Abstract
Primary immunodeficiency is a group of disorders associated with susceptibility to infectious agents and the development of various comorbidities. Many primary immunodeficiencies are complicated by immune dysregulation, autoinflammation, or autoimmunity which impacts multiple organ systems. Major advances in the treatment of these disorders have occurred over the last half-century, and deeper molecular understanding of many disorders combined with clinically available genetic testing is allowing for use of precision therapy for several primary immunodeficiencies. Patients with antibody deficiencies who rely on immunoglobulin replacement therapy now have many treatment options with products that are much safer and better tolerated compared to the past. Newborn screening for severe combined immunodeficiency, now implemented throughout the USA and in many countries worldwide, has lowered the age at which many patients are diagnosed with these diseases. Early diagnosis of severe combined immunodeficiency allows infants to proceed to definitive therapy such as stem cell transplantation or gene therapy prior to facing potentially life-threatening infections. While stem cell transplantation continues to carry significant risks, knowledge gained over recent decades is allowing for improved survival with less toxicity and less graft versus host disease.
Keywords: Primary immunodeficiency, Immunoglobulin, Stem cell transplant, Gene therapy, Newborn screening
Introduction
The recognition and treatment of infectious diseases have undergone incredible changes since the first recognition of microbes as being the cause of human disease and the subsequent development of antimicrobial medications. Newly emerging infections can impact all of us to varying degrees, as we experienced during the global pandemic in 2020 caused by the novel coronavirus SARS-CoV-2. However, it has been clear for a very long time that individuals might respond differently to the same pathogen. Even in biblical times, it was noted that individuals had unique susceptibilities to microbes and had outward signs of a possible underlying disorder. While there is no evidence that he suffered from a primary immunodeficiency, the biblical character Job with his cutaneous boils and disfigurement has long been used among immunologists to describe one of these illnesses. Limited treatments would have existed during those times. Now, these fascinating disorders which impact our ability to ward off illness are now recognized as primary immunodeficiency diseases or PID/PI. Great strides in understanding these diseases have been gained over time, allowing us to recognize and classify these disorders, diagnose patients earlier in life, and tailor the treatment to the specific risks associated with the underlying immunologic defect. While immunologists have always recognized that primary immunodeficiencies affect all ages, this concept has never been more important than in the current era as genetic testing is now uncovering primary immunodeficiencies in patients who fall well beyond the prototypical young child with recurrent or opportunistic infections. Genetic testing may uncover primary immunodeficiencies in patients of any age who present with non-infectious symptomatology such as refractory cytopenias, inflammatory bowel disease, susceptibility to malignancies, and granulomatous (sarcoid-like) inflammation among other autoimmune conditions. Identification of the molecular defect in such patients may allow for targeted treatments which are effective in controlling the dysregulated immune response without the wide-sweeping side effects which would otherwise occur with use of prolonged systemic steroids and other traditional antiinflammatory drugs. For example, patients with CTLA-4 deficiency may have a common variable immunodeficiency (CVID)–like presentation along with severe symptoms of autoimmunity and immune dysregulation and may experience significant improvement when treated with CTLA-4 IgG fusion protein. Patients with STAT1-gain of function (GOF) or STAT3-GOF present with varying degrees of autoimmunity and may be treated with JAK inhibitors. The lymphoproliferation associated with the combined immunodeficiency activated PI3K delta syndrome (APDS) has been successfully treated with PI3Kδ inhibitors [1]. All of these conditions encompass both susceptibility to infections and immune dysregulation and may be classified as primary immunodysregulatory disorders (PIRDs). Through increased understanding of the molecular pathways of these disorders and increased availability of clinical genetic testing, patients with these disorders may now benefit from precision therapy.
Classification of Primary Immunodeficiency
Various classification schemata have been used over the years to describe the different forms of immunodeficiency, usually in the context of the type of immune cell affected by the disease. Currently, the International Union of Immunological Societies stratifies over 400 primary immunodeficiencies into ten categories: (1) immunodeficiencies affecting cellular and humoral immunity; (2) combined immunodeficiencies with associated or syndromic features; (3) predominantly antibody deficiencies; (4) diseases of immune dysregulation; (5) congenital defects of phagocyte number, function, or both; (6) defects in intrinsic and innate immunity; (7) autoinflammatory disorders; (8) complement deficiencies; (9) bone marrow failure disorders; (10) phenocopies of PID [2]. While a comprehensive list of primary immunodeficiencies is beyond the scope of this article, for illustrative purposes, Table 1 lists a few representative disorders from each category. Worldwide, the distribution of the forms of primary immunodeficiency within treatment centers is very similar. Antibody deficiencies make up most patients identified with an underlying immunodeficiency. Other forms frequently seen include phagocytic defects and combined T and B cell disorders. Complement deficiencies are seen less commonly.
Table 1.
