Abstract
Spinal neuroinflammation plays a critical role in the genesis of neuropathic pain. Accumulating data suggest that abscisic acid (ABA), a phytohormone, regulates inflammatory processes in mammals. In this study, we found that reduction of the LANCL2 receptor protein but not the agonist ABA in the spinal cord is associated with the genesis of neuropathic pain. Systemic or intrathecal administration of ABA ameliorates the development and pre-existence of mechanical allodynia and heat hyperalgesia in animals with partial sciatic nerve ligation (pSNL). LANCL2 is expressed only in microglia in the spinal dorsal horn. Pre-emptive treatment with ABA attenuates activation of microglia and astrocytes, ERK activity, and TNFα protein abundance in the dorsal horn in rats with pSNL. These are accompanied by restoration of spinal LANCL2 protein abundance. Spinal knockdown of LANCL2 gene with siRNA recapitulates the behavioral and spinal molecular changes induced by pSNL. Activation of spinal toll-like receptor 4 (TLR4) with lipopolysaccharide leads to activation of microglia, and over production of TNFα, which are concurrently accompanied by suppression of protein levels of LANCL2 and peroxisome proliferator activated-receptor γ. These changes are ameliorated when ABA is added with LPS. The anti-inflammatory effects induced by ABA do not requires Gi protein activity. Our study reveals that the ABA/LANCL2 system is a powerful endogenous system regulating spinal neuroinflammation and nociceptive processing, suggesting the potential utility of ABA as the management of neuropathic pain.
Keywords: lanthionine synthetase C-like protein 2, neuroinflammation, nociception, cytokine, PPARγ
Introduction
Treatment of neuropathic pain caused by injury or dysfunction in the nervous system remains a clinical challenge due to lack of potent and safe analgesics. 1 It is widely appreciated that neuropathic pain is a reflection of aberrant neuronal activity along the pain signaling pathway including neurons in the spinal dorsal horn.2,3 Previous studies reported by others4-6 and us7-10 have demonstrated that neuroinflammation in the spinal dorsal horn plays a critical role in the aberrant spinal neuronal activation and genesis of neuropathic pain. Identifying signaling molecules controlling neuroinflammation would provide novel molecular targets for the development of novel analgesics.
Neuroinflammation is characterized by infiltration of leukocytes, activation of microglia and astrocytes, and over-production of pro-inflammatory cytokines.4-6 Pro-inflammatory cytokines like tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β) enhance activation of spinal neurons along the pain transmission pathway. Numerous studies show that neuropathic pain in animals is ameliorated by blocking receptors on microglia or intracellular signaling pathways involving in production of inflammatory cytokines, or blocking inflammatory cytokine receptors. For example, selective inhibition of microglia by minocycline reduces mechanical allodynia in animals with nerve injury.7,11 Chronic pain induced by nerve injury can be ameliorated by blocking chemokine receptors (e.g. CCR2, CX3CR1), purinergic receptors (P2X4R, P2X7R, P2Y12, and P2Y13), toll like receptor 4 (TLR4),5,12-14 and colony-stimulating factor 1 (CSF1) receptors15,16 on microglia. Many intracellular signaling molecules like MAP kinases, and NFκB are critical for production of inflammatory cytokines.4-6 Mechanical allodynia in animals with nerve injury is reduced when animals are treated with IL-1β or TNFα receptor blockers.4-6 Despite such extensive studies, much less is known about endogenous signaling molecules that exert inhibitory effects on neuroinflammation.
