Abstract
Background
Extreme telomere length has been previously reported to be associated with increased risk of gastric cancer. However, evidence from prospective studies on a relative large sample size with long-term follow-up to further corroborate previous study findings is meager.
Methods
The association between peripheral blood leukocyte telomere length and risk of gastric adenocarcinoma was prospectively examined in a cohort of 26,540 middle-aged or older Chinese nested in the Singapore Chinese Health Study. Telomere length was determined using a validated qPCR-based method. The Cox proportional regression method was used to estimate hazard ratio (HR) and its 95% confidence interval (CI) of gastric adenocarcinoma associated with telomere length after adjustment for potential confounders. Restricted cubic spline analysis was applied to assess the nonlinear relationship between telomere length and gastric cancer risk.
Results
A U-shaped association was found between telomere length and risk of gastric adenocarcinoma (Pnonlinearity = 0.020). Compared with the second quintile of telomere length, a statistically significant higher risk of gastric adenocarcinoma was associated with either the lowest quintile (HR = 1.63, 95% CI, 1.07–2.47) or the highest quintile (HR = 1.55, 95% CI, 0.97–2.47) of telomere length. This U-shaped relationship was more apparent in men and younger individuals.
Conclusions
This is the first prospective study demonstrating a higher risk of gastric cancer to be associated with either extremely short or extremely long telomere length. Short and long telomere length may function differently in the early and late stages of gastric carcinogenesis.
Keywords: Gastric adenocarcinoma, Telomere length, Prospective cohort study
Introduction
Telomeres are tandem nucleotide repeats of TTAGGG located at the end of eukaryotic chromosomes [1]. Telomeres are important in maintaining chromosome stability by preventing degradation, atypical combination, and chromosome end fusion [2]. Progressive shortening of telomeres occurs as a consequence of somatic cell division and is associated with increasing age [3]. Several other factors have also been identified related to decreasing telomere length, including cigarette smoking [4] and oxidative stress [5]. When telomeres are shortened to a critical point, irreversible cellular growth arrest and replicative senescence or apoptosis could occur [6]. Telomere shortening could further lead to chromosomal instability and the initiation of oncogenesis.
Based on a meta-analysis of 16 case–control and 11 prospective studies, short telomeres were associated with higher risk of several cancers including bladder, esophagus, stomach, ovary, head and neck, and kidney [7]. Two earlier studies, both with a retrospective study design, showed that short telomeres were significantly associated with a higher risk of gastric cancer [3, 8]. A more recent case–control study among a Han Chinese population found a U-shaped relationship of telomere length to the risk of gastric cancer, wherein either short or long telomeres were associated with a higher risk of gastric cancer [9]. However, telomere length in retrospective studies, in which biospecimens for measurement of telomere length were collected after cancer diagnosis, may be biased because development and progression of the disease itself may influence telomere length. A prospective cohort study of more than 40,000 participants reported a null association between telomere length and gastric cancer risk in an European population [10]. There has been no prospective study of telomere length and gastric cancer risk in an Asian population, which have a higher risk for gastric cancer compared to Western populations.
Utilizing the resources of the Singapore Chinese Health Study, we prospectively examined the relationship between telomere length in peripheral blood leukocytes at baseline and the risk of developing gastric cancer in middle-aged or older Chinese men and women in Singapore.
Methods and materials
Study design and population
The details of study design and subject enrollment of the Singapore Chinese Health Study have been previously described [11]. In brief, we enrolled 63,257 Chinese men and women aged 45–74 years, from 1993 through 1998, who were living in the government housing estates and belonged to one of two major Chinese dialect groups in Singapore: the Hokkiens, who originated from the southern part of Fujian Province, and the Cantonese, who came from the central region of Guangdong Province. At the time of recruitment, each subject was interviewed in person by a trained interviewer to obtain information on demographics, body weight and height, lifetime use of tobacco (cigarettes and water-pipe), current physical activity, menstrual/reproductive history (women only), occupational exposure, medical history, and family history of cancer. Information on current diet and consumption of beverages was assessed via a 165-item food frequency questionnaire that had been validated against a series of 24-h dietary recall interviews [11] and selected biomarker studies [12, 13] conducted on random subsets of cohort participants. Blood and urine samples were initially collected from a 3% random sample of cohort participants in 1994–1999 and extended to all survivors of the entire cohort in 2000–2005. Overall, 28,346 subjects provided baseline blood samples. The present study has been approved by the Institutional Review Board of the National University of Singapore and the University of Pittsburgh (Pittsburgh, PA, USA).
Case ascertainment
Incident cases of cancer and death among the cohort participants were identified through the periodic record linkage analysis with the databases of the nationwide Singapore Cancer Registry and the Birth and Death Registry. Since 1968, the Singapore Cancer Registry has provided information on cancer trend and patterns [14] and has been shown to be complete in recording of incident cancer cases [15]. To date, only 47 (<1%) cohort participants are known to be lost to follow-up because of migration from Singapore. Gastric adenocarcinoma was defined using the International Classification of Disease Oncology, 3rd edition (ICD-O-3) as primary site of C16.0–C16.9 and histology type of 8140/3–8560/3. As of December 2015, after an average 11.8 years of follow-up after their baseline blood collection, 220 patients among 26,761 subjects, after excluding 1,585 participants with a history of cancer at baseline, developed gastric adenocarcinoma.
