Abstract
The transmission of SARS-CoV-2 has caused serious health crises globally. So far, 7 vaccines that are already being assessed in Phase IV clinical trials are, Comirnaty/ Pfizer; Spikevax/Moderna (m RNA vaccine); Vaxzevria or Covishield; Ad26.COV2.S; Ad5-nCoV (adenoviral vector-based vaccine); CoronaVac and BBIBP-CorV (inactivated virus vaccine). Besides, there are about 280 vaccines that are undergoing preclinical and clinical trials including Sputnik-V, Covaxin or BBV152, and NVX-CoV2373. These vaccines are being studied for their immunological responses and efficiency against COVID-19, and have been reported to demonstrate effective T and B cell responses. However, the long-lasting immunity of these vaccine regimens still needs to be investigated. An in-depth understanding of the vaccine efficacy and immune control mechanism is imperative for the rational purposing and implementation of the vaccines. Hence, in this review, we have comprehensively discussed the immune response induced in COVID-19 patients, as well as in the convalescent individuals to avoid reinfection. Moreover, we have also summarized the immunological responses and prophylactic efficacy of various COVID-19 vaccine regimens. In this context, this review can give insights into the development of effective vaccines against SARS-CoV-2 and its variants in the future.
Keywords: COVID-19, convalescent individuals, immune response, clinical trial, vaccine regimens
Introduction
The novel coronavirus, now called severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), recently has been the main reason behind the extreme health crisis all around the globe. On March 11, 2020, WHO considered it a global pandemic. This particular beta coronavirus which uses ACE-2 (angiotensin-converting enzyme 2) receptor to enter the human host was initially reported in the Wuhan city of Hubei province, China [1, 2]. COVID-19 occurs in two phases, the first phase includes an increase in the levels of SARS-CoV-2 infection and transmission, where the RBD region of the S1 subunit binds with the ACE-2 receptor of the host cell and blocks the RAS system, further TMPRSS2 cleaves the S2 subunit at some definite regions, resulting in the activation and internalization of the virus inside the host cell. While the second phase is associated with a drastic increase in the levels of cytokines, impairment of the tissues due to increased SARS-CoV-2 organotropism, aggressive inflammation, and systemic failure, probably as a result of unusual RAS system signaling that comprises the ACE / AT1R / ANGII and ACE-2/ANG (1–7)/MASR proteins [3].
The SARS-CoV-2 associated with the COVID-19 disease display various symptoms from cough, fever, dyspnea, and respiratory failure to sometimes no symptoms at all [4]. To overcome the severity of this disease various vaccines have been approved viz., m RNA vaccines (Comirnaty/ BNT162b2/ Pfizer and Spikevax/ mRNA-1273/ Moderna) adenovirus-based vaccine (Vaxzevria /AstraZeneca/ Covishield, Sputnik V, Ad5-nCoV), Inactivated vaccine (BBIBP-CorV, Covaxin), etc. Vaccines works on the principle of immunological memory which helps an individual to overcome a particular infection for several years, it works by inducing the development of memory B and T cells for that particular virus which further stimulates an efficient immune response in a short span to clear the virus as soon as it enters the host, thus preventing the reinfection [4]. The reinfection can be prevented for several years as in the case of diseases like chickenpox, measles, and mumps, by exploiting the natural immunity to generate extensively stable neutralizing antibodies [5]. In the case of COVID- 19 infection, vaccines have played a critical role in preventing this pandemic to a great extent, further individuals with reports of the previous infection, and unvaccinated individuals displayed a higher rate of reinfection [6]. Hence it was declared by the CDC that all the eligible individuals should be offered COVID-19 vaccines [6]. However, the vaccinated individuals can be reinfected due to the high rate of mutations in the original strain of SARS-CoV-2 [7]. Furthermore, the reinfection in fully vaccinated individuals also depends on the immunological response of the host towards the vaccine. In the present review, we have extensively discussed various immunological responses in COVID-19 affected and recovered individuals. Moreover, we have also presented the protective efficacy and immunological responses of the various vaccine regimens against COVID-19.
Disease Progression and Protection in Patients
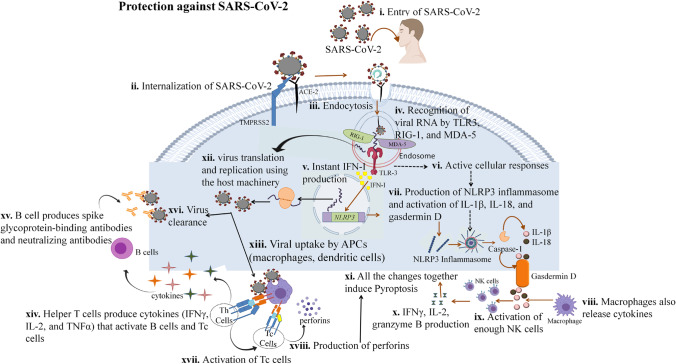
Both innate and adaptive immunity, in general, intend to impede the viral infection and remove the infected cells. However, the immune responses in mild and severe SARS-CoV-2 patients are different. Despite the variation present among these patients, now it is evident, that following the initial infection a range of consequences can be observed. Although some patients with milder symptoms have been reported to experience a wide range of distressing symptoms for several months following the initial infection [8, 9]. This condition when the symptoms persist even after three months post-infection is referred to as long COVID syndrome [9]. Autonomic dysfunction expressed as postural orthostatic tachycardia syndrome, consistent fatigue, aberrant thermoregulation, myalgia, digestive problems, and skin manifestations are among the symptoms of this disease [9]. Another condition that has been reported post-SARS-CoV-2 infection is the Multisystem inflammatory syndrome (MIS). This condition can occur after 2–6 weeks post-SARS-CoV-2 infection and was first identified in children (MIS-C) [8, 10, 11], and lately in youngsters (MIS-A) [12]. However, with aging, there are more complications associated with the immune response viz., weakened non-lymphoid and lymphoid tissues that play a crucial role in the host immunity. There is a decline in the levels of naive T and B cells with the degeneration of the primary lymphoid organs, consequently lesser amount of T and B cells reach the secondary lymphoid organ which is the site for antigen encounter. Moreover, the extrapulmonary and pulmonary organs have been reported to be accumulated by the proinflammatory cells and mediators [13]. The progression of COVID-19 infection associated with the compromised innate and adaptive immunity has been described in Fig. 1.
Fig. 1.
Immune responses and protection against SARS-CoV-2: (i, ii, iii) SARS-CoV-2 binds to the ACE-2 receptor and gets internalized via endocytosis with the help of TMPRSS2. (iv) Inside the host cell Viral RNA is recognized by TLR3 and some sensors of viral infection (MDA 5 and RIG- I), (v) which induce production of IFN-I, and (vi) activation of various cellular responses (ROS production, calcium influx), (vii) leading to the production and activation of NLRp3 inflammasome, which further activates caspases that cleaves pro-IL-1β, pro-IL-18, and gasdermin D, leading to their activation (IL-1β, IL-18, and gasdermin D). (viii, ix) Macrophages also produce cytokines that activate enough amount of NK cells, (x, xi) leading to the production of IFNγ, IL-2, and granzyme B, which causes pyroptosis of the infected cell. (xii) However, the viral RNA can also use the host machinery to form new virions, (xiii) which can be recognized by the APCs leading to their presentation to the T cells, which activates Th and Tc cells. (xiv) Th cells produce IFNγ, IL-2, and TNF α, (xv, xvi) which activate the B cells to produce spike-specific and neutralizing antibodies resulting in the virus clearance. (xvii) The cytokines produced from Th cells also induce the Tc cells to produce perforins, (xviii) which leads to pyroptosis of the infected cell and protection against the disease.
