Abstract
Obstructive sleep apnoea (OSA) is strongly associated with cardiovascular disease (CVD). However, evidence supporting this association in the Asian population is scarce. Given the differences in the epidemiology of CVD and cardiovascular risk factors, as well as differences in the availability of healthcare resources between Asian and Western countries, an Asian Pacific Society of Cardiology (APSC) working group developed consensus recommendations on the management of OSA in patients with CVD in the Asia-Pacific region. The APSC expert panel reviewed and appraised the available evidence using the Grading of Recommendations Assessment, Development, and Evaluation system. Consensus recommendations were developed and put to an online vote. Consensus was reached when 80% of votes for a given recommendation were in support of ‘agree’ or ‘neutral.’ The resulting statements provide guidance on the assessment and treatment of OSA in patients with CVD in the Asia-Pacific region. The APSC hopes for these recommendations to pave the way for screening, early diagnosis and treatment of OSA in the Asia-Pacific region.
Keywords: Obstructive sleep apnoea, cardiovascular disease, Asia-Pacific, consensus
Obstructive sleep apnoea (OSA) is strongly associated with cardiovascular disease (CVD).[1] A few studies in Asia have reported an OSA prevalence ranging from 4.1% to 7.5% in men and from 2.1% to 4.5% in women, with an overall prevalence of 8.5% in the general adult population aged >18 years.[2,3] Furthermore, a multi-ethnic southeast Asian country has shown an increase in prevalence to 30%. [4] The influence of ethnicity on prevalence has also been demonstrated.[4,5]
The reported prevalence rates of OSA in patients with coronary heart disease, stroke, heart failure (HF) and arrhythmia are as high as 65%, 75%, 55% and 50%, respectively.[6–10] In studies on Asian patients with coronary artery disease undergoing percutaneous coronary intervention or surgical revascularisation, the prevalence of OSA is around 45–50%.[11,12] However, the prevalence of OSA among Asian patients with other forms of CVD is less well established.
Given the limited published clinical evidence and country-specific guidelines on the management of OSA in patients with CVD in the Asia-Pacific region, the Asian Pacific Society of Cardiology (APSC) developed consensus recommendations to guide general cardiologists, internal medicine specialists practicing cardiology and sleep physicians in the Asia-Pacific region on the diagnosis and treatment of OSA in patients with CVD. These recommendations are intended to improve screening, early diagnosis and treatment throughout the Asia-Pacific region.
Methods
The APSC convened an expert consensus panel to review the literature on the screening, assessment and treatment of OSA, discuss gaps in current management, determine areas where further guidance is needed and develop consensus recommendations on the diagnosis and management of OSA in patients with CVD. The 16 panel experts were members of the APSC who were nominated by national societies and endorsed by the APSC's consensus board or invited international experts. The expert consensus panel comprised cardiologists and sleep specialists from Japan, Myanmar, Singapore, Taiwan, Thailand and Vietnam.
After a comprehensive literature search, applicable articles were reviewed and appraised using the Grading of Recommendations Assessment, Development, and Evaluation system, as follows:
High (authors have high confidence that the true effect is similar to the estimated effect.
Moderate (authors believe that the true effect is probably close to the estimated effect).
Low (true effect might be markedly different from the estimated effect).
Very low (true effect is probably markedly different from the estimated effect). [13]
Using these levels of evidence, the authors adjusted the level of evidence if the estimated effect when applied to the Asia-Pacific region might differ from the published evidence because of various factors such as ethnicity, cultural differences and/or healthcare systems and resources.
The available evidence was then discussed during a consensus meeting held in April 2021. Consensus recommendations were developed during the meeting, which were then put to an online vote. Each recommendation was voted on by each panel member using a three-point scale (agree, neutral, or disagree). Consensus was reached when 80% of votes for a given recommendation were agree or neutral. In the case of non-consensus, the recommendations were further discussed via email and revised accordingly until the criteria for consensus were fulfilled.
Consensus Recommendations
Screening and Diagnosis
Recommendation 1. Any one of the following conditions should prompt the clinician to screen patients for OSA:
hypertension;
type 2 diabetes;
obesity;
coronary artery disease;
stroke;
HF; or
arrhythmia.
Level of evidence: Very low.
Level of consensus: 81.25% agree; 12.5% neutral; 6.25% disagree.
Recommendation 2. Symptoms of OSA or screening questionnaires, such as STOP-Bang, may be used to screen for OSA in patients with CVD.