The International Union of Immunological Societies classification of primary immunodeficiencies. A few representative immunodeficiencies are listed in each category for illustrative purposes only. A comprehensive list of disorders is available in the 2019 IUIS publication [2]
| Category | Subcategory | Example |
|---|---|---|
| 1. Immunodeficiencies affecting cellular and humoral immunity | 1.a. SCID, defined by CD3 T cell lymphopenia | IL2RG |
| JAK-3 def | ||
| ADA def | ||
| RAG 1&2 def | ||
| 1.b. Combined immunodeficiencies generally less profound than SCID | DOCK8 def | |
| MHC I&II def* | ||
| CARD11 def | ||
|
CD40 ligand def (X-linked hyper IgM) | ||
| 2. Combined immunodeficiencies with associated or syndromic features | Wiskott-Aldrich | |
| Ataxia-telangiectasia | ||
| Chr22q11.2 deletion syndrome | ||
| 3. Predominantly antibody deficiencies | 3.a. Hypogammaglobulinemia | X-linked agammaglobulinemia |
| CVID | ||
| 3.b. Other antibody deficiencies | Selective IgA def | |
| Specific antibody def | ||
| 4. Diseases of immune dysregulation | 4.a. HLH & EBV susceptibility | |
| XLP 1&2 | ||
| Chediak Higashi | ||
| 4.b. Syndromes with autoimmunity and others | ALPS* | |
| APECED | ||
| IPEX | ||
| CTLA-4 deficiency | ||
| STAT3 GOF | ||
| 5. Congenital phagocyte defects | 5.a. Neutropenia (without anti-PMN) | Elastase def |
| Shwachman-Diamond | ||
| 5.b. Functional defects | LAD* | |
| CGD* | ||
| 6. Defects in intrinsic and innate immunity | 6.a. Bacterial and parasitic infections | STAT1 GOF |
| CARD9 | ||
| 6.b. Mycobacterial and viral | IFNGR1&2 | |
| WHIM | ||
| 7. Autoinflammatory disorders | Familial Mediterranean fever | |
| Mevalonate kinase def | ||
| Familial cold autoinflammatory syn | ||
| 8. Complement deficiencies | C5–C9 def (disseminated Neisserial infection) | |
| C1q,r,s (SLE-like syn) | ||
| Factor H&I def (atypical hemolytic uremic syn) | ||
| 9. Bone marrow failure | Fanconi anemia* | |
| Dyskeratosis congenita* | ||
| 10. Phenocopies of PID | Autoantibody to IL-17 or IL-22 (chronic mucocutaneous candidiasis) |
SCID severe combined immunodeficiency, CVID common variable immunodeficiency, HLH hemophagocytic lymphohistiocytosis, EBV Epstein-Barr virus, XLP X-linked lymphoproliferative disease, ALPS autoimmune lymphoproliferative syndrome, APECED autoimmune polyendocrinopathy with candidiasis and ectodermal dystrophy, IPEX immune dysregulation polyendocrinopathy enteropathy X-linked, LAD leukocyte adhesion deficiency, CGD chronic granulomatous disease, GOF gain of function, WHIM warts, hypogammaglobulinemia, infections, and myelokathexis, SLE systemic lupus erythematosus
*Multiple molecular causes exist for the condition
While it was typical to group patients together by immunologic phenotype, identifying the underlying genetic cause of the deficiency has become more commonplace over the last 10 years or so. Costs associated with these tests have decreased significantly while the number of disorders tested with a single sample has increased. At the time of this publication, one commercial lab offers a genetic panel test for 474 known causes of immunodeficiency using a single sample [3]. With so many newly recognized disorders, the treatment of primary immunodeficiency is more complex than in the past. Many of these forms of primary immunodeficiency have unique susceptibilities and require individualized treatment regimens; thus, it is impossible to discuss all in this review. However, we can focus on a few of the treatments for the more frequently seen forms of immunodeficiency.
Use of Antimicrobial Therapy in Primary Immunodeficiency
Antimicrobial therapy is important for the treatment of all forms of immunodeficiency. Severe forms of immunodeficiency usually require antimicrobial prophylaxis regimens based on the known risk for infection, and a detailed description of these regimens is beyond the scope of this brief review. Infants with severe combined immunodeficiency (SCID) are typically managed with multiple forms of antimicrobial prophylaxis while moving toward definitive treatment such as stem cell transplant or gene therapy. Infants with SCID should receive prophylaxis against Pneumocystis jirovecii pneumonia (PJP) with trimethoprim/sulfamethoxazole using a dosage of 5 mg/kg/day of trimethoprim by mouth 3 times weekly, although alternative regimens exist [4]. Because infants with SCID are so profoundly immunocompromised, many centers also give prophylaxis against viral and fungal infections, using acyclovir and fluconazole, respectively. The RSV monoclonal antibody Palivizumab is also recommended [5]. If an infant with SCID develops signs or symptoms of infection, both common and opportunistic pathogens must be considered and aggressive broad-spectrum antimicrobials are needed until the source of the infection can be identified.
More commonly, the treatment of primary immunodeficiencies that cause various levels of hypogammaglobulinemia also consists of the provision of antimicrobials to clear intercurrent infections. For antibody deficiencies, these infections are typically caused by encapsulated bacteria such as Streptococcus pneumoniae, Haemophilus influenzae, or other typical bacteria; therefore, it is important to select an antibiotic that will overcome known mechanisms of resistance to these drugs. Most immunologists treat patients with antibody deficiencies more aggressively than normal individuals, often prescribing antimicrobials at higher doses or for longer durations than is typically recommended for routine use. While data supporting this practice is lacking, the most recent Practice Parameter for the Diagnosis and Management of Primary Immunodeficiency states: “The standard dose and duration of antimicrobial regimens might not be adequate to eradicate infections in immunocompromised hosts. Early combined antimicrobial therapy and prolonged courses should be considered.” [4]. Empiric therapy based on suspected organisms is the norm. However, the selection of an antimicrobial based on the culture of an adequate sample (i.e., sputum or tissue sample) is considered the gold standard and the preferred approach when possible.
Antimicrobial prophylaxis is also sometimes used to prevent infections with organisms that typically affect patients with antibody deficiency. Again, no published studies with large numbers of patients exist but experience in other disease states with similar risks of infection has given immunologists some evidence by which to choose a treatment regimen. For children, daily amoxicillin at doses of 20 mg/kg once or twice daily, trimethoprim/sulfamethoxazole given either daily or three times weekly at 5 mg/kg, or azithromycin 5 mg/kg thrice weekly has been reported to be beneficial to patients [4, 6]. For adults, often used regimens include either amoxicillin 500–1000 mg daily or twice daily, trimethoprim/sulfamethoxazole 160 mg daily or twice daily, or azithromycin 500 mg once weekly or 250 mg every other day [4]. In some cases of mild hypogammaglobulinemia or other disorders with less risk of invasive infection, a clinical response to antimicrobial prophylaxis can be employed to avoid the need for IgG replacement. Conversely, failure to respond to prophylactic regimens may prove the need for IgG replacement and better define a patient’s individual risk for infection.
