Abstract
We investigated the presence of endophytic rhizobia within the roots of the wetland wild rice Oryza breviligulata, which is the ancestor of the African cultivated rice Oryza glaberrima. This primitive rice species grows in the same wetland sites as Aeschynomene sensitiva, an aquatic stem-nodulated legume associated with photosynthetic strains of Bradyrhizobium. Twenty endophytic and aquatic isolates were obtained at three different sites in West Africa (Senegal and Guinea) from nodal roots of O. breviligulata and surrounding water by using A. sensitiva as a trap legume. Most endophytic and aquatic isolates were photosynthetic and belonged to the same phylogenetic Bradyrhizobium/Blastobacter subgroup as the typical photosynthetic Bradyrhizobium strains previously isolated from Aeschynomene stem nodules. Nitrogen-fixing activity, measured by acetylene reduction, was detected in rice plants inoculated with endophytic isolates. A 20% increase in the shoot growth and grain yield of O. breviligulata grown in a greenhouse was also observed upon inoculation with one endophytic strain and one Aeschynomene photosynthetic strain. The photosynthetic Bradyrhizobium sp. strain ORS278 extensively colonized the root surface, followed by intercellular, and rarely intracellular, bacterial invasion of the rice roots, which was determined with a lacZ-tagged mutant of ORS278. The discovery that photosynthetic Bradyrhizobium strains, which are usually known to induce nitrogen-fixing nodules on stems of the legume Aeschynomene, are also natural true endophytes of the primitive rice O. breviligulata could significantly enhance cultivated rice production.
Bacteria of the genera Rhizobium, Bradyrhizobium, Sinorhizobium, Allorhizobium, Mesorhizobium, and Azorhizobium, commonly known as rhizobia, have great environmental and agricultural importance because their symbioses with legumes are responsible for most of the atmospheric nitrogen fixed on earth. These microorganisms are soil bacteria able to elicit the formation of new organs, called nodules, on most species of the family Fabaceae and on the nonleguminous Parasponia, in which they reduce atmospheric nitrogen to ammonia to the benefit of the host plant. In the absence of leguminous plants, populations of rhizobia are commonly found in soils where they can survive saprophytically. Recently, the natural habitat of rhizobia was extended to a third niche, the roots of gramineous plants. Rhizobium leguminosarum bv. trifolii was shown to be naturally present inside the roots of rice grown in rotation with clover in Egypt, without forming any nodule-like structure (44). Such habitats inside roots of cereals and grass plants have already been identified as an important reservoir for various N2-fixing endophytic bacteria, known as plant growth-promoting rhizobacteria (PGPR), such as Acetobacter diazotrophicus (11, 36) and Herbaspirillum seropedicae (5, 7, 22) in sugar cane, Azoarcus spp. (34, 35) in Kallar grass (Leptochloa fusca), and Azospirillum spp. (6) in maize and rice. Like most of these PGPR on their homologous gramineous host plants, R. leguminosarum was shown to efficiently promote rice growth after field inoculation (44).
Rice (Oryza sativa L.) is the most important food crop in developing countries. The high-yielding rice varieties of the “Green Revolution” have resulted in large increases in rice production but require large amounts of nitrogen fertilizers, which contribute to nitrate contamination of soils and groundwater supplies, often leading to health hazards and environmental pollution. Therefore, endophytic rhizobia, known to directly supply biologically fixed nitrogen to the legume plant, may also have great potential to improve sustainable rice production.
In order to discover other natural rice-rhizobium associations, we screened sustainable primitive rice production systems where N fertilizer has never been applied. In Africa, the wetland wild rice Oryza breviligulata, which is the ancestor of the African cultivated rice Oryza glaberrima (38), has been harvested and consumed in the Sahelian and Sudanian regions for more than 10,000 years. O. breviligulata grows spontaneously in temporary ponds, wetland plains, and river deltas of the semiarid and semihumid regions of Africa. It grows in 0.5- to 1.5-m-deep water, where it floats and forms numerous nodal and aquatic roots. This primitive rice species is frequently found growing in association with several aquatic legumes belonging to the genera Aeschynomene and Sesbania (1, 14, 19). Among these aquatic species, Aeschynomene indica and Aeschynomene sensitiva form stem nodules with photosynthetic Bradyrhizobium strains (26), and Sesbania rostrata forms stem nodules with Azorhizobium caulinodans (29). We therefore screened three O. breviligulata growing sites in West Africa (two sites in Senegal and one in Guinea) for natural associations of photosynthetic bradyrhizobia and/or azorhizobia with the rice roots.
MATERIALS AND METHODS
Isolation of endophytic rhizobia from wild rice.
Young nodal roots of 10 different O. breviligulata plants per site were collected at the flowering stage in wild-paddy fields of Senegal and Guinea (West Africa) where the water was 30 cm to 1 m deep. Roots were cleaned thoroughly with tap water, rinsed with sterile deionized water, drained on absorbent paper, and cut into 3- to 5-cm-long sections. Five grams of sections from each individual collected plant was then transferred to a sterile 250-ml Erlenmeyer flask containing 50 ml of sterile water, shaken for 15 min, and washed twice in 50 ml of sterile distilled water. Sections were then aseptically transferred to another sterile 250-ml Erlenmeyer flask and surface sterilized as follows. They were placed in 96% ethanol for 1 min, washed with sterile distilled water, then sterilized with 0.1% HgCl2 for 5 min, and washed six times with sterile distilled water. As a control to check for superficial contamination, for each individual plant, 200 μl of water from the final rinse was inoculated on plates containing tryptone-glucose-yeast extract agar (TY) medium (Difco, Detroit, Mich.). No individual root sample resulting in contamination on TY plates was retained for isolation of endophytic rhizobia. Sections were aseptically crushed in a Waring blender containing 100 ml of sterile water, and 0.5 ml of the root homogenate was then aseptically inoculated into 4-day-old young seedlings of A. sensitiva, S. rostrata, and Acacia albida grown in Gibson tubes (41) (see below). Enumeration of rhizobia from surface-sterilized root homogenates, non-surface-sterilized roots, or rice field water was performed by the plant infection most-probable-number (MPN) technique as described by Brockwell (10).
Legume cultivation and rhizobium isolation from nodules.
Seeds of A. sensitiva, A. indica, Aeschynomene afraspera, S. rostrata, and F. albida were scarified, surface sterilized for 45 min with concentrated sulfuric acid, and rinsed six times with sterile distilled water. Seeds were incubated to germinate in sterile petri dishes on 0.8% agar yeast extract-mannitol (YEM) medium (41). After 24 to 48 h, seeds showing no contamination were transferred to Gibson tubes containing nitrogen-free Jensen seedling slant agar (23) and grown under continuous light (20 W/m2) at 28°C. Two days later, legume species (eight replications for each treatment) were aseptically inoculated with rhizobial cultures or root homogenate. Three-week-old nodulated plants were collected, and nodules were picked, washed, and surface sterilized by immersion in HgCl2 (0.1%) for 5 min. From this stage, the nodules were manipulated aseptically. Nodules were rinsed six times in sterile distilled water and then crushed in a drop of sterile water, and the suspension was streaked onto YEM plates. After 1 week of incubation at 28°C under aerobic conditions, colonies were checked for purity by repeated streaking on YEM medium and by microscopic examination of living cells. Isolates were checked for nodulation on their original host plants.