Level of evidence: Low.
Level of consensus: 87.5% agree; 12.5% neutral; 0% disagree.
Recommendation 3. Patients with the following conditions should be referred to a sleep specialist for sleep testing:
resistant hypertension;
AF requiring cardioversion/ablation; or
unexplained pulmonary hypertension.
Level of evidence: Moderate.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Recommendation 4. In patients suspected of OSA after screening with significant cardiopulmonary disease (HF, congenital heart disease or complicated valvular disease), polysomnography (Level I) should be used to diagnose OSA. Level III or Level IV sleep studies should not be used to diagnose OSA in these patients because of the complexity of the clinical scenario, which may lead to misdiagnosis.
Level of evidence: Very low.
Level of consensus: 87.5% agree; 12.5% neutral; 0% disagree.
Recommendation 5. In patients with a high pre-test probability of moderate to severe OSA without significant cardiopulmonary disease or stroke, home sleep apnoea testing (Level III or IV) or polysomnography (Level I/II) may be used to diagnose OSA.
Level of evidence: Moderate.
Level of consensus: 93.75% agree; 0% neutral; 6.25% disagree.
Recommendation 6. Sleep studies conducted on patients with CVD should be scored and reported by an adequately trained sleep specialist.
Level of evidence: Very low.
Level of consensus: 87.5% agree; 12.5% neutral; 0% disagree.
Because of the association between OSA and CVD, it is not surprising that patients with certain CVDs and/or cardiovascular risk factors have a disproportionately high prevalence of OSA (Figure 1).[6–10] Hence, the panel voted to screen patients with these conditions, namely hypertension, type 2 diabetes, obesity, coronary artery disease, stroke, HF and arrhythmia. This recommendation is in line with the 2009 recommendations from the Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine (AASM).[14] Certain physical features should also prompt physicians to screen for OSA (Figure 1). It should be noted that the Asia-Pacific classification of BMI has a lower cut-off value for the obesity category (25 kg/m2) than the WHO's classification (30 kg/m2). [15]
Figure 1: Patient Characteristics That May Suggest Obstructive Sleep Apnoea.
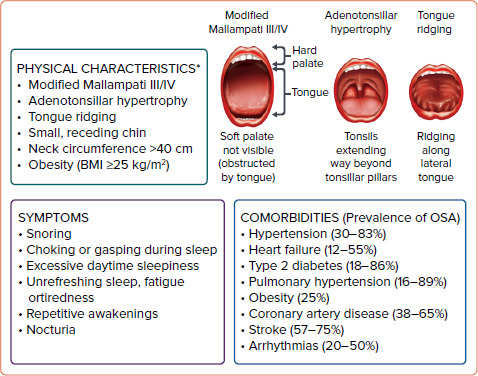
*Not all of these physical characteristics need to be present to suspect OSA. OSA = obstructive sleep apnoea. Level of consensus: 100% agree; 0% neutral; 0% disagree. Sources: Javaheri et al. 2017, Doumit and Prasad 2016, Garvey et al. 2015, Romero-Corral et al. 2010 and Kholdani et al. 2015.[6–10]
However, some expert panel members noted that screening patients with these fairly prevalent conditions may lead to cardiologists needing to screen a high proportion of patients in daily practice. Others noted that the benefit of screening varies across the conditions, with some conditions, such as hypertension, obesity, coronary artery disease, HF and AF (rather than other arrhythmias), having stronger evidence of benefit.
A number of screening questionnaires are available that can be used to screen patients for OSA. During the expert panel meeting, most of the panellists reported that STOP-Bang is one of the most commonly used questionnaires at their respective institutions, largely because of its ease of administration compared with other questionnaires, such as the Berlin questionnaire (Tables 1, 2 and 3).[16] STOP-Bang was also shown to have the best diagnostic accuracy for OSA.[17]
Table 1: STOP-Bang Questionnaire.
| Question | Response |
|---|---|
| STOP | |
| Snoring? Do you snore loudly (loud enough to be heard through closed doors or your bed-partner elbows you for snoring at night)? | Yes or No |
| Tired? Do you often feel tired, fatigued, or sleepy during the daytime (such as falling asleep during driving or talking to someone)? | Yes or No |
| Observed? Has anyone observed you stop breathing or choking/gasping during your sleep? | Yes or No |
| Pressure? Do you have or are being treated for high blood pressure? | Yes or No |
| BANG | |
| BMI? BMI more than 35 kg/m2? | Yes or No |
| Age? Age older than 50 years? | Yes or No |
| Neck size large? Is your shirt collar 16 inches/40 cm or larger (measured around the Adam's apple)? | Yes or No |
| Gender? Are you a man? | Yes or No |
Source: Used with permission from http://www.stopbang.ca/osa/screening.php[52]
Table 2: STOP-Bang Questionnaire Interpretation.