Immunoglobulin Replacement
The Nobel Prize in Medicine was presented to Emile von Behring in 1901 in recognition of his work to show that the serum of rabbits immunized against tetanus had protective qualities for animals who received a passive transfer of this serum. His work is felt to be one of the first in the field of antibody replacement. The concept was applied to the treatment of tetanus in humans during the first World War [7]. As researchers began to understand that these protective antibodies were a distinct subset of proteins within the serum, efforts were initiated to separate them for widespread human use. The fractionation method of serum which allows for the use of purified IgG was developed by Cohn in the 1930s and 1940s, and this technique is still employed today [8, 9].
The first immunoglobulin products obtained for clinical use were not as purified or stable as those available today. Intramuscular or subcutaneous use of these products was the norm. The products were relatively impure compared to today’s standards, and this was likely the cause of significant discomfort during administration. Intravenous (IV) use was not possible due to aggregates and other impurities. Reactions to IV use of these preparations led to hypotension and shock [10].
Prior to the first highly purified licensed IgG product designed for IV use, management of antibody deficiencies consisted of treatment of infections with antimicrobials and provision of immunoglobulin via intramuscular (IM) or subcutaneous (SC) routes. The first use of immunoglobulin to treat a patient with antibody deficiency occurred in 1952 by Col Ogden Bruton, when he used subcutaneous immunoglobulin to successfully treat a boy with X-linked agammaglobulinemia [11]. Over the next 30 years or so, replacement of IgG intramuscularly was standard practice for patients with varying degrees of hypogammaglobulinemia. The dosing was limited due to side effects associated with IM administration. At the time, typical monthly doses ranged from 100 to 200 mg/kg [12]. Compared to today’s practices, these doses are significantly lower and not adequate for protection from all infections.
As the manufacturing processes and distribution of these lifesaving products improved over the next 25–50 years, the methods for infusion of IgG changed. The first product licensed for use intravenously was made available in 1981 [13]. This allowed for infusions of larger doses of gamma-globulin to be given to patients at relatively fast rates. Tolerability of the infusions was a limiting factor when selecting a dosing regimen. One issue with these early IVIG preparations was the development of renal complications. Higher doses, the presence of comorbidities, and the use of products stabilized with sucrose or maltose were associated with these rare complications [14].
As suggested earlier, the intravenous route has been associated with an increased risk of intolerance and systemic reactions. These adverse reactions are most often related to the rate of infusion. However, it is unclear what exactly causes these adverse events. Examples of adverse events commonly seen include headache, malaise, and fatigue. Fever, rash, and other complaints occur less often. Various interventions have been employed to mitigate these symptoms from adjustment of infusion rate, changes to the prescribed product, and premedication with antipyretics or antihistamines. While these are often successful, none have consistently changed outcomes across all patients, and it is sometimes difficult to predict who might be at higher risk for these adverse events. Currently, most immunologists would consider alternative infusion methods such as the subcutaneous route in cases where simple interventions failed to ameliorate symptoms [15].
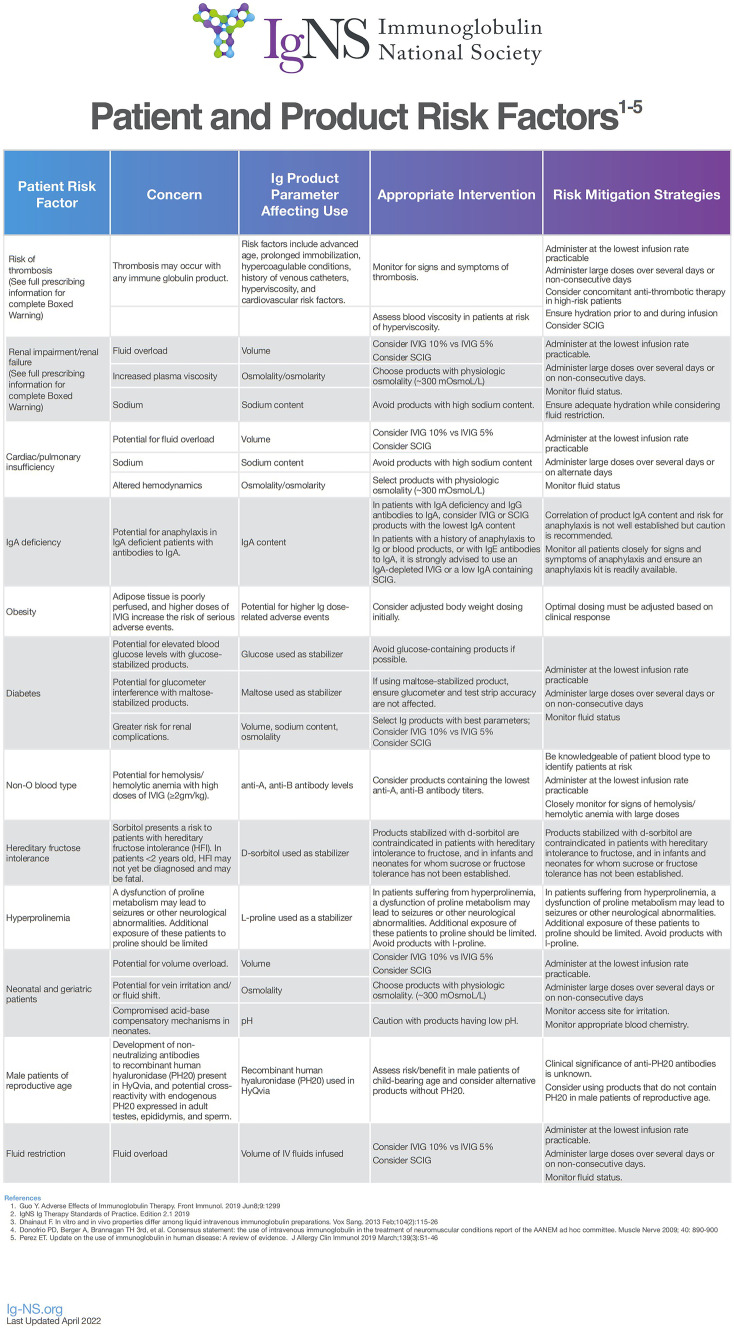
One current strategy regarding the management of intolerance or adverse events involves understanding the profiles of patients who might be at higher risk for these complications and choosing a dose or mode of administration that might reduce the risk. For example, patients with a history of migraine headache are felt to be at higher risk of developing headache during or after infusion with IVIG. Therefore, lower dosing or slower infusions would be a first-line intervention. Infusion of IVIG imparts a known risk for thromboembolism. Studies have revealed that Factor XIa activity in the product is a significant contributor to thrombosis, and measures have been implemented to reduce Factor XIa activity in immunoglobulin products [16]. Patient characteristics including older age, prior history of thromboembolism, hypercoagulable state, and alcohol abuse also increase the risk of experiencing thromboembolic events with IVIG infusion [16]. Therefore, patients with an increased risk for these events should receive IgG at slower rates or via the subcutaneous route to allow for slow absorption into the bloodstream via the lymphatics [4]. Multiple products are currently available for use intravenously and no one product has been shown to be better tolerated or more efficacious than others. Figure 1 describes patient and product risk factors that may impact the tolerability of immunoglobulin infusions.
