Abstract
Purpose of review:
To discuss how nutritional management could be optimized to promote protective metabolism in sepsis and associated acute kidney injury.
Recent findings:
Recent evidence suggests that sepsis is a metabolically distinct critical illness and that certain metabolic alterations, such as activation of fasting metabolism, may be protective in bacterial sepsis. These findings may explain the lack of survival benefit in recent randomized controlled trials of nutrition therapy for critical illness. These trials are limited by cohort heterogeneity, combining both septic and non-septic critical illness, and the use of inaccurate caloric estimates to determine energy requirements. These energy estimates are also unable to provide information on specific substrate preferences or the capacity for substrate utilization. As a result, high protein feeding beyond the capacity for protein synthesis could cause harm in septic patients. Excess glucose and insulin exposures suppress fatty acid oxidation, ketogenesis and autophagy, of which emerging evidence suggest are protective against sepsis associated organ damage such as acute kidney injury.
Summary:
Distinguishing pathogenic and protective sepsis-related metabolic changes are critical to enhancing and individualizing nutrition management for critically ill patients.
Keywords: Critical illness, nutrition, sepsis, metabolism, acute kidney injury
Introduction
The most recent clinical consensus defines sepsis as life-threatening organ dysfunction owing to dysregulated host response to infection [1]. Sepsis remains a global health problem with over 31 million sepsis cases and 5 million deaths worldwide annually [2]. In the United States, sepsis accounts for 50% of in-hospital deaths even though it accounts for only 10% of hospitalizations [3]. Moreover, it remains the most expensive hospital diagnosis [4]. Despite broad-spectrum antibiotics and life-supporting technologies, therapeutics to improve sepsis outcomes remain limited. We have long appreciated the hallmark characteristics of metabolic derangements in sepsis, including hyperglycemia resulting from gluconeogenesis and insulin resistance, hyperlipidemia from lipolysis, and enhanced protein catabolism [5]. While many of these metabolic derangements have traditionally been viewed as entirely pathologic, emerging evidence suggests a complex relationship between host defense and metabolism. Host defense consists of both disease resistance, which involves pathogen clearance, and disease tolerance, in which physiologic responses are activated to limit tissue damage [6-8]. Some metabolic alterations resulting from the immune response to an infection may reflect protective defense mechanisms involved in disease tolerance. Thus, current attempts to reverse metabolic derangements associated with sepsis may be counterproductive. For example, the loss of muscle mass from increased protein catabolism is associated with poor outcomes in sepsis [9]. However, the proteolytic response in sepsis could be an essential adaptive response to fuel the liver’s synthesis of acute phase proteins [10], many of which are important in host defense. Similarly, changes in glucose metabolism are considered pathologic given the association of hyperglycemia with increased mortality in critical illness [11]. However, it has been proposed that the purpose of this change in glucose metabolism is to redirect glucose utilization, including fueling the immune system for the initial antimicrobial response [12]. While lipolysis and the resulting hyperlipidemia could be considered maladaptive [13-16], there is evidence that lipoproteins are capable of binding and neutralizing endotoxin [17]. Moreover, the mobilized lipid substrates could be used as fuel for fatty acid oxidation (FAO) [5]. Many of our current approaches to minimize the consequences of these metabolic changes are indirect rather than targeting the root cause. Moreover, correction of abnormal clinical metabolic metrics by any means possible, without full consideration of the potential adverse consequences, could lead to harm. With a better understanding of metabolic disease tolerance pathways, we may need to revise our current management of septic patients to limit organ damage such as acute kidney injury (AKI) and optimize survival.
Clinical trials for nutritional therapy in critical illness: Interpretations and limitations
The provision of nutrition is a major factor contributing to the metabolic state of a septic patient. Many recent clinical trials have addressed timing, route, and caloric content of nutritional therapy in critically ill patients without any significant effect on survival (Table). The EDEN and PermiT trials showed that low calorie, trophic feeds are safe, but do not improve survival [18, 19]. Alternatively, the TARGET trial suggests feeding more also does not improve outcomes [20]. The CALORIES and NUTRIREA-2 trials showed that the route of early feeding, enteral versus parenteral, did not alter survival [21, 22]. However, the occurrence of severe gastrointestinal adverse effects observed in NUTRIREA-2 led to concerns about excessive enteral feeding in mechanically ventilated patients on vasopressors. Finally, if patients cannot tolerate enteral feeds, the EPaniC trial suggests the addition of parenteral feeds could cause harm if given too early [23]. Overall, the lack of mortality benefit in all these trials has left significant equipoise on how best to deliver nutritional therapy to critically ill patients.
Table 1.
Recent Intensive Care Unit Nutrition Therapy Clinical Trials
| Author Study Year |
Study population | Study regimen/Intervention | Primary Outcomes | Secondary Outcomes | |
|---|---|---|---|---|---|
| Rice (EDEN) 2012 [18] | Mechanically ventilated patients within 48 hours of acute lung injury | Trophic1 | Full2 | No difference in ventilator free days | Full feeding group with increased GI adverse effects (vomiting, residuals) and increased insulin use. |
| Arabi (PermiT) 2015 [19] | Patients fed enterally within 48 hours of ICU admission | Permissive underfeeding3 | Standard feeding4 | No difference in 90-day all-cause mortality | Standard feeding group with more incident RRT, higher blood glucose, and increased insulin use. |
| Chapman (TARGET) 2018 [20] | Mechanically ventilated ICU patients | Energy dense enteral5 | Routine enteral6 | No difference in 90-day all-cause mortality | Energy dense group had increased GI adverse effects (vomiting, residuals) and increased insulin use. |
| Harvey (CALORIES) 2014 [21] | ICU patients requiring nutritional support7 | Enteral8 | Parenteral8 | No difference in 30-day all-cause mortality | Enteral group had increased GI adverse effects (vomiting) and increased rates of hypoglycemia. |
| Reignier (NUTRIREA-2) 2018 [22] | Mechanically ventilated ICU patients requiring vasoactive therapy and nutritional support | Enteral9 | Parenteral9 | No difference in 28-day all-cause mortality | Enteral group had increased GI adverse effects (vomiting, diarrhea, bowel ischemia, and acute colonic pseudo-obstruction), increased rates of hypoglycemia, and less insulin use. |
| Casaer (EPanIC) 2011 [23] | Nutritionally at risk ICU patients (not meeting caloric goals with enteral nutrition) | Early PN initiation10 | Late PN initiation initiated on day 810,11 | Early PN had increased ICU length of stay. | Early PN group had increased ICU infections, longer hospital length of stay, and longer duration of MV and RRT. |
20 kcal/h
25-30 kcal/kg/d of nonprotein calories and 1.2-1.6 g/kg/d of protein
40-60% estimated caloric needs
70-100% estimated caloric needs
Enteral feed with 1.5kcal/mL at a target rate of 1 ml/kg calculated ideal body weight/hr.