| Interpretation | |
|---|---|
| OSA: Low risk | Yes to 0–2 questions |
| OSA: Intermediate risk: | Yes to 3–4 questions |
| OSA: High risk: |
|
OSA = obstructive sleep apnoea. Source: Used with permission from http://www.stopbang.ca/osa/screening.php[52]
Table 3: STOP-Bang Diagnostic Accuracy for Detecting Moderate to Severe Obstructive Sleep Apnoea (Apnoea–Hypopnoea Index >15) and Severe Obstructive Sleep Apnoea (Apnoea–Hypopnoea Index >30).
| Moderate to Severe OSA (AHI >15) | Severe OSA (AHI >30) |
|---|---|
| Sensitivity: 92.9% (95% CI [84.1–97.6]) | Sensitivity: 100% (95% CI [91.0–100.0]) |
| Specificity: 43.0% (95% CI [33.5–52.9]) | Specificity: 37.0% (95% CI [28.90–45.6]) |
| PPV: 51.6% (95% CI [42.5–60.6]) | PPV: 31.0% (95% CI [23.0–39.8]) |
| NPV: 90.2% (95% CI [78.6–96.7]) | NPV: 100% (95% CI [93.0–100.0]) |
AHI = apnoea–hypopnoea index; NPV = negative predictive value; OSA = obstructive sleep apnoea; PPV = positive predictive value. Source: Chung et al. 2008.[16] Adapted with permission from Wolters-Kluwer.
A study conducted on a multi-ethnic Asian population also concluded that the STOP-Bang questionnaire could be used as a screening tool among Asians in view of its moderate sensitivity and high negative predictive value for patients with moderate-to-severe OSA and severe OSA.[18]
The benefit of interventions in patients without OSA symptoms, such as excessive daytime sleepiness, remains unclear. A rapid and easy-to-perform screening test is needed; thus, the panellists also voted that checking for symptoms of OSA is a reasonable screening tool for daily practice (Figure 1).
Patients with resistant hypertension, AF requiring cardioversion/ablation, or unexplained pulmonary hypertension have an exceptionally high risk of severe cardiovascular adverse events. In these patients, treatment for OSA, if present, has been shown to confer benefit.[19–23] Hence, the panel voted in favour of early referral to a sleep specialist for prompt evaluation in such patients. Several panellists also noted that after thorough evaluation, sleep specialists may be able to identify underlying causes of these conditions without needing to proceed to sleep testing or to identify patients who would not benefit from further sleep testing.
The 2020 European Society of Cardiology guidelines on the management of AF recommended that OSA treatment should be optimised to reduce AF recurrences and improve AF treatment results, with a Class IIB recommendation (level of evidence C), and stated: “It remains unclear how and when to test for OSA and implement OSA management in the standard work-up of AF patients.”[24] In the multidisciplinary clinical management strategy proposed by Tietjens et al., diagnostic sleep testing for all patients with recurrent AF following either cardioversion or ablation, including those without symptoms of sleep-disordered breathing (SDB), as well as AF patients who are persistently symptomatic, challenging to pharmacologically rate control or managed via rhythm control strategies, when there is a suspicion for sleep apnoea based on comprehensive sleep assessment.[19]
The APSC consensus agrees with these recommendations and acknowledged the importance of an accurate diagnosis in these patients. Hence, the panel voted in favour of an early referral to a sleep specialist for all AF patients requiring cardioversion or ablation (e.g. those persistently symptomatic despite initial rate/rhythm control strategies), regardless of the presence of clinically suspected SDB.