Abscisic acid (ABA) was originally discovered in plants and considered as a phytohormone for its role in orchestrating numerous physiological processes, including growth, development, and stress responses to adverse environments.17,18 ABA in plants exerts its function via binding G-protein coupled receptor (GCR2).16,17 ABA was later found to be also present in a wide range of animals including rodents 19 and humans. 20 ABA is found in many tissues in the body, such as blood, brain, heart, lung, and kidney. 19 In humans and rodents, ABA can be obtained through dietary sources like fruits and vegetables, and produced endogenously through the carotenoid biogenesis pathway. 21 In vitro studies show that ABA is endogenously produced in many cellular types in humans and rodents, including granulocytes, monocytes, macrophages, and fibroblasts. 21 In mammals, ABA binds to lanthionine synthetase C-like protein 2 (LANCL2), which has high homology to the ABA receptor GCR2 in plants. 22 LANCL2 belongs to the LANCL protein family, which includes LANCL1, LANCL2 and LANCL3. 23 Previous reports demonstrate that inflammation signaling pathways in mammalian cells or tissues are regulated by the ABA/LANCL2 system.22,24 Currently, little is known about the role of the ABA/LANCL2 system in the genesis of neuropathic pain.
In the present study, we demonstrated that impairment of the ABA/LANCL2 system in the spinal cord contributes to dysregulation of inflammatory processes in the spinal cord and the hind paw hypersensitivity to heat and mechanical stimulation in animals with partial sciatic nerve ligation. ABA treatment attenuates spinal inflammation and chronic pain by ameliorating LANCL2 protein expression.
Methods
Animals
Adult male Sprague-Dawley rats (weight range 160–200 g) were purchased from Harlan Laboratories (Indianapolis, IN). Two hundred and eight animals were used in this study. All studies were approved by the Institutional Animal Care and Use Committees at the University of Georgia and Mercer University, and were fully compliant with the National Institutes of Health Guidelines for the Use and Care of Laboratory Animals.
Partial sciatic nerve ligation
To induce neuropathic pain caused by nerve injury, partial sciatic nerve ligation was made in animals. This animal model mimics neuropathic pain induced by nerve compression seen in patients in clinics. 25 Animals were randomly divided into partial sciatic nerve ligation (pSNL) or sham-operated groups. Briefly, under isoflurane (2–3%) anesthesia, the left sciatic nerve at the upper thigh was exposed and ligated approximately to two-thirds the thickness of the sciatic nerve with a 5-0 silk suture as previously described. 25 Following surgery, the wound was closed with skin staples. In sham-operated rats, the left sciatic nerve was exposed but not ligated.
Behavior tests
Measurement of mechanical thresholds of hind paw withdrawal responses: Rats were placed on a wire mesh, loosely restrained under a plexiglass cage (12 × 20 × 15 cm3) and allowed to acclimate for a minimum of 30 min. A series of von Frey monofilaments (bending force: 0.6 g, 1.0 g, 1.4 g, 2.0 g, 4.0 g, 6.0 g, 8.0 g, 10.0 g, 15.0 g, 26.0 g) were tested in ascending order to evoke hind paw withdrawal responses. Each von Frey filament was applied 5 times to the mid-plantar area of the hind paw ipsilateral to the operated side from beneath for about 1s.26-28 Only a quick retraction of the paw was considered as a withdrawal response. The percentage of withdrawal responses for each von Frey filament was determined. The 50% mechanical withdrawal threshold was defined as the lowest force that evoked a response-frequency greater than 50%. This value was averaged across all animals in each group to yield the group response threshold.29,30
Measurement of thermal thresholds of hind paws withdrawal responses: Rats were placed on a glass surface at 30oC while loosely constrained in a Plexiglass cage (12 × 20 × 15 cm), and allowed to acclimate for a minimum of 30 min. To test the thermal sensitivity, a radiant heat beam was directed from below to the mid-plantar surface of the hind paw for rats to evoke a withdrawal response. The latency of paw withdrawal responses, i.e. the time between the stimulus onset and paw withdrawal responses, was recorded. 31 A cutoff time of 20s was used to avoid damage to the skin. Three latencies of hind paw withdrawal responses were obtained from the hind paw with an interval of at least 3 min. The three latencies obtained from each paw were averaged and used for analysis.