Innate Immunity in Disease Progression and Protection
SARS-COV-2 or the novel coronavirus enters the human host via ACE-2 receptors [14]. As soon as it enters the host, it starts replicating, subsequently transmitting the infection to other cells, which induces pyroptosis in the infected cells, and consequently, the release of the DAMPs (Damage-associated molecular patterns) [15]. These DAMPs are recognized by the PRRs (Pattern recognition receptors) viz., TLRs (Toll-like receptors) TLR3, TLR7, TLR8, TLR9, and some sensors of viral infection (MDA-5 and RIG- I), which leads to the production of the type I IFN (interferon) i.e, IFN-α and IFN-β that are protective interferon against any viral infection [9, 16, 17]. These responses, further result in the transcription of the NLRP3 gene (NLR family pyrin domain containing 3), and induction of various cellular responses (Calcium influx, aggregation of protein, ROS production, and release of danger-associated pattern), which together activates various inflammasome complexes particularly the NLRP3 inflammasomes [18]. Moreover, these inflammasomes induce the activation of caspase-1 dependent cleavage, which results in the activation of the proinflammatory cytokines (IL-1β and IL-18), and gasdermin-D (pore-creating protein) mediated pyroptosis, thus the magnitude of NLRP3 activation can be related to the severity of the novel coronavirus [19]. In addition, an increased amount of the enzyme Lactate dehydrogenase (released during pyroptosis) has also been observed in the blood of severely infected COVID-19 patients [9].
Some studies have suggested that SARS-CoV-2 is susceptible to the type-I IFN response [20, 21]. Besides, further investigations are required to understand the role of various genes that regulate the induction of the IFN response. The probable reason behind the less severe symptoms or no symptoms at all is because in such individuals type I interferons are released as soon as the DAMPs are recognized, resulting in viral inhibition [22]. In elderly people or people with weak immune conditions, IFN-I production is delayed, which results in the activation of various inflammatory cells viz., neutrophils, monocytes, and macrophages. Subsequently, a large number of proinflammatory cytokines are released by these cells, this condition is termed as the cytokine storm that has been reported to cause severe damage to the lung alveoli resulting in a condition named severe acute respiratory syndrome. This condition further results in a decrease in blood pressure, and consequently damages multiple organs [23]. In a study involving 5,279 patients, it was observed that C reactive protein and D- dimer were elevated, in the early stages of the patient that required ventilator support or were deceased due to this virus [24].
The granular lymphocytes include the Natural killer or NK cells that are involved in the eradication of the cells infected with the virus. People severely infected with COVID-19 were reported to have decreased quantity of NK cells in the PBMC (Peripheral blood mononuclear cells) in contrast to the people of the same age that were not severely infected [25–27]. In general, NK cells are absent from the lung cells, but SARS-CoV-2 infection in the lungs induces the macrophages and monocytes to release the chemoattractants, as a result, NK cells migrate towards these chemoattractants and infiltrate into the lungs via CXCR3 receptor (chemokine receptor) [15, 28, 29]. However, the cytotoxicity of NK cells may decrease most likely due to the declined levels of granzyme B in severely infected COVID-19 patients [30].
Adaptive Immune Response during Infection and Protection
Adaptive immunity includes the B and T lymphocytes that correspond to the humoral and cell-mediated immune response. As soon as the virus enters the host, the viral peptides are presented by the MHC-I of the nucleated cells to the TCR (T cell receptor) of cytotoxic T (Tc or CD8 T cells) cells, causing apoptosis of these cells. Moreover, the proteins secreted by these viruses are also presented by the MHC II molecule to the helper T cells (Th or CD4 T cells), resulting in the secretion of IL-2 and IL-6 molecules which leads to the proliferation of the virus-specific B lymphocyte, consequently, forming the plasma and memory B cells. The plasma B cells further secrete the IgG, IgM, and IgA antibodies to neutralize the virus [31]. However these responses build up in their fair share of time and in the case of SARS-CoV-2, this type of antibody response takes about 19 days to develop following the symptom onset, which is trailed by the seroconversion (the development of specific antibodies in the blood serum as a result of infection or immunization, including vaccination) of IgM and IgG antibodies [32]. Besides, the spike-specific IgA and IgM antibodies were also reported to develop in COVID-19 patients. The IgA antibodies started expanding in the initial week and at 20-22 days these antibodies reached their highest concentration, and the IgM antibodies were at their peak by 10-12 days and then declined 18 days after the symptoms occurred [33]. In addition, following the symptom onset, an increase in the spike-specific IgG titers was observed during the initial three weeks, and later a decrease was observed from the eighth week in some COVID-19 affected individuals [34]. However, reports suggest that in 2-4 months, the patients with moderate COVID-19 symptoms, showed a swift decrease in the level of spike-RBD-specific IgG titers, signifying that the humoral immunity induced by SARS-CoV-2 is not long-term [35, 36]. A very similar observation was reported against the nucleocapsid protein of SARS-CoV-2 [37]. Furthermore, lower IgG titers were found to be associated with an elevated level of virus clearance compared to the high IgG titers [38]. Moreover, 3-4 weeks following the acute SARS-CoV-2 infection, symptomatic SARS-CoV-2 individuals have been reported to show an elevated level of IgG titer compared to the asymptomatic individuals [39].
Various studies have suggested a decline in the count of CD4 and CD8 T cells in COVID-19 patients [40–42]. In acute COVID-19 infections, CD8 T cells declined drastically [41, 42], but in mild infections, the level of both the CD8 and CD4 cells was slightly higher or normal [1, 43]. There have been studies that reported the increased expression of NKG2A in cytotoxic T and NK cells but decreased level of granzyme b [44]. Besides, the proportion of NKG2A+ cytotoxic lymphocytes was reported to decline in SARS-CoV-2 patients, which means that the expression of NKG2A can be associated with the impaired cytotoxic lymphocyte function and increased progression of SARS-CoV-2 in the initial stages [15, 44]. The studies conducted so far suggest that SARS-CoV-2 infection is associated with both reduced levels and impaired function of T lymphocytes. But, to understand the definite T cell response in SARS-CoV-2 infection more studies involving the apparent CD4 and CD8 T cell responses, and understanding the effector and central memory T cell generation, are required. Hence, the virus-specific cytotoxic T cell response is crucial in understanding the cell-mediated destruction of SARS-CoV-2 which is difficult to measure.
Immunological Responses in COVID-19 Recovered Patients
Immunological memory plays a crucial role to recognize and elicit an immune response to the previously encountered pathogen effectively and quickly. Therefore, comprehending whether any immunological response occurs against SARS-CoV-2 would be crucial for overcoming this pandemic [45]. Various investigations have suggested that individuals who recovered from this disease were found to have an elevated level of neutralizing antibodies (NA), Th1 cytokine-producing CXCR5+ circulating TFH cells (T follicular helper cells), proliferating CXCR3+ CD4+ memory cells, CXCR5- non-TFH cells, IFN-g-producing CD8+ T cells, proliferating CXCR3+ CD4+ memory cells and IgG+ classical MBCs (memory B cells) with BCRs (B cell receptors) that produced neutralizing antibodies [45]. Moreover, there have been reports which suggest that specific memory Tc cells were also present in the convalescent patients. These components of the immune system have been crucial against many other human viral diseases [5, 46, 47]. In addition, an elevated level of plasma and memory RBD-specific IgG+ antibodies were maintained for at least three months post-COVID in some patients [48–50]. Neutralizing antibodies that prevent the virus from binding to the host receptor also play an important role in combating SARS-CoV-2 [51]. In addition, the neutralizing antibodies were found to be at their peak in the serum of the patients even after 3 to 5 weeks from their recovery, and the magnitude of this peak can be associated with the disease severity [52], however, these antibodies decay rapidly in the patients that recovered in a short period [45, 53–55]. When the half-life of these neutralizing antibodies was analyzed for initial 70 days after infection an early half-life of about 55 days was observed [56]. Moreover, when this analysis was done for the initial 8 months after the infection half-life of about 90 days was observed [57]. Therefore, the short half-life of serological antibodies and short life span of antibody-secreting cells is the reason behind the rapid decay. However, longer-term follow-up studies will unveil further slowing of decay of the titers to reach a stable level analogous to the humoral immune responses elicited in other viral pathogens [5, 58]. These neutralizing antibodies even in their lowest concentration (where they cannot restrain the viral entry or its early replication) can act by slowing down the division and the severity caused by the virus [59]. However, understanding how neutralizing antibodies work against the SARS-CoV-2 infection needs to be further investigated.
