
- Barrett LF. 2017b. The theory of constructed emotion: an active inference account of interoception and categorization. Soc. Cogn. Affect. Neurosci 12(1):1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Adolphs R, Marsella S, Martinez AM, Pollak SD. 2019. Emotional expressions reconsidered: challenges to inferring emotion from human facial movements. Psychol. Sci. Public Interest 20(1):1–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Bliss-Moreau E. 2009. Affect as a psychological primitive. Adv. Exp. Soc. Psychol 41:167–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Finlay BL. 2018. Concepts, goals, and the control of survival-related behaviors. Curr. Opin. Behav. Sci 24:172–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, Hamilton P. 2016. An active inference theory of allostasis and interoception in depression. Philos. Trans. R. Soc. B 371(1708):20160011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Mesquita B, Ochsner KN, Gross JJ. 2007. The experience of emotion. Annu. Rev. Psychol 58:373–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB. 2013. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol 23(3):361–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK. 2015. Interceptive predictions in the brain. Nat. Rev. Neurosci 16(7):419–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW. 1983. Ad hoc categories. Mem. Cogn 11(3):211–27 [DOI] [PubMed] [Google Scholar]
- Barsalou LW. 2003. Situated simulation in the human conceptual system. Lang. Cogn. Proc 18(5/6):513–62 [Google Scholar]
- Barsalou LW. 2008. Grounded cognition. Annu. Rev. Psychol 59:617–45 [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Simmons KW, Barbey AK, Wilson CD. 2003. Grounding conceptual knowledge in modalityspecific systems. Trends Cogn. Sci 7(2):84–91 [DOI] [PubMed] [Google Scholar]
- Bayer S, Altman J. 2004. Development of the telencephalon neural stem cells, neurogenesis, and neuronal migration. In The Rat Nervous System, ed. Paxinos G, pp. 27–73. San Diego, CA: Elsevier. 3rd ed. [Google Scholar]
- Bechtel W, Bich L. 2021. Grounding cognition: heterarchical control mechanisms in biology. Philos. Trans. R. Soc. B 376(1820):20190751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Frazier CRM, Zhuang X. 2012. Putting desire on a budget: dopamine and energy expenditure, reconciling reward and resources. Front. Integr. Neurosci 6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Davidson G, Niv Y. 2021. A model of mood as integrated advantage. Psychol. Rev In press. 10.1037/rev0000294 [DOI] [PMC free article] [PubMed]
- Berkes P, Orbán G, Lengyel M, Fiser J. 2011. Spontaneous cortical activity reveals hallmarks of an optimal internal model of the environment. Science 331(6013):83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra de Pontes AL, Engelberth RCGJ, da Silva Nascimento E Jr., Cavalcante JC, de Oliveira Costa MSM, et al. 2010. Serotonin and circadian rhythms. Psychol. Neurosci 3(2):217–28 [Google Scholar]
- Blessing WW.1997.Inadequate frameworks for understanding bodily homeostasis.Trends Neurosci.20(6):235–39 [DOI] [PubMed] [Google Scholar]
- Brand JG, Cagan RH, Naim M. 1982. Chemical senses in the release of gastric and pancreatic secretions. Annu. Rev. Nutr 2:249–76 [DOI] [PubMed] [Google Scholar]
- Buckner RL. 2012. The serendipitous discovery of the brain’s default network. NeuroImage 62(2):1137–45 [DOI] [PubMed] [Google Scholar]
- Cacciola A,Bertino S, Basile GA,Di Mauro D,Calamuneri A,et al.2019.Mapping the structural connectivity between the periaqueductal gray and the cerebellum in humans. Brain Struct. Funct 224(6):2153–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X,Padoa-Schioppa C.2012.Neuronal encoding of subjective value in dorsal and ventral anterior cingulate cortex. J. Neurosci 32(11):3791–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. 1989. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J. Comp. Neurol 287(4):393–421 [DOI] [PubMed] [Google Scholar]