Measurement of ABA concentrations in the spinal dorsal horn
Behavioral tests were performed in animals 10 days after surgery to confirm the development of mechanical allodynia in animals with pSNL and normal mechanical thresholds in animals with sham operation. Animals were then anesthetized with urethane (1.3–1.4 g/Kg, i.p.) and spinal cord were then exposed. Spinal dorsal quadrant in L4-5 spinal segment ipsilateral to the operation side were collected. Measurement of ABA was performed using an ABA ELISA kit (MyBiosource; MBS 2,000,214) according to the manufacturer’s protocol. Fresh tissue was homogenized with ice-cold 80% methyl alcohol and shaken on a shaker for 24 h at 4°C. The supernatant was collected, additional alcohol added to the pellet, and shaken for 1 hour at 4°C. The total supernatant was collected and evaporated on a rotary evaporator. Petroleum ether was added to the liquid and mixed. After the liquid became layered, the top layer of petroleum ether was removed by pipetting, then the bottom methyl alcohol layer was collected and used immediately. Positive controls of 100, 33.33, 11.11, 3.7, and 1.23 ng/mL ABA, as well as a negative control of the diluent were used to create a standard concentration curve. Samples (50 μL) were added to the wells of a 96 well plate. Detection Reagent A (50 μL) was added, gently shaken by hand, covered with plate sealer, and set in an incubator for 1 hour at 37°C. The solution was aspirated and washed with 350 μL of 1 x Wash Solution four times. Detection Reagent B (100 μL) was added to each well, covered, and incubated for 30 min at 37°C. Aspiration and wash process was repeated five times. Substrate Solution (90 μL) was added to each well, covered, and incubated for 15 min away from light. Stop Solution (50 μL) was added and immediately run on a microplate reader at 450 nm.
Drug administration
ABA was dissolved in DMSO and then mixed with sterile saline with DMSO concentration less than 1% in the final solution. For systemic administration, ABA (20 mg/kg, in a volume of 1 mL) or equal volume of saline was injected intraperitoneally. For pre-emptive treatment, the intraperitoneal administration was made 30 min prior to pSNL or sham surgery on day 0, and then daily up to day 9. When behavior tests and the drug administration were conducted on the same day, behavior tests were completed prior to drug treatment. For intrathecal (i.t.) administration, a polyethylene (PE-10) catheter that ended at the spinal L4 segment was intrathecally placed as previously described.32,33 Rats were anesthetized with 2–3% isoflurane, and a PE-10 catheter was carefully inserted into the lumbar subarachnoid space through the space between the fifth and sixth lumbar vertebrae. The muscles were then sutured in layers and the skin edges were closed with skin staples. Rats with hind limb paresis or paralysis after surgery were excluded. Successful catheter implantation was confirmed by hind limb paralysis after lidocaine (2%, 5 μL) was injected via the implanted catheter. ABA (at a dosage of 1.5 μg or 15 μg/rat, in a volume of 10 μL) or saline (10 μL) was injected into the spinal lumbar enlargement through a pre-implanted intrathecal catheter, followed by 10 μL of saline to flush.
In vivo drug incubation
The L4–L5 spinal cord was exposed by laminectomy and the spinal dura was excised in rats anesthetized with urethane (1.3–1.5 g/kg, i.p.). The rate of heart beat and breathing, and the core temperature of the animals were constantly monitored and maintained in normal limits. 34 Tested drug(s) or vehicle (saline) was applied onto the L4-L5 spinal segment through a piece of cotton soaked with the drug(s) in saline at 35°C for two to 3 hours. Immediately after treatment, the dorsal half of the L4-L5 spinal segment was isolated and frozen in liquid nitrogen and stored at −80°C for later use.
