Abstract
PURPOSE:
This study compared the efficacy and safety of suberoylanilide hydroxamic acid (SAHA) and mitomycin C (MMC) up to 4 months in the prevention of corneal haze induced by photorefractive keratectomy (PRK) in rabbits in vivo.
METHODS:
Corneal haze in rabbits was produced with −9.00 diopter PRK. A single application of SAHA (25 μM) or MMC (0.02%) was applied topically immediately after PRK. Effects of the two drugs were analyzed by slit-lamp microscope, specular microscope, TUNEL assay, and immunofluorescence.
RESULTS:
Single topical adjunct use of SAHA (25 μM) or MMC (0.02%) after PRK attenuated more than 95% corneal haze and myofibroblast formation (P < .001). SAHA did not reduce keratocyte density, cause keratocyte apoptosis, or increase immune cell infiltration compared to MMC (P < .01 or .001). Furthermore, SAHA dosing did not compromise corneal endothelial phenotype, density, or function in rabbit eyes, whereas MMC application did (P < .01 or .001).
CONCLUSIONS:
SAHA and MMC significantly decreased corneal haze after PRK in rabbits in vivo. SAHA exhibited significantly reduced short- and long-term damage to the corneal endothelium compared to MMC in rabbits. SAHA is an effective and potentially safer alternative to MMC for the prevention of corneal haze after PRK. Clinical trials are warranted.
Corneal haze formation involves changes in extracellular matrix deposition and keratocyte proliferation, migration, apoptosis, and differentiation of fibroblasts and myofibroblasts.1–4 Transforming growth factor beta-1 (TGF-β1) has been shown to play a central role in myofibroblast generation and corneal haze formation.5 Myofibroblasts are opaque cells with high contractibility, show de novo expression of alpha-smooth muscle actin (α-SMA), and synthesize extracellular matrix protein. Accumulating literature reveals that myofibroblasts are responsible for corneal haze/fibrosis, but the exact mechanism leading to fibrosis is still unclear.6–8 Agents capable of limiting keratocyte proliferation or differentiation after photorefractive keratectomy (PRK) are effective in preventing myofibroblast formation and corneal haze appearance in preclinical animal models and human patients.8–17
We have previously reported that the histone deacetylase inhibitors trichostatin A and suberoylanilide hydroxamic acid (SAHA) effectively reduce corneal haze in vivo in rabbits with minimal toxicity.15–17 Subsequently, we identified the mechanism through which SAHA reduces TGFβ1-induced myofibroblast formation, collagen synthesis, and corneal fibrosis using an in vitro model.18 In this study, we compared in vivo long-term safety and efficacy of SAHA with mitomycin C (MMC) for preventing corneal haze after PRK using an established in vivo rabbit model and no-drug treatment control.
MATERIALS AND METHODS
A total of 45 New Zealand white rabbits weighing between 2 and 3 kg were used. The Institutional Animal Care and Use Committee of the Harry S. Truman Memorial Veterans’ Hospital and University of Missouri–Columbia approved the study. All animals were treated humanely and in accordance with the tenets of the Association for Research in Vision and Ophthalmology’s (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. General anesthesia in rabbits was given by an intramuscular injection of ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (10 mg/kg). Local anesthesia was given with two to three drops of topical 0.5% proparacaine hydrochloride (Alcon Laboratories, Inc., Fort Worth, TX). The rabbits were killed by an intravenous injection of Beuthanasia-D (Merck & Co., Inc., Madison, NJ) (150 mg/kg) in rabbits under general anesthesia.
Corneal Haze Production in Rabbit Eyes
PRK was used to produce corneal haze in rabbits by performing −9.00 diopter (D) ablation with the Summit Apex excimer laser (Model: SVS APEX Plus ER; Alcon Laboratories, Inc.) as reported previously.9 Briefly, the rabbits were anesthetized and local anesthesia of the cornea was achieved through the application of topical ophthalmic 0.5% proparacaine hydrochloride (Alcon Laboratories, Inc.). A wire eyelid speculum was placed and corneal epithelium was removed by gentle scraping with a surgical Beaver blade #64 (BD Biosciences, Franklin Lakes, NJ). Spherical laser ablation of −9.00 diopters, with a 6-mm diameter optical zone, was performed by programing the laser (421 pulses for a depth of 108 μm). This treatment is known to produce significant haze in rabbit corneas with 100% reproducibility, as reported previously.6,19 This technique consistently produces corneal haze and myofibroblasts in the rabbit cornea, which peaks at 4 weeks after PRK.6,10,15,16 Only one eye of each animal was used for experimentation.
SAHA and MMC Treatment Regimen
A 10-mM stock solution of SAHA (Cayman Chemical Company, Ann Arbor, MI) was prepared using dimethyl sulfoxide (DMSO), and diluted to 25 μM with balanced salt solution (BSS) eye drops (Alcon Laboratories, Inc., Fort Worth, TX). For vehicle control, the same volume of DMSO was diluted with BSS. The 0.02% MMC solution was prepared at the Harry S. Truman VA Hospital Pharmacy, Columbia, Missouri, using 5 mg/mL powder (Accord Healthcare, Inc., Durham, NC) diluted with sterile normal saline. This solution was stable for 1 week at room temperature but was used within 48 hours after preparation. After PRK, rabbits were divided into three groups: one received a single topical application of SAHA (25 μM) for 5 minutes (n = 15) (SAHA group), one received a single topical application of MMC (0.02%) for 1 minute (n = 15) (MMC group), and one received a single topical application of vehicle for 5 minutes (n = 15) (vehicle group). Thereafter, eyes were washed profusely with BSS. The contralateral eye served as naive control. All rabbits received clinical eye examinations.
