Abstract
Introduction
Active disease in inflammatory bowel disease patients during pregnancy is associated with poor maternal and fetal outcomes. Objective evaluation of disease activity is a core strategy in IBD, and during pregnancy noninvasive modalities are preferred. We aimed to evaluate feasibility and accuracy of intestinal ultrasound (IUS) to objectify disease activity throughout pregnancy.
Methods
Pregnant patients with known IBD were included and followed throughout pregnancy for clinical disease activity, with fecal calprotectin (FCP) and with IUS every trimester. Feasibility of IUS was assessed for all colonic segments and terminal ileum (TI). Intestinal ultrasound outcomes to detect active disease and treatment response were compared with clinical scores combined with FCP.
Results
In total, 38 patients (22 CD, 16 UC) were included, with 27 patients having serial IUS. Feasibility of IUS decreases significantly in third trimester for TI (first vs third trimester: 91.3% vs 21.7%, P < .0001) and sigmoid (first vs third trimester: 95.6% vs 69.5%, P = .023). Intestinal ultrasound activity showed moderate to strong correlation with clinical activity (r = 0.60, P < .0001) and FCP (r = 0.73, P < .0001). Throughout pregnancy, IUS distinguished active from quiescent disease with 84% sensitivity and 98% specificity according to FCP combined with clinical activity. IUS showed disease activity in >1 segment in 52% of patients and detected treatment response with 80% sensitivity and 92% specificity.
Conclusions
IUS is feasible and accurate throughout pregnancy, although visualization of the sigmoid and TI decreases in the third trimester. IUS provides objective information on disease activity, extent, and treatment response, even during second and third trimester, and offers a noninvasive strategy to closely monitor patients during pregnancy.
Keywords: inflammatory bowel disease, pregnancy, intestinal ultrasound, noninvasive treatment monitoring
Introduction
Inflammatory bowel disease (IBD) frequently affects women in their fertile years. Active disease during pregnancy is associated with unfavorable outcomes such as persistent active disease throughout pregnancy, preterm delivery, low birthweight, and the baby being small for gestational age. Therefore, obtaining and maintaining remission is a key strategy to improve both maternal and fetal outcomes.1–3
In the nonpregnant IBD patient, disease activity is closely monitored with clinical scores, biochemical parameters, cross-sectional imaging modalities, and endoscopy.2 However during pregnancy, clinical activity indices become even less reliable as symptoms might also be pregnancy-related.4,5 In addition, biochemical parameters such as C-reactive protein (CRP), hemoglobin and albumin are known to fluctuate throughout pregnancy and are not accurate to determine disease activity.4–6 Fecal calprotectin (FCP) is noninvasive and more accurate to monitor disease activity during pregnancy. However, it fails to provide information on disease extent, location, or complications.4,7,8 Other objective measures such as endoscopy and magnetic resonance imaging are accurate but invasive or provide less accurate depiction of the large bowel9,10 and, hence, are less attractive in a pregnant patient, especially for close monitoring.2,3 Consequently, there is a lack of objective measures to determine and closely monitor disease activity in pregnant IBD patients.
Intestinal ultrasound (IUS) is a noninvasive, accurate, low-cost cross-sectional imaging modality to determine disease activity, disease extent, and complications in the colon and small bowel in the nonpregnant IBD patient.10–15 Furthermore, it allows frequent use to closely monitor disease activity and determine treatment response.12,16,17 Although there is accumulating data in nonpregnant IBD patients, data on feasibility and accuracy during pregnancy are scarce.18,19 Additionally, accuracy of IUS to detect treatment response in pregnant IBD patients remains unresolved.
In this study, we aimed to follow IBD patients throughout their pregnancy with regular IUS in each trimester to determine feasibility and accuracy when evaluated against a composite standard of clinical activity and fecal calprotectin.
Methods
This was a longitudinal prospective cohort study. All pregnant patients 18 years of age and older with IBD, visiting the IBD pregnancy clinic in our center between October 2018 and December 2019, were eligible for inclusion. Patients with previous pouch surgery were excluded. Patients visited the clinic every trimester as part of routine care. Clinical scores, serum and fecal inflammatory parameters, and IUS were performed at every visit. All patients gave informed consent. This study was assessed and approved by the medical ethical committee of the Amsterdam University Medical Center.
Procedures
For all patients, medical history, demographic data, disease phenotype, and current medical treatment were collected. At every visit, the Harvey-Bradshaw Index (HBI) and Simple Clinical Colitis Activity Index (SCCAI) were documented. Furthermore CRP, albumin, leukocytes, thrombocytes, hemoglobin, and FCP levels were registered. All clinical and biochemical measurements were performed within 7 days from IUS.