Bacterial strains and culture growth conditions.
The bacterial isolates used in this study are listed in Table 1. All Bradyrhizobium strains were maintained on YEM medium. All strains were stored at −80°C on YEM medium adjusted to 20% (vol/vol) glycerol. Representative and type strains of Bradyrhizobium japonicum and Bradyrhizobium elkanii and various clusters of Bradyrhizobium strains previously described (13, 15, 26, 27) were included in this study (Table 2).
TABLE 1.
Photosynthetic endophytic and aquatic strains used in the present study
| Bacterial straina | Source | Geographic origin | Nodulation specificityb
|
Crt typec | ||
|---|---|---|---|---|---|---|
| Aeschynomene elaphroxylon (group I) | A. afraspera (group II) | A. sensitiva (group III) | ||||
| ORS402 | Rice root | Joal (Senegal) | 0 | 0 | E | W |
| ORS404 | Water | Joal (Senegal) | 0 | 0 | E | DP |
| ORS405 | Water | Joal (Senegal) | 0 | 0 | I | W |
| ORS406 | Water | Joal (Senegal) | 0 | 0 | I | W |
| ORS408 | Water | Joal (Senegal) | 0 | 0 | E | DP |
| ORS2005 | Rice root | Joal (Senegal) | 0 | 0 | E | O |
| ORS2006 | Rice root | Joal (Senegal) | 0 | 0 | E | DP |
| ORS2007 | Rice root | Joal (Senegal) | 0 | 0 | E | O |
| ORS2008 | Rice root | Joal (Senegal) | 0 | 0 | E | DP |
| ORS2009 | Rice root | Joal (Senegal) | 0 | 0 | E | DP |
| ORS2010 | Water | Joal (Senegal) | 0 | 0 | E | DP |
| ORS2011 | Rice root | Joal (Senegal) | 0 | 0 | E | O |
| ORS2012 | Rice root | Joal (Senegal) | 0 | 0 | E | DP |
| ORS2013 | Water | Joal (Senegal) | 0 | 0 | E | DP |
| ORS2014 | Rice root | Joal (Senegal) | 0 | 0 | E | O |
| ORS2019 | Water | Joal (Senegal) | 0 | 0 | E | O |
| STM478 | Rice root | Kapachez (Guinea) | 0 | 0 | E | DP |
| STM481 | Rice root | Kapachez (Guinea) | 0 | 0 | E | DP |
| STM482 | Rice root | Kapachez (Guinea) | 0 | 0 | E | DP |
| STM515 | Rice root | Kapachez (Guinea) | 0 | 0 | E | DP |
Designations: ORS, collection of the Institut de Recherche pour le Développement, Montpellier, France; STM, collection of the Laboratoire des Symbioses Tropicales et Méditerranéennes, Montpellier, France. Bacterial strains listed here are new isolates.
0, no nodulation; I, ineffective root nodulation; E, effective root nodulation.
Crt, carotenoid; W, white (strain lacking Bchl a and carotenoid); DP, dark pink; O, orange.
TABLE 2.
Reference strains used in this studya
| Bacterial strain | LMG no. | Original host plant | Geographic origin | Reference or source | Crt type spectrumb |
|---|---|---|---|---|---|
| Photosynthetic Bradyrhizobium spp. | |||||
| ORS266 | 15442 | Aeschynomene tambacoundensis | Senegal | 26 | LP |
| ORS276 | 12185 | Aeschynomene sensitiva | Senegal | 26 | |
| ORS278 | 12187 | Aeschynomene sensitiva | Senegal | 26 | O |
| ORS279 | 12188 | Aeschynomene sensitiva | Senegal | 26 | |
| ORS280 | 15411 | Aeschynomene indica | Senegal | 26 | |
| ORS287 | 15378t1 | Aeschynomene afraspera | Senegal | 26 | LP |
| ORS288 | 12189 | Aeschynomene afraspera | Senegal | 26 | |
| ORS292 | 12205 | Aeschynomene sensitiva | Senegal | 26 | |
| ORS294 | 12192 | Aeschynomene sensitiva | Senegal | 26 | LP |
| ORS296 | 12194 | Aeschynomene sensitiva | Senegal | 26 | |
| ORS297 | 12195 | Aeschynomene sensitiva | Senegal | 26 | LP |
| ORS299 | 15399 | Aeschynomene sensitiva | Senegal | 26 | |
| ORS306 | 11797 | Aeschynomene indica | Senegal | 1 | LP |
| ORS308 | 15401 | Aeschynomene afraspera | Senegal | 3 | LP |
| ORS324 | 8295 | Aeschynomene afraspera | Senegal | 2 | LP |
| ORS331 | 11799 | Aeschynomene tambacoundensis | Senegal | 2 | LP |
| ORS337 | 8299t2 | Aeschynomene afraspera | Senegal | 3 | |
| ORS342 | 15383 | Aeschynomene indica | Senegal | 26 | |
| ORS344 | 12198 | Aeschynomene indica | Senegal | 26 | |
| ORS348 | 12200 | Aeschynomene sp. | Senegal | 26 | |
| ORS352 | 15384 | Aeschynomene afraspera | Senegal | 1 | LP |
| ORS359 | 12201 | Aeschynomene sensitiva | Senegal | 26 | LP |
| ORS362 | 10305 | Aeschynomene afraspera | Senegal | 2 | |
| ORS364 | 11802 | Aeschynomene nilotica | Senegal | 2 | LP |
| ORS368 | 15387 | Aeschynomene indica | Senegal | 26 | |
| ORS371 | 11804 | Aeschynomene indica | Senegal | 26 | DP |
| ORS377 | 15420 | Aeschynomene elaphroxylon | Senegal | 26 | W |
| ORS378 | 15421 | Aeschynomene elaphroxylon | Senegal | 26 | W |
| ORS379 | 15422 | Aeschynomene elaphroxylon | Senegal | 26 | W |
| ORS381 | 15423 | Aeschynomene elaphroxylon | Senegal | 26 | W |
| ORS384 | 15390 | Aeschynomene indica | Senegal | 26 | LP |
| ORS385 | 15391 | Aeschynomene indica | Senegal | 26 | |
| ORS386 | 11815 | Aeschynomene indica | Senegal | 26 | LP |
| ORS392 | 12205 | Aeschynomene indica | Senegal | 26 | O |
| ORS400 | 12207 | Aeschynomene indica | Senegal | 26 | |
| ORS29 | 15165 | Unknown | Senegal | 13 | |
| ORS31 | 15167 | Indigofera tinctoria | Senegal | 13 | |
| ORS87 | 15177 | Tephrosia purpurea | Senegal | 13 | |
| ORS90 | R-2196 | Tephrosia purpurea | Senegal | 13 | |
| ORS110 | 10666 | Acacia albida | Senegal | 15 | |
| ORS122 | 10678 | Acacia albida | Senegal | 15 | |
| ORS123 | 10679 | Acacia albida | Senegal | 15 | |
| ORS124 | 10680 | Acacia albida | Senegal | 15 | |
| ORS127 | 10683 | Acacia albida | Senegal | 15 | |
| ORS133 | 10689 | Acacia albida | Senegal | 15 | |
| ORS140 | 10696 | Acacia albida | Senegal | 15 | |
| ORS167 | 10710 | Acacia albida | Senegal | 15 | |
| ORS169 | 10712 | Acacia albida | Senegal | 15 | |
| ORS175 | 10718 | Acacia albida | Senegal | 15 | |
| ORS182 | 10721 | Acacia albida | Senegal | 15 | |
| ORS183 | 10722 | Acacia albida | Senegal | 15 | |
| ORS523 | R-2194 | Indigofera senegalensis | Senegal | 13 | |
| ORS520 | R-2192 | Indigofera senegalensis | Senegal | 13 | |
