Abstract
Background:
The efficacy of virtual reality for people living with a terminal illness is unclear.
Aim:
To determine the feasibility and effectiveness of virtual reality use within a palliative care setting.
Design:
Systematic review and meta-analysis. PROSPERO (CRD42021240395).
Data sources:
Medline, Embase, AMED, PsycINFO, CINAHL, Cochrane Central Register of Controlled Trials and Web of Science were searched from inception to March 2021. Search terms included ‘virtual reality’ and ‘palliative care’. Eligibility: (1) adult (>18 years old) with a terminal illness (2) at least one virtual reality session and (3) feasibility data and/or at least one patient outcome reported. The ROB-2 and ROBINS tools assessed risk of bias. The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) tool assessed the quality of the evidence. Standardised mean differences (Hedges’s g) were calculated from the pre- and post-data. A DerSimonian-Laird random effects model meta-analysis was conducted.
Results:
Eight studies were included, of which five were in the meta-analysis. All studies had at least some concern for risk of bias. Virtual reality statistically significantly improved pain (p = 0.0363), tiredness (p = 0.0030), drowsiness (p = 0.0051), shortness of breath (p = 0.0284), depression (p = 0.0091) and psychological well-being (p = 0.0201). The quality of the evidence was graded as very low due to small sample sizes, non-randomisation methods and a lack of a comparator arm.
Conclusions:
Virtual reality in palliative care is feasible and acceptable. However, limited sample sizes and very low-quality studies mean that the efficacy of virtual reality needs further research.
Keywords: Palliative care, virtual reality, technology, electronics, medical
What is already known on this topic?
Virtual reality is available as a technology in clinical practice without specific indications or measurement of clinical benefit.
There is limited evidence as to the efficacy of its use within a palliative population.
What this paper adds?
This review highlights the limited and often very low-quality evidence about efficacy of virtual reality in palliative care.
The data from this review suggests that the technology is generally well tolerated with some possible therapeutic potential.
Implications for practice, theory or policy
This review highlights the methodological and clinical challenges that need to be addressed in order to fully understand the efficacy of virtual reality in a palliative care setting. Higher quality and larger studies, with a comparator arm, exploring the use of virtual reality in palliative care settings is critical.
Introduction
Hand-held technology has rapidly improved to become one of the main methods of communication and accessing information in daily life. Prior to COVID-19, healthcare services were already being digitalised. 1 The public were becoming more familiar with using different technology as part of their routine healthcare – be that the management of an illness (e.g. diabetes) or by video calling a primary care provider. Since the COVID-19 pandemic, digitalisation of healthcare will continue and it is important to understand the future applications as well as benefits and possible harms.
The specific technology focus of this review is virtual reality. Over the last two decades, it has become more portable and accessible (in terms of cost and availability). Large technology companies such as Google and Facebook have invested in virtual reality and are currently developing better virtual reality equipment and platforms. More pertinently to healthcare, virtual reality has been used to help train surgeons to operate by visualising the complex vascular supplies around tumours, 2 and in simulations around end-of-life care. 3 Virtual reality immerses the individual in a three-dimensional world (with experiences such as underwater diving, rollercoasters) often by using a headset, sometimes with handheld remotes. This immersion experience can trigger similar physical and emotional responses akin to being physically in the location being viewed 4 ; for this reason, the therapeutic benefit of virtual reality has been researched.
There have been four Cochrane reviews investigating the potential therapeutic benefits of virtual reality: in paediatric pain, 5 rehabilitation following a stroke 6 and for people with Parkinson Disease, 7 also in treatment compliance for serious mental illness. 8 All reported that it was difficult to make recommendations for clinical practice due to low quality studies and low strength of evidence; all advocated for larger trials. Multiple systematic reviews have also been conducted exploring the efficacy of virtual reality in different settings. Pain is a common symptom that has been addressed in virtual reality research; in paediatric populations, 9 adult populations10,11 and during specific interventions or procedures.12–14 Two systematic reviews looked more globally at the effect of virtual reality on common physical and mental health issues in any setting (e.g. anxiety, pain, depression).15,16
There has been a mini-review looking at the evidence of virtual reality for people living with dementia 17 and for people undergoing cancer treatment 18 however, to date, there has been no review to date that has evaluated the effectiveness of virtual reality specifically in a palliative care population. People living with a terminal illness often have multiple and complex physical and mental health needs19,20 and virtual reality may have a role as an adjuvant non-pharmacological contribution to the management of complex symptoms. This review aims to determine the extent of the evidence regarding the efficacy of virtual reality within palliative care. Due to the novel nature of the technology, the review focuses on the feasibility and acceptability of the technology, as well as identifying reported physical and psychological effects.
Methods
The protocol for this review was registered prospectively with PROSPERO (CRD42021240395, 3rd March 2021). This review was conducted using the Cochrane handbook for conducting the systematic reviews 21 and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. 22 Ethical approval was not required for this review.
Aim
The overall aim of the review was to determine the feasibility and effectiveness of virtual reality use within a palliative care setting.
The objectives were:
To describe the virtual reality technology that has been used in a palliative care setting.
To describe the feasibility and acceptability of the technology.
To explore the efficacy of virtual reality in a palliative care setting.
Eligibility criteria
Studies were included if they reported on the use of virtual reality in a palliative population. To define this population, we included any study that described the participant group as having an illness that was no longer curative or not receiving curative treatment; synonyms of this included ‘end of life’, ‘palliative’ and ‘terminal’.
Inclusion criteria
Human adults (over 18 years of age).