Slit-lamp and Specular Biomicroscopy
Slit-lamp (SL15; Kowa Company Ltd., Nagoya, Japan), stereo (MZ16F; Leica, Heerbrugg, Switzerland), and specular (NSP-8800; Konan, Irvine, CA) microscopes were used to evaluate ocular health, corneal haze, and corneal endothelial cells in anesthetized animals. Grading of clinical corneal haze was done using the Fantes scale20 by three researchers (AS, MW, and SG) in a masked manner as reported earlier.6,10,15,16 A high performance digital imaging system (VK2; Kowa Company Ltd.) was used for corneal image analysis.
Cornea Collection, Immunofluorescence, and TUNEL Assay
Rabbits from each group were killed 3 days (n = 3), 1 month (n = 6), and 4 months (n = 6) after PRK. Corneas were snap frozen, sectioned (7 μm), and stored at −80°C as reported earlier.6,10 Immunofluorescence was used to detect myofibroblast marker α-SMA using monoclonal antibody (M0851; Dako, Santa Clara, CA). TUNEL assay (ApopTag, S7165; Billerica, MA) detected apoptosis and DAPI-stained nuclei determined cellular density. Sections were viewed and photographed under a fluorescence microscope (Leica, Buffalo Grove, IL) equipped with a digital camera system (SpotCam RT KE; Diagnostic Instruments, Sterling Heights, MI).
Quantification and Statistical Analyses
Stained proteins in corneal sections were quantified using Image J software (National Institutes of Health, Bethesda, MD) in six randomly selected areas. Standard error means were calculated following methods reported previously.6,15 One-way analysis of variance and the Wilcoxon ranked-sum test were used for statistical analysis. A P value of less than .05 was considered significant.
RESULTS
In Vivo Efficacy Studies of SAHA and MMC
Figure A (available in the online version of this article) presents biomicroscopy images showing levels of corneal haze in live rabbits. A single application of SAHA or MMC significantly decreased corneal haze after PRK in rabbits compared to corneas with no drug treatment (P < .001). Haze score at 4 months in rabbit corneas treated with BSS was elevated (2.7 ± 0.4) compared to corneas treated with SAHA (0.9 ± 0.1) and MMC (0.85 ± 0.15) (Figure 1).
Figure A.
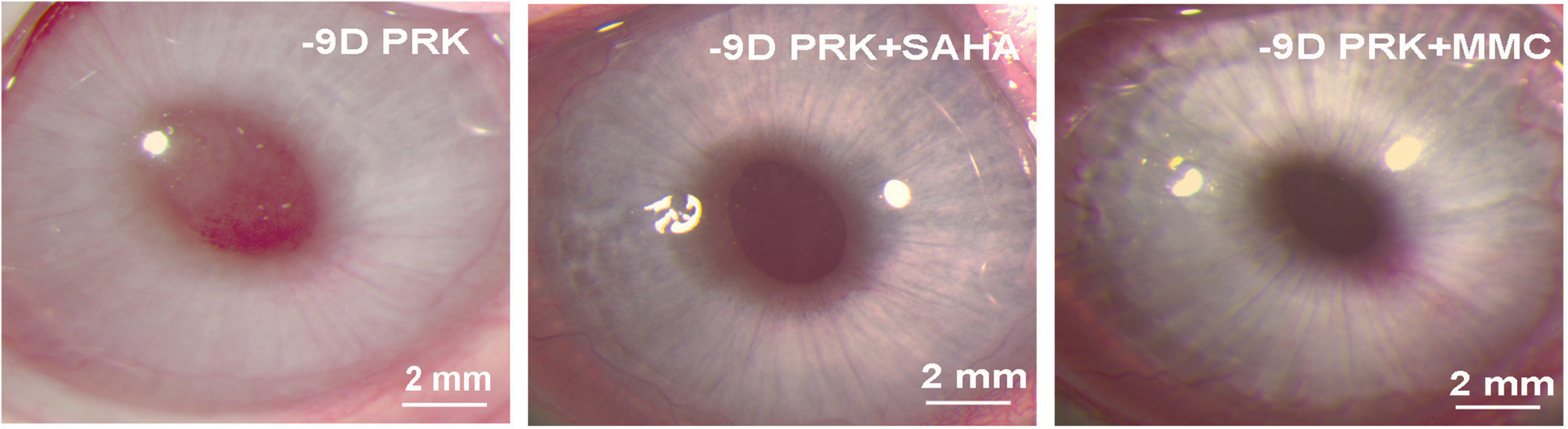
Effect of suberoylanilide hydroxamic acid (SAHA) or mitomycin C (MMC) application on corneal haze in vivo. Representative stereomicroscopy images showing corneal haze levels and its quantification noted at 4 months in −9.00 diopter photorefractive keratectomy (PRK) performed on rabbit corneas that received either balanced salt solution (control), SAHA (25 μM), or MMC (0.02%). Each group had 6 eyes for slit-lamp biomicroscopy. The central scar was considerably reduced and the overall corneal clarity appeared improved in corneas treated with SAHA or MMC.
Figure 1.
