Abstract
Background and Aims
A rapid increase in the use of telemedicine for delivering healthcare has occurred since the onset of the Covid-19 pandemic. There is evidence for using telemedicine to facilitate cancer care delivery for patients with hepatocellular carcinoma (HCC). Examining how telemedicine can be used to communicate multidisciplinary tumor board (MTB) recommendations for HCC has not been studied. This study has two specific aims: (1) to evaluate the patient perspective of the MTB review process and identify best strategies for communicating treatment recommendations for HCC and (2) to pilot test a telemedicine intervention following MTB review to assess patient feasibility and satisfaction with using telemedicine to facilitate treatment decision-making and treatment referral.
Methods
We conducted a mixed-methods study. First, semi-structured qualitative interviews were conducted among patients diagnosed with HCC who were discussed in MTB review at one of three VA Medical Centers (VAMC). We collected information about the MTB process from the patient perspective and identified strategies for improving communication and delivery of care. Rapid qualitative analysis was used to inform intervention development. Using our qualitative data, a MTB telemedicine pilot intervention was developed and implemented to assess the feasibility of using this approach for patients with HCC.
Results
Almost all patients (94%) in the pilot study would recommend telemedicine to other patients with HCC, and half of the patients (50%) preferred telemedicine over in-person visits. Many patients (81%) found communication through telemedicine an acceptable platform to deliver difficult cancer information. Overall, patients felt they understood their treatment recommendations and found them clear and useful. Further, patients reported that they enjoyed being included in the decision-making process and appreciated being able to have family members easily join them for the telemedicine visit.
Conclusions
Using telemedicine to communicate treatment recommendations following MTB review was found to be feasible and an acceptable alternative to an in-person visit for patient with HCC. Future studies could include expanding this approach for communicating MTB recommendations to patients with other types of cancers.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12029-022-00844-w.
Keywords: Liver cancer, Multidisciplinary tumor board, Telemedicine, VA
Introduction
A rapid increase in the use of telemedicine for delivering healthcare has occurred since the onset of the Covid-19 pandemic. The use of telemedicine for patient care was previously met with a number of barriers, including reimbursement, physician usability and comfort, and a steep learning curve [1–4]. Telemedicine is now widely utilized and offers several advantages for patients, including eliminating travel time and associated costs, reducing time off from work, and increased access to care [5–10]. Telemedicine has also demonstrated high levels of satisfaction by physicians including reducing patient no-show rates, lowering operating costs, and increasing efficiency in the utilization of resources [11–15]. Before the pandemic, telemedicine for oncology services was uncommon [16]. Given the need to continue providing oncology care in the midst of a pandemic, access to and coverage for telemedicine services have been expanded. Fifty states and Washington, DC now provide better reimbursement for telemedicine services [15–20].
Hepatocellular cancer (HCC) is a complex cancer associated with a poor prognosis [21]. Delays for HCC diagnosis have been reported in nearly half of patients, and treatment is often underutilized in patients with HCC [22, 23]. Due to its difficult diagnosis and management treatment algorithm, multidisciplinary care for these patients is often needed. There is a growing body of evidence for using telemedicine to facilitate cancer care delivery for patients with hepatocellular carcinoma (HCC). Recent studies have shown that telemedicine is an effective way to remotely evaluate HCC patients for liver transplantation [24, 25]. Further, virtual multidisciplinary tumor boards (MTB), which involve a team of specialists providing recommendations for care, have been shown to be effective with completing HCC treatment evaluations in a shorter amount of time [26]. However, examining how telemedicine can be used to communicate MTB treatment recommendations to patients with HCC has not been studied.
To fill this knowledge gap, we conducted a mixed-methods study among patients with HCC who underwent review by MTB. Our study had two specific aims: (1) to evaluate the patient perspective of the MTB review process and identify best strategies for communicating treatment recommendations for HCC and (2) to pilot test a telemedicine intervention following MTB review to assess feasibility and satisfaction with using telemedicine to facilitate treatment decision-making and treatment referral.
Methods
Study Design
We performed a mixed-methods study, specifically a sequential explanatory design, among patients with HCC who were diagnosed in the Department of Veterans Affairs Medical Center and underwent review by a MTB. First, we conducted semi-structured qualitative interviews among patients diagnosed with HCC (N = 30) at three Veterans Affairs Medical Centers (VAMC) from 2017 to 2018 who were recently evaluated by the HCC MTB to collect information about the MTB process from the patient perspective and identify strategies for improving communication and delivery of care. Then, based on data collected from our patient qualitative interviews, we used a participatory framework and developed and implemented a pilot intervention among a different cohort of patients with HCC (N = 16) at one of the three sites, the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC), from 2019 to 2020 to assess feasibility and satisfaction with telemedicine follow-up visits following MTB review.
