Abstract
Abstract
Lower respiratory tract infections (LRTIs) are a leading cause of morbidity and mortality in children. The ability of healthcare providers to diagnose and prognose LRTIs in the pediatric population remains a challenge, as children can present with similar clinical features regardless of the underlying pathogen or ultimate severity. Metabolomics, the large-scale analysis of metabolites and metabolic pathways offers new tools and insights that may aid in diagnosing and predicting the outcomes of LRTIs in children. This review highlights the latest literature on the clinical utility of metabolomics in providing care for children with bronchiolitis, pneumonia, COVID-19, and sepsis.
Impact
This article summarizes current metabolomics approaches to diagnosing and predicting the course of pediatric lower respiratory infections.
This article highlights the limitations to current metabolomics research and highlights future directions for the field.
Introduction
Lower respiratory tract infections (LRTIs) cause an estimated 700,000 deaths and 60.6 disability-adjusted life years annually in children under 5 years worldwide.1 LRTIs consist of infections below the larynx, frequently including pneumonia and bronchiolitis in children.2 The majority of LRTIs in pediatric patients are caused by viruses, however, determining the underlying pathogens of LRTIs remains a challenge with currently available criteria, such as fever, white blood cell count, and radiographic imaging.3 For example, reports of chest radiographs for pediatric pneumonia have high inter-observer variability,4 and both viral and bacterial LRTIs can cause high fever.3 Additionally, there is currently no standard approach to predicting the severity of a child’s LRTI based on signs, symptoms, and common laboratory tests with great accuracy.5 Given the difficulties described above, new clinical tools are needed to help guide management and predict which children will experience severe disease.
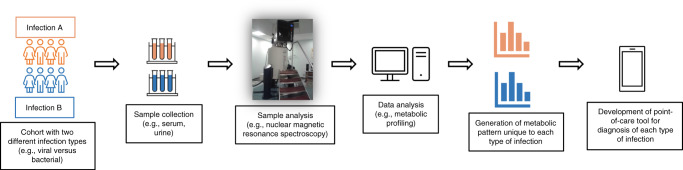
An emerging branch of the “omics” field that may aid the care of LRTIs is metabolomics. Metabolomics studies the metabolism of organisms, and experiments are designed to generate “snapshots” of the active metabolic pathways in an organism at a given moment in time. Metabolomic experiments can be either targeted, where a set of predefined molecules are identified from a sample, or untargeted, where all the molecules in a sample are identified and analyzed.6 The techniques most used to identify metabolites from a biological sample are mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy.7–11 With MS, chemical compounds are ionized and separated based on their mass-to-charge ratio. NMR generates spectral intensities based on the magnetic properties of molecules (Table 1). Each metabolite has its own unique mass-to-charge ratio and spectral intensity. Data analysis focuses on identifying and determining the relative abundance of metabolites in a sample. In the context of metabolomics in LRTIs, biological fluids such as serum, urine, or nasopharyngeal aspirates can be collected and analyzed via either MS and/or NMR. The identified metabolites are then compared and used to generate metabolic profiles of patients based on the infecting pathogen and severity of the disease (Fig. 1). Challenges of metabolomics include timing of sample collection, as whether an individual is early in the disease course or late,12 and whether any treatment has been received can influence the metabolic profile and make comparisons difficult.13
Table 1.
A comparison of nuclear magnetic resonance (NMR) spectroscopy, gas chromatography mass spectrometry (GC-MS), and liquid chromatography mass spectrometry (LC-MS) for metabolomics experiments.
| Analytical technique | NMR | GC-MS | LC-MS |
|---|---|---|---|
| Sample preparation | Minimal preparation required (e.g., ultrafiltration) | Derivatization and extraction required | Extraction required |
| Separation | Differences in magnetic frequency | Differences in volatility and mass | Differences in polarity and mass |
| Number of detectable metabolites/metabolic features | 30–100 | 100–500 | 1000+ |
| Sensitivity | Low | High | Highest |
| Reproducibility | High | Average | Average |
Fig. 1. Schematic representation of the design of a metabolomics study for pediatric lower respiratory infections.
Cohorts with two different infection types are recruited, and then a sample is collected from each participant. The sample is then analyzed using either nuclear magnetic resonance spectroscopy or mass spectrometry to detect all metabolites. The data is then analyzed to generate a metabolic profile for each infection type, which can then be used clinically to help manage respiratory infections in children.
This review builds on a previous one on the metabolomics of respiratory diseases in children up to 2011, highlighting the progress that has been made in the field.14 We feature new literature on the clinical utility of metabolomics for managing LRTIs and associated complications in children. Specifically, we focus on bronchiolitis, pneumonia, and coronavirus disease 2019 (COVID-19), as these are currently the most common LRTIs in pediatric patients. Sepsis was also included in this review as it is a life-threatening complication of LRTIs wherein prompt initiation of treatment is critical for survival.15 Neonatal sepsis was excluded from this review as this topic has been discussed extensively elsewhere.16–18
Bronchiolitis
Bronchiolitis is the most common LRTI in young children, accounting for 18% of hospitalizations in infants <1 year of age.19 The most common viruses associated with bronchiolitis are respiratory syncytial virus (RSV) and rhinovirus (RV).20,21 Predicting which children will experience severe disease is currently difficult, although some factors such as age and clinical status may help predict disease severity.22 Additionally, some evidence suggests bronchiolitis is a risk factor for developing asthma later in life.23,24
Distinguishing between bronchiolitis and bacterial LRTIs remains a challenge in pediatrics. Adamko et al. made the first attempt to use metabolomics as a tool to differentiate bronchiolitis from bacterial LRTIs in the pediatric population (Table 2).25 The final model consisted of 17 metabolites (when available, lists of significant metabolites that are >10 can be found in the Supplementary Tables), and when tested with age-matched samples, it correctly identified 90% of those with a viral infection (Supplemental Table S1).25 As this was a pilot study, further validation is needed in a larger cohort.
Table 2.