Intestinal Ultrasound
Intestinal ultrasound was performed at every visit by 3 ultrasonographers (F.V., K.G., and E.W.) with a Philips EPIQ 5G machine with a convex 5-1, linear 5-12, and linear 4-18 probe. All ultrasonographers were trained in an international curriculum for IUS prior to study commencement. After the procedure, all cine-loops and IUS parameters were scored by 1 ultrasonographer (F.V.). Patients were not fasting and did not receive bowel preparation.
First, the size of the uterus was measured along the longitudinal and cross-sectional axis. The bowel was evaluated following a standardized approach. The rectum was visualized with the convex probe in cross-sectional and longitudinal plane. Subsequently, using the linear probe, the sigmoid colon was identified as the colonic segment crossing the left iliopsoas and iliac vessels. Next, the descending colon, transverse colon, ascending colon, and terminal ileum (TI) were evaluated. Feasibility was scored per segment and was scored as feasible when (1) the bowel segment could be identified; (2) the lumen could be identified; (3) the anterior wall could be identified; and (4) a bowel wall thickness (BWT) measurement could be performed. Bowel wall thickness was measured from the lumen-mucosa interface to the muscularis propria-serosa interface, and the average of 2 measurements in a longitudinal and cross-sectional plane was used. Color Doppler Intensity (CDI) was scored according to a modified Limberg score (Table 1).11,14 Wall layer stratification, fatty wrapping, and preserved haustration were scored as absent or present (Table 1).
Table 1.
Intestinal Ultrasound Parameters and Cut-off Values Per Parameter
| IUS parameter | Technique/categories | Pathologic |
|---|---|---|
| BWT | (1x longitudinal plane + 1 x cross-sectional plane)/2 | BWT >2.0 mm (ileum), BWT >3.0 mm (colon), BWT >5.0 mm (rectum) |
| Color Doppler Intensity | 0: absent; | Grade ≥2 |
| 1: small spots (single vessels) within the wall | ||
| 2: long stretches within the wall | ||
| 3: long stretches extending into the mesentery | ||
| Wall layer stratification | 0: preserved | Grade 1 |
| 1: loss | ||
| Loss of haustration | 0: preserved | Grade 1 |
| 1: loss | ||
| Fatty wrapping | 0: absent | Grade 1 |
| 1: present |
Abbreviations: IUS, intestinal ultrasound; BWT, bowel wall thickness).
Definitions
A composite reference standard was created to define disease activity, primarily relying on FCP. If FCP was ≥250 µg/g (in the absence of other underlying causes such as infections), the disease was considered active, and FCP <100 µg/g was considered inactive disease for both CD and UC. When FCP was <250 µg/g but ≥100 µg/g, clinical disease activity HBI score (in CD) or SCCAI (in UC) was used to determine disease activity, with HBI ≥4 and SCCAI ≥3 reflecting disease activity.20,21 For IUS, active disease was defined as a BWT >2.0 mm for the TI, >3.0 mm for the colonic segments, and ≥1 for abnormal IUS features (Table 1).22 For the rectum, a BWT >5.0 mm was defined as active disease. Intestinal ultrasound remission was defined as a normal BWT in all visualized segments (BWT ≤2.0 mm for TI and ≤3.0 mm for the colon), with the absence of any other IUS features indicating active disease (Table 1).
Statistical Analysis
All data were reported as means with standard deviation (SD) or proportions of the total cohort. Dichotomous data were compared using a χ 2 test. Accuracy was reported as sensitivity, specificity, and overall accuracy. Continuous data were compared using an independent t test when equally distributed. For not equally distributed data, the Mann-Whitney U test was applied. For continuous data among more than 2 groups, a Kruskal-Wallis test was used. Correlation was analysed with a Spearman rank correlation coefficient and agreement between IUS, and the reference standard to determine disease activity was assessed using Cohen kappa statistics.23,24 A P value of ≤0.05 was considered statistically significant. All data were analyzed using SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA).
Results
A total of 38 patients were included, and 76 IUS examinations were performed. One IUS examination was performed in 11 patients, 16 patients underwent 2 IUS examinations, and 11 patients underwent 3 IUS examinations in different trimesters during follow-up in pregnancy. One patient had a miscarriage during the first trimester. In 6 patients (16%), clinical active disease was present while becoming pregnant (Table 2). In addition, 8 patients (21%) relapsed during the first trimester, and 4 (11%) relapsed during the second or third trimester based on evaluation with the reference standard. Conversely, 10 out of 18 patients (56%) reached remission during pregnancy while having active disease in first and/or second trimester.
Table 2.
