Abstract
Three-dimensional (3D) printing uses a process of adding material in a layer-by-layer fashion to form the end product. This technology is advancing rapidly and is being increasingly utilized in the medical field as it becomes more accessible and cost-effective. It has an increasingly important role in ophthalmology and eyecare as its current and potential applications are extensive and slowly evolving. Three-dimensional printing represents an important method of manufacturing customized products such as orbital implants, ocular prostheses, ophthalmic models, surgical instruments, spectacles and other gadgets. Surgical planning, simulation, training and teaching have all benefitted from this technology. Advances in bioprinting seem to be the future direction of 3D printing with possibilities of printing out viable ocular tissues such as corneas and retinas in the future. It is expected that more ophthalmologists and other clinicians will use this technology in the near future.
Keywords: 3D printing, bioprinting, cornea, ophthalmology, retina
Introduction
Three-dimensional (3D) printing, also known as additive manufacturing (AM), utilizes a process of adding material in a layer-by-layer fashion to form the end product. Three-dimensional printing has advanced rapidly over the recent years, allowing for a wider range of technologies, materials and applications to be realized.1 –7 One of the key areas where 3D printing has demonstrated a wide range of applications is the medical sector.8,9 In the literature, the use of 3D printing for medical applications was reported in areas such as medical instruments, 10 pharmaceuticals, 11 diagnostics, 12 orthopaedics,13,14 drug delivery,15,16 cardiology, 17 dentistry, 18 response to pandemics, 19 general surgery, 20 spinal surgery, maxillofacial surgery, neurosurgery and cardiac surgery. 21 In the field of ophthalmology and eyecare, the role of 3D printing is evolving, particularly after the introduction of high-resolution pico- to micrometre scale 3D printers. The most common applications are production of orbital implants, ocular prostheses, intraocular devices, ophthalmic models and surgical instruments. Other uses include pre-operative surgical planning, simulation, training and teaching.22,23
A PubMed search was carried out using the terms ‘3D printing’, ‘three-dimensional printing’, ‘ophthalmology’ and ‘bioprinting’. The reference list in each relevant article was inspected for additional relevant publications. This review aims at presenting a comprehensive account of recent developments and applications in 3D printing of ophthalmology. The review starts with an overview of 3D printing principles, followed by a detailed review of recent applications in ophthalmology and eyecare.
Principles of 3D printing
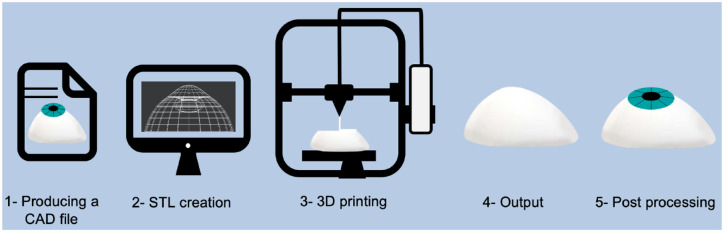
Three-dimensional printing is the process of joining materials to make parts from 3D model data, usually layer upon layer. There are several approaches to 3D printing, 24 but all of them follow five principal steps as described below and shown in Figure 1:
Figure 1.
An illustration of the five core steps of 3D printing.
Production of a digital model: A digital model of the target part is produced, either by using computer-aided design (CAD) tools or by reverse engineering an existing part via 3D scanning methods [e.g. computed tomography (CT)-scanning or magnetic resonance imaging (MRI) images].
Conversion of model into a print file: In this step, the CAD file is converted into a format that the 3D printer can read. This is most commonly a Standard Triangle Language (STL) file. This file undergoes manipulation by a slicer programme that slices the design into layers used to print up the product.
Three-dimensional printing: The physical part is printed, and there are several different 3D printing technologies that could be used and are discussed later in this section.
Print removal: Once printing is completed, the printed part is removed from the printer. This could be as simple as detaching the print from a build platform but can be more complicated depending on the type of technology used.
Post processing: The printed part could be post-processed using different methods to fulfil functional or aesthetic requirements.
Categories of 3D printing technologies
There are seven standard 3D printing technologies. 24 For the purpose of this review, a summary of the commonly used processes in ophthalmology applications are presented here.25,26
Stereolithography
Stereolithography (SLA) is a vat-polymerisation technology, where a vat of liquid photopolymer resin is selectively exposed to a curing radiation following a CAD-controlled process. A build platform within the vat is altered in height during the process, thus additional layers are built on top of each other. At the end, the vat is drained of liquid resin, and the object is removed. A variant SLA process is digital light processing (DLP), which uses a digital light projector that flashes a two-dimensional (2D) image of each layer at once. This allows DLP to achieve much faster printing times compared with SLA, which uses a single-point source.
Fused filament fabrication
Fused filament fabrication (FFF), also known as fused deposition modelling (FDM), is a material-extrusion technology. In this process, a thermoplastic material is heated to a semi-molten state and extruded at a precise location on the build platform, where the material solidifies as it cools. Once a layer is complete, the build platform moves down allowing the process to be repeated, thus building up the part layer by layer until complete.
Selective laser sintering
Selective laser sintering (SLS) uses a printing process called powder bed fusion (PBF), where a thermal energy source is used to selectively induce fusion between powder particles at a specific location inside a build area to create a solid object. Un-sintered powder stays in place to support the part as it is being built, thus eliminating the need for support structures.
Table 1 summarizes the characteristics and capabilities of some 3D printing technologies.25 –28
Table 1.