Enteral feed with 1.0 kcal/mL at a target rate of 1 ml/kg calculated ideal body weight/hr.
Cohort included 83% mechanically ventilated and 83% requiring vasoactive agents.
Energy target of 25 kcal/kg of actual body weight/d.
20-25 kcal/kg of actual body weight/d first 7 days, then 25-30 kcal/kg of actual body weight/d from day 8 to extubation.
Caloric goal included protein energy and were based on corrected ideal body weight, age, and sex.
If enteral nutrition was insufficient after 7 days in the ICU, parenteral nutrition was initiated on day 8 to reach the caloric goal.
Intensive Care Unit (ICU), Mechanical ventilation (MV), Gastrointestinal (GI), Renal Replacement Therapy (RRT), Parenteral Nutrition (PN)
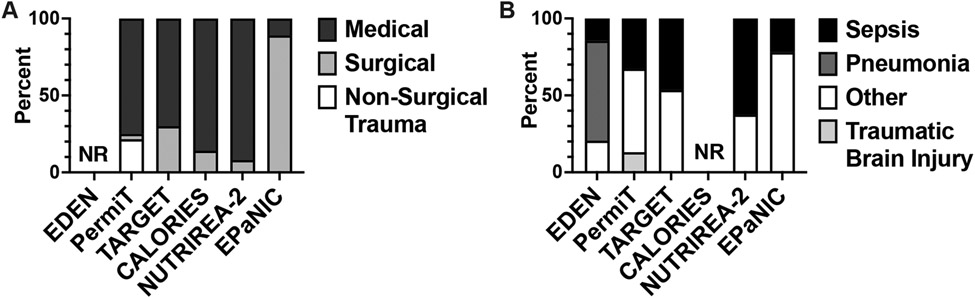
There are several limitations of these trials precluding applicability to critically ill septic patients. First, energy expenditure (EE) and therefore presumed energy requirements were estimated, not measured. It is well established that equations using static anthropometric measurements such as height and weight to estimate EE are inaccurate in critically ill patients, including those with AKI [24-27]. EE is also known to be dynamic, classically described by Cuthbertson in 1942 as the Ebb and Flow energy phases of hemorrhagic shock [28], and variable depending on the presence and severity of sepsis [29]. Thus, multiple guidelines recommend the use of indirect calorimetry, the gold standard, to measure EE in critically ill patients, including those with AKI, to guide nutritional therapy [30-32]. However, current commercially available indirect calorimeters can be inaccurate and are expensive, limiting routine clinical use. Fortunately, a new generation of indirect calorimeters that are more accurate and cost effective are in development [33]. As nonprotein calories are rarely differentiated, another layer of complexity is the calorie source, which is not fully addressed in these trials. Moreover, we do not know whether substrate preferences differ among various critical illnesses and across distinct phases of disease. Thus, differential metabolic downstream consequences of delivered carbohydrates and fat calories are not clear. Second, as is common in most intensive care unit (ICU) clinical trials, cohort heterogeneity is significant (Table, Figure 1A). These trials include a mix of medical, surgical, and neurological ICU patients. Another important clinical parameter with significant metabolic implications is the presence of sepsis. In fact, the composition of septic patients varied across these trials (Figure 1B). The causative pathogen was also not reported. The type of sepsis may be significant as preclinical studies suggest metabolic determinants of survival differ between bacterial and viral septicemia [34]. Thus, the imprecision of EE estimates without differentiating specific calories and the heterogeneity of critically ill patients included in these trials limit the clinical applicability to septic patients.
Figure 1. Cohort heterogeneity in intensive care unit nutrition therapy clinical trials.
A. Study population by intensive care unit setting by percentage. B. Study population by type of critical illness by percentage. NR, not reported.
Protein catabolism: How to limit negative protein balance?
Critical illness is often associated with increased protein catabolism, leading to net negative nitrogen balance and loss of muscle mass, which are associated with increased mortality [9]. Pharmacologic and nutritional approaches have attempted to minimize negative nitrogen balance in critically ill patients with minimal benefit. For example, growth hormone is effective in improving nitrogen balance in critically ill patients, but at the cost of increasing mortality, incident sepsis, hyperglycemia and insulin use [35]. Nutritional approaches with high protein feeds which minimize negative nitrogen balance and improve outcomes have been supported by several observational studies [36]. However, in meta-analyses of randomized controlled trials (RCTs), high protein feeding in critically ill patients has not shown benefit [37, 38]. The association of higher protein intake with better outcomes seen in observational studies are limited by potential confounders. Patients who have a better prognosis will survive long enough to achieve nutritional targets and may receive more attention to optimize their nutritional support, introducing potential immortal time and indication bias. As some RCTs were confounded by differences in calorie intake, ongoing RCTs are addressing this to test the efficacy of higher protein nutrition in the critically ill at a fixed caloric intake [39, 40]. While these studies are ongoing, there remains the possibility that excess dietary protein could be harmful in critically ill patients.
In the example of continuous renal replacement therapy (CRRT), amino acid losses are known to occur [41, 42]. While iatrogenic losses should be replaced, the overall benefit of high protein feeds to minimize negative protein balance in AKI patients on CRRT is unclear. One RCT examined the use of indirect calorimetry to guide nutritional therapy and escalating dietary protein in AKI patients on CRRT [43]. While patients with increased nitrogen balance had improved survival and higher protein intake associated with increased nitrogen balance, higher protein intake itself was not associated with improved survival. This would suggest that the true relationship between nitrogen balance and survival involves another factor that minimizes negative nitrogen balance, such as resolution of the underlying critical illness. Similarly in a post-hoc analysis of the RENAL study, higher dietary protein intake was associated with higher rates of mortality [44, 45]. While the total dietary protein intake in both groups were relatively low, it is important to note that the higher protein intake group had significantly more septic patients. In other studies, the relative proportion of septic patients in a cohort appears to correlate with worse outcomes associated with higher dietary protein intake. For example, in small RCTs examining the effect of high protein intake on limiting muscle loss, studies with few (less than 10%) or unreported septic patients showed improved outcomes [46, 47], while one RCT with a high percentage (80%) of septic patients showed no effect [48]. Similarly, in an observational study in which half the cohort was septic, higher protein delivery was associated with increased muscle wasting [49]. In burn patients, sepsis is a primary determinant of protein catabolism [50]. Similarly, in amino acid balance studies, only patients who recovered from sepsis could achieve net protein synthesis with the provision of high protein nutrition [51, 52]. While critically ill non-septic and septic patients both have increased protein catabolism, the presence of sepsis appears to be a primary driver of outcomes and not nitrogen balance per se. Therefore, negative nitrogen balance is more likely to be reflective of the inflammatory state, similar to decreased serum visceral proteins such as albumin and pre-albumin, which are no longer recommended to guide nutritional therapy [53]. Thus, there is a critical need for methods to accurately measure protein utilization to guide nutritional delivery rather than targeting nitrogen balance.