Palliative participant group (or a synonym of palliative, i.e. ‘not curable’, ‘terminal’, ‘stage 4’).
Participants completed at least one virtual reality session.
Outcome measures reported included at least one of the following: feasibility, acceptability efficacy (through a validated measure) on physical and/or psychological symptoms.
Randomised Control Trial (RCT), a non-RCT or a pre-post design.
English language.
Studies that were solely qualitative were excluded from this review. Mixed method studies were included as long as they met the criteria above. Studies were excluded if they did not meet the inclusion criteria.
Data sources
The following electronic databases were searched from inception up until 26th March 2021: Medline (OVID), Embase (OVID), AMED (OVID), PsycINFO (OVID), CINAHL (EBSCOhost), Cochrane Central Register of Controlled Trials (CENTRAL) and Web of Science.
Search strategy
The search strategy was developed in consultation with a specialist librarian at the University College London. Search terms combined two concepts: (1) ‘Palliative care’ and (2) ‘Virtual reality’. Relevant key concepts were identified from a previous review in palliative care, 23 a recent Cochrane review 5 and searched using Mesh terms in PubMed and equivalent terms in other databases, with tailored searches being developed for each database (see e.g. search strategy in Supplemental Material 1). The search strategy was piloted and refined, particularly the search terms for ‘virtual reality’, initially to balance sensitivity (retrieving a high number of relevant articles) and specificity (retrieving a low number of irrelevant articles) of searches. In addition to searching the databases, the lead author (JM) also screened the reference lists of included papers for relevant articles. JM also contacted the authors of the included studies for any unreported data, unpublished or ongoing work.
Selection process
At the first stage of screening, two reviewers (NW and JM) independently reviewed the titles and abstracts of studies identified from the database searches. Reviewers screened against the following criteria: (1) the study reported using virtual reality technology and (2) participants were described as receiving palliative care. For the second stage, the same two reviewers conducted full-text screening. Reviewers screened against the full inclusion criteria (see Inclusion criteria). At both stages of screening, any disagreement on included studies was resolved by liaising a third reviewer (PS).
Data collection process
The research team developed a data extraction form to code the demographic, methodological and outcome variables extracted from each study, with data extraction performed by NW and JM independently.
Data extraction
Final data to be included in the analysis were confirmed by both NW and JM. Information on study characteristics were extracted, including authors, country, year, sample size, design, setting, recruitment. Participant characteristics were extracted including age, gender, diagnosis. virtual reality characteristics were also extracted, including virtual reality type, dosing, comparison group, virtual reality results (feasibility, acceptability, efficacy). Feasibility and acceptability data were extracted for all included studies, while efficacy data were extracted for studies that reported quantitative data using validated measures.
Risk of bias assessment
Two reviewers (NW and JM) independently assessed the quality of the included studies, and any disagreement was resolved through revision and discussion. The Cochrane risk-of-bias tool for randomised trials version 2 (RoB 2) 24 was used to assess the quality of RCTs. RoB 2 contains five domains of bias: randomisation, deviations from the intended interventions, missing outcome data, measurement of the outcome and reporting results. Judgement about the risk of bias for each domain was either ‘Low’, ‘Some concerns’, ‘High’ risk of bias. Non-randomised control trials were assessed using the Cochrane Risk of Bias in non-randomised studies – of Interventions (ROBIN-I). 25 The tool contains seven domains: confounding, participant selection, classification of intervention, deviations from the intended interventions, missing data, measurement of outcomes and reporting results. Judgement for each domain was rated as either ‘Low’, ‘Moderate’, ‘Serious’ and ‘Critical’. An overall risk of bias judgement was made based on judgement for the seven individual domains.
No study was excluded based on their quality score, but they are reported for transparency.
Data synthesis and analysis
A summary of the study characteristics (e.g. study design, setting), demographics of the patient population (e.g. age, gender, diagnosis) and details about the delivery of virtual reality (e.g. frequency, length, content, follow-up, experience) were described.
Outcome data were organised in the following domains: (a) feasibility, (b) acceptability and usability and (c) efficacy.
Data from the RCTs were not combined due to using different comparator arms. A meta-analysis was completed instead using the pre-post study data. A meta-analysis was performed using the outcome measures reported in the studies, these were: Pain, Anxiety, Depression, Psychological wellbeing and other physical symptoms (tiredness, drowsiness, nausea, appetite and shortness of breath). The meta-analysis was performed if more than one study reported the outcome of interest, by any scale. We calculated the standardised mean differences (Hedges’s g) comparing the pre- and post-data scores. Statistical heterogeneity was assessed with the I 2 statistic (an I 2 value equal or more than 50% would have been considered as substantial heterogeneity 26 ). As the patient populations were quite variable in disease type and age a DerSimonian-Laird random effects model meta-analysis was conducted using STATA version 17.0. Publication bias was assessed using funnel plots per outcome.
Quality of the evidence
The Grading of Recommendations, Assessment, Develop-ment and Evaluations (GRADE) framework 27 was used to assess the quality of the evidence available. The GRADE profiler (GRADEPRO) allowed us to create a summary table of the findings. Two reviewers (NW and JM) independently rated the certainty of the evidence for each domain. The evidence was downgraded by one level for serious (or by two for very serious) risk of bias, indirectness of evidence, imprecision of effect estimates or potential publication bias. Studies that were observational in design started as low quality. The quality of evidence was independently checked by a third reviewer (VV).