Baseline characteristics
| Age (years; mean ± SD) | 29.87 ± 4.96 |
|---|---|
| Disease (number of patients/percentages) | |
| Crohn’s Disease | 22 (58%) |
| Ileal (L1) | 4 (18%) |
| Colonic (L2) | 5 (23%) |
| Ileocolonic (L3) | 13 (59%) |
| Ulcerative colitis | 16 (42%) |
| Proctitis (E1) | 3 (19%) |
| Left-sided (E2) | 7 (44%) |
| Pancolitis (E3) | 6 (37%) |
| Previous surgery for Crohn’s Disease | |
| Treatment at baseline | 7 (32%) |
| No medication | 5 (13%) |
| Corticosteroids | 2 (5%) |
| Aminosalicylates | 12 (32%) |
| Thiopurines | 11 (29%) |
| Anti-TNF-α | 15 (39%) |
| Vedolizumab | 4 (11%) |
| Ustekinumab | 3 (7.9%) |
| Clinical remission at start of pregnancy (HBI < 4 or SCCAI < 3) |
32 (84%) |
| Weeks pregnant at baseline (median and range) | 11 (5–33) |
Abbreviations: SD, standard deviation; IBD, inflammatory bowel disease; TNF-α, tumor necrosis factor; HBI, Harvey-Bradshaw Index; SCCAI, Simple Clinical Colitis Activity Index.
Feasibility of IUS
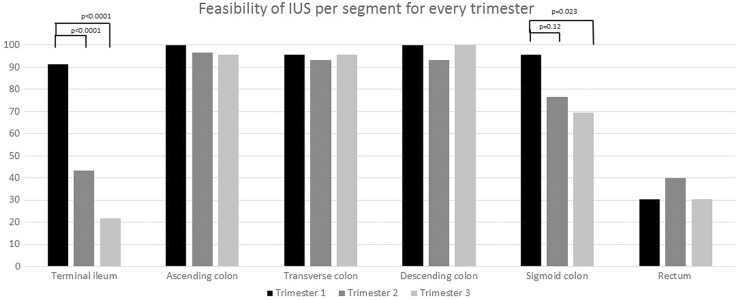
A total of 23 patients (12 CD, 11 UC) underwent an IUS in the first trimester, 30 (16 CD, 14 UC) in the second trimester, and 23 (12, CD, 11 UC) in the third trimester. All 3 patients with inadequate visibility of the bowel segments in the first trimester had a body mass index (BMI) ≥29.5. A significant decrease in feasibility was found in the second and third trimester for the terminal ileum (first trimester 91.3 % vs second trimester 43.3%, vs third trimester 21.7%; P < .0001) and third trimester for the sigmoid colon (first trimester trimester 95.6% vs second trimester 76.7%, P = 0.12; and first trimester 95.6% vs third trimester 69.5%, P = .023; Fig. 1). In addition, the mean size of the uterus was significantly lower when IUS was feasible for the terminal ileum (12.59 ± 6.89 cm vs 18.12 ± 5.30 cm, P = .001) and sigmoid colon (14.46 ± 6.52 cm vs 20.41 ± 5.49 cm, P = .024) but not for the other segments. The mean size of the uterus highly correlated with progression of pregnancy in weeks (r = 0.906, P < .0001), and pregnancy duration was significantly lower when the terminal ileum (15.21 ± 7.06 weeks vs 26.00 ± 8.32 weeks, P < .0001) and sigmoid colon (19.26 ± 9.64 weeks vs 25.29 ± 6.62 weeks, P = .009) were visible.
Figure 1.
Feasibility per trimester per segment.
Accuracy of IUS
For 68 out of 76 (89%) IUS examinations (37 CD, 31 UC), corresponding FCP values and clinical scores were available (first trimester, 22; second trimester, 26; third trimester, 20). Intestinal ultrasound disease activity and disease activity according to the reference standard showed almost perfect agreement (κ = 0.84, P < .0001). To determine disease activity throughout pregnancy, IUS showed a 92% (95% CI, 82.0%–99.8%) accuracy with 84% and 98% sensitivity and specificity, respectively (Table 3; AUROC, 0.926; 95% CI, 0.844–1.000, P < .0001). For CD and UC, accuracy was 94% and 89%, respectively (CD AUROC, 0.939; 95% CI, 0.842–1.000, P < .0001; AUROC UC, 0.889; 95% CI, 0.723–1.000, P = .001). Accuracy per trimester is shown in Table 4 and in Supplementary Table 1. Two of 3 patients with a known proctitis in medical history and active disease according to the reference standard had quiescent disease on IUS.
Table 3.
Disease activity on IUS vs reference standard
| Active disease according to reference standard | Quiescent disease according to reference standard | Total | |
|---|---|---|---|
| Active disease on IUS | 21 | 1 | 22 |
| Quiescent disease on IUS | 4 | 42 | 46 |
| Total | 25 | 43 | 68 |
Active disease is fecal calprotectin ≥250 µg/g or fecal calprotectin ≥100 µg/g and HBI ≥4/SCCAI ≥3
Table 4.