- Cavalieri M, Ropele S, Petrovic K, Pluta-Fuerst A, Homayoon N, et al. 2010. Metabolic syndrome, brain magnetic resonance imaging, and cognition. Diabetes Care 33(12):2489–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan O, Inouye K, Akirav E, Park E, Riddell MC, et al. 2005. Insulin alone increases hypothalamo-pituitaryadrenal activity, and diabetes lowers peak stress responses. Endocrinology 146(3):1382–90 [DOI] [PubMed] [Google Scholar]
- Chanes L, Barrett LF. 2016. Redefining the role of limbic areas in cortical processing. Trends Cogn. Sci 20(2):96–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clore GL, Clore GL, Ortony A. 2008. Appraisal theories: how cognition shapes affect into emotion. In Handbook of Emotions, ed. Lewis M, Haviland-Jones JM, Barrett LF, pp. 628–42. New York: Guilford. 3rd ed. [Google Scholar]
- Cohen AH.1992.The role of heterarchical control in the evolution of central pattern generators. Brain Behav. Evol 40(2/3):112–24 [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, Weber B. 2008. Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. NeuroImage 39(3):1396–407 [DOI] [PubMed] [Google Scholar]
- Conant RC, Ashby WR. 1970. Every good regulator of a system must be a model of that system. Int. J. Syst. Sci 1(2):89–97 [Google Scholar]
- Cowen AS, Keltner D. 2017. Self-report captures 27 distinct categories of emotion bridged by continuous gradients. PNAS 114(38):E7900–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig ADB. 2003. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol 13(4):500–5 [DOI] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, et al. 2014. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137(Part 8):2382–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Capuron L, eds. 2017. Inflammation-Associated Depression: Evidence, Mechanisms and Implications. Cham, Switz.: Springer [Google Scholar]
- Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. 2014. The neuroimmune basis of fatigue. Trends Neurosci. 37(1):39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denève S. 2008. Bayesian spiking neurons. I: Inference. Neural Comput. 20(1):91–117 [DOI] [PubMed] [Google Scholar]
- Denève S, Alemi A, Bourdoukan R. 2017. The brain as an efficient and robust adaptive learner. Neuron 94(5):969–77 [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. 2008. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat. Rev. Neurosci 9(10):788–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG,Beevers CG,Haigh EAP,Beck AT.2011.Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci 12(8):467–77 [DOI] [PubMed] [Google Scholar]
- Edelman GM, Gally JA. 2001. Degeneracy and complexity in biological systems. PNAS 98(24):13763–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose SA, Meyer PM, Powell LH, Pandey D, Torrens JI, et al. 2004. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care 27(12):2856–62 [DOI] [PubMed] [Google Scholar]
- Fattal O, Link J, Quinn K, Cohen BH, Franco K.2007. Psychiatric comorbidity in 36 adults with mitochondrial cytopathies. CNS Spectr. 12(6):429–38 [DOI] [PubMed] [Google Scholar]
- Feillet CA. 2010. Food for thoughts: feeding time and hormonal secretion. J. Neuroendocrinol 22(6):620–28 [DOI] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, et al. 2007. Effects of interferon-α on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol. Psychiatry 62(11):1324–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandino L, Binder JR, Desai RH, Pendl SL, Humphries CJ, et al. 2016. Concept representation reflects multimodal abstraction: a framework for embodied semantics. Cereb. Cortex 26(5):2018–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried E. 2017. Moving forward: how depression heterogeneity hinders progress in treatment and research. Expert Rev. Neurother 17(5):423–25 [DOI] [PubMed] [Google Scholar]
- Frijda NH. 1986. The Emotions. Cambridge, UK: Cambridge Univ. Press [Google Scholar]
- Friston K. 2003. Learning and inference in the brain. Neural Netw. 16(9):1325–52 [DOI] [PubMed] [Google Scholar]
- Friston K. 2010. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci 11(2):127–38 [DOI] [PubMed] [Google Scholar]
- Gans ROB. 2006. The metabolic syndrome, depression, and cardiovascular disease: interrelated conditions that share pathophysiologic mechanisms. Med. Clin. N. Am 90(4):573–91 [DOI] [PubMed] [Google Scholar]