Fig. 1.
Characteristics of patients and/or IgG products that may impact tolerability of immunoglobulin infusion. With permission from Ig Clinicians Quick Reference published by Ig-NS (Immunoglobulin National Society)
Subcutaneous immunoglobulin (SCIG) has advantages over IVIG in regard to systemic tolerability although clinical efficacy is similar. The frequency of local injection site reactions is high when initiating therapy, although these decrease over time [17]. Limitations of SCIG include the need for multiple needle sticks with some products, frequent therapy sessions, and self-administration of the drug. The first licensed product for subcutaneous use in the USA was approved in 2006 [17]. Prior to 2006, the subcutaneous route was infrequently used by immunologists in the USA although immunologists in other countries did so. Now, multiple products are available in varying concentrations of 10–20% and this route is commonly prescribed to patients who need immunoglobulin replacement.
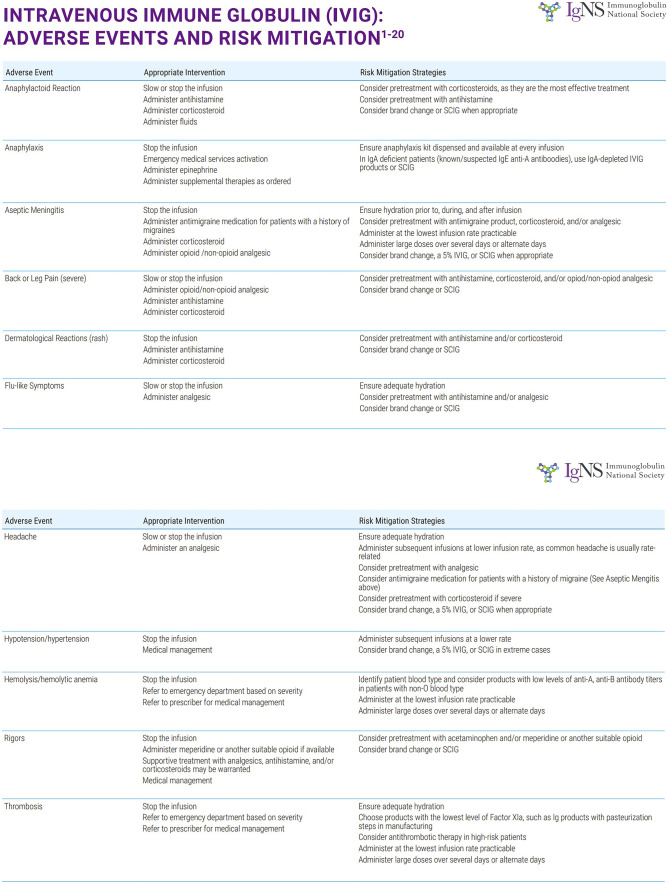
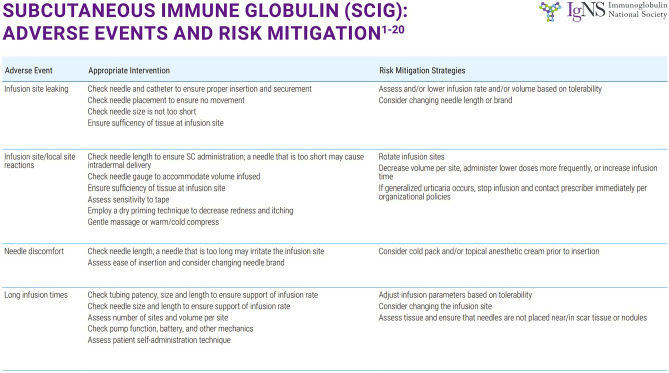
More recently, recombinant human hyaluronidase has been used to facilitate infusions of 10% immunoglobulin. The trials leading to US Food and Drug Administration (FDA) approval in 2014 showed tolerability of up to 600 ml of 10% IgG solution in a single subcutaneous site with low rates of systemic adverse events. While this infusion method is generally well tolerated, it has a higher rate of systemic adverse events compared with the subcutaneous infusion. The systemic reaction rate was significantly less than IVIG, however [18]. Figures 2 and 3 show the common adverse events associated with IVIG and SCIG as well as suggested mitigation techniques for each.
Fig. 2.
Adverse events associated with IVIG and mitigation techniques recommended by Ig-NS (Immunoglobulin National Society). With permission Ig Clinicians Quick Reference 2020 Edition 1.0 www.Ig-NS.org
Fig. 3.
Adverse events associated with SCIG and mitigation techniques recommended by Ig-NS (Immunoglobulin National Society). With permission Ig Clinicians Quick Reference 2020 Edition 1.0 www.Ig-NS.org
Currently, there are multiple products available for use in the USA and worldwide, some being specific to one of the three modes of administration routinely used. Other products can be used interchangeably between administration routes. Consideration of a patient’s diagnosis, comorbidities, and preferences are all important when determining product selection, dosing, infusion method, and site of care. It is important to understand the differences in the available products in terms of their chemical properties, stabilizers used, and dosing/administration limitations. When choosing therapy, it is often helpful to reference the currently available IgG products that are FDA-approved for use in the USA. For example, the Immunoglobulin National Society (Ig-NS), an organization that works with healthcare providers involved in the care of patients receiving immunoglobulin replacement, has compiled a detailed chart of these products and this is available online [19].
