Summary
Glioma is a highly fatal cancer with prognostically significant molecular subtypes and few known risk factors. Multiple studies have implicated infections in glioma susceptibility, but evidence remains inconsistent. Genetic variants in the human leukocyte antigen (HLA) region modulate host response to infection and have been linked to glioma risk. In this study, we leveraged genetic predictors of antibody response to 12 viral antigens to investigate the relationship with glioma risk and survival. Genetic reactivity scores (GRSs) for each antigen were derived from genome-wide-significant (p < 5 × 10−8) variants associated with immunoglobulin G antibody response in the UK Biobank cohort. We conducted parallel analyses of glioma risk and survival for each GRS and HLA alleles imputed at two-field resolution by using data from 3,418 glioma-affected individuals subtyped by somatic mutations and 8,156 controls. Genetic reactivity scores to Epstein-Barr virus (EBV) ZEBRA and EBNA antigens and Merkel cell polyomavirus (MCV) VP1 antigen were associated with glioma risk and survival (Bonferroni-corrected p < 0.01). GRSZEBRA and GRSMCV were associated in opposite directions with risk of IDH wild-type gliomas (ORZEBRA = 0.91, p = 0.0099/ORMCV = 1.11, p = 0.0054). GRSEBNA was associated with both increased risk for IDH mutated gliomas (OR = 1.09, p = 0.040) and improved survival (HR = 0.86, p = 0.010). HLA-DQA1∗03:01 was significantly associated with decreased risk of glioma overall (OR = 0.85, p = 3.96 × 10−4) after multiple testing adjustment. This systematic investigation of the role of genetic determinants of viral antigen reactivity in glioma risk and survival provides insight into complex immunogenomic mechanisms of glioma pathogenesis. These results may inform applications of antiviral-based therapies in glioma treatment.
Keywords: glioma, human leukocyte antigen, Epstein-Barr virus, Merkel cell polyomavirus, polygenic risk score
Introduction
Studies linking viruses and cancer date back over 100 years1 and laid the foundation for understanding oncogenes.2 It has also become increasingly clear, as well evidenced by the current pandemic, that host genetics play an important role in response to viruses.3,4 To date, seven viruses have been accepted to be tumor-initiating in humans: Epstein-Barr virus (EBV), hepatitis B virus (HBV), human papillomavirus (HPV), human T-lymphotropic virus-1 (HTLV-1), hepatitis C (HCV), Kaposi’s sarcoma herpesvirus (HHV-8), and Merkel cell polyomavirus (MCV).5 Recent analyses have shown that infections account for approximately 13% of human cancers worldwide.6 However viruses have not been definitively implicated in the etiology of glioma despite decades of suggestive associations.7, 8, 9, 10, 11
Glioma (MIM: 137800) is a highly fatal brain cancer with a paucity of known risk factors, and exposure to ionizing radiation is an accepted causal factor.12 A history of previous infection with Varicella-Zoster virus (VZV) has been the only infectious agent consistently linked to adult glioma, conferring an estimated 20% decrease in risk.10,13 A suite of other viruses have been associated with the risk and grade of glioma, including EBV, MCV, John Cunningham virus (JCV), BK virus (BKV), human Cytomegalovirus (CMV), and human herpesvirus-6 (HHV-6), but with discordant results.11,14, 15, 16 The identification of key somatic molecular alterations (e.g., IDH mutation [MIM: 147700], 1p/19q chromosomal arm codeletion, TERT mutation [MIM :187270]), which drastically affect glioma prognosis, have uncovered subtype-specific risk factors.17,18 Recently, several studies/clinical trials have suggested a prognostic benefit of antiviral medications in the treatment of glioma.19, 20, 21
Genetic host response to viral infection could play a role in elucidating potential links between viruses and cancer incidence and prognosis. Studies have demonstrated significant heritable components (32%–48%) of antibody response to many viruses and have identified genetic loci within host genes related to cell entry, cytokine production, and immune response.22, 23, 24 Genetic variants of class I and II human leukocyte antigen (HLA) genes contribute the most important identified components of genetic determinants of response to viral antigens. Class II genes each encode half of a heterodimeric class II HLA protein, which presents extra-cellularly derived peptides to CD4+ helper T cells. The immune response is triggered when a CD4+ T cell recognizes the combination of a class II HLA protein and its bound peptide. It is well studied that CD4+ T cells have an important role in creating and sustaining effective anti-tumor immunity.25
The HLA region of the genome is considered the most polymorphic region of the human genetic system26 where polymorphisms have been shown to alter the risk and progression of disease in a variety of autoimmune (notably HLA class II) and malignant conditions.27,28 Certain HLA haplotypes have shown non-additive epistatic effects on glioma risk,29 yet no germline variants within the HLA have been directly identified as risk loci in a glioma genome-wide association study (GWAS).
In this study, we leveraged previously published genome-wide SNP associations with viral antibody response30 to generate genetically inferred antigen reactivity profiles in glioma cases and controls and evaluated their association with risk and survival by major glioma subtypes. We further conducted imputed HLA gene association analysis with glioma risk and survival and our findings suggest a convergence of genetic mechanisms regulating host immune response to viral challenge and glioma development and progression.
Subjects and methods
Ethics
Collection of affected individual samples and associated clinicopathological information was undertaken with written informed consent and relevant ethical review board approval at the respective study centers in accordance with the tenets of the Declaration of Helsinki. Specifically, informed consent and ethical board approval was obtained from the UCSF Committee on Human Research (USA) and the Mayo Clinic Office for Human Research Protection (USA). The diagnosis of glioma (ICDO-3 codes 9380–9480 or equivalent) was established through histology in all cases in accordance with World Health Organization guidelines.
Study populations
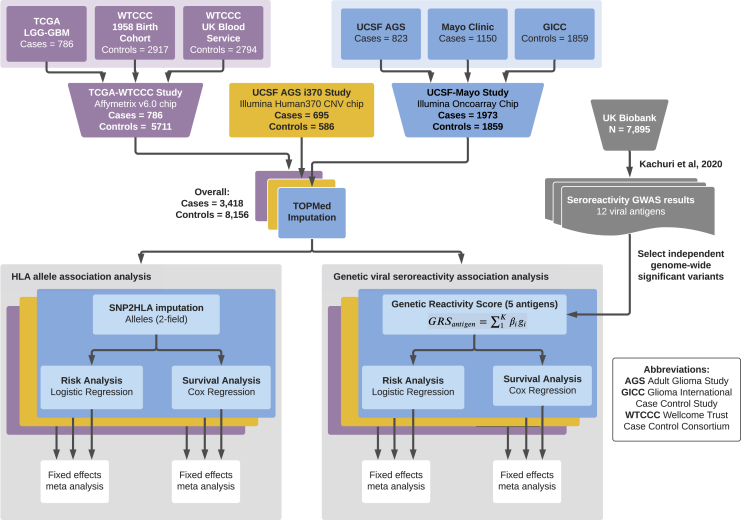
We analyzed three glioma case-control sets assembled on the basis of genotyping platform and study population for a total sample size of 3,418 cases and 8,156 controls (Figure 1, Table 1). The first set included 1,973 cases from the Mayo Clinic and University of California San Francisco (UCSF) Adult Glioma Study and 1,859 controls from the Glioma International Case-Control Study (GICC) who were genotyped on the Illumina OncoArray, as previously described.18,31, 32, 33, 34 The second dataset (AGS-i370) included 659 cases and 586 controls from the UCSF Adult Glioma study genotyped on the Illumina HumanHap370duo panel.32 The third dataset included 786 glioma cases from The Cancer Genome Atlas (TCGA) with available molecular data genotyped on the Affymetrix 6.0 array. Cancer-free controls were assembled from two Wellcome Trust Case Control Consortium (WTCCC) studies genotyped using the Affymetrix 6.0 array: 2,917 controls from the 1958 British Birth cohort and 2,794 controls from the UK Blood Service control group.