Commonly used 3D printing technologies in ophthalmology applications and their characteristics.
| Technology | Printing process | Typical layer thickness (µm) | Dimensional accuracy | Materials | Pros | Cons |
|---|---|---|---|---|---|---|
| SLA | Vat polymerization | 25–100 | ±0.15% to ±0.5% | Photopolymer resin | • Accuracy • High resolution resulting in smooth surface finish |
• Slow printing speed • Post processing required to remove support structures • Handling and storage of chemicals |
| DLP | Vat polymerization | 25–100 | ±0.15% to ±0.5% | Photopolymer resin | • Quicker higher printing speed than SLA due to area curing | • Post processing required to remove support
structures • Handling and storage of chemicals |
| FFF | Material extrusion | 50–400 | ±0.5% | Thermoplastic filaments or granules | • Strong prints • Low-cost printer • Low material cost |
• Slow printing speed • Lower accuracy • Requires support structure for overhang features less than 45° |
| SLS | Power bed fusion | 100 | ±0.3% | Thermoplastic powder | • Strong prints • No support structures needed • Wide range of materials available |
• Skilled operator required • Rough surface finish • Laborious process in terms of powder cleaning and recycling |
DLP, digital light processing; FFF, fused filament fabrication; SLA, stereolithography; SLS, selective laser sintering.
Advantages and limitations of 3D printing
Some of the key advantages of 3D printing are:
Unique shapes and designs: 3D printing enables the production of complex geometries, such as assemblies and lattices.
Design flexibility and customisation: 3D printing enables mass customisation, where the same 3D printer could be used to build an almost limitless variety of designs.
Reduced product development costs: building within a 3D printer machine is generally performed in a single step, which reduces the number of iterative design stages. 29
Material variety: 3D-printable materials include polymers, metals, ceramics and composites.
Some of the main 3D printing limitations are:
Limited throughput: The layer-by-layer approach in 3D printing results in a generally long printing cycle, which tends to increase with increasing part size and complexity.
Limited build volume: Most commercially available 3D printer systems have relatively small build volumes, which severely limits production capacity for relatively larger parts.
Directional properties: Due to the layer-by-layer approach in 3D printing, dimensions and mechanical properties tend to be different in the z-direction (build direction) compared with the x- and y-directions.
Limited range of materials for medical applications: Only a small number of materials are classified as safe for medical applications, especially for placing inside the body. 29
Advancements in 3D printing for healthcare applications
Recent developments in 3D printing technologies have focused on overcoming general technical challenges in 3D printing, such as process throughput or build volume. When it comes to healthcare applications, certain developments have made it possible for the technology to find wider applications in this sector. For example, the resolution of 3D printers has improved drastically in the last few decades, with some 3D printers capable of printing resolutions from pico- to micrometre scale features. 30 In addition, it is now possible to 3D print parts with a range of materials, including biomaterials, such as bioprinting living cells and growth factors 31 with potential applications in ophthalmology, such as corneas. 32 Three-dimensional printing of smart materials [also known as four-dimensional (4D) printing] has allowed for building parts that exhibit changes in functionality, property and shape as a function of time, including smart biomaterials, 33 with applications including implants, medical devices and complex surgery. 34 In healthcare, the translation of medical imaging such as CT and MRI from virtual images to physical models has allowed surgeons and physicians to enhance visualization of lesions, planning surgical procedures and communication with patients.35,36 Many hospitals around the world are slowly adopting and integrating the use of 3D printing technology for various uses such as education, surgical planning and medical research. 35 It has also been shown to be promising in pharmaceutical applications including the development of multifunctional drug delivery systems. 37
Applications of 3D printing in ophthalmology and eye care
Surgical applications
Three-dimensional printing technology is useful for objects that are produced in small series and require a high degree of customization. The benefit of utilizing 3D technology as opposed to other techniques is that it avoids the need to build a mould for every production series, thus having the potential for unlimited customized design possibilities at a reasonable cost. 38
Orbital implants
Three-dimensional printing technology is becoming widely used to perform more accurate orbital wall reconstruction surgery for orbital blowout fractures39,40 and developmental anomalies. 41 In the traditional surgical method, the surgeon inspects the fracture site and visually excises a 2D orbital implant that approximately corresponds to the anatomy of the fracture site. However, 3D printing enables the manufacture of implants that precisely fit the size, shape and contour of each individual fracture site. In their study, Kang et al. 42 have shown a novel approach of 3D printing templates that mould customized orbital implants. The study showed excellent outcomes in all 11 patients.
Three-dimensional printing is also utilized in printing orbital implant spheres for evisceration and enucleation. 43 Three-dimensional printing technology allows for the customization of implant formats and sizes allowing for varying eye socket dimensions. Kormann et al. 44 tested the biocompatibility of using photocurable resin to 3D print orbital implants for 11 patients who underwent evisceration. None of the patients showed any signs of local inflammation or change in implant size at 12 months post-operatively. Nahumi et al. 45 described a case report for a patient with fronto-orbital fibrous dysplasia who had primary reconstruction surgery repair with the aid of 3D-printed customized polyetheretherketone (PEEK) implantation.
Ocular prostheses
Making customized ocular prosthesis for patients who have lost their eyes is time-consuming, labour-intensive and expensive. It requires manual artistic work that can usually only be done by a skilled ocularist or craftsman. Three-dimensional printing technology gives the ability to manufacture high-quality, custom-made ocular prostheses that are quicker and cheaper to produce. 46 In 2016, the first 3D-printed ocular prosthesis was successfully fitted for a 68-year-old man. 47 The fitting method was done through a cone beam CT scan of the patient in order to design a digital 3D model of the anophthalmic cavity. This is different from the conventional method of creating an impression mould as it does not require injecting impression material into the anophthalmic cavity. Also, with conventional methods, it is not possible to store data pertaining to the previously created ocular prostheses, and reproducing the original work requires the same amount of time, effort and cost in case of damage.
