Abstract
Background/Aim: Triple-negative breast cancers represent 15% of all mammary malignancies and encompass several entities with different genomic characteristics. Among these, luminal androgen receptor (LAR) tumors express the androgen receptor (AR) and are characterized by a genomic profile which resembles luminal breast cancers. Moreover, LAR malignancies are usually enriched in PIK3CA, KMTC, CDH, NF1, and AKT1 alterations. Still, molecular features, clinical behavior and prognosis of this variant remain controversial, while identification of effective treatments represents an unmet medical need. Additionally, the predictive role of the AR is unclear.
Materials and Methods: We performed an extensive next generation sequencing analysis using a commercially available panel in a cohort of patients with LAR breast cancer followed at two local Institutions. We next employed bioinformatic tools to identify signaling pathways involved in LAR pathogenesis and looked for potentially targetable alterations.
Results: Eight patients were included in the study. In our cohort we found 26 known genetic alterations (KGAs) in 15 genes and 64 variants of unknown significance (VUS) in 59 genes. The most frequent KGAs were single nucleotide variants in PIK3CA, HER2, PTEN and TP53. Among VUS, CBFB, EP300, GRP124, MAP3K1, RANBP2 and TSC2 represented recurrently altered genes. We identified five signaling pathways (MAPK, PI3K/AKT, TP53, apoptosis and angiogenesis) involved in the pathogenesis of LAR breast cancer. Several alterations, including those in PIK3CA, ERBB2 and PI3K/AKT/mTOR signaling, were potentially targetable.
Conclusion: Our findings confirm a role for PI3K/AKT/mTOR signaling in the pathogenesis of LAR breast cancers and indicate that targeting this pathway, along with ERBB2 mutations, may represent an additional therapeutic strategy which deserves further exploration in larger studies.
Keywords: Triple-negative breast cancer, luminal androgen receptor, next generation sequencing, genomic alterations, predictive tools
Triple-negative breast cancers (TNBC) are characterized by the lack of estrogen (ER) and progesterone receptors (PgR) in the absence of epidermal growth factor receptor 2 (HER2) amplification (1). They account for 15% of all breast cancers and are usually associated with an aggressive clinical behavior and a poor prognosis (2,3). These unfavorable features, along with the absence of viable therapeutic targets, fostered increasing efforts aimed at understanding the molecular characteristics of this disease. In the last few years, massive parallel sequencing and “omics” technologies have partially clarified the biological bases of this breast cancer variant revealing an unexpected heterogeneity (4,5). Indeed, while some TNBC harbor a limited number of somatic mutations, others display a high number of genetic alterations affecting different signaling pathways (6,7).
Based on their molecular profile, their chemosensitivity and on the presence of potential therapeutic targets, six TNBC subtypes have been identified: basal-like 1, basal-like 2, immunomodulatory, mesenchymal, mesenchymal stem-like and luminal androgen receptor (LAR). The latter tumors are characterized by the expression of the androgen receptor (AR) and by an apocrine histological appearance. The gene expression profile of these tumors resembles ER-positive breast cancers (e.g. FOXA1, GATA3, SPDEF and XBP1 hyperexpression) (8). Additionally, LAR breast malignancies are usually enriched in PIK3CA, KMTC, CDH, NF1, and AKT1 mutations (9,10). Still, the prognosis and clinical behavior of LAR breast tumors remain undefined, with conflicting outcomes emerging from the available literature (11-14). Similarly, the predictive role of the AR is unclear. Several clinical trials tested anti-androgen compounds in LAR breast cancer patients (15,16). Given the frequent presence of PIK3CA mutations among these tumors, ongoing studies are also exploring PI3K inhibitors or combining anti-androgen therapies with cyclin-dependent kinase 4/6 inhibitors (17,18). However, to date none of these targeted approaches has shown significant efficacy in LAR tumors.
Herein we describe the molecular findings emerging from a comprehensive next-generation sequencing (NGS) analysis in a cohort of LAR breast cancer patients. We also report the results of in silico analyses performed to identify the activated pathways in these tumors and the relative potentially actionable alterations.
Materials and Methods
Patient samples. Patients diagnosed between 2014 and 2019 with TNBC expressing AR (i.e., LAR breast cancers) were considered eligible for the study if formalin-fixed paraffine-embedded (FFPE) samples from the primary tumor were available. Subjects were followed either in the Oncology Unit of the Policlinico “G. Rodolico - San Marco” or at the “Humanitas Medical Care” Center in Catania. All patients gave written informed consent in accordance with the Declaration of Helsinki.
Next-generation sequencing. Nucleic acids were isolated from FFPE samples containing ≥50% tumor cells. Comprehensive genomic profiling was performed using a hybrid capture-based (7) platform (FoundationOne™, Foundation Medicine Inc., Cambridge, MA, USA) which identifies single nucleotide substitutions (SNV), insertions and deletions (indels), copy number alterations (CNAs), and rearrangements. The platform interrogates the coding sequence of 315 cancer-related genes and introns from 28 genes often rearranged in solid tumors to a median depth of coverage greater than 500× (19).
Immunohistochemistry for the Androgen Receptor. Immunohisto-chemical staining was performed on representative 5-micron thick sections using antibodies against the androgen receptor (DAKO AR441 clones; 1:75 dilution; pretreatment with citric buffer, pH=6.2; HRP detection; DAB chromogen). Nuclear staining for the androgen receptor in ≥1% of tumor cells was considered positive.
Bioinformatic analysis and literature search. Known genetic alterations and VUS alterations were annotated and reported by FoundationOne patient reports.