- García-Cabezas MÁ, Zikopoulos B, Barbas H. 2019. The structural model: a theory linking connections, plasticity, pathology, development and evolution of the cerebral cortex. Brain Struct. Funct 224(3):985–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee H. 2018. Across the Bridge: Understanding the Origin of the Vertebrates. Chicago: Univ. Chicago Press. Illus. ed. [Google Scholar]
- Gendron M, Mesquita B, Barrett LF. 2020. The brain as a cultural artifact. In Culture, Mind, and Brain: Emerging Concepts, Models, and Applications, ed. Kirmayer LJ, Worthman CM, Kitayama S, Lemelson R, Cummings CA, pp. 188–222. New York: Cambridge Univ. Press [Google Scholar]
- Goldberg D. 2011. The heterogeneity of “major depression.” World Psychiatry 10(3):226–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. 2003. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat 26(4):317–30 [DOI] [PubMed] [Google Scholar]
- Hasler G,van der Veen JW,Tumonis T,Meyers N,Shen J,Drevets WC.2007.Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 64(2):193–200 [DOI] [PubMed] [Google Scholar]
- Hassabis D,Maguire EA.2009.Theconstruction system of the brain.Philos. Trans. R. Soc. B 364(1521):1263–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse TM,Porter JH.2015.A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp. Clin. Psychopharmacol 23(1):1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoemann K, Gendron M, Barrett LF. 2017. Mixed emotions in the predictive brain. Curr. Opin. Behav. Sci 15:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoemann K, Khan Z, Feldman MJ, Nielson C, Devlin M, et al. 2020. Context-aware experience sampling reveals the scale of variation in affective experience. Sci. Rep 10:12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hroudová J, Fišar Z, Kitzlerová E, Zvěřová M, Raboch J. 2013. Mitochondrial respiration in blood plateletš of depressive patients. Mitochondrion 13(6):795–800 [DOI] [PubMed] [Google Scholar]
- Hutchinson JB,Barrett LF.2019.The power of predictions: an emerging paradigm for psychological research. Curr. Dir. Psychol. Sci 28(3):280–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Amaral DG. 2012. Hippocampal formation. In The Human Nervous System, ed. Mai JK, Paxinos G, pp. 896–942. Amsterdam: Elsevier. 3rd ed. [Google Scholar]
- Kan C, Silva N, Golden SH, Rajala U, Timonen M, et al. 2013. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care 36(2):480–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Komura Y, Shipp S, Friston K. 2015. Cerebral hierarchies: predictive processing, precision and the pulvinar. Philos. Trans. R. Soc. B 370(1668):20140169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. 2009. Preaching to the converted? From constructivism to neuroconstructivism. Child Dev. Perspect 3(2):99–102 [Google Scholar]
- Katsumi Y, Kamona N, Zhang J, Bunce JG, Hutchinson JB, et al. 2021. Functional connectivity gradients as a common neural architecture for predictive processing in the human brain. bioRxiv 456844. 10.1101/2021.09.01.456844 [DOI]
- Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, Blazer DG. 1996. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US national comorbidity survey. Br. J. Psychiatry 168(Suppl. 30):17–30 [PubMed] [Google Scholar]
- Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, et al. 2017. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat. Hum. Behav 1(5):0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A, Cai W, Cappellucci L, Ghazarian A, Collins WR, et al. 2015. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. PNAS 112(11):3463–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. 2008. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. NeuroImage 42(2):998–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Wittmann MK, Behrens TEJ, Boorman ED, Mars RB, Rushworth MFS. 2016. Value, search, persistence, and model updating in anterior cingulate cortex. Nat. Neurosci 19(10):1280–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren T, Yifa R, Amer M, Krot M, Boshnak N, et al. 2021.Insular cortex neurons encode and retrieve specific immune responses. Cell 184(24):5902–15.e17 [DOI] [PubMed] [Google Scholar]