Surgical planning
An example of pre-operative surgical planning is the use of 3D-printed models of eyes in patients with intraocular tumours such as uveal melanomas. In one study, 3D printing has been used to aid clinicians in planning stereotactic radiosurgery (SRS). SRS is a treatment option for uveal melanoma, one of the most aggressive types of intraocular tumours. Precise planning of stereotactic coordinates of radiation beams of SRS is crucial. This makes the printing of 3D models of the tumour useful as it can provide additional information and better localization of the lesion inside the globe to maximize the accuracy of the treatment. 48
Intraocular devices
In an ophthalmic surgical setting, a 3D pupil expansion device, called Canabrava’s Ring, was the first intraocular device manufactured using 3D printing technology. This device achieves a pupillary dilation of 6.5 mm. It also allows cataract surgeries to be performed using standard techniques. 49
In their study, Navajas and Ten Hove 50 used an SLS 3D printer to manufacture a customized trocar system for vitreoretinal surgery. The printed devices were evaluated and tested on pigs’ eyes. This was the first study to demonstrate the feasibility of printing trocar systems using commercially available 3D printing technology. The printed products had some limitations including minimum size achievable and mechanical strength. However, with improving 3D technology, the tensile strength of the materials used and using 3D printers with higher resolutions, these limitations could be overcome. In the future, 3D printing will allow surgical instruments to be inexpensively customized and printed, therefore tailoring it to the surgeon’s needs and preferences. 38
Intraocular lenses (IOLs) are another example of personalized items that have benefitted from advancements in 3D printing technology. Debellemanière et al. 51 have attempted a reproduction of Ridley’s lens using 3D printing. Although the printed lens showed good transparency, it did not meet the current IOL standards due to suboptimal optical quality and surface roughness of the printed IOL. This advancement, nevertheless, could have a vital impact by offering complete customization of future IOL design.
Ruzza et al. 52 have used 3D printing technology to develop and print a smart storage glide that is capable of storing and delivering posterior lenticules needed for Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) surgery. The 3D-printed smart storage glide was clinically validated and ensured the safe delivery of the pre-cut tissues allowing the surgeon to perform the keratoplasty more easily.
Bioprinting and tissue engineering
Three-dimensional bioprinting is a 3D printing technology whereby cells are combined with a suitable biomaterial and deposited within micrometre precision. This is done layer by layer to generate tissue constructs for a variety of applications including, but not limited to, tissue engineering. 53 Bioprinting has already been widely applied to construct functional tissues such as vasculature, muscle, cartilage and bone. 54
The goal of tissue engineering is to produce 3D artificial tissues or organs consisting of scaffold, cells and microenvironment that mimic the real environment of the human body. Generating scaffolds is a key element for tissue engineering as they provide the necessary support and physical structure for transplanted cells to attach, grow and maintain their physiological functions. The disadvantages of conventional methods of manufacturing 3D scaffolds such as electrospinning and freeze-drying include lack of precise control of internal structure features and topology. Three-dimensional printing is increasingly being recognized as a potential solution to manufacture complex tissue engineering scaffolds. Using this technology, shape, size, porosity and interconnectivity of tissue engineered scaffolds can be controlled with very high precision. 30 This can also be a very effective way to study disease progression, mechanisms of drug action and application in tissue and organ transplants.
Three-dimensional printing has a potentially revolutionary role in the production of tissue engineered scaffolds, as it creates the most suitable scaffolds through simple and effective porosity dimensions that cannot be achieved using traditional techniques.
In the last few decades, the development of 3D bioprinting, aiming to reconstruct tissues and organs with designed complex geometries, demonstrated the immense potential and promising results in regenerative medicine. Three-dimensional bioprinting is an emerging technology that can be harnessed for the manufacture of biological tissue for clinical applications.
Cornea
There is a severe shortage of donor cornea as the main source for cornea transplantation is based on donations. 55 Three-dimensional bioprinting can be a promising approach for corneal grafts. The advantages of this technology in corneal regeneration enable personalized corneal implants and single- or multi-layer corneal equivalents with controllable structure and designed refractive ability. 32
Isaacson et al. 56 produced corneal structures that resembled the structure of the native human stroma using 3D digital human cornea models and a suitable support structure. These were bioprinted with collagen-based bio-ink containing corneal keratocytes. Keratocytes showed high cell viability 1 day and 7 days post printing. Although their study had many limitations, they have demonstrated the feasibility of using 3D bioprinting to create an artificial biological cornea for regenerative medicine application.
In another study, Zhang et al. 57 combined DLP and extrusion bioprinting into an integrated 3D cornea bioprinting system. A personalized corneal substitute was designed based on CT scan of a natural cornea and mathematical modelling. Using their method, it was feasible to manufacture geometry controllable biosynthetic corneas with controllable thickness and curvature.
Kim et al. 58 used 3D bioprinting processes to deposit and densely arrange cultured human corneal endothelial cells that overexpress RNase 5 on amniotic membranes. RNase 5 is well known for promoting cell survival. These 3D bioprinted grafts were transplanted on rabbit models. The results showed that the bioprinted corneal endothelium with cultured human corneal endothelial cells easily survives and functions as a corneal endothelium in vivo, and that overexpression of RNase 5 can be an option to obtain higher graft cellularity to enhance the function of transplanted grafts.
In another study, Kim et al. 59 developed a bio-ink that can optically and biochemically provide a cornea-mimicking microenvironment. The developed bio-ink did not have any cytotoxic effects on encapsulated cells and has been tested in mice and rabbits. The authors argue that their bio-ink can be applied to 3D cell printing technology to support the progress of cornea tissue engineering applications.
Retina
As various diseases related to blindness come from damage to the retina, the development of retinal alternatives is one of the promising treatments on 3D printing technology.