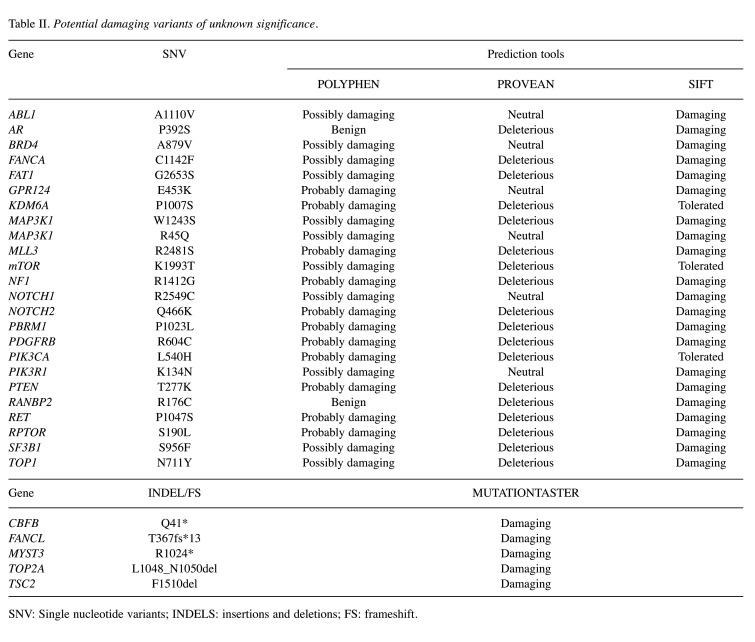
We evaluated SNV, indel and frameshift variants of VUS using different online in silico prediction tools. Polymorphism Phenotyping v2 (PolyPhen-2), Protein Variation Effect Analyzer (PROVEAN) and Sorting Intolerant from Tolerant (SIFT) were employed to predict the potential impact of SNVs on protein structure and function (20-22). Only SNVs considered not neutral by at least 2 of the 3 prediction tools (thereafter indicated as possibly damaging) were included into further functional analyses. Insertions, deletions and frameshift variants were studied using the MutationTaster tool (23). In this case, alterations which may lead to a dysfunctional protein will be here indicated as possibly damaging. Two authors (M.M. and S.R.V) performed a literature search on PubMed looking at genes with potentially damaging VUS to confirm their possible implication in breast carcinogenesis.
To understand the integrated biological significance of known genomic alterations (KGAs) and variants of unknown significance (VUS) we interrogated two annotation tools, Database for Annotation, Visualization and Integrated Discovery (DAVID) and Protein ANalysis THrough Evolutionary Relationships (PANTHER). The DAVID bioinformatics system analyzes a gene list using functional classifications, functional annotation charts or clustering and functional annotation tables (http://david.niaid.nih.gov). The PANTHER program is part of the Gene Ontology Reference Genome Project designed to classify proteins and genes with high-throughput analysis and exploits a database containing 20851 proteins directly associated with 165 metabolic and signaling pathways (www.pantherdb.org). We used the KEGG tool for the DAVID analysis and the CellDesigner tool in PANTHER to generate a pathway alteration status from the list of mutated genes.
Results
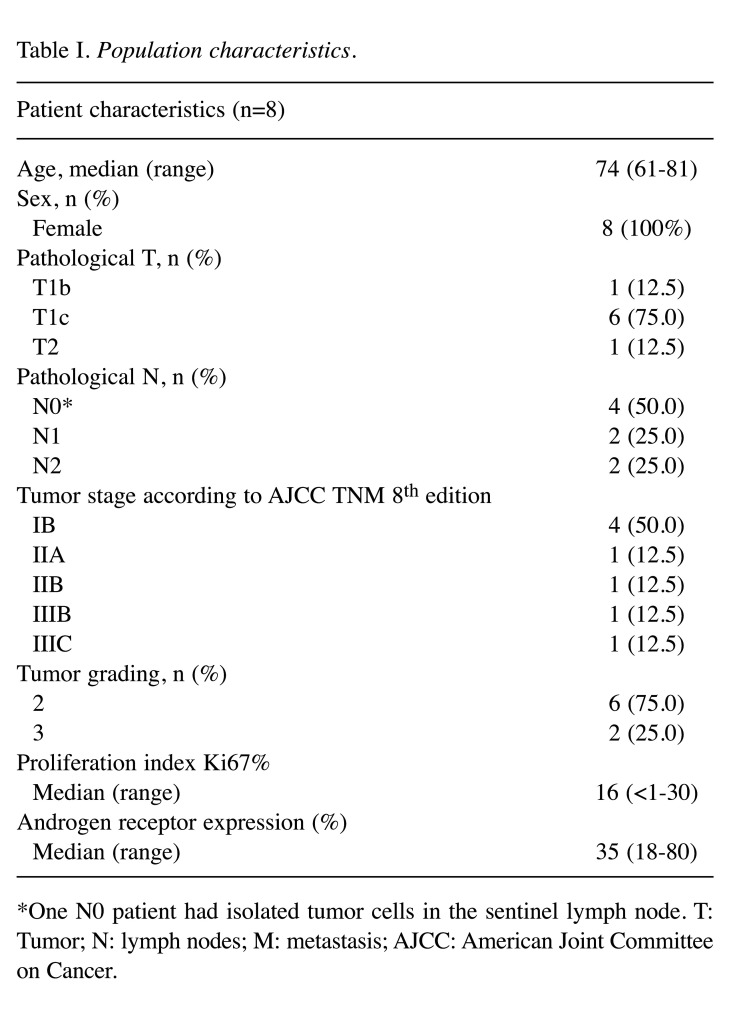
Population characteristics. Eleven patients diagnosed with LAR breast cancer satisfied the eligibility criteria for the study. Tumor tissue was obtained at the moment of primary surgery in all subjects. Three samples failed NGS analysis due to inadequate quality and were therefore excluded. Table I summarizes the main clinical and pathological features of the included cases. Median age at diagnosis was 74 years (range=61-81 years). All patients were female and displayed localized disease at diagnosis. Tumor size was ≤20 mm (i.e., pT1) in 7 cases and nodal status was negative in 4 patients. Cancer stages according to the AJCC TNM 8th edition were as follows: 4 IB, 1 IIA, 1 IIB, 1 IIIB and 1 IIIC. Tumor grading, defined according to the Elston-Ellis modified Scarff-Bloom-Richardson system, was intermediate (G2) in 6 cases and high (G3) in 2 subjects. Median Ki67% proliferation index was 16% (range=<1-30%), with 5 patients below the 20% threshold defined by the Sant Gallen criteria (24,25). Median androgen receptor expression was 35% (range=18-80%).
Table I. Population characteristics.
*One N0 patient had isolated tumor cells in the sentinel lymph node. T: Tumor; N: lymph nodes; M: metastasis; AJCC: American Joint Committee on Cancer.