Figure 1.
Summary of data processing and analysis
Our analysis consisted of three glioma case-control datasets. The first dataset (purple) included 786 glioma cases from The Cancer Genome Atlas (TCGA) genotyped on the Affymetrix 6.0 array and cancer-free controls assembled from two Wellcome Trust Case Control Consortium (WTCCC) studies genotyped with the Affymetrix 6.0 array: 2,917 controls from the 1958 British Birth cohort and 2,794 controls from the UK Blood Service control group. The second dataset (yellow) included 659 cases and 586 controls from the University of California, San Francisco (UCSF) Adult Glioma Study (AGS) genotyped on the Illumina HumanHap370duo panel.32 The third set (blue) included 1,973 cases from the Mayo Clinic and UCSF AGS and 1,859 controls from the Glioma International Case-Control Study (GICC) who were genotyped on the Illumina OncoArray, as previously described.18,31, 32, 33, 34 The three resulting case-control datasets were processed through quality controls as described in the main text and imputed with the TOPMed imputation server. SNP2HLA was used to impute HLA alleles from SNP data. Risk and survival analyses were performed separately on each study's imputed HLA alleles, on multiple glioma molecular subtypes, and a fixed-effects meta-analysis was performed to aggregate results. Separately, genetic reactivity scores to five viral antigens were created with previously published GWAS data. For cases and controls across the three studies, a genetic reactivity score (GRS) for antibody response to each of the five antigens was calculated for each individual with the available sequencing data. Risk and survival analyses were performed separately on each study with each GRS as a predictor, with results aggregated via a fixed effects inverse-variance-weighted meta-analysis for each subset of glioma patients and controls based on molecular subtype.
Table 1.
Clinical and molecular summary of the three case-control datasets
|
UCSF-Mayo cases N = 1,973 |
GICC controls N = 1,859 |
AGS i370 cases N = 659 |
AGS i370 controls N = 586 |
TCGA cases N = 786 |
WTCCC controls N = 5,711 |
|
|---|---|---|---|---|---|---|
| Age | ||||||
| <40 | 983 (50%) | 538 (29%) | 101 (15%) | 85 (15%) | 213 (27%) | 0 (0%) |
| 40–59 | 476 (24%) | 555 (30%) | 329 (50%) | 252 (43%) | 279 (36%) | 0 (0%) |
| ≥60 | 514 (26%) | 766 (41%) | 229 (35%) | 249 (42%) | 245 (31%) | 0 (0%) |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 49 (6%) | 5,711 (100%) |
| Sex | ||||||
| Female | 822 (42%) | 744 (40%) | 229 (35%) | 280 (48%) | 328 (42%) | 2,819 (49%) |
| Male | 1,151 (58%) | 1,115 (60%) | 430 (65%) | 306 (52%) | 458 (58%) | 2,892 (51%) |
| IDH mutation status | ||||||
| Mutant | 588 (30%) | N/A | 111 (17%) | N/A | 375 (48%) | N/A |
| Wild type | 699 (35%) | N/A | 416 (63%) | N/A | 364 (46%) | N/A |
| Missing | 686 (35%) | N/A | 132 (20%) | N/A | 47 (6%) | N/A |
| Molecular subtype based on IDH mutation and 1p/19q codeletion | ||||||
| MT-codel | 244 (12%) | N/A | 9 (1%) | N/A | 143 (18%) | N/A |
| MT-noncodel | 291 (15%) | N/A | 94 (14%) | N/A | 230 (29%) | N/A |
| WT-noncodel | 507 (26%) | N/A | 416 (63%) | N/A | 357 (46%) | N/A |
| Missing/other | 905 (47%) | N/A | 140 (22%) | N/A | 56 (7%) | N/A |
N/A, not applicable to controls.
Molecular subtype information (IDH mutation and 1p/19q codeletion status) was downloaded from Ceccarelli et al.35 Table S1 for the third dataset and was provided directly from the UCSF AGS and Mayo Clinic for the first and second datasets.
Quality control and imputation
Standard quality control procedures were implemented prior to imputation. Analyses were restricted to individuals of predominantly (>70%) European ancestry, determined with ADMIXTURE36 and the HapMap 3 reference populations. Within each ancestral group, we removed samples with excess heterozygosity (>3 standard deviations [SD] from mean), <95% call rates, and discordant self-reported and genetically inferred sex. Relatedness checks were performed within each study with KING37 (kinship > 0.12), filtering out up to second-degree relations and retaining the samples with higher call rate. TCGA blood samples were preferentially chosen when both blood and tumor sequencing data were available for the same affected individual (693 European samples had both blood and tumor samples available). SNPs with <95% call rate were removed, along with variants deviating from Hardy-Weinberg equilibrium (p < 10−6) or at a low minor allele frequency (MAF < 0.005). Samples genotyped on the same platform (i.e., Affymetrix 6.0 for TCGA and WTCCC) were imputed together with the multi-ethnic TOPMed reference panel (ver. r2).
Statistical analysis
Genetically predicted viral antigen response
We calculated genetic reactivity scores (GRSs) to obtain genetically inferred viral antigen response profiles in each of the glioma datasets. For each GRS, candidate variants and corresponding effect sizes were obtained from genome-wide summary statistics for seroreactivity to 12 viral antigens previously identified in Kachuri and Francis et al. (2020)30 in 7,895 randomly selected individuals of European descent from the UK Biobank (UKB) cohort (aged 40–69) with serological measures of immunoglobulin G (IgG) antibody response,38 a stable biomarker of lifetime exposure to common viruses. For each antigen, we preferentially selected independent SNPs (linkage disequilibrium, LD, r2 < 0.01 within 500 kb) with the lowest p value among genome-wide-significant variants (p < 5 × 10−8). LD proxies (r2 > 0.9) were obtained for variants unavailable in the target glioma datasets. For each individual, an antigen-specific GRS was calculated as a weighted sum with weights (β) corresponding to a standard deviation increase in antibody response:
| GRSAntigen = β1xSNP1+ … +βkxSNPk. |
Each GRS was calculated with a minimum of four variants with MAF ≥ 0.01 and imputation quality (R2 > 0.8) and then standardized within each dataset.
Antigen GRS associations with glioma susceptibility
GRS associations with disease risk were examined for glioma overall and for molecular subtypes defined by the specific tumor alterations: IDH mutation status and 1p/19q chromosomal arm codeletion. For each GRS, odds ratios (ORs) were estimated with logistic regression models adjusted for age, sex, and the first ten genetic ancestry principal components (PCs). Models in the UCSF-Mayo dataset were further adjusted for contributing site. GRS associations from each study were meta-analyzed with a fixed-effects inverse-variance-weighted approach. Heterogeneity in study-specific GRS associations was assessed with Cochran’s Q test.