Telomere length measurement
Genomic DNA was extracted from peripheral blood samples using QIAamp 96 DNA Blood kits (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol, with the following modifications. First, protease incubation was increased from 10 to 20 min to ensure effective digestion of protein. Second, DNA was eluted from 96-well filter plates using two serial 200-μl aliquots of Qiagen AE buffer (eluates were combined for a total elution volume of 400 μl).
The quantitative polymerase chain reaction (PCR) method developed by Cawthon [16] was applied to quantify relative telomere length determined by the ratio of telomere repeat copy number (T) to a single-copy gene for albumin (S) on all subjects. The details of the assay were described previously [17]. Briefly, each 10-μl experimental sample contained 20 ng DNA diluted in pure water and was aliquoted into the reaction well of a 96-well plate compatible with the Bio-Rad MyiQ Single Color Real-Time PCR Detection system. A standard DNA curve was drawn based on five concentrations (1.85–150 ng/μl by threefold incremental increase) of reference DNA sample. All experimental DNA samples were assayed in duplicate, and the average value of the two was used for final analysis for each subject. The mean percentage of coefficients of variability was 3.5%.
Helicobacter pylori infection status testing
To further examine the association of telomere length with gastric cancer risk after adjusting for H. pylori infection status, a nested case–control study was conducted in a subset of cases and their matched controls (128 gastric adenocarcinoma cases and 383 individually matched controls) whose serological H. pylori infection status was determined by the presence or absence of 116-kDa CagA in serum. The details of this case–control study have been previously reported [18].
Statistical analysis
The present analysis included 26,540 subjects after excluding 221 subjects with missing telomere length measurements caused by assay problems. Baseline demographic and lifestyle information was compared by quintiles of telomere length. The Cox proportional hazard regression method was used to estimate hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) of developing gastric adenocarcinoma for different quintiles of telomere length. Person-years of follow-up were computed from the date of blood sample collection to the date of gastric cancer diagnosis, death, migration out of Singapore, or December 31, 2015, whichever occurred first. We used the information updated in the follow-up I interview on body weight and height (for calculating body mass index), smoking status, number of cigarettes per day, and years of smoking (for calculating pack-years of smoking), and alcohol consumption for the present analysis because the collection of blood for measurement of telomere length was done immediately after the follow-up I interview. The proportional hazards assumption was examined by testing the significance of Pearson’s correlation coefficient between Schoenfeld residuals of the relative telomere length and ranked survival time [19]. We found no violation of proportional hazards assumption. To examine the nonlinear relationship between telomere length and risk of gastric adenocarcinoma, we used the restricted cubic spline technique [20], with predefined knots at the 5th, 30th, 70th, and 95th percentile of log-transformed measured telomere length.
To adjust for potential confounding effects, age, sex, dialect group, year of recruitment, level of education (no formal education, primary education, or secondary education or higher), smoking status (never-smoker, former smoker, or current smoker), and alcohol consumption (nondrinkers, <7 or ≥7 drinks per week) were included in all regression models. Further adjustment for other covariates such as intake of vegetables and fruit (<7 servings/week, 7–14 servings/week, >14 servings/week), family history of cancer (yes or no), and history of gastric/duodenal ulcer (yes or no) did not meaningfully alter the association. Thus, the results presented were not adjusted for these additional variables.
Stratified analysis was conducted by gender and median age (67 years) at blood collection from subjects who later developed gastric cancer during the follow-up period. We also examined the associations between telomere length and risk of gastric adenocarcinoma stratified by the duration of follow-up (e.g., <5 or ≥5 years) to assess if subclinical symptoms and progression of gastric adenocarcinoma had any impact on the observed association in the overall data.
The conditional logistic regression method was used to further investigate the potential confounding effect of H. pylori infection on the association between telomere length and gastric adenocarcinoma risk in a nested case–control study within the cohort. This analysis included a subset of the study population (128 cases and 383 controls) with known serological status of CagA, a marker for H. pylori infection.
All statistical analysis was performed in the SAS 9.4 software package (SAS Institute). All P values reported are two sided. P values <0.05 were considered statistically significant.
Results
The comparison of baseline characteristics of 26,540 subjects by quintiles of peripheral blood leukocyte telomere length is shown in Table 1. Overall, the mean of relative telomere length was 1.02 with a standard deviation (SD) of 0.23. Compared to those in the lowest quintile, individuals in the highest quintile of telomere length were younger, were less likely to smoke cigarettes, or consumed more alcoholic beverages. Ever-smokers in the highest quintile of telomere length also reported fewer pack-years of smoking than their counterparts in the lowest quintile of telomere length.