Results
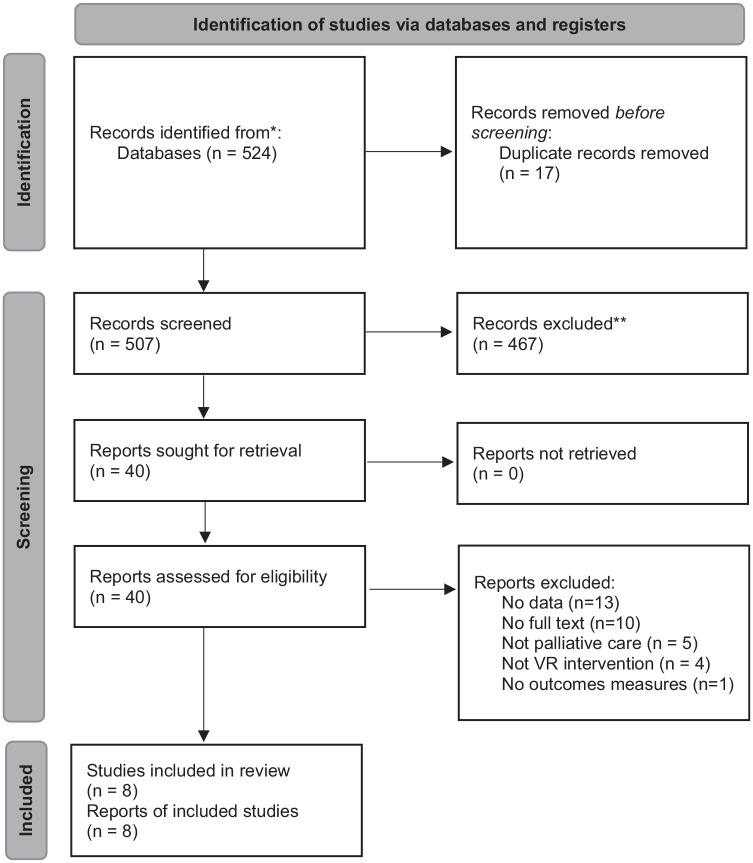
A total of 524 published articles were retrieved from the database searches (See Figure 1). Following de-duplication, 507 studies were included in the title and abstract screening. Forty studies were included for full-text screening, of which 33 were excluded. After contacting the authors of abstracts and included papers, one additional paper was identified that was in press. 28 Eight studies28–35 were included in the final review, of which five28,29,33–35 were included in a meta-analysis.
Figure 1.
PRISMA flowchart.
Study characteristics
Characteristics of included studies are shown in Table 1. All studies were conducted between 2012 and 2021. Six studies29–34 were non-randomised studies and two studies28,35 were randomised controlled trials (RCTs). The RCTs compared the virtual reality to guided imagery (meditation) or to a different virtual reality experience. One study 30 had no baseline data.
Table 1.
Study and participant characteristics.
| Study characteristics | Participant characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Country | Year | Setting | Comparator | Total Sample size (n) | Diagnosis | Gender | Age | ||
| Male | Female | |||||||||
| n (%) | n (%) | Mean (SD) | ||||||||
| Baños et al. 25 | Spain | 2012 | Inpatient hospital | None | 19 | Cancer | 19 (100) | 10 (53) | 9 (47) | 60.9 (14.5) |
| Brungardt et al. 26 | USA | 2020 | Inpatient hospital | None | 23 | Cancer Heart failure end-stage renal | 14 (61), 7 (30), 2 (9) | 11 (48) | 12 (52) | 47.7 (17.1) |
| Dang et al. 27 | USA | 2020 | Ambulatory care unit | None | 12 | Cancer | 12 (100) | 5 (42) | 7 (58) | 24–65+a |
| Ferguson et al. 28 | USA | 2020 | Multiple | None | 25 | Dementia | 25 (100) | 3 (12) | 22 (88) | 85 (8.9) |
| Groninger et al. 24 | USA | 2021 | Inpatient hospital | Guided-imagery | 88 | Heart failure | 88 (100) | 44 (50) | 44 (50) | 56 (13.2) |
| Johnson et al. 32 | USA | 2020 | Hospice | None | 12 | Cancer heart failure, bronchiectasis, Pneumonia | 8 (67), 2 (17), 1 (8), 1 (8) | 4 (33) | 8 (67) | 72 (16) |
| Niki et al. 33 | Japan | 2019 | Palliative care wards | None | 20 | Cancer | 20 (100) | 14 (70) | 6 (30) | 72.3 (11.9) |
| Perna et al. 31 | UK | 2021 | Hospice inpatient | Non-personalised, VR | 20 | Cancer, other | 15 (75), 5 (25) | 6 (30) | 14 (70) | 66a |
Age range/Perna et al. did not report SD.
Participant characteristics
There were 225 participants in total, with demographic data reported for 219 participants. One study 35 only reported the demographic data of those who completed all sessions (n = 20/26). There were 97/219 (44%) males and 122/219 (56%) females. The mean age ranged from 47.4 to 85 years: ranging between 20 and up to 103 years of age.
In total, 3/8 (37.5%) studies included only oncology patients, 3/8 (37.5%) studies included patients with diverse types of advanced diseases, 1/8 (12.5%) studies included patients with advanced heart failure and 1/8 (12.5%) studies included only patients with dementia. See Table 1 for more detail.
Quality appraisal
All studies had at least some concerns for risk of bias. The six non-randomised observational studies all had serious or critical risks in the domains of confounding and outcome measurements. One RCT 35 had some concerns for bias in the domains of the randomisation process, deviations from intended interventions and outcome measurements. The remaining RCT 28 had some concerns for bias in the domain of deviations from intended intervention (See Supplemental Material 2).