Sensitivity and specificity per trimester for disease activity on IUS vs reference standard
| Trimester 1 (n = 22) | Trimester 2 (n = 23) | Trimester 3 (n = 20) | |
|---|---|---|---|
| Sensitivity | 83.3% | 92.3% | 66.6% |
| Specificity | 93.8% | 100% | 100% |
| AUROC (95% CI) | 0.891 (0.702–1.000), P = .006 | 0.923 (0.802–1.000), P < .0001 | 0.813 (0.591–1.000), P = .021 |
Active disease defined as fecal calprotectin ≥250 µg/g or fecal calprotectin ≥100 µg/g and HBI ≥4/SCCAI ≥3
Furthermore, disease activity on IUS correlated moderately with clinical disease activity scores (ρ = 0.60, P < .0001), strongly with FCP (ρ = 0.73, P < .0001) and poorly with hemoglobin (ρ = −0.27, P = .031). Other biochemical parameters did not show a significant correlation. Fecal calprotectin levels were significantly increased in those patients with active disease on IUS compared with quiescent disease on IUS (mean 927.21 ± 1133.6 mg/g vs 72.73 ± 131.53 mg/g, P = .001).
Disease Activity on IUS
In 16 patients, 21 IUS examinations showed active disease, and 65% of these patients were known to have CD. In the most affected segment, mean BWT was 5.07 mm ± 0.96 mm. In addition, 53% had increased CDS, 25% had loss of WLS, 35% showed the presence of fatty wrapping, and 76.5% had loss of haustrations when the colon was affected. In 52% of the IUS examinations, multiple segments were affected with, the sigmoid colon being the most affected segment in 41% of examinations.
Treatment Decision After IUS
Treatment decisions were documented after each of the 76 IUS examinations. In 13 cases (7 CD, 6 UC), treatment was escalated based on active disease on IUS (Table 5), and in 9 cases (6 CD, 3 UC), current treatment was continued as clinical scores or FCP did not deteriorate (n = 7) or was interpreted as fibrosis on IUS (n = 2). In 54 cases, IUS did not show disease activity. In 4 of these cases (2 CD, 2 UC), disease activity was present according to the reference standard; the 2 UC patients had increase of symptoms and increase of FCP, consequently escalating treatment with rectal treatment for proctitis. The other 2 cases continued the current treatment, as no deterioration of disease was found according to clinical scores and FCP.
Table 5.
Reason for treatment escalation after regular follow-up
| Patient | Age | Week of Pregnancy | Montreal Classification Disease | Clinical rRmission at start pregnancy or previous visit during pregnancy | Maintenance Treatment Prior to Pregnancy | HBI/SCCAI | FCP (µg/g) | IUS Findings | Most Affected Segment | Treatment Decision |
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 29 | 9 | CD L3 | No | No treatment | 11 | 1400 | Disease activity in TI, transverse and descending colon | TI | Start prednisone |
| 1b | 29 | 14 | CD L3 | No | No treatment | 5 | 1100 | No disease activity in colon, TI still present disease activity | TI | Taper Prednisone, start Azathioprine 75 mg |
| 2 | 27 | 11 | UC E2 | Yes | Oral mesalazine | 7 | 751 | Disease activity in sigmoid, rectum not visualized | Sigmoid | Start beclomethasone enema |
| 3 | 30 | 9 | CD L3 | Yes | Oral mesalazine and mercaptopurine | 7 | 2270 | Disease activity in, descendens and sigmoid colon. Normal TI | Colon descendens | Start infliximab |
| 4 | 31 | 22 | CD L1 | No | Infliximab every 6 weeks | 2 | 1593 | Disease activity in TI | TI | Intensify infliximab to every 4 weeks |
| 5 | 27 | 18 | UC E1 | No | Oral mesalazine | 2 | 2000 | No disease activity on IUS | n.a. | Start mesalazine suppository |
| 6 | 31 | 26 | UCE1 | Yes | Mesalazine suppository | 7 | 5436 | Normal sigmoid, rectum 6 mm wall thickness | Rectum | Increase mesalazine suppository dosage |