Worthington et al. 60 have demonstrated the feasibility of creating 3D-printed scaffolds for retinal progenitor cells. This could facilitate in vitro studies of photoreceptor cell behaviour, disease pathogenesis and novel treatments for retinal degeneration. In another recent article, 61 a high-resolution 3D-printed extracellular matrix was developed that is compatible with pluripotent stem cells and early retinal cells. This biopolymer-based scaffold supported the growth and attachment of retinal cells. Authors of the study anticipate that this approach will enable more efficient and accurate retinal disease modelling and therapeutic testing in vitro than current techniques allow.
Wang et al. 62 have synthesized chemically modified hyaluronic acid hydrogel which resembled hydrogels within the native retina. This hydrogel was suitable for 3D bioprinting of a retinal structure and improved the differentiation of retinal progenitor cells into photoreceptors with the support of retinal-pigment epithelium (RPE).
Kim et al. 63 have fabricated a Bruch’s membrane-mimetic substance using 3D printing technology. The structure and extracellular matrix components were very similar to those of the natural Bruch’s membrane, which enabled the growth and maturation of retinal-pigment cells (RPE). This could potentially be used in the future for RPE transplantation to treat a wide variety of diseases such as age-related macular degeneration, retinitis pigmentosa and Stargardt disease.
Lorber et al. 64 have shown that two types of retinal cells (retinal ganglion cells and glial cells) can be successfully printed using 3D printing. These cells remained viable and did not change their phenotypic features as a result of the printing process. This is a promising step towards creating a functional retinal graft. However, there are many issues that remain to be addressed before printing a functional retina. More research is needed to explore whether other retinal cells can be printed as well and if a complex retinal structure can be created using 3D printing.
Tarsal plate
Tarsal plate regeneration has always been a problem in the treatment on eyelid defects such as those occurring from tumour invasion of the tarsal plate. Three-dimensional printing technology has been applied for the first time in 2020 for the manufacture of tarsal plate scaffolds using poly-caprolactone. 65 These 3D-printed scaffolds were coated with adipose-derived mesenchymal stromal cells, and sebocytes were seeded on the scaffolds so that natural lipids were secreted for replacing meibocytes. In vitro experiments of the study showed excellent biocompatibility of the scaffolds with sebocytes. In vivo experiments revealed excellent sebocytes proliferation on the scaffolds and secreted abundant neutral lipid. This shows that 3D printing techniques can be promising in the field of tarsal plate tissue engineering and eyelid construction.
Non-surgical applications
Customized spectacles and prescription lenses
Three-dimensional printing technology has been used to produce customized spectacles for the first time in 2018.66 –68 The customized spectacles were manufactured for a child with facial abnormalities due to Goldenhar syndrome. 68 This was achieved by taking surface topography images of the patient’s face to produce a 3D digital model, that was 3D printed. Customized spectacles were designed using the face software data of the child taking all measurements into consideration. The customized spectacles were 3D printed using SLA apparatus. The customized 3D-printed spectacles have superior optical alignment, and greater comfort and cosmesis. As a significant number of children with facial deformities require spectacle correction, it is essential to provide appropriate frames for this group of patients. Because of their uniquely irregular anatomy, commercially available spectacles fit poorly. The 3D printing technique described herein may offer a novel and accurate option. Similarly, in another study, CT scans of patients with craniofacial abnormalities were used to produce a digital model and make custom-printed 3D spectacles. 69
On a more commercial scale, Luxexcel became the first company in the world to 3D print prescription lenses. 70 These lenses have already started to be produced at high volume and have passed the American Food and Drug Administration (FDA), International Organization for Standardization (ISO) and the American National Standards Institute (ANSI) standards. This new development can enable the integration of smart technology within the 3D-printed lenses to produce smart interactive prescription glasses. This can potentially include the integration of liquid crystal displays (LCDs) in the prescription lenses. The company believes that there will be a mass demand for these smart glasses in the next decade.
Smartphone adaptors
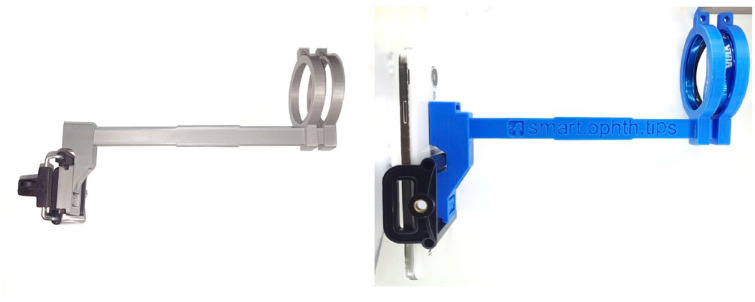
Three-dimensional printing technology is used to manufacture many prototype devices and gadgets. In ophthalmology, it has been utilized to produce smartphone lens holders which can take high-quality fundus photos.71,72 An example of these adaptors is shown in Figure 2. This low-cost adaptor that easily couples lenses to smartphones can make teleophthalmology increasingly accessible. The adaptors are continuously improving in design and functionality. For example, a hand-held, battery-powered 3D-printed optical and hardware system built around a smartphone called CellScope Retina was recently produced. 73 This was capable of capturing wide-field, 100° images of a broad variety of retinal pathologies.
Figure 2.
Smartphone lens holder produced using 3D technology.
Other prototypes produced for use in ophthalmology include slit-lamp adaptors for smartphones, and an example is shown in Figure 3.
Figure 3.

Slit-lamp adaptor for smartphones produced using 3D printing technology.