Virtual reality intervention characteristics
Table 2 lists the characteristics of the virtual reality technology used by the studies. Two studies28,30 employed the same virtual reality headset, however there was no overall consistency in the technology or virtual reality platform used. Five studies (63%) adopted a 30-min single virtual reality session as the intervention,30–34 one study used a single 10-min virtual reality intervention session 28 ; two studies completed multiple virtual reality sessions of either four 30-min virtual reality sessions over a week 29 or a 4-min virtual reality session once a week for 4 weeks. 35
Table 2.
Characteristics of virtual reality intervention.
| Authors | Intervention | Comparator | Technology | Duration of treatment | Follow-up |
|---|---|---|---|---|---|
| Randomised controlled trials | |||||
| Groninger et al. 28 | Guided walk-in virtual environment with narration | Active control (guided imagery) | Oculus Go VR headset | One 10-min session | Same day |
| Perna et al. 35 | Personalised virtual reality experience based on participants preference | Non-personalised virtual reality experiences | Google Daydream headset; Google Pixel XL smartphone and headphones. | Four 4-min/week VR sessions for 4 weeks | None |
| Non-randomised controlled trials | |||||
| Baños et al. 29 | Navigation through virtual environment to induce joy and relaxation | Pre-post data | LCD screen connected to a computer; headphone, keyboard, mouse | Four 30-min sessions/1 week | 4 times/week |
| Brungardt et al. 30 | Virtual-based music therapy with customised soundtrack | None | Oculus Go VR headset | One approx. 30-min session | Same day |
| Dang et al. 31 | Virtual reality-based life review using synchronised personalised avatar | Pre-post data | MoCap (Motion capture device); VocingHan hardware; Logitech wireless headset | One approx. 30-min session | 1-month |
| Ferguson et al. 32 | Virtual reality-based 360° beach viewing | Pre-post data | Lenovo’s Mirage Solo VR headset with business edition | One 30-min session | 3–5 h after invention (behavioural changes only) |
| Johnson et al. 33 | Virtual reality still images/animated videos viewing using one or more Virtual reality applications in Oculus Library | Pre-post data | Samsung Gear VR | One 30-min session | None |
| Niki et al. 34 | Virtual reality travel to the destination according to participants’ wishes | Pre-post data | VR headset HTC VIVE and VR software Google Earth VR | One 30-min session (time shortened or extended as needed) | None |
Outcomes used for virtual reality in palliative care
Table 3 summarises the outcome domains and measures reported in all included studies. All 8 (100%) studies included one or more acceptability measures of the virtual reality intervention; 5/8 (62.5%) studies reported usability measures and 4/8 (50%) reported feasibility measures; 7/8 studies (88%) reported at least one psychological and/or physical outcome measure.
Table 3.
Specific outcomes reported and measures used.
| Authors | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baños et al. 29 | Brungardt et al. 30 | Dang et al. 31 | Ferguson et al. 32 | Groninger et al. 28 | Johnson et al. 33 | Niki et al. 34 | Perna et al. 35 | |
| Domains | ||||||||
| Feasibility | ✓ | ✓ | ✓ | ✓ | ||||
| Acceptability | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Usability | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Pain | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Mood | ✓ a | |||||||
| Anxiety | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Depression | ✓ | ✓ | ✓ | ✓ | ||||
| Psychological wellbeing | ✓ | ✓ | ✓ | ✓ | ||||
| Other physical symptoms | ✓ b | ✓ d | ✓ c | ✓ d | ✓ d | ✓ d | ||
| Othere | ✓ | ✓ | ✓ | |||||
Consisted of 7 items: joy, sadness, anxiety, relax, vigour (1 ‘not at all’ to 7 ‘completely’), general mood (scale of 1–7 where 7 was equivalent to positive mood and well-being) and subjective mood change (from −3 ‘much worse’ to +3 ‘much better’).
Consisted of fatigue, pain and physical discomfort (0 ‘not at all’ to 10 ‘very much so’).
Subdomains of the FACIT-Pal-14: shortness of breath, distress (0 ‘not at all’ to 4 ‘very much’).
As measured by the ESAS-r.
Dang et al., included measures of Health related quality of life, symptom burden and spiritual wellbeing; Ferguson et al., measured behavioural changes after the virtual reality session; Groninger et al. also measured quality of life.
Seven out of the eight included studies (88%) reported on the impact of virtual reality on physical and/or psychological domains.28,29,31–35 The 8 h study reported only on the acceptability and feasibility of using virtual reality 30 using a numerical rating scale. See Table 3 for more details.
One study 32 reported the behavioural change of the participants between 3 and 5 h after the intervention, through a qualitative interview. The remaining seven studies provided quantitative data, using the following measures: ESAS-r,36-37 FACIT-Pal-14, 38 FACIT-Sp, 39 visual analogue scales and numerical rating scales Supplemental Material 5.
Feasibility
Recruitment
Available recruitment information for included studies is in Table 4. Three (37.5%) studies reported a recruitment goal,28,31,35 of which 2/3 (67%) reached their sample target within the recruitment period. Six (75%) studies reported a recruitment period, which ranged from 1 month up to 20 months. One study 35 mentioned potential recruitment barriers, which was not having an assigned researcher to conduct the research.
Table 4.