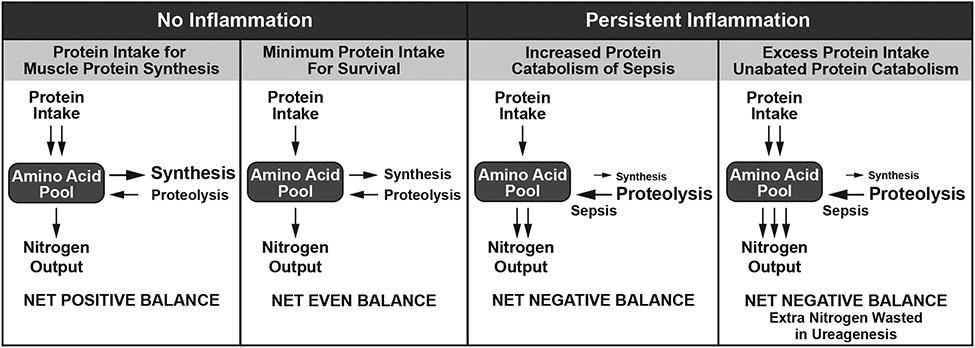
Unfortunately, the inability to accurately measure the capacity for protein utilization is a major limitation in the clinical management of protein catabolism. Dietary protein requirements are derived from nitrogen balance estimates which compare urinary and other bodily losses against dietary intake. As nitrogen balance estimates have many limitations even for healthy individuals at equilibrium [54], use of these balance estimates to calculate dietary protein needs in critically ill septic patients is highly problematic. As there is no ability to store protein, dietary protein not utilized for synthesis must be catabolized and excreted primarily as urea. In sepsis, high, insuppressible rates of protein catabolism will result in increased urea production. As a result, calculation of estimated protein requirements with ongoing catabolism will continually increase with rising urinary urea excretion in the absence of appropriate protein utilization (Figure 2). In a pre-specified analysis of the EPaNIC trial, timing of parenteral nutrition did not change the incidence of AKI [55]. However, early parenteral nutrition slowed renal recovery potentially due to increased ureagenesis, which prolonged RRT. It was also estimated that 63% of extra nitrogen intake was net wasted in ureagenesis. Excess dietary protein, beyond the capacity of utilization, is not only wasteful, it may also be deleterious. In fact, an unpublished subgroup analysis of an observational study suggesting a benefit of high protein intake in critically ill patients, the survival benefit was only observed in non-septic patients, while there was a trend toward increased mortality in septic patients [56, 57]. Osmotic urea diuresis, of which high dietary protein intake is a major cause, is a common cause of hypernatremia, which increases mortality in critically ill patients [58, 59]. High protein intake can also suppress autophagy [60], a regulated cellular mechanism that removes unfolded or misfolded proteins and damaged organelles, resulting in recycling of nutrients and cell survival. In preclinical sepsis models, autophagy has been shown to be protective, improving survival and organ function [61, 62]. This is particularly relevant in septic AKI, where inhibition of autophagy will increase septic AKI, while augmenting autophagy will limit septic kidney damage [63-67]. As a result, focusing on nitrogen balance alone to guide dietary protein interventions without considering capacity for utilization and potential adverse effects may inadvertently delay kidney recovery in critically ill patients.
Figure 2. Nitrogen balance in health and disease.
In the absence of inflammation, positive nitrogen balance and muscle protein synthesis are possible. Excess dietary protein in the presence of ongoing inflammation due to diseases such as sepsis, will lead to wasted nitrogen in ureagenesis and could cause harm.
Glucose versus fatty acid oxidation
It is well-appreciated that hyperglycemia associates with poor outcomes in critically ill patients [68]. However, glycemic control with insulin in the ICU remains controversial. Much of the controversy stems from the inability to reproduce the landmark Leuven I trial [69]. Subsequently, intensive glycemic control in the NICE-SUGAR multi-center RCT [70] led to increased mortality. While hypoglycemia has been cited to be the most likely cause of increased mortality, patients in the intensive control group also received more of both insulin and glucose. While hypoglycemic events are detrimental and are likely contributing to mortality, the exposure of excess insulin and glucose could also cause harm in sepsis.
Sepsis is associated with the development of anorexia, which is a highly conserved component of sickness behavior. Anorexia associated with sepsis contributes to activation of fasting metabolism. Normal fasting and starvation adaptation includes induction of FAO, gluconeogenesis, ketogenesis, and autophagy. Fasting metabolism is a primary mechanism by which caloric restriction and intermittent fasting promotes longevity and mitigates diseases [71-73]. It has been proposed that fasting metabolism is dysfunctional in sepsis [14, 74-77], and this abnormal fasting response is a driver of morbidity and mortality. While the quality, magnitude, and kinetics of the fasting metabolic pathways may differ in sepsis compared to normal fasting, many of these pathways are intact in septic patients and in preclinical models of sepsis [34, 78-80]. However, these pathways are suppressed by the provision of glucose even at hypocaloric levels [34, 81, 82]. For example, patients with sepsis exhibit a significant switch in global metabolism from glucose oxidation to FAO. This is evident in a lower respiratory quotient, reflective of increased lipid metabolism, seen in critically ill patients with sepsis compared to those without sepsis [83]. Moreover, septic patients have low glucose oxidation which cannot be induced with a hyperglycemic glucose clamp [81]. Instead, glucose infusion in septic patients will increase insulin levels while suppressing FAO [84] and ketogenesis [81, 82]. These data suggest FAO may be an adaptive response and the preferred metabolic state in sepsis.