Participants
For both components of the study, patients were recruited using convenience sampling and enrolled based on availability and accessibility. HCC was confirmed by the presence of at least one positive imaging within 90 days prior to MTB review. Eligible patients met the following criteria: (1) confirmed HCC diagnosis by imaging or biopsy; (2) received care at a VAMC; and (3) evaluated by MTB for HCC treatment recommendations. For the intervention component, we excluded patients who did not have Internet access.
Part 1: Qualitative Study Among Patients with HCC Who Were Seen by MTB
Study Procedures
Patients at each of the three sites were initially identified by medical record review. Prior to recruitment, study staff confirmed patient eligibility with their hepatologist or primary physician. Eligible patients were recruited by telephone.
For those who agreed to participate, the consent process was completed by phone, and they were scheduled for an interview. Semi-structured qualitative interviews were conducted by project coordinators with qualitative interviewing experience and lasted approximately 30–45 min. Most interviews were completed by phone; only three interviews were completed in person due to patient preference. All interviews were audio-recorded and transcribed. Based on recommendations from a qualitative methodologist, the sample size for the qualitative component of the study was determined to achieve data saturation.
Data Analysis
We used rapid qualitative analysis to identify key themes across sites, synthesize data by interview domains, and inform intervention development [27, 28]. Transcript summary templates that reflected and condensed major topics of the interview guide into key domains were developed. Two qualitative analysts independently reviewed all data and met regularly to compare summaries and resolve discrepancies.
To optimize validity, a qualitative methodologist reviewed a subset of the analysts’ work for accuracy. Transcript summary domains were placed within a matrix to review findings across the dataset. Domain summaries were created that identified relevant themes across the three sites and sub-themes within sites that informed tumor board intervention. Patient and clinical domains and themes were reviewed by an expert panel. Key focus areas were identified for the pilot intervention in patient experiences, preferences, and expectations.
Part 2: Pilot Study of MTB Telemedicine Intervention for HCC
Study Intervention
Prior to the study intervention, information regarding MTB recommendations and next steps was communicated with patients primarily through telephone, and sometimes during in-person clinical visits. Information was generally conveyed by either a physician assistant, nurse practitioner, or physician.
Results from our qualitative study were used to inform and develop our pilot intervention. Our pilot intervention consisted of a structured protocol to schedule a telemedicine visit with a hepatologist within 7 days following MTB review, a telemedicine visit with the HCC patient to discuss MTB recommendations and next steps using a standardized format, and a direct referral to a specialist for treatment immediately following the telemedicine visit. To support the long-term sustainability of the intervention, existing clinic equipment for live video technology, such as computers and video cameras, was used to deliver the intervention.
Study Procedures
We identified patients with HCC who were scheduled for MTB review and evaluated the medical record for preliminary eligibility. We used the convenience sampling method, and our target enrollment was 20 patients based on recommendations from a qualitative methodologist. This a priori sample size was selected based on the complexity and desired level of depth for our research questions. For patients who met the initial eligibility criteria, we contacted their primary physician or hepatologist to confirm their eligibility for the study. Patients were then contacted by telephone and those who consented to participate in the pilot intervention were scheduled for a telemedicine visit with their hepatologist within 7 days following the date of their MTB review. Concurrently, an appointment was scheduled with the study coordinator to complete the patient survey by phone. The survey focused on key study domains, including patient satisfaction and preference, use of technology, ability to communicate with the physician, and appropriateness of receiving healthcare information using telemedicine. The approximate time to complete the survey was 30 min and patients received a $20 incentive.
Intervention Protocol
Approximately 24 h prior to the telemedicine visit, patients were contacted by phone to remind them about the visit, answer questions, and address any technology-related issues. VA Video Connect (VVC), a platform that allows Veterans and their caregivers to quickly and easily meet with VA healthcare physicians through live video on any computer, tablet, or mobile device with an Internet connection, was used for the telemedicine follow-up visit [29]. Patients were not provided any tutorials, coaching, or additional information on how to use VVC.
The structure and content of the telemedicine visit did not differ from the telephone and in-person clinical visits. During the telemedicine visit, the hepatologist and patient discussed the following topics: (1) overview of the MTB discussion and treatment recommendations, (2) benefits and risks associated with treatment alternatives, and (3) patient preferences related to treatment. The goal of the visit was to decide on a treatment plan that was aligned with patient preferences and to determine next steps. Immediately following the visit, the physician entered specialty referrals into the electronic health system for follow-up by the tumor board coordinator, who then communicated with the patient to facilitate coordination of appointments.
Data Collection
On the day following the telemedicine visit, the study coordinator contacted the patient to complete the survey. Information related to patient clinical characteristics and HCC treatment was ascertained from the VA electronic health record at the time of the intervention and at 6 months following the telemedicine visit. Electronic health record information was obtained from the Computerized Patient Record System (CPRS) at the MEDVAMC in Houston, TX. Patient clinical characteristics included HCC stage at the time of the tumor board review, cirrhosis status, and presence of liver-related comorbidities. Information on referrals for HCC treatment and receipt of treatment was captured at 6 months following the telemedicine visit. Patients living in rural areas were identified using the 2013 Rural–Urban Continuum Codes [30]. Patient demographics such as age, gender, race/ethnicity, geographic area, and clinical characteristics such as liver disease etiology were also obtained.