In the future, teleophthalmology can also be utilized by healthcare staff with minimal training in specialized ophthalmology to remotely capture and share high-quality retinal images in order to enhance multidisciplinary communication between healthcare providers. During the COVID-19 pandemic in 2020, this received more interest in view of the isolation, shielding and social-distancing measures needed.
Moisture chamber spectacles
Moisture chamber spectacles were made using 3D technology for selective patients with chronic dry eyes to improve symptoms of ocular discomfort. In a cross-over study, personalized moisture chamber spectacles (PMCS) were 3D printed and compared with commercially available uniform moisture chamber spectacles (UMCS). The results show statistically significant improvement in periocular humidity in PMCS compared with UMCS. 74 This could be useful in reducing the use of topical lubricants in severe dry eye diseases.
Contact lens fitting
Three-dimensional printing technology can be used in the simulation fitting of rigid gas permeable contact lenses for patients with high degree of refractive error, keratoconus and corneal transplantation. In one study, a 3D model of the corneal anterior surface was 3D printed using digital data obtained from corneal topography. The 3D model was used as a contact lens trial until a fit is achieved before doing the final trial on the patient. 75 This method is safer and can reduce the number of trials patients have to have, thus reducing patient discomfort and minimizing the risk of corneal damage and infection.
Eyelid crutches
Three-dimensional printing technology has been used to produce inexpensive eyelid crutches to help patients with significant ptosis that has reoccurred after surgical interventions such as chronic progressive external ophthalmoplegia. The 3D-printed eyelid crutches were cheaper to produce than traditional ones and were easily removable and more adjustable, and thus have the potential for large-scale production. 76
Simulation, training and teaching applications
The development of 3D printing technology allowed the manufacture of several models for ophthalmic training and simulation. This is becoming popular in ophthalmology, as it is in other surgical specialities, with the expectation that surgery is taught initially in a simulated environment before moving on to patients. Furthermore, simulation is attracting more interest both in the introduction of new techniques and in the assessment of procedural competence. Maintenance of surgical competence would be another very important benefit for trainees and their seniors alike whenever there is a gap in performing any given surgery. This was brought to the fore by the severe effect the COVID-19 pandemic in 2020 had on surgical activity worldwide.
Simulation
Trainees have performed wet-lab bony orbital decompressions and simulated upcoming surgeries on 3D-printed orbits. 77
In another study, Jagan et al. 78 have designed a novel 3D-printed silicone eye which was used for simulating various strabismus surgeries. In their multicentre study, a validated questionnaire was developed to compare the fidelity of practicing strabismus surgery with their 3D-printed silicone eye model to that with a rabbit head. The 3D-printed model had statistically significant better results for anatomical accuracy and position of eyes. The 3D-printed model also had a conjunctiva and sclera that mimicked real human tissue.
A 3D-printed iris has been used to simulate an anterior chamber in a new wet-lab model for teaching Descemet Membrane Endothelial Keratoplasty (DMEK). 79 This method permits the performance of all surgical steps via simulation. It also has the option of practicing various surgical difficulties by changing the depth of the anterior chamber.
Education
Three-dimensional-printed models of choroidal vessels and choroidal tumours have been produced based on optical coherence tomography (OCT) images. 80 Thirteen choroidal 3D models were printed using different techniques including SLS, SLA and FFF. Models were magnified 70–100 times. These models have shed new light onto the 3D architecture of the choroidal vessels and the interactions of choroidal tumours and the surrounding vascular networks. This will be useful as tactile models can be used in patient education about their medical condition. This advancement can also potentially represent a new way to monitor tumour size, growth or response to therapy in the future.
The pterygopalatine fossa is poorly visualized in cadaveric dissections and is one of the most complicated anatomical regions. The 2D textbook schematics do not allow full appreciation of its structure and communicating channels. Bannon et al. 81 have produced a low-cost ‘negative space’ model of the pterygopalatine fossa using open source software and 3D printing technology. This could be a useful aid for understanding the complex anatomy of the pterygopalatine fossa. This 3D model can also be replicated in an affordable manner by other ophthalmology departments. 82
Future directions and conclusion
Three-dimensional printing is an evolving technological advance that is being increasingly utilized in the medical field. It is revolutionizing medical and surgical procedures as it becomes widely accessible, especially as it can be very cost-effective. Three-dimensional printing has an increasingly important role in ophthalmology and its current and potential applications are slowly increasing. It represents an important method of manufacturing customized products such as orbital implants, ocular prostheses, ophthalmic models, surgical instruments, spectacles and other gadgets. Surgical planning, simulation, training and teaching have all benefitted from the evolving 3D technology. It is anticipated that more ophthalmologists and other clinicians will use this technology in the near future.
Advances in bioprinting seem to be the future direction of 3D printing with possibilities of printing out viable ocular tissues such as corneas and retinas. The hope has been raised that creating a functional retina to cure blindness is within reach. It is necessary to continuously improve existing technologies and materials and develop new printing equipment, in order to realize the construction of fully functional artificial ocular tissues in the future. The additional prospects of 4D printing and tissue regeneration (especially the retina) bode very well for an exciting future for this technology.
Acknowledgments
We thank Dr Ahmed Ateya for providing us with Figure 2 and Figure 3 of this manuscript.
Footnotes
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Yazan Fakhoury: Formal analysis; Investigation; Project administration; Writing – original draft.
Abdallah Ellabban: Conceptualization; Methodology; Supervision; Writing – original draft.
Usama Attia: Formal analysis; Writing – original draft.
Ahmed Sallam: Methodology; Writing – review & editing.
Samer Elsherbiny: Conceptualization; Methodology; Supervision; Writing – review & editing.