Administration of siRNA
LANCL2 small interfering RNA (LANCL2 siRNA, Santa Cruz Biotechnology, Inc. Ca.) and scrambled siRNA (Control siRNA, Santa Cruz Biotechnology, Inc. Ca.) were administered directly into the intrathecal space through lumbar puncture. Injections were made into the intrathecal space in rats anesthetized with 2% isoflurane using a 0.5 inch 27 gauge needle connected to a Hamilton syringe as previously described.30,35 LANCL2 siRNA and Control siRNA were prepared immediately prior to the intrathecal administration by mixing the RNA solution (100 μM) with transfection reagent (iFect), in a ratio of 1:5 as described in the iFect siRNA transfection kit. 36 LANCL2 siRNA (2 μg) and an equal amount of Control siRNA in a volume of 10 μl were intrathecally injected at 10:00 a.m. and 10:00 p.m. for two consecutive days. The hind paw withdrawal response to mechanical stimuli and the withdrawal response latency to thermal stimuli were measured prior to the initial lumbar injection and 12 h following the last lumbar puncture. The dorsal spinal cord at the L4 to L5 region was removed after the behavioral tests for western blotting.
Immunohistochemical studies
Immunocytochemistry was used to determine the cellular location of LANCL2 in the spinal cord of four rats. Rats were deeply anesthetized with urethane (1.3–1.5 g/kg, i.p) and perfused intracardially as previously described. 37 The L4-L5 spinal cord was removed, post-fixed for 24 h at 4°C in the same fixative, cryoprotected in 15% sucrose in 0.1M PBS for 24 h at 4°C, and then placed in 30% sucrose in 0.1M PBS solution at 4°C. Serial transverse sections (30 μm thick) were cut on a freezing microtome at −20°C and collected in 0.1M PBS and processed as previously described. 37 Sections were incubated overnight at 4°C with rabbit anti-LANCL2 (1:200, Invitrogen) for 24 h, followed by incubation with either mouse anti-GFAP (a marker for astrocytes, 1: 500, Cell Signaling), mouse anti-Iba1 (a marker for microglia, 1:250, Santa Cruz), mouse anti-NeuN (a marker for neurons, 1:500, Cell Signaling) antibodies for 12 h. The sections were washed 3 times in 0.1M PBS and incubated for 2 h at room temperature with the corresponding Texas Red antibody (1:500 Vector Laboratories), and Alexa Fluor 488 antibody (1:500 Life Technologies). After rinsing three times with 0.1M PBS, the sections were mounted onto gelatin-coated slides, air-dried, and cover-slipped with Vectashield mounting medium (Vector Laboratories). For each cellular marker, four nonadjacent sections per rat were randomly selected. The immunostaining for each antibody was recorded on an Olympus BX43 microscope with an Olympus U-CMAD3 camera. Images were processed using the Olympus-cellSens Dimensions software.
Western blot experiments
Animals were deeply anesthetized with urethane (1.3–1.5 g/kg, i.p.). The L4 to L5 spinal segment was exposed. The dorsal half (in the siRNA and in vivo drug incubation experiments) or dorsal quadrant of the spinal cord ipsilateral to the surgery side at the L4 to L5 spinal segment was removed as previously described.37,38 The spinal tissue was quickly frozen in liquid nitrogen and stored at −80°C for later use. Frozen tissues were homogenized as previously described.37,38 Protein concentrations were determined using Nanodrop 1000. Protein samples were electrophoresed in SDS polyacrylamide gels and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA). The membranes were blocked with milk and incubated overnight at 4°C with anti-LANCL2 (1:750, Bioss Antibodies), anti-phospho-ERK (1:1000, Cell Signaling), anti-ERK (1:1000, Cell Signaling), anti-GFAP (1:2000, Cell Signaling), anti-Iba-1 (1:200, Abcam), anti-TNFα (1:200, Millipore), PPARγ (1:50, Santa Cruz) primary antibody, and anti-β-Actin (1:2000, Cell Signaling) or GAPDH (1:5000, Proteintech, Rosemont, IL) primary antibody as a loading control. The blots were then incubated for 1 h at room temperature (RT) with the corresponding HRP-conjugated secondary antibody (1:5000; Santa Cruz Biotechnology, CA, USA), visualized in ECL solution (SuperSignal West Chemiluminescent Substrate, Pierce, Rockford, IL, USA), and exposed on the Odyssey Fc Imaging System (LI-COR Biosciences). The intensity of immunoreactive bands was quantified using ImageJ 1.46 software (NIH). The ratio of each protein immunoreactivity over the loading control protein β-Actin or GAPDH was calculated.