Recruitment information.
| Authors | Recruitment | Retention | |||||
|---|---|---|---|---|---|---|---|
| Time (months) | Target | Screened | Eligible | Consented | Rate (%) | Reasons for attrition | |
| n (%) | |||||||
| Randomised control trials | |||||||
| Groninger et al. 28 | 17 | 128 | nr | nr | 94 | 94 | nr |
| Perna et al. 35 | 20 | 26 | nr | 26 | 26 (100) | 77 | Illness (n = 5), death (n = 1) |
| Non-randomised control trials | |||||||
| Baños et al. 29 | nr | nr | nr | 26 | 20 (77) | 55 | Discharge (n = 4), high physical discomfort (n = 2), presence of other worries (n = 1), voluntary withdrawal (n = 1), clinical deterioration (n = 1) |
| Brungardt et al. 30 | 5 | nr | 33 | 28 | 23 (82) | 74 | Not feeling well (n = 3), delirium (n = 2), not available (n = 1) |
| Dang et al. 31 | 1 | 12 | nr | 17 | 12 (71) | 92 | Did not want to talk about feelings or share stories (n = 1) |
| Ferguson et al. 32 | nr | nr | nr | nr | 25 | 100 | |
| Johnson et al. 33 | 7 | nr | nr | nr | 12 | 100 | |
| Niki et al. 34 | 5 | nr | nr | nr | 20 | 100 | |
nr: not reported.
Retention
Among the four studies29–31,35 that had information on participant consent, the proportion of participants who consented to participant when approached ranged from 71% up to 100%. The proportion of participants who completed the studies ranged from 55% up to 100%. Deterioration due to ill health was one of the main reasons for leaving the trial. See Table 4 for more detail.
Acceptability and usability
All included studies except Perna et al. 35 included one or more general measure of participant satisfaction; with most participants reported being moderately satisfied with the virtual reality intervention. Perna et al. 35 measured acceptability in terms of attrition data, which was surpassed (over 60% completed).
Four studies (50%)29,30,32,33 reported difficulties in using the virtual reality, including unfamiliarity with the software and hardware, difficulty wearing the headset at a comfortable position, difficulty making mouse movement, involuntary keyboard strokes, difficulties getting used to the button configuration of the remote controller and not able to see the image clearly. Brungardt et al. 30 reported a mean SUS score of 80.4 (SD 13.8) suggesting that participants were happy with the usability of the virtual reality equipment.
Ferguson et al. 32 reported that 22/25 participants had a PAINAD score of 0 at baseline and 23/25 had a PAINAD score of 0 5 min after the virtual reality experience. Four studies (50%)29,31–33 reported that participants experienced some discomfort using the device, including uncomfortable position, physical challenges and not getting used to wearing the virtual reality headset. One study 29 reported that participants required the assistance of a clinician in using the virtual reality device due to symptom severity and high level of discomfort. Adverse events of the virtual reality were reported in 2/8 (25%) studies,29,33 including tiredness, worsening of existing dizziness and sore shoulders due to repeated adjustment of the virtual reality headset.
Six studies (75%)28–33 indicated that participants had positive attitudes towards the virtual reality session, perceived the intervention as beneficial were willing to repeat the intervention again or recommend to others.
Efficacy of virtual reality in palliative care
Groninger et al. 28 and Perna et al. 35 were the RCTs included in this review. Groninger et al. 28 reported that patients in both groups experienced a significant reduction in pain scores; those who completed the virtual reality session compared to the guided imagery had significantly lower pain scores (−2.9 ± 2.6 vs −1.3 ± 1.8, p = 0.0153). Perna et al. 35 reported no difference in using personalised versus non-personalised virtual reality experiences.
Meta-analysis
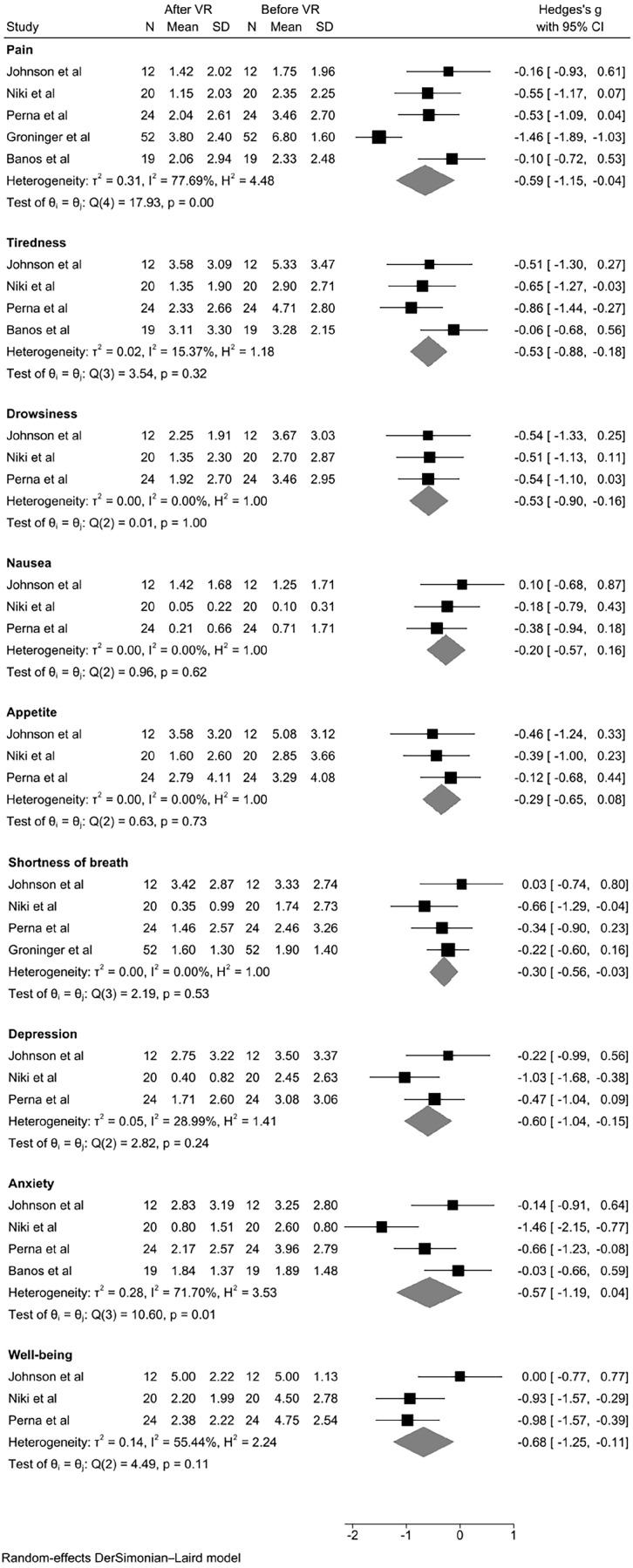
Six studies reported data on the same patient outcomes 28,29,31,33–35; one study did not report the study data in enough detail and did not respond to an email request prior to the analysis 31 ; therefore five studies were included in the meta-analysis. Four of these used the ESAS-r as the outcome measure. For this reason, we used the ESAS-r domains to structure the meta-analysis. The funnel plot (see Supplemental Material 3) indicates no evidence of publication bias.