Table 1.
Distribution of selected baseline characteristics of all participants by relative telomere length: The Singapore Chinese Health Study, 1993–2015
| Characteristics | Relative telomere length in quintile |
||||
|---|---|---|---|---|---|
| 1st (lowest) | 2nd | 3rd | 4th | 5th (highest) | |
|
| |||||
| n | 5038 | 5038 | 5038 | 5038 | 5038 |
| Age in years, mean (SD) | 65.9 (7.8) | 63.8 (7.6) | 62.7 (7.5) | 61.5 (7.2) | 60.1 (6.9) |
| Body mass index (BMI) (kg/m2), mean (SD) | 23.1 (3.5) | 23.2 (3.5) | 23.3 (3.5) | 23.4 (3.5) | 23.3 (3.5) |
| Female, % | 44.8 | 50.2 | 53.2 | 59.1 | 62.2 |
| Education level, % | |||||
| No formal education | 22.5 | 21.1 | 20.5 | 20.6 | 19.4 |
| Primary school | 47.6 | 44.6 | 45.4 | 45.0 | 44.1 |
| ≥Secondary level | 29.9 | 34.3 | 34.2 | 34.4 | 36.5 |
| Smoking status, % | |||||
| Never-smoker | 60.5 | 65.4 | 68.3 | 72.0 | 74.4 |
| Former smoker | 21.6 | 17.4 | 15.3 | 13.5 | 12.4 |
| Current smoker | 17.9 | 17.2 | 16.4 | 14.4 | 13.2 |
| Pack-years smoking among ever-smokers, mean (SD) | 33.6 (29.1) | 30.8 (28.6) | 30.5 (27.5) | 28.1 (24.9) | 27.9 (25.9) |
| Alcohol consumption status, % | |||||
| Non-drinkers | 87.9 | 88.5 | 88.5 | 89.1 | 89.0 |
| <7 drinks/week | 8.4 | 8.4 | 8.5 | 8.4 | 8.4 |
| ≥7 drinks/week | 3.7 | 3.1 | 3.0 | 2.5 | 2.6 |
| Ethanol intake (g/day) among drinkers, mean (SD) | 10.6 (16.5) | 9.2 (15.2) | 10.2 (16.9) | 7.9 (13.6) | 8.6 (15.0) |
| Daily sodium intake (mg), mean (SD) | 1122.5 (583) | 1136.9 (599.5) | 1149.7 (595.8) | 1142.3 (596.5) | 1162.8 (618.3) |
| Food consumption in grams, mean (SD) | |||||
| Total vegetables | 112.2 (63.9) | 115.9 (64.5) | 116.3 (64.7) | 116.7 (64) | 119.6 (65.9) |
| Total fruits | 205.6 (165.3) | 216.4 (171.7) | 210.3 (166.1) | 214.9 (167.4) | 221.4 (172.9) |
| Total red meat | 30.7 (23.5) | 31.1 (24.5) | 31.7 (25.1) | 31.2 (24.3) | 31.4 (24.6) |
| Physical activity (hours/week), mean (SD) | 5.0 (6.2) | 4.7 (6.0) | 4.9 (6.3) | 4.7 (6.0) | 4.7 (6.3) |
After 314,226 person-years of follow-up (mean, 11.8 years per subject), a total of 265 incident cases of gastric cancer were identified. Among them, 17 cases were sarcoma, 12 lymphomas, and 16 malignancies with unspecified histology: all of these were excluded from the present analysis. Thus, the present study included 220 cases of gastric adenocarcinoma. The mean follow-up time from baseline to gastric adenocarcinoma diagnosis was 6.13 years (SD 4.01). Subsites of the stomach included 28 (12.7%) cardia, 145 (65.9%) non-cardia, and 47 (21.4%) unknown.
The restricted cubic spline analysis revealed a U-shaped relationship between telomere length and risk of gastric adenocarcinoma (Fig. 1). The nonlinear relationship was statistically significant (P value for nonlinearity test = 0.020). Both the lowest and highest quintile of telomere lengths were associated with statically significant or borderline significant higher risk of gastric adenocarcinoma (Table 2). Compared with the 2nd quintile, HRs (95% CI) for the lowest and highest quintile of telomere length were 1.63 (1.07–2.47) and 1.55 (0.97–2.47), respectively, after adjustment for age, sex, education, interview year, dialect group, smoking status, and alcohol intake. The U-shaped association was stronger among males than among females (P-interaction = 0.027), and was confined to the younger age group (age <67 years) (P-interaction = 0.018) (Table 3). The U-shaped relationship was present in the entire cohort with shorter duration (≤5 years) and with longer duration (>5 years) of followup (Table 4). We also examined the association between telomere length and risk of gastric cardia or non-cardia cancer. The multivariate-adjusted HRs (95% CIs) of cardia cancer for the lowest and highest quartile of telomere length were 2.65 (0.72–9.86) and 2.84 (0.73–11.10), respectively. The corresponding figures for non-cardia cancer were 1.15 (0.74–1.79) and 1.26 (0.78–2.05). However, the difference in the risk estimates for cardia and non-cardia cancer was not statistically significant because of the small sample size.