Materials
Abscisic acid was purchased from PhytoTechnology Laboratories (Overland Park, KS). LANCL2 siRNA and Control siRNA were obtained from Santa Cruz Biotechnology. The siRNA vehicle, i-Fect, in the siRNA experiments was obtained from Neuromics (Edina, MN).
Data Analysis
All data are presented as the mean ± standard error (SE). One- or two-way analysis of variance (ANOVA) with repeated measures was used to detect differences in mean nociceptive behaviors between rats receiving different treatments. A Bonferroni post-hoc test was performed to determine sources of the differences. When applicable, Student’s t-tests were used to make comparison between groups (non-paired) or within the same group (paired). A p value less than 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software Inc.).
Results
Nerve injury does not alter ABA levels but reduces LANCL2 protein levels in the spinal dorsal horn
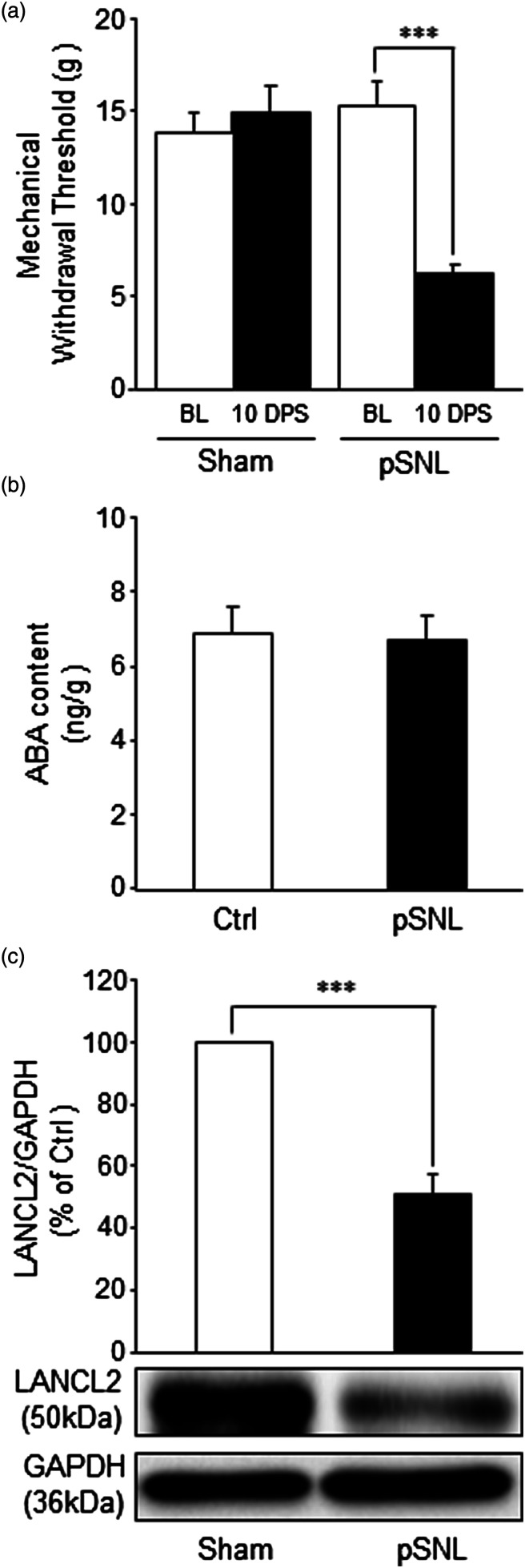
To determine whether the ABA/LANCL2 system is present in the spinal cord and altered under neuropathic pain conditions, two groups of rats were used: sham-operated group and nerve-injury (pSNL) group. Hind paw mechanical thresholds ipsilateral to the operation side were measured on day 10 post surgery39-41 to determine the development of neuropathic pain. The mechanical thresholds in the nerve-injury group were significantly reduced (p < 0.001) from 15.33 ± 1.27 g (mean ± SE, n = 15) prior to surgery to 6.26 ± 0.33 g (n = 15) 10 days post-surgery, while the mechanical threshold in sham-operated rats was not significantly altered (from 13.73 ± 1.18 g to 14.87 ± 1.36 g, n = 15) (Figure 1(a)). Immediately after completion of the nociceptive behavior test, the ipsilateral spinal dorsal horn of rats was prepared for either measuring ABA levels or LANCL2 protein expression in the spinal dorsal horn. Using