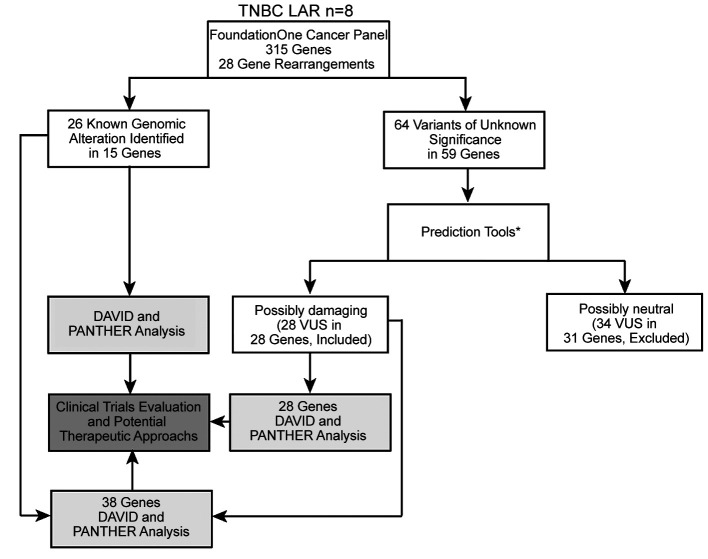
Study workflow. We retrospectively collected tumor specimens to perform NGS analysis as detailed above. Twenty-six KGAs in 15 genes and 64 VUS in 59 genes emerged from sequencing. We then sought the potential biological significance of the above-mentioned VUS using in silico prediction tools (PolyPhen2, PROVEAN, SIFT, MutationTaster). This analysis identified 28 not repetitive genes with possibly damaging mutations (Table II). A literature search confirmed that all 28 genes had been previously correlated with breast cancer. Next, two functional annotation tools (DAVID and PANTHER) were used to pair biological alterations with signaling pathways considering the 15 genes with KGAs, the 28 genes with possibly damaging VUS, or their combination which totaled 38 genes as 5 were common between KGA and VUS. We then analyzed the results to identify potentially actionable therapeutic targets in our population (Figure 1).
Table II. Potential damaging variants of unknown significance.
SNV: Single nucleotide variants; INDELS: insertions and deletions; FS: frameshift.
Figure 1. Flow diagram of the study. Tumor samples from 8 luminal androgen receptor breast cancers underwent Next Generation Sequencing using the FoundationOne Cancer Panel, which interrogates 315 genes as well as introns of 28 genes involved in rearrangements. Twenty-six known genomic alterations (KGAs) and 64 variants of unknown significance (VUS) were identified. Variants of unknown significance were stratified according to their possibly damaging role as indicated by in silico prediction tools. Genes with KGAs, possibly damaging VUS and their combination, which totaled 38 genes as 5 were common between KGA and VUS, were analyzed with functional annotation tools (DAVID and PANTHER) and the results were matched with potential therapeutic targets. *Confirmed implication in breast cancer according to a literature search.
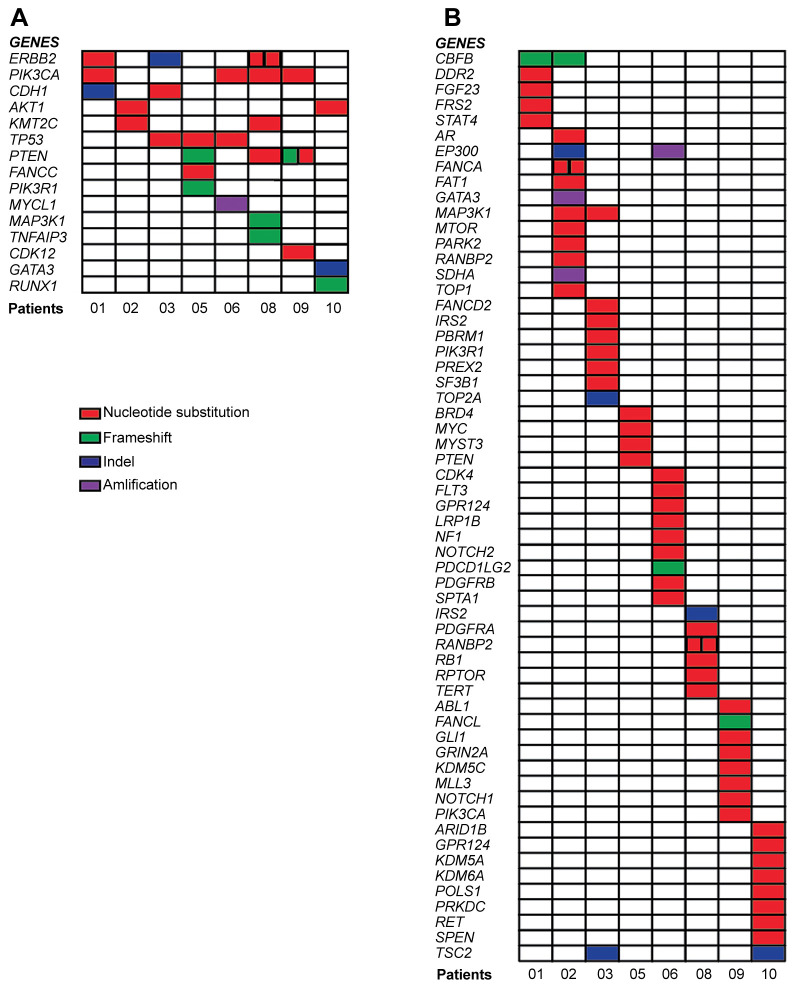
Identification of somatic molecular alterations by NGS. Detailed NGS findings are illustrated in Figure 2. Among the 15 genes presenting KGAs, PIK3CA was altered in 4 patients, ERBB2, PTEN and TP53 in 3 patients, AKT1, CDH1 and KTM2C in 2 patients and the remaining genes in only one individual. Two alterations were recurrent, namely PIK3CA H1047R (patients 01, 08 and 09) and AKT E17K (patients 02 and 079). Of note, patient 08 harbored a double SNV in ERBB2 (I767M, S310F), while subject 09 presented two alterations in PTEN (C136R, S10fs*14) (Figure 2A). In the VUS dataset 6 (CBFB, EP300, GRP124, MAP3K1, RANBP2 and TSC2) of the 59 included genes were altered in more than one patient. Only one VUS was recurrent (TSC2 F1510del in subjects 03 and 10), while 2 individuals presented a double SNV in the same gene, namely FANCA A1132V and C1142F in patient 02 and RUNBP2 F3085L and R176C in patient 08.
Figure 2. Tile plot of the identified genomic alterations. Every colored square represents a specific gene alteration in each patient, indicated with a progressive number. Panel A displays known genomic alterations (KGAs) while panel B shows the variants of unknown significance (VUS) (B).