Patients with significant cardiopulmonary disease (HF with reduced ejection fraction, congenital heart disease or complicated valvular disease) are at a high risk of central sleep apnoea (CSA) or mixed sleep apnoea.[6,25] Polysomnography (Level I) rather than home sleep apnoea testing (HSAT) should be the diagnostic test of choice if patients have coexisting and significant cardiopulmonary disease, a history of stroke, or suspected non-respiratory sleep disorders (Table 4). This recommendation is in line with AASM and American Heart Association (AHA)/American College of Cardiology (ACC) guidelines.[18,26] Other conditions that may affect the accuracy of HSAT include potential respiratory muscle weakness due to neuromuscular conditions, chronic opiate use and environmental or personal factors that may interfere with the conduct and interpretation of HSAT.[26]
Table 4: American Academy of Sleep Medicine Classification of Sleep Apnoea Evaluation.
| Level | Level I: Standard Polysomnography | Level II: Comprehensive Portable Polysomnography | Level III: Modified Portable Sleepapnoea Testing | Level IV: Continuous (Single or Dual) Bioparameter Recording |
|---|---|---|---|---|
| Minimum recording channels | EEG, EOG, chin EMG, ECG, airflow, respiratory effort and oxygen saturation. | Same as for Level I except heart rate instead of ECG is acceptable. | Recording of ventilation (at least two channels of respiratory movement, or respiratory movement and airflow), ECG or heart rate and oxygen saturation. | Only one or two physiological variables need to be recorded. |
| Other characteristics | Body position must be documented or objectively measured. Leg movement recording (EMG or motion sensor) is desirable but optional. | |||
| Personnel and ability to intervene | Trained personnel must be in constant attendance and able to intervene. | Personnel are needed for preparation. Ability to intervene is not required for all studies. | Personnel are needed for preparation. Ability to intervene is not required for all studies. | Personnel are needed for preparation. Ability to intervene is not required for all studies. |
EOG = electrooculogram; EMG = electromyogram.
In contrast, among patients with a high pre-test probability of moderate to severe OSA (including those with very severe OSA) after screening but without significant cardiopulmonary disease or stroke, both HSAT (Level III or IV) and polysomnography (Level I) may be used to diagnose OSA.[27,28] In this context, clinicians may use the AASM definition of high pre-test probability of moderate to severe OSA, which includes patients with daytime hypersomnolence and at least two of the following: habitual loud snoring, witnessed apnoea or gasping/choking, or diagnosed hypertension.[26]
Finally, because of the complexity of interpreting sleep studies, such tests conducted on patients with CVD should be scored and reported by a qualified or adequately trained sleep specialist.[29] The expert panel also reported that at some centres in the region, sleep tests are scored by a registered sleep technologist and interpreted by a sleep specialist.
Figure 2 summarises the screening and testing pathway for patients with CVD. However, one panellist cautioned that some patients with HF may require further sleep testing, even with a negative OSA screening questionnaire or in the absence of signs and symptoms of OSA.
Figure 2: Proposed Algorithm on Screening and Sleep Testing of Obstructive Sleep Apnoea in Cardiovascular Disease Patients.
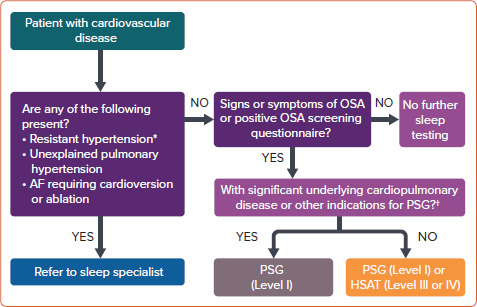
*Above-goal blood pressure despite therapy with three or more oral antihypertensive agents (commonly including a long-acting calcium channel blocker, a renin-angiotensin system blocker and a diuretic); or adequate blood pressure control requiring four or more agents.[53] †Potential respiratory muscle weakness caused by neuromuscular disorders, documented awake hypoventilation or suspected sleep-related hypoventilation, chronic opioid use, severe insomnia, or suspected sleep-related movement disorder such as restless leg syndrome. HSAT = home sleep apnoea test; OSA = obstructive sleep apnoea; PSG = polysomnography. Level of consensus: 93.75% agree; 6.25% neutral; 0% disagree.
Treatment and Referral
Recommendation 7. All patients undergoing OSA treatment should also undergo lifestyle modification and educational and behavioural interventions for OSA, as well as for weight loss if they are classified as overweight or obese.
Level of evidence: Very low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Recommendation 8. OSA treatment improves daytime sleepiness and cognitive function. In observational studies, continuous positive airway pressure (CPAP) therapy for OSA is strongly associated with reduced rates of adverse cardiovascular events, but randomised control trial evidence to support CPAP therapy in non-sleepy OSA patients with CVD is inconclusive.
Level of evidence: Low.
Level of consensus: 93.75% agree; 6.25% neutral; 0% disagree.