Various dosing regimens have been suggested over the years with the intention of preventing infection. Previously, targeting a particular trough level measured just before an IV infusion was used to adjust therapy. Patients with underlying pulmonary disease and recurrent cases of pneumonia may need higher dosing regimens as these have been shown to reduce the incidence of pneumonia [20]. As physicians, however, we are often reminded to treat the patient and not necessarily a number or lab value. The concept of a biologic trough was introduced by Bonagura et al. [21], in which it was recognized that each patient seemed to require different serum IgG concentrations to remain infection-free. It is now more commonplace for physicians to adjust dosing based on clinical response to prevent breakthrough infections during therapy.
Other parameters of therapy such as the mode of administration or site of care are more subjective in nature. A careful selection can reduce the burden of care by matching the properties of the infusion to the needs of the patient. It is important that prescribers of immunoglobulin understand the differences between the various modes of administration. IVIG is typically given in a healthcare setting every 3–4 weeks. Home treatment is standard for both subcutaneous immunoglobulin (SCIG) and facilitated subcutaneous immunoglobulin (fSCIG) administration, although the latter may also be routinely given in physician offices or infusion centers. The length of each infusion session for each mode of administration also differs, with IVIG typically taking several hours to infuse compared to SCIG infusions that might last 1 h or less depending on the product selected. SCIG can be given as frequently as daily, or every 2 weeks.
Deciding upon a mode of administration, the product used, and the site of care requires that both the patient and the physician understand the choices available. The inclusion of shared decision-making (SDM) has become widely used for several disease states including the treatment of primary immunodeficiency. SDM is especially important at the onset of therapy because it can lead to better engagement of the patient in his or her healthcare decisions and improve adherence to therapy. Patients should understand however that treatment plans can change as needed to meet their needs. For example, it is not uncommon for pediatric patients to receive IVIG during early childhood when their parents might feel uncomfortable placing subcutaneous needles each week for therapy sessions. As the child becomes older, a change to subcutaneous therapy at home might fit the family’s lifestyle better. Subcutaneous administration also allows flexibility for college students and working adults. It is important to revisit treatment decisions every so often to ensure that the mode of administration and site of care is appropriate for the patient.
Newborn Screening for Severe Combined Immunodeficiencies
While not a definitive treatment for primary immunodeficiency per se, it would be remiss to not discuss newborn screening (NBS) for T cell lymphopenia, as it has been a major advancement impacting the success of SCID treatment. Prior to the implementation of NBS, the diagnosis of patients with immunodeficiency relied on physicians and other healthcare providers having a suspicion that an individual might be affected due to recurrent or unusual infections or failure to thrive or by having a family history of the disease. Unfortunately, patients affected by SCID almost always appear normal in the first few weeks or months after birth. Only after infections occur or if the infant fails to gain weight or develop appropriately would someone suspect an underlying disease. SCID is a disease amenable to screening for several reasons. Patients otherwise appear normal, fatality is almost certain without treatment, a low-cost validated test is available, and treatment of the disease is highly successful [22, 23]. Before the implementation of NBS with the method developed in 2005, a CBC was often recommended as a screening method to identify infants with SCID but some individuals were missed due to relatively high numbers of B or NK cells or the presence of maternally engrafted T cells [23].
The assay employs the use of the dried blood spot obtained on every newborn for screening of other diseases. This RT-PCR-based assay measures the presence of T cell receptor excision circles (TRECs) which are representative of the presence of naïve T cells that have recently emigrated from thymus. Low or absent TRECs suggest significant T cell lymphopenia and must be confirmed by flow cytometry. It is now routinely performed in all 50 states, in Puerto Rico, and in other nations worldwide.
NBS was intended to identify infants with SCID who are known to be at high risk for early death due to opportunistic infection. After it was implemented in larger pilot studies and then as routine screening for the general population, it was shown to also identify other causes of non-SCID T cell lymphopenia (both mild and severe). Immunodeficiencies such as DiGeorge syndrome and ataxia-telangiectasia are sometimes identified, and novel mutations in other genes have been discovered in patients with low or absent TRECS. Syndromes such as trisomy 21 and Noonan syndrome can also cause low TRECs. Secondary causes of lymphopenia also cause abnormal results on the TREC assay [24].
Once a newborn with SCID has been identified by NBS, treatment must begin immediately. Whereas previous management for SCID differed between centers, there is now some consensus regarding initial management. Isolation of the patient and family should occur as soon as possible to prevent infection. Infants must also undergo confirmatory testing. This is best done at a center where experts skilled at interpretation of the results are available. Antibiotic and antifungal prophylaxis should begin with medications tailored to the age and clinical status of the patient. Other supportive treatments may be indicated such as the provision of immunoglobulin replacement and transfusion of leukoreduced, irradiated, cytomegalovirus (CMV)-negative red blood cells (RBCs) if significant anemia is present [5].
As previously mentioned, NBS allows for the identification of patients with SCID prior to the onset of infections in many cases. NBS has lowered the age of diagnosis of SCID, and we have a better understanding of the true prevalence of the various forms of SCID. In one series, 19% of patients identified with SCID had mutations in IL2RG whereas 10% were ADA-SCID. The overall prevalence of these diseases is more common than previously thought, affecting approximately 1/50,000–1/60,000 live births [25].
Hematopoietic Stem Cell Transplant
As mentioned earlier, for a disease to qualify for implementation of newborn screening, there must be a proven successful treatment for the underlying disease. Hematopoietic stem cell transplant (HSCT or SCT) was first used as a curative therapy for a patient with SCID in 1968 by Dr. Robert A. Good. The unique situation in which the recipient was unable to reject the bone marrow of his HLA-compatible sibling was a first in regard to the treatment of non-malignant diseases with HSCT [26]. Since that time, the use of SCT for the treatment of SCID and other forms of primary immunodeficiency has become the standard of care when a compatible match is available.