| ORS935 | 15269 | Rhynchosia minima | Senegal | 13 | |
| ORS937 | 15271 | Rhynchosia minima | Senegal | 13 | |
| ORS976 | R-2197 | Indigofera senegalensis | Senegal | 13 | |
| ORS979 | 15275 | Indigofera senegalensis | Senegal | 13 | |
| ORS980 | 15276 | Indigofera senegalensis | Senegal | 13 | |
| ORS984 | 15279 | Indigofera senegalensis | Senegal | 13 | |
| ORS1217 | 15301 | Indigofera senegalensis | Senegal | 13 | |
| ORS1218 | R-2200 | Indigofera senegalensis | Senegal | 13 | |
| ORS1219 | 15302 | Indigofera senegalensis | Senegal | 13 | |
| ORS1228 | 15304 | Indigofera astragalina | Senegal | 13 | |
| ORS1810 | 15242 | Crotalaria lathyroïdes | Senegal | 13 | |
| ORS1813 | 15244 | Crotalaria hyssopifolia | Senegal | 13 | |
| ORS1814 | 15245 | Crotalaria hyssopifolia | Senegal | 13 | |
| ORS1819 | 15249 | Crotalaria retusa | Senegal | 13 | |
| ORS1820 | 15250 | Indigofera hirsuta | Senegal | 13 | |
| ORS1823 | 15367 | Indigofera hirsuta | Senegal | 13 | |
| ORS1824 | 15153 | Indigofera hirsuta | Senegal | 13 | |
| ORS1844 | 15266 | Chamaecrista sp. | Senegal | 13 | |
| ORS1848 | 15373 | Indigofera hirsuta | Senegal | 13 | |
| ORS1849 | 15374 | Indigofera hirsuta | Senegal | 13 | |
| ORS1898 | 15699 | Tephrosia bracteolata | Senegal | 13 | |
| ORS1903 | 15702 | Tephrosia villosa | Senegal | 13 | |
| BTAi1 | 11795 | Aeschynomene indica | United States | 16 | LP |
| BR3606 | 9959 | Acacia mollissima | Brazil | 27 | |
| BR3621 | 9966 | Acacia mangium | Brazil | 27 | |
| CB756 | 8319 | Macrotylona africanum | Zimbabwe | ||
| NZP2192 | 6161 | Lotus corniculatus | New Zealand | ||
| NZP2309 | 6128 | Lotus pedunculatus | Australia | ||
| NZP2314 | 6129 | Lotus pedunculatus | Australia | ||
| MSDJ718 | Lupinus luteus | France | |||
| TAL309 | 8316 | ||||
| Nonphotosynthetic Bradyrhizobium spp. | |||||
| ORS272 | 15408 | Aeschynomene schimperi | Senegal | 26 | |
| ORS313 | 15416 | Aeschynomene afraspera | 2 | ||
| ORS317 | 15147 | Aeschynomene afraspera | 2 | ||
| ORS354 | 15424 | Aeschynomene afraspera | 2 | ||
| Bradyrhizobium japonicum | |||||
| USDA135 | 8321 | Glycine max | United States | ||
| USDA110 | Glycine max | United States | |||
| MAR1589 | 14312 | Arachis hypogaea | |||
| NZP5533 | 6136 | Glycine max | United States | ||
| NZP5549T | 6138T | Glycine max | Japan | ||
| Bonnier 3.1 | 4252 | Glycine max | |||
| 1BOa2 | 4262 | Albizia julibrissin | |||
| Bradyrhizobium liaoningense | |||||
| SFI 2062 | 18231 | Glycine max | China | 43 | |
| SFI 2281 | 18230 | Glycine max | China | 43 | |
| Bradyrhizobium elkanii | |||||
| NZP 5532 | 6135 | Glycine max | United States | 24 | |
| NZP 5531T | 6134T | Glycine max | United States | 24 |
Photosynthetic strains are in boldface. Designations: ORS, collection of the Institut de Recherche pour le Développement, Montpellier, France; LMG, collection of bacteria of the Laboratorium voor Microbiologie, University of Ghent, Ghent, Belgium; BR, strain from the Centro Nacional de Pesquisa em Biologia do Solo (CNPBS), Seropédica, and Emprasa Brasiliera de Pesquisa Agropequaria (EMBRAPA), Rio de Janeiro, Brazil; INPA, National Institute of Amazonia Research, Manaus, Brazil; NZP, Culture Collection of the Department for Scientific and Industrial Research, Biochemistry Division, Palmerston North, New Zealand; USDA, U.S. Department of Agriculture, Beltsville, Md.; TAL, Nitrogen Fixation in Tropical Agricultural Legumes (NifTAL), University of Hawaii, Paia; MSDJ, Institut National de la Recherche Agronomique (INRA), Microbiologie des Sols, Dijon, France; MAR, Soil Productivity Research Laboratory, Marondera, Zimbabwe; SFI, Soils and Fertilizers Institute, Chinese Academy of Agricultural Sciences, Beijing, People's Republic of China.
Crt, carotenoid; W, white (strain lacking Bchl a and carotenoid); DP, dark pink; O, orange; LP, light pink.
Rice cultivation and nitrogen fixation activity.
Wild rice seeds were hulled, placed in 96% ethanol for 15 min, rinsed twice with sterile water, sterilized for 25 min in 0.1% HgCl2, and rinsed six times in sterile distilled water. Seeds were germinated in the dark at 30°C for 2 days on plates containing semisolid TY medium (0.8% agar-agar [wt/vol]). Plantlets with a 1- to 2-cm-long root showing no contamination were soaked for 5 min in an axenic mid-log-phase rhizobial culture. The inoculated rootlet was packed between two sterile filter papers in a large petri plate containing Jensen nitrogen-free medium. The plants were grown at 28°C for 3 to 4 weeks in growth chambers. Nitrogen-fixing activity was estimated by measurement of the acetylene-reducing activity of roots by the method of Hardy et al. (21).
Greenhouse rice inoculation experiments.
Two-week-old rice plantlets grown on sterile sand in a nursery and previously inoculated were transplanted into pots containing 5 kg of local unsterilized sandy soil (psamment) with 93% sand. The first control consisted of noninoculated, non-N-fertilized plantlets, and the second control comprised noninoculated but N-fertilized plantlets that received 5 mM KNO3 added in two equal doses 15 days after transplanting and at the mid-tillering stage. The experiment was arranged in a randomized design with six replicates for each treatment. Pots were watered with tap water and, every 2 weeks, with nitrogen-free Jensen's plant growth medium, to maintain a 2-cm waterhead above the soil surface. Experiments were performed in a greenhouse between June and October at 27 to 34°C with a day length of about 12 h. All O. breviligulata plants were harvested 115 days after transplanting. Grain, shoot, and leaf yields were evaluated.
Microscopic examination of endophytes within rice roots.