Recommendation 9. Patients with HF and OSA may undergo CPAP therapy, which has been shown to improve ventricular function, symptoms and quality of life.
Level of evidence: Moderate.
Level of consensus: 93.75% agree; 6.25% neutral; 0% disagree.
Recommendation 10. Patients undergoing rhythm control for AF who have moderate to severe OSA should undergo CPAP therapy to reduce the risk of AF recurrence.
Level of evidence: Low.
Level of consensus: 93.75% agree; 6.25% neutral; 0% disagree.
Recommendation 11. Patients with CVD and OSA whose OSA symptoms persist despite treatment or who are non-adherent to OSA therapy should be reviewed by a sleep specialist.
Level of evidence: Very low.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
All patients with CVD and OSA should be educated with regard to their diagnosis, risk factors and the natural history and consequences of OSA.[15,30] They should also be educated on the impact of their treatment and encouraged to continue treatment. Furthermore, all patients should be educated on lifestyle modifications, sleep hygiene and behavioural interventions that may help to minimise the impact of OSA. These include interventions to help reduce weight in patients who are classified as overweight or obese; improved sleep positions in cases of positional OSA; and the avoidance of alcohol and medications that may worsen OSA (e.g. benzodiazepines, barbiturates, other anti-epileptic drugs, sedative antidepressants, antihistamines and opiates). When such medications are necessary, their use should be closely monitored and the dose carefully titrated, if possible.
Weight loss and exercise should be recommended to all patients with OSA who are classified as overweight or obese.[14,30–35] Weight loss has been shown to improve overall health and metabolic parameters, decrease the apnoea–hypopnoea index (AHI), reduce blood pressure and improve quality of life for patients with OSA, although weight loss alone rarely leads to complete remission of OSA. Given that obesity and inactivity are cardiovascular risk factors, weight loss and exercise are also recommended in patients with CVD and OSA. Exercise may also modestly improve OSA, even in the absence of significant weight loss. A 2014 meta-analysis found that a supervised exercise program significantly improved AHI (mean change, −6 events/hour), sleep efficiency, subjective sleepiness and cardiorespiratory fitness, even without substantial weight loss.[36]
In a randomised clinical trial (RCT), 2,717 adults aged between 45 and 75 years with moderate to severe OSA and coronary or cerebrovascular disease were randomised to undergo CPAP plus usual care or usual care alone. The study found that the primary composite endpoint of death from cardiovascular causes, MI, stroke, hospitalisation for unstable angina, HF, or transient ischemic attack was not significantly reduced by CPAP therapy. However, CPAP therapy significantly reduced daytime sleepiness (p<0.001) and significantly improved the physical and mental subscales of the 36-Item Short Form Health Survey of the Medical Outcomes Study.[37] It should be noted that this study excluded patients with severe daytime sleepiness (Epworth Sleepiness Scale score of >15), although these patients are most likely to benefit from CPAP therapy.
Some observational studies have suggested that CPAP therapy may be effective in reducing cardiovascular outcomes (e.g. all-cause mortality, cardiovascular mortality, MI, stroke, repeat revascularisation) in patients with coronary artery disease, including after percutaneous coronary intervention.[38–41] However, this benefit has not been confirmed in RCTs. A meta-analysis of nine RCTs (n=3,314) on adult patients with polysomnography-diagnosed OSA and any CVD found that the primary outcomes (i.e. all-cause death, cardiovascular death, acute MI, stroke and any major cardiovascular event) were not significantly reduced in patients undergoing CPAP therapy (pooled RR 0.93; 95% CI [0.70–1.24]; I2 49%).[42] Possible reasons for this lack of benefit in the primary outcome include the overall low adherence to CPAP therapy, the inclusion of patients with low levels of symptoms or a heterogeneous pool of patients with different disease phenotypes and arterial oxygen desaturation and the potential lack of efficacy of CPAP in reducing recurrent cardiovascular events in patients with advanced or symptomatic atherosclerotic vascular disease.[43–45]