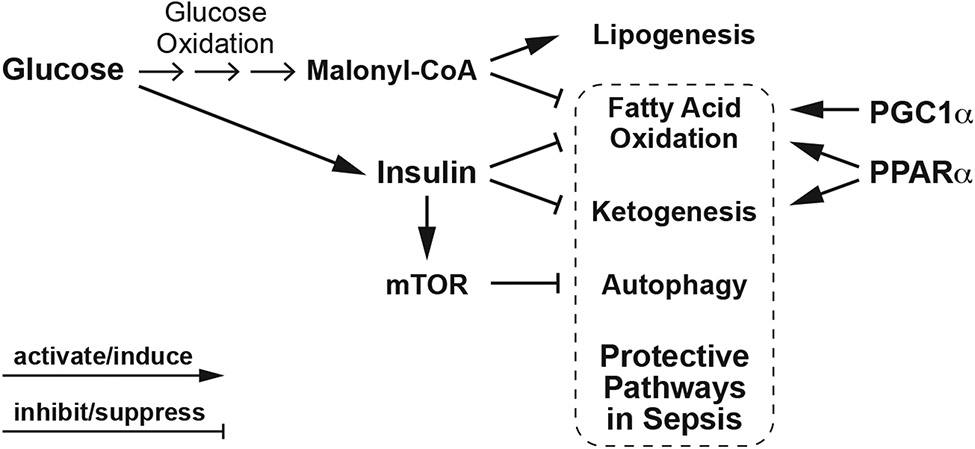
Growing preclinical evidence supports a role for peroxisomal proliferator-activated receptor (PPARα) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) as protective metabolic mediators in sepsis, limiting organ damage including AKI and improving survival [79, 85-92]. PPARα and PGC1α promote FAO, while PPARα also activates ketogenesis. Glucose and insulin could suppress metabolic pathways activated by PPARα and PGC1α. Glucose oxidation inhibits FAO through the metabolic intermediate, malonyl-CoA, and insulin promotes lipogenesis while inhibiting FAO and ketogenesis (Figure 3) [93]. Insulin will also suppress autophagy through activating mammalian target of rapamycin (mTOR), the main inhibitor of autophagy (Figure 3). One RCT of intensive glycemic control using insulin observed that increased insulin exposure associated with suppression of autophagy in critically ill patients [94]. A recent retrospective analysis of type 2 diabetic hospitalized patients with confirmed COVID-19 reported a significant relationship of insulin treatment with increased mortality, mechanical ventilation, and AKI [95]. Thus, the clinical consequences of suppressing FAO, ketogenesis and autophagy by insulin could be significant.
Figure 3. Metabolic effects of glucose and insulin.
Glucose oxidation promotes lipogenesis and inhibits fatty acid oxidation. In response to elevated blood glucose levels, secreted insulin regulates glucose storage while inhibiting fatty acid oxidation, ketogenesis, and autophagy through the activation of mTOR, a strong inhibitor of autophagy. PPARα and PGC1α promote fatty acid oxidation, while PPARα activates both fatty acid oxidation and ketogenesis. Fatty acid oxidation, ketogenesis, and autophagy have all been proposed as protective pathways in sepsis. Thus, excess glucose or carbohydrate nutritional delivery and the resulting obligate insulin requirements could lead to suppression of these protective metabolic pathways.
The possibility of glucose and insulin-regulated metabolism interfering with protective metabolic pathways in sepsis raises concerns of whether the way we feed and medicate septic patients could be counterproductive and potentially harmful. Commercially available enteral nutrition formulations are generally high in carbohydrate content. Many intravenous medications are delivered in dextrose containing solutions and parenteral nutrition is also formulated with significant glucose content. This high carbohydrate load ultimately will lead to increased obligate insulin requirements. In studies examining the effect of high versus low calorie or protein nutritional delivery on nitrogen balance in AKI patients on CRRT, the average glucose administered is close to 400 grams or more per day [96-98]. This amount of glucose will not only obligate more insulin, but it is also more than the carbohydrate oxidation capacity of AKI patients. One indirect calorimetry study found that AKI patients, both septic and non-septic, were not given enough lipid to support measured lipid oxidation rates, while carbohydrates were given more than actual glucose oxidation rates [99]. Thus, alternative approaches could include limiting the glycemic load or changing the delivery of feeds to allow for adequate periods of fasting capable of enhancing FAO, ketogenesis and autophagy [100].
Other metabolic considerations in sepsis:
Intermittent feeding.
Prior work has extensively examined the timing of feeding initiation in ICU patients, however an organized approach to comparing an intermittent versus continuous feeding protocol and by extension the importance of fasting periods in critically ill patients has yet to be undertaken. Several small non-inferiority RCTs reported favorably on clinical endpoints such as gastrointestinal distress and aspiration pneumonia for those receiving intermittent feeds compared to those continuously fed [100, 101]. However, the small sample sizes, lack of harder endpoints, inconsistent reporting on metabolic variables, and heterogeneity in feed and insulin administration protocols limit our ability to make broader conclusions about the effect of fasting duration on optimizing metabolism to survive critical illnesses such as sepsis. In contrast to outpatient intermittent fasting regimens using at least 16-18 hours of fasting [71], typical intermittent feeding protocols for critical illness have at most 8 hours of fasting [102]. The optimal duration of the fasting state to promote the protective effects of autophagy, mitochondrial biogenesis, muscle protein synthesis and ketogenesis still lacks consensus, especially in septic patients. Van Dyck et al reported that 12 hours of fasting in critically ill patients was insufficient to alter autophagic marker expression levels in blood samples [103], suggesting a longer interval of fasting may be necessary. However, improved methods of measuring autophagy are needed as static blood expression levels may not be sufficient to determine autophagic flux or tissue specific autophagy [104]. Moreover, feed composition may still be a critical factor independent of fasting duration.
Underlying metabolic disease.
An additional complicating factor is the increasing prevalence of obesity, diabetes mellitus, sarcopenia, and cancer among critically ill patients. Common to these diseases and disorders is the presence of metabolic dysregulation. While the prognostic relationships are evident, the mechanistic effects of these underlying metabolic diseases on the acute phase of sepsis are not as clear. Moreover, metabolic inflexibility could render septic patients with these co-morbidities less likely to respond to metabolic and nutritional interventions [105, 106].
Immune system and the microbiome.
Immunometabolism is an important component of host defense [107-109]. Metabolic determinants of successful host response are complex with both infection- and immune cell type-specificity. It is unknown whether nutritional and metabolic interventions optimal for the immune system could conflict with peripheral organ metabolic response under stress conditions [108]. Nutritional exposures and medications, especially antibiotics, will also alter the gut microbiota and intestinal integrity, of which both impact inflammation and host responses to sepsis [110, 111]. Balance of these potentially conflicting metabolic demands needs to be considered when designing appropriate nutritional interventions.