ELISA techniques, we found that ABA was present in both sham-operated and pSNL rats. Rats in the pSNL group had ABA levels at 6.72 ± 0.65 ng/g (n = 11) in the spinal dorsal horn, which were similar to the ABA levels (6.89 ± 0.74 ng/g, n = 11) in the sham-operated group (Figure 1(b)). Protein expression of LANCL2 in the spinal dorsal horn was measured using western blots. We found that while both sham-operated and pSNL rats had protein expression of LANCL2 in the spinal dorsal horn, the protein expression of LANCL2 in pSNL rats was significantly reduced (p < 0.01, n = 4) in comparison with that in the sham operated group (n = 4; Figure 1(c)). These data indicate that of the ABA/LANCL2 system protein expression of LANCL2 but not the level of ABA in the spinal dorsal horn is associated with for peripheral nerve injury, and the reduction of LANCL2 is not due to changes of ABA levels in the spinal dorsal horn. These findings also suggest that a functional deficiency of ABA/LANCL2 signaling may contribute to the genesis of neuropathic pain in rats.
Figure 1.
Nerve injury does not alter abscisic acid contents but reduces protein levels of LANCL2 in the spinal dorsal horn. (a) Shows the mean (+SEM) of mechanical withdrawal thresholds at baseline (BL) and 10 days post-surgery (10 DPS) in the sham operated group (n = 15) and pSNL group (n = 15). (b) Shows the levels of ABA (ng/g of tissue, mean + SE) measured with ELISA in the spinal dorsal horn ipsilateral to the operation site in the sham-operated rats (n = 11) and pSNL-rats (n = 11). Protein expression levels (% of control; mean + SE) of LANCL2 measured with western blots in the spinal dorsal horn ipsilateral to the operation site in the sham-operated rats (n = 4) and pSNL-rats (n = 4) are shown in (c). Samples of LANCL2 protein molecule expression in each group are shown below. ***: p < 0.001.