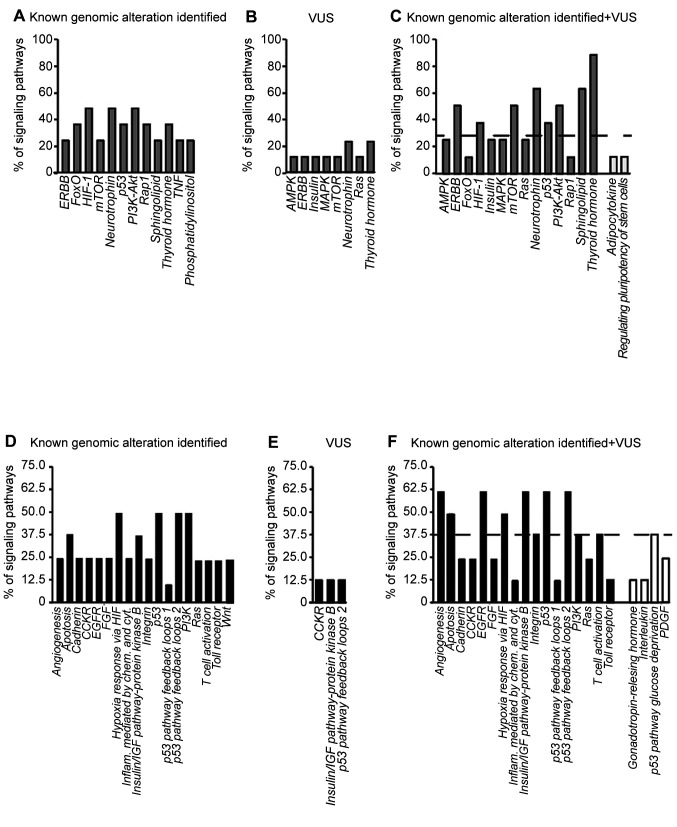
Co-analysis of KGAs and VUS identifies novel pathways in LAR breast cancer. We next evaluated whether the detected alterations cluster in known signaling pathways, using two different classification systems (DAVID and PANTHER) to analyze KGAs, possibly damaging VUS or their combination (Figure 3). Clustering genes with KGAs, similar pathways emerged from the DAVID and PANTHER analysis (Figure 3A and D). Additionally, several retrieved pathways can be traced back to broader networks. For example, HIF-1 and Hypoxia response via HIF are both involved in angiogenesis and apoptosis while FoxO also contributes to cell death. Functional analysis of genes with possibly damaging VUS provided a smaller number of activated pathways, with different results from the DAVID and the PANTHER tools (Figure 3B and E). However, the retrieved pathways were partly superimposable with those observed for gene with KGAs. Lastly, we ran a combined analysis for genes with KGAs and possibly damaging VUS (Figure 3C and F).
Figure 3. Signaling pathways activated by known genomic alterations, possibly damaging variants of unknown significance (VUS), and their combinations. Bars represent the activated signaling pathways according to known genomic alterations (A-D), possibly damaging VUS (B-E) or both (C-F), analyzed using the DAVID (gray bars) or PANTHER (black bars) tools. The height of each bar varies according to the different rate of pathway involvement. White bars in the C and F panels indicate pathways identified exclusively by the combination of known genomic alterations and possibly damaging VUS. Dashed lines indicate the median rate of pathway involvement.
To compare the results from the DAVID and PANTHER analyses, we arbitrarily set a threshold defined as the median rate of pathway involvement and considered significant pathways only whose contribution was above the threshold. Among them, PI3K-Akt, p53, apoptosis, angiogenesis and MAPK signaling were the most represented. Additional activated pathways emerged from the combined analysis (Figure 3C and F, white bars). Although these pathways were mainly below our defined threshold, they were all implicated in breast cancer biology according to pre-existing evidence (2,50). These results suggest that genes with potentially damaging VUS might provide additional information on the mutational landscape of LAR breast cancer.
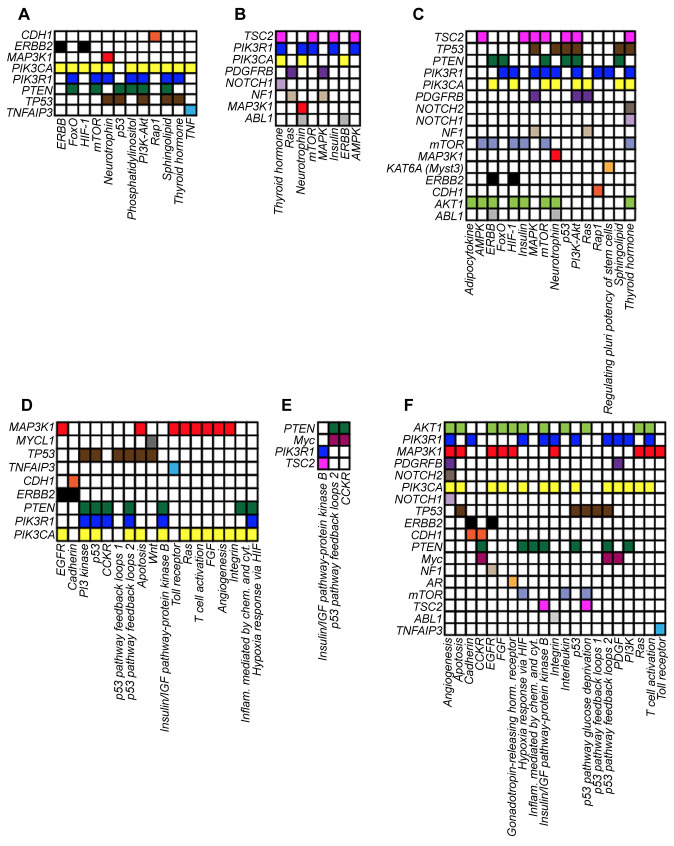
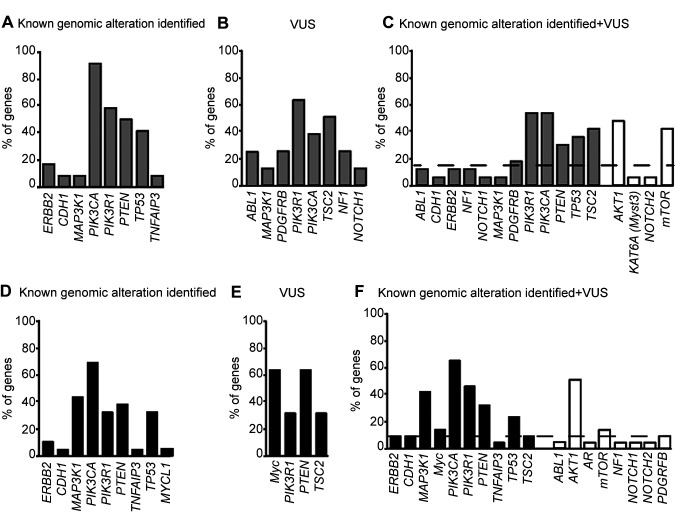
Evaluation of the activated pathways identifies genes involved in LAR breast tumorigenesis. We next wanted to investigate the genes involved in the retrieved pathways. To this end, we examined the list of genes with KGAs, possibly damaging VUS and their combination, included in each activated pathway (Figure 4) and calculated the percentage of pathway contribution for each gene.