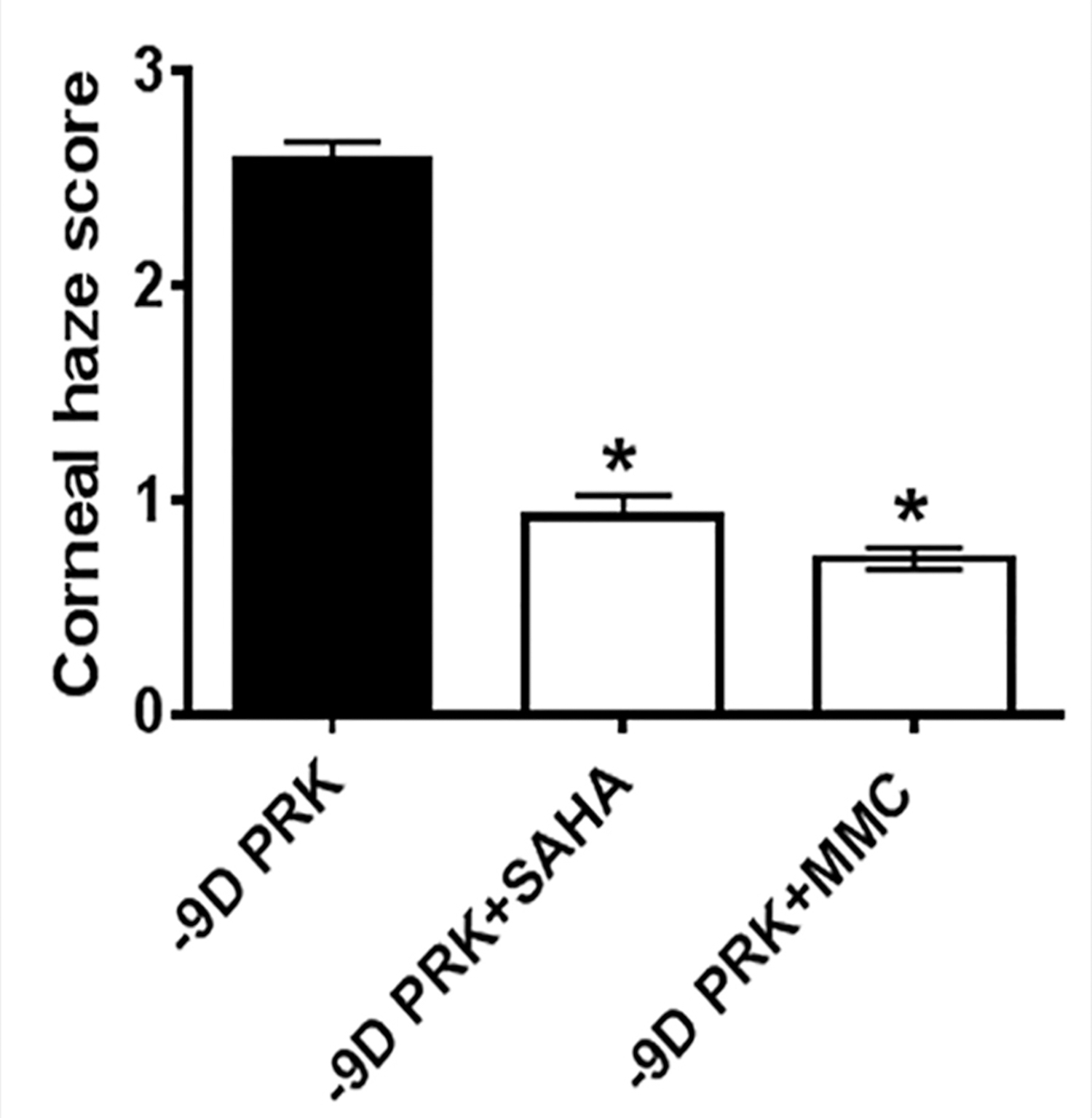
Haze quantification showed that a single 5-minute topical application of suberoylanilide hydroxamic acid (SAHA) or mitomycin C (MMC) significantly decreased corneal haze following photorefractive keratectomy (PRK) (P < .001 for both). D = diopters
Figure 2 shows the inhibitory effects of SAHA and MMC in the development of myofibroblasts after PRK in rabbit corneas at 1 month (Figures 2A–2C) and 4 months (Figures 2D–2F) measured with α-SMA immunofluorescence. Rabbit corneas that received a single topical application of SAHA (Figures 2B and 2E) or MMC (Figures 2C and 2F) after PRK showed significantly fewer α-SMA+ cells (70% to 93%; P < .001) in the stroma at 1 and 4 months compared to the BSS controls (Figures 2A and 2D).
Figure 2.
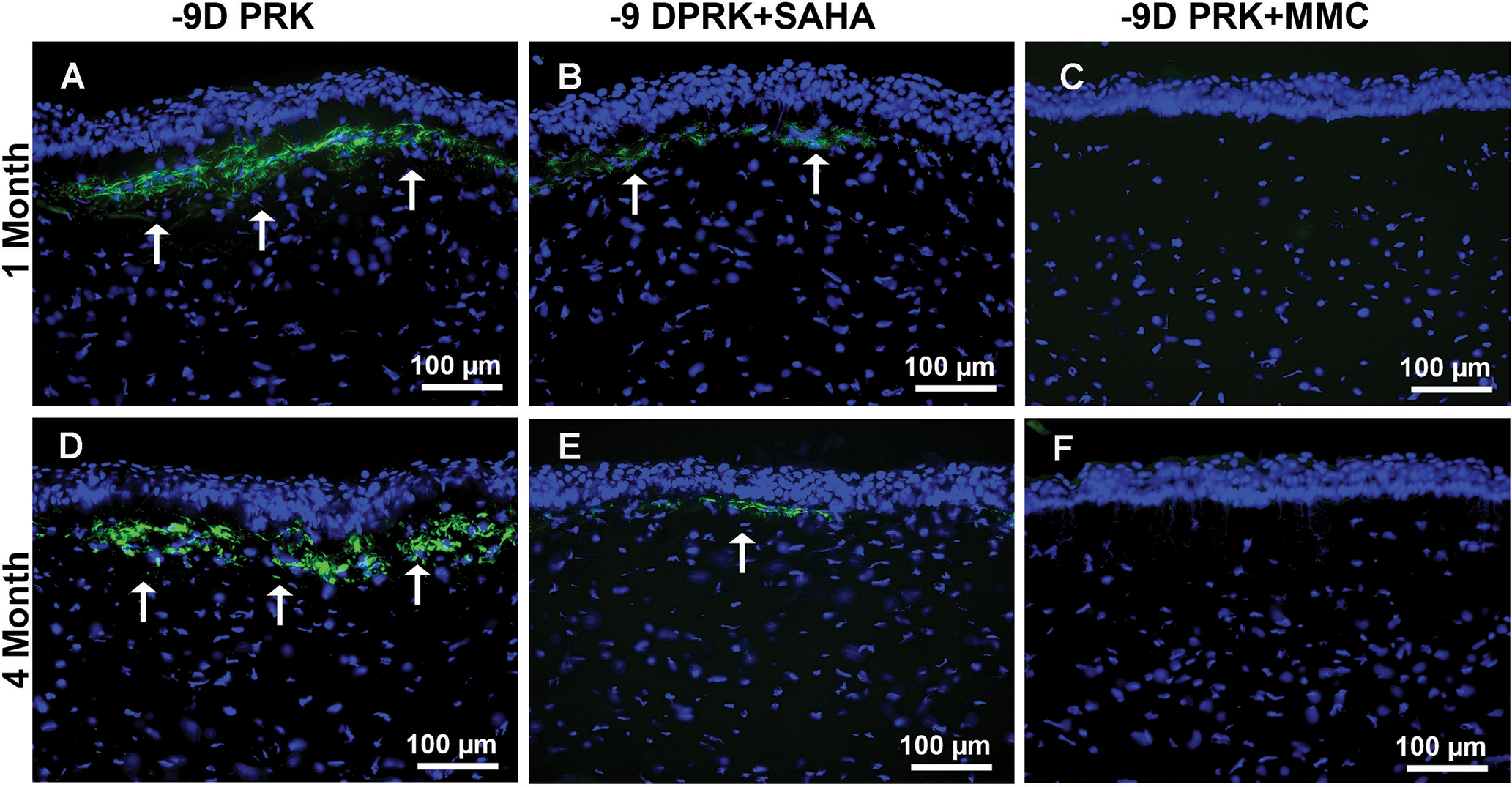
Effect of suberoylanilide hydroxamic acid (SAHA) and mitomycin C (MMC) on the attenuation of myofibroblasts after photorefractive keratectomy (PRK). Representative fluorescence images of rabbit corneas that underwent −9.00 diopter (D) PRK followed by balanced salt solution, or SAHA (25 μM), or MMC (0.02%) treatment at 1 and 4 months after treatment. Myofibroblasts (arrows) for α-smooth muscle actin in the subepithelial stroma (green). SAHA (B and E) and MMC (C and F) treatment significantly decreased α-SMA compared to control corneas (A and D). Scale bar = 100 μm (Blue = DAPI-stained nuclei, Green = α-SMA+ cells)
In Vivo Safety Analyses of SAHA and MMC