Memory B cells play a crucial role by acting rapidly and activating the protective antibodies as they re-encounter SARS-CoV-2. Various studies insinuate that the SARS-CoV-2 specific memory B cells accumulate for some months following the initial infection [45, 56, 57, 60, 61]. The expanding levels of antibody somatic mutations propose that the continuous activity in the germinal center drives the affinity maturation of the antibody reactions with time [60]. The steady maintenance of the affinity developed by the memory B cells could help in eliminating subsequent diseases, albeit the memory B cells' protective nature in SARS-CoV-2 resistance is yet to be resolved.
There have been studies that described the T cell response against the spike protein of SARS-CoV-2 or any other antigen of this particular virus [56, 57]. Comparable half-lives of about 120-139 days for spike-specific CD4+ memory T cell reactions and a high recurrence of convalescent people that possess CD4+ T cell responses for about 6 months following the symptom onset were observed in some studies [56, 57]. Moreover, CD8 + T cell responses are also activated during the SARS-CoV-2 infection, and in comparison to the CD4+ T cell, they exhibit a longer half-life [56, 57]. Even though the role of T cells to overcome the SARS-COV-2 infection is unclear, however, some studies report strong T cell responses due to mild infection [62]. In addition, the progression of the germinal center B cells is supported by the CD4+ T follicular helper cells (TFH), which further activate the antibody responses. TFH cells responses specific for the spike protein of SARS-CoV-2, precisely the TFH cells with CCR6 phenotype, are associated with the production of the neutralizing antibodies in patients that overcame the infection [63]. However, the lymph nodes present at the site of infection are yet to be studied for particular phenotype and presence of the TFH cells, moreover, the factors involved in the production of these TFH cells are still unknown, but they play a crucial role in the generation of sustained antibody response, consequently developing immunity. Thus, T-cell responses to the SARS-CoV-2 antigens are probably present in ample amounts in convalescent people, to trigger an enhanced response during reinfection. The function of T-cell responses in human re-exposure to SARS-CoV-2 is unknown, but by correlating with various other viral infections, these cells may limit the division of this particular virus within the upper respiratory tract which would decrease viral load and serious illness during re-exposure [57, 64]. However, it is not clear which response, either humoral or cellular response is sufficient to develop protection against SARS-CoV-2 reinfection.
Various Vaccine Regimens against SARS-CoV-2
SARS-CoV-2 vaccines have been reported to induce an efficient immune response in order to overcome and reduce the mortality caused by the COVID-19 infection. Different vaccine candidates under clinical and preclinical trials are the m RNA vaccines, viral vector-based vaccines, inactivated pathogen vaccines, etc. (Table 1). So far, 7 vaccines are already being assessed in Phase IV clinical trials (Fig. 2), namely: 1) Comirnaty, a nucleoside modified m RNA vaccine developed by Pfizer/BioNTech and Fosun Pharma; 2) Spikevax, an m RNA vaccine that is encapsulated in the lipid nanoparticle developed by Moderna and National Institute of Allergy and Infectious diseases; 3) Vaxzevria, a recombinant ChAdOx1 adenoviral vector encoding spike protein of SARS-CoV-2 developed by AstraZeneca and University of Oxford; 4) CoronaVac, an inactivated virus vaccine, produced in Vero cells developed by Sinovac Research and Development Co. 5) Ad5-nCoV, a non-replicating adenovirus type 5 vector vaccine, developed by CanSino Biological Inc./Beijing Institute of Biotechnology, 6) Ad26.COV2.S, a recombinant, replication-incompetent adenovirus type 26 vectored vaccine encoding the spike protein, developed by Johnson and Johnson, and 7) BBIBP-CorV, an inactivated SARS-CoV-2 vaccine, developed by Sinopharm, China National Biotec Group Co, and the Beijing Institute of Biological Products. In addition, over 280 vaccines are still in preclinical and clinical development. Vaccines indeed are reliable for maintaining high level of safety and efficacy against a range of diseases. However, pain at the site of injection, malaise, and fever due to systemic or local inflammatory responses are among the most common side effects, although the frequency of these adverse effects varies with age [65]. There have been rare cases of severe side effects reported in response to the SARS-CoV-2 vaccines viz., only less than 0.001% of individuals were reported to display myocarditis, and anaphylaxis [65]. Similarly, in the case of adenoviral vectored vaccine thrombocytopenia syndrome, Guillain Barre syndrome, and capillary leak syndrome were observed in very few individuals [65]. Moreover, various adverse effects of these vaccine regimens have been described in detail in Fig. 3.
Table 1.
COVID-19 vaccines currently distributed worldwide
| S.No. | Vaccine | Manufacturer/ WHO EUL holder | Platform | Dose | Immunological Response | Clinical Trial | References |
|---|---|---|---|---|---|---|---|
| 1. | BNT162b2/ Comirnaty | Pfizer/BioNTech + Fosun Pharma | mRNA (nucleoside-modified RNA) encoding the SARS-CoV-2 full-length spike, with two proline mutations to lock it in the prefusion conformation) | 2 doses (3 weeks apart) |
Neutralizing antibodies were produced in all the participants. Virus-specific Th1 and CD8+ T cell responses were reported. 95% efficiency reported. |
[4, 66, 67https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.] | |
| 2. | mRNA-1273/ Spikevax | Moderna + National Institute of Allergic and Infectious diseases | mRNA (encodes SARS-CoV-2 prefusion-stabilized full- length spike protein) | 2 doses (4 weeks apart) |
Neutralizing antibodies were produced in all the participants. Active CD4+ T cell responses were reported and CD8 T cell response was reported only in 100 μg dose. 94% efficiency reported. |
[4, 66, 67https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines] | |
| 3. | AZD122 (ChAdOx1-S)/ Vaxzevria /CoviShield | AstraZeneca + University of Oxford | Adenoviral vector (replication-deficient chimpanzee adenovirus vector the S protein of SARS-CoV-2) | 2 doses (4 weeks apart) |
Neutralization antibodies increased in participants that received the booster dose. T cell response observed in all participants. |
[4, 66, 67https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines] | |
| 4. | Ad26.COV2. S | Janssen Pharmaceutical by Johnson & Johnson | Adenoviral vector (recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector the S protein of SARS-CoV-2) | 1 dose |
Neutralizing antibodies reported in 92% of the participants. Besides robust CD8+ T cell response was also reported. CD4+ T cell responses also reported in 80% of the participants. |
EUCTR2021-002327-38-NL |
[4, 66, 67https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines] |
| 5. | Sputnik V (rAd26-S + rAd5-S) | Gamaleya Research Institute of Epidemiology and Microbiology | Adenoviral vector (recombinant adenovirus type 26 and recombinant adenovirus type 5 vectors the S protein of SARS-CoV-2) | 2 doses (3 weeks apart) |
Neutralizing antibodies produced in all the participants. CD4+ and CD8+ T cell responses observed in all the participants. 91.4% efficiency reported. |
[4, 66, 67https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines] | |
| 6. | CoronaVac | Sinovac Research and Development Co. | Inactivated whole virion (inactivated whole virion with aluminium hydroxide adjuvant) | 2 doses (2 weeks apart) |
Neutralizing antibodies reported in 97.4% of the participants that received the booster dose. T cell responses not reported. |
[4, 66, 67https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines] | |
| 7. | NVX-CoV2373 | Novavax | Protein subunit (wild-type SARS-CoV-2 spike glycoprotein) | 2 doses (3 weeks apart) |
Elevated neutralizing antibody titer in all participants. CD4+ T cell response also reported in all the participants. |
[4, 66, 67https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines] | |
| 8. | Covaxin (BBV152B) | Bharat Biotech International Limited | Inactivated virus | 2 doses (4 weeks apart) |
Neutralizing antibodies present and robust Humoral immune response reported. Virus specific CD4+ and CD8+ responses were reported. |
CTRI/2020/11/028976 |
[68, https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.] |
| 9. | Ad5-nCoV | CanSino Biologics | Viral vector (adenovirus type-5 vector containing the S protein of SARS-CoV-2) | 1 dose |
Neutralizing antibodies produced in 97% of the participants. T cell responses observed in 88% of the participants |
[69, https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines] | |
| 10. | BBIBP-CorV | Sinopharm + China National Biotec Group Co + Beijing Institute of Biological Products | Inactivated virus | 2 doses |
Strong humoral immune response were reported with elevated neutralizing antibody titre. 79.34% efficiency reported. |
NCT04863638 | [70, https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines] |
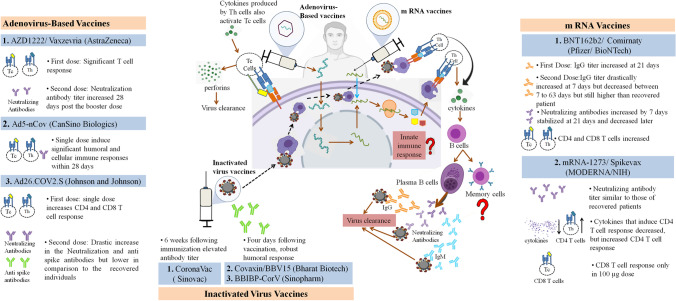
Fig. 2.
