Abstract
Distal wire perforation is an infrequent percutaneous coronary intervention (PCI) complication, which may progress to one of the fearful conditions, cardiac tamponade, and rarely to iatrogenic pericarditis. We described 2 cases of acute pericarditis and cardiac tamponade following distal guidewire coronary artery perforation that was successfully managed with pericardiocentesis, anti-inflammatory agents, and meticulous follow-up. Although uncommon, acute traumatic pericarditis may also be considered as a complication after a complex PCI.
Keywords: Iatrogenic pericarditis, cardiac tamponade, percutaneous coronary intervention, distal wire perforation
Introduction
Post cardiac injury syndrome (PCIS) accounts for one-fifth of patients with pericarditis. This pericardial inflammation with pericardial effusion could be caused by the injury of the myocardium, epicardium, pericardium, or accumulation of blood and debris from tissue damage. 1 Distal wire perforation is a rare but fearful complication during percutaneous coronary intervention (PCI). It occurs 0.35% of the PCI procedure. The outcome varies from asymptomatic to cardiac tamponade, emergent coronary bypass graft (CABG) surgery, to death. 2 We describe 2 cases of distal wire perforation induced cardiac tamponade and acute pericarditis.
Case Series
Patient 1
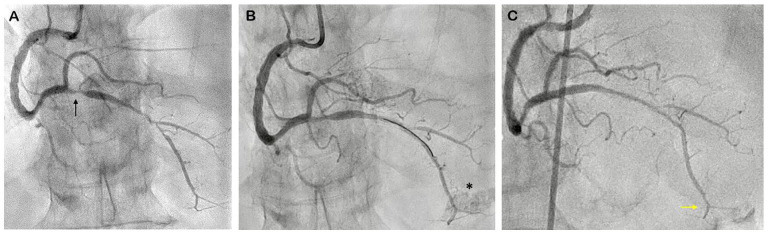
A 70-year-old male came to our hospital with typical chest pain despite optimal medical therapy. He was planned for staged PCI to RCA and had a history of PCI at the left coronary artery (LCA) 3 months ago. Angiography showed a patent stent in situ at LAD and LCx and severe stenosis at the ostial-distal right posterior descending artery (RPDA) (Figure 1A). Following wiring with Runthrough NS (Terumo, Japan) floppy guidewire (GW), intravascular ultrasound (IVUS) was performed as PCI guidance. Stenting of the target lesion was carried out after adequate lesion preparation using a cutting balloon. Final contrast injection revealed contrast staining (Figure 1B, Supplemental Video 1) at distal RPDA. Bedside echocardiography did not find any pericardial effusion. Hence, the patient was transferred to Cardiac Intensive Care Unit (CICU) for close observation.
Figure 1.
Case 1: Coronary Angiography of RCA: (A) pre PCI angiogram showed severe stenosis at ostial-distal RPDA (black arrow), (B) contrast staining at distal RPDA (asterisk), and (C) angiography evaluation post pericardiocentesis showed spontaneous sealed perforation (yellow arrow).
Abbreviations: PCI, percutaneous coronary intervention; RPDA, right posterior descending artery.
One hour after the procedure, the patient developed hypotension with echocardiography showed mild to moderate pericardial effusion with tamponade physiology and pericardial clot at right ventricle free wall (Figure 2). Emergent pericardiocentesis was performed, followed by angiography evaluation which showed spontaneous closure of the perforation (Figure 1C). Two days later, the electrocardiogram (ECG) showed inferoanteroseptal ST elevation with PR depression (Spodick’s sign) (Figure 3A and B) with echocardiography finding of mild pericardial effusion (Supplemental Video 2) and elevated leucocyte 10.420/uL. He was diagnosed with acute pericarditis due to iatrogenic trauma and given high dose aspirin 800 mg 3 times daily, tapering off every week, and colchicine 0.5 mg once daily for 3 months. During the 3-month follow-up, the patient was asymptomatic. ECG was within the normal limit (Figure 3C), and no pericardial effusion was found on echocardiography evaluation.
Figure 2.
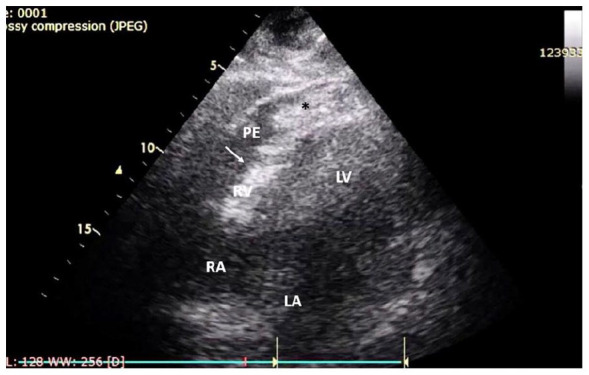
Case 1: Echocardiogram revealed cardiac tamponade. Echocardiography from subxiphoid view showed mild to moderate (8-14 mm) pericardial effusion with RV collapse (white arrow), pericardial clot at RV free wall (asterisk, *).