- Kragel PA, Bianciardi M, Hartley L, Matthewson G, Choi J-K, et al. 2019. Functional involvement of human periaqueductal gray and other midbrain nuclei in cognitive control. J. Neurosci 39(31):6180–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Hassabis D, McClelland JL. 2016. What learning systems do intelligent agents need? Complementary learning systems theory updated. Trends Cogn. Sci 20(7):512–34 [DOI] [PubMed] [Google Scholar]
- Kurby CA, Zacks JM.2008.Segmentation in the perception and memory of events.Trends Cogn. Sci 12(2):72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS.1993.Frompsychologicalstresstotheemotions:ahistoryofchangingoutlooks.Annu. Rev. Psychol 44:1–228434890 [Google Scholar]
- Lebrecht S, Bar M, Barrett L, Tarr M. 2012. Micro-valences: perceiving affective valence in everyday objects. Front. Psychol 3:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire LA, Cao C, Yoon PH, Long J, Levine M. 2021. The hypothalamus predates the origin of vertebrates. Sci. Adv 7(18):eabf7452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ma T, Hoemann K, Lyons SH, Fugate JMB, Brown EN, et al. 2021. Professional actors demonstrate variability,not stereotypical expressions,when portraying emotional states in photographs.Nat. Commun 12:5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE, Wegener G. 2020. Inflammation, insulin resistance and neuroprogression in depression. Acta Neuropsychiatr. 32(1):1–9 [DOI] [PubMed] [Google Scholar]
- Li B-J,Friston K,Mody M,Wang H-N,Lu H-B,Hu D-W.2018.A brain network model for depression: from symptom understanding to disease intervention. CNS Neurosci. Ther 24(11):1004–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, et al. 2013. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. PNAS 110(24):9950–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF. 2016. The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb. Cortex 26(5):1910–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. 2011. Common and distinct networks underlying reward valence and processingstages:a meta-analysis offunctional neuroimagingstudies.Neurosci. Biobehav. Rev 35(5):1219–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. 2000. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 23(7):934–42 [DOI] [PubMed] [Google Scholar]
- Lyra e Silva ND,Lam MP,Soares CN,Munoz DP,Milev R,De Felice FG.2019.Insulin resistance as a shared pathogenic mechanism between depression and type 2 diabetes. Front. Psychiatry 10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Berk M, Goehler L, Song C, Anderson G, et al. 2012. Depression and sickness behavior are Janusfaced responses to shared inflammatory pathways. BMC Med. 10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareschal D,Johnson MH,Sirois S,Spratling MW,Thomas MSC,Westermann G.2007.Neuroconstructivism: How the Brain Constructs Cognition. Oxford, UK: Oxford Univ. Press; [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Guest PC, Harris LW, Vanattou-Saifoudine N, Webster MJ, et al. 2012. Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl. Psychiatry 2:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr E. 1974. Teleological and teleonomic, a new analysis. In Methodological and Historical Essays in the Natural and Social Sciences, ed. RS Cohen MW Wartofsky, pp. 91–117. Berlin: Springer [Google Scholar]
- McIntyre RS, Soczynska JK, Konarski JZ, Woldeyohannes HO, Law CWY, et al. 2007. Should depressive syndromes be reclassified as “Metabolic Syndrome Type II”? Ann. Clin. Psychiatry 19(4):257–64 [DOI] [PubMed] [Google Scholar]
- McKellar S, Loewy AD. 1981. Organization of some brain stem afferents to the paraventricular nucleus of the hypothalamus in the rat. Brain Res. 217(2):351–57 [DOI] [PubMed] [Google Scholar]
- Morán MA, Mufson EJ, Mesulam MM. 1987. Neural inputs into the temporopolar cortex of the rhesus monkey. J. Comp. Neurol 256(1):88–103 [DOI] [PubMed] [Google Scholar]