Fig. 1.
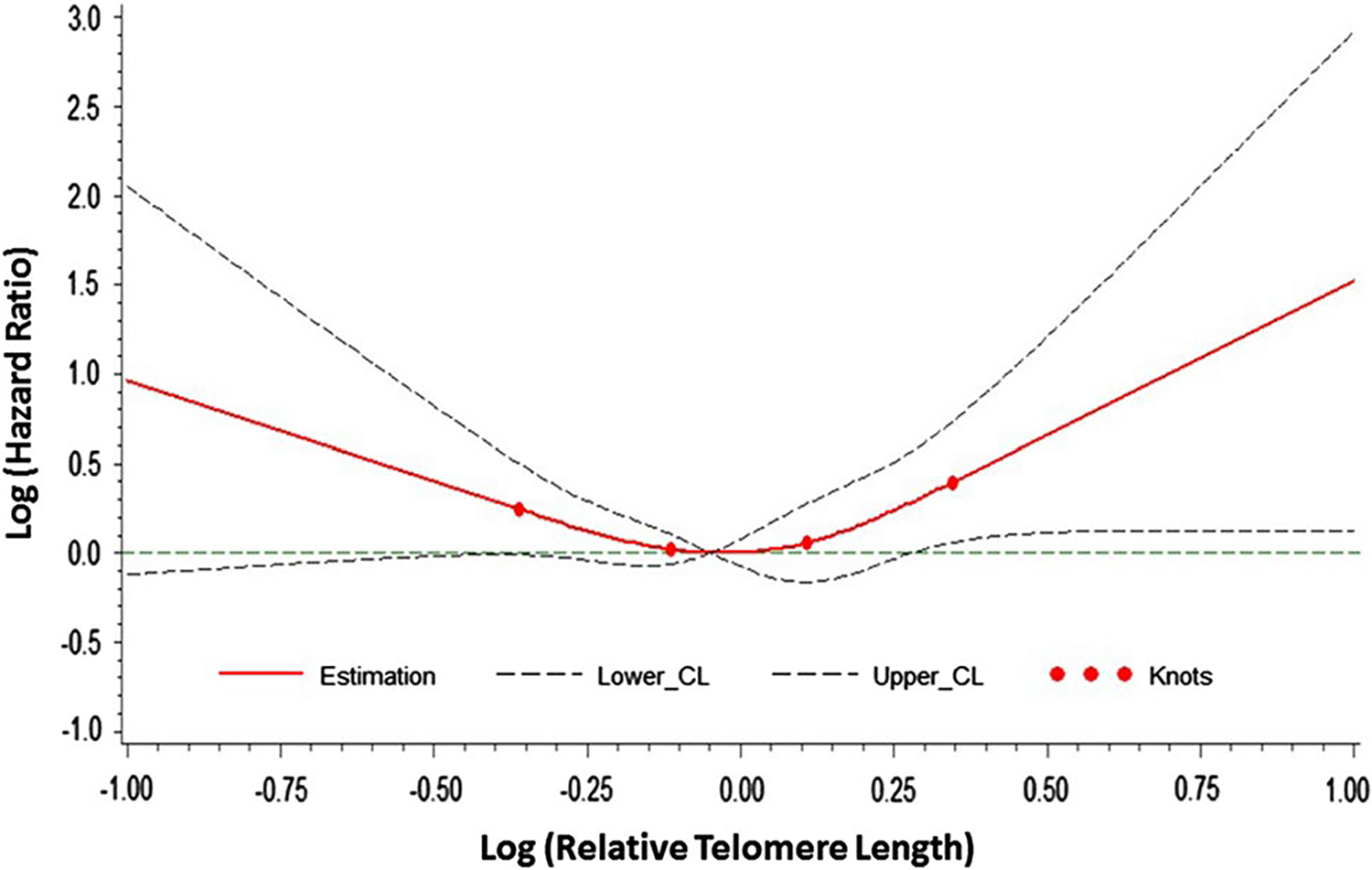
Fitted restricted cubic spline curve for the hazard ratio of gastric adenocarcinoma associated with relative telomere length at 4 knots located at the 5th, 30th, 70th, and 95th percentile
Table 2.
Relative telomere length and gastric cancer risk among all subjects: The Singapore Chinese Health Study, 1993–2015
| Relative telomere length in quintile | Cases | Person-years | HR (95% CI)a |
|---|---|---|---|
|
| |||
| 1st (lowest) | 65 | 59,791 | 1.63 (1.07–2.47) |
| 2nd | 34 | 62,053 | 1.00 (ref.) |
| 3rd | 41 | 63,024 | 1.30 (0.82–2.04) |
| 4th | 41 | 64,003 | 1.45 (0.92–2.29) |
| 5th (highest) | 39 | 65,355 | 1.55 (0.97–2.47) |
| P nonlinear b | 0.020 | ||
HR hazard ratio, CI confidence interval
Model adjusted for age at baseline interview (in years), baseline interview year (1993–1995, 1996–1998), father’s dialect (Cantonese, Hokkien), gender, education (no formal education, primary education, ≥secondary education), alcohol intake (never-drinker, <7 drinks/week, ≥7 drinks week), and smoking status (never-smoker, former smoker, current smoker)
Based on RCS nonlinearity test
Table 3.