Figure 4. Heatmap of altered genes involved in the activated signaling pathways of the eight LAR-TNBC patients. Panels display the involvement of the known genomic alterations (A-D), possibly damaging VUS (B-E) or both (C-F) in modulating the signaling pathways reported in Figures 3 and 4. Each gene is indicated with a different color.
As expected, the DAVID or PANTHER tools provided a superimposable set of genes involved in the activated pathways with KGAs (Figure 5A and D), while the gene sets were mostly different when considering pathways with possibly damaging VUS (Figure 5B and E). When we investigated activated pathways considering both KGAs and possibly damaging VUS, we found that PIK3CA, PIK3R1, PTEN and TP53 were above the threshold according to both the DAVID and PANTHER tools (Figure 5C and F). Additional genes emerged from the combined analysis (Figure 5C and F, white columns) but only AKT1 and mTOR were above our predefined threshold. These findings confirm that the TP53, PI3K-Akt and its downstream target mTOR are strongly involved in LAR breast carcinogenesis.
Figure 5. Implication of known genomic alterations, variants of unknown significance (VUS), and their combination in activated pathways. Bars represent the known genomic alterations (A-D), possibly damaging VUS (B-E) or their combination (C-F) involved in the activated pathways reported in Figure 3 and generated by the DAVID (gray bars) or PANTHER (black bars) tools. The height of each bar varies according to the different rate of gene involvement in the different pathway. White columns in C and F panels indicate genes identified exclusively by the combination of known genomic alterations and possibly damaging VUS. The dashed line identifies the median rate gene involvement in the different pathways.
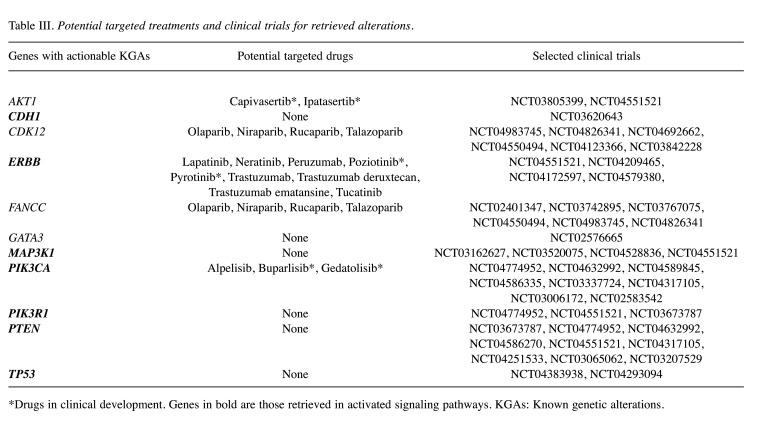
Evaluation of potential therapeutic approaches. Lastly, we matched genes with KGAs with potential targeted therapies and performed an online search of ongoing clinical trials that may be suitable for LAR breast cancer patients displaying the given molecular alterations (Table III). The clinical trial search was performed using the My Cancer Genome (https://www.mycancergenome.org) and ClinicalTrials.gov (https://clinicaltrials.gov) websites.
Table III. Potential targeted treatments and clinical trials for retrieved alterations.
*Drugs in clinical development. Genes in bold are those retrieved in activated signaling pathways. KGAs: Known genetic alterations.
Five altered genes (AKT1, CDK12, ERBB2, FANCC and PIK3CA) were potentially actionable, of which 4 (CDK12, ERBB2, FANCC and PIK3CA) with commercially available drugs. Among these, ERBB2 and PIK3CA were the only genes retrieved in activated pathways. Alterations in all 5 genes may represent eligibility criteria for clinical trials. In addition, CDH1, GATA3, MAP3K1, PIK3R1, PTEN and TP53 presented KGAs which cannot be targeted with available molecules but may candidate LAR breast cancer patients to ongoing studies. With the lone exception of GATA3, all these genes were found in activated pathways.
Discussion
Pursuing the goal of precision medicine for the treatment of cancer patients represents an imperative, especially for malignancies with unfavorable outcomes and limited therapeutic options (26-29). TNBC displays the worse prognosis among all breast cancer variants and the search for actionable targets is an urgent medical need. The dissection of TNBC molecular features unraveled a considerable heterogeneity encompassing at least 6 distinct pathological entities (10,30). Among these, the LAR subtype is characterized by expression of the androgen receptor, which represents an appealing therapeutic target (31). However, the use of anti-androgen compounds has generated inconsistent and often suboptimal results in LAR breast cancer patients, while alternative targeted treatments have yet to be identified (15,16,32). In the present study we performed an extensive genomic sequencing in a small cohort of LAR breast cancer patients, searching for molecular alterations and activated pathways to leverage as therapeutic targets.
Clinical-pathological features of our cohort are in line with those previously reported. Indeed, while median age (i.e., 74 years) is higher compared to that observed in TNBC (33), it mirrors the results of previous reports on LAR breast tumors (10,34). Older age at diagnosis is usually associated with endocrine-sensitive breast cancer and our results reinforce the correlation between LAR and ER-positive tumors (35). Likewise, in our population median Ki67 proliferation index was below the 20% threshold, as expected from pre-existing evidence on LAR cancers. Of note, AR seems to play an anti-proliferative role that may explain the low proliferation rate observed in most of these tumors (36,37).
Results of our NGS analysis are in line with previous characterizations of LAR tumors, which typically harbor SNVs rather than amplifications and indels (10). According to TCGA, TP53 is by far the most altered gene in TNBC (80%), followed by PIK3CA (9%). However, a specific analysis for LAR tumors was not included in TCGA (4). More recently, Bareche et al. carried out an integrative analysis combining somatic SNVs, CNV and gene expression profiles 550 TNBC derived from Molecular Taxonomy of Breast Cancer International Consortium (METABRIC), specifically addressing at the different TNBC subtypes. According to their results, LAR breast tumors display a distinct molecular profile compared to the other molecular subtypes, with PIK3CA, KMT2C, CDH1, NF1 and AKT1 being the most frequently mutated genes (4,10). Consistently, all these SNVs, except for NF1, were present in our cohort. However, we found a higher incidence of TP53 as well as PTEN alterations. This discrepancy is also concordant with the results of Weismann et al. showing that apocrine TNBC displays a lower TP53 mutational rate compared with other TNBCs and may be explained by the small number of patients in our study (34).