The 2017 AHA/ACC guidelines on HF identified CPAP therapy as a reasonable treatment strategy (class IIb) to improve sleep quality and daytime sleepiness in patients with CVD and OSA.[46] Furthermore, CPAP therapy has beneficial haemodynamic effects, such as diminished systemic venous return, right ventricular preload and left ventricular afterload, as well as improved pulmonary total vascular resistance and ventricular diastolic function.[47,48]
Patients with paroxysmal AF, including those undergoing rhythm control strategies, such as cardioversion or ablation and moderate to severe OSA should undergo CPAP therapy to reduce the risk of recurrence. Multiple observational studies have assessed the ability of CPAP therapy to reduce the AF burden after ablation or cardioversion. Although limited by methodological issues and small sample sizes, these studies largely support the use of CPAP therapy in reducing the burden of AF.[49] Furthermore, data from 1,841 patients with OSA and AF in the ORBIT-AF registry showed that patients who undergo CPAP therapy are less likely to progress to more permanent forms of AF than patients who do not undergo CPAP therapy (p=0.021).[50]
The proposed management of patients with CVD after HSAT in patients with a high pre-test probability is outlined in Figure 3. Figure 4 shows the proposed management of patients with CVD after polysomnography. In this algorithm, the first-line treatment for mild OSA is conservative management, which includes a combination of weight loss, positional sleep therapy, optimised treatment for nasal obstruction and oromyofunctional therapy. One panellist noted that oromyofunctional therapy requires adequate training of healthcare professionals, which may limit its implementation in some areas of the Asia-Pacific region. Some expert panel members emphasised that co-management with a sleep specialist is encouraged in patients with moderate to severe OSA, or for patients with mild OSA if the attending cardiologist has inadequate expertise in its treatment. They also noted that a referral to an otorhinolaryngologist or dental sleep specialist for alternative OSA therapies may be needed in some patients with moderate to severe OSA. Finally, they emphasised the complexity of treating patients with CSA or mixed SDB; hence, referral to a sleep specialist is recommended. One panellist dissented, arguing that the treatment of CSA or mixed SDB using oxygen or pharmacotherapy remains controversial. The medical treatment of any associated CVD in patients with CSA or mixed SDB should also be optimised to improve outcomes.
Figure 3: Proposed Management of Cardiovascular Disease Patients after the Home Sleep Apnoea Test in Patients with High Pre-test Probability of Obstructive Sleep Apnoea.
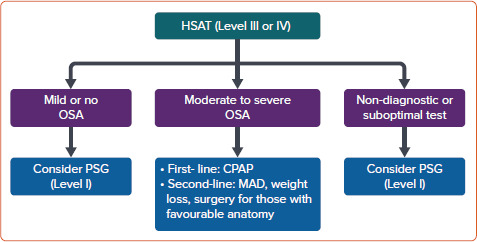
CPAP = continuous positive airway pressure; HSAT = home sleep apnoea test; MAD = mandibular advancement device; OSA = obstructive sleep apnoea; PSG = polysomnography. Level of consensus: 93.75% agree; 0% neutral; 6.25% disagree.
Figure 4: Proposed Management of Cardiovascular Disease Patients after Polysomnography.
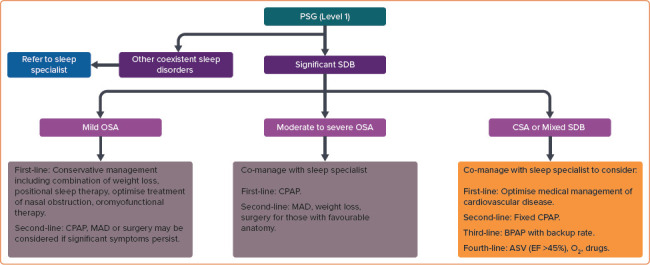
ASV = adaptive servo ventilation; BPAP = bilevel positive airway pressure; CPAP = continuous positive airway pressure; CSA = central sleep apnoea; EF = ejection fraction; MAD = mandibular advancement device; OSA = obstructive sleep apnoea; PSG = polysomnography; SDB = sleep-disordered breathing. Level of consensus: 87.5% agree; 6.25% neutral; 6.25% disagree.
Limitations
As a result of the obesity epidemic, the prevalence of OSA in Asia has increased in recent decades. However, the awareness of OSA in patients in Asia is low.[51] These consensus recommendations aim to guide practising cardiologists and internal medicine specialists practicing cardiology on the management of patients with CVD with regard to OSA screening, diagnosis treatment and referral in the Asia-Pacific region. The 11 recommendations presented in this paper aim to guide clinicians based on the most up-to-date evidence. However, given the varied clinical situations and healthcare resources present in the region, these recommendations should not replace clinical judgement.
Conclusion
Management of patients with CVD and OSA should be individualised and should consider the patient's symptoms, clinical characteristics and comorbidities, as well as patients' and caregivers' concerns and preferences. Clinicians should also be aware of the challenges that may limit the applicability of these consensus recommendations, such as limited access to specific interventions and technologies, limited availability of resources, accepted local standards of care, cultural factors and individualised expertise in OSA management.