When a fully HLA-matched sibling donor is used for SCT, no conditioning with chemotherapy is required for most forms of SCID. The use of a matched sibling donor is also associated with higher survival rates, fewer complications such as graft versus host disease (GVHD), and more complete B cell engraftment [27]. Unfortunately, most patients do not have an available HLA identical sibling, and so other donor sources must be used despite their potential for increased risk of graft rejection and complications.
Mismatched related donors (MMRD) can be used and are the most common donor source in some series. Success with these transplants is higher at centers with experience and has continued to improve with time [28]. Matched unrelated donors (MUD) and umbilical cord blood (UCB) are also used. Transplantation with UCB is limited by the fixed number of donor cells in the umbilical cord and is not usually adequate for larger patients. For infant recipients, this limitation of UCB is less problematic due to their low body weight. In one study, the overall survival after UCB transplant for SCID was similar to other donor sources. The degree of HLA match impact survival [29]. It is difficult to compare outcomes based on a donor source alone, since many factors impact success. Most importantly, there is increased survival when infants are treated with SCT at < 3.5 months of age. If an infant with SCID is transplanted at an older age, they can also do well if there is no evidence of active infection [27]. Age at transplant is, nonetheless, an important factor since younger patients with infection do better than older patients with infections [30]. This lends further importance to early diagnosis via NBS as a way to improve the outcomes of patients with SCID.
Conditioning with chemotherapy before transplant has been a topic of great debate when treating patients with SCID using donor sources other than an HLA-matched sibling. Two studies have shown that the use of a conditioning regimen led to better T cell reconstitution [27, 30]. In another study, the use of reduced-intensity conditioning was associated with better overall survival when compared to myeloablative conditioning (94% vs 53%) [31].
HSCT is also curative for the treatment of chronic granulomatous disease (CGD), a primary immunodeficiency causing recurrent deep-seated infections and inflammatory complications in multiple organs. Significant conditioning before transplantation is required and often comorbidities exist in the recipient that can impact the development of post-transplant complications [32]. In recent years, HSCT for CGD has been increasingly successful as shown by a multicenter study describing transplantation using reduced-intensity conditioning and matched unrelated or sibling donors [33]. Other forms of immunodeficiency can also be definitively treated with SCT including Wiskott-Aldrich syndrome, X-linked hyper IgM syndrome, and X-linked lymphoproliferative disease [34].
New techniques in the approach to both host conditioning and graft preparation could allow for successful SCT while essentially eliminating toxicity. Taking advantage of the fact that CD45 is present on the surface of all nucleated hematopoietic cells and their precursors [35], the use of an anti-CD45 antibody is a promising non-chemotherapy conditioning tool [36]. Graft manipulation, specifically ex vivo alpha/beta T cell depletion, is currently being used to decrease GVHD [36]. With this approach, the gamma/delta T cells are allowed to remain in the graft, with the benefit of antiviral activity. As they are MHC-independent, the gamma/delta T cells do not mediate GVHD [36, 37].
Approaches have also been developed to combat the often devastating post-transplant complication of severe viral infection. Viruses such as cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus, among others, are potentially fatal in the post-transplant period and may be extremely challenging to control with conventional antiviral treatments alone. One immunologic method of treatment is donor lymphocyte infusion (DLI), although this approach is limited by lack of specificity against the virus and risk for GVHD [36]. A newer approach uses virus-specific T lymphocytes (VSTs) with the goal of isolating donor T cells with activity against certain virus(es) of interest without including alloreactive T cells [38]. This technology has also been used to isolate third-party VSTs for “off-the-shelf” availability for prophylaxis or treatment of viral infections [38]. The use of VSTs appears to be effective, but remains available in the USA only in clinical trials.
Gene Therapy
Current approaches to gene therapy (GT) for primary immunodeficiencies use autologous stem cells harvested from an affected patient. These cells then undergo genetic transformation with the wildtype or normal gene, and are re-infused into the patient where they differentiate into immune cells that express the normal gene [39]. Gene therapy has been used to treat patients with T cell deficiencies such as IL2RG deficiency and ADA-SCID and can correct the immunologic defect [40]. A benefit of gene therapy is that a suitable donor is not necessary; therefore, it is a possible treatment for patients lacking either a sibling donor or a well-matched unrelated donor. Unfortunately, gene therapy is not yet available for most patients. Initial trials of GT for the treatment of ADA-SCID were not entirely successful, most likely due to the lack of pretreatment conditioning [41]. The addition of pretreatment conditioning with a gamma-retroviral vector was later shown to be successful. Currently, an approved product (Strimvelis) is available in the EU for the treatment of ADA-SCID [42]. In the USA, we are awaiting an approved GT treatment product for patients who lack an adequately matched stem cell donor.
Gene therapy for IL2RG SCID was also developed, and gamma-retroviral vectors were used in the initial clinical trials in 1999. GT was successful in the correction of the immunologic defect, but it was noted that T cell leukemia developed in 25% of study participants due to the integration of the vector close to protooncogenes. Alternative lentiviral vectors felt to have less risk of insertional mutagenesis are now being used in the context of clinical trials at several centers and these complications have not been observed [43].
Currently, there are multiple clinical trials ongoing for the use of gene therapy in various primary immunodeficiencies including SCID, Wiskott-Aldrich syndrome, and chronic granulomatous disease [34]. It is hoped that it will be a safe and viable therapy for widespread use in our patients without an acceptable stem cell donor.
Summary
Significant advances in the treatment of primary immunodeficiency have occurred during the last century. Patients now have choices regarding immunoglobulin replacement and infusion modes of administration that are flexible and can be adjusted to their needs and comorbidities. Diagnosis of patients via NBS has led to the ability to treat the most fragile patients early with increasing success. When a stem cell donor is identified in a timely manner, many forms of primary immunodeficiency can be cured by hematopoietic stem cell transplantation although there is still a significant risk for complications. For those infants lacking an adequate stem cell donor, autologous gene therapy is becoming a viable alternative with approved products being currently available or on the horizon.