Figure B (available in the online version of this article) shows the effects of SAHA and MMC application on the rabbit corneal endothelial cell phenotype and density in vivo at 1 and 4 months after −9.00 D PRK. As an indicator of safety, these images show that SAHA treatment does not alter endothelial cell phenotype or density. At 1 month after PRK, rabbit eyes treated with BSS or SAHA exhibited the classic endothelial polygonal mosaic phenotype with few occasional large normal hexagonal endothelial cells (Figures BA–BB), whereas corneas treated with MMC exhibited significantly compromised endothelial cell phenotype and density (Figure BC). A similar pattern was noted at 4 months, where corneas treated with BSS or SAHA showed normal endothelium (Figures BD–BE) and corneas treated with MMC still had compromised endothelial cells (Figure BF).
Figure B.
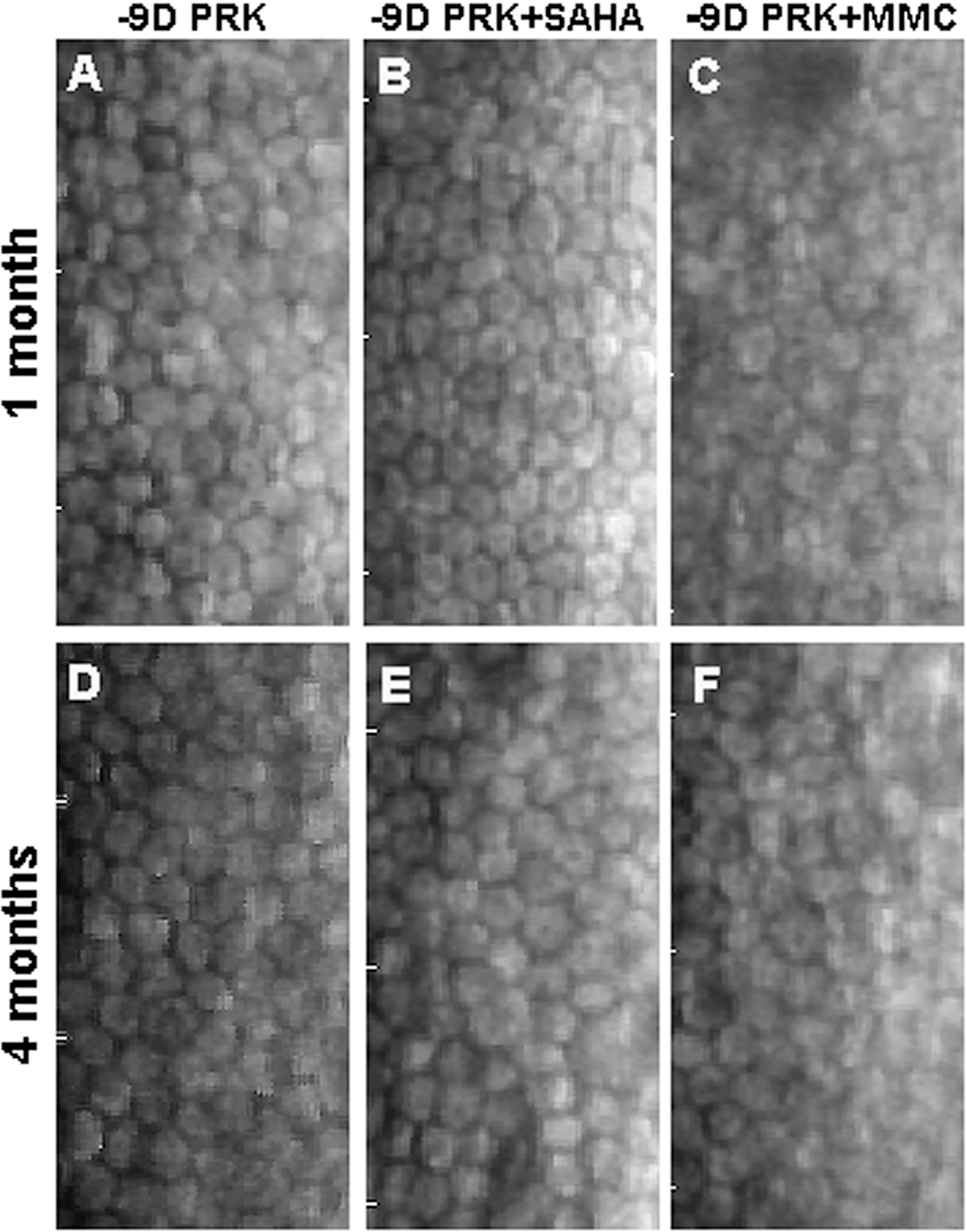
Effect of suberoylanilide hydroxamic acid (SAHA) and mitomycin C (MMC) on corneal endothelial cells. Representative specular biomicroscopy images of endothelial cells in the rabbit corneas at 1 and 4 months collected from eyes treated with balanced salt solution (control), SAHA (25 μM), or MMC (0.02%) after −9.00 diopter (D) photorefractive keratectomy (PRK). Corneas treated with SAHA (B and E) showed typical hexagonal corneal endothelial morphology and density similar to control (A and D). Conversely, corneas treated with MMC (C and F) showed markedly damaged corneal endothelial with compromised cell size and density at 1 and 4 months.