Data Analysis
Descriptive analyses of survey data and information collected from CPRS were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC). Statistically significant differences in proportions were determined using the chi-square and continuity-adjusted chi-square tests to prevent overestimation of statistical significance for small data. A significance level of p < 0.05 was used as an indicator of statistically significant differences. The study protocol was approved by the Baylor College of Medicine Institutional Review Board and the Michael E. DeBakey VA Research & Development Committee.
Results
Rapid Qualitative Analysis
Key domains obtained from the patient interviews included (1) patient confidence in tumor board recommendations; (2) patient understanding of tumor board recommendations; (3) communication of tumor board recommendations to patients; and (4) patient concerns about receiving healthcare. Among patients who recalled discussions with their physicians about tumor board recommendations (n = 23), the majority felt comfortable, hopeful, and more confident that a team of physicians discussed their case. The predominant context of this communication was in person, which patients preferred over telephone discussion. Most patients felt the recommendations were communicated in understandable, lay language. However, not all patients interviewed felt they had a say in treatment decisions after their case was discussed at MTB, but most were satisfied with treatment results and were confident in their physicians’ recommendations.
When asked about the preferred method of communication in patient interviews, patients did not endorse using secure messaging through the electronic patient portal as a mechanism for providing tumor board recommendations. Patients stated they do not actively use the electronic patient portal and discussed having security concerns regarding their health information on the Internet. Patients also mentioned that letters providing tumor board recommendations are not a preferred form of communication. They preferred a specialist, such as an oncologist, a hepatologist, or a physician, from the transplant team to communicate tumor board recommendations with them directly.
Among patients who recalled that physicians discussed their case at MTB, a majority felt reassured they were receiving good care after learning about these discussions. Among patients who did not recall MTB discussions, these patients also felt reassured they were receiving good care after learning about MTB discussions and preferred receiving tumor board recommendations in person. These patients had mixed feelings about using secure messaging through the online patient portal as the primary form of communication for their cancer care. Themes and illustrative quotes identified from the rapid qualitative analysis are reported in Table 1.
Table 1.
Summary of patient interview domains, themes, and illustrative quotes
| Domain | Theme | Illustrative quote |
|---|---|---|
| Patients’ confidence in MTB | 1. Patients said they felt comfortable and confident and hopeful with the treatment plan when their case was discussed by a group of physicians | 1. “It made me comfortable that they came up with three choices and that it was discussed by more than one doctor.” |
| 2. Patients said they felt they were receiving better care because of a multidisciplinary viewpoint when their case was discussed by a group of physicians | 2. “I feel that a group of doctors is the way to go because they bring everything to the table, they can see what is the best for the Veteran to get back to health, and they can discuss my how my other problems may affect which treatment plan.” | |
| Patient understanding of MTB recommendations | 1. Patients felt MTB recommendations were very clear | 1. “They told me what to expect, what was going to happen, how the procedure was going to work, how the radiation was going to get administered and how it was going to affect the tumor.” |
| 2. Patients found MTB recommendations helpful | 2. “The information they gave me was about the treatment and about having cancer of the liver, and I found it useful.” | |
| 3. Patients liked that they were able to ask a lot of questions about the different treatment options following MTB recommendations | 3. “If the doctors have the possibility of different options, they should explain to you all the different options…” | |
| 4. Patients wanted to be included in the treatment decision-making process | 4. “I definitely felt I was part of the decision-making process for my treatment. If I had any questions or concerns, I would address it prior to the treatment.” | |
| Communication of MTB recommendations to patients | 1. There is variation in the method of communication of MTB recommendations | 1. “During the course of my treatments they have delivered the results in different various ways by either telling me verbally, in written form, or a phone call. They have also shown me images on the computer.” |
| 2. Patients expressed they would have liked to receive MTB recommendations in person and face-to-face | 2. “I think MTB recommendations should at least have a face-to-face consultation.” | |
| 3. Patients reported mixed feelings about using the patient portal to communicate MTB recommendations | 3. “I don’t think MTB recommendations should be on my [patient portal]; they should be kept in person with the doctor.” | |
| Patient concerns about receiving healthcare | 1. Patients emphasized concerns about adverse side effects from treatment recommended by MTB | 1. “I was concerned about how sick the procedure would make me…” |
| 2. Patients reported spending a significant time on transportation for in-person visits | 2. “I live in Port Arthur and now I’ve been to Houston about 10 times, hey, I would just rather [have] that information, instead of go all the way back to my doctor.” | |
| 3. Patients expressed concerns about wait-times for scheduling an in person appointment | 3. “Every time I made an appointment to see somebody it was like a two month wait.” |
MTB multidisciplinary tumor board
Pilot Intervention Results
A total of 16 HCC patients had a telemedicine visit after their MTB review and completed the patient survey. All patients were male, and more than half were 50 to 65 years old and living in metropolitan areas. Half of patients in this study were White non-Hispanic. Most patients had cirrhosis and hepatitis C virus and were within Milan criteria. Demographic features and clinical characteristics of these patients are reported in Table 2. Approximately 82% of patients reported they had prior experience using video conferencing for healthcare visits. When patients were asked about their personal comfort using telemedicine after the intervention, all but one patient (n = 15) rated their comfort as “excellent” or “very good” or “good.”