Antigen GRS associations with glioma survival
The association between each antigen-GRS and overall survival was assessed with a Cox proportional hazards regression model with follow-up time calculated from the date of first surgery to either date of death or last known contact. The latter censored at that date. Analyses were conducted for glioma overall and molecular subtypes with a minimum of 50 cases and 20 events (deaths). Proportionality assumptions were checked within each dataset via examination of Kaplan-Meier curves. Hazard ratios (HRs) were estimated with Cox models adjusted for age, sex, ten genetic PCs, and study site (if applicable). Associations with survival in each study were combined via fixed-effects meta-analysis. For each nominally significant (p < 0.05) glioma-GRS association, survival differences were further assessed with Kaplan-Meier curves by comparing mortality trajectories in affected individuals with high genetically predicted immune reactivity (top 20%) to the remainder.
Regional HLA analyses of glioma risk and survival
Classical HLA alleles were imputed for samples in all cohorts at two-field resolution with SNP2HLA39 and the Type 1 Diabetes Genetics Consortium (T1DGC) reference panel of 2,767 unrelated individuals. Associations were tested for 77 alleles (MAF ≥ 0.01) with imputation quality > 0.4 across eight genes: HLA-A (MIM: 142800), HLA-B (MIM: 142830), HLA-C (MIM: 142840), HLA-DPA1 (MIM: 142880), HLA-DPB1 (MIM: 142858), HLA-DQA1 (MIM: 146880), HLA-DQB1 (MIM: 604305), and HLA-DRB1(MIM: 142857).
Subtype-specific associations with risk were estimated with logistic regression models. HLA allele associations with mortality were assessed with SPACox,40 an extension of the Cox model with improved type I error control in high-dimensional settings. Study-specific risk and survival associations were combined in a meta-analysis. Associations for each HLA allele were considered statistically significant if p < 6.5 × 10−4 (0.05/77 alleles).
Results
The creation of a GRS was attempted for all antigens with genome-wide-significant seroreactivity-associated variants (p < 5 × 10−8) as reported in Kachuri and Francis et al. (2020).30 This included 12 antigens: four EBV antigens (EA-D, EBNA, p18, and ZEBRA) and antigens for BKV, HHV7, HSV1, JCV, MCV, VZV, CMV, and HHV6. An antigen-specific GRS was considered successfully created if at least four independent SNPs passed LD clumping and thresholding and were present (or via proxy SNP) in the three glioma study datasets. GRSs were successfully generated for EBV EA-D, EBV EBNA, EBV p18, EBV ZEBRA, and MCV. Only one independent SNP remained for each of HSV1, BKV, JCV, and VZV after accounting for long-range LD in the HLA region, although numerous SNPs met genome-wide significance in the previous seroreactivity study. Three SNPs remained for HHV7, each on a different chromosome (6, 11, and 17). CMV and HHV6 each had only one significantly associated SNP in the previously published GWAS and therefore a GRS was not considered for either. Complete information of variants considered for all 12 viral antigens, including nearest gene, allele frequencies, and corresponding glioma risk associations, are reported in Table S1. Allele frequencies of all considered SNPs were similar between our datasets and the UK Biobank cohort (maximum frequency difference = 0.0401). LD correlations for all associated chromosome 6 variants are available in a heatmap in Figure S1.
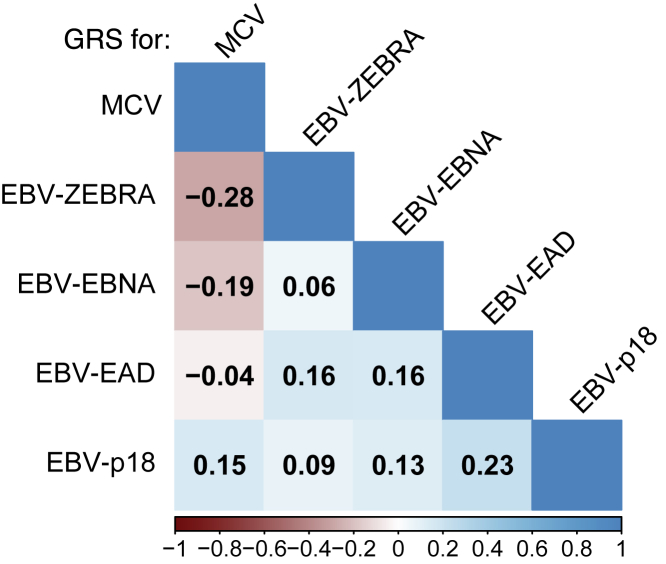
The variants included in each of the five created GRSs were overwhelmingly located in the HLA region, and few predictors were located elsewhere across the genome: rs67886110 in 3q25.1 an eQTL for MED12L (MIM: 611318) and P2RY12 (MIM: 600515) (EBV EBNA), rs7618405 in 3p24.3 (MCV), and rs7444313 in 5q31.2 near TMEM173 (MIM: 612374) (MCV). Of the 38 SNPs included across the five GRSs, two had a significant association (Bonferroni-corrected: p < 1.3 × 10−3) with overall glioma risk: rs9265517 in HLA-B (EBV EBNA), p = 3.08 × 10−4 and rs9268847 near HLA-DRB9 (MCV), p = 2.86 × 10−4. Figure S2 visualizes the correlation between the effect-increasing GRS alleles and each imputed HLA allele (two field resolution) from UCSF-Mayo cases and controls. Notably, the GRS for MCV was inversely correlated with GRSs for EBV ZEBRA (Pearson's r = −0.28, p = 3.89 × 10−70) and EBV EBNA (r = −0.19, p = 6.28 × 10−31) (Figure 2).
Figure 2.
GRS correlations within the UCSF-Mayo cases and controls
Pearson correlations between genetically predicted antigen responses (via GRS) as computed in the UCSF-Mayo glioma cases and controls. Values were printed in each block if and only if the associated correlation test p value was less than 0.01.