Acknowledgements
The authors thank the Immunoglobulin National Society (Ig-NS) for providing figures for this publication.
Declarations
Ethical Approval
Not applicable/no human subjects.
Informed Consent
Not applicable/no human subjects.
Conflict of Interest
Kenneth Paris MD: Dr. Paris has been a member of advisory boards and a speaker for Takeda Pharmaceuticals regarding the treatment and diagnosis of primary immunodeficiency. He has participated in clinical trials with Takeda regarding immunoglobulin products and with Chiesi Pharmaceuticals regarding enzyme replacement for ADA-SCID. He is also on the Medical Advisory Committee for the Immunoglobulin National Society who provided figures for this manuscript. Luke Wall MD: Dr. Wall has participated in clinical trials with Takeda Pharmaceuticals regarding immunoglobulin products, and with Chiesi Pharmaceuticals regarding enzyme replacement for ADA-SCID.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kenneth Paris, Email: kparis@lsuhsc.edu.
Luke A. Wall, Email: lwall@lsuhsc.edu
References
- 1.Chellapandian D, Chitty-Lopez M, Leiding JW. Precision therapy for the treatment of primary immunodysregulatory diseases. Immunology and Allergy Clinics. 2020;40(3):511–526. doi: 10.1016/j.iac.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Bousfiha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C (2020) Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol 11:1–6. (PMID: 32048120; PMCID: PMC7082388) [DOI] [PMC free article] [PubMed]
- 3.Invitae. Test Catalog: Primary Immunodeficiency Gene Panel. Retrieved May 8, 2022, from https://www.invitae.com/en/providers/test-catalog/test-08100
- 4.Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, Keller M, Kobrynski LJ, Komarow HD, Mazer B, Nelson RP., Jr Practice parameter for the diagnosis and management of primary immunodefieiency. Journal of Allergy and Clinical Immunology. 2015;136(5):1186–1205. doi: 10.1016/j.jaci.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 5.Dorsey MJ, Dvorak CC, Cowan MJ, Puck JM. Treatment of infants identified as having severe combined immunodeficiency by means of newborn screening. J Allergy Clin Immunol. 2017;139(3):733–742. doi: 10.1016/j.jaci.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballow M, Paris K, de la Morena M (2018) Should antibiotic prophylaxis be routinely used in patients with antibody-mediated primary immunodeficiency? J Allergy Clin Immunol Pract 6(2):421–426. 10.1016/j.jaip.2017.11.024 (Epub 2017 Dec 21. PMID: 29274825) [DOI] [PubMed]
- 7.Eibl MM (2008) History of immunoglobulin replacement. Immunol Allergy Clin North Am 28(4):737–64, viii. 10.1016/j.iac.2008.06.004 (PMID: 18940572) [DOI] [PubMed]
- 8.Cohn EJ. Blood proteins and their therapeutic value. Science. 1945;101(2612):51–56. doi: 10.1126/science.101.2612.51. [DOI] [PubMed] [Google Scholar]
- 9.Cohn EJ, Luetscher JL, Oncley, SH et al (1940) Preparation and properties of serum and plasma proteins. III. Size and charge of proteins separating upon equilibration across membranes with ethanol - water mixtures of controlled pH, ionic strength and temperature. J Am Chem Soc 62(12):3396–3400. 10.1021/ja01869a032
- 10.Barandun S, Kistler P, Jeunet F, Isliker H. Intravenous administration of human gamma-globulin. Vox Sang. 1962;7:157–174. doi: 10.1111/j.1423-0410.1962.tb03240.x. [DOI] [PubMed] [Google Scholar]
- 11.Bruton OC. Agammaglobulinemia Pediatrics. 1952;9(6):722–728. doi: 10.1542/peds.9.6.722. [DOI] [PubMed] [Google Scholar]
- 12.MRC Working Party on Hypogammaglobuinaemia. Hypogammaglobulinaemia in the UK. London
- 13.Ochs HD, Buckley RH, Pirofsky B, Fischer SH, Rousell RH, Anderson CJ, Wedgwood RJ. Safety and patient acceptability of intravenous immune globulin in 10% maltose. Lancet. 1980;2(8205):1158–1159. doi: 10.1016/S0140-6736(80)92594-5. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) (1999) Renal insufficiency and failure associated with immune globulin intravenous therapy - United States, 1985–1998. Morb Mortal Wkly Rep 48(24):518–21 (PMID: 10401909) [PubMed]
- 15.Wasserman RL. Personalized therapy: immunoglobulin replacement for antibody deficiency. Immunol Allergy Clin North Am. 2019;39(1):95–111. doi: 10.1016/j.iac.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Sridhar G, Ekezue BF, Izurieta HS, Selvam N, Ovanesov MV, Divan HA, Liang Y, Golding B, Forshee RA, Anderson SA, Menis M. Immune globulins and same-day thrombotic events as recorded in a large health care database during 2008–2012. Transfusion. 2014;54(10):2553–2565. doi: 10.1111/trf.12663. [DOI] [PubMed] [Google Scholar]
- 17.Ochs HD, Gupta S, Kiessling P, Nicolay U, Berger M (2006) Subcutaneous IgG Study Group. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol 26(3):265–73. 10.1007/s10875-006-9021-7 (PMID: 16783465) [DOI] [PubMed]
- 18.Wasserman RL, Melamed I, Stein MR, Gupta S, Puck J, Engl W, Leibl H, McCoy B, Empson VG, Gelmont D, Schiff RI; IGSC, 10% with rHuPH20 study group. Recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulins for primary immunodeficiency. J Allergy Clin Immunol. 2012 Oct;130(4):951–7.e11. 10.1016/j.jaci.2012.06.021 (Epub 2012 Jul 28. PMID: 22846381) [DOI] [PubMed]
- 19.IG Therapy Products and Risk Factor Chart (2022) Immunoglobulin National Society. May 2, 2022, from https://ig-ns.org/product/ig-therapy-products-and-risk-factor-chart/