Table 2.
Patient characteristics for MTB and telemedicine follow-up visit (N = 16)
| Variable | Percentage |
|---|---|
| Gender (male) | 100.0 |
| Age at HCC diagnosis | |
| 50–65 years | 56.3 |
| > 65 years | 43.8 |
| Race/ethnicity | |
| White non-Hispanic | 50.0 |
| Other | 50.0 |
| Geographic area | |
| Metropolitan counties | 81.2 |
| Nonmetropolitan counties | 18.8 |
| Liver disease etiology | |
| Cirrhosis | 75.0 |
| Hepatitis C | 68.8 |
| Diabetes | 50.0 |
| Milan criteria (yes) | 68.8 |
| Child–Pugh-Turcotte | |
| Class A | 81.2 |
| Class B | 18.8 |
| Class C | 0.0 |
Almost all patients (94%) felt comfortable asking questions during their telemedicine visit for MTB recommendations, and 81% felt the length of time to convey questions or concerns while speaking with the hepatologist was either “excellent” or “very good.” Nearly 70% of patients were “completely satisfied” with the care they received during the telemedicine visit, and a quarter of patients reported they were “somewhat satisfied.” When asked about the visual quality of their telemedicine visit, half of the patients reported the visual quality was “excellent.” Overall, patients felt the quality of the telemedicine visit as a whole was “excellent,” “very good,” or “good,” and 88% of patients found telemedicine to be very efficient (n = 14).
Approximately 25% of patients reported they traveled to MEDVAMC more than 5 times in the past 12 months for in-person follow-up visits before the survey. Almost half of the patients (44%) traveled 3 to 5 times to MEDVAMC in the past 12 months for in-person follow-up visits. We found that 38% of patients spent 1 to 3 h traveling for in-person follow-up visits and almost half of patients (44%) spent more than 3 h traveling. Half of the patients received VA travel pay within 12 months before their telemedicine visit. This program reimburses for mileage and other travel expenses to and from approved healthcare appointments within 12 months before their telemedicine visit.
Most patients (75%) stated they felt they understood the tumor board recommendations and their treatment plan after the telemedicine follow-up visit. Approximately 70% of patients felt confident in their physicians’ treatment recommendations, while 31% were unsure. When patients were asked how confident they were in receiving the treatment discussed during the visit, 69% of patients reported they were confident.
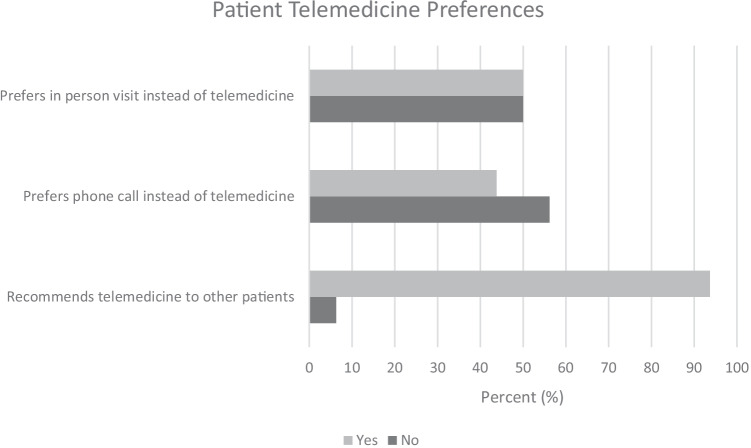
Half of the patients reported that a family member, friend, or relative was able to join them during the telemedicine visit. Regarding patient telemedicine preferences, half of the patients said they prefer in-person visits instead of telemedicine. When asked if patients preferred a phone call instead of a video visit, more than half (56%) said they preferred telemedicine. When patients were asked whether they would recommend telemedicine to other patients with HCC who were reviewed by MTB, almost all patients (94%; n = 15) said they would recommend telemedicine to other patients (Fig. 1). Finally, most patients (81%) reported telemedicine was an acceptable form of delivering bad news.
Fig. 1.
Patient telemedicine preferences (N = 16)
When assessing receipt of treatment, almost all patients (94%) received the treatment recommended by MTB. Regarding types of recommended treatment received, 38% of patients received curative or local–regional treatment (surgical resection, microwave ablation, or transcatheter arterial chemoembolization (TACE)), 38% of patients received active surveillance with follow-up magnetic resonance imaging (MRI), and 19% received palliative care. One patient sought and received treatment outside of the VA. Of the patients that received the recommended treatment, only one patient had a 3-month delay in treatment receipt due to Covid-19 concerns.