SARS-CoV-2 Vaccines in development: Adenoviral vector vaccine (Vaxzevria, Ad5-nCov, and Ad26.COV2.S) - The adenovirus is designed to contain information encoding the wild type spike protein. As this modified adenovirus DNA enters the host cell, it uses the host machinery to translate the viral antigens which are presented to the T cells, leading to the activation of both Th and Tc cells. The Th cells produce cytokines that activate the B cells and Tc cells. The B cells differentiate into plasma B cells and memory B cells. The plasma B cells produce spike-specific IgG and IgM antibodies along with the neutralizing antibodies that play a major role in virus clearance. The Tc cells produce perforins that are also involved in virus clearance. mRNA-based vaccines (Comirnaty, and Spikevax)- The vaccine consists of an mRNA encapsulated in a lipid nanoparticle and has the information required to synthesize a stable prefusion of the spike protein. This m RNA when inside the host cells uses its machinery to translate the viral antigens, which are presented to the T cells, and thus activate the adaptive immune response against the Spike protein. Inactivated virus vaccine (CoronaVac, Covaxin, and BBIBP-CorV) - The inactivated whole virus when inside the host cell are presented by the APCs to the T cells which activate the adaptive immune response. The role of innate immune response and memory B cells in virus clearance still needs to be investigated.
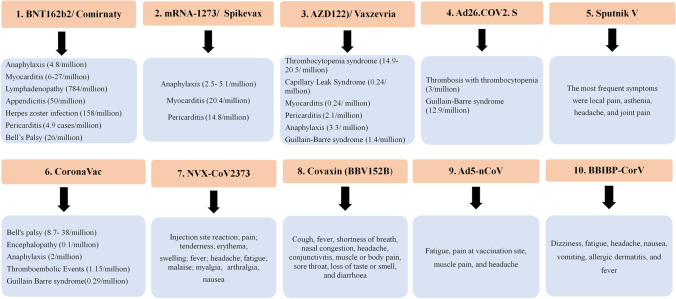
Fig. 3.
Main adverse effects of various SARS-CoV-2 vaccine regimens. As illustrated, there have been rare cases of these severe side effects.
BNT162b2/ Comirnaty (Pfizer/ BioNTech)
An American company, Pfizer in association with a German-based company BioNTech and Shanghai-based Fosun Pharma developed Comirnaty which is an mRNA vaccine, formulated in the lipid nanoparticles [71]. So far, millions of people around the world have administered this vaccine and it has been reported to be about 95% effective in preventing COVID-19 in volunteers with and without prior signs of COVID-19 [71]. There have been studies that suggest the occurrence of sufficient T and B cell responses against this vaccine in humans [72], but the occurrence of the innate immune response still needs to be studied. The mRNA encoding the spike protein of SARS-CoV-2 has alterations that make the spike protein stable in an antigenically preferred, prefusion conformation [71]. Lipid nanoparticles prevent the degradation of this non-replicating RNA and release it into the host cells when injected intramuscularly. As it enters the host cell, translation occurs and SARS-CoV-2 spike protein is formed, which is further expressed on the host cell’s surface. Hence in response to this brief expression of the spike protein, neutralizing antibody response and cell-mediated immune responses are stimulated, in order to provide protection against the SARS-CoV-2 infection [71]. In a non-random, open-phase I / II (NCT04380701) study that was conducted in Germany, Comirnaty has been reported to elicit a potent antibody reaction in healthy individuals from 19 to 55 years of age [73]. Patients were divided into different groups to receive various concentrations of the vaccine (1 μg, 10 μg, 20 μg, or 30 μg) in two doses over a gap of 21-days. The geometric mean concentration (GMC) of the spike-specific IgG was reported to increase in all these dose groups at 21 days following the initial administration, and, a strong improvement in response was observed at 7 days following the next dose. Although following the second dose the GMC of the Spike-specific antibodies dropped from 7 to 63 days but were still significantly higher in comparison to the sera of SARS-CoV-2 recovered patients. Moreover, after 7 days following the second dose (10–30 μg) of Comirnaty an elevation in the levels of SARS-CoV-2 50% neutralizing geometric mean titer (GMT) was observed. When a follow-up was done for 63 days from the second dose it was observed that about 21 days following the second dose the GMT declined slightly before stabilization [73].
The Comirnaty immunized serum has been reported to neutralize all the strains, specifically the modified SARS-CoV-2 with the spike mutations (including N501Y) that were present in the highly transmissible South African and United Kingdom variants [74, 75], and induced a dramatic increase in the spike-specific CD4 and CD8 T cells response in most of the immunized individuals [73].
The ability of a vaccine to elicit a specific antibody response against its epitopes is not a mere criterion to demonstrate its effectiveness to develop immunity against SARS-CoV-2. Hence, the efficacy and safety of Comirnaty were validated in a broad range of multinational, placebo-controlled, observer-blinded, efficacy trials (NCT04368728) with 43,548 participants [76]. In terms of efficacy, among participants with no previous proof of infection, 8 cases were reported to be infected after 7 days from the second dose of the vaccine, and 162 cases were reported from the placebo group. Overall, Comirnaty has shown 95% efficiency in the prevention of COVID-19. Interestingly, between the first and second doses, 39 cases of COVID-19 were reported from the Comirnaty vaccinated group, and 82 cases of COVID-19 were observed in the placebo group, hence, a 52% efficacy was observed in this period. This signifies that the vaccine provides early protection, which occurs 12 days from the first dose. In addition, the individuals vaccinated with Comirnaty were assessed for the NA GMT against the South African (Beta) and the United Kingdom (Alpha) variant of SARS-CoV-2. The virus was designed to include subsequent mutations in their spike protein: N501Y in Alpha and Beta variants; 69/70-deletion + N501Y + D614G in Alpha mutant; and E484K + N501Y + D614G in Beta mutant. It was observed that the mutant type induced the neutralization titer similar to that of the wild type in a similar duration i.e., two to three weeks post-immunization. However, neutralization GMT against SARS- CoV-2 Beta mutant with three mutations (E484K + N501Y + D614G) was less in comparison to that against the N501Y mutant or Alpha mutant with three mutations (69/70 deletion + N501Y + D614G) [74]. Apart from these variants, WHO recognized Omicron as the variant of concern, hence it is crucial to develop a vaccine against this particular variant as it is distantly related to the previously reported variants [77]. Moreover, a study has been conducted on the health care workers, who received a third dose (booster) following 6-9 months of the second dose. It has been reported that cross-protective neutralizing antibodies were present against the Omicron and other variants of SARS-CoV-2 in the individuals vaccinated with Comirnaty, but the titer of these antibodies was less in Comirnaty vaccinated individuals compared to individuals vaccinated with Spikevax [77, 78]. Moreover, the cellular response against SARS-CoV-2 has also been roughly estimated and it was observed that in 95.2% of the participants RBD-specific CD4+ T cell lymphocytes were activated and the magnitude of this T cell response was directly proportional to the GMC of the anti-RBD antibodies and the GMT of NA. Moreover, in 76.2% of the immunized individuals, a definite CD8+ T cell response was observed which was directly proportional to the CD4+ T response but this was not the case for GMT of NA. Both of the RBD-specific T lymphocytes (CD4+ and CD8+) released IFNg+ and IL-2. In addition, the intracellular cytokine examination revealed the presence of the functional and pro-inflammatory response in both CD4+ and CD8+ T lymphocytes, with a Th1 orientation consisting in the production of TNF, IL-1b, and IL-12p70, but neither IL-4 nor IL-5 [73, 79].