Pre-emptive systemic treatment with ABA attenuates the development of mechanical allodynia and thermal hyperalgesia induced by nerve injury
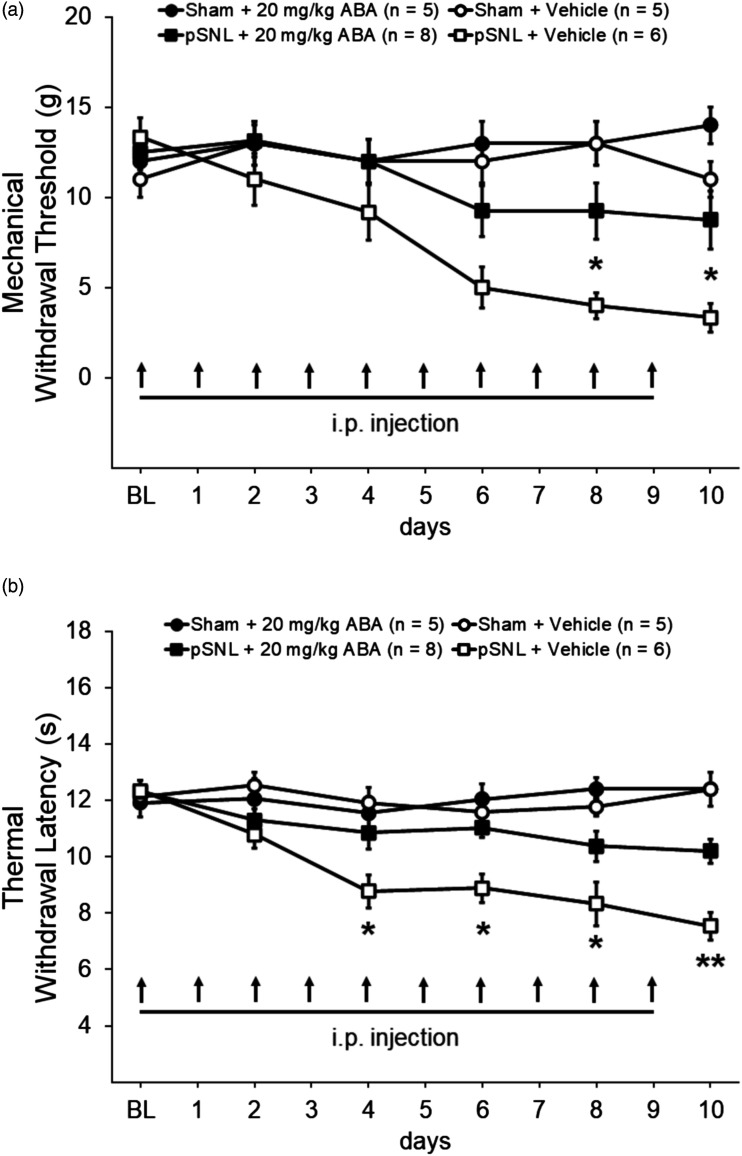
To determine whether deficient function of the ABA/LANCL2 system contributes to the development of mechanical allodynia and thermal hyperalgesia, ABA was used to enhance the activation of LANCL2. Rats were randomly assigned into four groups: Sham + Vehicle, Sham + ABA, pSNL + Vehicle, and pSNL + ABA. Rats in the Sham + ABA and the pSNL + ABA group were treated with ABA at a dose of 20 mg/kg (in a volume of 1 mL).42,43 ABA at this dose reportedly reduces inflammation in adipose tissues in obese mice 44 and neuroinflammation in the cortex in a Alzheimer’s mouse model. 45 ABA administered intraperitoneally passes the blood brain barrier and reaches a peak level in the CNS within 30 min 43 . As shown in Figure 2(a), the mechanical withdrawal threshold obtained from the pSNL + Vehicle group on days 6–10 following nerve injury were significantly reduced (n = 6, p < 0.01 to 0.001) in comparison to their baseline measurement prior to surgery. In comparison with the pSNL + Vehicle group, the mechanical withdrawal threshold in the pSNL + ABA group was elevated from day 4 to day 6, and became statistically significantly higher from day 8 to day 10 (n = 8, p < 0.05). These results indicate that daily systemic treatment with ABA attenuates the development of mechanical allodynia. The effects of ABA were also determined on the thermal sensitivity in the same four groups of rats described above. Prior to surgery, the withdrawal response latencies to radiant heat stimuli were comparable across all four groups as shown in Figure 2(b). The pSNL + Vehicle group had significantly (p < 0.01 to 0.001) decreased withdrawal response latencies to radiant heat stimuli on days 4–10 compared to their readings prior to the surgery. In comparison to the pSNL + Vehicle group (n = 6), the withdrawal response latencies to radiant heat stimuli for the pSNL + ABA group (n = 8) was significantly longer from day 4 to day 10 (p < 0.05 to 0.01) (Figure 2(b)). Through the same 10-day period, the withdrawal response latencies to radiant heat stimuli or mechanical thresholds were not significantly altered in the Sham + Vehicle group (n = 5) and the Sham + ABA group (n = 5). These data demonstrate that insufficient activation of the ABA/LANCL2 system plays a crucial role in the development of mechanical allodynia and heat hyperalgesia induced by nerve injury.