ORCID iDs: Yazan Fakhoury  https://orcid.org/0000-0002-1749-3732
https://orcid.org/0000-0002-1749-3732
Usama Attia  https://orcid.org/0000-0002-6329-6385
https://orcid.org/0000-0002-6329-6385
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Availability of data and materials: Not applicable.
Contributor Information
Yazan Fakhoury, Medical Doctor, St James’s University Hospital, Beckett St, Harehills, Leeds, LS9 7TF, UK.
Abdallah Ellabban, Hull University Teaching Hospitals NHS Trust, Kingston upon Hull, UK; Suez Canal University, Ismailia, Egypt.
Usama Attia, Manufacturing Technology Centre (MTC), Coventry, UK.
Ahmed Sallam, Jones Eye Institute, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Samer Elsherbiny, Machen Eye Unit, Warwick Hospital, South Warwickshire NHS Foundation Trust, Warwick, UK; Warwick Medical School, University of Warwick, Coventry, UK.
References
- 1. Nadagouda MN, Rastogi V, Ginn M. A review on 3D printing techniques for medical applications. Curr Opin Chem Eng 2020; 28: 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bozkurt Y, Karayel E. 3D printing technology; methods, biomedical applications, future opportunities and trends. J Mater Res Technol 2021; 14: 1430–1450. [Google Scholar]
- 3. Kumar R, Kumar M, Chohan JS. The role of additive manufacturing for biomedical applications: a critical review. J Manuf Process 2021; 64: 828–850. [Google Scholar]
- 4. Jadhav A, Jadhav VS. A review on 3D printing: an additive manufacturing technology. Mater Today Proc. Epub ahead of print 10 March 2022. DOI: 10.1016/J.MATPR.2022.02.558. [DOI] [Google Scholar]
- 5. Praveena BA, Lokesh N, Buradi A, et al. A comprehensive review of emerging additive manufacturing (3D printing technology): methods, materials, applications, challenges, trends and future potential. Mater Today Proc 2022; 52: 1309–1313. [Google Scholar]
- 6. Sheoran AJ, Kumar H, Arora PK, et al. Bio-medical applications of additive manufacturing: a review. Procedia Manuf 2020; 51: 663–670. [Google Scholar]
- 7. Al-Dulimi Z, Wallis M, Tan DK, et al. 3D printing technology as innovative solutions for biomedical applications. Drug Discov Today 2021; 26: 360–383. [DOI] [PubMed] [Google Scholar]
- 8. Yan Q, Dong H, Su J, et al. A review of 3D printing technology for medical applications. Engineering 2018; 4: 729–742. [Google Scholar]
- 9. Javaid M, Haleem A. Additive manufacturing applications in medical cases: a literature based review. Alex J Med 2018; 54: 411–422. [Google Scholar]
- 10. Culmone C, Smit G, Breedveld P. Additive manufacturing of medical instruments: a state-of-the-art review. Addit Manuf 2019; 27: 461–473. [Google Scholar]
- 11. Varghese R, Sood P, Salvi S, et al. 3D printing in the pharmaceutical sector: advances and evidences. Sensors Int 2022; 3: 100177. [Google Scholar]
- 12. Yang J, Cheng Y, Gong X, et al. An integrative review on the applications of 3D printing in the field of in vitro diagnostics. Chinese Chem Lett 2022; 33: 2231–2242. [Google Scholar]
- 13. Javaid M, Haleem A. Current status and challenges of additive manufacturing in orthopaedics: an overview. J Clin Orthop Trauma 2019; 10: 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar L, Haleem A, Javaid M. Impact of three dimensional printing in orthopedics. Glob Health J 2021; 5: 178–182. [Google Scholar]
- 15. Durga Prasad Reddy R, Sharma V. Additive manufacturing in drug delivery applications: a review. Int J Pharm 2020; 589: 119820. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Zhang Y, Aghda NH, et al. Emerging 3D printing technologies for drug delivery devices: current status and future perspective. Adv Drug Deliv Rev 2021; 174: 294–316. [DOI] [PubMed] [Google Scholar]
- 17. Lindquist EM, Gosnell JM, Khan SK, et al. 3D printing in cardiology: a review of applications and roles for advanced cardiac imaging. Ann 3D Print Med 2021; 4: 100034. [Google Scholar]
- 18. Bhargav A, Sanjairaj V, Rosa V, et al. Applications of additive manufacturing in dentistry: a review. J Biomed Mater Res B Appl Biomater 2018; 106: 2058–2064. [DOI] [PubMed] [Google Scholar]
- 19. Vakharia VN, Khan S, Marathe K, et al. Printing in a pandemic: 3D printing solutions for healthcare during COVID-19. A protocol for a PRISMA systematic review. Ann 3D Print Med 2021; 2: 100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krauel L, Valls-Esteve A, Tejo-Otero A, et al. 3D-printing in surgery: beyond bone structures. A review. Ann 3D Print Med 2021; 4: 100039. [Google Scholar]
- 21. Tack P, Victor J, Gemmel P, et al. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online 2016; 15: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang W, Zhang X. 3D printing: print the future of ophthalmology. Investig Ophthalmol Vis Sci 2014; 55: 5380–5381. [DOI] [PubMed] [Google Scholar]
- 23. Akkara J, Kuriakose A. The magic of three-dimensional printing in ophthalmology. Kerala J Ophthalmol 2018; 30: 209. [Google Scholar]
- 24. ISO/ASTM 52900:2015. Additive manufacturing – general principles – terminology. [Google Scholar]
- 25. Ngo TD, Kashani A, Imbalzano G, et al. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos Part B Eng 2018; 143: 172–196. [Google Scholar]
- 26. Lee JY, An J, Chua CK. Fundamentals and applications of 3D printing for novel materials. Appl Mater Today 2017; 7: 120–133. [Google Scholar]