At days 15 and 30 after inoculation with a lacZ-tagged strain of ORS278 (strain M10) (E. Giraud and L. Hannibal, personal communication), roots were histochemically treated as described earlier by Boivin et al. (8) and screened under an Olympus SZH10 stereomicroscope. Control roots inoculated with the wild-type strain ORS278 and the Bradyrhizobium strain Aust13c originally isolated from Acacia mangium (20) were also examined. Transverse sections (40 μm thick) were made using a Leica VT1000S Vibratome. Microscopic preparations were examined with an Olympus Provis microscope.
Photosynthetic pigment determination.
Cultures were grown at 30°C for 7 days under aerobic conditions on a cycle of 15 h of light and 9 h of darkness. Bacteriochlorophyll a (Bchl a) was extracted under dim light with cold acetone-methanol (7:2, vol/vol) at 4°C for 30 min (25). The supernatant was analyzed using a Beckman DU40 spectrophotometer. Absorption spectra were obtained by scanning over a wavelength range from 350 to 800 nm. Carotenoids were further purified and analyzed by high-pressure liquid chromatography (HPLC) or thin-layer chromatography (TLC) as previously described (25).
16S amplified ribosomal DNA restriction analysis (ARDRA).
Strains were grown at 28°C for 5 days on YEM. Total DNA was purified by a slightly modified technique from Pitcher et al. (31) described by Doignon-Bourcier et al. (13). The primers and the general conditions for 16S rRNA gene PCR amplification were those described by Doignon-Bourcier et al. (13) except that amplifications were carried out in a Gene Amp PCR System 2400 (Perkin-Elmer). Endonuclease restriction of PCR products was performed with HinfI, DdeI, MwoI, AluI, and HhaI. Restricted DNA was then analyzed by horizontal agarose gel electrophoresis using Metaphor agarose (FMC Bioproducts). Clustering was obtained by restriction profile analyses using the GelCompar 4.2 software package (40). The Dice coefficient (Tol, 0.3%; Opt, 0.50%; minimum area, 0.0%) and UPGMA (unweighted pair group method using average linkage) clustering were used.
Analysis of the 16S rRNA genes.
We amplified the nearly full-length 16S rDNA of six photosynthetic strains by using the two specific oligonucleotide primers FGPS6, 5′-GGAGAGTTAGATCTTGGCTCAG-3′ (sense; positions 6 to 27 by Escherichia coli numbering), and FGPS1509, 5′-AAGGAGGGGATCCAGCCGCA-3′ (antisense; positions 1540 to 1521 by E. coli numbering), according to the method of Normand et al. (30). PCR products were purified with a QIAquick Gel extraction Kit (Qiagen, Courtaboeuf, France). The ABI Prism BigDye Terminator Cycle sequence kit (Applied Biosystems, Foster City, Calif.) was used to directly sequence the purified PCR product. Sequencing reactions were analyzed on an Applied Biosystems model 310 DNA sequencer.
DNA sequence analysis.
The six 16S rDNA sequences obtained were compared to the GenBank database by using the algorithm BLASTN (4) to identify the most similar 16S rDNA sequences. An alignment was performed, using the PILEUP program (12), with a set of sequences of representatives of the most closely related genera identified. A phylogenetic tree was constructed by the neighbor-joining method (37), and a bootstrap confidence analysis was performed on 1,000 replicates to determine the reliability of the tree topology obtained (18).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the 16S rRNA gene sequences of strains ORS2005, ORS2006, ORS2011, ORS2012, STM481, and ORS278 are AF230718, AF230719, AF230720, AF230721, AF239254, and AF239255, respectively.
RESULTS
Isolation of endophytic rhizobia from wild rice.
Nitrogen-fixing nodules were formed within 2 weeks on the roots of A. sensitiva, used as a trap legume host and previously inoculated with crushed surface-sterilized nodal roots of O. breviligulata. Thirteen endophytic rhizobial isolates were independently obtained from these Aeschynomene nodules (Fig. 1 and Table 1). Of these, nine isolates (ORS2005, ORS2006, ORS2007, ORS2008, ORS2009, ORS2011, ORS2012, ORS2014, and ORS402) were obtained from two distant temporary ponds of the coastal region of Joal and Nianing in Senegal. The other four isolates (STM478, STM481, STM482, and STM515) were obtained from deep-water wild rice growing in the Baga wetland coastal region of Guinea, located 600 km south of the Senegalese sampling sites. Both of the other legumes used as trap hosts for rice endophytic rhizobia, S. rostrata and A. albida, failed to develop any nodules upon inoculation with crushed rice roots.
FIG. 1.
Isolation of endophytic and aquatic Bradyrhizobium strains.
Isolation of aquatic rhizobia from rice field water.
In order to compare the endophytic and aquatic rhizobia, water samples from the two wild rice growing sites in Senegal were also tested for the presence of free rhizobia (Fig. 1). Six isolates (ORS404, ORS405, ORS406, ORS408, ORS2010, and ORS2013) were obtained from nodules of A. sensitiva previously inoculated with water samples. One isolate (ORS2019) was obtained by direct streaking of the water sample onto YEM plates and was selected as a result of its pink pigmentation.
Symbiotic characterization of endophytic and aquatic rhizobial isolates.
To determine to which specificity group the endophytic and aquatic rhizobia belonged, we performed root inoculation tests with representative plants of different nodulation groups as defined by Alazard (1). The 20 endophytic and aquatic isolates showed a very specific nodulation pattern: they nodulated only A. sensitiva and A. indica, two species belonging to the most specific Aeschynomene cross-inoculation group (Table 1). Nodules were effective, except for those induced by both aquatic isolates, ORS405 and ORS406, that did not fix nitrogen. None of the isolates induced nodulation on A. afraspera, which belongs to another Aeschynomene cross-inoculation group, or on A. albida, a nonspecific host known to be nodulated by a broad range of typical promiscuous “cowpea” Bradyrhizobium strains.
Photosynthetic properties of endophytic and aquatic rhizobia.
All isolates developed typical slow-growing Bradyrhizobium colonies on YEM plates. With the exception of isolates ORS402, ORS405, and ORS406, which formed white colonies, all isolates synthesized pink or orange pigments on yeast-mannitol solid or liquid culture medium (Table 1), particularly when exposed to light, suggesting the presence of carotenoids and photosynthetic pigments (17, 25). All pigmented isolates produced an absorbance peak at 770 nm, characteristic of Bchl a, and peaks around 400 to 500 nm, corresponding to carotenoids. No Bchl a or carotenoids were observed in isolates ORS402, ORS405, and ORS406.
All pigmented isolates (ORS404, ORS408, ORS478, ORS481, ORS482, ORS515, ORS2005, ORS2006, ORS2007, ORS2008, ORS2009, ORS2010, ORS2011, ORS2012, ORS2013, ORS2014, and ORS2019), and one nonpigmented isolate (ORS402) gave a fragment of the expected size (926 bp) using the primers defined from the pufLM sequence of Bradyrhizobium sp. strain ORS278 as described by Molouba et al. (26). The sequences of the amplified products obtained with two representative endophytic isolates (ORS2005 and ORS2011) were homologous to those of the pufLM genes. No PCR products were obtained with isolates ORS405 and ORS406.
ARDRA.