mRNA-1273/ Spikevax (MODERNA/NIH)
Moderna Therapeutics an American company from Cambridge, Boston in association with the National Institute of Allergy and Infectious Diseases (NIAID) created the first vaccine candidate for clinical trials in 63 days from the SARS-CoV-2 genome sequencing. The vaccine consists of an mRNA encapsulated in a lipid nanoparticle and has the information required to synthesize a stable prefusion of the spike protein. This m RNA when inside the host cell uses its machinery to translate the viral antigens, which are presented to the T cells and recognized directly by the host B cells, thereby activating the adaptive immune response against the spike protein. The initial phase of administration of mRNA 1273 began with 45 healthy volunteers aged 18-55 years. They initially were administered at different doses of 25 μg, 100 μg, and 250 μg. The second shot was given 28 days following the initial one. The reports from the Phase I study demonstrated that the production of the neutralizing antibody titers and the dose-dependent humoral immune responses were similar to that in the convalescent individuals [80]. Doses of 25 μg and 100 μg were reported to induce CD4+ T cell response but the levels of cytokines involved in the expression of Th 2 cells were in low concentration (which were found to be detrimental during SARS and MERS vaccine development efforts) [81, 82]. Moreover, the CD8+ T lymphocyte was only activated by a 100 μg dose. There have been no stage 4 adverse effects (disabling or life-threatening) reported for this vaccine and is well tolerated. A small phase I trial that included 40 older people in two age groups (56-70 years and 71 years or older) was also conducted. The volunteers received the highest tolerated doses i.e., 25 μg or 100 μg of Spikevax. These individuals generated a similar immune response to that of the 18-55 age group. Hence, it signifies that Spikevax can induce immunogenicity even in the lesser immunocompetent and most vulnerable age groups. A dose of 100 μg has been shown to elicit strong cell-mediated and humoral immune responses, thus employing it in phase IV vaccine studies. Moreover, the efficacy and safety of this vaccine candidate were confirmed to be 94.1% [83]. Moreover, when the third dose of this vaccine was administered as a booster dose to the health care workers, 6 to 9 months after the second dose, the spikevax has been reported to be effective against Omicron, and other variants of SARS-CoV-2, and the concentration of the cross-neutralizing antibodies were higher in individuals vaccinated with Spikevax, compared to that of the Comirnaty vaccinated individuals [77, 84].
AZD1222/ Vaxzevria (AstraZeneca/Oxford University) or Covishield
Vaxzevria, manufactured by Oxford University and the British pharmaceutical company AstraZeneca is a viral vector vaccine. This vaccine candidate was among the first that started the clinical trials. It is the only vaccine to use an incapacitated chimpanzee adenovirus (ChAdOx1), therefore there would be no existing immunity in humans against this vector due to little or no exposure to this simian virus. The ChAdOx1 vector is designed to contain information encoding the wildtype spike protein [66]. A phase I study was conducted where patients were parted into two groups, a group with a large number of participants that received a single dose of Vaxzevria and a small group with 10 participants who received two doses 28 days apart. The mean neutralization titer showed an elevation in the second group that received the booster dose [85]. The antibody titers were comparable with that of the average convalescent samples, however, the comparative study was difficult due to the lack of data reporting the exact titers of the convalescent individuals. Interestingly, IFN-γ ELISPOT assays also suggest that this vaccine also induces a significant T-cell immune response [4]. Moreover, the presence of anti-spike IgA and IgG were reported in the sera of vaccinated individuals, i.e., post-vaccination robust B cell responses were observed [86]. Cytokines like IL-2 and IFN-γ were found to be elevated in individuals who received ChAdOx1 in comparison to the controls, moreover, a decrease in the level of IL-13 and IL-4 was observed [87]. The increase in the level of Th1 cytokines viz., IFN-γ, IL-2, and TNF-α in comparison to the Th2 cytokines have also been reported, and it has also been demonstrated that Vaxzevria generated the Th1 response predominantly [87]. Phase I/II trials of Vaxzevria have also been conducted in the population aged 18- 55 years, and it has been reported to be well tolerated in these individuals, further Vaxzevria also elicited a strong cellular and neutralizing antibody response against the S protein of SARS-COV-2 [86]. Moreover, in Phase II/III clinical trial involving volunteers of different age groups viz., 18 to 55 years 56 to 69 years, and 70 years and older, it has been reported that spike-specific T cell responses were induced and reached their peak on the 14th-day post-vaccination. In addition to this the spike specific neutralizing antibody response was maintained and peaked at 28 days post the booster dose, in all the age groups. Moreover, it has been reported to be well tolerated in all age groups [88].
CoronaVac (Sinovac Research and Development Co)
Inactivated vaccines have been extensively used for more than a decade. The infectivity of the virus is inactivated which makes it harmless along with maintaining its immunogenic nature to induce an immune response [67]. CoronaVac, the formaldehyde-inactivated SARS-CoV-2 vaccine, developed by the China-based Sinovac Research and Development Co. is presently being evaluated in Phase IV of the clinical trial. CoronaVac has been observed to induce 92.4% seroconversion in some individuals following 2 weeks and 97.4% following 4 weeks of the second dose [89]. In addition, 6 weeks following the immunization also an elevated titer of antibodies was observed [90]. In Chile, Phase III clinical trial in health care workers, suggested an elevated level of seroconversion in the neutralizing and anti-S1-RBD IgG antibodies, along with a strong T cell response in these individuals [91]. Coronavac is highly efficient in preventing the occurrence of COVID-19 disease in individuals between 18 to 59 years of age [92]. Moreover, around 14 days after vaccination, 89.7% of the volunteers were reported to produce anti-RBD antibodies, and about 92% of the seropositive (The presence of detectable levels of a specific marker within the serum is considered seropositivity, while the absence of such levels is considered seronegativity) individuals were reported to develop protective neutralizing antibodies. Further, the mean neutralizing antibody titer was evaluated in these volunteers and it was found that the neutralizing antibodies were more significant for the P.1 and P.2 variants in comparison to the B.1.128 variant [92]. Further, Phase I/II trials were conducted in the older aged group i.e., 60 years and older with two different doses viz., 3 μg and 6 μg, in a two-dose regimen (0 and 28 days apart). The neutralizing antibody response was much stronger in the 3 and 6 ug group compared to the group that received a dose of 1·5 μg. Moreover, these responses were similar to the immune responses developed in the adults (18–59 years ) that received a similar dose [93].
Ad5-nCov (CanSino Biologics)
Ad5-nCoV developed by the Chinese company CanSino Biologics from Tianjin, in association with the Institute of Biology of China’s Academy of Military Medical Sciences is an adenoviral vector vaccine. This vaccine uses the human adenovirus serotype 5 vector (Ad5) to express the S protein in the host cells [66]. As the vector is a human adenovirus, there may be a pre-existing immunity already present against this vector, which can also hinder the development of an immune response against the presented antigen [66]. In a Phase II trial (double-blinded randomized placebo-controlled), the volunteers between 18 to 83 years of age received, either of the two doses (5 × 1010 or 1× 1011) of this particular vaccine or placebo, and 28 days post-vaccination more than 95% of the individuals from both the dose groups were observed to produce the anti-RBD and neutralizing antibodies against SARS-CoV-2 [69]. Further, in both the dose groups, specific T cell responses were confirmed via the interferon-γ ELISpot assay [69].