Discussion
The Covid-19 pandemic has brought transformative changes in healthcare, and telemedicine has now emerged as a necessary clinical innovation to provide expanded access to specialty health services. We found that almost all patients would recommend telemedicine to other patients with HCC, and half of the patients preferred telemedicine over in-person visits. Both findings in our rapid qualitative analysis and pilot intervention are very similar to findings in the literature, where numerous studies report high patient satisfaction with telemedicine across various specialty health areas [9, 12, 31–34]. We believe this is due to conveniences related to reduced or eliminated travel distance, frequency, and associated costs, which is required for conventional in-person clinic visits. Reduced travel time and associated travel costs have been consistently reported in the literature as the leading reason for patient satisfaction with telemedicine [8, 10, 34–36].
Previous studies have indicated that physician and patient communication regarding news about a life-threatening cancer diagnosis and subsequent treatment options should be done face-to-face and in person, since this form of communication is considered more compassionate in terms of psychosocial context [37–40]. Loss of non-verbal communication, such as body language and facial expressions, makes it difficult for physicians to show empathy and support, which is important to patients when receiving bad news [41, 42]. However, our pilot intervention suggests that a majority of patients found communication through telemedicine an acceptable platform to deliver difficult cancer information. This is likely due to the video component of the telemedicine visits that allows physicians to express non-verbal cues during the visit. Patients felt that using secure messaging through the electronic patient portal to share MTB recommendations was not acceptable. Patients expressed that they valued shared decision-making with their physician regarding treatment decisions, which has been reported in the literature [37, 43–45].
Previous studies suggested that the presence of a family member who is able to be present to provide emotional support when the patient received bad news is very important and affects health outcomes, including cancer outcomes [37, 39, 46–49]. In our study, half of the patients reported that a family member, friend, or relative was able to join them during the telemedicine visit. Telemedicine allows the flexibility for family members whether at home or living far away to join the telemedicine visit, participate in the discussion, and provide emotional support [46, 49].
We found that many patients in both components of our study felt they understood their MTB treatment recommendations, which is contrary to findings in literature [34, 50–52]. Patients in our study reported that their treatment recommendations from MTB were clear and useful, and that they liked being included in the decision-making process. In addition, most patients in our study were confident in their physicians’ recommendations.
We suggest further research in the role of expanding patient-centered care and MTB treatment decision-making, so patients can be empowered to make better-informed treatment decisions. This area of research should be expanded to broader populations, including other cancers.
Limitations
We acknowledge the limitations of our study. For both components of the study, selection bias may have subsequently affected patient satisfaction with MTB and acceptability of telemedicine services since we used a convenience sampling approach to recruit patients. In addition, the sample size for our pilot intervention survey was small, and we did not encounter any females that were eligible to enroll, which could reduce the validity and generalizability of our findings. Unfortunately, only 1.5% of Veterans in the USA are female [53]. There is also a gender disparity in hepatocellular carcinoma, where men have a much higher prevalence rate compared to women [54]. Future research investigating how telemedicine can be used to communicate MTB treatment recommendations to patients with HCC is needed in a larger, broader population.
We excluded patients who did not have Internet access and understand this could create biased estimates, which could have impacted findings. Research has shown that those without Internet access have considerable demographic and socioeconomic differences [55–57]. People who do not have Internet access are more likely to be 65 years and older, black, and are more likely to live alone [55–57]. Further, this population is much less affluent and has less education [55–57]. It is possible that these patients who were excluded from the study may have disliked receiving MTB treatment recommendations through a telemedicine visit for various reasons (i.e., high costs of paying for the Internet and obtaining devices needed for a telemedicine visit, difficulty hearing or seeing, problems with speaking or making oneself understood) [55–57].
Lastly, our pilot intervention was delivered at only one VA facility. Future studies are needed to understand whether findings from this study are generalizable in other settings.
Conclusion
To our knowledge, this is the first study to report how telemedicine can be used to communicate MTB treatment recommendations to patients with HCC in a standardized format. Patients are open to telemedicine as a form of communication for complex cancer information, whether MTB treatment recommendations or even if patients are receiving bad news. Future research is needed in broader populations to facilitate patient-centered care regarding MTB treatment decision-making, so patients can be empowered to be engaged in their healthcare and participate in shared decision-making.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contribution
Conception and design: Drs. Davila, Sada, and Hernaez. Analysis and interpretation of the data: Drs. Choi, Davila, Sada, Hernaez, and Sansgiry. Drafting of the article: Drs. Choi, Davila, and Sada. Final approval of the article: Drs. Choi, Davila, Sada, Hernaez, Kaplan, and Taddei. Administrative, technical, or logistic support: Jason Aguilar and Michael Strayhorn. Collection and assembly of data: Drs. Davila, Sada, Hernaez, Kaplan, Taddei, and Sansgiry.