Summary of metabolomics studies on bronchiolitis and outcomes explored.
| Reference | Metabolomics analysis | Sample type | Study cohorts (number of study participants) | Age range of study participants | Outcome | Result |
|---|---|---|---|---|---|---|
| Adamko et al.25 | 1H-NMR | Urine |
Healthy controls (N = 37) RSV infection (N = 55) Non-RSV virus infection (N = 16) Bacterial infection (N = 24) |
Children any age | Healthy controls vs. RSV infection |
R2 = 0.86 Q2 = 0.76 |
| Length of hospital stay (>7days vs. 4–7 days vs. <4 days) |
R2 = 0.83 Q2 = 0.81 |
|||||
| RSV infection vs. bacterial infection |
R2 = 0.75 Q2 = 0.61 |
|||||
| Turi et al.27 | 1H-NMR | Urine |
Healthy controls (N = 60) RSV infection (N = 70) RV infection (N = 10) |
Infants | RSV infection vs. RV infection | No significant metabolites after FDR adjustment |
| Citrate: cis-aconitate ratio to distinguish between RSV infection and healthy controls | AUROC = 0.84 | |||||
| Stewart et al.28 | UPLC-MS/MS | Nasopharyngeal aspirates |
RV bronchiolitis (N = 26) RSV bronchiolitis (N = 80) |
Infants | RV bronchiolitis vs. RSV bronchiolitis | P < 0.001 |
| Stewart et al.29 | UPLC-MS/MS | Nasopharyngeal aspirates |
Bronchiolitis patients requiring use of PPV (N = 25) Bronchiolitis patients not requiring use of PPV (N = 119) |
<1 year | Bronchiolitis patients requiring PPV vs. bronchiolitis patients not requiring PPV |
Sensitivity = 0.84 Specificity = 0.86 |
| Stewart et al.30 | UPLC-MS/MS | Serum |
RSV requiring PPV (N = 38) RSV not requiring PPV (N = 102) |
<1 year | RSV requiring PPV vs. RSV not requiring PPV | FDR < 0.1 |
| Hasegawa et al.33 | UPLC-MS/MS | Nasopharyngeal aspirates |
Bronchiolitis patients with low free 25OHD levels (N = 71) Bronchiolitis patients with normal free 25OHD levels (N = 73) |
<1 year | Bronchiolitis patients requiring PPV vs. not requiring PPV based on 20 metabolites associated with vitamin D pathways | AUROC = 0.95 |
| Hasegawa et al.34 | UPLC-MS/MS | Serum |
Bronchiolitis patients with low free 25OHD levels (N = 70) Bronchiolitis patients with normal free 25OHD levels (N = 70) |
<1 year | Bronchiolitis patients requiring PPV vs. not requiring PPV based on 20 metabolites associated with vitamin D pathways | AUROC = 0.92 |
| Zhang et al.35 | UPLC-MS/MS | Serum |
RSV infection with subsequent wheezing (N = 26) RSV infection without subsequent wheezing (N = 48) |
<6 months | Infants with vs. infants without subsequent wheeze | 24 significantly different metabolites between two groups (P < 0.05) |
| Barlotta et al.36 | UPLC-MS/MS | Urine |
Bronchiolitis patients that develop recurrent wheeze (N = 17) Bronchiolitis patients that do not develop recurrent wheeze (N = 24) Healthy infants (N = 24) |
<1 year | Bronchiolitis vs. healthy controls | AUROC = 0.99 |
| Recurrent wheeze vs. no recurrent wheeze | AUROC = 0.87 | |||||
| Zhu et al.37 | LC-MS/MS | Nasopharyngeal aspirates |
Bronchiolitis patients with metabotype A (N = 79) Bronchiolitis patients with metabotype B (N = 72) Bronchiolitis patients with metabotype C (N = 363) Bronchiolitis patients with metabotype D (N = 177) Bronchiolitis patients with metabotype E (N = 227)a |
<1 year | Development of asthma in metabotype B patients (high proportion of corticosteroid use and parental asthma) vs. metabotype A patients (clinically classic bronchiolitis) | ORadj = 2.18; 95% CI = 1.03–4.71; P = 0.04; E = 2.32 |
| Fujiogi et al.39 | UPLC-MS/MS | Serum |
Infants hospitalized with RSV bronchiolitis (N = 63) Infants hospitalized with RV-A bronchiolitis (N = 21) Infants hospitalized with RV-C bronchiolitis (N = 29) |
<1 year | 23 metabolites differentiating RSV from RV-A bronchiolitis | FDR < 0.05 for each metabolite |
| 20 metabolites differentiating RSV from RV-C bronchiolitis | FDR < 0.05 for each metabolite | |||||
| Fujiogi et al.40 | UPLC-MS/MS | Serum and nasopharyngeal aspirates | Infants hospitalized for bronchiolitis (N = 140) | <1 year | Identified several modules in both serum and nasopharyngeal metabolomes that were associated with disease severity and asthma development | Please see the original article for the results of individual modules |
AUROC area under the receiver operating characteristic, CI confidence interval, FDR false discovery rate, 1H NMR proton nuclear magnetic resonance spectroscopy, ORadj adjusted odds ratio, PPV positive pressure ventilation, Q2 predictive ability of the model, R2 goodness of fit of the model, RSV respiratory syncytial virus, RV rhinovirus, RV-A rhinovirus A, RV-C rhinovirus C, UPLC-MS/MS ultra-performance liquid chromatography coupled with tandem mass spectrometry.
aSee the original article for metabotype descriptions.
Recent evidence suggests that children may face different short-term and long-term outcomes based on the specific virus they are infected with, including length of hospital stay and risk for future asthma.20,26 As such, some metabolomics studies have focused on identifying unique metabolic profiles for the different viruses that cause bronchiolitis.27,28 Turi et al. were unable to generate a significant model to discern differences between children with laboratory-confirmed RSV and non-RSV infection.27 On the other hand, Stewart et al. identified 26 significant metabolites with an adjusted P value of <0.05 which differentiated between bronchiolitis caused by two common viruses, RSV and RV (Supplemental Table S2).28 The authors did not generate any clinical models to determine if these metabolites could distinguish between the two viruses with high sensitivity and specificity. Differences in results between the studies may be due to the sample type that was analyzed, as Turi et al. used urine samples, while Stewart et al. used nasopharyngeal samples.27,28
Metabolomics has also been applied to identify pathways associated with increased severity in children with bronchiolitis.25,29,30 Using the length of hospital stay as a marker of severity, Adamko et al. separated study cohorts with a hospital stay of >7 days from those with a stay <3 days using 17 metabolites (Supplemental Table S3).25 Another research group used positive pressure ventilation (PPV) as the severity marker for two studies.29,30 The first study reported 25 metabolites, generating a strong statistical model (sensitivity = 84%, specificity = 86%) for differentiating between patients requiring PPV and those not requiring PPV (Supplemental Table S4).29 Twenty other metabolites were identified in the authors’ second study distinguishing between patients requiring PPV from those not requiring PPV (Supplemental Table S5).30 This second study did not provide any clinical parameters such as sensitivity or specificity for the metabolic model. No significant overlap between the key metabolites and their associated pathways was identified in the two studies from the same group.29,30 The most likely explanation for the various results between the two studies is the different biofluids used; the first study analyzed nasopharyngeal samples, while the second study used the serum. As mentioned by the authors, whether the serum metabolome is representative of respiratory tract diseases requires further investigation.30
A factor with mixed evidence on its association with bronchiolitis severity is vitamin D deficiency.31,32 Hasegawa et al. analyzed nasopharyngeal samples of children with severe bronchiolitis that had low levels of vitamin D and compared them to severe bronchiolitis cases with sufficient vitamin D levels. The group identified the top 20 metabolites associated with lower serum vitamin D levels (Supplemental Table S6).33 These 20 metabolites distinguished between those needing PPV and those not with an area under the receiver operating characteristic (AUROC, a value between 0 and 1, i.e., how well a model differentiates between two groups, with a higher number suggesting better separation) of 0.95. In their final analysis, nine of these metabolites were significantly associated with a higher risk for PPV (Supplemental Table S6). In other words, metabolites associated with low serum vitamin D levels were also associated with a higher risk for PPV use. A couple of months later, the same group published a short report looking at the serum metabolomes of bronchiolitis patients based on their vitamin D levels.34 Again, the authors identified the top 20 metabolites associated with serum vitamin D levels and found nine of them to be significantly associated with PPV use; three of which were associated with a higher risk of PPV and six associated with a lower risk of PPV (Supplemental Table S7).34 The only overlapping metabolite amongst the top 20 metabolites associated with serum vitamin D levels in both studies was sphingomyelin (d18:1/18:1;d18:2/18:0). This metabolite was associated with a higher risk of PPV in both studies. The most likely explanation for only one common metabolite across the studies is the different biofluids used (nasopharyngeal vs. serum samples).