Relative telomere length and gastric cancer risk in subgroups stratified by median age and gender: The Singapore Chinese Health Study, 1993–2015
| Relative telomere length in quintile | Cases | Person-years | HR (95% CI)a |
|---|---|---|---|
|
| |||
| Age <67 years (n = 18,293) | |||
| 1st (lowest) | 30 | 44,681 | 3.02 (1.46–6.25) |
| 2nd | 12 | 45,485 | 1.00 (ref.) |
| 3rd | 15 | 45,747 | 1.73 (0.80–3.75) |
| 4th | 27 | 45,709 | 2.29 (1.09–4.79) |
| 5th (highest) | 24 | 46,526 | 2.79 (1.35–5.76) |
| P non-linear b | 0.007 | ||
| Age ≥67 years (n = 8,247) | |||
| 1st (lowest) | 26 | 16,662 | 1.13 (0.68–1.89) |
| 2nd | 20 | 17,279 | 1.00 (ref.) |
| 3rd | 26 | 17,170 | 1.14 (0.64–2.02) |
| 4th | 23 | 17,349 | 1.11 (0.59–2.06) |
| 5th (highest) | 17 | 17,620 | 0.87 (0.42–1.83) |
| P non-linear b | 0.449 | ||
| Male (n = 12,234) | |||
| 1st (lowest) | 47 | 31,406 | 1.74 (1.04–2.92) |
| 2nd | 21 | 29,425 | 1.00 (ref.) |
| 3rd | 25 | 27,903 | 1.33 (0.74–2.38) |
| 4th | 23 | 25,261 | 1.49 (0.82–2.70) |
| 5th (highest) | 24 | 23,602 | 1.88 (1.04–3.39) |
| P non-linear b | 0.009 | ||
| Female (n = 14,306) | |||
| 1st (lowest) | 18 | 28,385 | 1.42 (0.69–2.89) |
| 2nd | 13 | 32,627 | 1.00 (ref.) |
| 3rd | 16 | 35,121 | 1.24 (0.60–2.58) |
| 4th | 18 | 38,742 | 1.36 (0.66–2.78) |
| 5th (highest) | 15 | 41,753 | 1.15 (0.54–2.44) |
| P non-linear b | 0.779 | ||
HR hazard ratio, CI confidence interval
Model adjusted for age at baseline interview (in years), baseline interview year (1993–1995, 1996–1998), father’s dialect (Cantonese, Hokkien), gender (only adjusted for age-stratified models), education (no formal education, primary education, ≥secondary education), alcohol intake (never-drinker, <7 drinks/week, ≥7 drinks week), and smoking status (never-smoker, former smoker, current smoker)
Based on RCS nonlinearity test
Table 4.
Relative telomere length and gastric cancer risk by duration of follow-up: The Singapore Chinese Health Study, 1993–2015
| Relative telomere length in quintile | Follow-up <5 years | Follow-up ≥5 years | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Cases | Person-years | HR (95% CI)a | Cases | Person-years | HR (95% CI)a | |
|
| ||||||
| 1st (lowest) | 33 | 25,002 | 1.73 (0.95–3.15) | 32 | 34,494 | 1.53 (0.86–2.73) |
| 2nd | 16 | 25,404 | 1.00 (ref.) | 18 | 36,314 | 1.00 (ref.) |
| 3rd | 19 | 25,482 | 1.31 (0.67–2.54) | 22 | 37,211 | 1.28 (0.69–2.39) |
| 4th | 15 | 25,566 | 1.18 (0.58–2.40) | 26 | 38,084 | 1.66 (0.91–3.04) |
| 5th (highest) | 15 | 25,611 | 1.37 (0.67–2.79) | 24 | 39,362 | 1.69 (0.91–3.13) |
| P nonlinear b | 0.004 | 0.037 | ||||
HR hazard ratio, CI confidence interval
Model adjusted for age at baseline interview (in years), baseline interview year (1993–1995, 1996–1998), father’s dialect (Cantonese, Hokkien), gender, education (no formal education, primary education, ≥secondary education), smoking status (never-smoker, former smoker, current smoker), and alcohol intake (never-drinker, <7 drinks/week, ≥7 drinks week)
Based on restricted cubic spline model
To examine whether H. pylori infection would modify the association between telomere length and risk of gastric adenocarcinoma, a nested case–control study was analyzed for those with serological status of CagA in serum. After adjusting for CagA positivity status, the U-shaped association remained unchanged (Table 5). The association attenuated slightly when analysis was restricted to subjects with serologically positive CagA status (Table 5).