Viral antigen GRS associations with glioma risk
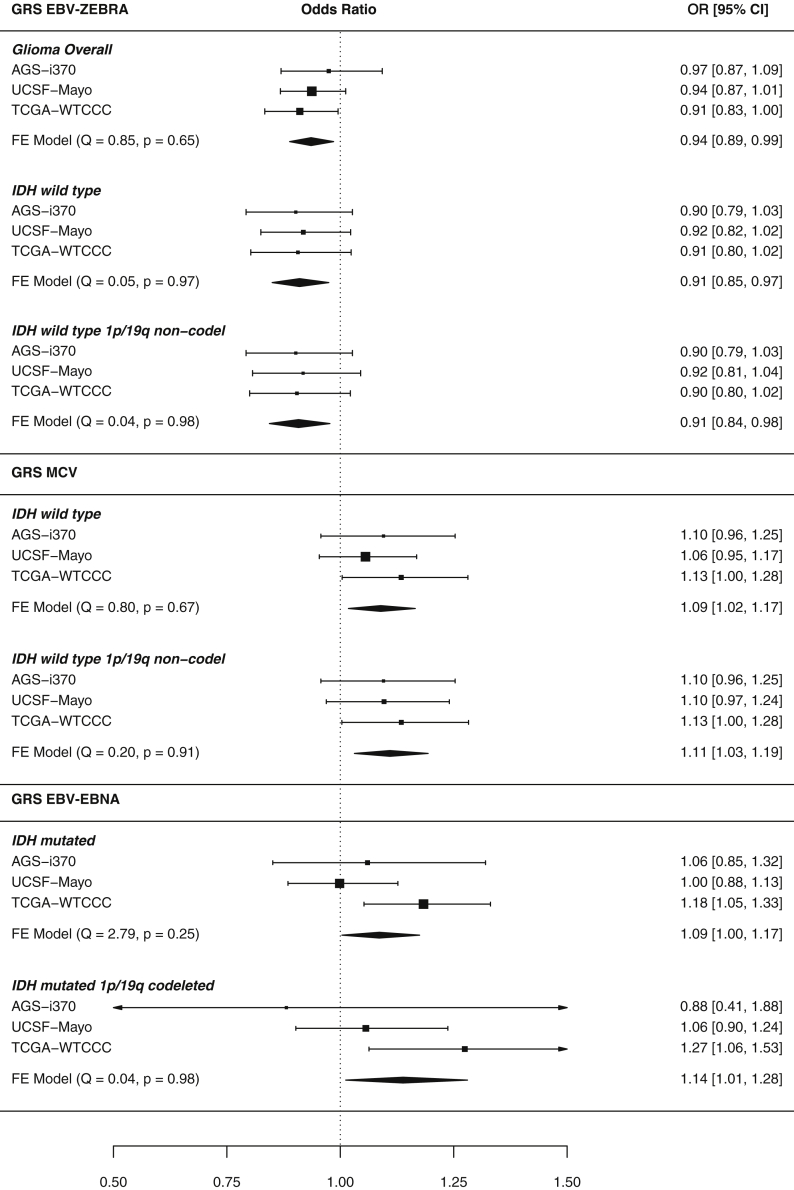
In the combined meta-analysis of three case-control studies, three GRSs reached at least nominal significance (p < 0.05), and some associations remained statistically significant after correction for the number of antigens tested (Bonferroni: p < 0.05/5 = 0.01) (Figure 3). Genetic predisposition to an increased serological response to EBV ZEBRA was inversely associated with risk of glioma overall (per 1 SD increase in GRS: odds ratio, ORZEBRA = 0.94, 95% confidence interval 0.89–0.99, p = 0.012, 3,418 cases). GRSEBNA was associated with an increased risk of IDH mutated gliomas (OREBNA = 1.09, 1.004–1.18, p = 0.040, 1,074 cases) and the magnitude of this effect persisted for IDH mutated 1p/19q codeleted gliomas (OREBNA = 1.14, 1.012–1.28, p = 0.031, 396 cases).
Figure 3.
Significant GRS-glioma subtype risk association meta-analysis forest plots
Forest plot meta-analysis results of GRS-glioma risk associations that were at least nominally statistically significant (p < 0.05). Response to antigens EBV ZEBRA, MCV, and EBV EBNA had associations that reached this threshold. Results are reported as odds ratios along with 95% confidence intervals. Briefly, each header indicates the studied viral antigen GRS, within are its association with molecular glioma subtypes reported with p < 0.05 and the 95% confidence interval of each study-specific effect. The diamond visualizes the 95% confidence interval for the fixed effect (FE) meta-analysis across all three studies. Each meta-analysis was tested for between-study heterogeneity (Q statistic), and p < 0.05 indicates evidence of study-specific associations.
We observed some evidence of antagonistic pleiotropy between genetic determinants of antibody response to EBV ZEBRA and MCV, which generalized across glioma subtypes. GRSZEBRA and GRSMCV were associated with susceptibility to IDH wild-type gliomas, but in opposite directions: higher genetically predicted reactivity to EBV ZEBRA was inversely associated with IDH wild type glioma risk (ORZEBRA = 0.91, 0.85–0.98, p = 0.0072, 1,479 cases), while increased predicted antibody response to MCV conferred an increased risk (ORMCV = 1.09, 1.02–1.17, p = 0.013). This pattern persisted for IDH wild-type 1p/19q non-codeleted gliomas (ORZEBRA = 0.91, 0.84–0.9, p = 0.0099; ORMCV = 1.11, 1.03–1.19, p = 0.0054, 1,280 cases). When GRSZEBRA and GRSMCV were tested together along with interaction term in a single logistic model, we observed no significant interaction (interaction term p = 0.34 in IDH wild-type risk). The general correlation between GRSZEBRA and GRSMCV (Pearson’s r = −0.28, p = 3.9 × 10−70 in UCSF-Mayo, Figure 2) suggests a possible shared underlying genetic mechanism between the response to the two antigens. This genetic correlation is most likely driven by the significant inverse relationship of the effect increasing allele of rs9268847 near HLA-DRB9 (MCV) with both rs9274728 near HLA-DQB1 (EBV ZEBRA) (Pearson's r = −0.38, p = 2.6 × 10−134) and rs7757696 near HLA-DQA1 (EBV ZEBRA) (r = −0.23, p = 3.1 × 10−45). The odds ratios were robust to the level of genetic ancestry controlled for, as measured by the number of genetic principal components included in the model (Table S6). Results reported here were adjusted for the first ten PCs, but analysis was conducted in parallel with 15 and 20 PCs.
Viral antigen GRS-survival associations
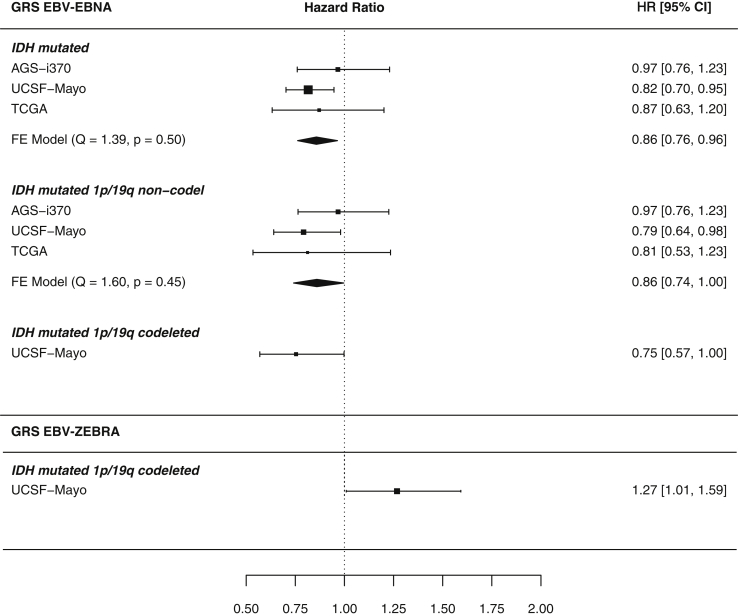
The number of available cases and deaths across the three studies is available in Table 2. Associations between genetically predicted viral antigen response profiles and survival were restricted to IDH mutated gliomas (Figure 4). GRSEBNA was associated with survival in 1,074 IDH mutated glioma cases (per 1 SD increase: hazard ratio, HR = 0.86, 0.76–0.96, p = 0.010, 325 events), suggesting that a higher genetically predicted reactivity to EBV EBNA improved duration of survival.
Table 2.