Figure C (available in the online version of this article) shows the effects of SAHA and MMC application on corneal keratocyte number and density in vivo at 1 month (Figures CA–CC) and 4 months (Figures CD–CF) after −9.00 D PRK analyzed with DAPI-staining. Corneas treated with MMC demonstrated significant keratocyte loss within the anterior stroma at 1 month (Figure CC) and 4 months (Figure CF) after PRK. Conversely, SAHA did not reduce the number or density of keratocytes in rabbit stroma at these time points (Figures CB and CE) and showed cellular density similar to corneas treated with BSS (Figures CA and CD).
Figure C.
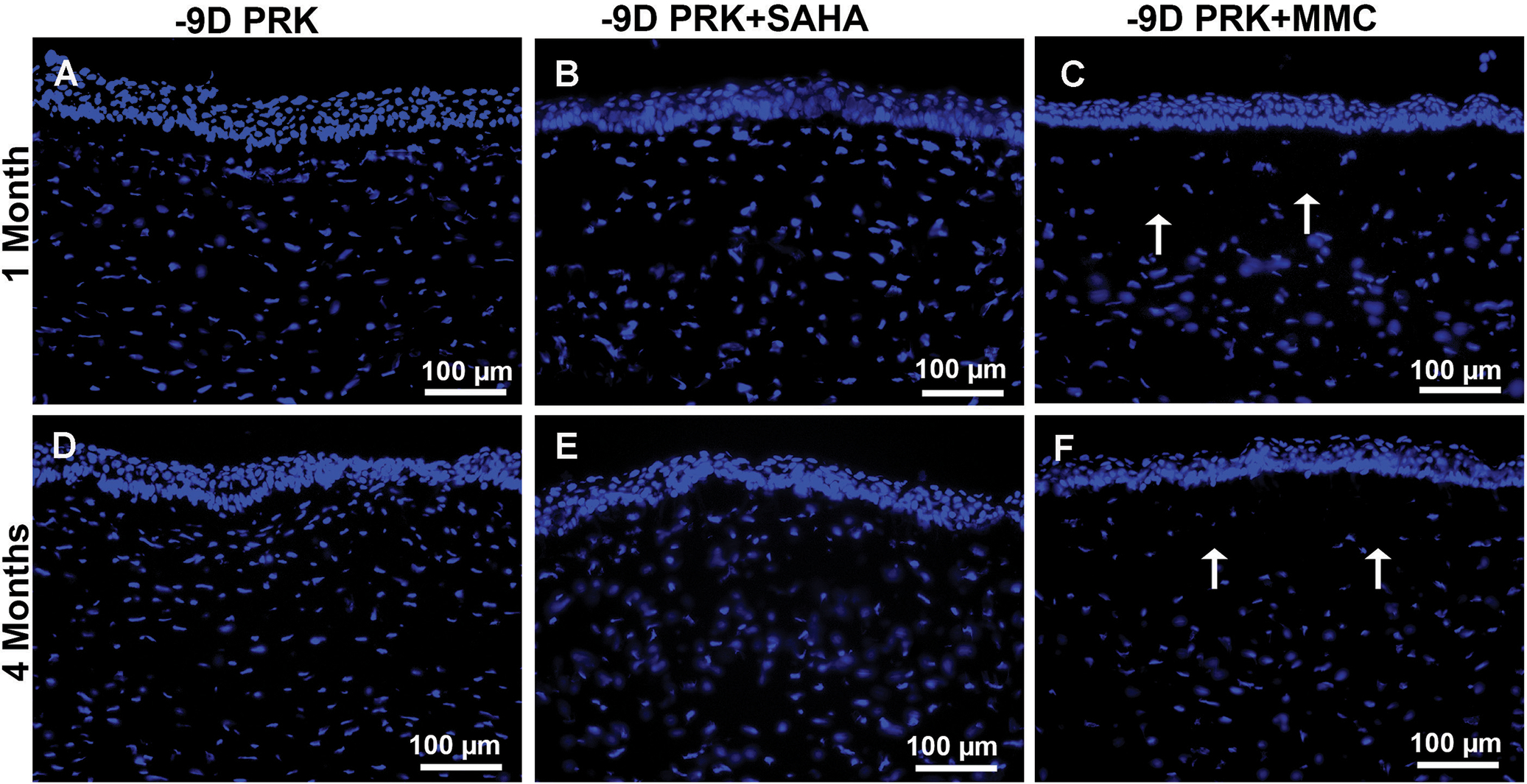
Effect of suberoylanilide hydroxamic acid (SAHA) and mitomycin C (MMC) on keratocyte number and density. Representative DAPI nuclear staining showing keratocyte population and density at 1 and 4 months in rabbit corneas treated with balanced salt solution (control), SAHA (25 μM), and MMC (0.02%) after photorefractive keratectomy (PRK). Control (A and C) or SAHA (B and D) treatment did not decrease keratocyte number and density in the stroma, whereas MMC treatment (C and F) caused significant keratocyte depletion of keratocyte number and density, especially in the anterior stroma as indicated by the arrows (C and F) at 1 and 4 months. Scale bar = 100 μm (Blue = DAPI-stained nuclei)
Figure 3 shows the effects of SAHA and MMC on corneal keratocyte apoptosis measured by TUNEL assay at 3 days (Figures 3A–3C), 1 month (Figures 3D–3F), and 4 months (Figures 3G–3I) after −9.00 D PRK. Treatment with BSS (Figure 3A) or SAHA (Figure 3B) did not induce apoptosis in any cell type, whereas treatment with MMC caused significant apoptosis in epithelial and keratocyte cells of the rabbit corneas collected at 3 days (Figure 3C). Similarly, the long-term toxicity studies at 1 and 4 months detected many TUNEL+ cells in the stroma and endothelium of corneas treated with MMC (Figures 3F and 3I), but not in corneas treated with SAHA (Figures 3E and 3H).