Acknowledgments
Medical writing support was provided by Ivan Olegario.
References
- 1.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012;126:1495–510. doi: 10.1161/CIRCULATIONAHA.111.070813. [DOI] [PubMed] [Google Scholar]
- 2.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duong-Quy S, Dang Thi Mai K, Tran Van N et al. Study of the prevalence of obstructive sleep apnoea syndrome in Vietnam. Rev Mal Respir. 2018;35:14–24. doi: 10.1016/j.rmr.2017.10.006. [in French] [DOI] [PubMed] [Google Scholar]
- 4.Tan A, Cheung YY, Yin J et al. Prevalence of sleep-disordered breathing in a multiethnic Asian population in Singapore: a community-based study. Respirology. 2016;21:943–50. doi: 10.1111/resp.12747. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Wang R, Zee P et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38:877–88. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaheri S, Barbe F, Campos-Rodriguez F et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69:841–58. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doumit J, Prasad B. Sleep apnea in type 2 diabetes. Diabetes Spectr. 2016;29:14–9. doi: 10.2337/diaspect.29.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis. 2015;7:920–9. doi: 10.3978/j.issn.2072-1439.2015.04.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711–9. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kholdani C, Fares WH, Mohsenin V. Pulmonary hypertension in obstructive sleep apnea: is it clinically significant? A critical analysis of the association and pathophysiology. Pulm Circ. 2015;5:220–7. doi: 10.1086/679995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CH, Sethi R, Li R et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133:2008–17. doi: 10.1161/circulationaha.115.019392. [DOI] [PubMed] [Google Scholar]
- 12.Koo CY, Aung AT, Chen Z et al. Sleep apnoea and cardiovascular outcomes after coronary artery bypass grafting. Heart. 2020;106:1495–502. doi: 10.1136/heartjnl-2019-316118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balshem H, Helfand M, Schünemann HJ et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Epstein LJ, Kristo D, Strollo PJ Jr et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. doi: 10.5664/jcsm.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia, 2000
- 16.Chung F, Yegneswaran B, Liao P et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. doi: 10.1097/aln.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 17.Chiu HY, Chen PY, Chuang LP et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth Sleepiness Scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70. doi: 10.1016/j.smrv.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Tan A, Yin JD, Tan LW et al. Predicting obstructive sleep apnea using the STOP-Bang questionnaire in the general population. Sleep Med. 2016;27–28:66–71. doi: 10.1016/j.sleep.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 19.Tietjens JR, Claman D, Kezirian EJ et al. Obstructive sleep apnea in cardiovascular disease: a review of the literature and proposed multidisciplinary clinical management strategy. J Am Heart Assoc. 2019;8:e010440. doi: 10.1161/JAHA.118.010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Cao Q, Guo Z, Dai Q. Continuous positive airway pressure in patients with obstructive sleep apnea and resistant hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 2016;18:153–8. doi: 10.1111/jch.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnbull F. Blood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–35. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 22.Arias MA, García-Río F, Alonso-Fernández A et al. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J. 2006;27:1106–13. doi: 10.1093/eurheartj/ehi807. [DOI] [PubMed] [Google Scholar]
- 23.Bradley TD, Logan AG, Kimoff RJ et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/nejmoa051001. [DOI] [PubMed] [Google Scholar]
- 24.Hindricks G, Potpara T, Dagres N et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 25.Oldenburg O, Lamp B, Faber L et al. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–7. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Kapur VK, Auckley DH, Chowdhuri S et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13:479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell AJ, Neill AM. Home set-up polysomnography in the assessment of suspected obstructive sleep apnea. J Sleep Res. 2011;20:207–13. doi: 10.1111/j.1365-2869.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- 28.Banhiran W, Durongphan A, Saleesing C, Chongkolwatana C. Diagnostic properties of the STOP-Bang and its modified version in screening for obstructive sleep apnea in Thai patients. J Med Assoc Thai. 2014;97:644–54. [PubMed] [Google Scholar]
- 29.BaHammam AS, Han F, Gupta R et al. Asian accreditation of sleep medicine physicians and technologists: practice guidelines by the Asian Society of Sleep Medicine. Sleep Med. 2021;81:246–52. doi: 10.1016/j.sleep.2021.02.041. [DOI] [PubMed] [Google Scholar]