Abbreviations: LA, left atrium; LV, left ventricle; PE, pericardial effusion; RA, right atrium; RV, right ventricle.
Figure 3.
Case 1: Serial ECG showed evolution and resolution of pericarditis: (A) normal sinus rhythm before PCI, (B) ECG 2 days post PCI showed Spodick’s sign with depressed TP segment and ST-elevation (black arrow) at inferoanteroseptal leads, and (C) normalized ECG after 1-week treatment of anti-inflammatory agents.
Abbreviations: ECG, electrocardiogram; PCI, percutaneous coronary intervention.
Patient 2
A 67-year-old male was referred for high-risk PCI from a district hospital with non-ST-elevation myocardial infarction (NSTEMI). He had left main trifurcation disease and refused CABG surgery. Angiogram showed severe stenosis at distal left main (Medina 1-1-1-0) moderate stenosis at ostial LAD and ostial Ramus (Figure 4A). Triple wiring was performed with BMW (Abbott, USA) GW to LAD, Runthrough NS (Terumo, Japan) GW to Ramus, and Sion (Asahi, Japan) GW to LCx, followed by Optic Coherence Tomography (OCT) study as PCI guidance. Provisional LM—LAD stenting was performed after lesion preparation with scoring and NC balloons. Final contrast injection showed TIMI flow 3 with residual contrast staining (Figure 4B) at distal LAD. A bedside echocardiogram showed no pericardial effusion, and due to stable hemodynamics, we decided for close observation.
Figure 4.
Case 2: Coronary angiogram: (A) pre PCI angiogram revealed LM trifurcation disease (black arrow), (B) contrast staining at distal LAD (yellow arrow), (C) angiogram after pericardiocentesis showed persistent contrast leakage (blue arrow), (D) prolonged balloon inflation was performed to seal the perforation, and (E) angiography evaluation revealed sealed perforation.
Abbreviations: LAD, left anterior descending; LM, left main; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
One hour after the procedure, the patient became hypotensive. Echocardiography revealed mild-moderate pericardial effusion with tamponade signs (Figure 5). Emergent pericardiocentesis was performed, followed by coronary angiogram, which revealed persistent contrast leakage at distal LAD (Figure 4C, Supplemental Video 3), therefore it was decided to perform prolonged balloon inflation with a 2.0/15 mm Ikazuchi (Kaneka, Japan) semi-compliant balloon for 15 minutes (Figure 4D). Contrast injection showed sealed perforation (Figure 4E), and the patient was then stabilized.
Figure 5.

Case 2: Echocardiogram revealed pericardial tamponade. Echocardiography from the parasternal short-axis view showed mild to moderate (8-18 mm) pericardial effusion with RV collapse (white arrow).
Abbreviations: Ao, aorta; LA, left atrium; PA, pulmonary artery; PE, pericardial effusion; RA, right atrium; RV, right ventricle.
Five days later, the patient had sharp chest pain typical of pericarditis. Recurrent pericardial effusion was found (Supplemental Video 4) from echocardiogram with elevated inflammatory biomarkers (leucocyte 14.750/uL, C-reactive protein 4.84 mg/dL) and widespread ST elevation with PR depression at anterior leads ECG (Figure 6A and B), which confirmed the diagnosis of acute pericarditis. He was treated with high dose aspirin 800 mg 3 times daily, colchicine 0.5 mg twice daily, and high dose methylprednisolone with tapering dose. During the three-month follow-up, the patient was asymptomatic. ECG was within the normal limit (Figure 6C), and no pericardial effusion was found on echocardiography evaluation.
Figure 6.
Case 2: Serial ECG showed evolution and resolution of pericarditis: (A) sinus rhythm with anterior ischemia before PCI, (B) ECG 5 days post PCI showed anterior ST elevation (black arrow) and PR segment depression, concurrent with elevated inflammatory marker and normal angiogram, the diagnosis of pericarditis was made, and (C) normalized ECG after 1-week treatment of anti-inflammatory agents.
Abbreviations: ECG, electrocardiogram; PCI, percutaneous coronary intervention.
Discussion
Stathopoulos et al 3 found that GW caused 42.6% of coronary perforations. The main predictors of distal wire perforations (DWP) are complex and prolonged procedures, >1 lesion per vessel, multivessel diseases, complex lesions, initial TIMI flow 0, use more than 1 guidewire, especially when using stiff, tapered, or hydrophilic wires with tip load 3 gr or more.2,4 However, soft-tip workhorse GW was associated with more than a third DWP, which may be related to it being the most used GW in PCI procedures, therefore having a higher prevalence of DWP. 3 Furthermore, the lack of meticulous attention to the tip of GW during the procedure and the use of intracoronary imaging might increase the risk of perforation.3,5 Our cases were complex and prolonged procedures, using more than 1 GW and intracoronary imaging, which all increased the risk for DWP.