| 7 | 31 | 19 | CD L2 | Yes | Ustekinumab | 2 | 433 | Disease activity limited to sigmoid colon | Sigmoid | Start beclomethasone/mesalazine enema |
| 8 | 34 | 24 | UC E3 | Yes | Oral mesalazine and mesalazine suppository | 7 | 850 | Normal sigmoid, rectum 6 mm wall thickness | Rectum | Start beclomethasone suppository |
| 9 | 32 | 20 | CD L3 | Yes | Oral mesalazine and azathioprine | 7 | 345 | Disease activity in ascendens and sigmoid colon. TI not visualized | Sigmoid | Increase azathioprine dosage |
| 10 | 29 | 22 | UC E2 | Yes | Oral mesalazine | 4 | 617 | Disease activity in descending and sigmoid colon. Rectum not visualized | Colon descendens | Start beclomethasone enema |
| 11a | 19 | 28 | CD L3 | No | Ustekinumab every 8 weeks | 7 | 467 | Disease activity in descending and sigmoid colon. Rectum and TI not visualized | Sigmoid | Start prednisone |
| 11b | 19 | 33 | CD L3 | No | Ustekinumab every 8 weeks and prednisone | 7 | 1084 | Disease activity in descending and sigmoid colon. No response to prednisone | Sigmoid | Intensify ustekinumab to every 4 weeks |
| 12 | 24 | 34 | UC E1 | Yes | Oral mesalazine and azathioprine | 3 | 108 | No disease activity on IUS, rectum and sigmoid not visualized | n.a. | Start mesalazine suppository |
| 13 | 29 | 32 | UC E2 | Yes | Oral mesalazine | 5 | 1980 | Disease activity in descendens and sigmoid colon | Descendens | Start budesonide and mesalazine enema |
Abbreviations: HBI, Harvey-Bradshaw Index; SCCAI, Simple Clinical Colitis Activity Index; FCP, fecal calprotectin; IUS, intestinal ultrasound; CD, Crohn’s disease; UC, ulcerative colitis; TI, terminal ileum; n.a., not applicable.
Follow-up During Pregnancy
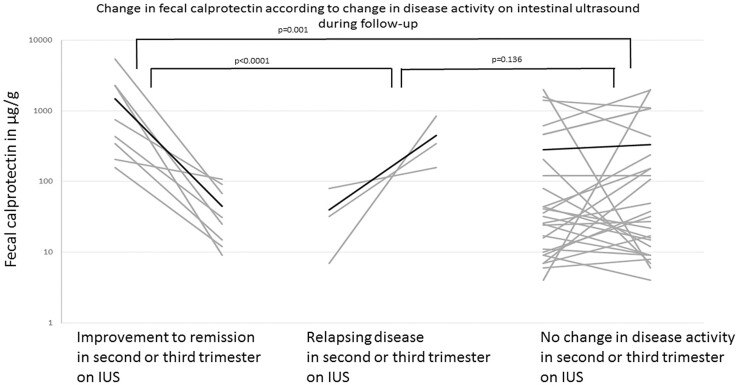
In 23 patients with an IUS in the first trimester, there was a follow-up IUS available in second and/or third trimester (37 IUS examinations) with corresponding FCP and clinical disease activity scores. At follow-up, 4 patients relapsed (2 CD, 2 UC), 10 improved to remission (6 CD, 4 UC), and 23 did not show change in disease activity (11 CD, 14 UC; 8 patients maintained active disease and 15 patients maintained remission) when evaluated according to the reference standard. In comparison, 3 patients relapsed (2 CD, 1 UC), 8 patients improved to remission (4 CD, 4 UC), and 26 did not show a change in disease activity (12 CD, 14 UC) when evaluated by IUS. Intestinal ultrasound showed 78% accuracy (95% CI, 61.1%–94.8%) to diagnose a change in disease pattern to remission or relapse at follow-up, with 82% sensitivity and 82% specificity (AUROC, 0.782; 95% CI, 0.585–0.979; P = .009). Intestinal ultrasound correlated strongly with the reference standard (ρ = 0.76, P < .0001) in detecting improvement to remission, with 80% sensitivity and 92% specificity, respectively. Furthermore, FCP levels decreased significantly when patients with active disease reached remission on IUS compared with the group with relapsing disease and no change in disease activity on IUS (mean, −1439 ± 1818 ug/g vs 91 ± 674 ug/g; P < .0001; Fig. 2). For the 4 relapsing patients, IUS showed moderate correlation (r = 0.48, P = .01) with the reference standard. Moreover, FCP levels significantly increased when IUS showed a relapse when compared with patients showing no change in disease activity or improvement to remission on IUS (mean, 411 ± 391 µg/g vs 297 ± 1216 µg/g, P = .038; Fig. 2). Because the number of relapsing patients was limited, determining sensitivity and specificity was not possible. Furthermore, in the patients not showing any change in disease activity at follow-up by IUS, FCP did not change significantly either (mean baseline, 280.92 ± 541.74 µg/g; mean follow-up, 334.46 ± 596.45 µg/g; P = 0.70).
Figure 2.