Bronchiolitis early in life has been identified as a potential risk factor for the development of recurrent wheeze and asthma.23,24 To our knowledge, four prospective studies have been conducted wherein the metabolic profiles of children with bronchiolitis were analyzed, and the children were then followed to determine if they developed a wheeze.27,35–37 Zhu et al. collected nasopharyngeal samples from 918 infants and clustered them into five different metabotypes. The metabotype rich in amino acids and low in polyunsaturated fatty acids was found to be associated with an increased risk for asthma at 5 years old. This finding did not maintain its significance when infants with previous breathing problems were excluded from the analysis.37 The goal of the remaining three studies was to identify metabolic pathways involved in the pathogenesis of wheeze following bronchiolitis and did not include clinical models for the prediction of wheeze.27,35,36 Nonetheless, significant metabolites were reported in two studies that were associated with wheeze 2–3 years after bronchiolitis (Supplemental Tables S8 and S9).35,36 There are a number of clinical tools that have been developed for predicting asthma in children based on features including age, sex, personal and family history of atopic conditions, and serum eosinophil and immunoglobulin E levels.38 Unfortunately, each clinical tool has its own drawbacks, and are often limited by poor negative likelihood ratios. Therefore, the metabolites mentioned above may serve as a valuable addition to clinical tools for predicting asthma in children.
Two of the most recent multicenter metabolomic studies on bronchiolitis have focused on multiple outcomes using both serum and nasopharyngeal metabolomes.39,40 In 2020, Fujiogi et al. analyzed the serum metabolomes of 113 infants with bronchiolitis. Their first outcome was to distinguish RSV bronchiolitis from both RV-A and RV-C bronchiolitis. They then took the metabolites that discriminated between virus types and looked for associations with clinical outcomes including PPV use, and the development of wheeze and asthma. The multivariable models identified 23 significant metabolites that differentiated RSV from RV-A infection, and 20 metabolites that differentiated RSV from RV-C infection (Supplemental Table S10). In the RSV vs. RV-A comparison, sphingolipids and carnitines were found to be associated with clinical outcomes such as PPV use and asthma.39 This finding fits with the fact that sphingolipids play a role in lung inflammation and immune signaling.41 In the RSV vs. RV-C comparison, metabolites involved in the plasmalogen pathway were associated with lower PPV use, and arginine was associated with a higher risk of asthma.39 Interestingly, arginine is an important substrate for nitric oxide synthase and arginase, which play a role in the regulation of airway smooth muscle tone, and arginine bioavailability has been associated with airflow abnormalities in severe asthma.42,43
The second recent study analyzed both the nasopharyngeal and serum metabolomes of infants with bronchiolitis and looked for subsequent associations with bronchiolitis severity and development of asthma.40 The group clustered the metabolites into modules based on their respective biological pathways, and then looked for correlations of entire modules with clinical outcomes. Several identified pathways from both the serum and nasopharyngeal samples were associated with the use of PPV, but no pathways from either group were associated with asthma development. The interesting aspect to this study is that an integrated analysis was conducted using both the serum and nasopharyngeal modules, to look for consistency between the two sample types. While some correlations were found between the two metabolomes, the underlying mechanisms explaining the interconnectedness of the serum and nasopharyngeal metabolomes remain to be elucidated. We direct the reader to the original article for in-depth descriptions of the complex integrated analyses.40 To our knowledge, this study was the first to investigate the relationship between the serum and nasopharyngeal metabolome in regards to infant bronchiolitis; a step in the right direction toward designing optimal metabolomics studies for diagnosing and prognosing bronchiolitis.
Pneumonia
Worldwide, almost 15% of deaths in children under 5 years of age are attributed to pneumonia.44 Pneumonia is a LRTI affecting the alveoli, and is caused by many different types of pathogens including bacteria, viruses, and fungi.45 The diagnosis of pediatric pneumonia is challenging as children often present with nonspecific signs including fever, cough, and tachypnea, also typical of other respiratory infections. Systematic reviews have been unable to identify any constellation of vital signs that can accurately diagnose pneumonia in children with fever and cough.46 Prospective cohort studies and literature reviews on currently used biomarkers to diagnose pneumonia such as white blood cell count, absolute neutrophil count, C-reactive protein, and procalcitonin (PCT) have failed to demonstrate significant differences across children with varying disease severity and etiologies of pneumonia.47,48
To date, one metabolomics study has focused on diagnosing pediatric pneumonia (Table 3).49 Laiakis et al. analyzed both urine and plasma samples collected from children with pneumonia and compared them to healthy controls. When using urine samples, the analysis resulted in an accuracy of 95%. The plasma samples had a slightly lower accuracy of 90%.49 Interestingly, the level of uric acid was found to be decreased in pneumonia patients when looking at urine samples but increased in plasma samples. The authors hypothesized that in severe pneumonia, there is increased reabsorption of uric acid by the kidneys, explaining why they found decreased levels of uric acid in the urine of children.49 This finding may suggest that various biofluids can produce different metabolic results, highlighting the importance of considering which biofluid was analyzed when comparing metabolomics-based models.
Table 3.