Functional analysis of VUS by in silico prediction tools revealed novel genes, such as CBFB, and EP300 that may be implicated in LAR tumorigenesis although additional studies are needed to confirm this evidence.
Several studies have investigated the more frequently activated pathways in LAR breast cancers, demonstrating a central role for PI3K and TP53 signaling (34,38). Moreover, estrogen and androgen response as well as adipogenesis, fatty acid metabolism and protein secretion pathways have also been implicated in LAR carcinogenesis (14). Our analysis corroborates a significant contribution of PI3K and TP53, while also including additional pathways, such as apoptosis, angiogenesis, integrin, ERBB, hypoxia and MAPK. The information emerging from combination of KGAs, and possibly damaging VUS implies that unknown variants might also contribute to LAR carcinogenesis. In any case, we confirm a pivotal role for PI3K signaling in our cohort. This correlation has already been observed in apocrine triple-negative breast cancers (34). Indeed, a mechanistic link between the PI3K pathway and AR signaling was initially demonstrated in prostate cancer, while preclinical evidence suggests an interplay between AR and PIK3CA in supporting LAR cancer pathogenesis (39,40).
Given the preeminent role of PI3K signaling in LAR breast cancers, targeting the components of this pathway presents a strong biological rationale. A study on patient-derived xenografts (PDX) of LAR TNBC resistant to anti-androgens showed a remarkable sensitivity towards PIK3CA and mToR inhibitors (41). Additional preclinical evidence demonstrated that dual blockade of AR and PI3K has a synergistic effect on AR-positive TNBC cell lines and PDX (38). Multiple clinical trials are testing PI3K/mToR/AKT inhibitors in patients harboring alterations in this pathway (Table III), with one specifically addressing LAR breast cancer patients by combining the PIK3CA inhibitor alpelisib with the anti-androgen enzalutamide (NCT03207529) (42).
HER2 represents a further potentially relevant target for patients with LAR breast cancer, since four ERBB2 mutations emerged in three subjects included in our cohort. Somatic alterations in ERBB2 are usually considered driver events in breast cancer and a consolidated body of evidence suggests that they may be effectively targeted with anti-HER2 agents even in absence of HER2 amplification (43,44). Among the ERBB2 mutations detected in our cohort, one was the 755-759 in frame deletion, which increases ERBB2 heterodimerization resulting in higher phosphorylation of EGFR and HER3 (44). This alteration confers sensitivity towards EGFR inhibitors and neratinib, but apparently induces resistance to lapatinib (44-47). Of the other ERBB2 mutations retrieved, two (S310F and S653C) are activating, while one (I767M) has no functional effect (44,48,49). However, HER2I767M responds to conventional anti-HER2 therapies (trastuzumab, lapatinib, neratinib) (44,50). Of note, while a trial is investigating combinations of AR and HER2 inhibitors in AR positive/HER2-amplified breast cancer (NCT02091960), no studies are available for HER2-mutated LAR TNBC.
In conclusion, despite the small number of patients included, our study defines a molecular portrait of LAR tumors which is in line with previous evidence. Functional analyses incorporating KGAs and VUS to explore activated pathways not only reinforce pre-existing knowledge, but also provide information concerning potential targeted treatments. Future studies are warranted to shed light on the clinical impact of these molecular alterations in LAR breast tumors.
Conflicts of Interest
The Authors declare no conflicts of interest in relation to this study.
Authors’ Contributions
Conceptualization: SS, SVR, MM, MC and PV; methodology: SS, MM, SVR, CF, GMV, CL, GM and FM; software: SS, SRV, CL, MM and GM; validation: SS, SRV and MM; formal analysis: SS, SVR, CL, GM and MM; investigation: SS, SVR, MM and PV; resources: CL, GM, KL, NI and RC; data curation: SS, SRV, MM, LM and PV; writing - original draft preparation: SS, SVR, LM and FM; writing - review and editing: SVR, FM, MM, PV, SS and LM; visualization: SS, SRV, MM, CL, GM, KL, FM, CF, GMV, ET, RC, NI, LM, MC and PV; supervision: PV. All Authors have read and agreed to the published version of the manuscript.
Acknowledgements
The Authors acknowledge Foundation Medicine® (Roche) for the donation of free molecular tests used to conduct the present research.