Summary of available cases and events for survival analyses
| Molecular subtype |
UCSF-Mayo |
TCGA |
AGS i370 |
|||
|---|---|---|---|---|---|---|
| cases | events | cases | events | cases | events | |
| Glioma | 1,973 | 1,218 | 786 | 310 | 659 | 592 |
| IDH mutateda | 588 | 201 | 375 | 50 | 111 | 74 |
| 1p/19q codeletedb | 244 | 64 | 143 | 13 | 9 | 1 |
| 1p/19q non-codeletedb | 291 | 117 | 230 | 36 | 94 | 69 |
| IDH wild typea | 699 | 594 | 364 | 228 | 416 | 402 |
| 1p/19q non-codeletedc | 507 | 458 | 357 | 224 | 416 | 402 |
Study/subtype combinations with less than 50 cases or 20 events were not utilized in the meta-analysis of survival associations.
Further subset of Glioma.
Further subset of IDH mutated.
Further subset of IDH wild type.
Figure 4.
Nominal GRS-glioma subtype survival association meta-analysis forest plots
Forest plot meta-analysis results of GRS-glioma survival associations that were at least nominally statistically significant (p < 0.05). Genetically inferred response to antigens EBV ZEBRA, MCV, and EBV EBNA had associations that reached this threshold. Results are reported as hazard ratios along with 95% confidence intervals. Briefly, each header indicates the studied viral antigen GRS, within are its association with molecular glioma subtypes reported with p < 0.05 and the 95% confidence interval of each study-specific effect. The diamond visualizes the 95% confidence interval for the fixed effect (FE) meta-analysis across included studies. Studies that had an insufficient number of cases/events in a subtype were not included in the meta-analysis. Each meta-analysis (where more than one study was included) was tested for between-study heterogeneity (Q statistic), and p < 0.05 indicates evidence of study-specific associations.
GRS for two EBV antigens were nominally associated with survival amongst 244 IDH mutated 1p/19q codeleted glioma cases, but in opposite directions (HREBNA = 0.75, 0.57–0.99, p = 0.048/HRZEBRA = 1.27, 1.01–1.6, p = 0.042, 64 events). This subtype-specific result was limited to the UCSF-Mayo dataset due to insufficient number of reported events (deaths) in IDH mutated 1p/19q codeleted glioma cases from TCGA and AGS-i370.
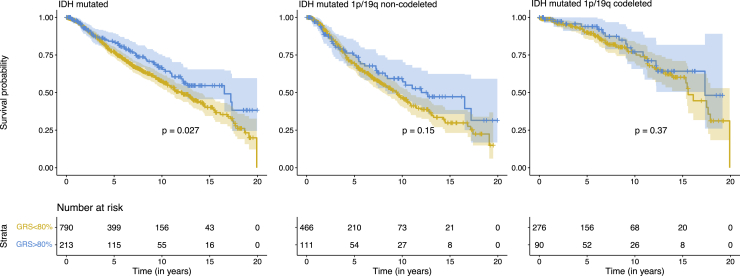
GRSEBNA was also associated with improved survival outcomes in 614 IDH mutated 1p/19q non-codeleted glioma cases (HR = 0.75, 0.74–0.997, p = 0.045, 222 events), suggesting the association of GRSEBNA is independent of 1p/19q status. Figure 5 visualizes the significant GRSEBNA associations with Kaplan-Meier survival curves.
Figure 5.
Kaplan Meier curves for significant GRSEBNA-glioma molecular subtype associations
Kaplan-Meier curves for subtypes where GRSEBNA was nominally associated with subtype-specific glioma survival differences (p < 0.05 via Cox regression). The second and third plots are distinct partitions of the IDH-mutated subgroup. To visualize, unnormalized GRSs across the included studies were binned on the basis of the case-specific 80th percentile score in the UCSF-Mayo dataset. p values included on each plot are results of a log-rank test for difference between the two curves. Below each set of curves provides the number of cases surviving beyond that time point for each of the two GRS groups. In all cases, the glioma cases with higher GRS for EBV EBNA had visually improved survival outcomes compared to the bottom 80%.
We did not detect any significant GRS associations in the analysis of glioma overall, which is consistent with the strong prognostic significance of molecular glioma subtypes.17,33 Also, as the prognosis in IDH wild-type gliomas is the poorest, we suspect our GRS instruments are underpowered to detect significant deviations over such short intervals. Full survival results are available in Figures S3 and S4.
HLA allele-glioma associations
Of the 77 HLA alleles imputed at two-field resolution, only HLA-DQA1∗03:01 reached Bonferroni-corrected significance (p < 6.5 × 10−4 after correcting for 77 HLA alleles tested) for association with glioma risk in a meta-analysis. The presence of an HLA-DQA1∗03:01 allele was associated with a decrease in overall glioma risk (OR = 0.84, p = 3.96 × 10−4). This allele was also nominally associated with risk of IDH wild-type (OR = 0.82, p = 1.4 × 10−3) and IDH wild-type 1p/19q non-codeleted gliomas (OR = 0.81, p = 2.6 × 10−3). These subtype-specific associations and direction of effect mirror those of GRSZEBRA presented above, suggesting HLA-DQA1∗03:01 as a potential shared marker for both glioma risk and EBV ZEBRA seroreactivity. Results of all HLA allele (one and two field resolution) associations with glioma risk tested in a meta-analysis are available in Table S2. Odds ratios for the association of HLA-DQA1∗03:01 were slightly attenuated when adding further genetic principal components to the model (Table S6).
Discussion
We investigated genetically predicted antibody response to twelve antigens for nine viruses in relation to glioma risk and prognosis in a meta-analysis of three patient cohorts with molecular subtype information. Our analysis discovered evidence that genetic predisposition to reactivity to specific viral antigens associated with susceptibility to glioma in a subtype-specific manner. This pattern of effect modification by glioma subtype also extends into prognosis, where we observed associations between viral antigen GRSs and survival among specific glioma subtypes.
One of our main findings is that genetic predisposition to increased seroreactivity to EBV ZEBRA was associated with a decreased overall glioma risk, with a significant decrease in IDH wild-type subtypes. Predicted reactivity to the MCV VP1 antigen mirrored the same IDH wild-type associations but in the opposite direction, where higher reactivity was associated with increased glioma risk. The significant inverse relationship between predicted reactivity to EBV ZEBRA and MCV VP1 highlights the possibility of shared genetic components of antibody response to the two antigens. We saw evidence that this relationship is driven by SNPs near class II HLA alleles. Understanding underlying mechanisms guided by these genetics is left as an open question. One possible link is that the class II HLA allele DQA1∗03:01 was associated with decreased glioma risk in the same subtypes associated with GRSs for EBV ZEBRA. In our previous UKB analysis,30 the presence of HLA-DQA1∗03:01 was positively associated with EBV ZEBRA seroreactivity measurements (β = 0.168, p = 1.3 × 10−16). Taken together, the associations between HLA-DQA1∗03:01, EBV ZEBRA, and glioma risk suggest possible shared underlying immunogenetic architecture. As HLA class II proteins present potentially antigenic peptides, functional genetic alterations can result in changes to the binding affinity of specific antigens, modulating the potential for recognition by CD4+ helper T cells. It is possible that the heterodimeric DQ proteins half-encoded by DQA1∗03:01 have improved binding or recognition of peptides presented by both EBV ZEBRA proteins and glioma (specifically IDH wild type), allowing for an increased immune response in both cases. As IDH wild type has generally been shown to serve as a marker for more severe glioma cases, the exact somatic tumor alterations leading to recognized peptide variants in these tumors is not clear and is an area that warrants future investigation. Further analysis of viral-tumor protein homology is needed to understand if this could be a possible connection.