Summary of metabolomics studies on pediatric pneumonia and outcomes explored.
| Reference | Metabolomics analysis | Sample type | Study cohorts (number of study participants) | Age range of study participants | Outcome | Test characteristics |
|---|---|---|---|---|---|---|
| Laiakis et al.49 | UPLC-TOFMS | Plasma and urine |
Severe pneumonia (N = 11) Healthy controls (N = 11) |
2–59 months | Diagnosis of pneumonia with the urine sample | Accuracy = 0.96 |
| Diagnosis of pneumonia with the plasma sample | Accuracy = 0.91 | |||||
| Del Borrello et al.51 | UPLC-MS | Urine |
CAP of pneumococcal origin (N = 12) CAP of viral origin (N = 15) |
1–14 years | Bacterial vs. viral pneumonia | AUROC = 0.87 |
| Chiu et al.57 | 1H-NMR | Pleural fluid |
CAP patients with complicated parapneumonic effusion (N = 18) CAP patients with noncomplicated parapneumonic effusion (N = 22) |
4.6 ± 3.4 years 3.9 ± 1.3 years |
Complicated vs. noncomplicated parapneumonic effusion |
R2 = 0.32 Q2 = 0.15 |
| Chiu et al.58 | 1H-NMR | Pleural fluid |
CAP patients with fibrinous parapneumonic effusion (N = 28) CAP patients with nonfibrinous parapneumonic effusion (N = 30) |
<18 years | Fibrinous vs. nonfibrinous parapneumonic effusions |
R2 = 0.38 Q2 = 0.32 |
AUROC area under the receiver operating characteristic, CAP community-acquired pneumonia, 1H NMR proton nuclear magnetic resonance spectroscopy, MRSA methicillin-resistant S. aureus, Q2 predictive ability of the model, R2 goodness of fit of the model, UPLC-MS ultra-performance liquid chromatography coupled to mass spectrometry, UPLC-TOFMS ultra-performance liquid chromatography coupled with time-of-flight mass spectrometry.
Up to 23% of pediatric appointments for respiratory conditions end in the inappropriate prescribing of antibiotics, ultimately contributing to emerging antibiotic resistance.50 Having the ability to accurately determine the underlying pathogen(s) of LRTIs may significantly reduce this trend. One metabolomics study thus far has focused on distinguishing between viral and S. pneumoniae pneumonia in children.51 Del Borrello et al. identified 93 metabolites, which generated a model with an AUROC of 0.87. Twenty of these metabolites were then identified using metabolite databases (Supplemental Table S11). The group reported a strong metabolic model for distinguishing between the two etiologies of pneumonia, showing promise for applying metabolomics-based models to diagnose pneumonia in children.51 A strength of the present study is the strict criteria used to exclude children that had bacterial and viral coinfection, and children that are carriers of S. pneumoniae.51 Excluding carriers of S. pneumoniae is important, as emerging evidence suggests that the host nasopharyngeal microbiome, particularly colonization with S. pneumoniae, contributes to the pathogenesis of viral LRTIs.52
Development of parapneumonic effusions (PPEs), which are a collection of fluid in the pleural space, can affect children with pneumonia.53 PPEs can be either complicated or noncomplicated, and the distinction between the two is based on the fluid’s pH, glucose, and lactate dehydrogenase levels.54 Uncontrolled inflammation in a complicated PPE can cause fibrin deposition, leading to loculations and further pleural fluid accumulation.55 It is important to distinguish between types of PPEs to avoid unnecessary treatments, as complicated and fibrinous PPEs are more likely to require procedural interventions and medications.56 There is currently one research group that has published metabolomics studies investigating PPEs in children with pneumonia.57,58 Using pleural fluid samples, the first study looked at complicated vs. noncomplicated PPEs in pediatric patients infected with S. pneumoniae.57 The second study aimed to identify metabolic patterns associated with PPEs that were fibrinous and nonfibrinous in children infected with both S. pneumoniae and M. pneumoniae.58 Interestingly, the metabolites associated with complicated PPEs in study one and fibrinous PPEs in study two belonged to the same metabolic pathways. These pathways included amino acid synthesis and anaerobic metabolism, particularly propanoate and butanoate metabolism. The authors attributed the increase in these pathways to the increased invasion of bacteria in the pleural space, as bacteria synthesize amino acids and S. pneumoniae is a known facultative anaerobe.59 The drawback to both studies is that they were limited by weaker R2 (goodness of fit of the model) and Q2 (predictive ability of the model) values (Table 3), possibly due to small sample sizes (Study #1: N = 40; Study #2: N = 58).57,58
Sepsis
Sepsis is a life-threatening complication of infection, wherein nearly half of all sepsis cases in children follow an initial respiratory infection.60 The timely recognition of sepsis and septic shock in children is of paramount importance. Children presenting with septic shock face two-fold increased odds of mortality for every hour that there is a delay in the initiation of antibiotics.15 Despite the release of new guidelines for pediatric sepsis as part of the Surviving Sepsis campaign in 2020, screening tools for the prompt diagnosis of sepsis with both high sensitivity and specificity are lacking.61 The gold standard for diagnosing sepsis is blood culture, however, cultures can take hours to days to yield results and up to 87% of pediatric sepsis cases are blood culture-negative.62,63 A benefit to metabolomics is that very small volume samples can be analyzed in less than an hour.6
One of the earliest studies that used metabolomics for pediatric sepsis focused on differentiating between septic shock and systemic inflammatory response syndrome (SIRS) and predicting mortality (Table 4).64 The analysis yielded a sensitivity and specificity of 0.78 and 0.72, respectively for differentiating between septic shock and SIRS.64 The mortality model was compared to commonly used predictors of mortality, including serum PCT concentration and the Pediatric Risk of Mortality III-Acute Physiology Score (PRISM III-APS). The sensitivity of the metabolic model for predicting survivorship was 0.80, as compared to sensitivities of 0.29 for PCT, and 0.70 for the PRISM III-APS.64 A later case study that examined urinary metabolites from an infant with fatal sepsis due to pneumonia identified three metabolites (3-hydroxybutyrate, glucose, and acetone) that overlapped with the results from the previously discussed study.65
Table 4.
Summary of metabolomics studies on pediatric sepsis and outcomes explored.
| Reference | Metabolomics analysis | Sample type | Study cohorts (number of study participants) | Age range of study participants | Outcome | Test characteristics |
|---|---|---|---|---|---|---|
| Mickiewicz et al.64 | 1H NMR | Serum |
Septic shock (n = 60) SIRS/no infection (n = 40) Healthy controls (n = 40) |
1 week–11 years | Septic shock vs. SIRS |
AUROC = 0.82 Sensitivity = 0.78 Specificity = 0.72 |
| Septic shock survivors vs. non-survivors |
AUROC = 0.91 Sensitivity = 0.80 Specificity = 0.90 |
|||||
| Ambroggio et al.65 | 1H-NMR | Urine |
Fatal MRSA pneumonia (N = 1) Influenza pneumonia (N = 4) Healthy controls (N = 7) |
8 months–11 years | Sepsis and severe disease | Case study—not applicable |
| Mickiewicz et al.66 | 1H NMR | Serum |
PICU sepsis care (n = 94) Non-PICU sepsis care (n = 81) Non-sepsis (n = 63) |
2–15 years | PICU sepsis care vs. non-PICU sepsis care |
Accuracy = 0.89 AUROC = 0.96 Sensitivity = 0.86 Specificity = 0.91 PPV = 0.92 NPV = 0.86 |
| Mickiewicz et al.67 | 1H NMR | Serum |
PICU sepsis care (n = 46) Non-PICU sepsis care (n = 58) Non-sepsis (n = 19) |
1 month–17 years | PICU-sepsis care vs. non-PICU sepsis care |
Accuracy = 0.86 AUROC = 0.93 Sensitivity = 0.84 Specificity = 0.89 PPV = 0.89 NPV = 0.84 |
| Grauslys et al.68 | 1H NMR | Plasma |
Bacterial infection (n = 25) Viral infection (n = 30) Controls: elective cardiac surgery without infection (n = 58) |
Birth–16 years | Bacterial vs. control | AUROC = 0.93 |
| Viral vs. control | AUROC = 0.84 | |||||
| Bacterial vs. viral | AUROC = 0.78 | |||||
| Sepsis with organ dysfunction vs. sepsis without organ dysfunction | AUROC = 0.73 | |||||
| Li et al.69 | HPLC-MS | Serum |
PICU sepsis (n = 84) Healthy controls (non-sepsis, n = 59) |
15 days–13 years | PICU sepsis vs. non-sepsis | Not reported |
AUROC area under the receiver operating characteristic, HPLC-MS high-performance liquid chromatography and mass spectrometry, 1H NMR proton nuclear magnetic resonance spectroscopy, PICU pediatric intensive care unit, SIRS systemic inflammatory response syndrome.