Per-patient change in FCP per change in disease activity on IUS in second and/or third trimester. Improvement to remission on IUS (n = 8, FCP baseline, mean, 1483.88 ± 1819.71 µg/g to FCP follow-up, mean, 44.88 ± 38.72 µg/g), relapsing disease on IUS (n = 3, FCP baseline, mean, 39.67 ± 37.10 µg/g to FCP follow-up, mean, 451.33 ± 357.56 µg/g), no change in disease activity (n = 26, FCP baseline, mean, 280.92 ± 541.74 µg/g to FCP follow-up, mean, 334.46 ± 596.45 µg/g). Both of those patients in whom change in disease activity was not detected by IUS exhibited proctitis. Black line is mean value.
Discussion
In this study, we show that IUS is feasible throughout pregnancy, although the terminal ileum is more challenging to visualize in the second and third trimester. This is in agreement with a previous study by Flanagan et al.18 Although a limited number of patients received IUS in their third trimester in this study, we continued to perform IUS in the third trimester and additionally found that visualization of the sigmoid colon is significantly less feasible in the third trimester, whereas other colonic segments remain unaffected. We also demonstrated high inverse correlation between the mean diameter of the uterus, the progression of pregnancy, and visualization of TI and sigmoid colon. The most notable decrease in feasibility for both segments was found for patients entering week 26 of their pregnancy. Although less feasible, IUS still allows visualization of most bowel segments and should not immediately be deemed impossible in late pregnancy.
The accuracy of IUS to detect disease activity had almost perfect agreement with the composite reference standard, defined by clinical scoring indices and FCP. Furthermore in all trimesters, specificity was higher than sensitivity, which is in concordance with previous results.18 Flanagan et al concluded that IUS could be used in pregnant IBD patients to reassure quiescent disease. In addition, a second study found IUS to detect subclinical inflammation in pregnant IBD patients.19 In general, high specificity makes it likely that active disease on IUS truly reflects active IBD. Therefore, in a “point of care” setting, IUS might play a pivotal role in patients with symptoms or mildly or moderately elevated biochemical parameters to confirm presence of disease activity with immediate effect on treatment decision-making.
Moreover, more than half of the patients with active disease on IUS had more than 1 segment affected. As already shown in nonpregnant IBD patients, IUS can determine disease extent, whereas clinical and biochemical parameters merely inform on disease presence.25,26 We showed poor or no significant correlation for serum markers, with IUS disease activity confirming the nonspecificity of these markers to determine disease activity during pregnancy.4 Fecal calprotectin predominantly informs on presence of disease activity, rather than disease location. However, disease extent is clinically relevant and guides treatment decision. In addition, in pregnant IBD patients, both mother and fetus are affected by the ongoing disease burden, and selecting the best suitable treatment with the least (potential) side effects is of major importance. Intestinal ultrasound, preferably in combination with FCP, could guide this treatment decision in the “point of care” setting.
In general, with regard to disease activity in the rectum, IUS is less feasible and accurate, which was consistent with the results in our cohort.27 Interestingly, the growing fetus did not directly affect visualization of the rectum. As the rectum is situated deep in the pelvis and the uterus expands more cranially, visualization of the rectum by transabdominal US is still possible in one-third of the patients regardless of trimester. However, other modalities such as perineal ultrasound might detect proctitis more accurately.27
Recently, IUS has been shown to be accurate in treatment follow-up both in CD and UC.12,16 In this study, IUS accurately detected patients responding to treatment initiated earlier on in pregnancy and found most IUS parameters to normalize when patients reached remission according to the reference standard. To our knowledge, this is the first study to evaluate treatment response during pregnancy in IBD patients.
In clinical practice, IUS—possibly in combination with FCP—could indicate early treatment response. As active disease during pregnancy is associated with adverse pregnancy outcomes, these patients might benefit from frequent IUS assessment in a close monitoring setting to subsequently determine treatment continuation or escalation.
This study has some limitations. First, there is a lack of an endoscopic reference standard to determine disease activity. However in clinical practice, endoscopy is only recommended during pregnancy if there is a strong indication and direct impact on treatment.3 Therefore, endoscopy was considered neither routinely feasible nor necessary. This was overcome by the use of a combined reference standard of clinical disease activity and FCP. To overcome a potential bias of a cutoff value and intra-individual variability, we created this combined reference standard: patients with no clinical activity but a high FCP and patients with clear clinical symptoms but mildly elevated FCP were both considered to have active disease. Although our reference standard lacks validation, the combination of clinical symptoms and fecal biomarkers are often used in clinical practice during pregnancy. Secondly, the ultrasonographer was not standardly blinded to clinical disease activity and FCP. Thirdly, we conducted this study in a high-volume IBD center with established “point of care” use of IUS with a dedicated pregnancy clinic for patients with IBD, and therefore, results may not be completely generalizable. At last, not all patients were followed-up during their whole pregnancy. This study describes a real-world follow-up of patients, with some of the patients referred for an expert opinion or some patients starting regular visits from second trimester onwards. However for every trimester, a similar number of IUS examinations was available; hence, analysis was possible per trimester without significant bias.