- Müller GB. 2017. Why an extended evolutionary synthesis is necessary. Interface Focus 7(5):20170015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Cidlowski JA. 2013. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J. Allergy Clin. Immunol 132(5):1033–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, et al. 2009. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol. Sci 20(11):1322–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. 2012. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem. Res 37(11):2496–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onat F, Çavdar S. 2003. Cerebellar connections: hypothalamus. Cerebellum 2(4):263–69 [DOI] [PubMed] [Google Scholar]
- Osgood CE,Miron MS,May WH.1975.Cross-Cultural Universals of Affective Meaning.Urbana: Univ. Ill. Press [Google Scholar]
- Ottowitz WE, Dougherty DD, Savage CR. 2002. The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harv. Rev. Psychiatry 10(2):86–99 [DOI] [PubMed] [Google Scholar]
- Papez JW. 1937. A proposed mechanism of emotion. Arch. Neurol. Psychiatry 38(4):725–43 [Google Scholar]
- Parent A. 1984. Functional anatomy and evolution of monoaminergic systems. Am. Zool 24(3):783–90 [Google Scholar]
- Paus T. 2001. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci 2(6):417–24 [DOI] [PubMed] [Google Scholar]
- Pavlov IP, Thompson WH. 1910. The Work of the Digestive Glands. London: C. Griffin [Google Scholar]
- Penninx BWJH, Lamers F, Milaneschi Y. 2018. Clinical heterogeneity in major depressive disorder. Eur. Neuropsychopharmacol 28(Suppl.):59–60 [Google Scholar]
- Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, et al. 2015. Mitochondrial functions modulate neuroendocrine, metabolic, inflammatory, and transcriptional responses to acute psychological stress. PNAS 112(48):E6614–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontzer H. 2015. Energy expenditure in humans and other primates: a new synthesis. Annu. Rev. Anthropol 44:169–87 [Google Scholar]
- Powley TL. 2000. Vagal input to the enteric nervous system. Gut 47(4):30–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. 2010. Neurocircuitry of mood disorders. Neuropsychopharmacology 35(1):192–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Norgren R. 2000. Projections of the parabrachial nucleus in the old world monkey. Exp. Neurol 165(1):101–17 [DOI] [PubMed] [Google Scholar]
- Quigley KS, Kanoski S, Grill WM, Barrett LF, Tsakiris M. 2021. Functions of interoception: from energy regulation to experience of the self. Trends Neurosci. 44(1):29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeder MB, Bjelland I, Emil Vollset S, Steen VM. 2006. Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: the Hordaland Health Study. J. Clin. Psychiatry 67(12):1974–82 [DOI] [PubMed] [Google Scholar]
- Richmond LL,Zacks JM.2017.Constructing experience: event models from perception to action.Trends Cogn. Sci 21(12):962–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, et al. 2014. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol. Psychiatry 76(12):963–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. 1996. Premotor cortex and the recognition of motor actions. Brain Res. Cogn 3(2):131–41 [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. 1979. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 179(1):3–20 [DOI] [PubMed] [Google Scholar]
- Rolls ET. 2015. Limbic systems for emotion and for memory, but no single limbic system. Cortex 62:119–57 [DOI] [PubMed] [Google Scholar]
- Rolls ET. 2019. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct 224(9):3001–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA. 1991. In defense of a prototype approach to emotion concepts. J. Personal. Soc. Psychol 60:37–47 [Google Scholar]
- Saper CB. 2002. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci 25:433–69 [DOI] [PubMed] [Google Scholar]
- Schiffer JA, Servello FA, Heath WR, Amrit FRG, Stumbur SV, et al. 2020. Caenorhabditis elegans processes sensory information to choose between freeloading and self-defense strategies. eLife 9:e56186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TL, Nihalani N, Jindal S, Virk S, Jones N. 2004. Psychiatric medication-induced obesity: a review. Obes. Rev 5(2):115–21 [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. 1994. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. J. Comp. Neurol 343(3):445–63 [DOI] [PubMed] [Google Scholar]