Predicting the trajectory of a patient’s sepsis is another way in which metabolomics can be utilized clinically. Studies from a group in Calgary, Canada, focused on predicting which patients will require care in a pediatric intensive care unit (PICU), as compared to those that can be managed in the pediatric emergency department (PED).66,67 The final analysis based on seven metabolites differentiated between PICU-sepsis and PED-sepsis for 1-month–17-year-old patients with an AUROC of 0.93. The seven metabolites were dimethylamine, mannose, 3-methyl-2-oxovalerate, 3-hydroxyisovalerate, alanine, O-acetylcholine, and acetate.67 Grauslys et al. also attempted to separate their cohort based on sepsis severity. Their analysis showed modest discrimination between patients with organ dysfunction and those without, with an AUROC of 0.73. The most significant metabolic discriminators were 2-hydroxyisovalerate, ornithine, isoleucine, creatinine, creatine phosphate, and citrate, all of which had higher levels in the group without organ dysfunction.68
Identifying the causative pathogen of a patient’s sepsis in a timely manner is important for deciding on treatment and improving antibiotic stewardship. To date, two metabolomics studies have attempted to predict the offending pathogen in children with sepsis.68,69 The model generated by Grauslys et al. differentiated between viral and bacterial infections with an AUROC of 0.78. The most significant metabolic discriminators included isoleucine, urea, creatinine, 2-hydroxyisovalerate, tyrosine, valine, creatine phosphate, and histidine. Li et al. identified three metabolites with levels that were higher in sepsis survivors infected with Staphylococcus aureus, as compared to infection with Candida albicans, and Pseudomonas aeruginosa. The three metabolites were cholic acid, isovalerylglycine, and histidine.69
While only a single case study has looked exclusively at sepsis caused by pneumonia,65 almost half of the children included in the other sepsis studies initially had pneumonia leading to sepsis.66 Given the significant proportion of pediatric sepsis patients that have an underlying LRTI, it is important to be able to predict which patients with LRTIs will develop sepsis and how severe their course will be. Future metabolomic studies could aim to identify metabolic patterns that can distinguish between children with LRTIs that develop sepsis and those that do not.
COVID-19
As of March 29, 2022, COVID-19 has claimed the lives of over 6.1 million people worldwide.70 COVID-19 is an acute respiratory illness caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The clinical presentation of COVID-19 can range from asymptomatic, to mild respiratory symptoms, to severe pneumonia and death.71 However, unlike the adult population, pediatric patients typically experience less severe disease and mortality rates from COVID-19.72 To date, one metabolomics study has focused on COVID-19 in children, with the goal of explaining why children experience less severe disease than adults (Table 5).73 The group compared the data they collected on healthy children and children with COVID-19 to data they had previously obtained from a cohort of healthy adults and adults with COVID-19.74,75 The final model consisted of five metabolites that could differentiate between healthy children and children with COVID-19, and children vs. adults with COVID-19, with an AUROC of 1. The five metabolites were the same for both comparisons and included methylmalonic acid, dihydroorotic acid, indoleacetaldehyde, tryptophan, and mannitol.73 This study was the first to our knowledge to compare children with COVID-19 to healthy children, and adults with COVID-19. The study provides insight into why children experience less severe disease, which may offer targets for novel treatments of COVID-19 in adults. Future studies regarding metabolomics and children with COVID-19 should focus on predicting which children will develop severe disease and multisystem inflammatory syndrome in children (MIS-C), a rare but serious complication of COVID-19.
Table 5.
Summary of metabolomics studies on pediatric COVID-19 and outcomes explored.
| Reference | Metabolomics analysis | Sample type | Study cohorts (number of study participants) | Age of study participants: median (IQR) | Outcome | Test characteristics |
|---|---|---|---|---|---|---|
| Wang et al.73 | LC-MS/MS | Plasma |
Children with COVID-19 (N = 18) Healthy children (N = 12) |
Children with COVID-19: 7 (5, 12) Healthy children: 6 (3, 7) |
Five metabolite model comparing children with COVID-19 and healthy children |
AUROC = 1 RMSE = 1.5 × 10−9 |
| Five metabolite model comparing children and adults with COVID-19 |
AUROC = 1 RMSE = 8.8 × 10−7 |
AUROC area under the receiver operating characteristic, COVID-19 coronavirus disease 2019, LC-MS/MS liquid chromatography coupled with tandem mass spectrometry, RMSE root mean square error.
Discussion
In 2011, Atzei et al. published a review on the metabolomics of common respiratory diseases in children, including pneumonia and bronchiolitis.14 At the time, one study was identified pertaining to pneumonia in children and zero studies were identified pertaining to bronchiolitis in children. A decade later, we have reviewed over 10 studies related to the clinical utility of metabolomics for diagnosing and prognosing pneumonia and bronchiolitis in children, plus additional studies on other respiratory infections and their complications. The field of metabolomics has gained notable attention over the last several years as a useful tool for improving how LRTIs in children are diagnosed and cared for.
One can conclude from this manuscript that there is a need for guidelines and quality control measures for metabolomics studies for pediatric LRTIs. Such guidelines should discuss the ideal biofluid for LRTIs (which is yet to be elucidated but should be minimally invasive to obtain and representative of both the infection itself and the host response). As already mentioned, different biofluids produce different results in metabolomics studies.29,30,49 Thus, it becomes difficult to compare results from metabolomics studies that have used different biofluids to generate a clinical tool for routine use. Additionally, as stated by Stewart et al., it remains unknown whether biofluids such as urine and serum are truly representative of a respiratory tract illness.30 It seems probable that a sample from the lower respiratory tract would be most ideal for studying the metabolomics of LRTIs in children. However, such samples can only be obtained through invasive procedures such as bronchoscopy with bronchoalveolar lavage. Other important points for guidelines to address include the timing of sample obtainment. Results can differ based on when a sample was collected, as demonstrated by Slupsky et al. who showed that urinary profiles of mice infected with S. pneumonia and S. aureus differ based on the amount of time that has passed since the initial infection.12 While individual studies discussed here had strict rules for timing of sample obtainment, the criteria differed across studies (i.e., on admission to PICU,68 within 24 h of hospitalization30). Additionally, even with criteria such as sample collection within 24 h of hospitalization, it is unlikely that children will be captured at the same timepoint in their illness, as some may present earlier or later than others. This point speaks to the challenge of using metabolomics for acute illnesses.