Table 5.
Relative telomere length and gastric adenocarcinoma risk among subjects with known serological status of Helicobacter pylori CagA: The Singapore Chinese Health Study, 1993–2015
| Relative telomere length in quintile | All subjects | H. pylori-positive onlyc | |||
|---|---|---|---|---|---|
|
|
|
||||
| Ca/Co | OR (95% CI)a | OR (95% CI)b | Ca/Co | OR (95% CI)a | |
|
| |||||
| 1st (lowest) | 37/98 | 2.00 (1.05–3.87) | 1.95 (1.00–3.81) | 37/85 | 1.92 (0.97–3.83) |
| 2nd | 18/87 | 1.00 (ref.) | 1.00 (ref.) | 18/74 | 1.00 (ref.) |
| 3rd | 29/78 | 2.06 (1.06–4.00) | 2.06 (1.05–4.05) | 29/64 | 2.02 (1.00–4.08) |
| 4th | 24/62 | 2.13 (1.05–4.32) | 2.20 (1.06–4.56) | 22/51 | 1.92 (0.89–4.12) |
| 5th (highest) | 20/58 | 2.13 (0.98–4.61) | 2.20 (1.00–4.84) | 17/50 | 1.64 (0.72–3.74) |
| P nonlinear d | 0.036 | 0.030 | 0.078 | ||
Ca cases, Co controls, OR odds ratio, CI confidence interval
Conditional logistic regression model adjusted for age at baseline interview (in years), baseline interview year (1993–1995, 1996–1998), father’s dialect (Cantonese, Hokkien), gender, and education (no formal education, primary education, ≥secondary education), smoking status (never-smoker, former smoker, current smoker), and alcohol intake (never-drinker, <7 drinks/week, ≥7 drinks/week)
Further adjusted for serum H. pylori CagA status (positive, negative)
H. pylori positive was defined by the presence of CagA in serum
Based on restricted cubic spline model
Discussion
In this prospective cohort study of 26,540 middle-aged or older Chinese men and women, we found that extreme short and long telomeres were associated with an 63% and 55% increased risk, respectively, of gastric adenocarcinoma after adjustment for multiple potential confounding factors. The U-shaped relationship was stronger among men than women and stronger in younger than older individuals. Addition of the H. pylori infection marker in a subgroup analysis did not materially alter the observed association. The U-shaped relationship was present in both cohorts with shorter and longer duration of follow-up. These robust results support a possible role of extreme short and long telomeres in the development of gastric adenocarcinoma.
Most early studies found that short telomeres were associated with increased risk of gastric cancer [7, 8]. These early studies were hospital based, with a relatively small sample size, and used a retrospective study design in which blood samples for telomere measures were collected after cancer diagnosis. The selection bias, random chance, and reverse causality, i.e., the impact of cancer development and progression on telomere length, cannot be ruled out. A more recent community-based case–control study in China that included 1136 patients with gastric cancer and 1012 control subjects free of cancer found a U-shaped relationship between telomere length and gastric cancer risk. Both the highest and lowest quintiles of telomere length were significantly associated with 78% and 281% higher risk, respectively, of gastric cancer compared with the fourth quintile [9]. Consistent with this large case–control study, the present study demonstrated a robust U-shaped relationship of telomere length to the risk of developing gastric adenocarcinoma.
Potential explanations for the U-shaped relationship between telomere length and the risk of gastric cancer could be that shortened telomeres may induce chromosomal instability, initiating carcinogenesis, whereas on the other hand long telomeres upregulate cell division and increase the likelihood of abnormalities, thus promoting cancer development [21]. Short telomeres could lead to chromosome instability, cell inflammation, and neoplastic changes in gastric mucosa cells during early carcinogenesis. A clinical study found that telomere length was significantly shorter in tumor cells of stage I gastric cancer than cells of adjacent non-cancer tissue [22]. In a matched case–control study using gastric mucosa biopsies from 217 gastric cancer patients and 102 controls, short telomeres were significantly associated with both chronic inflammation (P = 0.002) and intestinal metaplasia (P < 0.001) [23]. In addition, short telomeres could induce epigenetic transformations. In an in vitro study of five gastric mucosa cell lines from cancer-free subjects, telomere shortening was associated with a 71% increased risk of hypermethylation in the regional promoter CpG island [24]. These molecular changes are generally associated with increased risk of cancer.
Telomere length determines the cellular lifespan and proliferative capacity. Each round of genome replication has the potential to introduce genetic mutations and chromosomal alterations, which may promote malignant transformation [25]. Excessively long telomeres could result in increased cell division [21]. For example, an increased activity of telomerase was reported in more than 85% of cancer cells [26]. In studies applying therapy to inhibit telomerase activity, such as antisense human telomerase reverse transcriptase (ahTERT), decreased tumor cell proliferative and invasive capability and partial reverse of malignant phenotypes were found [27, 28]. In a recent experiment, Bull et al. demonstrated that early increase in telomere length was significantly associated with increased levels of folic acid deficiency-induced global DNA hypomethylation and markers of chromosome instability [29], both of which are key molecular events in carcinogenesis [30, 31]. It is possible that global DNA hypomethylation may be involved in the association between longer telomeres and the risk of gastric cancer.