- Sennesh E,Theriault J,Brooks D,van de Meent JW,Barrett LF,Quigley KS 2022. Interoception as modeling, allostasis as control. Biol. Psychol 167:108242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S, Adams RA, Friston KJ. 2013. Reflections on agranular architecture: predictive coding in the motor cortex. Trends Neurosci. 36(12):706–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel EH, Sands MK, Van den Noortgate W, Condon P, Chang Y, et al. 2018. Emotion fingerprints or emotion populations? A meta-analytic investigation of autonomic features of emotion categories. Psychol. Bull 144(4):343–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Leslie SM, Packer MM, Zaiko YV, Phillips OR, et al. 2019. Brain and behavioral correlates of insulin resistance in youth with depression and obesity. Horm. Behav 108:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P. 2012. Allostasis: a model of predictive regulation. Physiol. Behav 106(1):5–15 [DOI] [PubMed] [Google Scholar]
- Sterling P, Laughlin S. 2015. Principles of Neural Design. Cambridge, MA: MIT Press [Google Scholar]
- Striedter GF, Northcutt RG. 2020. Brains Through Time: A Natural History of Vertebrates. Oxford, UK: Oxford Univ. [Google Scholar]
- Suarez AN, Liu CM, Cortella AM, Noble EE, Kanoski SE. 2020. Ghrelin and orexin interact to increase meal size through a descending hippocampus to hindbrain signaling pathway. Biol. Psychiatry 87(11):1001–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. 2006. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol 6(10):772–83 [DOI] [PubMed] [Google Scholar]
- Tingley D, Buzsáki G. 2018. Transformation of a spatial map across the hippocampal-lateral septal circuit. Neuron 98(6):1229–42.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger JW, Livingston JN, Moss AM. 1991. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog. Neurobiol 36(5):343–62 [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. 2013. Network hubs in the human brain. Trends Cogn. Sci 17(12):683–96 [DOI] [PubMed] [Google Scholar]
- Versteeg RI,Koopman KE,Booij J,Ackermans MT,Unmehopa UA,et al.2017.Serotonin transporter binding in the diencephalon is reduced in insulin-resistant obese humans. Neuroendocrinology 105(2):141–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Kang J, Johnson TD, Nichols TE, Satpute AB, Barrett LF. 2015. A Bayesian model of categoryspecific emotional brain responses. PLOS Comput. Biol 11(4):e1004066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdermann M,Berger I,Scriba LD,Santambrogio A,Schlinkert P,et al.2021.Insulin and obesity transform hypothalamic-pituitary-adrenal axis stemness and function in a hyperactive state. Mol. Metab 43:101112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ. 1998. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr. Dir. Psychol. Sci 7(6):177–88 [Google Scholar]
- Witham E, Comunian C, Ratanpal H, Skora S, Zimmer M, Srinivasan S. 2016. C. elegans body cavity neurons are homeostatic sensors that integrate fluctuations in oxygen availability and internal nutrient reserves. Cell Rep. 14(7):1641–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabut JM, Crane JD, Green AE, Keating DJ, Khan WI, Steinberg GR. 2019. Emerging roles for serotonin in regulating metabolism: new implications for an ancient molecule. Endocr. Rev 40(4):1092–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Tversky B. 2001. Event structure in perception and conception. Psychol. Bull 127(1):3–21 [DOI] [PubMed] [Google Scholar]
- Zhang J, Abiose O, Katsumi Y, Touroutoglou A, Dickerson BC, Barrett LF. 2019. Intrinsic functional connectivity is organized as three interdependent gradients. Sci. Rep 9:15976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Scholtens LH, Wei Y, van den Heuvel MP, Chanes L, Barrett LF. 2020. Topography impacts topology: Anatomically central areas exhibit a “high-level connector”profile in the human cortex.Cereb. Cortex 30(3):1357–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo C, Li G, Lin X, Jiang D, Xu Y, et al. 2019. The rise and fall of MRI studies in major depressive disorder. Transl. Psychiatry 9:335. [DOI] [PMC free article] [PubMed] [Google Scholar]