References
- 1.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 3.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, Harris L, Hait W, Toppmeyer D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24(36):5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, Bashashati A, Prentice LM, Khattra J, Burleigh A, Yap D, Bernard V, McPherson A, Shumansky K, Crisan A, Giuliany R, Heravi-Moussavi A, Rosner J, Lai D, Birol I, Varhol R, Tam A, Dhalla N, Zeng T, Ma K, Chan SK, Griffith M, Moradian A, Cheng SW, Morin GB, Watson P, Gelmon K, Chia S, Chin SF, Curtis C, Rueda OM, Pharoah PD, Damaraju S, Mackey J, Hoon K, Harkins T, Tadigotla V, Sigaroudinia M, Gascard P, Tlsty T, Costello JF, Meyer IM, Eaves CJ, Wasserman WW, Jones S, Huntsman D, Hirst M, Caldas C, Marra MA, Aparicio S. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486(7403):395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng CK, Schultheis AM, Bidard FC, Weigelt B, Reis-Filho JS. Breast cancer genomics from microarrays to massively parallel sequencing: paradigms and new insights. J Natl Cancer Inst. 2015;107(5):djv015. doi: 10.1093/jnci/djv015. [DOI] [PubMed] [Google Scholar]
- 7.Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB, Martin S, Wedge DC, Van Loo P, Ju YS, Smid M, Brinkman AB, Morganella S, Aure MR, Lingjærde OC, Langerød A, Ringnér M, Ahn SM, Boyault S, Brock JE, Broeks A, Butler A, Desmedt C, Dirix L, Dronov S, Fatima A, Foekens JA, Gerstung M, Hooijer GK, Jang SJ, Jones DR, Kim HY, King TA, Krishnamurthy S, Lee HJ, Lee JY, Li Y, McLaren S, Menzies A, Mustonen V, O’Meara S, Pauporté I, Pivot X, Purdie CA, Raine K, Ramakrishnan K, Rodríguez-González FG, Romieu G, Sieuwerts AM, Simpson PT, Shepherd R, Stebbings L, Stefansson OA, Teague J, Tommasi S, Treilleux I, Van den Eynden GG, Vermeulen P, Vincent-Salomon A, Yates L, Caldas C, van’t Veer L, Tutt A, Knappskog S, Tan BK, Jonkers J, Borg Å, Ueno NT, Sotiriou C, Viari A, Futreal PA, Campbell PJ, Span PN, Van Laere S, Lakhani SR, Eyfjord JE, Thompson AM, Birney E, Stunnenberg HG, van de Vijver MJ, Martens JW, Børresen-Dale AL, Richardson AL, Kong G, Thomas G, Stratton MR. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47–54. doi: 10.1038/nature17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9(2):176–198. doi: 10.1158/2159-8290.CD-18-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bareche Y, Venet D, Ignatiadis M, Aftimos P, Piccart M, Rothe F, Sotiriou C. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol. 2018;29(4):895–902. doi: 10.1093/annonc/mdy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He J, Peng R, Yuan Z, Wang S, Peng J, Lin G, Jiang X, Qin T. Prognostic value of androgen receptor expression in operable triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol. 2012;29(2):406–410. doi: 10.1007/s12032-011-9832-0. [DOI] [PubMed] [Google Scholar]
- 12.Zuo T, Wilson P, Cicek AF, Harigopal M. Androgen receptor expression is a favorable prognostic factor in triple-negative breast cancers. Hum Pathol. 2018;80:239–245. doi: 10.1016/j.humpath.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Riaz N, Idress R, Habib S, Lalani EN. Lack of androgen receptor expression selects for basal-like phenotype and is a predictor of poor clinical outcome in non-metastatic triple negative breast cancer. Front Oncol. 2020;10:1083. doi: 10.3389/fonc.2020.01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Yu D, Kwon Y, Lee KS, Sim SH, Kong SY, Lee ES, Park IH, Park C. Genomic characteristics of triple-negative breast cancer nominate molecular subtypes that predict chemotherapy response. Mol Cancer Res. 2020;18(2):253–263. doi: 10.1158/1541-7786.MCR-19-0453. [DOI] [PubMed] [Google Scholar]
- 15.Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, Blackwell K, Rugo H, Nabell L, Forero A, Stearns V, Doane AS, Danso M, Moynahan ME, Momen LF, Gonzalez JM, Akhtar A, Giri DD, Patil S, Feigin KN, Hudis CA, Traina TA, Translational Breast Cancer Research Consortium (TBCRC 011) Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res. 2013;19(19):5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O’Shaughnessy J, Gradishar W, Schmid P, Winer E, Kelly C, Nanda R, Gucalp A, Awada A, Garcia-Estevez L, Trudeau ME, Steinberg J, Uppal H, Tudor IC, Peterson A, Cortes J. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018;36(9):884–890. doi: 10.1200/JCO.2016.71.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann BD, Abramson VG, Sanders ME, Mayer EL, Haddad TC, Nanda R, Van Poznak C, Storniolo AM, Nangia JR, Gonzalez-Ericsson PI, Sanchez V, Johnson KN, Abramson RG, Chen SC, Shyr Y, Arteaga CL, Wolff AC, Pietenpol JA, Translational Breast Cancer Research Consortium TBCRC 032 IB/II multicenter study: Molecular insights to AR antagonist and PI3K inhibitor efficacy in patients with AR+ metastatic triple-negative breast cancer. Clin Cancer Res. 2020;26(9):2111–2123. doi: 10.1158/1078-0432.CCR-19-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asghar US, Barr AR, Cutts R, Beaney M, Babina I, Sampath D, Giltnane J, Lacap JA, Crocker L, Young A, Pearson A, Herrera-Abreu MT, Bakal C, Turner NC. Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast cancer. Clin Cancer Res. 2017;23(18):5561–5572. doi: 10.1158/1078-0432.CCR-17-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, Schnall-Levin M, White J, Sanford EM, An P, Sun J, Juhn F, Brennan K, Iwanik K, Maillet A, Buell J, White E, Zhao M, Balasubramanian S, Terzic S, Richards T, Banning V, Garcia L, Mahoney K, Zwirko Z, Donahue A, Beltran H, Mosquera JM, Rubin MA, Dogan S, Hedvat CV, Berger MF, Pusztai L, Lechner M, Boshoff C, Jarosz M, Vietz C, Parker A, Miller VA, Ross JS, Curran J, Cronin MT, Stephens PJ, Lipson D, Yelensky R. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel SM, Koringa PG, Reddy BB, Nathani NM, Joshi CG. In silico analysis of consequences of non-synonymous SNPs of Slc11a2 gene in Indian bovines. Genom Data. 2015;5:72–79. doi: 10.1016/j.gdata.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi Y, Sims GE, Murphy S, Miller JR, Chan AP. Predicting the functional effect of amino acid substitutions and indels. PLoS One. 2012;7(10):e46688. doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11(4):361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 24.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnedos M, Gligorov J. St Gallen International Consensus Guidelines in early breast cancer: experts to prevent patients’ overtreatment and breaking the bank. Ann Oncol. 2019;30(10):1533–1535. doi: 10.1093/annonc/mdz292. [DOI] [PubMed] [Google Scholar]