- 30.Randerath WJ, Verbraecken J, Andreas S et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J. 2011;37:1000–28. doi: 10.1183/09031936.00099710. [DOI] [PubMed] [Google Scholar]
- 31.Qaseem A, Holty JE, Owens DK et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471–83. doi: 10.7326/0003-4819-159-7-201310010-00704. [DOI] [PubMed] [Google Scholar]
- 32.Kuna ST, Reboussin DM, Strotmeyer ES et al. Effects of weight loss on obstructive sleep apnea severity. Ten-year results of the Sleep AHEAD study. Am J Respir Crit Care Med. 2021;203:221–9. doi: 10.1164/rccm.201912-2511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster GD, Borradaile KE, Sanders MH et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansson K, Neovius M, Lagerros YT et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ. 2009;339:b4609. doi: 10.1136/bmj.b4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuomilehto HP, Seppä JM, Partinen MM et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–7. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 36.Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192:175–84. doi: 10.1007/s00408-013-9511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEvoy RD, Antic NA, Heeley E et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–31. doi: 10.1056/nejmoa1606599. [DOI] [PubMed] [Google Scholar]
- 38.Milleron O, Pillière R, Foucher A et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25:728–34. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Cassar A, Morgenthaler TI, Lennon RJ et al. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:1310–4. doi: 10.1016/j.jacc.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Zhang Y, Dong Z et al. Effect of continuous positive airway pressure on long-term cardiovascular outcomes in patients with coronary artery disease and obstructive sleep apnea: a systematic review and meta-analysis. Respir Res. 2018;19:61. doi: 10.1186/s12931-018-0761-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peker Y, Thunström E, Glantz H et al. Outcomes in coronary artery disease patients with sleepy obstructive sleep apnoea on CPAP. Eur Respir J. 2017;50:1700749. doi: 10.1183/13993003.00749-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.da Silva Paulitsch F, Zhang L. Continuous positive airway pressure for adults with obstructive sleep apnea and cardiovascular disease: a meta-analysis of randomized trials. Sleep Med. 2019;54:28–34. doi: 10.1016/j.sleep.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 43.Resano-Barrio MP, Arroyo-Espliguero R, Viana-Llamas MC, Mediano O. Obstructive sleep apnoea syndrome: continuous positive airway pressure therapy for prevention of cardiovascular risk. Eur Cardiol. 2020;15:e65. doi: 10.15420/ecr.2020.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pack AI, Magalang UJ, Singh B et al. Randomized clinical trials of cardiovascular disease in obstructive sleep apnea: understanding and overcoming bias. Sleep. 2021;44:zsaa229. doi: 10.1093/sleep/zsaa229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynor A, McArdle N, Shenoy B et al. Continuous positive airway pressure and adverse cardiovascular events in obstructive sleep apnea: are participants of randomized trials representative of sleep clinic patients? Sleep. 2022;45:zsab264. doi: 10.1093/sleep/zsab264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yancy CW, Jessup M, Bozkurt B et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017;23:628–51. doi: 10.1016/j.cardfail.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Kato T, Suda S, Kasai T. Positive airway pressure therapy for heart failure. World J Cardiol. 2014;6:1175–91. doi: 10.4330/wjc.v6.i11.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shim CY, Kim D, Park S et al. Effects of continuous positive airway pressure therapy on left ventricular diastolic function: a randomised, sham-controlled clinical trial. Eur Respir J. 2018;51:1701774. doi: 10.1183/13993003.01774-2017. [DOI] [PubMed] [Google Scholar]
- 49.Patel N, Donahue C, Shenoy A et al. Obstructive sleep apnea and arrhythmia: a systemic review. Int J Cardiol. 2017;228:967–70. doi: 10.1016/j.ijcard.2016.11.137. [DOI] [PubMed] [Google Scholar]
- 50.Holmqvist F, Guan N, Zhu Z et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation – results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647–54e2. doi: 10.1016/j.ahj.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 51.Sia CH, Hong Y, Tan LWL et al. Awareness and knowledge of obstructive sleep apnea among the general population. Sleep Med. 2017;36:10–7. doi: 10.1016/j.sleep.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 52.University Health Network, University of Toronto. STOP-Bang Questionnaire. 2012. http://www.stopbang.ca/osa/screening. php (accessed 13 April 2022)
- 53.Carey RM, Calhoun DA, Bakris GL et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–90. doi: 10.1161/hyp.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]