- 20.Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol. 2010;137(1):21–30. doi: 10.1016/j.clim.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Bonagura VR, Marchlewski R, Cox A, Rosenthal DW. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol. 2008;122(1):210–212. doi: 10.1016/j.jaci.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 22.Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115(2):391–398. doi: 10.1016/j.jaci.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Buckley RH (2012) The long quest for neonatal screening for severe combined immunodeficiency. J Allergy Clin Immunol 129(3):597–604; quiz 605–6. 10.1016/j.jaci.2011.12.964 (Epub 2012 Jan 24. PMID: 22277203; PMCID: PMC3294102) [DOI] [PMC free article] [PubMed]
- 24.Puck JM. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia. Immunol Rev. 2019;287(1):241–252. doi: 10.1111/imr.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwan A, Abraham RS, Currier R, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312(7):729–738. doi: 10.1001/jama.2014.9132.Erratum.In:JAMA.2014Nov26;312(20):2169.Bonagura,VincentR[Added]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2(7583):1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 27.Pai SY, Logan BR, Griffith LM, Buckley RH, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371(5):434–446. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gennery AR, Slatter MA, Grandin L, Taupin P, Cant AJ et al (2010) Inborn errors working party of the european group for blood and marrow transplantation; european society for immunodeficiency. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol 126(3):602–10.e1–11. 10.1016/j.jaci.2010.06.015 (Epub 2010 Jul 31. PMID: 20673987) [DOI] [PubMed]
- 29.Fernandes JF, Rocha V, Labopin M, Neven B, Moshous D, Gennery AR et al (2012) Eurocord and inborn errors working party of european group for blood and marrow transplantation. Transplantation in patients with SCID: mismatched related stem cells or unrelated cord blood? Blood 119(12):2949–55. 10.1182/blood-2011-06-363572 (Epub 2012 Feb 3. PMID: 22308292) [DOI] [PubMed]
- 30.Haddad E, Landais P, Friedrich W, Gerritsen B, Cavazzana-Calvo M, Morgan G, Bertrand Y, Fasth A, Porta F, Cant A, Espanol T, Müller S, Veys P, Vossen J, Fischer A. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood. 1998;91(10):3646–3653. [PubMed] [Google Scholar]
- 31.Rao K, Amrolia PJ, Jones A, Cale CM, Naik P, King D, Davies GE, Gaspar HB, Veys PA. Improved survival after unrelated donor bone marrow transplantation in children with primary immunodeficiency using a reduced-intensity conditioning regimen. Blood. 2005;105(2):879–885. doi: 10.1182/blood-2004-03-0960. [DOI] [PubMed] [Google Scholar]
- 32.Cole T, Pearce MS, Cant AJ, Cale CM, Goldblatt D, Gennery AR. Clinical outcome in children with chronic granulomatous disease managed conservatively or with hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2013;132(5):1150–1155. doi: 10.1016/j.jaci.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Güngör T, Teira P, Slatter M, Stussi G, Stepensky P, Moshous D et al (2014) Inborn Errors Working Party of the European Society for Blood and Marrow Transplantation. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet 383(9915):436–48. 10.1016/S0140-6736(13)62069-3 (Epub 2013 Oct 23. PMID: 24161820) [DOI] [PubMed]
- 34.Pai SY. Treatment of primary immunodeficiency with allogeneic transplant and gene therapy. Hematology Am Soc Hematol Educ Program. 2019;2019(1):457–465. doi: 10.1182/hematology.2019000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penninger JM, Iriel-Sasaki J, Sasaki T, Oliveira-dos-Santos AJ. CD45: new jobs for an old acquaintance. Nat Immunol. 2001;2(5):389–396. doi: 10.1038/87687. [DOI] [PubMed] [Google Scholar]
- 36.Shamriz O, Chandrakasan S. Update on advances in hematopoietic cell transplantation for primary immunodeficiency disorders. Immunology and Allergy Clinics. 2019;39(1):113–128. doi: 10.1016/j.iac.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, Pende D, Falco M, Handgretinger R, Moretta F, Lucarelli B. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood. 2014;124(5):822–826. doi: 10.1182/blood-2014-03-563817. [DOI] [PubMed] [Google Scholar]
- 38.Naik S, Nicholas SK, Martinez CA, Leen AM, Hanley PJ, Gottschalk SM, Rooney CM, Hanson IC, Krance RA, Shpall EJ, Cruz CR (2016) Adoptive immunotherapy for primary immunodeficiency disorders with virus-specific T lymphocytes. J Allergy Clin Immunol 137(5):1498–505. (PMID: 26920464; PMCID: PMC4860050) [DOI] [PMC free article] [PubMed]
- 39.Fox TA, Booth C (2020) Gene therapy for primary immunodeficiencies. Br J Haematol. 10.1111/bjh.17269 (Epub ahead of print. PMID: 33336808) [DOI] [PubMed]
- 40.Aiuti A, Ficara F, Cattaneo F, Bordignon C, Roncarolo MG. Gene therapy for adenosine deaminase deficiency. Curr Opin Allergy Clin Immunol. 2003;3(6):461–466. doi: 10.1097/00130832-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Kohn DB, Hershfield MS, Carbonaro D, Shigeoka A, Brooks J, et al. T lymphocytes with a normal ADA gene accumulate after transplantation of transduced autologous umbilical cord blood CD34+ cells in ADA-deficient SCID neonates. Nat Med. 1998;4(7):775–780. doi: 10.1038/nm0798-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aiuti A, Roncarolo MG, Naldini L. Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products. EMBO Mol Med. 2017;9(6):737–740. doi: 10.15252/emmm.201707573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mamcarz E, Zhou S, Lockey T, Abdelsamed H, Cross SJ, Kang G, et al. Lentiviral gene therapy combined with low-dose busulfan in infants with SCID-X1. N Engl J Med. 2019;380(16):1525–1534. doi: 10.1056/NEJMoa1815408. [DOI] [PMC free article] [PubMed] [Google Scholar]