Ad26.COV2.S (Johnson, and Johnson)
Johnson and Johnson Pharmaceuticals has a subsidiary viz., Israel-based Janssen Pharmaceuticals that develops the Ad26.COV2.S vaccine. This vaccine is an adenovirus 26-based viral vector vaccine (replication-defective) that expresses the stable SARS-CoV-2 prefusion S protein. CanSino differs from Ad26.COV2.S in the adenovirus serotype [66]. Since very few individuals have been exposed to the Ad26 serotype in comparison to the ubiquitous Ad5 serotype, therefore, the already present immunoreactivity against this vector must not reduce the immunogenicity of the developed vaccine. Secondly, this vaccine requires a single dose [66], and stimulates the induction of anti-spike antibodies and neutralizing antibodies, which have been reported to display lower mean titers than in the recovered individuals [94]. A single dose of this vaccine has been reported to induce the production of CD4 and CD8 T lymphocytes. Moreover, further studies are being conducted regarding the response developed due to the second dose and this might drastically increase the level of the nAb titers, similar to the other vaccines [4]. Hence, all of the viral vector vaccine candidates show similarities in terms of safety and induced immunogenicity [4]. Although, in contrast to the RNA-based and the adjuvant protein-based vaccines, they are slightly inferior [4]. In addition, Phase I/II trials involving 805 volunteers between 18 to 55 years, and more than 65 years were conducted at different dose levels in one or two-dose schedules including a high dose of 1×1011 viral particles or a low dose of 5×1010 viral particles per milliliter or placebo. Both low and high doses showed similar effects. In individuals aged 18 to 55 years the immunogenicity was observed to decline following the second dose. In addition, on 29 days following the initial dose neutralizing antibodies were observed in more than 90% of the volunteers and by day 57 this value reached 100 % (independent of dose and age group). Moreover, the levels of antibodies were reported to increase and were stable till day 71, and following the second dose, these levels were further reported to increase. Besides, in around 76- 83% of the cases from the individuals aged 18 to 55 years and 60- 67% of the cases from 60 and above age group were reported to display CD4+ T-cell response, further, in this age group a clear skewing towards the robust CD8+ T-cell response was observed [95].
BBIBP-CorV (Sinopharm)
BBIBP-CorV is an inactivated SARS-CoV-2 vaccine (Vero cell) developed by Sinopharm, China National Biotec Group Co, and the Beijing Institute of Biological Products [70]. This vaccine induces an effective immune response of about 79.34% [66] and is tolerable in healthy individuals. Moreover, a strong humoral immune response is induced even on the fourth day from the first dose of the vaccine, and 100 % seroconversion was reported on the 42nd day [70]. The neutralizing antibody titer was elevated in the individuals that were administered with the BBIP-CorV in the two-dose schedule of 0 and 21 and 0 and 28 days in comparison to the two-dose schedule of 0 and 14 days and the single-dose schedule [70]. Phase I/ II trial study showed that this vaccine was immunogenic, safe, and well-tolerated in healthy individuals. Moreover neutralizing antibody responses were reported in all the recipients from the two age groups between 18- 59 and more than 60 years at different doses of 2 μg, 4 μg, and 8 μg [70]. However, more studies need to be reported to give insights into how this inactivated vaccine is being evaluated for the control and prevention of COVID-19.
Some Other Vaccine Regimens
Sputnik V or Gam-COVID-Vac developed by Gamaleya Research Institute of epidemiology and microbiology Moscow, Russia is a viral vector vaccine, which uses different adenoviral vectors in the prime dose (Ad26) and the booster dose (Ad5) [66]. A phase I and II trial that included 38 participants suggested that this vaccine can induce a strong humoral and cell-mediated immune response (Tc and Th cell activation) in all the volunteers [96]. Further, a study including 22,714 participants reported the efficiency of this vaccine to be 91.4%, where about 20 individuals in the placebo group were reported to be severely infected with COVID-19 and none of the severe cases were reported in the vaccinated individuals, concluding that the vaccine is 100% efficient against severe cases of COVID-19 [97]. Moreover, Covaxin or BBV152, developed by the Bharat Biotech International Limited, along with the National Institute of Virology of Indian Council of Medical Research, India is an inactivated whole virus vaccine. To prepare this vaccine β-propiolactone has been used to inactivate the whole virus, which was further propagated in the CCL81 Vero cell lines [66]. Clinical trials have been successfully conducted in India, and it has been reported to induce a strong humoral response four days following the initial immunization. Moreover, seroconversion has also been reported 42 days following the immunization [68]. Further, NVX-CoV2373 is a subunit vaccine that is developed by Novavax from Maryland, it is the recombination of the Spike protein and saponin-based Matrix-M1 adjuvant [98]. Novaxax induces an elevation in the level of B, T, NK, and dendritic cells in the draining lymph nodes. Moreover, an elevated level of spike-specific antibodies and neutralizing antibodies have also been reported following the initial dose of immunization [99, 100].
Conclusion
In this review, first, we have extensively discussed the levels of IFN-I response, degree of antibodies produced, and CD8/ CD4 T cell responses in mild and acute SARS-CoV-2 infection. These variations in acute and mild infections can aid in the development of effective remedies and vaccine design to overcome this pandemic. Moreover, we have also discussed the immunity developed in recovered patients. The short lifespan of the neutralizing antibodies in the recovered individuals suggests that the reinfection may be frequent in the upcoming months or years. However, strong B and T cell responses are induced during the infection, hence these responses will surely aid in the mitigation of the deadly SARS-CoV-2 transmission, and inhibit the reinfection in long term.
Vaccines play a central role in the development of herd immunity. In the context of COVID-19, the major characteristics, immunological responses, and efficacy of the available vaccines have been described extensively in this review (Table 1). Almost all of these vaccine candidates provide protection against this infection by inducing an effective neutralizing antibody response. However, the effects of these neutralizing antibodies are not long-lasting, so rapid and efficient vaccination programs are essential to inhibit the transmission of SARS-CoV-2. The efficacy and safety profile of the vaccine candidates that are in Phase IV of the clinical trial are being carefully monitored by the global scientific community, and are reported to be highly effective against this particular infection, viz., m RNA based vaccines like Comirnaty and Spikevax were 95% and 94% efficient respectively, and the viral vector vaccine like sputnik V, was 91.4% effective, moreover, the inactivated pathogen vaccine like BBIBP-CorV was 79.34% effective [66, 67, 71, 83, 97]. Due to the effectiveness of these vaccine candidates, the COVID-19 restrictions are slowly being removed in the majority of the countries where the vaccination program has reached its final stage, which might be a promising approach in bringing this global pandemic to an end. Whether these vaccines are effective against the variants of the SARS-CoV-2 still needs much investigation. However, m RNA vaccines and AZD1222 displayed high protective efficacy against Alpha, Beta, and Gamma variants of SARS-CoV-2 [101, 102]. Further Ad26.COV2.S and m RNA vaccines resulted in a lower possibility of viral culture positivity, and a rapid decrease of the viral load for the delta and a range of other variants [65].
SARS-CoV-2 vaccines came into the market as an emergency, hence, the PPP (public-private partnership) could be helpful for the distribution of various vaccines and to conduct the immunization program around the globe to overcome this deadly pandemic. The declarations by the scientific community rely on reports that involve various statistical and biological approaches in their studies. Moreover, Scientific research should be transparent and reproducible. The global distribution of the vaccines and the international collaboration to develop these vaccines in different countries is a great step in terms of humanity and will surely play a vital role to end this pandemic. In addition, vaccination campaigns are really important in order to induce herd immunity, which could mitigate the threat of COVID-19 pandemic. Hence, rapid production and supply of various vaccines are crucial[103]. Besides, mixing different vaccine to achieve an elevated level of immunity can also be an approach to overcome the shortage of vaccines in poor countries. In addition, the mixing of different vaccines can also help to overcome the havoc caused by the new emerging variants or the variants of concern (VOC) that are becoming partially resistant to the vaccines available in the market today [104]. There have been reports which suggest that the mixing of vaccines has induced an elevation in the levels of neutralizing antibodies, along with strong IgG response, and robust cellular immunity [105–107] . Hence, this mix and match strategy is being applied in both the developing and developed countries to vaccinate the individuals of their country with an effective vaccination strategy [108]. There have been studies where the mixing of the Vaxzevria and Comirnaty vaccine induced a more robust immune response compared to that induced by the two doses of either of these vaccines [105, 109–111]. Further, a single dose of Vaxzevria when followed by a booster dose of Pfizer reported to induce an 11.5 times elevation in the level of IgG and spike specific IgA immunoglobulins compared to the individuals who received the two doses of Vaxzevria [112]. Moreover, humoral and cell-mediated immune responses particularly the levels of spike specific IgG, neutralizing antibodies, spike specific CD4, and CD8 T cells were reported to increase significantly in the individuals who received the first dose of Vaxzevria followed by the second dose of an mRNA vaccine viz., Comirnaty or Spikevax [106]. A phase II trial in Spain when Vaxzevria was given as a first dose, followed by the booster dose of the Comirnaty vaccine, resulted in 150 times elevation in the levels of antibodies after fourteen days of the booster dose [105]. Besides, these mix and match strategies along with boosting the immune response showed the least side effects [109, 110]. Furthermore, the fractional dosing (half or lesser dose) of different vaccine regimens would speed up the vaccination program in underdeveloped countries [103]. In addition, the fractional dosing strategy might be more economical particularly when the worldwide stock of these vaccines is restricted or in the early phase of development for the new emerging variants that could pose an increased threat to the global public health, hence providing insights into the development of a potential cost-effective vaccine against SARS-CoV-2.