Interestingly, a higher genetically predicted response to EBV EBNA was nominally associated with increased risk in IDH mutated/1p/19q codeleted gliomas. Still, reactivity toward EBV EBNA was more strongly associated with improved survival in IDH mutated gliomas. This discrepancy between the disease-promoting and pro-survival associations of GRSEBNA may suggest the presence of different biologic pathways after initiation of disease. It may also point toward EBV latency-mediated treatment effects. Previous studies show that the EBV latency type predicts the relative amount of induced reactivation generated by cytotoxic chemotherapy drugs.41 Therefore, individuals who react strongly to EBV EBNA antigens may exhibit a different pattern of reactivation when treated with temozolomide. Further research is required to elucidate this putative association, yet it is clear that EBV lytic/latent cycling is a critical aspect of other germline interactions and disease risk.
Epstein-Barr virus was the first recognized human oncovirus42 and exists in two distinct life cycles within a host: a lytic phase of active infection where new viruses are produced and a latent phase where the virus remains dormant to avoid detection by the host. Distinct sets of antigens are produced during these two phases, two of such are EBNA, during the latent (inactive) phase of infection, and the ZEBRA protein, which initiates a change from latent to lytic gene expression. Past evidence suggests processes during the latent stage are responsible for the virus’ oncogenic properties via mechanisms promoting cell growth and preventing cell apoptosis (reviewed in Akhtar et al., 201843). Recent work has also implicated the ZEBRA protein as oncogenic with evidence demonstrating its ability to deregulate immune surveillance and promote immune escape.44
In contrast, MCV is the most recently recognized human oncovirus, first described in 2008 and later accepted as a causal agent for Merkel cell carcinoma,45 a neuroendocrine carcinoma. Previous studies have identified the presence of MCV DNA in glioma patients and have drawn an association with infection and increased risk of glioma.15,16 It has been shown that both the large T and small T antigens of MCV are oncoproteins that target tumor suppressor proteins such as pRB. Although the exact latency mechanism in polyomaviruses is unknown, it has been suggested that complex protein-mediated latency may be critical to the MCV life cycle.46
In interpreting our findings, several limitations should be acknowledged. The UKB GWAS used to develop antigen GRSs was restricted to individuals of predominantly European ancestry and we would expect low portability to other populations considering the high level of polymorphism in the HLA region. Therefore, our analyses of glioma susceptibility were also restricted to European ancestry subjects. Furthermore, sample size in other ancestry groups was insufficient for a meta-analysis (African: 73 cases/29 controls, Asian: 48/49, American: 24/24, admixed: 131/68).
The shared genetic architecture between the viruses studied here, as seen in the correlations in Figures 2 and S1, may result in a lack of specificity. This limitation in our study may be applicable in other viruses that share the same underlying genetic programming of antigen response, particularly VZV, which is associated with a unique pattern of LD spanning a large region of the HLA.30 Furthermore, the clumping and thresholding approach to GRS development may not be optimal for regions with complex LD structure, such as HLA. An approach that can appropriately account for the long-range correlation structure and capture non-linear interactions, such as haplotype effects, may improve future studies that use genetically inferred immune responses. Similarly, the complex LD patterns present challenges in disentangling the specific function of individual SNPs located within the HLA region.
The magnitude and direction of associations with glioma risk observed for GRS and HLA alleles were robust to the number of genetic ancestry principal components included in the models. Including up to 20 principal components did not result in an appreciable change in effect size for GRS associations. The OR for HLA-DQA1∗03:01 increased slightly from 0.82 to 0.89 (Table S6) and become less precise. Analyses of the HLA region are complicated by the highly polymorphic HLA variation that is driven by demographic factors associated with fine-scale geographic variation.47 Additional studies leveraging targeted HLA sequencing approaches can provide further clarity into the genetic determinants of antigen response and glioma risk/survival.
We could only study the single SNP-glioma relationships (Table S1) for variants associated with reactivity to several antigens of interest (BKV, HSV-1, JCV, VZV, HHV-7, HHV-6, CMV) because of the limited number reaching genome-wide significance.30 Although the GRS-glioma association results presented here require further replication in independent affected individual populations, our findings are intriguing and suggest previous inconsistencies in observational epidemiologic viral association studies may be partially due to individual differences in genetic factors that affect antigen reactivity. Further, our approach focused on the genetic underpinnings of antibody response in the adaptive immune response to common viral infections as measured by IgG levels, which is only one pathway utilized by the adaptive immune system. Another arm of the adaptive immune system, T cell-mediated immunity, is not accounted for in this study. The construction of genetic instruments that capture the role of this arm in viral response would complement the current study well. Differences in the innate immune system, such as the efficiency of natural killer cells, could also provide additional associations between viral infections and glioma. Lastly, there is inherent noise in the seroreactivity GWAS used here, as the time between infection and IgG measurement was unknown in the UK Biobank cohort. As we restricted our analysis to common viruses, it is however a safe assumption that most people were exposed early in life (before the age of 20), and all viruses we considered set up some form of permanent latency in their hosts.
This study directly examined the underlying genetic architecture of antigen reactivity to common viruses and glioma risk and survival. We observed important associations between programmed reactivity to viruses and glioma etiology and prognosis. This methodology is not limited to the study of glioma, as the GRS instruments proposed here can readily be applied to cohorts of any cancer type. This offers a unique approach for future studies to reinvestigate the genetic contributions of long-running epidemiologic associations between viruses and cancer and possibly clarify effects of viral therapies in their treatment.
Acknowledgments
Work at University of California, San Francisco was supported by the National Institutes of Health (grant numbers T32CA151022, K99CA246076, R01CA52689, P50CA097257, R01CA126831, R01CA139020, R01AI128775, and R25CA112355), the National Brain Tumor Foundation, the Stanley D. Lewis and Virginia S. Lewis Endowed Chair in Brain Tumor Research, the Robert Magnin Newman Endowed Chair in Neuro-oncology, and by donations from families and friends of John Berardi, Helen Glaser, Elvera Olsen, Raymond E. Cooper, and William Martinusen. This publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI grant number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute, Cancer Registry of Greater California. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors.
Author contributions
G.G., L.K., and S.S.F. conceived of the study and wrote main drafts of the manuscript. G.G., L.K., G.W., S.J.M., N.K., and S.S.F. conducted and advised on informatic and statistical analyses along with result interpretations. H.M.H., A.M.M., T.R., P.B., J.K.W., J.E.E., R.B.J., and M.W. were involved in primary data collection. All authors contributed and reviewed the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: May 11, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2022.04.011.
Contributor Information
Geno Guerra, Email: geno.guerra@ucsf.edu.
Stephen S. Francis, Email: stephen.francis@ucsf.edu.
Supplemental information
All antigen-associated SNPs considered, basic variant information and beta/SE/p-value for a meta-GWAS for the association of the SNPs for each glioma subtype.
All HLA alleles/genes which were imputed via SNP2HLA, beta/SE/p-value for associations in a meta-analysis of risk analysis of each of the HLA alleles on each glioma subtype.