Conclusion
Sepsis is a distinct metabolic entity compared to other critical illnesses. As such, the optimal metabolic and nutritional management will likely differ between septic and non-septic critically ill patients. Thus, it is critical to understand the metabolic determinants of host defense and disease tolerance. Growing evidence suggests that a switch towards a metabolic state favoring FAO, ketogenesis and autophagy may be protective in bacterial sepsis. In fact, several lines of preclinical evidence support FAO and autophagy as mechanisms to limit septic AKI. Development of bedside methods to accurately measure substrate preferences and capacity for utilization is needed to avoid excess nutrient delivery that could cause harm, while optimizing metabolic pathways that promote survival.
Key points:
Metabolic alterations associated with sepsis may represent protective defense mechanisms.
Without fully understanding sepsis pathophysiology, iatrogenic interventions meant to correct abnormal clinical parameters such as hyperglycemia and negative nitrogen balance could lead to harm.
Similar to decreased visceral proteins, negative nitrogen balance likely reflects ongoing inflammation and would not serve as an appropriate target for nutrition therapy.
Excess glucose and insulin exposure could lead to inhibition of fatty acid oxidation and autophagy, metabolic pathways that support survival and limit AKI in preclinical sepsis models.
Development of bedside methods to accurately measure substrate preferences and capacity for utilization is critically needed.
Financial support and sponsorship:
S.C.H. was supported by the NIH (grants K08DK110424 and R35GM137984) and the American Society of Nephrology Carl W. Gottschalk Research Scholar Grant. A.H.V was supported by the NIH (grant T32DK007257).
Abbreviations:
- AKI
acute kidney injury
- CRRT
continuous renal replacement therapy
- EE
energy expenditure
- FAO
fatty acid oxidation
- mTOR
mammalian target of rapamycin
- NR
not reported
- PGC1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PPARα
peroxisomal proliferator-activated receptor
- RCTs
randomized controlled trials
Footnotes
Conflicts of interest: none.
References and recommended reading:
- 1.Singer M, Deutschman CS, Seymour CW et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016; 315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NK et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016; 193:259–272. [DOI] [PubMed] [Google Scholar]
- 3.Liu V, Escobar GJ, Greene JD et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014; 312:90–92. [DOI] [PubMed] [Google Scholar]
- 4.Torio C, Moore B. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013. HCUP Statistical Brief #204. Agency for Healthcare Research and Quality, Rockville, MD. 2016. Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.pdf. [PubMed] [Google Scholar]
- 5.Tappy L, Chioléro R. Substrate utilization in sepsis and multiple organ failure. Crit Care Med. 2007; 35:S531–4. [DOI] [PubMed] [Google Scholar]
- 6.Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012; 30:271–294. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012; 335:936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huen SC. Metabolism as Disease Tolerance: Implications for Sepsis-Associated Acute Kidney Injury. Nephron. 2021; 1–4. *Brief review on the importance of considering metabolism as a mechanism of disease tolerance in sepsis and the relevance in sepsis associated acute kidney injury.
- 9.Zhang J, Huang Y, Chen Y, Shen X, Pan H, Yu W. Impact of Muscle Mass on Survival in Patients with Sepsis: A Systematic Review and Meta-Analysis. Ann Nutr Metab. 2021; 77:330–336. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006; 84:475–482. [DOI] [PubMed] [Google Scholar]
- 11.Mesotten D, Preiser JC, Kosiborod M. Glucose management in critically ill adults and children. Lancet Diabetes Endocrinol. 2015; 3:723–733. [DOI] [PubMed] [Google Scholar]
- 12.Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response. Crit Care. 2013; 17:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallin JI, Kaye D, O’Leary WM. Serum lipids in infection. N Engl J Med. 1969; 281:1081–1086. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann RL, Matson CF, Beisel WR. Hypertriglyceridemia produced by endotoxin: role of impaired triglyceride disposal mechanisms. J Infect Dis. 1976; 133:548–555. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi O, Sakaguchi S. Alterations of lipid metabolism in mice injected with endotoxin. Microbiol Immunol. 1979; 23:71–85. [DOI] [PubMed] [Google Scholar]
- 16. Barker G, Leeuwenburgh C, Brusko T, Moldawer L, Reddy ST, Guirgis FW. Lipid and Lipoprotein Dysregulation in Sepsis: Clinical and Mechanistic Insights into Chronic Critical Illness. J Clin Med. 2021; 10:1693. *Comprehensive review on changes in lipid metabolism during sepsis.
- 17.Harris HW, Gosnell JE, Kumwenda ZL. The lipemia of sepsis: triglyceride-rich lipoproteins as agents of innate immunity. J Endotoxin Res. 2000; 6:421–430. [PubMed] [Google Scholar]
- 18.National Heart L, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Rice TW, Wheeler AP et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012; 307:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arabi YM, Aldawood AS, Haddad SH et al. Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. N Engl J Med. 2015; 372:2398–2408. [DOI] [PubMed] [Google Scholar]
- 20.TARGET Investigators for the ANZICS Clinical Trials Group, Chapman M, Peake SL et al. Energy-Dense versus Routine Enteral Nutrition in the Critically Ill. N Engl J Med. 2018; 379:1823–1834. [DOI] [PubMed] [Google Scholar]
- 21.Harvey SE, Parrott F, Harrison DA et al. Trial of the route of early nutritional support in critically ill adults. N Engl J Med. 2014; 371:1673–1684. [DOI] [PubMed] [Google Scholar]
- 22.Reignier J, Boisramé-Helms J, Brisard L et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet. 2018; 391:133–143. [DOI] [PubMed] [Google Scholar]
- 23.Casaer MP, Mesotten D, Hermans G et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011; 365:506–517. [DOI] [PubMed] [Google Scholar]
- 24.Oshima T, Berger MM, De Waele E et al. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin Nutr. 2017; 36:651–662. [DOI] [PubMed] [Google Scholar]
- 25.de Góes CR, Berbel-Bufarah MN, Sanches AC, Xavier PS, Balbi AL, Ponce D. Poor Agreement between Predictive Equations of Energy Expenditure and Measured Energy Expenditure in Critically Ill Acute Kidney Injury Patients. Ann Nutr Metab. 2016; 68:276–284. [DOI] [PubMed] [Google Scholar]
- 26.Góes CR, Balbi AL, Ponce D. Evaluation of Factors Associated with Hypermetabolism and Hypometabolism in Critically Ill AKI Patients. Nutrients. 2018; 10:E505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabatino A, Theilla M, Hellerman M et al. Energy and Protein in Critically Ill Patients with AKI: A Prospective, Multicenter Observational Study Using Indirect Calorimetry and Protein Catabolic Rate. Nutrients. 2017; 9:E802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuthbertson DP. Post-shock metabolic response. The Lancet. 1942; 239:433–437. [Google Scholar]
- 29.Kreymann G, Grosser S, Buggisch P, Gottschall C, Matthaei S, Greten H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Crit Care Med. 1993; 21:1012–1019. [DOI] [PubMed] [Google Scholar]
- 30.McClave SA, Taylor BE, Martindale RG et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016; 40:159–211. [DOI] [PubMed] [Google Scholar]
- 31.Singer P, Blaser AR, Berger MM et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019; 38:48–79. [DOI] [PubMed] [Google Scholar]
- 32. Fiaccadori E, Sabatino A, Barazzoni R et al. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin Nutr. 2021; 40:1644–1668. **Recent comprehensive guidelines on nutrition therapy for hospitalized patients with kidney disease.