Acknowledgments
The authors are thankful to the Department of Zoology, SSJ University, Almora (Uttarakhand), India for providing the facility for this work. This work is supported by DST-FIST grant SR/FST/LS- I/2018/131to the Department of Zoology.
Declarations
Conflict of Interest
The authors have declared no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu B, Han J, Cheng X, Yu L, Zhang L, Wang W, et al. Reduced numbers of T cells and B cells correlates with persistent SARS-CoV-2 presence in non-severe COVID-19 patients. Sci Rep. 2020;10:17718. doi: 10.1038/s41598-020-73955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. Gallagher T, editor. J Virol. 2020:94. [DOI] [PMC free article] [PubMed]
- 3.Trougakos IP, Stamatelopoulos K, Terpos E, Tsitsilonis OE, Aivalioti E, Paraskevis D, et al. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J Biomed Sci. 2021;28:9. doi: 10.1186/s12929-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grigoryan L, Pulendran B. The immunology of SARS-CoV-2 infections and vaccines. Semin Immunol. 2020;50:101422. doi: 10.1016/j.smim.2020.101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination — Kentucky, may–June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1081–1083. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman S, Rahman MM, Miah M, Begum MN, Sarmin M, Mahfuz M, et al. COVID-19 reinfections among naturally infected and vaccinated individuals. Sci Rep. 2022;12:1438. doi: 10.1038/s41598-022-05325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludvigsson JF. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2021;110:914–921. doi: 10.1111/apa.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 10.Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020;25. [DOI] [PMC free article] [PubMed]
- 11.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ . 2020;369:m2094. [DOI] [PMC free article] [PubMed]
- 12.Morris SB, Schwartz NG, Patel P, Abbo L, Beauchamps L, Balan S, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection - United Kingdom and United States, march-august 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajaj V, Gadi N, Spihlman AP, Wu SC, Choi CH, Moulton VR. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front Physiol. 2021;11:1793. doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra KP, Singh AK, Singh SB. Hyperinflammation and immune response generation in COVID-19. Neuroimmunomodulation. 2020;27:80–86. doi: 10.1159/000513198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim YX, Ng YL, Tam JP, Liu DX. Human coronaviruses: a review of virus-host interactions. Diseases. 2016;4:26. doi: 10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. 2020;180:1044–1066. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieto-Torres JL, Verdiá-Báguena C, Jimenez-Guardeño JM, Regla-Nava JA, Castaño-Rodriguez C, Fernandez-Delgado R, et al. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues TS, de Sá KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021;218. [DOI] [PMC free article] [PubMed]
- 20.Lokugamage KG, Hage A, Schindewolf C, Rajsbaum R, Menachery VD. SARS-CoV-2 is sensitive to type I interferon pretreatment. bioRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 21.Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antivir Res. 2020;179:104811. doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Mol Oral Microbiol. 2010;25:25–37. doi: 10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in new York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song C-Y, Xu J, He J-Q, Lu Y-Q. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv. 2020;2020.03.05.20031906.
- 26.Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu L, Tong Y, Shen G, Fu A, Lai Y, Zhou X, et al. Immunodepletion with Hypoxemia: A Potential High Risk Subtype of Coronavirus Disease 2019. medRxiv. 2020;2020.03.03.20030650.
- 28.Chu H, Chan JF-W, Wang Y, Yuen TT-T, Chai Y, Hou Y, et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. 2020;71:1400–1409. doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. medRxiv. 2020;2020.02.23.20026690.
- 30.Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martínez-Colón GJ, McKechnie JL, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuby J. Kuby immunology. New York: W.H.Freeman; 2013. [Google Scholar]
- 32.Long Q-X, Liu B-Z, Deng H-J, Wu G-C, Deng K, Chen Y-K, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 33.Padoan A, Sciacovelli L, Basso D, Negrini D, Zuin S, Cosma C, et al. IgA-ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: a longitudinal study. Clin Chim Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams ER, Ainsworth M, Anand R, Andersson MI, Auckland K, Baillie JK, et al. Antibody testing for COVID-19: a report from the national COVID scientific advisory panel. Wellcome Open Res. 2020;5:139. doi: 10.12688/wellcomeopenres.15927.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Röltgen K, Wirz OF, Stevens BA, Powell AE, Hogan CA, Najeeb J, et al. SARS-CoV-2 antibody responses correlate with resolution of RNAemia but are short-lived in patients with mild illness. medRxiv. 2020.
- 37.Liu T, Wu S, Tao H, Zeng G, Zhou F, Guo F, et al. Prevalence of IgG antibodies to SARS-CoV-2 in Wuhan – implications for the ability to produce long-lasting protective antibodies against SARS-CoV-2. medRxiv. 2020;2020.06.13.20130252-2020.06.13.20130252.
- 38.Tan W, Lu Y, Zhang J, Wang J, Dan Y, Tan Z, et al. Viral Kinetics and Antibody Responses in Patients with COVID-19. medRxiv. 2020;2020.03.24.20042382-2020.03.24.20042382.
- 39.Long Q-X, Tang X-J, Shi Q-L, Li Q, Deng H-J, Yuan J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 40.Nie S, Zhao X, Zhao K, Zhang Z, Zhang Z, Zhang Z. Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID-19): a retrospective study. medRxiv . 2020;2020.03.24.20042283.
- 41.Wenjun W, Xiaoqing L, Sipei W, Puyi L, Liyan H, Yimin L, et al. The definition and risks of Cytokine Release Syndrome-Like in 11 COVID-19-Infected Pneumonia critically ill patients: Disease Characteristics and Retrospective Analysis. medRxiv. 2020;2020.02.26.20026989.
- 42.Zheng H-Y, Zhang M, Yang C-X, Zhang N, Wang X-C, Yang X-P, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thevarajan I, Nguyen THO, Koutsakos M, Druce J, Caly L, van de Sandt CE, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodda LB, Netland J, Shehata L, Pruner KB, Morawski PA, Thouvenel CD, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184:169–183.e17. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 47.Morita R, Schmitt N, Bentebibel S-E, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isho B, Abe KT, Zuo M, Jamal AJ, Rathod B, Wang JH, et al. Evidence for sustained mucosal and systemic antibody responses to SARS-CoV-2 antigens in COVID-19 patients. medRxiv. 2020;2020.08.01.20166553.
- 49.Perreault J, Tremblay T, Fournier M-J, Drouin M, Beaudoin-Bussières G, Prévost J, et al. Longitudinal analysis of the humoral response to SARS-CoV-2 spike RBD in convalescent plasma donors. bioRxiv. 2020;2020.07.16.206847.