Figure 3.
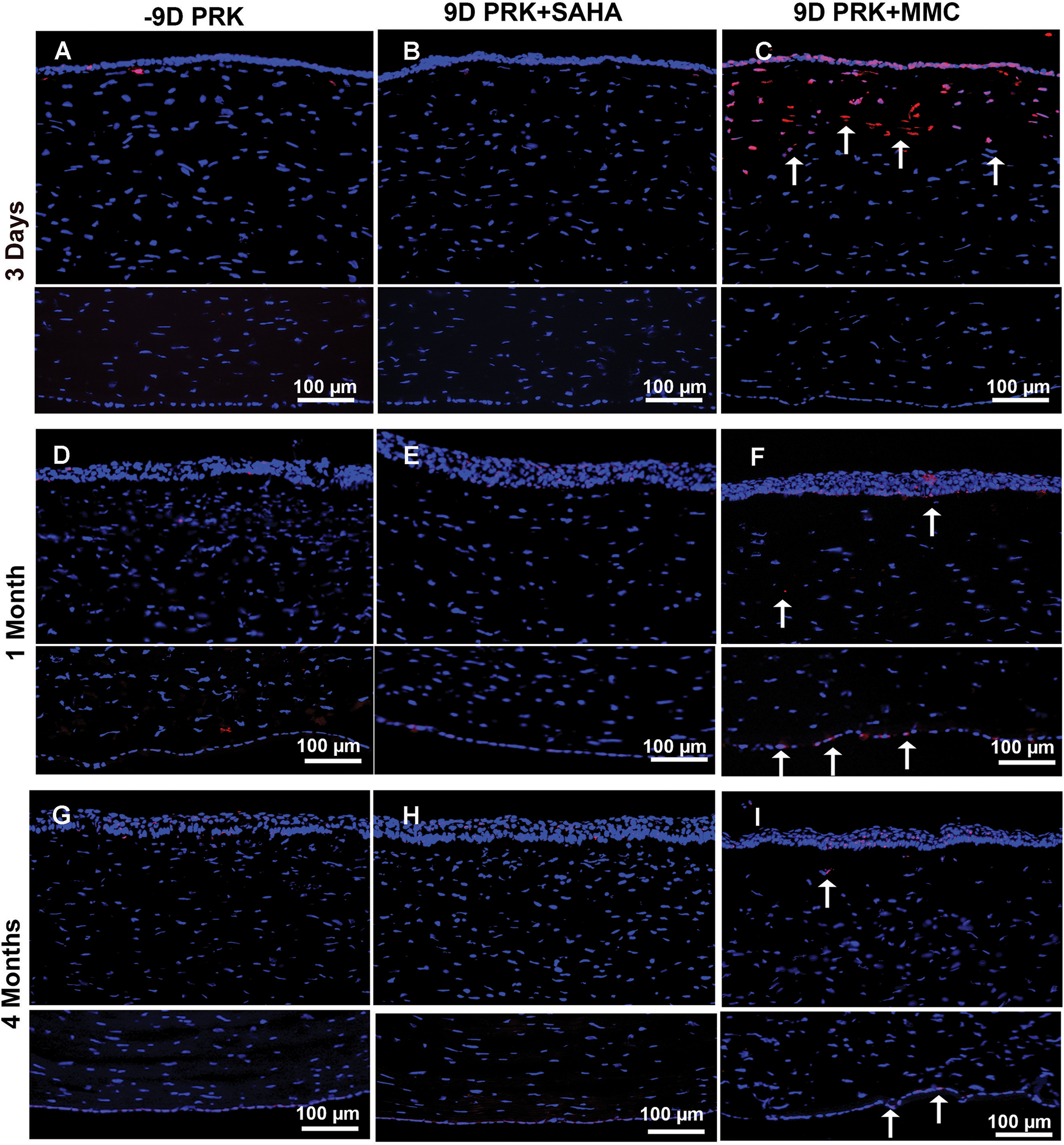
Effect of suberoylanilide hydroxamic acid (SAHA) and mitomycin C (MMC) treatment on keratocyte and endothelial loss. Representative images showing TUNEL staining in rabbit corneas collected 3 days, 1 month, and 4 months after −9.00 diopter (D) photorefractive keratectomy (PRK) and balanced salt solution (BSS), SAHA (25 μM), or MMC (0.02%) single adjunct topical application. BSS (A, D, and G) or SAHA (B, E, and H) treatment did not induce apoptosis in corneal keratocyte or endothelial cells as shown by detection of no TUNEL+ cells. Conversely, MMC treatment (C, F, and I) provoked significant apoptosis in keratocyte and epithelial cells but none in endothelial cells at 3 days (C), with noticeable apoptosis in endothelial cells and some in keratocytes at 1 (F) and 4 (I) months as shown by arrows. These data suggest that MMC caused significant damage to anterior stroma and restricted keratocyte revival in this region lost in the first 3 days of MMC application. Nuclei are stained blue with DAPI. TUNEL+ cells are stained red (arrows). Scale bar = 100 μm
DISCUSSION
Adjunct therapy is needed to reduce corneal haze after PRK, especially with higher diopter treatments. Our group has previously demonstrated that histone deacetylase inhibitors effectively reduce corneal haze and scarring in vivo in the rabbit cornea without causing significant acute side effects.15–18 In this study, we found adjunct topical SAHA and MMC application after PRK significantly prevented corneal haze and decreased the pro-fibrotic biomarkers in vivo in rabbits. Although haze inhibition by SAHA was less than that by MMC, this difference was statistically insignificant and appeared clinically irrelevant based on the slit-lamp subjective analysis. The most remarkable findings of the current study were the detection of significantly reduced cytotoxicity and enhanced safety profile by SAHA compared to MMC. SAHA application demonstrated markedly improved keratocyte viability and phenotype, reduced keratocyte and endothelial apoptosis, and strikingly better endothelial cellular morphology. These results suggest that topical adjunct SAHA application after PRK would be a safer alternative to MMC in preventing corneal haze after PRK.