- 33. De Waele E, Jonckheer J, Wischmeyer PE. Indirect calorimetry in critical illness: a new standard of care. Curr Opin Crit Care. 2021; 27:334–343. *A recent review and perspective on the use of indirect calorimetry to guide nutritional therapy in critical illness. There is a description of the development of newer generation indirect calorimeters with improved accuracy and cost effectiveness.
- 34.Wang A, Huen SC, Luan HH et al. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell. 2016; 166:1512–1525.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takala J, Ruokonen E, Webster NR et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999; 341:785–792. [DOI] [PubMed] [Google Scholar]
- 36.Heyland DK, Stapleton R, Compher C. Should We Prescribe More Protein to Critically Ill Patients. Nutrients. 2018; 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies ML, Chapple LS, Chapman MJ, Moran JL, Peake SL. Protein delivery and clinical outcomes in the critically ill: a systematic review and meta-analysis. Crit Care Resusc. 2017; 19:117–127. [PubMed] [Google Scholar]
- 38. Lee ZY, Yap CSL, Hasan MS et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021; 25:260. *Meta-analysis of randomized controlled trials comparing higher versus lower protein nutritional therapy in critically ill patients found no difference in outcomes.
- 39.Heyland DK, Patel J, Bear D et al. The Effect of Higher Protein Dosing in Critically Ill Patients: A Multicenter Registry-Based Randomized Trial: The EFFORT Trial. JPEN J Parenter Enteral Nutr. 2019; 43:326–334. [DOI] [PubMed] [Google Scholar]
- 40.Arabi YM, Al-Dorzi HM, Tamim H et al. Replacing protein via enteral nutrition in a stepwise approach in critically ill patients: A pilot randomized controlled trial (REPLENISH pilot trial). Clin Nutr ESPEN. 2021; 44:166–172. [DOI] [PubMed] [Google Scholar]
- 41.Oh WC, Mafrici B, Rigby M et al. Micronutrient and Amino Acid Losses During Renal Replacement Therapy for Acute Kidney Injury. Kidney Int Rep. 2019; 4:1094–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stapel SN, de Boer RJ, Thoral PJ, Vervloet MG, Girbes ARJ, Oudemans-van Straaten HM. Amino Acid Loss during Continuous Venovenous Hemofiltration in Critically Ill Patients. Blood Purif. 2019; 48:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheinkestel CD, Kar L, Marshall K et al. Prospective randomized trial to assess caloric and protein needs of critically Ill, anuric, ventilated patients requiring continuous renal replacement therapy. Nutrition. 2003; 19:909–916. [DOI] [PubMed] [Google Scholar]
- 44.Bellomo R, Cass A, Cole L et al. RENAL Replacement Therapy Study Investigators. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009; 361:1627–1638. [DOI] [PubMed] [Google Scholar]
- 45.Bellomo R, Cass A, Cole L et al. Daily protein intake and patient outcomes in severe acute kidney injury: findings of the randomized evaluation of normal versus augmented level of replacement therapy (RENAL) trial. Blood Purif. 2014; 37:325–334. [DOI] [PubMed] [Google Scholar]
- 46.Ferrie S, Allman-Farinelli M, Daley M, Smith K. Protein Requirements in the Critically Ill: A Randomized Controlled Trial Using Parenteral Nutrition. JPEN J Parenter Enteral Nutr. 2016; 40:795–805. [DOI] [PubMed] [Google Scholar]
- 47.Fetterplace K, Deane AM, Tierney A et al. Targeted Full Energy and Protein Delivery in Critically Ill Patients: A Pilot Randomized Controlled Trial (FEED Trial). JPEN J Parenter Enteral Nutr. 2018; 42:1252–1262. [DOI] [PubMed] [Google Scholar]
- 48. Dresen E, Weißbrich C, Fimmers R, Putensen C, Stehle P. Medical high-protein nutrition therapy and loss of muscle mass in adult ICU patients: A randomized controlled trial. Clin Nutr. 2021; 40:1562–1570. *Randomized controlled trial including a large proportion of patients with sepsis showing no benefit of high protein nutrition on minimizing muscle mass loss.
- 49.Puthucheary ZA, Rawal J, McPhail M et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013; 310:1591–1600. [DOI] [PubMed] [Google Scholar]
- 50.Hart DW, Wolf SE, Chinkes DL et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000; 232:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cerra FB, Siegel JH, Coleman B, Border JR, McMenamy RR. Septic autocannibalism. A failure of exogenous nutritional support. Ann Surg. 1980; 192:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leverve X, Guignier M, Carpentier F, Serre JC, Caravel JP. Effect of parenteral nutrition on muscle amino acid output and 3-methylhistidine excretion in septic patients. Metabolism. 1984; 33:471–477. [DOI] [PubMed] [Google Scholar]
- 53. Evans DC, Corkins MR, Malone A et al. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr Clin Pract. 2021; 36:22–28. **Important position paper from ASPEN recommending against using visceral proteins such as albumin and pre-albumin as markers of nutrition or to guide nutritional therapy. Decreased serum levels of visceral proteins are more consistent with ongoing inflammation than nutritional status or nutritional adequacy.