- 27. Redwood B, Schöffer F, Garret B. The 3D printing handbook: technologies, design and applications. Amsterdam: 3D Hubs B.V., 2017. [Google Scholar]
- 28. Tofail SAM, Koumoulos EP, Bandyopadhyay A, et al. Additive manufacturing: scientific and technological challenges, market uptake and opportunities. Mater Today 2018; 21: 22–37. [Google Scholar]
- 29. Gibson I, Rosen DW, Stucker B. Additive manufacturing technologies – rapid prototyping to direct digital manufacturing. New York: Springer, 2010. [Google Scholar]
- 30. Li J, Chen M, Fan X, et al. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med 2016; 14: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng 2015; 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang B, Xue Q, Li J, et al. 3D bioprinting for artificial cornea: challenges and perspectives. Med Eng Phys 2019; 71: 68–78. [DOI] [PubMed] [Google Scholar]
- 33. Lui YS, Sow WT, Tan LP, et al. 4D printing and stimuli-responsive materials in biomedical aspects. Acta Biomater 2019; 92: 19–36. [DOI] [PubMed] [Google Scholar]
- 34. Javaid M, Haleem A. 4D printing applications in medical field: a brief review. Clin Epidemiol Glob Health 2019; 7: 317–321. [Google Scholar]
- 35. Abdullah KA, Reed W. 3D printing in medical imaging and healthcare services. J Med Radiat Sci 2018; 65: 237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Squelch A. 3D printing and medical imaging. J Med Radiat Sci 2018; 65: 171–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jamróz W, Szafraniec J, Kurek M, et al. 3D printing in pharmaceutical and medical applications – recent achievements and challenges. Pharm Res 2018; 35: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kent C. 3-D printing technology in ophthalmology. Review of Ophthalmology, 10 April 2018, https://www.reviewofophthalmology.com/article/3d-printing-technology-in-ophthalmology (accessed 13 May 2020).
- 39. Fan B, Chen H, Sun YJ, et al. Clinical effects of 3-D printing-assisted personalized reconstructive surgery for blowout orbital fractures. Graefes Arch Clin Exp Ophthalmol 2017; 255: 2051–2057. [DOI] [PubMed] [Google Scholar]
- 40. Nekooei S, Sardabi M, Razavi ME, et al. Implantation of customized, preshaped implant for orbital fractures with the aid of three-dimensional printing. Middle East Afr J Ophthalmol 2018; 25: 56–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mourits DL, Wolff J, Forouzanfar T, et al. 3D orbital reconstruction in a patient with microphthalmos and a large orbital cyst – a case report. Ophthalmic Genet 2016; 37: 233–237. [DOI] [PubMed] [Google Scholar]
- 42. Kang S, Kwon J, Ahn CJ, et al. Generation of customized orbital implant templates using 3-dimensional printing for orbital wall reconstruction. Eye 2018; 32: 1864–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dave TV, Gaur G, Chowdary N, et al. Customized 3D printing: a novel approach to migrated orbital implant. Saudi J Ophthalmol 2018; 32: 330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kormann RB, Mörschbächer R, Moreira H, et al. A three-dimensional printed photopolymer resin implant for orbital rehabilitation for evisceration. Arq Bras Oftalmol 2019; 82: 471–475. [DOI] [PubMed] [Google Scholar]
- 45. Nahumi N, Shohet MR, Bederson JB, et al. Frontorbital fibrous dysplasia resection and reconstruction with custom polyetherlatone alloplast. J Craniofac Surg 2015; 26: e720–e722. [DOI] [PubMed] [Google Scholar]
- 46. Ko JS, Kim SH, Baek SW, et al. Semi-automated fabrication of customized ocular prosthesis with three-dimensional printing and sublimation transfer printing technology. Sci Rep 2019; 9: 2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ruiters S, Sun Y, de Jong S, et al. Computer-aided design and three-dimensional printing in the manufacturing of an ocular prosthesis. Br J Ophthalmol 2016; 100: 879–881. [DOI] [PubMed] [Google Scholar]
- 48. Furdová A, Sramka M, Thurzo A, et al. Early experiences of planning stereotactic radiosurgery using 3D printed models of eyes with uveal melanomas. Clin Ophthalmol 2017; 11: 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Canabrava S, Diniz-Filho A, Schor P, et al. Production of an intraocular device using 3D printing: an innovative technology for ophthalmology. Arq Bras Oftalmol 2015; 78: 393–394. [DOI] [PubMed] [Google Scholar]
- 50. Navajas EV, Ten Hove M. Three-dimensional printing of a transconjunctival vitrectomy trocar-cannula system. Ophthalmologica 2017; 237: 119–122. [DOI] [PubMed] [Google Scholar]
- 51. Debellemanière G, Flores M, Montard M, et al. Three-dimensional printing of optical lenses and ophthalmic surgery: challenges and perspectives. J Refract Surg 2016; 32: 201–204. [DOI] [PubMed] [Google Scholar]
- 52. Ruzza A, Parekh M, Ferrari S, et al. Preloaded donor corneal lenticules in a new validated 3D printed smart storage glide for Descemet stripping automated endothelial keratoplasty. Br J Ophthalmol 2015; 99: 1388–1395. [DOI] [PubMed] [Google Scholar]
- 53. Mandrycky C, Wang Z, Kim K, et al. 3D bioprinting for engineering complex tissues. Biotechnol Adv 2016; 34: 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang Y, Zhang XF, Gao G, et al. 3D bioprinting and the current applications in tissue engineering. Biotechnol J 2017; 12: 1600734. [DOI] [PubMed] [Google Scholar]
- 55. Gain P, Jullienne R, He Z, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol 2016; 134: 167–173. [DOI] [PubMed] [Google Scholar]