Figure 2 reports the forest plot of the studies, by patient outcomes. Further meta-analyses indicated that the following domains showed significant differences between the pre- and post-data; pain (p = 0.0363), tiredness (p = 0.0030), drowsiness (p = 0.0051), shortness of breath (p = 0.0284), depression (p = 0.0091), psychological well-being (p = 0.0201).
Figure 2.
Forest plot.
Other measures
Spiritual wellbeing using the FACIT-Sp
Dang et al. 31 reported that there were no significant difference pre- and post-intervention for spiritual wellbeing.
Quality of life
Groninger et al. 28 reported a significant improvement in total FACIT-Pal-14 scores in both the virtual reality and guided imagery groups. Dang et al. 31 reported no significant difference in EORTC QLQ-C30 scores. 40
GRADE evidence statement
Supplemental Material 5 shows the GRADE quality of evidence assessment and summary of findings. We judged the quality of the evidence for virtual reality on outcomes measured in patient outcomes as very low. Our confidence in the effect estimate is limited. We downgraded the certainty of evidence due to the risk of bias, imprecision and due to the observational design of four out of the five studies.
Discussion
Main findings
Findings from the studies included in this review suggest that recruitment to a virtual reality trial in palliative care was possible. It also shows that people who are living with a terminal illness enjoyed using virtual reality technology with few to no adverse reactions noted. The meta-analysis on the efficacy of virtual reality on patient outcomes suggests that there could be a therapeutic benefit to virtual reality, however the quality of the evidence was rated as low to very low due to the small sample sizes, and the study design in that that there was often no comparator arm.
Strengths and weaknesses
This is the first rigorous systematic review to investigate the use of virtual reality in palliative care; however, there are a few areas of caution to consider when interpreting the results. Firstly, six out of the eight studies included were feasibility studies with no control group. Secondly, as virtual reality is an emerging technology, there was no agreed methodology across the studies including: the equipment used, the procedures employed (e.g. how many sessions, number of follow-ups), the type of virtual reality experience (the earliest study in 2012 used a computer to watch the experience, whereas the later studies published between 2019 and 2021 employed headsets that either had an inbuilt virtual reality experience or used a smartphone), the quality of the experience (this was often not described although one study did describe the challenge of sourcing a high quality experience from the internet 35 ), and the outcomes used to measure the efficacy of the virtual reality. No study addressed the cost-effectiveness of the virtual reality compared to the efficacy. Only studies reported in English were included in this review, which could mean that some studies were omitted in other languages.
What this study adds
This review reports the same as previous systematic reviews published looking at virtual reality in other settings; that further higher quality research is needed to offer definitive recommendations for clinical practice. A heterogeneous mix of outcome measures, study designs and virtual reality equipment limits the generalisability of the findings. No study in this review discussed capturing the efficacy of virtual reality on chronic and acute pain; only two studies completed more than one virtual reality session.29,35 Previous research has focussed on the impact of virtual reality on acute pain (i.e. during a procedure) however, patients under palliative care often experience chronic pain too. More research is needed to fully capture how virtual reality might best support people living with a terminal illness.
Virtual reality is an emerging technology with potential in multiple settings. It offers the opportunity for individualised care which can be readily accessed by the patient, at any time. As the technology is developing and we are becoming more familiar with using technology as part of our routine healthcare, it is vital to determine the efficacy of such methods. Additionally, if virtual reality is to become a routine part of healthcare, it is important that the appropriate policy measures are taken to ensure that the platforms and experiences are monitored for content and quality, as often the poor quality can lead to negative experiences (such as nausea or headaches).
Further research is needed to understand the efficacy of virtual reality in a palliative care setting. This review highlights the methodological and clinical challenges that need to be addressed. Methodologically, more rigorous study designs and standardised outcome measures are needed to improve the quality of the evidence. Clinically, more exploration into acute pain versus chronic pain versus disease progression within palliative care is needed to fully understand where the therapeutic benefit is of using virtual reality for people living with a terminal illness.