- 54.Tomé D, Bos C. Dietary protein and nitrogen utilization. J Nutr. 2000; 130:1868S–73S. [DOI] [PubMed] [Google Scholar]
- 55.Gunst J, Vanhorebeek I, Casaer MP et al. Impact of early parenteral nutrition on metabolism and kidney injury. J Am Soc Nephrol. 2013; 24:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weijs PJ, Stapel SN, de Groot SD et al. Optimal protein and energy nutrition decreases mortality in mechanically ventilated, critically ill patients: a prospective observational cohort study. JPEN J Parenter Enteral Nutr. 2012; 36:60–68. [DOI] [PubMed] [Google Scholar]
- 57.Weijs PJ. Fundamental determinants of protein requirements in the ICU. Curr Opin Clin Nutr Metab Care. 2014; 17:183–189. [DOI] [PubMed] [Google Scholar]
- 58.Lindner G, Schwarz C, Funk G-C. Osmotic diuresis due to urea as the cause of hypernatraemia in critically ill patients. Nephrology Dialysis Transplantation. 2012; 27:962–967. [DOI] [PubMed] [Google Scholar]
- 59.Lindner G, Funk G-C, Schwarz C et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis. 2007; 50:952–957. [DOI] [PubMed] [Google Scholar]
- 60.Gunst J, Vanhorebeek I, Thiessen SE, Van den Berghe G. Amino acid supplements in critically ill patients. Pharmacol Res. 2018; 130:127–131. [DOI] [PubMed] [Google Scholar]
- 61.Escobar DA, Botero AM, Gomez H, Zuckerbraun BS. Autophagy: Chapter 17. Sepsis-Induced Autophagy is a Protective Mechanism against Cell Death. Elsevier Inc. Chapters; 2013:25. [Google Scholar]
- 62.Yin X, Xin H, Mao S, Wu G, Guo L. The Role of Autophagy in Sepsis: Protection and Injury to Organs. Front Physiol. 2019; 10:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howell GM, Gomez H, Collage RD et al. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PLoS One. 2013; 8:e69520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Escobar DA, Botero-Quintero AM, Kautza BC et al. Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. J Surg Res. 2015; 194:262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mei S, Livingston M, Hao J, Li L, Mei C, Dong Z. Autophagy is activated to protect against endotoxic acute kidney injury. Sci Rep. 2016; 6:22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leventhal JS, Ni J, Osmond M et al. Autophagy Limits Endotoxemic Acute Kidney Injury and Alters Renal Tubular Epithelial Cell Cytokine Expression. PLoS One. 2016; 11:e0150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sunahara S, Watanabe E, Hatano M et al. Influence of autophagy on acute kidney injury in a murine cecal ligation and puncture sepsis model. Sci Rep. 2018; 8:1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.editor. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clinic Proceedings 78(12); 2003; Elsevier; 2003. [DOI] [PubMed] [Google Scholar]
- 69.van den Berghe G, Wouters P, Weekers F et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001; 345:1359–1367. [DOI] [PubMed] [Google Scholar]
- 70.NICE-SUGAR, Study-Investigators, Finfer S et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009; 360:1283–1297. [DOI] [PubMed] [Google Scholar]
- 71.de Cabo R, Mattson MP. Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med. 2019; 381:2541–2551. [DOI] [PubMed] [Google Scholar]
- 72.Patterson RE, Sears DD. Metabolic Effects of Intermittent Fasting. Annu Rev Nutr. 2017; 37:371–393. [DOI] [PubMed] [Google Scholar]
- 73. Pak HH, Haws SA, Green CL et al. Fasting drives the metabolic, molecular and geroprotective effects of a calorie-restricted diet in mice. Nat Metab. 2021; 3:1327–1341. *Preclinical models of caloric restriction have an element of prolonged periods of fasting. This preclinical study shows that the benefit of calorie restriction on longevity and metabolism is from periods of fasting, not caloric restriction itself.
- 74.Lanza-Jacoby S, Rosato E, Braccia G, Tabares A. Altered ketone body metabolism during gram-negative sepsis in the rat. Metabolism. 1990; 39:1151–1157. [DOI] [PubMed] [Google Scholar]
- 75.Eaton S, Pierro A. Carnitine and Fatty Acid Oxidation in Sepsis. Monatshefte für Chemie - Chemical Monthly. 2005; 136:1483–1492. [Google Scholar]
- 76.Van Wyngene L, Vanderhaeghen T, Timmermans S et al. Hepatic PPARα function and lipid metabolic pathways are dysregulated in polymicrobial sepsis. EMBO Mol Med. 2020; 12:e11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vandewalle J, Libert C. Sepsis: a failing starvation response. Trends Endocrinol Metab. 2022; S1043–2760(22)00006. *Review and perspective comparing the rapid sepsis-induced energy imbalance and starvation response to a normal fasting metabolism. The authors propose that survival from sepsis is dependent on maintaining a fasting metabolic state while still receiving nutritional support to provide energy for recovery.
- 78.Goossens C, Weckx R, Derde S et al. Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones. Crit Care. 2019; 23:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paumelle R, Haas JT, Hennuyer N et al. Hepatic PPARα is critical in the metabolic adaptation to sepsis. J Hepatol. 2019; 70:963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Bruyn A, Gunst J, Goossens C et al. Effect of withholding early parenteral nutrition in PICU on ketogenesis as potential mediator of its outcome benefit. Crit Care. 2020; 24:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.White RH, Frayn KN, Little RA, Threlfall CJ, Stoner HB, Irving MH. Hormonal and metabolic responses to glucose infusion in sepsis studied by the hyperglycemic glucose clamp technique. JPEN J Parenter Enteral Nutr. 1987; 11:345–353. [DOI] [PubMed] [Google Scholar]
- 82.Beylot M, Guiraud M, Grau G, Bouletreau P. Regulation of ketone body flux in septic patients. Am J Physiol. 1989; 257:E665–74. [DOI] [PubMed] [Google Scholar]
- 83.Giovannini I, Boldrini G, Castagneto M et al. Respiratory quotient and patterns of substrate utilization in human sepsis and trauma. JPEN J Parenter Enteral Nutr. 1983; 7:226–230. [DOI] [PubMed] [Google Scholar]
- 84.Sidossis LS, Wolfe RR. Glucose and insulin-induced inhibition of fatty acid oxidation: the glucose-fatty acid cycle reversed. Am J Physiol. 1996; 270:E733–8. [DOI] [PubMed] [Google Scholar]
- 85.Tran M, Tam D, Bardia A et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011; 121:4003–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Drosatos K, Khan RS, Trent CM et al. Peroxisome proliferator-activated receptor-γ activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circ Heart Fail. 2013; 6:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xia C, Dong R, Chen C, Wang H, Wang DW. Cardiomyocyte specific expression of Acyl-coA thioesterase 1 attenuates sepsis induced cardiac dysfunction and mortality. Biochem Biophys Res Commun. 2015; 468:533–540. [DOI] [PubMed] [Google Scholar]
- 88.Standage SW, Bennion BG, Knowles TO et al. PPARα augments heart function and cardiac fatty acid oxidation in early experimental polymicrobial sepsis. Am J Physiol Heart Circ Physiol. 2017; 312:H239–H249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwaki T, Bennion BG, Stenson EK et al. PPARα contributes to protection against metabolic and inflammatory derangements associated with acute kidney injury in experimental sepsis. Physiol Rep. 2019; 7:e14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cui H, Xie N, Banerjee S, Ge J, Guo S, Liu G. Impairment of Fatty Acid Oxidation in Alveolar Epithelial Cells Mediates Acute Lung Injury. Am J Respir Cell Mol Biol. 2019; 60:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Léveillé M, Besse-Patin A, Jouvet N et al. PGC-1α isoforms coordinate to balance hepatic metabolism and apoptosis in inflammatory environments. Mol Metab. 2020; 34:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Toro J, Manrique-Caballero CL, Gómez H. Metabolic Reprogramming and Host Tolerance: A Novel Concept to Understand Sepsis-Associated AKI. J Clin Med. 2021; 10:4184. *Comprehensive review on disease tolerance and metabolic programs that limit injury or promote recovery from sepsis-associated acute kidney injury.