- 56. Isaacson A, Swioklo S, Connon CJ. 3D bioprinting of a corneal stroma equivalent. Exp Eye Res 2018; 173: 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang B, Xue Q, Hu HY, et al. Integrated 3D bioprinting-based geometry-control strategy for fabricating corneal substitutes. J Zhejiang Univ Sci B 2019; 20: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim KW, Lee SJ, Park SH, et al. Ex vivo functionality of 3D bioprinted corneal endothelium engineered with ribonuclease 5-overexpressing human corneal endothelial cells. Adv Healthc Mater 2018; 7: e1800398. [DOI] [PubMed] [Google Scholar]
- 59. Kim H, Park MN, Kim J, et al. Characterization of cornea-specific bioink: high transparency, improved in vivo safety. J Tissue Eng 2019; 10: 2041731418823382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Worthington KS, Wiley LA, Kaalberg EE, et al. Two-photon polymerization for production of human iPSC-derived retinal cell grafts. Acta Biomater 2017; 55: 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shrestha A, Allen BN, Wiley LA, et al. Development of high-resolution three-dimensional-printed extracellular matrix scaffolds and their compatibility with pluripotent stem cells and early retinal cells. J Ocul Pharmacol Ther 2020; 36: 42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang P, Li X, Zhu W, et al. 3D bioprinting of hydrogels for retina cell culturing. Bioprinting 2018; 11: e00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim J, Park JY, Kong JS, et al. Development of 3D printed Bruch’s membrane-mimetic substance for the maturation of retinal pigment epithelial cells. Int J Mol Sci 2021; 22: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lorber B, Hsiao WK, Martin KR. Three-dimensional printing of the retina. Curr Opin Ophthalmol 2016; 27: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen L, Yan D, Wu N, et al. 3D-printed poly-caprolactone scaffolds modified with biomimetic extracellular matrices for tarsal plate tissue engineering. Front Bioeng Biotechnol 2020; 8: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schubert C, van Langeveld MC, Donoso LA. Innovations in 3D printing: a 3D overview from optics to organs. Br J Ophthalmol 2014; 98: 159–161. [DOI] [PubMed] [Google Scholar]
- 67. Gwamuri J, Wittbrodt BT, Anzalone NC, et al. Reversing the trend of large scale and centralization in manufacturing: the case of distributed manufacturing of customizable 3-D-printable self-adjustable glasses. Chall Sustain 2014; 2: 30–40. [Google Scholar]
- 68. Ayyildiz O. Customised spectacles using 3-D printing technology. Clin Exp Optom 2018; 101: 747–751. [DOI] [PubMed] [Google Scholar]
- 69. Brodie FL, Nattagh K, Shah V, et al. Computed tomography–based 3D modeling to provide custom 3D-printed glasses for children with craniofacial abnormalities. J AAPOS 2019; 23:165–167.e1. [DOI] [PubMed] [Google Scholar]
- 70. Luxexcel. Luxexcel’s 3D printed prescription lenses for smart glasses, https://www.luxexcel.com (accessed 21 December 2020).
- 71. Myung D, Jais A, He L, et al. 3D printed smartphone indirect lens adapter for rapid, high quality retinal imaging. J Mob Technol Med 2014; 3: 9–15. [Google Scholar]
- 72. Arshad J, Helms R, Orge F, et al. Educational value of a 3D printer in ophthalmology training. J Acad Ophthalmol 2018; 10: e69–e71. [Google Scholar]
- 73. Kim TN, Myers F, Reber C, et al. A smartphone-based tool for rapid, portable, and automated wide-field retinal imaging. Transl Vis Sci Technol 2018; 7: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moon CH, Kim JY, Kim MJ, et al. Effect of three-dimensional printed personalized moisture chamber spectacles on the periocular humidity. J Ophthalmol 2016; 2016: 5039181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhao F, Zhao G, Weijie F, et al. Application of 3D printing technology in RGPCL simulation fitting. Med Hypotheses 2018; 113: 74–76. [DOI] [PubMed] [Google Scholar]
- 76. Sun MG, Rojdamrongratana D, Rosenblatt MI, et al. 3D printing for low cost, rapid prototyping of eyelid crutches. Orbit 2019; 38: 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Scawn RL, Foster A, Lee BW, et al. Customised 3D printing: an innovative training tool for the next generation of orbital surgeons. Orbit 2015; 34: 216–219. [DOI] [PubMed] [Google Scholar]
- 78. Jagan L, Turk W, Petropolis C, et al. Validation of a novel strabismus surgery 3D-printed silicone eye model for simulation training. J AAPOS 2020; 24: 3.e1–3.e6. [DOI] [PubMed] [Google Scholar]
- 79. Famery N, Abdelmassih Y, El-Khoury S, et al. Artificial chamber and 3D printed iris: a new wet lab model for teaching Descemet’s membrane endothelial keratoplasty. Acta Ophthalmol 2019; 97: e179–e183. [DOI] [PubMed] [Google Scholar]
- 80. Maloca PM, Tufail A, Hasler PW, et al. 3D printing of the choroidal vessels and tumours based on optical coherence tomography. Acta Ophthalmol 2019; 97: e313–e316. [DOI] [PubMed] [Google Scholar]
- 81. Bannon R, Parihar S, Skarparis Y, et al. 3D printing the pterygopalatine fossa: a negative space model of a complex structure. Surg Radiol Anat 2018; 40: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McMenamin PG, Quayle MR, McHenry CR, et al. The production of anatomical teaching resources using three-dimensional (3D) printing technology. Anat Sci Educ 2014; 7: 479–486. [DOI] [PubMed] [Google Scholar]