Supplemental Material
Supplemental material, sj-jpg-3-pmj-10.1177_02692163221099584 for How effective is virtual reality technology in palliative care? A systematic review and meta-analysis by Jiping Mo, Victoria Vickerstaff, Ollie Minton, Simon Tavabie, Mark Taubert, Patrick Stone and Nicola White in Palliative Medicine
Supplemental material, sj-jpg-4-pmj-10.1177_02692163221099584 for How effective is virtual reality technology in palliative care? A systematic review and meta-analysis by Jiping Mo, Victoria Vickerstaff, Ollie Minton, Simon Tavabie, Mark Taubert, Patrick Stone and Nicola White in Palliative Medicine
Supplemental material, sj-pdf-1-pmj-10.1177_02692163221099584 for How effective is virtual reality technology in palliative care? A systematic review and meta-analysis by Jiping Mo, Victoria Vickerstaff, Ollie Minton, Simon Tavabie, Mark Taubert, Patrick Stone and Nicola White in Palliative Medicine
Supplemental material, sj-pdf-2-pmj-10.1177_02692163221099584 for How effective is virtual reality technology in palliative care? A systematic review and meta-analysis by Jiping Mo, Victoria Vickerstaff, Ollie Minton, Simon Tavabie, Mark Taubert, Patrick Stone and Nicola White in Palliative Medicine
Acknowledgments
The authors would like to thank the UCL specialist librarian for their guidance on the search strategy. We would like to thank Dr Bridget Candy for her methodology expertise.
Footnotes
Authorship: The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors made a substantial contribution to the concept and design of the review. JP, NW and VV conducted the review and data interpretation. All authors contributed to the drafting of the manuscript and approved the final draft.
Data management and sharing: All data are reported within the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Marie Curie I-CAN-CARE Program grant (MCCC-FPO-16-U). Professor Stone is supported by the Marie Curie Chair’s grant (MCCC-509537). Nicola White, Victoria Vickerstaff and Patrick Stone are partly supported by the UCLH NIHR Biomedical Research Centre.
Research ethics and patient consent: No ethical approval was required for this systematic review.
ORCID iDs: Ollie Minton  https://orcid.org/0000-0002-4258-8995
https://orcid.org/0000-0002-4258-8995
Nicola White  https://orcid.org/0000-0002-7438-0072
https://orcid.org/0000-0002-7438-0072
Supplemental material: Supplemental material for this article is available online.
References
- 1. Pillai AS, Mathew PS. Impact of virtual reality in healthcare: a review. In: Guazzaroni G. (ed.) Virtual and augmented reality in mental health treatment. Hershey, PA: IGI Global, 2019, pp.17–31. [Google Scholar]
- 2. Lee C, Wong GKC. Virtual reality and augmented reality in the management of intracranial tumors: a review. J Clin Neurosci 2019; 62: 14–20. [DOI] [PubMed] [Google Scholar]
- 3. Taubert M, Webber L, Hamilton T, et al. Virtual reality videos used in undergraduate palliative and oncology medical teaching: results of a pilot study. BMJ Support Palliat Care 2019; 9: 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tieri G, Morone G, Paolucci S, et al. Virtual reality in cognitive and motor rehabilitation: facts, fiction and fallacies. Expert Rev Med Devices 2018; 15: 107–117. [DOI] [PubMed] [Google Scholar]
- 5. Lambert V, Boylan P, Boran L, et al. Virtual reality distraction for acute pain in children. Cochrane Database Syst Rev 2020; 10: CD010686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laver KE, Lange B, George S, et al. Virtual reality for stroke rehabilitation. Stroke 2018; 49: e160–e161. [Google Scholar]
- 7. Dockx K, Bekkers EM, Van den Bergh V, et al. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst Rev 2016; 12: CD010760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Välimäki M, Hätönen HM, Lahti ME, et al. Virtual reality for treatment compliance for people with serious mental illness. Cochrane Database Syst Rev (10) 2014: CD009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eijlers R, Utens EMWJ, Staals LM, et al. Systematic review and meta-analysis of virtual reality in pediatrics: effects on pain and anxiety. Anesth Analg 2019; 129: 1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mallari B, Spaeth EK, Goh H, et al. Virtual reality as an analgesic for acute and chronic pain in adults: a systematic review and meta-analysis. J Pain Res 2019; 12: 2053–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dascal J, Reid M, IsHak WW, et al. Virtual reality and medical inpatients: a systematic review of randomized, controlled trials. Innov Clin Neurosci 2017; 14: 14–21. [PMC free article] [PubMed] [Google Scholar]
- 12. Scapin S, Echevarría-Guanilo ME, Boeira Fuculo Junior PR, et al. Virtual reality in the treatment of burn patients: a systematic review. Burns 2018; 44: 1403–1416. [DOI] [PubMed] [Google Scholar]
- 13. Chow H, Hon J, Chua W, et al. Effect of virtual reality therapy in reducing pain and anxiety for cancer-related medical procedures: a systematic narrative review. J Pain Symptom Manag 2021; 61: 384–394. [DOI] [PubMed] [Google Scholar]
- 14. Morris LD, Louw QA, Grimmer-Somers K. The effectiveness of virtual reality on reducing pain and anxiety in burn injury patients: a systematic review. Clin J Pain 2009; 25: 815–826. [DOI] [PubMed] [Google Scholar]
- 15. Ioannou A, Papastavrou E, Avraamides MN, et al. Virtual reality and symptoms management of anxiety, depression, fatigue, and pain: a systematic review. SAGE Open Nurs 2020; 6: 2377960820936163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maples-Keller JL, Bunnell BE, Kim S-J, et al. The use of virtual reality technology in the treatment of anxiety and other psychiatric disorders. Harv Rev Psychiatry 2017; 25: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. D’Cunha NM, Nguyen D, Naumovski N, et al. A mini-review of virtual reality-based interventions to promote well-being for people living with dementia and mild cognitive impairment. Gerontology 2019; 65: 430–440. [DOI] [PubMed] [Google Scholar]
- 18. Pittara M, Matsangidou M, Stylianides K, et al. Virtual reality for pain management in cancer: a comprehensive review. IEEE Access 2020; 8: 225475–225489. [Google Scholar]
- 19. Potter J, Hami F, Bryan T, et al. Symptoms in 400 patients referred to palliative care services: prevalence and patterns. Palliat Med 2003; 17: 310–314. [DOI] [PubMed] [Google Scholar]
- 20. Finucane AM, Swenson C, MacArtney JI, et al. What makes palliative care needs “complex”? A multisite sequential explanatory mixed methods study of patients referred for specialist palliative care. BMC Palliat Care 2021; 20: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JPT, Thomas J, Chandler J, et al. (eds). Cochrane handbook for systematic reviews of interventions version 6.3, www.training.cochrane.org/handbook (2022, accessed February 2022).