We found a stronger association between telomere length and risk of gastric adenocarcinoma in men than in women. One of the possible explanations is that the present study had 75% more gastric cancer cases in men (n = 140) than in women (n = 80), thus provided a greater statistical power for subgroup analysis in men than in women.
The present study shows that the U-shaped relationship of telomere length to gastric adenocarcinoma was more apparent in younger individuals. Inconsistent data have been reported by previous studies. Although some studies found an association between shorter telomeres and higher risk of gastric cancer in both younger and older groups [3], other studies found the association only among older people [8]. Our study finding of both extremely short and extremely long telomeres in relationship to increased risk of gastric adenocarcinoma among younger people differs from those findings from previous studies. The underlying reason for a more pronounced association in younger people is not clear. Telomeres shorten with increasing age. Extremely short telomeres among young people may indicate some genetic variants in the genes for short telomeres. A meta-analysis of genome-wide association studies on 21 cohorts of 48,000 individuals have identified multiple single-nucleotide polymorphisms (SNPs) on candidate genes such as telomerase reverse transcriptase (TERT) and telomerase RNA component (TERC) in relationship to telomere shortening [32]. On the other hand, extremely long telomeres among young individuals might indicate a systematic disruption of telomere length maintenance and thus escape from programmed cell death. A null association for older subjects may result from the increased background risk of gastric cancer because of aging, thus masking the effect of telomere-related effect on gastric cancer risk.
The present study has several strengths. The prospective study design, in which blood samples for telomere measurements were collected, on average, 6 years before the diagnosis of gastric cancer, minimized the potential impact of the presence and progression of disease on telomere length. With quantification of telomere length for all participants of the entire cohort, the large samples (26,500 subjects) with relatively long follow-up (up to 12 years and more than 300,000 cumulative person-years) provided a sufficient sample size and produced robust results across different subgroups of study population. A comprehensive assessment of subject demographic characteristics, lifestyle factors, dietary habits, family history of cancer, and medical history allowed for adjustment for potential confounding effects of these factors on the association between telomere length and risk of gastric cancer. Furthermore, the serological status of CagA, an established marker for H. pylori infection that is an important risk factor for gastric cancer, has been determined in more than 50% of cases from a previous study, which allowed us to rule out the potential confounding effect of H. pylori on the observed association.
However, our study had several limitations. First, information on the stage of gastric cancer at diagnosis was not available to us because the Singapore Cancer Registry did not routinely collect and record this information. Thus, we could not investigate the association between telomere length and risk of gastric cancer by stage separately. Telomere length was measured in peripheral blood leukocytes, which is not the target tissue. However, a previous study showed a significant positive correlation between telomere length in peripheral blood leukocytes and other tissue types such as muscle cells, fat, and skin [33]. Future studies are warranted to evaluate the clinical utility of telomere length in identifying individuals at high risk of gastric cancer or in evaluating the prognosis of patients after diagnosis of gastric cancer.
In conclusion, our study demonstrates that both extremely short and extremely long telomeres are associated with a significantly higher risk of gastric adenocarcinoma in this middle-aged or older Chinese population in Singapore. These results support a possible biological mechanism whereby both shortening and elongation of telomere length are involved in the development of gastric adenocarcinoma.
Acknowledgements
We thank Siew-Hong Low of the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study and the Singapore Cancer Registry for assistance with the identification of cancer outcomes. This study was funded by National Institute of Health (NIH)/National Cancer Institute (NCI) Grant number: R01 CA144034 and UM1 CA182876 (to J.-M. Yuan). W-PK is supported by the National Medical Research Council, Singapore (NMRC/CSA/0055/2013).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
Informed consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all participants.
Ethical approval The Singapore Chinese Health Study was approved by the Institutional Review Boards at the National University of Singapore and the University of Pittsburgh (Pittsburgh, PA, USA).