Other potential explanations regarding the inconsistencies in results across studies may be due to factors that are already known to influence metabolomics results. Studies suggest that inconsistencies in the way samples are handled can lead to substantial variation in metabolic results.76,77 Furthermore, the time of day that a sample is collected can lead to different metabolic profiles being detected.78 Other factors such as environmental conditions and lifestyle habits are all known to play a role in the human metabolome.79 The studies reviewed here were conducted in a number of different countries, where the protocols used for sample handling, and the environment and lifestyle of the children included are all likely to differ, contributing to variation in reported results.
Despite the progress that has been made, metabolomics has not yet made its way into the clinic as a routinely used tool for children with LRTIs. One of the biggest barriers to the widespread use of metabolomics is cost. For example, the machinery necessary to conduct NMR spectroscopy can cost upwards of US$800k.80 One possible solution to this problem is through collaboration with biomedical engineers to aid in the development of point-of-care metabolomics tests. Such a tool has already been developed for the diagnosis of prostate cancer.81 Using four metabolites detected in human plasma, this point-of-care device detected prostate cancer with a sensitivity of 94% and a specificity of 70%. Ensuring equitable access to such point-of-care tests for LRTIs in children will be the final challenge for metabolomics technologies, as half of the childhood deaths due to pneumonia occur in just five low-resource countries worldwide.82
Conclusions
LRTIs and their subsequent complications are a leading cause of death in children worldwide.1 Current diagnostic strategies lack the sensitivity and specificity to make timely and accurate diagnoses that allow for optimal care of children with LRTIs. Metabolomics is a novel tool with the potential to improve the care of children with LRTIs and subsequent complications. However, before metabolomics becomes a widely available technology for improving outcomes in children with LRTIs, more large-scale studies are required to validate current findings, and guidelines are needed to produce more consistent results. Nonetheless, metabolomics holds promise as a clinical tool to 1 day improve physicians’ ability to diagnose and prognose LRTIs in children and improve health outcomes worldwide.
Supplementary information
Acknowledgements
The authors thank Nicole Dunnewold, MLIS and Diane Lorenzetti MLS, Ph.D. from the Health Sciences Library at the University of Calgary for their assistance with this review.
Author contributions
E.W. drafted the manuscript, collected the data, performed data analysis and data interpretation, revised the manuscript, and approved the final version of the manuscript to be published. B.M. conceptualized and designed the study, performed data interpretation, revised and critically reviewed the manuscript for important intellectual content, and approved the final version of the manuscript to be published. H.J.V. participated in data interpretation, revised and critically reviewed the manuscript for important intellectual content, and approved the final version of the manuscript to be published. G.C.T. conceptualized and designed the study, supervised data collection and data interpretation, revised and critically reviewed the manuscript for important intellectual content, and approved the final version of the manuscript to be published. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
No financial assistance was received in support of this manuscript.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed for this review.
Competing interests
B.M. and H.J.V. hold patent US 8969017 “Metabolite biomarkers for diagnosis and prognosis of pediatric septic shock.”
Ethics approval and consent to participate
Patient consent was not required as patients were not recruited for the completion of this manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41390-022-02162-0.
References
- 1.Troeger C, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the global burden of disease study 2015. Lancet Infect. Dis. 2017;17:1133–1161. doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boloursaz MR, et al. Epidemiology of lower respiratory tract infections in children. J. Compr. Pediatr. 2013;4:93–98. doi: 10.17795/compreped-10273. [DOI] [Google Scholar]
- 3.Elliott, S. P. & Ray, C. G. Viral infections of the lower respiratory tract. Pediatr. Respir. Med. 481–489 (2008).
- 4.Elemraid MA, et al. Accuracy of the interpretation of chest radiographs for the diagnosis of paediatric pneumonia. PLoS ONE. 2014;9:e106051. doi: 10.1371/journal.pone.0106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards G, et al. Predicting poor outcomes in children aged 1–12 with respiratory tract infections: a systematic review. PLoS ONE. 2021;16:e0249533. doi: 10.1371/journal.pone.0249533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emwas A-HM. The strengths and weaknesses in NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Metabonomics Methods Mol. Biol. 2015;1277:161–193. doi: 10.1007/978-1-4939-2377-9_13. [DOI] [PubMed] [Google Scholar]
- 7.Dieterle F, et al. NMR and MS methods for metabonomics. Methods Mol. Biol. 2011;691:385–415. doi: 10.1007/978-1-60761-849-2_24. [DOI] [PubMed] [Google Scholar]
- 8.Dunn WB, Broadhurst DI, Atherton HJ, Goodacre R, Griffin JL. Systems level studies of mammalian metabolomes: the roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011;40:387–426. doi: 10.1039/B906712B. [DOI] [PubMed] [Google Scholar]
- 9.Gowda GA, Djukovic D. Overview of mass spectrometry-based metabolomics: opportunities and challenges. Methods Mol. Biol. 2014;1198:3–12. doi: 10.1007/978-1-4939-1258-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama Y, Tamada Y, Tsugawa H, Bamba T, Fukusaki E. Novel strategy for non-targeted isotope-assisted metabolomics by means of metabolic turnover and multivariate analysis. Metabolites. 2014;4:722–739. doi: 10.3390/metabo4030722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Courant F, Antignac JP, Dervilly-Pinel G, Le Bizec B. Basics of mass spectrometry based metabolomics. Proteomics. 2014;14:21–22. doi: 10.1002/pmic.201400255. [DOI] [PubMed] [Google Scholar]
- 12.Slupsky CM, et al. Streptococcus pneumoniae and Staphylococcus aureus pneumonia induce distinct metabolic responses. J. Proteome Res. 2009;8:3029–3036. doi: 10.1021/pr900103y. [DOI] [PubMed] [Google Scholar]
- 13.Kaddurah-Daouk R, Kristal BS, Weinshilboum RM. Metabolomics: a global biochemical approach to drug response and disease. Annu. Rev. Pharmacol. Toxicol. 2008;48:653–683. doi: 10.1146/annurev.pharmtox.48.113006.094715. [DOI] [PubMed] [Google Scholar]
- 14.Atzei A, et al. Metabolomics in paediatric respiratory diseases and bronchiolitis. J. Matern. Fetal Neonatal Med. 2011;24(Suppl 2):59–62. doi: 10.3109/14767058.2011.607012. [DOI] [PubMed] [Google Scholar]