- 22. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 74: 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. White N, Reid F, Harris A, et al. A systematic review of predictions of survival in palliative care: how accurate are clinicians and who are the experts? PLoS One 2016; 11: e0161407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deeks JJ, Higgins JP, Altman DG. Chapter 10: analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane handbook for systematic reviews of interventions cochrane, 2021. [Google Scholar]
- 27. Guyatt G, Oxman Ad, Fau-Akl EA, Akl Ea, Fau-Kunz R, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64(4): 383–394. [DOI] [PubMed] [Google Scholar]
- 28. Groninger H, Stewart D, Fisher JM, et al. Virtual reality for pain management in advanced heart failure: a randomized controlled study. Palliat Med 2021; 35: 2008–2016. [DOI] [PubMed] [Google Scholar]
- 29. Baños RM, Espinoza M, García-Palacios A, et al. A positive psychological intervention using virtual reality for patients with advanced cancer in a hospital setting: a pilot study to assess feasibility. Support Care Cancer 2013; 21: 263–270. [DOI] [PubMed] [Google Scholar]
- 30. Brungardt A, Wibben A, Tompkins AF, et al. Virtual reality-based music therapy in palliative care: a pilot implementation trial. J Palliat Med 2021; 24: 736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dang M, Noreika D, Ryu S, et al. Feasibility of delivering an avatar-facilitated life review intervention for patients with cancer. J Palliat Med 2021; 24: 520–526. [DOI] [PubMed] [Google Scholar]
- 32. Ferguson C, Shade MY, Blaskewicz Boron J, et al. Virtual reality for therapeutic recreation in dementia hospice care: a feasibility study. Am J Hosp Palliat Care 2020; 37: 809–815. [DOI] [PubMed] [Google Scholar]
- 33. Johnson T, Bauler L, Vos D, et al. Virtual reality use for symptom management in palliative care: a pilot study to assess user perceptions. J Palliat Med 2020; 23: 1233–1238. [DOI] [PubMed] [Google Scholar]
- 34. Niki K, Okamoto Y, Maeda I, et al. A novel palliative care approach using virtual reality for improving various symptoms of terminal cancer patients: a preliminary prospective, multicenter study. J Palliat Med 2019; 22: 1490. [DOI] [PubMed] [Google Scholar]
- 35. Perna L, Lund S, White N, et al. The potential of personalized virtual reality in palliative care: a feasibility trial. Am J Hosp Palliat Care 2021; 38: 1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991; 7: 6–9. [PubMed] [Google Scholar]
- 37. Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manag 2017; 53: 630–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeng L, Bedard G, Cella D, et al. Preliminary results of the generation of a shortened quality-of-life assessment for patients with advanced cancer: the FACIT-Pal-14. J Palliat Med 2013; 16: 509–515. [DOI] [PubMed] [Google Scholar]
- 39. Bredle JM, Salsman JM, Debb SM, et al. Spiritual well-being as a component of health-related quality of life: the functional assessment of chronic illness therapy—spiritual well-being scale (FACIT-Sp). Religions 2011; 2: 77–94. [Google Scholar]
- 40. Fayers P, Bottomley A. Quality of life research within the EORTC—the EORTC QLQ-C30. Eur J Cancer 2002; 38(Suppl 4): S125–S133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-3-pmj-10.1177_02692163221099584 for How effective is virtual reality technology in palliative care? A systematic review and meta-analysis by Jiping Mo, Victoria Vickerstaff, Ollie Minton, Simon Tavabie, Mark Taubert, Patrick Stone and Nicola White in Palliative Medicine
Supplemental material, sj-jpg-4-pmj-10.1177_02692163221099584 for How effective is virtual reality technology in palliative care? A systematic review and meta-analysis by Jiping Mo, Victoria Vickerstaff, Ollie Minton, Simon Tavabie, Mark Taubert, Patrick Stone and Nicola White in Palliative Medicine
Supplemental material, sj-pdf-1-pmj-10.1177_02692163221099584 for How effective is virtual reality technology in palliative care? A systematic review and meta-analysis by Jiping Mo, Victoria Vickerstaff, Ollie Minton, Simon Tavabie, Mark Taubert, Patrick Stone and Nicola White in Palliative Medicine
Supplemental material, sj-pdf-2-pmj-10.1177_02692163221099584 for How effective is virtual reality technology in palliative care? A systematic review and meta-analysis by Jiping Mo, Victoria Vickerstaff, Ollie Minton, Simon Tavabie, Mark Taubert, Patrick Stone and Nicola White in Palliative Medicine