References
- 1.Blackburn EH. Telomeres and telomerase: the means to the end (Nobel lecture). Angew Chem Int Ed Engl. 2010;49(41):7405–21. [DOI] [PubMed] [Google Scholar]
- 2.Karlseder J Telomere repeat binding factors: keeping the ends in check. Cancer Lett. 2003;194(2):189–97. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Bao G, Huo T, Wang Z, He X, Dong G. Constitutive telomere length and gastric cancer risk: case-control analysis in Chinese Han population. Cancer Sci. 2009;100(7):1300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–4. [DOI] [PubMed] [Google Scholar]
- 5.von Zglinicki T Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27(7):339–44. [DOI] [PubMed] [Google Scholar]
- 6.Baird DM. Mechanisms of telomeric instability. Cytogenet Genome Res. 2008;122(3–4):308–14. [DOI] [PubMed] [Google Scholar]
- 7.Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomark Prev. 2011;20(6):1238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou L, Savage SA, Blaser MJ, Perez-Perez G, Hoxha M, Dioni L, et al. Telomere length in peripheral leukocyte DNA and gastric cancer risk. Cancer Epidemiol Biomark Prev. 2009;18(11):3103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du J, Zhu X, Xie C, Dai N, Gu Y, Zhu M, et al. Telomere length, genetic variants and gastric cancer risk in a Chinese population. Carcinogenesis (Oxf). 2015;36(9):963–70. [DOI] [PubMed] [Google Scholar]
- 10.Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Bojesen SE. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013;105(7):459–68. [DOI] [PubMed] [Google Scholar]
- 11.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, et al. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39(2):187–95. [DOI] [PubMed] [Google Scholar]
- 12.Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, et al. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomark Prev. 1998;7(9):775–81. [PubMed] [Google Scholar]
- 13.Seow A, Shi CY, Franke AA, Hankin JH, Lee HP, Yu MC. Isoflavonoid levels in spot urine are associated with frequency of dietary soy intake in a population-based sample of middle-aged and older Chinese in Singapore. Cancer Epidemiol Biomarkers Prev. 1998;7(2):135–40. [PubMed] [Google Scholar]
- 14.Singapore Cancer Registry Interim Report. Trends in Cancer incidence in Singapore 2010–2014. May 26, 2015. https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library/cancer-trends-2010-2014_interim-annual-report_final-(public)9efe07a5c9d76bafab5af000014cdee.pdf?sfvrsn=0. Accessed 17 Feb 2016.
- 15.Forman DBF, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R, Ferlay J (eds) (2014) Cancer incidence in five continents. Vol. X. IARC Scientific Publication No. 164. Lyon: International Agency for Research on Cancer [Google Scholar]
- 16.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan J-M, Beckman KB, Wang R, Bull C, Adams-Haduch J, Huang JY, et al. Leukocyte telomere length in relation to risk of lung adenocarcinoma incidence: findings from the Singapore Chinese Health Study. Int J Cancer (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ainslie-Waldman CE, Koh WP, Jin A, Yeoh KG, Zhu F, Wang R, et al. Coffee intake and gastric cancer risk: the Singapore Chinese health study. Cancer Epidemiol Biomark Prev. 2014;23(4):638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrell FE, Lee KL. Verifying assumptions of the Cox proportional hazards model. In: Proceedings of the eleventh annual SAS user’s group international conference. Cary, NC: SAS Institute; 1986. p. 823–828. [Google Scholar]
- 20.Desquilbet L, Mariotti F. Dose–response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57. [DOI] [PubMed] [Google Scholar]
- 21.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11(5):319–30. [DOI] [PubMed] [Google Scholar]
- 22.Mu Y, Zhang Q, Mei L, Liu X, Yang W, Yu J. Telomere shortening occurs early during gastrocarcinogenesis. Med Oncol. 2012;29(2):893–8. [DOI] [PubMed] [Google Scholar]
- 23.Tahara T, Shibata T, Kawamura T, Horiguchi N, Okubo M, Nakano N, et al. Telomere length shortening in gastric mucosa is a field effect associated with increased risk of gastric cancer. Virchows Arch. 2016;469(1):19–24. [DOI] [PubMed] [Google Scholar]
- 24.Tahara T, Shibata T, Okubo M, Kawamura T, Horiguchi N, Ishizuka T, et al. Demonstration of potential link between Helicobacter pylori-related promoter CpG island methylation and telomere shortening in human gastric mucosa. Oncotarget. 2016;7(28):43989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347(6217):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33(5):787–91. [DOI] [PubMed] [Google Scholar]
- 27.Yang SM, Fang DC, Yang JL, Liang GP, Lu R, Luo YH, et al. Effect of antisense human telomerase RNA on malignant phenotypes of gastric carcinoma. J Gastroenterol Hepatol. 2002;17(11):1144–52. [DOI] [PubMed] [Google Scholar]
- 28.Yang SM, Fang DC, Yang JL, Chen L, Luo YH, Liang GP. Antisense human telomerase reverse transcriptase could partially reverse malignant phenotypes of gastric carcinoma cell line in vitro. Eur J Cancer Prev. 2008;17(3):209–17. [DOI] [PubMed] [Google Scholar]
- 29.Bull CF, Mayrhofer G, O’Callaghan NJ, Au AY, Pickett HA, Low GK, et al. Folate deficiency induces dysfunctional long and short telomeres; both states are associated with hypomethylation and DNA damage in human WIL2-NS cells. Cancer Prev Res (Phila). 2014;7(1):128–38. [DOI] [PubMed] [Google Scholar]
- 30.Ehrlich M DNA methylation in cancer: too much, but also too little. Oncogene. 2002;21(35):5400–13. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson LR, Chen H, Collins AR, Connell M, Damia G, Dasgupta S, et al. Genomic instability in human cancer: molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin Cancer Biol. 2015;35((suppl)):S5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422–7 (7e1–2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]