In summary, IUS could assure quiescent disease and confirm active disease in a noninvasive “point of care” setting in most pregnant IBD patients. Additionally, IUS is of merit in objectifying disease extent and treatment response, thereby guiding a more personalized treatment decision. However, IUS has its limitations in confirming quiescent disease in TI and sigmoid colon in the third trimester and in disease limited to the rectum.
In conclusion, there is emerging evidence on good feasibility and high accuracy for IUS for IBD patients during pregnancy. Being aware of the advantages and limitations of IUS, further incorporation of this noninvasive, safe, and highly accessible technique is warranted, preferably in a “point of care” setting.
Supplementary Material
Contributor Information
Floris De Voogd, Amsterdam University Medical Center, Department of Gastroenterology and Hepatology, University of Amsterdam, Amsterdam, the Netherlands.
Harshad Joshi, Sir H. N. Reliance Hospital and Research Centre, Mumbai, India.
Elsa Van Wassenaer, Amsterdam University Medical Center, Department of Pediatric Gastroenterology, Emma Children’s Hospital, University of Amsterdam, Amsterdam, the Netherlands.
Steven Bots, Amsterdam University Medical Center, Department of Gastroenterology and Hepatology, University of Amsterdam, Amsterdam, the Netherlands.
Geert D’Haens, Amsterdam University Medical Center, Department of Gastroenterology and Hepatology, University of Amsterdam, Amsterdam, the Netherlands.
Krisztina Gecse, Amsterdam University Medical Center, Department of Gastroenterology and Hepatology, University of Amsterdam, Amsterdam, the Netherlands.
Author Contributions
F.V. (conceptualization, collection of data, methodology, statistical analysis, writing). H.J. (conceptualization, review final manuscript). E.W. (collection of data, review final manuscript). S.B. (conceptualization, collection of data, review final manuscript). G.D. (conceptualization, review final manuscript, supervision). K.G. (conceptualization, collection of data, review final manuscript, supervision). All authors approved the final version of this manuscript.
Funding
No funding to report.
Conflicts of Interest
F.V. received honoraria and/or speaker fees from AbbVie and Janssen. H.J. reports no conflicts of interest; E.W. reports no conflict of interest. S.B. received speaker fees from Abbvie, Merck Sharp & Dome, Takeda, Jansen Cilag, Pfizer, and Tillotts. K.G. received consultancy fees and/or speaker’s honoraria from Amgen, AbbVie, Biogen, Boehringer Ingelheim, Ferring, Hospira, Immunic Therapeutics, Janssen, MSD, Pfizer, Sandoz, Samsung Bioepis, Takeda, Tigenix, and Tillotts. G.D. has served as advisor for AbbVie, Ablynx, Amakem, AM Pharma, Avaxia Biologics, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion Healthcare, Cosmo, Covidien, Ferring, Dr Falk Pharma, Engene, Galapagos, Gilead, GlaxoSmithKline, Hospira, Immunic, Johnson and Johnson, Lycera, Mediametrics, Millennium/Takeda, Mitsubishi Pharma, Merck Sharp & Dohme, Mundipharma, Novo Nordisk, Pfizer, Prometheus Laboratories/Nestle, Protagonist, Receptos, Robarts Clinical Trials, Salix, Sandoz, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant, and Vifor and received speaker fees from AbbVie, Ferring, Johnson and Johnson, Merck Sharp & Dohme, Mundipharma, Norgine, Pfizer, Shire, Millennium/Takeda, Tillotts, and Vifor outside of the submitted work.