- 15.Han YY, et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112:793–799. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 16.Dessi A, et al. New diagnostic possibilities in systemic neonatal infections: metabolomics. Early Hum. Dev. 2014;90(Suppl 1):S19–S21. doi: 10.1016/S0378-3782(14)70007-6. [DOI] [PubMed] [Google Scholar]
- 17.Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin. Chim. Acta. 2015;451:46–64. doi: 10.1016/j.cca.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Ng S, et al. Precision medicine for neonatal sepsis. Front. Mol. Biosci. 2018;5:70. doi: 10.3389/fmolb.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA., Jr Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132:28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansbach JM, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch. Pediatr. Adolesc. Med. 2012;166:700–706. doi: 10.1001/archpediatrics.2011.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calvo C, et al. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three-year prospective study. Acta Paediatr. 2010;99:883–887. doi: 10.1111/j.1651-2227.2010.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voets S, van Berlaer G, Hachimi-Idrissi S. Clinical predictors of the severity of bronchiolitis. Eur. J. Emerg. Med. 2006;13:134–138. doi: 10.1097/01.mej.0000206194.85072.33. [DOI] [PubMed] [Google Scholar]
- 23.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Björkstén B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. doi: 10.1542/peds.95.4.500. [DOI] [PubMed] [Google Scholar]
- 24.Sigurs N, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 25.Adamko DJ, Saude E, Bear M, Regush S, Robinson JL. Urine metabolomic profiling of children with respiratory tract infections in the emergency department: a pilot study. BMC Infect. Dis. 2016;16:439. doi: 10.1186/s12879-016-1709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson DJ, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turi KN, et al. Using urine metabolomics to understand the pathogenesis of infant respiratory syncytial virus (RSV) infection and its role in childhood wheezing. Metabolomics. 2018;14:135. doi: 10.1007/s11306-018-1431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart CJ, et al. Respiratory syncytial virus and rhinovirus bronchiolitis are associated with distinct metabolic pathways. J. Infect. Dis. 2018;217:1160–1169. doi: 10.1093/infdis/jix680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart CJ, et al. Associations of nasopharyngeal metabolome and microbiome with severity among infants with bronchiolitis. A multiomic analysis. Am. J. Respir. Crit. Care Med. 2017;196:882–891. doi: 10.1164/rccm.201701-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart CJ, et al. Serum metabolome is associated with the nasopharyngeal microbiota and disease severity among infants with bronchiolitis. J. Infect. Dis. 2019;219:2005–2014. doi: 10.1093/infdis/jiz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golan-Tripto I, et al. Vitamin D deficiency in children with acute bronchiolitis: a prospective cross-sectional case- control study. BMC Pediatr. 2021;21:211. doi: 10.1186/s12887-021-02666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alakaş Y, Celiloğlu C, Tolunay O, Matyar S. The relationship between bronchiolitis severity and vitamin D status. J. Trop. Pediatr. 2021;67:fmab081. doi: 10.1093/tropej/fmab081. [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa K, et al. Circulating 25-hydroxyvitamin D, nasopharyngeal airway metabolome, and bronchiolitis severity. Allergy. 2018;73:1135–1140. doi: 10.1111/all.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasegawa K, et al. Serum 25-hydroxyvitamin D, metabolome, and bronchiolitis severity among infants-a multicenter cohort study. Pediatr. Allergy Immunol. 2018;29:441–445. doi: 10.1111/pai.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, et al. Serum metabolomic profiling reveals important difference between infants with and without subsequent recurrent wheezing in later childhood after RSV bronchiolitis. APMIS. 2021;129:128–137. doi: 10.1111/apm.13095. [DOI] [PubMed] [Google Scholar]
- 36.Barlotta A, et al. Metabolomic profiling of infants with recurrent wheezing after bronchiolitis. J. Infect. Dis. 2019;219:1216–1223. doi: 10.1093/infdis/jiy659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu, Z. et al. Metabolome subtyping of severe bronchiolitis in infancy and risk of childhood asthma. J. Allergy Clin. Immunol. 149, 102–112 (2021). [DOI] [PMC free article] [PubMed]
- 38.Castro-Rodriguez JA, Cifuentes L, Martinez FD. Predicting asthma using clinical indexes. Front. Pediatr. 2019;7:320–320. doi: 10.3389/fped.2019.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujiogi M, et al. Respiratory viruses are associated with serum metabolome among infants hospitalized for bronchiolitis: a multicenter study. Pediatr. Allergy Immunol. 2020;31:755–766. doi: 10.1111/pai.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujiogi M, et al. Integrated associations of nasopharyngeal and serum metabolome with bronchiolitis severity and asthma: a multicenter prospective cohort study. Pediatr. Allergy Immunol. 2021;32:905–916. doi: 10.1111/pai.13466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghidoni R, Caretti A, Signorelli P. Role of sphingolipids in the pathobiology of lung inflammation. Mediators Inflamm. 2015;2015:487508–487508. doi: 10.1155/2015/487508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296:L911–L920. doi: 10.1152/ajplung.00025.2009. [DOI] [PubMed] [Google Scholar]
- 43.Lara A, et al. Alterations of the arginine metabolome in asthma. Am. J. Respir. Crit. Care Med. 2008;178:673–681. doi: 10.1164/rccm.200710-1542OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 45.Torres A, et al. Pneumonia. Nat. Rev. Dis. Prim. 2021;7:25. doi: 10.1038/s41572-021-00259-0. [DOI] [PubMed] [Google Scholar]
- 46.Shah SN, Bachur RG, Simel DL, Neuman MI. Does this child have pneumonia?: the rational clinical examination systematic review. JAMA. 2017;318:462–471. doi: 10.1001/jama.2017.9039. [DOI] [PubMed] [Google Scholar]
- 47.Florin TA, et al. Biomarkers and disease severity in children with community-acquired pneumonia. Pediatrics. 2020;145:e20193728. doi: 10.1542/peds.2019-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas J, Pociute A, Kevalas R, Malinauskas M, Jankauskaite L. Blood biomarkers differentiating viral versus bacterial pneumonia aetiology: a literature review. Ital. J. Pediatr. 2020;46:4. doi: 10.1186/s13052-020-0770-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laiakis EC, Morris GA, Fornace AJ, Howie SR. Metabolomic analysis in severe childhood pneumonia in the Gambia, West Africa: findings from a pilot study. PLoS ONE. 2010;5:e12655. doi: 10.1371/journal.pone.0012655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053–1061. doi: 10.1542/peds.2011-1337. [DOI] [PubMed] [Google Scholar]