Guidewire-induced coronary perforation allows slow accumulation of blood loss, resulting in a delayed tamponade (up to 3 days), 3 as occurred in our cases. The development of tamponade increased long-term mortality up to threefold. 3 To date, there is no standardized management for distal wire perforation. Overall, the clinician should pay attention to the patient’s hemodynamics. Therefore, a repeated echocardiogram is particularly important. When there is a hemodynamic compromise, pericardial effusion with tamponade should be suspected. Urgent pericardiocentesis simultaneously with prolonged balloon inflation should be performed. If distal perforation persists, proceed with coil or other materials for distal embolization. Emergent cardiac surgery may be required if the percutaneous methods fail. Observation for 48 to 72 hours with repeated echo evaluation is needed. 5
There are several methods to prevent distal wire perforation, including making a loop in the distal portion of the wire to keep it in the main large vessel (reduce the risk of wire dislodgement into small branches), exchange the stiff wire for workhorse wire after crossing the lesion, be aware of the distal tip of the wire at all the times and always take the shots to look at the distal end after the wire has been retracted.4,6
Although rare, DWP may damage mesothelial cells and blood in the pericardial space, initiating an autoimmune response, triggering cardiac antigen-antibody production, immune complex deposition in the pericardium, and producing inflammation. PCIS may be diagnosed if fulfilled 2 out of 5 clinical criteria: (i) fever without another etiology, (ii) pleuritic or pericarditic chest pain, (iii) pericardial or pleural rubs, (iv) pericardial effusion, and/or (v) pleural effusion with elevated C-Reactive Protein (CRP). Our cases were diagnosed with iatrogenic pericarditis due to recurrent pericardial effusion without visible leaks, ECG changes consistent with pericarditis and elevated CRP. 7
The mainstay treatment for iatrogenic pericarditis is high-dose anti-inflammatory agents such as aspirin/Non-Steroid Anti-Inflammatory Drugs (NSAIDs) and colchicine. 7 Both patients were given high-dose aspirin and colchicine. Due to recurrent pericardial effusion, the second patient has added a steroid. Recurrence of PCIS ranging from around 10% to 15% with 2.8% risk of constrictive pericarditis over 72 months. Hence, long-term follow-up of the symptoms, echocardiography, and laboratory (CRP) are needed. 1
Finally, despite the incidence of PCIS after distal wire perforation is unknown, the clinician must aware of this possible complication. Early detection and meticulous follow-up are essential to manage the PCIS in order to prevent long-term consequences.
Conclusions
Despite rare, distal wire perforation could lead to a catastrophic outcome and sequelae. Acute traumatic pericarditis should be considered as a complication after a complex PCI. Meticulous attention to any signs, symptoms, and effective management of coronary perforation and its subsequent complication are essential to reduce long-term mortality.
Supplemental Material
Supplemental material, sj-docx-1-cic-10.1177_11795468221108211 for Iatrogenic Pericarditis and Cardiac Tamponade Following Distal Wire Perforation: A Serial Case Report by Achmad Fauzi Yahya, Minsy Titi Sari and Aninka Saboe in Clinical Medicine Insights: Cardiology
Footnotes
Author Contributions: Conceptualization, data curation, writing original draft, review & editing and supervision: AFY; Conceptualization, data curation, writing original draft: MTS; Conceptualization, data curation, review & editing and supervision: AS.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Minsy Titi Sari  https://orcid.org/0000-0002-9082-4737
https://orcid.org/0000-0002-9082-4737
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Sasse T, Eriksson U. Post-cardiac injury syndrome: aetiology, diagnosis, and treatment. ESC E-J Cardiol Pract. 2017;15:21-31. [Google Scholar]
- 2. Teis-Soley A, Fernández-Nofrerías E, Rodríguez-Leor O, et al. Coronary artery perforation by intracoronary guidewires: risk factors and clinical outcomes. Rev Española Cardiol (English Ed.). 2010;63:730-734. [DOI] [PubMed] [Google Scholar]
- 3. Stathopoulos I, Panagopoulos G, Kossidas K, Jimenez M, Garratt K. Guidewire-induced coronary artery perforation and tamponade during PCI: in-hospital outcomes and impact on long-term survival. J Invasive Cardiol. 2014;26:371-376. [PubMed] [Google Scholar]
- 4. Lindsay A, Chitkara K, Di Mario C. Complications of Percutaneous Coronary Intervention: The Survival Handbook. Springer; 2016. [Google Scholar]
- 5. Deb T, Maddury J, Sahoo PK. Coronary artery perforation. Indian J Cardiovasc Dis Women WINCARS. 2019;4:110-120. [Google Scholar]
- 6. Khalil I. Coronary artery intervention techniques. In: Gaze DC, Kibel A, eds. Cardiac Diseases. IntechOpen; 2020:205-241. [Google Scholar]
- 7. Malik J, Zaidi SMJ, Rana AS, Haider A, Tahir S. Post-cardiac injury syndrome: an evidence-based approach to diagnosis and treatment. Am Heart J Plus Cardiol Res Pract. 2021;12:100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cic-10.1177_11795468221108211 for Iatrogenic Pericarditis and Cardiac Tamponade Following Distal Wire Perforation: A Serial Case Report by Achmad Fauzi Yahya, Minsy Titi Sari and Aninka Saboe in Clinical Medicine Insights: Cardiology