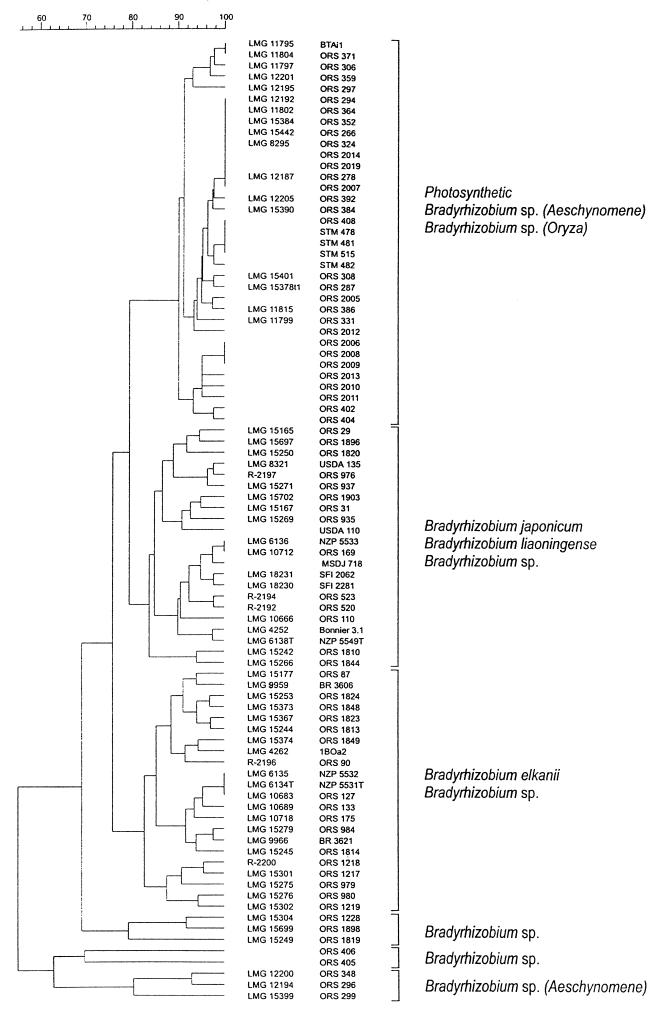
We also performed ARDRA to compare the 20 new isolates with Bradyrhizobium strains representative of the ARDRA groups previously described (13, 26). The results are shown as a dendrogram in Fig. 2. At a mean Dice similarity coefficient (SD) of 76%, we distinguished three main clusters. All the endophytic and aquatic isolates, except ORS405 and ORS406, grouped in the same major cluster together with photosynthetic Bradyrhizobium sp. strains from Aeschynomene. The two other main clusters, containing the type strains of the three named Bradyrhizobium species, were previously described by Doignon-Bourcier et al. (13).
FIG. 2.
Dendrogram based on UPGMA clustering of Dice correlation values (SD) of normalized and combined ARDRA patterns of new isolates and reference Bradyrhizobium strains obtained with the restriction enzyme combination HinfI, DdeI, MwoI, AluI, and HhaI. The scale represents SD values converted to percentages.
16S rRNA gene sequence analysis.
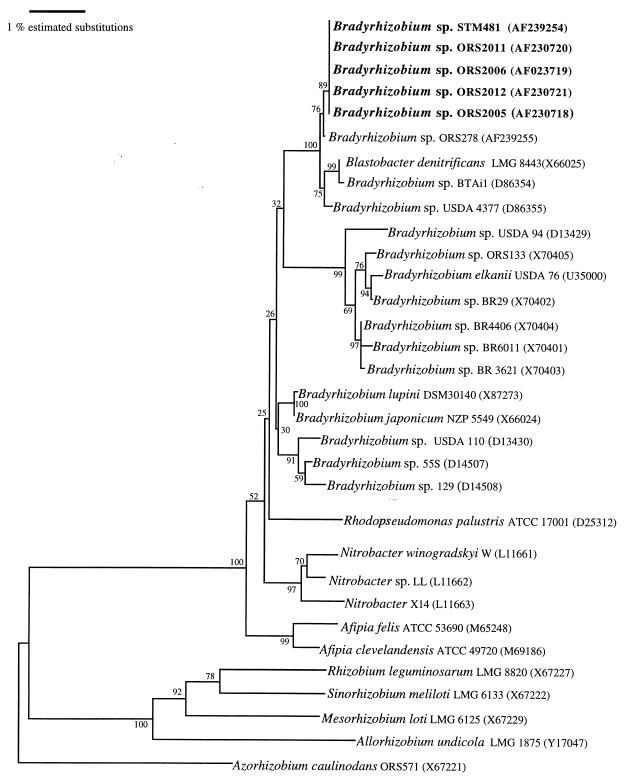
We determined the 16S rRNA gene sequences of the representative endophytic strains ORS2005, ORS2006, ORS2011, ORS2012, and STM481. All five sequences were identical and were 2, 6, 9, and 10 bp different from those of ORS278, USDA4377, BTAi1, and Blastobacter denitrificans strain LMG8443, respectively. A phylogenetic tree was constructed to determine the position of these isolates among other Bradyrhizobium strains (Fig. 3). All the endophytes formed a separate branch together with the photosynthetic Bradyrhizobium sp. strains ORS278, BTAi1, and USDA4377 from Aeschynomene and the aquatic strain B. denitrificans (LMG8443). This grouping was supported by a bootstrap value of 100% and was distinct from the five other well-separated clusters including, respectively, B. japonicum, B. elkanii, Rhodopseudomonas palustris, Nitrobacter winogradskyi, and Afipia felis (Fig. 3).
FIG. 3.
Neighbor-joining dendrogram of 16S rDNA sequences showing the position of endophytic strains (boldfaced) isolated from wild rice among bradyrhizobia and closely related taxa. Bootstrap values, expressed as percentages of 1,000 replications, are given at the branching points. Numbers in parentheses are the accession numbers of the sequences used. The bar represents 1 estimated substitution per 100 nucleotide positions.
Numeration of endophytic and aquatic rhizobia.
We found a population density of ∼5.0 × 106 endophytic rhizobia per g (fresh weight) of surface-sterilized rice roots. When the roots were not surface sterilized, the total population density of rhizobia reached ∼2.5 × 107 rhizobia per g of roots. Populations of aquatic rhizobia able to nodulate Aeschynomene were evaluated as ∼1.1 × 102 rhizobia per ml of water.
Inoculation with endophytic rhizobia of the wild rice grown in a greenhouse.
Sixteen rice endophytic and aquatic isolates and two photosynthetic Bradyrhizobium strains from Aeschynomene were inoculated on rice plants grown in sandy soil without added N fertilizer. After growth, the dry weights of shoots and leaves and of grains were determined; results are shown in Table 3. The endophytic isolate ORS2011 and the Aeschynomene photosynthetic Bradyrhizobium strain ORS278 produced the highest effects of inoculation, giving 20% increases in both shoot and grain yields. Inoculation with most other isolates, including strain BTAi1, resulted in dry weights of shoots and leaves similar to those of the N-fertilized control. With half of these isolates, however, no inoculation effect was observed on grain yields, which remained very low, as in the noninoculated, nonfertilized control.
TABLE 3.