- 93.McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980; 49:395–420. [DOI] [PubMed] [Google Scholar]
- 94.Vanhorebeek I, Gunst J, Derde S et al. Insufficient activation of autophagy allows cellular damage to accumulate in critically ill patients. J Clin Endocrinol Metab. 2011; 96:E633–45. [DOI] [PubMed] [Google Scholar]
- 95. Yu B, Li C, Sun Y, Wang DW. Insulin Treatment Is Associated with Increased Mortality in Patients with COVID-19 and Type 2 Diabetes. Cell Metab. 2021; 33:65–77.e2. *Retrospective cohort study found that insulin treatment in hospitalized type 2 diabetic patients with confirmed COVID-19 was associated with mortality, acute kidney injury and mechanical ventilation even with propensity-score matching to control for baseline comorbidities and severity of disease, stratification based on glucose control at admission, and analysis of patients without hypoglycemic episodes.
- 96.Bellomo R, Tan HK, Bhonagiri S et al. High protein intake during continuous hemodiafiltration: impact on amino acids and nitrogen balance. Int J Artif Organs. 2002; 25:261–268. [DOI] [PubMed] [Google Scholar]
- 97.Scheinkestel CD, Adams F, Mahony L et al. Impact of increasing parenteral protein loads on amino acid levels and balance in critically ill anuric patients on continuous renal replacement therapy. Nutrition. 2003; 19:733–740. [DOI] [PubMed] [Google Scholar]
- 98.Fiaccadori E, Maggiore U, Rotelli C et al. Effects of different energy intakes on nitrogen balance in patients with acute renal failure: a pilot study. Nephrol Dial Transplant. 2005; 20:1976–1980. [DOI] [PubMed] [Google Scholar]
- 99.Hellerman M, Sabatino A, Theilla M, Kagan I, Fiaccadori E, Singer P. Carbohydrate and Lipid Prescription, Administration, and Oxidation in Critically Ill Patients With Acute Kidney Injury: A Post Hoc Analysis. J Ren Nutr. 2019; 29:289–294. [DOI] [PubMed] [Google Scholar]
- 100. Gunst J, Casaer MP, Langouche L, Van den Berghe G. Role of ketones, ketogenic diets and intermittent fasting in ICU. Curr Opin Crit Care. 2021; 27:385–389. *Review and perspective on the evidence for dietary interventions to promote fasting metabolism including ketogenesis in critically ill patients.
- 101. Thong D, Halim Z, Chia J, Chua F, Wong A. Systematic review and meta-analysis of the effectiveness of continuous vs intermittent enteral nutrition in critically ill adults. JPEN J Parenter Enteral Nutr. 2021; *This meta-analysis found a lack of difference between continuous versus intermittent enteral nutrition in critically ill patients. This study also highlights the heterogeneity and high risk of bias in these trials.
- 102.Ichimaru S. Methods of Enteral Nutrition Administration in Critically Ill Patients: Continuous, Cyclic, Intermittent, and Bolus Feeding. Nutr Clin Pract. 2018; 33:790–795. [DOI] [PubMed] [Google Scholar]
- 103.Van Dyck L, Vanhorebeek I, Wilmer A et al. Towards a fasting-mimicking diet for critically ill patients: the pilot randomized crossover ICU-FM-1 study. Crit Care. 2020; 24:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bensalem J, Hattersley KJ, Hein LK et al. Measurement of autophagic flux in humans: an optimized method for blood samples. Autophagy. 2021; 17:3238–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goodpaster BH, Sparks LM. Metabolic Flexibility in Health and Disease. Cell Metab. 2017; 25:1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shoemaker ME, Pereira SL, Mustad VA et al. Differences in muscle energy metabolism and metabolic flexibility between sarcopenic and nonsarcopenic older adults. J Cachexia Sarcopenia Muscle. 2022; *This study shows that sarcopenic older adults have decreased metabolic flexibility. These finding have significant implications on whether critically ill patients with baseline sarcopenia will respond to nutritional or metabolic interventions.
- 107.Koutroulis I, Batabyal R, McNamara B, Ledda M, Hoptay C, Freishtat RJ. Sepsis Immunometabolism: From Defining Sepsis to Understanding How Energy Production Affects Immune Response. Crit Care Explor. 2019; 1:e0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lercher A, Baazim H, Bergthaler A. Systemic Immunometabolism: Challenges and Opportunities. Immunity. 2020; 53:496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Makowski L, Chaib M, Rathmell JC. Immunometabolism: From basic mechanisms to translation. Immunol Rev. 2020; 295:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Otani S, Coopersmith CM. Gut integrity in critical illness. J Intensive Care. 2019; 7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Reintam Blaser A, Hiesmayr M. Enteral feeding, even when the gut does not feel very good. Curr Opin Clin Nutr Metab Care. 2022; 25:122–128. *This review discusses the role of intestinal integrity, microbiota, and the gut-brain axis in nutrition therapy during critical illness.