- 51.Del Borrello G, et al. New insights into pediatric community-acquired pneumonia gained from untargeted metabolomics: a preliminary study. Pediatr. Pulmonol. 2020;55:418–425. doi: 10.1002/ppul.24602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasegawa K, et al. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur. Respir. J. 2016;48:1329–1339. doi: 10.1183/13993003.00152-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fletcher MA, Schmitt HJ, Syrochkina M, Sylvester G. Pneumococcal empyema and complicated pneumonias: global trends in incidence, prevalence, and serotype epidemiology. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:879–910. doi: 10.1007/s10096-014-2062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Light RW. Parapneumonic effusions and empyema. Proc. Am. Thorac. Soc. 2006;3:75–80. doi: 10.1513/pats.200510-113JH. [DOI] [PubMed] [Google Scholar]
- 55.Chung CL, Chen CH, Sheu JR, Chen YC, Chang SC. Proinflammatory cytokines, transforming growth factor-beta1, and fibrinolytic enzymes in loculated and free-flowing pleural exudates. Chest. 2005;128:690–697. doi: 10.1016/S0012-3692(15)50413-3. [DOI] [PubMed] [Google Scholar]
- 56.Gayretli-Aydın ZG, et al. Evaluation of complicated and uncomplicated parapneumonic effusion in children. Turk. J. Pediatr. 2016;58:623–631. doi: 10.24953/turkjped.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 57.Chiu CY, et al. Metabolomic profiling of infectious parapneumonic effusions reveals biomarkers for guiding management of children with streptococcus pneumoniae pneumonia. Sci. Rep. 2016;6:24930. doi: 10.1038/srep24930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiu CY, et al. Metabolomics reveals anaerobic bacterial fermentation and hypoxanthine accumulation for fibrinous pleural effusions in children with pneumonia. J. Proteome Res. 2019;18:1248–1254. doi: 10.1021/acs.jproteome.8b00864. [DOI] [PubMed] [Google Scholar]
- 59.Bridy-Pappas AE, Margolis MB, Center KJ, Isaacman DJ. Streptococcus pneumoniae: description of the pathogen, disease epidemiology, treatment, and prevention. Pharmacotherapy. 2005;25:1193–1212. doi: 10.1592/phco.2005.25.9.1193. [DOI] [PubMed] [Google Scholar]
- 60.Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis. Pediatr. Crit. Care Med. 2013;14:686–693. doi: 10.1097/PCC.0b013e3182917fad. [DOI] [PubMed] [Google Scholar]
- 61.Weiss SL, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr. Crit. Care Med. 2020;21:e52–e106. doi: 10.1097/PCC.0000000000002198. [DOI] [PubMed] [Google Scholar]
- 62.Patel K, McElvania E. Diagnostic challenges and laboratory considerations for pediatric sepsis. J. Appl. Lab. Med. 2019;3:587–600. doi: 10.1373/jalm.2017.025908. [DOI] [PubMed] [Google Scholar]
- 63.Hazwani TR, et al. Association between culture-negative versus culture-positive sepsis and outcomes of patients admitted to the pediatric intensive care unit. Cureus. 2020;12:e9981. doi: 10.7759/cureus.9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mickiewicz B, Vogel HJ, Wong HR, Winston BW. Metabolomics as a novel approach for early diagnosis of pediatric septic shock and its mortality. Am. J. Respir. Crit. Care Med. 2013;187:967–976. doi: 10.1164/rccm.201209-1726OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ambroggio L, et al. Emerging biomarkers of illness severity: urinary metabolites associated with sepsis and necrotizing methicillin-resistant Staphylococcus aureus pneumonia. Pharmacotherapy. 2017;37:1033–1042. doi: 10.1002/phar.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mickiewicz B, et al. Development of metabolic and inflammatory mediator biomarker phenotyping for early diagnosis and triage of pediatric sepsis. Crit. Care. 2015;19:320. doi: 10.1186/s13054-015-1026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mickiewicz B, et al. Biomarker phenotype for early diagnosis and triage of sepsis to the pediatric intensive care unit. Sci. Rep. 2018;8:16606. doi: 10.1038/s41598-018-35000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grauslys A, et al. Title NMR-based metabolic profiling provides diagnostic and prognostic information in critically ill children with suspected infection. Sci. Rep. 2020;10:20198. doi: 10.1038/s41598-020-77319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li G-B, et al. Plasma metabolic profiling of pediatric sepsis in a chinese cohort. Front. Cell Dev. Biol. 2021;9:643979. doi: 10.3389/fcell.2021.643979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization. WHO coronavirus (Covid-19) dashboard. https://covid19.who.int (2022).
- 71.de Souza, T. H., Nadal, J. A., Nogueira, R. J. N., Pereira, R. M. & Brandão, M. B. Clinical manifestations of children with COVID-19: a systematic review. Pediatr. Pulmonol. 55, 1892–1899 (2020). [DOI] [PMC free article] [PubMed]
- 72.Rabinowicz S, Leshem E, Pessach IM. COVID-19 in the pediatric population-review and current evidence. Curr. Infect. Dis. Rep. 2020;22:29. doi: 10.1007/s11908-020-00739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang C, et al. Multi-omic profiling of plasma reveals molecular alterations in children with COVID-19. Theranostics. 2021;11:8008–8026. doi: 10.7150/thno.61832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu D, et al. Plasma metabolomic and lipidomic alterations associated with COVID-19. Natl Sci. Rev. 2020;7:1157–1168. doi: 10.1093/nsr/nwaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shu T, et al. Plasma proteomics identify biomarkers and pathogenesis of COVID-19. Immunity. 2020;53:1108.e5–1122.e5. doi: 10.1016/j.immuni.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rist MJ, et al. Influence of freezing and storage procedure on human urine samples in nmr-based metabolomics. Metabolites. 2013;3:243–258. doi: 10.3390/metabo3020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamlage B, et al. Quality markers addressing preanalytical variations of blood and plasma processing identified by broad and targeted metabolite profiling. Clin. Chem. 2014;60:399–412. doi: 10.1373/clinchem.2013.211979. [DOI] [PubMed] [Google Scholar]
- 78.Ang JE, et al. Identification of human plasma metabolites exhibiting time-of-day variation using an untargeted liquid chromatography-mass spectrometry metabolomic approach. Chronobiol. Int. 2012;29:868–881. doi: 10.3109/07420528.2012.699122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kastenmüller G, Raffler J, Gieger C, Suhre K. Genetics of human metabolism: an update. Hum. Mol. Genet. 2015;24:R93–R101. doi: 10.1093/hmg/ddv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pinu FR, Goldansaz SA, Jaine J. Translational metabolomics: current challenges and future opportunities. Metabolites. 2019;9:108. doi: 10.3390/metabo9060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Annese VF, et al. A monolithic single-chip point-of-care platform for metabolomic prostate cancer detection. Microsyst. Nanoeng. 2021;7:21. doi: 10.1038/s41378-021-00243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McAllister DA, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob. Health. 2019;7:e47–e57. doi: 10.1016/S2214-109X(18)30408-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed for this review.