Effect of inoculation with endophytic and nonendophytic photosynthetic Bradyrhizobium strains on shoot and grain yields of O. breviligulata grown in a greenhouse
| Straina | Dry wtb (g) of:
|
|
|---|---|---|
| Shoots and leaves | Grain | |
| ORS405 | 19.7†,‡ | 1.9‡ |
| ORS2019 | 16.7‡ | 3.5‡ |
| ORS404 | 20.7†,‡ | 4.5‡ |
| ORS2012 | 21.4†,‡ | 4.6‡ |
| ORS402 | 23.0† | 4.9‡ |
| ORS408 | 26.4† | 5.0‡ |
| ORS2014 | 24.3† | 6.1‡ |
| ORS2008 | 23.6† | 6.8†,‡ |
| ORS2010 | 20.5†,‡ | 7.4† |
| ORS406 | 22.4† | 7.5† |
| ORS2005 | 22.4† | 8.1† |
| ORS2009 | 23.2† | 8.6† |
| ORS2013 | 21.8†,‡ | 8.7† |
| ORS2007 | 24.3† | 9.4*,† |
| ORS2006 | 27.5*,† | 9.6*,† |
| ORS2011 | 29.7* | 10.3* |
| ORS278 | 30.1* | 12.0* |
| BTAiI | 23.4† | 7.2† |
| Control (noninoculated) | 16.7‡ | 5.0‡ |
| Control (N fertilization) | 23.0† | 8.5† |
ORS strains are from the collection of the Institut de Recherche pour le Développement, Montpellier, France.
Values with different symbols differ significantly at the P < 0.01 level. Analysis of variance was carried out by Fisher's test (39). Six replicates were performed per treatment.
Colonization and infection of wild rice roots by photosynthetic rhizobia.
Because the photosynthetic Bradyrhizobium strain ORS278 was the strain with the highest inoculation effect on the growth and grain yield of rice grown in a greenhouse, we used a marked derivative of ORS278 (strain M10) to trace the infection process in rice roots. Strain M10 was constructed independently from this work by insertion of a lacZ reporter gene in the photosynthetic puf gene (Giraud and Hannibal, personal communication). The lacZ reporter gene was shown to be strongly expressed under the culture conditions we used for rice growth. The wild-type strain ORS278 and the nonphotosynthetic Bradyrhizobium strain Aust13c from A. mangium were used as controls. Fifteen days after inoculation of O. breviligulata with strain M10, histochemical staining of β-galactosidase activity revealed a strong bacterial colonization of the root cap which exhibited a dense blue staining (Fig. 4A). This colonization was particularly dense at the surface of the mucilage drop that covers the root apex (Fig. 4B). Senescent root cells, sloughed off the root cap, often exhibited a hairbrush shape (Fig. 4C), due to polar attachment of bacterial cells covering their surfaces. Polar attachment also occurred with the wild-type strain ORS278. By contrast, despite colonization of the mucilage, we never observed such polar attachment with the nonphotosynthetic Bradyrhizobium strain Aust13c (data not shown). Thirty days after inoculation, clusters of sloughed root cap cells were still observed far from the root tip, embedded in mucilage and densely colonized by Bradyrhizobium (Fig. 4E). An intense bacterial colonization of the root surface originated from these clusters (Fig. 4E). Bacteria appeared lined up along the borders of adjacent epidermal cells (Fig. 4G). Bacterial invasion developed deeper in the intercellular spaces as revealed by cross sections (Fig. 4H). A few intracellular infections were also regularly observed in epidermal cells filled with bradyrhizobia (Fig. 4I). In the zone of emergence of lateral roots, dense bacterial proliferation was observed in the fissures caused by protruding lateral roots (Fig. 4D), where bacterial proliferation commonly reached four to five cell layers deep (Fig. 4F).
FIG. 4.
Stereo- and light microscopic observations of O. breviligulata seedlings inoculated with a lacZ-tagged Bradyrhizobium ORS278 strain (M10). (A to C) Fifteen days after inoculation; (D to I) 30 days after inoculation. (A) Stereomicroscopic observation from the root apex to the root hair zone. An intense blue coloration occurs on the root cap surface, revealing a dense bacterial colonization. (B) Longitudinal section of the root apex showing the dense colonization of the mucilage drop that covers the root cap, viewed by bright-field microscopy. (C) A sloughed root cap covered with Bradyrhizobium cells attached in a polar way, observed by phase-contrast microscopy. (D) Colonization of the cracks at the emergence sites of lateral roots, observed by stereomicroscopy. (E) Cross section of the elongation zone of a root with densely colonized root cap remnants, from which the bacteria colonize the root surface, observed by bright-field microscopy. (F) Deep colonization of cracks at the emergence of a lateral roots, as seen in a cross section by bright-field microscopy. (G) Colonization of the root surface along borders of epidermal cells, observed by bright-field microscopy. (H) Cross section of a root with proliferation in the intercellular space between two epidermal cells, observed by bright-field microscopy. (I) Intracellular colonization of an epidermal cell by ORS278, observed by bright-field microscopy.
Nitrogen-fixing activity in the rice rhizosphere.
A low but significant level of nitrogen-fixing activity, as measured by the acetylene reducing activity, was detected (1.7 nmol of C2H4/h/plant) on 4-week-old rice plants artificially grown in growth chambers in large petri dishes and inoculated with the photosynthetic strain ORS278. No acetylene reducing activity was detected with the noninoculated rice control.
DISCUSSION
Photosynthetic Bradyrhizobium strains are known to specifically induce nitrogen-fixing nodules on stems and roots of aquatic legumes of the genus Aeschynomene (26). We report here for the first time that these photosynthetic symbiotic bacteria also form a natural endophytic association with the wild rice species O. breviligulata. The surfaces and interiors of the rice roots, which we found densely colonized by Aeschynomene-nodulating Bradyrhizobium strains, therefore appear in nature as unexpected niches, much more abundant than root and stem nodules of the aquatic legumes. Photosynthetic Bradyrhizobium strains were also directly isolated from water, thus confirming their aquatic character (9), which could result in a bacterial selective advantage in the absence of rice or legume hosts. Yanni et al. (44) previously reported endophytic association between another rhizobial species, R. leguminosarum bv. trifolii, and rice grown in Egypt in rotation with the legume Trifolium alexandrinum. Our results with O. breviligulata thus suggest that rhizobia could be usual endophytic bacteria of various primitive and cultivated rice species.
Growth stimulation of different cereals such as wheat or rice following seed inoculation with nonphotosynthetic rhizobia such as R. leguminosarum (44) or Azorhizobium (42) strains has been previously reported. We therefore conducted greenhouse experiments to measure plant growth responses to inoculation with photosynthetic endophytic isolates. Inoculation with both the photosynthetic isolate ORS2011 and the Aeschynomene photosynthetic strain ORS278 resulted in statistically significant increases in shoot and grain yields, indicating their potential ability to enhance rice production. As we detected a nitrogen-fixing activity in inoculated rice plants, nitrogen fixation could contribute to this plant growth-promoting response. Nitrogenase activity had previously been detected in wheat inoculated with Azorhizobium strain ORS571, but only when succinate was added to the plant growth medium (42). Photosynthetic Bradyrhizobium strains are known to fix nitrogen under free-living conditions (2). However, we cannot conclude that the low N2 fixation activity detected would support on its own a major role in the increase in shoot and grain yields. It has been shown that rhizobia can secrete indoleacetic acid and gibberellic acid phytohormones (44). The free N2-fixing strains ORS2011 and ORS278 could thus represent an interesting model to determine the respective roles of N2 fixation and phytohormone production in the significant benefit to rice.