Data and code availability
Genotype data of control samples from the 1958 British Birth Cohort and UK Blood Service Control Group were made available from the Wellcome Trust Case Control Consortium (WTCCC) and downloaded from the European Genotype Archive under accession numbers WTCCC2: EGAD00000000021 and WTCCC2: EGAD00000000023, respectively. Genotype data of glioma cases from The Cancer Genome Atlas (TCGA) were obtained from Database of Genotypes and Phenotypes (dbGaP) (dbGaP: phs000178). Genotype data of glioma samples from Mayo Clinic and control samples from the Glioma International Case Control Study (GICC) are available from dbGaP under accession dbGaP: phs001319.v1.p1. Genotype data from the University of California San Francisco Adult Glioma Study (AGS) are available under dbGap: phs001497.v2.p1. The code supporting the current study have not been deposited in a public repository because no novel methods have been developed but are available from the corresponding author on request.
References
- 1.Rous P. A transmissible avian neoplasm. (Sarcoma of the common fowl.) J. Exp. Med. 1910;12:696–705. doi: 10.1084/jem.12.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duesberg P.H., Vogt P.K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc. Natl. Acad. Sci. U S A. 1970;67:1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature. ;600(7889):472-477Published online July 8, 2021. doi:10.1038/s41586-021-03767-x [DOI] [PMC free article] [PubMed]
- 4.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., Downey P., Elliott P., Green J., Landray M., et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mui U.N., Haley C.T., Tyring S.K. Viral oncology: molecular biology and pathogenesis. J. Clin. Med. 2017;6:E111. doi: 10.3390/jcm6120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Martel C., Georges D., Bray F., Ferlay J., Clifford G.M. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob. Health. 2020;8:e180–e190. doi: 10.1016/S2214-109X(19)30488-7. [DOI] [PubMed] [Google Scholar]
- 7.Neves A.M., Thompson G., Carvalheira J., Trindade J.C., Rueff J., Caetano J.M., Casey J.W., Hermouet S. Detection and quantitative analysis of human herpesvirus in pilocytic astrocytoma. Brain Res. 2008;1221:108–114. doi: 10.1016/j.brainres.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Chauvin C., Suh M., Remy C., Benabid A.L. Failure to detect viral genomic sequences of three viruses (herpes simplex, simian virus 40 and adenovirus) in human and rat brain tumors. Ital. J. Neurol. Sci. 1990;11:345–357. doi: 10.1007/BF02335937. [DOI] [PubMed] [Google Scholar]
- 9.Cobbs C.S., Harkins L., Samanta M., Gillespie G.Y., Bharara S., King P.H., Nabors L.B., Britt W.J. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–3350. [PubMed] [Google Scholar]
- 10.Wrensch M., Weinberg A., Wiencke J., Masters H., Miike R., Barger G., Lee M. Does prior infection with varicella-zoster virus influence risk of adult glioma? Am. J. Epidemiol. 1997;145:594–597. doi: 10.1093/oxfordjournals.aje.a009155. [DOI] [PubMed] [Google Scholar]
- 11.Strojnik T., Duh D., Lah T.T. Prevalence of neurotropic viruses in malignant glioma and their onco-modulatory potential. In Vivo. 2017;31:221–230. doi: 10.21873/invivo.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bondy M.L., Scheurer M.E., Malmer B., Barnholtz-Sloan J.S., Davis F.G., Il'yasova D., Kruchko C., McCarthy B.J., Rajaraman P., Schwartzbaum J.A., et al. On behalf of the Brain Tumor Epidemiology Consortium Brain tumor epidemiology: consensus from the brain tumor epidemiology Consortium. Cancer. 2008;113:1953–1968. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amirian E.S., Scheurer M.E., Zhou R., Wrensch M.R., Armstrong G., Lachance D., Olson S.H., Lau C.C., Claus E.B., Barnholtz-Sloan J., et al. History of chickenpox in glioma risk: a report from the glioma international case–control study ( GICC ) Cancer Med. 2016;5:1352–1358. doi: 10.1002/cam4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kofman A., Marcinkiewicz L., Dupart E., Lyshchev A., Martynov B., Ryndin A., Kotelevskaya E., Brown J., Schiff D., Abounader R. The roles of viruses in brain tumor initiation and oncomodulation. J. Neurooncol. 2011;105:451–466. doi: 10.1007/s11060-011-0658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limam S., Missaoui N., Bdioui A., Yacoubi M.T., Krifa H., Mokni M., Selmi B. Investigation of simian virus 40 (SV40) and human JC, BK, MC, KI, and Wu polyomaviruses in glioma. J. Neurovirol. 2020;26:347–357. doi: 10.1007/s13365-020-00833-4. [DOI] [PubMed] [Google Scholar]
- 16.Egan K.M., Kim Y., Bender N., Hodge J.M., Coghill A.E., Smith-Warner S.A., Rollison D.E., Teras L.R., Grimsrud T.K., Waterboer T. Prospective investigation of polyomavirus infection and the risk of adult glioma. Sci. Rep. 2021;11:9642. doi: 10.1038/s41598-021-89133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molinaro A.M., Taylor J.W., Wiencke J.K., Wrensch M.R. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 2019;15:405–417. doi: 10.1038/s41582-019-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckel-Passow J.E., Drucker K.L., Kollmeyer T.M., Kosel M.L., Decker P.A., Molinaro A.M., Rice T., Praska C.E., Clark L., Caron A., et al. Adult diffuse glioma GWAS by molecular subtype identifies variants in D2HGDH and FAM20C. Neuro-Oncol. 2020;22:1602–1613. doi: 10.1093/neuonc/noaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpin F., Casaos J., Sesen J., Mangraviti A., Choi J., Gorelick N., Frikeche J., Lott T., Felder R., Scotland S.J., et al. Use of an anti-viral drug, Ribavirin, as an anti-glioblastoma therapeutic. Oncogene. 2017;36:3037–3047. doi: 10.1038/onc.2016.457. [DOI] [PubMed] [Google Scholar]
- 20.Stragliotto G., Pantalone M.R., Rahbar A., Bartek J., Söderberg-Naucler C. Valganciclovir as add-on to standard therapy in glioblastoma patients. Clin. Cancer Res. 2020;26:4031–4039. doi: 10.1158/1078-0432.CCR-20-0369. [DOI] [PubMed] [Google Scholar]
- 21.Piper K., Foster H., Gabel B., Nabors B., Cobbs C. Glioblastoma mimicking viral encephalitis responds to acyclovir: a case series and literature review. Front. Oncol. 2019;9:8. doi: 10.3389/fonc.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besson C., Amiel C., Le-Pendeven C., Plancoulaine S., Bonnardel C., Ranque B., Abbed K., Brice P., Ferme C., Carde P., et al. Strong correlations of anti–viral capsid antigen antibody levels in first-degree relatives from families with epstein-barr virus–related lymphomas. J. Infect. Dis. 2009;199:1121–1127. doi: 10.1086/597424. [DOI] [PubMed] [Google Scholar]
- 23.Kenney A.D., Dowdle J.A., Bozzacco L., McMichael T.M., St Gelais C., Panfil A.R., Sun Y., Schlesinger L.S., Anderson M.Z., Green P.L., et al. Human genetic determinants of viral diseases. Annu. Rev. Genet. 2017;51:241–263. doi: 10.1146/annurev-genet-120116-023425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkataraman T., Valencia C., Mangino M., Morgenlande W., Clipman S.J., Liechti T., Valencia A., Christofidou P., Spector T., Roederer M., et al. Antiviral antibody epitope selection is a heritable trait. Genetics. 2021 doi: 10.1101/2021.03.25.436790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tay R.E., Richardson E.K., Toh H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms. Cancer Gene Ther. 2021;28:5–17. doi: 10.1038/s41417-020-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiina T., Hosomichi K., Inoko H., Kulski J.K. The HLA genomic loci map: expression, interaction, diversity and disease. J. Hum. Genet. 2009;54:15–39. doi: 10.1038/jhg.2008.5. [DOI] [PubMed] [Google Scholar]