- 50.Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 1979;2020(370):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cromer D, Juno JA, Khoury D, Reynaldi A, Wheatley AK, Kent SJ, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol 2021 21:6. 2021;21:395–404. [DOI] [PMC free article] [PubMed]
- 52.Röltgen K, Powell AE, Wirz OF, Stevens BA, Hogan CA, Najeeb J, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5. [DOI] [PMC free article] [PubMed]
- 53.Crawford KHD, Dingens AS, Eguia R, Wolf CR, Wilcox N, Logue JK, et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;223:197–205. doi: 10.1093/infdis/jiaa618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu J, Liang B, Chen C, Wang H, Fang Y, Shen S, et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat Commun. 2021;12:1813. doi: 10.1038/s41467-021-22034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beaudoin-Bussières G, Laumaea A, Anand SP, Prévost J, Gasser R, Goyette G, et al. Decline of humoral responses against SARS-CoV-2 spike in convalescent individuals. mBio. 2020;11:1–7. doi: 10.1128/mBio.02590-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheatley AK, Juno JA, Wang JJ, Selva KJ, Reynaldi A, Tan H-X, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021;12:1162. doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371. [DOI] [PMC free article] [PubMed]
- 58.Antia A, Ahmed H, Handel A, Carlson NE, Amanna IJ, Antia R, et al. Heterogeneity and longevity of antibody memory to viruses and vaccines. PLoS Biol. 2018;16:e2006601. doi: 10.1371/journal.pbio.2006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021 27:7. 2021;27:1205–11. [DOI] [PubMed]
- 60.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartley GE, Edwards ESJ, Aui PM, Varese N, Stojanovic S, McMahon J, et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci Immunol. 2020;5. [DOI] [PMC free article] [PubMed]
- 62.Moderbacher CR, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Juno JA, Tan H-X, Lee WS, Reynaldi A, Kelly HG, Wragg K, et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med. 2020;26:1428–1434. doi: 10.1038/s41591-020-0995-0. [DOI] [PubMed] [Google Scholar]
- 64.Hufford MM, Kim TS, Sun J, Braciale TJ. The effector T cell response to influenza infection. Curr Top Microbiol Immunol. 2015;386:423–455. doi: 10.1007/82_2014_397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28:202–221. doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6:28. doi: 10.1038/s41541-021-00292-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.García-Montero C, Fraile-Martínez O, Bravo C, Torres-Carranza D, Sanchez-Trujillo L, Gómez-Lahoz AM, et al. An updated review of SARS-CoV-2 vaccines and the importance of effective vaccination programs in pandemic times. Vaccines (Basel). 2021;9. [DOI] [PMC free article] [PubMed]
- 68.Loo K-Y, Letchumanan V, Ser H-L, Teoh SL, Law JW-F, Tan LT-H, et al. COVID-19: insights into potential vaccines. Microorganisms. 2021;9:1–19. doi: 10.3390/microorganisms9030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamb YN. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs . Drugs; 2021;81:495–501. [DOI] [PMC free article] [PubMed]
- 72.Pulendran B, Arunachalam PS. Systems biological assessment of human immunity to BNT162b2 mRNA vaccination. Res Sq. 2021.
- 73.Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. 2020;2020.12.09.20245175-2020.12.09.20245175.
- 74.BioNTech. An In Vitro Study Shows Pfizer-BioNTech COVID-19 Vaccine Elicits Antibodies that Neutralize SARS-CoV-2 with a Mutation Associated with Rapid Transmission | pfpfizeruscom. 2021.
- 75.Xie X, Liu Y, Liu J, Zhang X, Zou J, Fontes-Garfias CR, et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat Med. 2021 27:4. 2021;27:620–1. [DOI] [PubMed]
- 76.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haveri A, Solastie A, Ekström N, Österlund P, Nohynek H, Nieminen T, et al. Neutralizing antibodies to SARS-CoV-2 omicron variant after third mRNA vaccination in health care workers and elderly subjects. European Journal of Immunology . 2022;0:1–9. [DOI] [PMC free article] [PubMed]
- 78.Falsey AR, Frenck RW, Walsh EE, Kitchin N, Absalon J, Gurtman A, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385:1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lombardi A, Bozzi G, Ungaro R, Villa S, Castelli V, Mangioni D, et al. Mini review immunological consequences of immunization with COVID-19 mRNA vaccines: preliminary results. Front Immunol. 2021;0:677. doi: 10.3389/fimmu.2021.657711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI. Insight. 2019;4. [DOI] [PMC free article] [PubMed]
- 82.Houser KV, Broadbent AJ, Gretebeck L, Vogel L, Lamirande EW, Sutton T, et al. Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody. PLoS Pathog 2017;13:e1006565. [DOI] [PMC free article] [PubMed]
- 83.Moderna. Moderna announces longer shelf life for its COVID-19 vaccine candidate at refrigerated temperatures. 2020.
- 84.Doria-Rose NA, Shen X, Schmidt SD, O’Dell S, McDanal C, Feng W, et al. Booster of mRNA-1273 strengthens SARS-CoV-2 omicron neutralization. medRxiv. 2021.
- 85.van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27:270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 88.Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, Zeng G, Pan H, Li C, Kan B, Hu Y, et al. Immunogenicity and Safety of a SARS-CoV-2 Inactivated Vaccine in Healthy Adults Aged 18-59 years: Report of the Randomized, Double-blind, and Placebo-controlled Phase 2 Clinical Trial. medRxiv. 2020;2020.07.31.20161216-2020.07.31.20161216.
- 90.Canedo-Marroquín G, Saavedra F, Andrade CA, Berrios RV, Rodríguez-Guilarte L, Opazo MC, et al. SARS-CoV-2: immune response elicited by infection and development of vaccines and treatments. Front Immunol 2020;11:569760. [DOI] [PMC free article] [PubMed]
- 91.Bueno SM, Abarca K, González PA, Gálvez NMS, Soto JA, Duarte LF, et al. Interim report: Safety and immunogenicity of an inactivated vaccine against SARS-CoV-2 in healthy chilean adults in a phase 3 clinical trial. medRxiv. 2021;2021.03.31.21254494.
- 92.Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–222. doi: 10.1016/S0140-6736(21)01429-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sadoff J, le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Safety and immunogenicity of the Ad26.COV2.S COVID-19 vaccine candidate: interim results of a phase 1/2a, double-blind, randomized, placebo-controlled trial. medRxiv. 2020;2020.09.23.20199604-2020.09.23.20199604.
- 95.Sadoff J, le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Logunov DY, Dolzhikova I, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sputnik V. Second interim analysis of clinical trial data showed a 91.4% efficacy for the Sputnik V vaccine on day 28 after the first dose; vaccine efficacy is over 95% 42 days after the first dose. 2020.
- 98.Belete TM. Review on up-to-date status of candidate vaccines for COVID-19 disease. Infect Drug Resist. 2021;14:151–161. doi: 10.2147/IDR.S288877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guebre-Xabier M, Patel N, Tian JH, Zhou B, Maciejewski S, Lam K, et al. NVX-CoV2373 vaccine protects cynomolgus macaque upper and lower airways against SARS-CoV-2 challenge. Vaccine. 2020;38:7892–7896. doi: 10.1016/j.vaccine.2020.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nasreen S, Chung H, He S, Brown KA, Gubbay JB, Buchan SA, et al. Effectiveness of COVID-19 vaccines against variants of concern in Ontario, Canada medRxiv 2021;2021.06.28.21259420.
- 102.Bernal JL, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv. 2021;2021.05.22.21257658. [DOI] [PMC free article] [PubMed]
- 103.Du Z, Wang L, Pandey A, Lim WW, Chinazzi M, Piontti APY, et al. Modeling comparative cost-effectiveness of SARS-CoV-2 vaccine dose fractionation in India. Nat Med. 2022. [DOI] [PMC free article] [PubMed]
- 104.Rashedi R, Samieefar N, Masoumi N, Mohseni S, Rezaei N. COVID-19 vaccines mix-and-match: the concept, the efficacy and the doubts. J Med Virol. 2022;94:1294–1299. doi: 10.1002/jmv.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Benning L, Töllner M, Hidmark A, Schaier M, Nusshag C, Kälble F, et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination induces strong humoral responses among health care workers. Vaccines (Basel) 2021;9:857. doi: 10.3390/vaccines9080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sesa G, Czabanowska K, Green M, Reid Professor J. Middleton J. The Importance of Health Communication during Emergencies: The Mix-and-Match Question; 2021. p. 1–12.
- 109.Vogel G. Mixing vaccines may boost immune responses. Science. 2021;372:1138. doi: 10.1126/science.372.6547.1138. [DOI] [PubMed] [Google Scholar]
- 110.Lewis D. Mix-and-match COVID vaccines: the case is growing, but questions remain. Nature. 2021;595:344–345. doi: 10.1038/d41586-021-01805-2. [DOI] [PubMed] [Google Scholar]
- 111.Hillus D, Schwarz T, Tober-Lau P, Hastor H, Thibeault C, Kasper S, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1-nCoV19 and BNT162b2: a prospective cohort study. medRxiv. 2021;2021.05.19.21257334. [DOI] [PMC free article] [PubMed]
- 112.Barros-Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov M, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]