Funding
This project was supported by the VA Health Services Research & Development Service (IIR-14–101, PI: J. Davila) and the facilities and resources of the Center for Innovations in Quality, Effectiveness and Safety (CIN 13–413) and the Michael E. DeBakey Veterans Affairs Medical Center, Houston, TX, USA. The views expressed in this article are those of the authors and do not necessarily represent the views of the funding institutions.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zachrison KS, Boggs KM, Hayden EM, Espinola JA, Camargo CA. Understanding barriers to telemedicine implementation in rural emergency departments. Ann Emerg Med. 2020;75(3):392–399. doi: 10.1016/j.annemergmed.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 2.Foster MV, Sethares KA. Facilitators and barriers to the adoption of telehealth in older adults: an integrative review. CIN - Comput Informatics Nurs. 2014;32(11):523–533. doi: 10.1097/CIN.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 3.Angood PB. Telemedicine, the Internet, and world wide web: overview, current status, and relevance to surgeons. World J Surg. 2001;25(11):1449–1457. doi: 10.1007/s00268-001-0130-4. [DOI] [PubMed] [Google Scholar]
- 4.Sauers-Ford HS, Hamline MY, Gosdin MM, et al. Acceptability, usability, and effectiveness: a qualitative study evaluating a pediatric telemedicine program. Acad Emerg Med. 2019;26(9):1022–1033. doi: 10.1111/acem.13763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gunter RL, Fernandes-Taylor S, Rahman S, et al. Feasibility of an image-based mobile health protocol for postoperative wound monitoring. J Am Coll Surg. 2018;226(3):277–286. doi: 10.1016/j.jamcollsurg.2017.12.013.Feasibility. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunter RL, Chouinard S, Fernandes-taylor S, et al. Current use of telemedicine for post-discharge surgical care: a systematic review. J Am Coll Surg. 2016;222(5):915–927. doi: 10.1016/j.jamcollsurg.2016.01.062.Current. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goedeke J, Ertl A, Zöller D, Rohleder S, Muensterer OJ. Telemedicine for pediatric surgical outpatient follow-up: a prospective, randomized single-center trial. J Pediatr Surg. 2019;54(1):200–207. doi: 10.1016/j.jpedsurg.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Qaderi SM, Vromen H, Dekker HM, Stommel MWJ, Bremers AJA, de Wilt JHW. Development and implementation of a remote follow-up plan for colorectal cancer patients. Eur J Surg Oncol. 2020;46(3):429–432. doi: 10.1016/j.ejso.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Le LB, Rahal HK, Viramontes MR, Meneses KG, Dong TS, Saab S. Patient satisfaction and healthcare utilization using telemedicine in liver transplant recipients. Dig Dis Sci. 2019;64(5):1150–1157. doi: 10.1007/s10620-018-5397-5. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen M, Waller M, Pandya A, Portnoy J. A review of patient and provider satisfaction with telemedicine. Curr Allergy Asthma Rep. 2020;20(11). 10.1007/s11882-020-00969-7. [DOI] [PMC free article] [PubMed]
- 11.Kahn JM. Virtual Visits — Confronting the challenges of telemedicine. N Engl J Med. 2015;372(18):1684–1685. doi: 10.1056/NEJMp1500533. [DOI] [PubMed] [Google Scholar]
- 12.Staicu ML, Holly AM, Conn KM, Ramsey A. The use of telemedicine for penicillin allergy skin testing. J Allergy Clin Immunol Pract. 2018;6(6):2033–2040. doi: 10.1016/j.jaip.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 13.Drerup B, Espenschied J, Wiedemer J, Hamilton L. Reduced no-show rates and sustained patient satisfaction of telehealth during the COVID-19 Pandemic. Telemed e-Health. 2021;00(00):1–7. doi: 10.1089/tmj.2021.0002. [DOI] [PubMed] [Google Scholar]
- 14.Greenhalgh T, Wherton J, Shaw S, Morrison C. Video consultations for Covid-19. BMJ. 2020;368(March):1–2. doi: 10.1136/bmj.m998. [DOI] [PubMed] [Google Scholar]
- 15.Kichloo A, Albosta M, Dettloff K, et al. Telemedicine, the current COVID-19 pandemic and the future: a narrative review and perspectives moving forward in the USA. Fam Med Community Heal. 2020;8(3):1–9. doi: 10.1136/fmch-2020-000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kircher SM, Mulcahy M, Kalyan A, Weldon CB, Trosman JR, Benson AB. Telemedicine in oncology and reimbursement policy during COVID-19 and beyond. J Natl Compr Canc Netw. 2020:1–7. 10.6004/jnccn.2020.7639. [DOI] [PubMed]
- 17.Gross GN. Coding telemedicine visits for proper reimbursement. Curr Allergy Asthma Rep. 2020;20(11). 10.1007/s11882-020-00970-0. [DOI] [PMC free article] [PubMed]
- 18.Center for Connected Health Policy. State Telehealth Laws and Medicaid Program Policies. West Sacramento, CA. 2022. https://www.cchpca.org/2022/05/Spring2022_ExecutiveSummaryfinal.pdf. Accessed 28 June 2022.