- 27.Bateman A.C., Howell W.M. Human leukocyte antigens and cancer: is it in our genes? J. Pathol. 1999;188:231–236. doi: 10.1002/(SICI)1096-9896(199907)188:3<231::AID-PATH325>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 28.Gebe J.A., Swanson E., Kwok W.W. HLA Class II peptide-binding and autoimmunity: gebe et al : HLA Class II peptide-binding and autoimmunity. Tissue Antigens. 2002;59:78–87. doi: 10.1034/j.1399-0039.2002.590202.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C., de Smith A.J., Smirnov I.V., Wiencke J.K., Wiemels J.L., Witte J.S., Walsh K.M. Non-additive and epistatic effects of HLA polymorphisms contributing to risk of adult glioma. J. Neurooncol. 2017;135:237–244. doi: 10.1007/s11060-017-2569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kachuri L., Francis S.S., Morrison M.L., Wendt G.A., Bosse Y., Cavazos T.B., Rashkin S.R., Ziv E., Witte J.S. The landscape of host genetic factors involved in immune response to common viral infections. Genome Med. 2020;12:93. doi: 10.1186/s13073-020-00790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melin B.S., Barnholtz-Sloan J.S., Wrensch M.R., Johansen C., Il'yasova D., Kinnersley B., Ostrom Q.T., Labreche K., Chen Y., Armstrong G., et al. GliomaScan Consortium Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat. Genet. 2017;49:789–794. doi: 10.1038/ng.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wrensch M., Jenkins R.B., Chang J.S., Yeh R.F., Xiao Y., Decker P.A., Ballman K.V., Berger M., Buckner J.C., Chang S., et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat. Genet. 2009;41:905–908. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckel-Passow J.E., Lachance D.H., Molinaro A.M., Walsh K.M., Decker P.A., Sicotte H., Pekmezci M., Rice T., Kosel M.L., Smirnov I.V., et al. Glioma groups based on 1p/19q, IDH , and TERT promoter mutations in tumors. N. Engl. J. Med. 2015;372:2499–2508. doi: 10.1056/NEJMoa1407279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins R.B., Wrensch M.R., Johnson D., Fridley B.L., Decker P.A., Xiao Y., Kollmeyer T.M., Rynearson A.L., Fink S., Rice T., et al. Distinct germ line polymorphisms underlie glioma morphologic heterogeneity. Cancer Genet. 2011;204:13–18. doi: 10.1016/j.cancergencyto.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceccarelli M., Barthel F.P., Malta T.M., Sabedot T.S., Salama S.R., Murray B.A., Morozova O., Newton Y., Radenbaugh A., Pagnotta S.M., et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164:550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander D.H., Lange K. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinformatics. 2011;12:246. doi: 10.1186/1471-2105-12-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manichaikul A., Mychaleckyj J.C., Rich S.S., Daly K., Sale M., Chen W.M. Robust relationship inference in genome-wide association studies. Bioinforma Oxf Engl. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mentzer A.J., Brenner N., Allen N., Littlejohns T.J., Chong A.Y., Cortes A., Almond R., Hill M., Sheard S., McVean G., et al. Infectious Diseases (except HIV/AIDS); 2019. Identification of Host-Pathogen-Disease Relationships Using a Scalable Multiplex Serology Platform in UK Biobank. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia X., Han B., Onengut-Gumuscu S., Chen W.M., Concannon P.J., Rich S.S., Raychaudhuri S., de Bakker P.I. Imputing amino acid polymorphisms in human leukocyte antigens. Tang J., editor. PLoS One. 2013;8:e64683. doi: 10.1371/journal.pone.0064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bi W., Fritsche L.G., Mukherjee B., Kim S., Lee S. A fast and accurate method for genome-wide time-to-event data analysis and its application to UK Biobank. Am. J. Hum. Genet. 2020;107:222–233. doi: 10.1016/j.ajhg.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phan A.T., Fernandez S.G., Somberg J.J., Keck K.M., Miranda J.L. Epstein–Barr virus latency type and spontaneous reactivation predict lytic induction levels. Biochem. Biophys. Res. Commun. 2016;474:71–75. doi: 10.1016/j.bbrc.2016.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epstein M.A., Achong B.G., Barr Y.M. Virus particles in cultured lymphoblasts from burkitt’s lymphoma. The Lancet. 1964;283:702–703. doi: 10.1016/S0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 43.Akhtar S., Vranic S., Cyprian F.S., Al Moustafa A.E. Epstein–barr virus in gliomas: cause, association, or artifact? Front. Oncol. 2018;8:123. doi: 10.3389/fonc.2018.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Germini D., Sall F.B., Shmakova A., Wiels J., Dokudovskaya S., Drouet E., Vassetzky Y. Oncogenic properties of the EBV ZEBRA protein. Cancers. 2020;12:1479. doi: 10.3390/cancers12061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang Y., Moore P.S. Merkel cell carcinoma: a virus-induced human cancer. Annu. Rev. Pathol. 2012;7:123–144. doi: 10.1146/annurev-pathol-011110-130227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwun H.J., Chang Y., Moore P.S. Protein-mediated viral latency is a novel mechanism for Merkel cell polyomavirus persistence. Proc. Natl. Acad. Sci. U S A. 2017;114:E4040–E4047. doi: 10.1073/pnas.1703879114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez-Mazas A., Buhler S., Nunes J.M. A new HLA map of europe: regional genetic variation and its implication for peopling history, disease-association studies and tissue transplantation. Hum. Hered. 2013;76:162–177. doi: 10.1159/000360855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All antigen-associated SNPs considered, basic variant information and beta/SE/p-value for a meta-GWAS for the association of the SNPs for each glioma subtype.
All HLA alleles/genes which were imputed via SNP2HLA, beta/SE/p-value for associations in a meta-analysis of risk analysis of each of the HLA alleles on each glioma subtype.
Data Availability Statement
Genotype data of control samples from the 1958 British Birth Cohort and UK Blood Service Control Group were made available from the Wellcome Trust Case Control Consortium (WTCCC) and downloaded from the European Genotype Archive under accession numbers WTCCC2: EGAD00000000021 and WTCCC2: EGAD00000000023, respectively. Genotype data of glioma cases from The Cancer Genome Atlas (TCGA) were obtained from Database of Genotypes and Phenotypes (dbGaP) (dbGaP: phs000178). Genotype data of glioma samples from Mayo Clinic and control samples from the Glioma International Case Control Study (GICC) are available from dbGaP under accession dbGaP: phs001319.v1.p1. Genotype data from the University of California San Francisco Adult Glioma Study (AGS) are available under dbGap: phs001497.v2.p1. The code supporting the current study have not been deposited in a public repository because no novel methods have been developed but are available from the corresponding author on request.