References
- 1. O’Toole A, Nwanne O, Tomlinson T. Inflammatory bowel disease increases risk of adverse pregnancy outcomes: a meta-analysis. Dig Dis Sci. 2015;60:2750–2761. [DOI] [PubMed] [Google Scholar]
- 2. van der Woude CJ, Ardizzone S, Bengtson MB, et al. ; European Crohn’s and Colitis Organization. . The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. j Crohns Colitis. 2015;9:107–124. [DOI] [PubMed] [Google Scholar]
- 3. Mahadevan U, Robinson C, Bernasko N, et al. . Inflammatory bowel disease in pregnancy clinical care pathway: a report from the american gastroenterological association IBD parenthood project working group. Inflamm Bowel Dis. 2019;25:627–641. [DOI] [PubMed] [Google Scholar]
- 4. Tandon P, Leung K, Yusuf A, Huang VW. Noninvasive methods for assessing inflammatory bowel disease activity in pregnancy: a systematic review. j Clin Gastroenterol. 2019;53:574–581. [DOI] [PubMed] [Google Scholar]
- 5. Winter RW, Friedman S. Update on pregnancy in patients with IBD. Curr Treat Options Gastroenterol. 2020;18:423–441. [Google Scholar]
- 6. Watts DH, Krohn MA, Wener MH, Eschenbach DA. C-reactive protein in normal pregnancy. Obstet Gynecol. 1991;77:176–180. [DOI] [PubMed] [Google Scholar]
- 7. Jha AK, Chaudhary M, Dayal VM, et al. . Optimal cut-off value of fecal calprotectin for the evaluation of ulcerative colitis: an unsolved issue? jgh Open. 2018;2:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Julsgaard M, Hvas CL, Gearry RB, et al. . Fecal calprotectin is not affected by pregnancy: clinical implications for the management of pregnant patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:1240–1246. [DOI] [PubMed] [Google Scholar]
- 9. Goodsall TM, Noy R, Nguyen TM, et al. . Systematic review: patient perceptions of monitoring tools in inflammatory bowel disease. j Can Assoc Gastroenterol. 2021;4:e31–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor SA, Mallett S, Bhatnagar G, et al. ; METRIC study investigators. . Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): a multicentre trial. Lancet Gastroenterol Hepatol. 2018;3:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Voogd F, Wilkens R, Gecse K, et al. . A reliability study-strong inter-observer agreement of an expert panel for intestinal ultrasound in ulcerative colitis. J Crohn’s Colitis. 2021;15(8):1284–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maaser C, Petersen F, Helwig U, et al. ; German IBD Study Group and the TRUST&UC study group; German IBD Study Group and TRUST&UC study group. . Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut. 2020;69:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maaser C, Sturm A, Vavricka SR, et al. ; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. . ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. j Crohns Colitis. 2019;13:144–164. [DOI] [PubMed] [Google Scholar]
- 14. Novak KL, Nylund K, Maaser C, et al. . Expert consensus on optimal acquisition and development of the international bowel ultrasound segmental activity score (IBUS-SAS): a reliability and inter-rater variability study on intestinal ultrasonography in Crohn’s Disease. J Crohn’s Colitis. 2021;15(4):609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Panés J, Bouzas R, Chaparro M, et al. . Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:125–145. [DOI] [PubMed] [Google Scholar]
- 16. Kucharzik T, Wittig BM, Helwig U, et al. ; TRUST study group. . Use of intestinal ultrasound to monitor Crohn’s disease activity. Clin Gastroenterol Hepatol. 2017;15:535–542.e2. [DOI] [PubMed] [Google Scholar]
- 17. Parente F, Molteni M, Marino B, et al. . Are colonoscopy and bowel ultrasound useful for assessing response to short-term therapy and predicting disease outcome of moderate-to-severe forms of ulcerative colitis?: a prospective study. Am j Gastroenterol. 2010;105:1150–1157. [DOI] [PubMed] [Google Scholar]
- 18. Flanagan E, Wright EK, Begun J, et al. . Monitoring inflammatory bowel disease in pregnancy using gastrointestinal ultrasonography. j Crohns Colitis. 2020;14:1405–1412. [DOI] [PubMed] [Google Scholar]
- 19. Leung Y, Shim HH, Wilkens R, et al. . The role of bowel ultrasound in detecting subclinical inflammation in pregnant women with Crohn’s disease. j Can Assoc Gastroenterol. 2019;2:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peyrin-Biroulet L, Panés J, Sandborn WJ, et al. . Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol. 2016;14:348–354.e17. [DOI] [PubMed] [Google Scholar]
- 21. Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey–Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8(4):357–363. [DOI] [PubMed] [Google Scholar]
- 22. Bots S, Nylund K, Löwenberg M, et al. . Ultrasound for assessing disease activity in IBD patients: a systematic review of activity scores. j Crohns Colitis. 2018;12:920–929. [DOI] [PubMed] [Google Scholar]
- 23. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 24. Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126:1763–1768. [DOI] [PubMed] [Google Scholar]
- 25. af Björkesten CG, Nieminen U, Turunen U, et al. . Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand j Gastroenterol. 2012;47:528–537. [DOI] [PubMed] [Google Scholar]
- 26. Jensen MD, Kjeldsen J, Nathan T. Fecal calprotectin is equally sensitive in Crohn’s disease affecting the small bowel and colon. Scand j Gastroenterol. 2011;46:694–700. [DOI] [PubMed] [Google Scholar]
- 27. Sagami S, Kobayashi T, Aihara K, et al. . Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis. Aliment Pharmacol Ther. 2020;51(12):1373–1383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.