By microscopic examination, we were able to distinguish two main stages of bradyrhizobial colonization of the rice root. The first step was the root cap colonization, followed by a strong bacterial multiplication covering large areas of the root surface. The root cap is known as one of the main sites of polysaccharide secretion by specialized plant cells completely embedded in a mucilage gel-like matrix which has been described as a substrate as well as a niche of proliferation for a wide range of soil microorganisms (32). As the root growth goes on, root cap cells are continuously renewed, and senescent cells are sloughed off along the root. This step, characterized by the polar attachment of the rhizobial cells to the sloughed cells, could be considered characteristic, since a nonphotosynthetic Bradyrhizobium strain from A. mangium showed no attachment to rice cells. Such oriented attachment has also been observed on the root hairs and root epidermis of rice artificially inoculated with A. caulinodans strain ORS571 (33). In O. breviligulata, attachment of photosynthetic Bradyrhizobium to root cap cells could facilitate the dispersal of the bacteria all along the root. We indeed observed an intense bacterial colonization of large areas of root surface, starting from the sloughed cells acting as an inoculant. Bacteria appeared lined up along the borders of epidermal root cells. Such a preferential location could result from a better bacterial proliferation favored by the presence of intercellular mucilage.
The second step of infection was the intercellular invasion of epidermal cells, which constituted the true endophytic stage. Simultaneously with bacterial root surface colonization, numerous lateral roots emerge, producing fissures in the root epidermis and underneath cell layers. These fissures are sites of intense intercellular bacterial proliferation. Bacteria invade the fissure via disjoined epidermis cells and migrate towards deeper layers of cortical cells, between which they form pockets of proliferating bacteria. Such an endophytic stage, in which bacterial cells are densely packed in a confined space, could constitute preferential sites of exchanges between the plant and the bacteria. These large intercellular pockets closely resemble the intercellular infection pockets formed during the early stages of nodulation by rhizobia in aquatic legumes such as S. rostrata (28) and A. afraspera (3). In these tropical legumes, infection starts intercellularly, directly through “crack entry” at the sites of emerging lateral roots, without formation of infection threads in root hairs as in Medicago or Trifolium. In S. rostrata, infection threads develop ultimately from these pockets and allow the release of the bacteria into the meristematic cells (28). The most primitive infection process is found in A. afraspera, where bacteria from the intercellular pockets directly invade the host cell by localized cell wall degradation, without any formation of infection threads (3). Further development of the nodule occurs by repeated division of the infected host legume cells. Interestingly, in O. breviligulata, photosynthetic bradyrhizobia are also present intracellularly in cortical cells. However, unlike in Aeschynomene, the number of invaded cells remained limited in O. breviligulata, and no division of these infected cells was observed. This intracellular invasion could thus be the ultimate stage of rice infection by Bradyrhizobium. Nevertheless, the infection process in the monocotyledonous plant O. breviligulata by the same photosynthetic bradyrhizobia seems to be very similar to the first stages of infection in the leguminous dicotyledonous plant Aeschynomene. Webster et al. (42) showed that colonization of rice and wheat roots by A. caulinodans strain ORS571 was nod gene independent. With the photosynthetic Bradyrhizobium strain ORS278, one hypothesis could also be that, in both A. sensitiva and O. breviligulata, expression of nod genes is not necessary for the first steps of infection involving primitive “crack entry” and direct intercellular invasion. Intracellular invasion in rice has also been reported with the endophytic bacterium Azoarcus sp. (34) and with A. caulinodans strain ORS571 (33). The reason why intracellular infection does not spread further in rice is not yet elucidated. One hypothesis could be that the endodermis constitutes a thick-walled boundary to infection. Alternatively, rice may lack part of the genetic program necessary for invaded cells to control the intracellular infection and subsequently be capable of repeated divisions. In conclusion, the O. breviligulata model, which offers a unique combination of a primitive rice species with primitive “crack entry” rhizobial infection, could represent a major step forward in achieving an efficient nitrogen-fixing endosymbiotic association between rice and rhizobia.
ACKNOWLEDGMENTS
This work was supported by a Ph.D. scholarship granted by IRD to Clémence Chaintreuil. Monique Gillis is indebted to the Fund for Scientific Research-Flanders (Belgium) and personnel grants.
We thank Catherine Boivin for comments on the manuscript and helpful discussions, and Mathieu Faye and Moustapha Ndiaye for technical assistance.
REFERENCES
- 1.Alazard D. Stem and root nodulation in Aeschynomene spp. Appl Environ Microbiol. 1985;50:732–734. doi: 10.1128/aem.50.3.732-734.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alazard D. La nodulation caulinaire dans le genre Aeschynomene. Ph.D. thesis. Lyon, France: University Claude Bernard-Lyon I; 1991. [Google Scholar]
- 3.Alazard D, Duhoux E. Development of stem nodules in a tropical forage legume, Aeschynomene afraspera. J Exp Bot. 1990;41:1199–1206. [Google Scholar]
- 4.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lippman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldani J I, Baldani V L D, Seldin L, Döbereiner J. Characterization of Herbaspirillum seropedicae gen. nov., sp. nov., a root-associated nitrogen-fixing bacterium. Int J Syst Bacteriol. 1986;36:86–93. [Google Scholar]
- 6.Bashan Y. Interaction of Azospirillum spp. in soils: a review. Biol Fertil Soils. 1999;29:246–256. [Google Scholar]
- 7.Boddey R M, Oliveira O C, Urquiaga S, Reis V M, de Olivares F L, Baldani V L D, Döbereiner J. Biological nitrogen fixation associated with sugar cane and rice: contributions and prospects for improvement. Plant Soil. 1995;174:195–209. [Google Scholar]
- 8.Boivin C, Camut S, Malpica C A, Truchet G, Rosenberg C. Rhizobium meliloti genes encoding catabolism of trigonelline are induced under symbiotic conditions. Plant Cell. 1990;2:1157–1170. doi: 10.1105/tpc.2.12.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boivin C, Ndoye I, Molouba F, de Lajudie P, Dupuy N, Dreyfus B L. Stem nodulation in legumes: diversity, mechanisms and unusual characters. Crit Rev Plant Sci. 1997;16:1–30. [Google Scholar]
- 10.Brockwell J. Experiments with crop and pasture legumes. Principle and practice. In: Bergersen F J, editor. Methods for evaluating biological nitrogen fixation. New York, N.Y: John Wiley & Sons, Inc.; 1980. pp. 417–488. [Google Scholar]