- 19.Volk J, Palanker D, O’Brien M, Goe CL. States’ actions to expand telemedicine access during COVID-19 and future policy considerations. Washington, DC; 2021. 10.26099/r95z-bs17.
- 20.Centers for Medicare & Medicaid Services. Telehealth For providers: what you need to know. U.S. Department of Health and Human Services. https://www.cms.gov/files/document/telehealth-toolkit-providers.pdf. Published 2021. Accessed 27 Sept 2021.
- 21.Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(Supplement 4):14–22. doi: 10.1634/theoncologist.2010-S4-14. [DOI] [PubMed] [Google Scholar]
- 22.Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther. 2013;38(7):703–712. doi: 10.1111/apt.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi DT, Davila JA, Sansgiry S, et al. Factors associated with delay of diagnosis of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konjeti VR, Heuman D, Bajaj JS, et al. Telehealth-based evaluation identifies patients who are not candidates for liver transplantation. Clin Gastroenterol Hepatol. 2019;17(1):207–209.e1. doi: 10.1016/j.cgh.2018.04.048. [DOI] [PubMed] [Google Scholar]
- 25.Binu JV, Love E, Dahmarn B, et al. Use of telehealth expedites evaluation and listing of patients referred for liver transplantation. Clin Gastroenterol Hepatol. 2020;18(8):1822–1830. doi: 10.1016/j.cgh.2019.12.021.Use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salami AC, Barden GM, Castillo DL, et al. Establishment of a regional virtual tumor board program to improve the process of care for patients with hepatocellular carcinoma. J Oncol Pract. 2015;11(1):e66–e74. doi: 10.1200/jop.2014.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor B, Henshall C, Kenyon S, Litchfield I, Greenfield S. Can rapid approaches to qualitative analysis deliver timely, valid findings to clinical leaders? A mixed methods study comparing rapid and thematic analysis. BMJ Open. 2018;8(10):5–7. doi: 10.1136/bmjopen-2017-019993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palinkas LA, Mendon SJ, Hamilton AB. Innovations in mixed methods evaluations. Annu Rev Public Health. 2019;40(1):423–442. doi: 10.1146/annurev-publhealth-040218-044215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Department of Veterans Affairs. VA Video Connect. https://mobile.va.gov/app/va-video-connect. Published 2021. Accessed 3 June 2021.
- 30.Economic Research Service. Rural-Urban Continuum Codes. U.S. Department of Agriculture. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/. Published 2020. Accessed 8 Sept 2021.
- 31.Piao C, Terrault NA, Sarkar S. Telemedicine: an evolving field in hepatology. Hepatol Commun. 2019;3(5):716–721. doi: 10.1002/hep4.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kane LT, Thakar O, Jamgochian G, et al. The role of telehealth as a platform for postoperative visits following rotator cuff repair: a prospective, randomized controlled trial. J Shoulder Elb Surg. 2020;29(4):775–783. doi: 10.1016/j.jse.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Brearly TW, Goodman CS, Haynes C, McDermott K, Rowland JA. Improvement of postinpatient psychiatric follow-up for veterans using telehealth. Am J Heal Pharm. 2020;77(4):288–294. doi: 10.1093/ajhp/zxz314. [DOI] [PubMed] [Google Scholar]
- 34.Donelan K, Barreto EA, Sossong S, et al. Patient and clinician experiences with telehealth for patient follow-up care. Am J Manag Care. 2019;25(1):40–44. [PubMed] [Google Scholar]
- 35.Huber M, Van Vliet M, Giezenberg M, et al. Towards a “patient-centred” operationalisation of the new dynamic concept of health: a mixed methods study. BMJ Open. 2016;6(1):1–11. doi: 10.1136/bmjopen-2015-010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buck S. Nine human factors contributing to the user acceptance of telemedicine applications: a cognitive-emotional approach. J Telemed Telecare. 2009;15(2):55–58. doi: 10.1258/jtt.2008.008007. [DOI] [PubMed] [Google Scholar]
- 37.Phyllis N. Butow, Kazemi JN, Beeney LJ, Griffin A-M, Dunn SM, Tattersall MHN. When the diagnosis is cancer: patient communication experiences and preferences. Cancer. 1996;77(12):2630–2637. 10.1002/(SICI)1097-0142(19960615)77:12%3C2630::AID-CNCR29%3E3.0.CO;2-S. [DOI] [PubMed]
- 38.Maguire P, Faulkner A. Communicate with cancer patients: 1. Handling bad news and difficult questions. BMJ. 1988;297:907. 10.1136/bmj.297.6653.907. [DOI] [PMC free article] [PubMed]
- 39.Ellis PM, Tattersall MHN. How should doctors communicate the diagnosis of cancer to patients? Ann Med. 1999;31(5):336–341. doi: 10.3109/07853899908995900. [DOI] [PubMed] [Google Scholar]
- 40.Collini A, Parker H, Oliver A. Training for difficult conversations and breaking bad news over the phone in the emergency department. Emerg Med J. 2021;38(2):151–154. doi: 10.1136/emermed-2020-210141. [DOI] [PubMed] [Google Scholar]
- 41.Fujimori M, Uchitomi Y. Preferences of cancer patients regarding communication of bad news: a systematic literature review. Jpn J Clin Oncol. 2009;39(4):201–216. doi: 10.1093/jjco/hyn159. [DOI] [PubMed] [Google Scholar]
- 42.Dosanjh S, Barnes J, Bhandari M. Barriers to breaking bad news among medical and surgical residents. Med Educ. 2001;35(3):197–205. doi: 10.1046/j.1365-2923.2001.00766.x. [DOI] [PubMed] [Google Scholar]
- 43.Levit L, Balogh E, Nass S, Ganz PA, Institute of Medicine (IOM). Delivering high-quality cancer care: charting a new course for a system in crisis. Challenges Aging Popul Board Heal Care Serv Inst Med. 2013;(September):412. http://www.nap.edu/catalog.php?record_id=18359. Accessed 27 Sept 2021.