The corneal wound healing response plays a central role in the outcome of refractive surgery. Pharmacologically broad-acting agents, specifically steroids and MMC, are most commonly used to control scarring after PRK. Increasingly precise targeted control of the corneal wound healing response will lead to faster recovery times, more accurate refractive outcomes, and decreased complication rates. In vitro analysis reveals that expression of α-SMA in stress fibers confers to the differentiated myofibroblast at least a two-fold stronger contractile activity compared with α-SMA–negative fibroblasts.21 We found many myofibroblasts after PRK expressing α-SMA in the anterior stroma of rabbit corneas. It is likely that these cells contribute to corneal scar formation and the refractive outcome of the procedure. The clearance of cells expressing α-SMA from the anterior stroma of corneas treated with MMC suggests the decrease in myofibroblasts is due to MMC toxicity. This toxicity is also responsible for a diminished keratocyte population available for conversion to myofibroblasts at the site of injury.
The literature suggests that some cells are vaporized instantly during laser photodisruption, whereas cells in close proximity go into a slow involution form of cell death, known as apoptosis.19 Myofibroblasts may undergo apoptosis or transdifferentiation back to a progenitor cell.22 The level of keratocyte apoptosis distribution and activated stromal keratocyte repopulation are likely contributors of corneal wound healing associated with variability and regression after PRK.
Previous studies demonstrated that topical application of MMC after PRK in rabbits not only decreased keratocyte density due to apoptosis at the wound site, but significantly delayed keratocyte repopulation and activation in the anterior stroma with normal epithelial cell differentiation.23 In this study, we observed a similar cytotoxicity pattern in rabbit corneas treated with MMC in which several TUNEL+ cells at shorter times and low DAPI-stained nuclei at longer times in the anterior stroma were observed. Contrary to this, SAHA treatment did not show such damage to the anterior stroma. These findings suggest SAHA has a superior safety profile to MMC in the treatment of corneal haze after PRK.
The corneal endothelial cells do not replicate in humans, and therefore their preservation is important for corneal transparency and normal functioning. In this study, topical application of SAHA did not cause apoptosis in endothelial cells, and also showed a typical polygonal morphology and cellular density similar to untreated control corneas up to 4 months, the longest tested time point. Conflicting literature on the effects of MMC on human corneal endothelial cells exists. A nonrandomized controlled trial showed that the prophylactic use of MMC (0.02%; 10 to 50 seconds) inhibited haze formation but caused significant loss of corneal endothelial cells.24 In contrast, other studies have shown that the administration of 0.02% MMC topically applied to the cornea for 1225 and 4026 seconds following PRK did not have a significant effect on qualitative morphometric parameters or quantitative endothelial cell density.
To the best of our knowledge, this is the first study to compare the efficacy and short- and long-term safety of MMC and SAHA. The results of our study suggest that single topical adjunct use of SAHA after −9.00 D PRK efficaciously prevents postoperative corneal haze without reducing keratocyte population or compromising corneal endothelial cells in vivo. We predict that SAHA may potentially offer an alternative to MMC for preventing corneal haze in patients undergoing PRK surgery. Human clinical trials are warranted.
Acknowledgments
Supported in part by the United States Veteran Health Affairs Merit 5I01BX000357 grant (RRM), the National Eye Institute, NIH 2RO1EY017294 grant (RRM), and the University of Missouri Ruth M. Karachi Missouri Ophthalmology Endowment fund (RRM).
The authors thank Mr. Matthew Faubion (medical student) and Mr. Nishant R. Sinha (medical student) for their assistance in fluorescence and quantification.
Footnotes
The authors have no financial or proprietary interest in the materials presented herein.
Contributor Information
Govindaraj Anumanthan, Harry S. Truman Memorial Veterans’ Hospital, Columbia, Missouri; One-health One-medicine Eye Research Center, Veterinary Medicine & Surgery and Biomedical Sciences, University of Missouri, Columbia, Missouri.
Ajay Sharma, Harry S. Truman Memorial Veterans’ Hospital, Columbia, Missouri; One-health One-medicine Eye Research Center, Veterinary Medicine & Surgery and Biomedical Sciences, University of Missouri, Columbia, Missouri; Chapman University, Irvine, California.