- 11.Cavalcante V, Döbereiner J. A new acid-tolerant nitrogen fixing bacterium associated with sugar cane. Plant Soil. 1988;108:23–31. [Google Scholar]
- 12.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doignon-Bourcier F, Sy A, Willems A, Torck U, Dreyfus B, Gillis M, de Lajudie P. Diversity of bradyrhizobia from 27 tropical Leguminosae species native of Senegal. Syst Appl Microbiol. 1999;22:647–661. doi: 10.1016/S0723-2020(99)80018-6. [DOI] [PubMed] [Google Scholar]
- 14.Dreyfus B, Dommergues Y R. Nitrogen-fixing nodules induced by Rhizobium on the stem of the tropical legume Sesbania rostrata. FEMS Microbiol Lett. 1981;10:313–317. [Google Scholar]
- 15.Dupuy N, Willems A, Pot B, Dewettinck D, Vandenbruaene I, Maestrojuan G, Dreyfus B, Kersters K, Collins M D, Gillis M. Phenotypic and genotypic characterization of bradyrhizobia nodulating the leguminous tree Acacia albida. Int J Syst Bacteriol. 1994;44:461–473. doi: 10.1099/00207713-44-3-461. [DOI] [PubMed] [Google Scholar]
- 16.Eaglesham A R J, Szalay A A. Aerial stem nodules on Aeschynomene spp. Plant Sci Lett. 1983;29:265–272. [Google Scholar]
- 17.Eaglesham A R J, Ellis J M, Evans W R, Fleischman D E, Hungria M, Hardy R W F. The first photosynthetic N2-fixing Rhizobium: characteristics. In: Gresshoff P M, Roth L E, Stacey G, Newton W L, editors. Nitrogen fixation: achievements and objectives. New York, N.Y: Chapman and Hall; 1990. pp. 805–811. [Google Scholar]
- 18.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:424–429. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 19.Fleischman D, Kramer D. Photosynthetic rhizobia. Biochim Biophys Acta. 1998;1364:17–36. doi: 10.1016/s0005-2728(98)00011-5. [DOI] [PubMed] [Google Scholar]
- 20.Galiana A, Chaumont J, Diem H G, Dommergues Y R. Nitrogen-fixing potential of A. mangium and A. auriculiformis seedlings inoculated with Bradyrhizobium and Rhizobium spp. Biol Fertil Soils. 1990;9:261–267. [Google Scholar]
- 21.Hardy R W F, Burn R C, Holten R D. Application of the acetylene-ethylene assay for measurement of N2 fixation. Soil Biol Biochem. 1973;43:47–87. [Google Scholar]
- 22.James E K, Olivares F L. Infection and colonization of sugar cane and other graminaceous plants by endophytic diazotrophs. Crit Rev Plant Sci. 1998;17:77–119. [Google Scholar]
- 23.Jensen H L. Nitrogen fixation in leguminous plants. I. General characters of root nodule bacteria isolated from species of Medicago and Trifolium in Australia. Proc Int Soc NSW. 1942;66:68–108. [Google Scholar]
- 24.Kuykendall L M, Saxena B, Devine T E, Udell S E. Genetic diversity in Bradyrhizobium japonicum Jordan 1982 and a proposal for Bradyrhizobium elkanii sp. nov. Can J Microbiol. 1992;38:501–503. [Google Scholar]
- 25.Lorquin J, Molouba F, Dreyfus B L. Identification of the carotenoid canthaxanthin from photosynthetic Bradyrhizobium strains. Appl Environ Microbiol. 1997;63:1151–1154. doi: 10.1128/aem.63.3.1151-1154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molouba F, Lorquin J, Willems A, Hoste B, Giraud E, Dreyfus B, Gillis M, de Lajudie P, Masson-Boivin C. Photosynthetic bradyrhizobia from Aeschynomene spp. are specific to stem-nodulated species and form a separate 16S ribosomal DNA restriction fragment length polymorphism group. Appl Environ Microbiol. 1999;65:3084–3094. doi: 10.1128/aem.65.7.3084-3094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreira F, Gillis M, Pot B, Kersters K, Franco A A. Characterization of rhizobia from different divergence groups of tropical Leguminosae by comparative polyacrylamide gel electrophoresis of their total proteins. Syst Appl Microbiol. 1993;16:135–146. [Google Scholar]
- 28.Ndoye I, de Billy F, Vasse J, Dreyfus B, Truchet G. Root nodulation of Sesbania rostrata. J Bacteriol. 1994;176:1060–1068. doi: 10.1128/jb.176.4.1060-1068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ndoye I, Dreyfus B, Becker M. Sesbania rostrata as green manure for lowland rice in Casamance (Senegal) Trop Agric. 1996;73:234–237. [Google Scholar]
- 30.Normand P, Cournoyer B, Simonet P, Nazaret S. Analysis of a ribosomal RNA operon in the actinomycete Frankia. Gene. 1992;111:119–124. doi: 10.1016/0378-1119(92)90612-s. [DOI] [PubMed] [Google Scholar]
- 31.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 32.Prin Y, Rougier M. Cytological and histochemical characteristics of axenic root surfaces of Alnus glutinosa. Can J Bot Fr. 1986;119:571–580. [Google Scholar]
- 33.Reddy P M, Ladha J K, So R, Hernandez R, Ramos M C, Angeles O R, Dazzo F B, De Bruijn F J. Rhizobial communication with rice roots: induction of phenotypic changes, mode of invasion and extent of colonization. Plant Soil. 1997;194:81–98. [Google Scholar]
- 34.Reinhold B, Hurek T. Life in grasses: diazotrophic endophytes. Trends Microbiol. 1998;6:139–144. doi: 10.1016/s0966-842x(98)01229-3. [DOI] [PubMed] [Google Scholar]
- 35.Reinhold B, Hurek T, Niemann E-G, Fendrik I. Close association of Azospirillum and diazotrophic rods with different root zones of Kallar grass. Appl Environ Microbiol. 1986;52:520–526. doi: 10.1128/aem.52.3.520-526.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rolfe B G, Djordevic M A, Weinman J J, Mathesius U, Pittock C, Gartner E, Ride K M, Dong Z M, McCully M, McIver J. Root morphogenesis in legumes and cereals and the effect of bacterial inoculation on root development. Plant Soil. 1997;194:131–134. [Google Scholar]
- 37.Saitou R R, Nei M. A neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;44:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 38.Second G. La domestication en régime autogame: exemple des riz (Oryza spp) Bull Soc Bot Fr Act Bot. 1986;133:35–44. [Google Scholar]
- 39.Snedecor G W, Cochran W E. Statistical methods. 8th ed. Ames, Iowa: Iowa State University Press; 1989. [Google Scholar]
- 40.Vauterin L, Vauterin P. Computer-aided objective comparison of electrophoresis patterns for grouping and identification of microorganisms. Eur Microbiol. 1992;1:37–41. [Google Scholar]
- 41.Vincent J M. A manual for the practical study of root nodule bacteria. Oxford, United Kingdom: Blackwell Scientific Publications Ltd.; 1970. [Google Scholar]
- 42.Webster G, Gough C, Vasse J, Batchelor C A, O'Callaghan K J, Kothari S L, Davey M R, Dénarié J, Cocking E C. Interactions of rhizobia with rice and wheat. Plant Soil. 1997;194:115–122. [Google Scholar]
- 43.Xu L M, Ge C, Cui Z, Li J, Fan H. Bradyrhizobium liaoningense sp. nov., isolated from the root nodules of soybeans. Int J Syst Bacteriol. 1995;45:706–711. doi: 10.1099/00207713-45-4-706. [DOI] [PubMed] [Google Scholar]
- 44.Yanni Y G, Rizk R Y, Corich V, Squartini A, Ninke K, Philip-Hollingsworth S, Orgambide G, De Bruijn F, Stolzfus J, Buckley D, Schmidt T M, Mateos P F, Ladha J K, Dazzo F B. Natural endophytic association between Rhizobium leguminosarum bv. trifolii and rice roots and assessment of its potential to promote rice growth. Plant Soil. 1997;194:99–114. [Google Scholar]