- 44.Heuser C, Diekmann A, Ernstmann N, Ansmann L. Patient participation in multidisciplinary tumour conferences in breast cancer care (pintu): a mixed-methods study protocol. BMJ Open. 2019;9(4):1–7. doi: 10.1136/bmjopen-2018-024621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jayadevappa R, Chhatre S. Patient centered care - a conceptual model and review of the state of the art. Open Health Serv Policy J. 2011;4:15–25. doi: 10.2174/1874924001104010015. [DOI] [Google Scholar]
- 46.Cao W, Qi X, Yao T, Han X, Feng X. How doctors communicate the initial diagnosis of cancer matters: cancer disclosure and its relationship with patients’ hope and trust. Psychooncology. 2017;26(5):640–648. doi: 10.1002/pon.4063. [DOI] [PubMed] [Google Scholar]
- 47.Dawson J, Pawar S. Breaking bad news in the mammography department: a patient perspective from primary care. J Med Imaging Radiat Sci. 2020;51(4):S23–S25. doi: 10.1016/j.jmir.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Hauk H, Bernhard J, McConnell M, Wohlfarth B. Breaking bad news to cancer patients in times of COVID-19. Support Care Cancer. 2021;29(8):4195–4198. doi: 10.1007/s00520-021-06167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andrew N, Barraclough KA, Long K, et al. Telehealth model of care for routine follow up of renal transplant recipients in a tertiary centre: a case study. J Telemed Telecare. 2020;26(4):232–238. doi: 10.1177/1357633X18807834. [DOI] [PubMed] [Google Scholar]
- 50.Yadav AK, Budhathoki SS, Paudel M, Chaudhary R, Shrivastav VK, Malla GB. Patients understanding of their diagnosis and treatment plans during discharge in emergency ward in a tertiary care centre: a qualitative study. J Nepal Med Assoc. 2019;57(219):357–360. doi: 10.31729/jnma.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cartwright LA, Dumenci L, Siminoff LA, Matsuyama RK. Cancer patients’ understanding of prognostic information. J Cancer Educ. 2014;29(2):311–317. doi: 10.1007/s13187-013-0603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almalki H, Absi A, Alghamdi A, Alsalmi M, Khan M. Analysis of patient-physician concordance in the understanding of chemotherapy treatment plans among patients with cancer. JAMA Netw Open. 2020;3(3):e200341. doi: 10.1001/jamanetworkopen.2020.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veterans’ employment and training service. 2019 Gender and veteran demographics. U.S. Department of Labor. https://www.dol.gov/agencies/vets/womenveterans/womenveterans-demographics. Published 2022. Accessed 25 May 2022.
- 54.Hefaiedh R, Ennaifer R, Romdhane H, et al. Gender difference in patients with hepatocellular carcinoma. Tunis Med. 2013;91(8–9):505–508. [PubMed] [Google Scholar]
- 55.Vogels EA. Some digital divides persist between rural, urban and suburban America. Pew Research Center. https://www.pewresearch.org/fact-tank/2021/08/19/some-digital-divides-persist-between-rural-urban-and-suburban-america/. Published 2021. Accessed 25 May 2022.
- 56.Atske S, Perrin A. Home broadband adoption, computer ownership vary by race, ethnicity in the U.S. Pew Research Center.
- 57.Roberts ET, Mehrotra A. Assessment of disparities in digital access among Medicare beneficiaries and implications for telemedicine. JAMA Intern Med. 2020;180(10):1386–1389. doi: 10.1001/jamainternmed.2020.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.