Michael Waggoner, Harry S. Truman Memorial Veterans’ Hospital, Columbia, Missouri; Mason Eye Institute, School of Medicine, University of Missouri, Columbia, Missouri.
Chuck W. Hamm, Mason Eye Institute, School of Medicine, University of Missouri, Columbia, Missouri.
Suneel Gupta, Harry S. Truman Memorial Veterans’ Hospital, Columbia, Missouri; One-health One-medicine Eye Research Center, Veterinary Medicine & Surgery and Biomedical Sciences, University of Missouri, Columbia, Missouri.
Nathan P. Hesemann, Harry S. Truman Memorial Veterans’ Hospital, Columbia, Missouri; Mason Eye Institute, School of Medicine, University of Missouri, Columbia, Missouri.
Rajiv R. Mohan, Harry S. Truman Memorial Veterans’ Hospital, Columbia, Missouri; One-health One-medicine Eye Research Center, Veterinary Medicine & Surgery and Biomedical Sciences, University of Missouri, Columbia, Missouri; Mason Eye Institute, School of Medicine, University of Missouri, Columbia, Missouri.
REFERENCES
- 1.Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. [DOI] [PubMed] [Google Scholar]
- 3.Mohan RR, Hutcheon AE, Choi R, et al. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. [DOI] [PubMed] [Google Scholar]
- 4.Stepp MA, Zieske JD, Trinkaus-Randal V, et al. Wounding the cornea to learn how it heals. Exp Eye Res. 2014;121:178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tandon A, Tovey JC, Sharma A, Gupta R, Mohan RR. Role of transforming growth factor beta in corneal function, biology and pathology. Curr Mol Med. 2010;10:565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tandon A, Sharma A, Rodier JT, Klibanov AM, Rieger FG, Mohan RR. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PLoS One. 2013;8:e66434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson SE. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp Eye Res. 2012;99:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan RR, Gupta R, Mehan MK, Cowden JW, Sinha S. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp Eye Res. 2010;91:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brart DP. Excimer laser surface ablation: a review of recent literature. Clin Exp Optom. 2014;97:12–17. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S, Rodier JT, Sharma A, et al. Targeted AAV5-Smad7 gene therapy inhibits corneal scarring in vivo. PLoS One. 2017;12:e0172928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Rodier JT, Tandon A, Klibanov AM, Mohan RR. Attenuation of corneal myofibroblast development through nanoparticle-mediated soluble transforming growth factor-β type II receptor (sTGFβRII) gene transfer. Mol Vis. 2012;18:2598–2607. [PMC free article] [PubMed] [Google Scholar]
- 12.Fink MK, Giuliano EA, Tandon A, Mohan RR. Therapeutic potential of Pirfenidone for treating equine corneal scarring. Vet Ophthalmol. 2015;18:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sy ME, Zhang L, Yeroushalmi A, Huang D, Hamilton DR. Effect of mitomycin-C on the variance in refractive outcomes after photorefractive keratectomy. J Cataract Refract Surg. 2014;40:1980–1984. [DOI] [PubMed] [Google Scholar]
- 14.Kim TI, Lee SY, Pak JH, Tchah H, Kook MS. Mitomycin C, ceramide, and 5-fluorouracil inhibit corneal haze and apoptosis after PRK. Cornea. 2006;25:55–60. [DOI] [PubMed] [Google Scholar]
- 15.Sharma A, Mehan MM, Sinha S, Cowden JW, Mohan RR. Trichostatin A inhibits corneal haze in vitro and in vivo. Invest Ophthalmol Vis Sci. 2009;50:2695–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tandon A, Tovey JC, Waggoner MR, et al. Vorinostat: a potent agent to prevent and treat laser-induced corneal haze. J Refract Surg. 2012;28:285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnelly KS, Giuliano EA, Sharma A, Mohan RR. Suberoylanilide hydroxamic acid (vorinostat): its role on equine corneal fibrosis and matrix metalloproteinase activity. Vet Ophthalmol. 2014;17:61–68. [DOI] [PubMed] [Google Scholar]
- 18.Gronkiewicz KM, Giuliano EA, Sharma A, Mohan RR. Molecular mechanisms of suberoylanilide hydroxamic acid in the inhibition of TGF-β1-mediated canine corneal fibrosis. Vet Ophthalmol. 2016;19:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Netto MV, Mohan RR, Sinha S, Sharma A, Gupta PC, Wilson SE. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, cellular proliferation, haze, and long-term keratocyte density in rabbits. J Refract Surg. 2006;22:562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantes FE, Hanna KD, Waring GO 3rd, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990;108:665–675. [DOI] [PubMed] [Google Scholar]
- 21.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maltseva I, Chan M, Kalus I, Dierks T, Rosen SD. The SULFs, extracellular sulfatases for heparan sulfate, promote the migration of corneal epithelial cells during wound repair. PLoS One. 2013;8:e69642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajan MS, O’Brart DP, Patmore A, Marshall J. Cellular effects of mitomycin-C on human corneas after photorefractive keratectomy. J Cataract Refract Surg. 2006;32:1741–1747. [DOI] [PubMed] [Google Scholar]
- 24.Nassiri N, Farahangiz S, Rahnavardi M, Rahmani L, Nassiri N. Corneal endothelial cell injury induced by mitomycin-C in photorefractive keratectomy: nonrandomized controlled trial. J Cataract Refract Surg. 2008;34:902–908. [DOI] [PubMed] [Google Scholar]
- 25.Goldsberry DH, Epstein RJ, Majmudar PA, et al. Effect of mitomycin C on the corneal endothelium when used for corneal subepithelial haze prophylaxis following photorefractive keratectomy. J Refract Surg. 2007;23:724–727. [DOI] [PubMed] [Google Scholar]
- 26.Zare M, Jafarinasab MR, Feizi S, Zamani M. The effect of Mitomycin-C on corneal endothelial cells after photorefractive keratectomy. J Ophthalmic Vis Res. 2011;6:8–12. [PMC free article] [PubMed] [Google Scholar]