- 26.Tirrò E, Massimino M, Romano C, Pennisi MS, Stella S, Vitale SR, Fidilio A, Manzella L, Parrinello NL, Stagno F, Palumbo GA, La Cava P, Romano A, Di Raimondo F, Vigneri PG. Chk1 inhibition restores inotuzumab ozogamicin citotoxicity in CD22-positive cells expressing mutant p53. Front Oncol. 2019;9:57. doi: 10.3389/fonc.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirosa MC, Leotta S, Cupri A, Stella S, Martino EA, Scalise L, Sapienza G, Calafiore V, Mauro E, Spadaro A, Vigneri P, Di Raimondo F, Milone G. Long-term molecular remission achieved by antibody anti-CD22 and ponatinib in a patient affected by Ph’+ acute lymphoblastic leukemia relapsed after second allogeneic hematopoietic stem cell transplantation: a case report. Chemotherapy. 2018;63(4):220–224. doi: 10.1159/000492941. [DOI] [PubMed] [Google Scholar]
- 28.Mehnert JM, Varga A, Brose MS, Aggarwal RR, Lin CC, Prawira A, de Braud F, Tamura K, Doi T, Piha-Paul SA, Gilbert J, Saraf S, Thanigaimani P, Cheng JD, Keam B. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced, PD-L1-positive papillary or follicular thyroid cancer. BMC Cancer. 2019;19(1):196. doi: 10.1186/s12885-019-5380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massimino M, Tirrò E, Stella S, Frasca F, Vella V, Sciacca L, Pennisi MS, Vitale SR, Puma A, Romano C, Manzella L. Effect of combined epigenetic treatments and ectopic NIS expression on undifferentiated thyroid cancer cells. Anticancer Res. 2018;38(12):6653–6662. doi: 10.21873/anticanres.13032. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann BD, Jovanović B, Chen X, Estrada MV, Johnson KN, Shyr Y, Moses HL, Sanders ME, Pietenpol JA. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS One. 2016;11(6):e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerratana L, Basile D, Buono G, De Placido S, Giuliano M, Minichillo S, Coinu A, Martorana F, De Santo I, Del Mastro L, De Laurentiis M, Puglisi F, Arpino G. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat Rev. 2018;68:102–110. doi: 10.1016/j.ctrv.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Bonnefoi H, Grellety T, Tredan O, Saghatchian M, Dalenc F, Mailliez A, L’Haridon T, Cottu P, Abadie-Lacourtoisie S, You B, Mousseau M, Dauba J, Del Piano F, Desmoulins I, Coussy F, Madranges N, Grenier J, Bidard FC, Proudhon C, MacGrogan G, Orsini C, Pulido M, Gonçalves A. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1) Ann Oncol. 2016;27(5):812–818. doi: 10.1093/annonc/mdw067. [DOI] [PubMed] [Google Scholar]
- 33.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K, Mitchell EP. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 34.Weisman PS, Ng CK, Brogi E, Eisenberg RE, Won HH, Piscuoglio S, De Filippo MR, Ioris R, Akram M, Norton L, Weigelt B, Berger MF, Reis-Filho JS, Wen HY. Genetic alterations of triple negative breast cancer by targeted next-generation sequencing and correlation with tumor morphology. Mod Pathol. 2016;29(5):476–488. doi: 10.1038/modpathol.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGuire A, Brown JA, Malone C, McLaughlin R, Kerin MJ. Effects of age on the detection and management of breast cancer. Cancers (Basel) 2015;7(2):908–929. doi: 10.3390/cancers7020815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNamara KM, Yoda T, Miki Y, Chanplakorn N, Wongwaisayawan S, Incharoen P, Kongdan Y, Wang L, Takagi K, Mayu T, Nakamura Y, Suzuki T, Nemoto N, Miyashita M, Tamaki K, Ishida T, Ohuchi N, Sasano H. Androgenic pathway in triple negative invasive ductal tumors: its correlation with tumor cell proliferation. Cancer Sci. 2013;104(5):639–646. doi: 10.1111/cas.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Losurdo A, De Sanctis R, Fernandes B, Torrisi R, Masci G, Agostinetto E, Gatzemeier W, Errico V, Testori A, Tinterri C, Roncalli M, Santoro A. Insights for the application of TILs and AR in the treatment of TNBC in routine clinical practice. Sci Rep. 2020;10(1):20100. doi: 10.1038/s41598-020-77043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehmann BD, Bauer JA, Schafer JM, Pendleton CS, Tang L, Johnson KC, Chen X, Balko JM, Gómez H, Arteaga CL, Mills GB, Sanders ME, Pietenpol JA. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014;16(4):406. doi: 10.1186/s13058-014-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, Scardino PT, Rosen N, Sawyers CL. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19(5):575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Romigh T, He X, Tan MH, Orloff MS, Silverman RH, Heston WD, Eng C. Differential regulation of PTEN expression by androgen receptor in prostate and breast cancers. Oncogene. 2011;30(42):4327–4338. doi: 10.1038/onc.2011.144. [DOI] [PubMed] [Google Scholar]
- 41.Coussy F, Lavigne M, de Koning L, Botty RE, Nemati F, Naguez A, Bataillon G, Ouine B, Dahmani A, Montaudon E, Painsec P, Chateau-Joubert S, Laetitia F, Larcher T, Vacher S, Chemlali W, Briaux A, Melaabi S, Salomon AV, Guinebretiere JM, Bieche I, Marangoni E. Response to mTOR and PI3K inhibitors in enzalutamide-resistant luminal androgen receptor triple-negative breast cancer patient-derived xenografts. Theranostics. 2020;10(4):1531–1543. doi: 10.7150/thno.36182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitale SR, Martorana F, Stella S, Motta G, Inzerilli N, Massimino M, Tirrò E, Manzella L, Vigneri P. PI3K inhibition in breast cancer: Identifying and overcoming different flavors of resistance. Crit Rev Oncol Hematol. 2021;162:103334. doi: 10.1016/j.critrevonc.2021.103334. [DOI] [PubMed] [Google Scholar]
- 43.Aung KL, Stockley TL, Serra S, Kamel-Reid S, Bedard PL, Siu LL. Testing ERBB2 p.L755S kinase domain mutation as a druggable target in a patient with advanced colorectal cancer. Cold Spring Harb Mol Case Stud. 2016;2(5):a001016. doi: 10.1101/mcs.a001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, Monsey J, Goel N, Aronson AB, Li S, Ma CX, Ding L, Mardis ER, Ellis MJ. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 46.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 47.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang T, Xu Y, Sheng S, Yuan H, Ouyang T, Li J, Wang T, Fan Z, Fan T, Lin B, Xie Y. HER2 somatic mutations are associated with poor survival in HER2-negative breast cancers. Cancer Sci. 2017;108(4):671–677. doi: 10.1111/cas.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Martino M, Zhuang D, Klatte T, Rieken M, Rouprêt M, Xylinas E, Clozel T, Krzywinski M, Elemento O, Shariat SF. Impact of ERBB2 mutations on in vitro sensitivity of bladder cancer to lapatinib. Cancer Biol Ther. 2014;15(9):1239–1247. doi: 10.4161/cbt.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deniziaut G, Tille JC, Bidard FC, Vacher S, Schnitzler A, Chemlali W, Trémoulet L, Fuhrmann L, Cottu P, Rouzier R, Bièche I, Vincent-Salomon A. ERBB2 mutations associated with solid variant of high-grade invasive lobular breast carcinomas. Oncotarget. 2016;7(45):73337–73346. doi: 10.18632/oncotarget.11819. [DOI] [PMC free article] [PubMed] [Google Scholar]