Figure 2.
Pre-emptive systemic treatment with ABA attenuates the development of mechanical allodynia and thermal hyperalgesia induced by nerve injury. Line plots show the hind paw mechanical withdrawal threshold (±SE) (a) and the withdrawal latency (mean ± SE) to heat stimuli (b) collected at baseline and then 2 days post-surgery (DPS), 4 DPS, 6 DPS, 8 DPS, and 10 DPS during 10 day period of daily intraperitoneal administration (i.p.) of the tested agents. Baseline indicates the measurement prior to undergoing pSNL or sham surgeries. Comparisons between the pSNL + ABA group and the pSNL + Vehicle group are labeled with *. *: p < 0.05; **: p < 0.01.
Systemic ABA treatment ameliorates pre-existing mechanical allodynia and thermal hyperalgesia induced by nerve injury
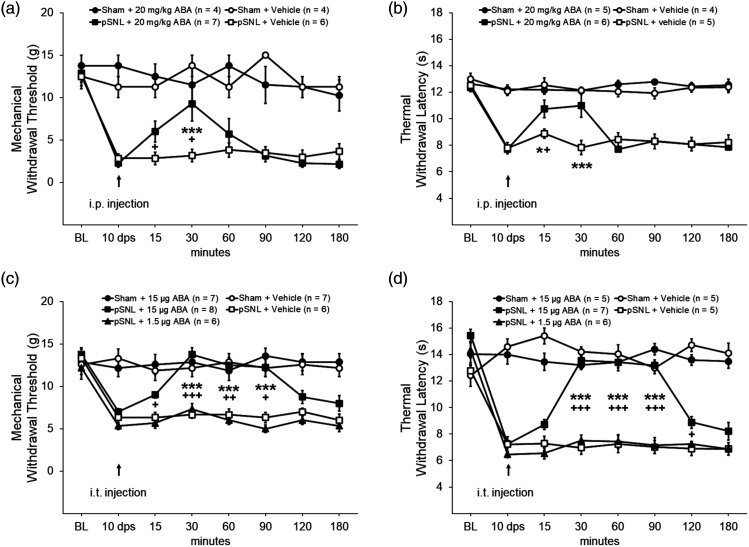
To determine whether the deficient function of the ABA/LANCL2 system contributes to the maintenance of mechanical allodynia and heat hyperalgesia, the effects of ABA on rats were determined in animals with established neuropathic pain 10 days after nerve injury. Rats were assigned into four groups: Sham + Vehicle, Sham + ABA, pSNL + Vehicle, and pSNL + ABA. After measuring mechanical and thermal thresholds of hind paw withdrawal responses, we performed either pSNL or sham surgery on the rats. Ten days post-surgery, we found mechanical thresholds and latencies for withdrawal responses were significantly (p < 0.001) reduced in rats receiving pSNL but not in rats with sham operation (Figure 3(a) and (b)), indicating development of allodynia and heat hyperalgesia. ABA at a dose of 20 mg/kg (in 1 mL saline) was intraperitoneally injected into the ABA treated groups. Vehicle (1 mL saline) was applied to the vehicle-treated group in the same fashion. As shown in Figure 3(a), systemic administration of ABA significantly (p < 0.01) raised the mechanical thresholds of hind paw withdrawal responses in the pSNL + ABA group (n = 7) at 15 min and 30 min after the injection in comparison with their own baseline. In comparison with pSNL rats treated with vehicle (n = 6), pSNL rats treated with ABA (n = 7) had significantly (p < 0.001) higher mechanical thresholds of withdrawal responses at 30 min after the injection. These effects dissipated 60 min after the injection. In rats used for measuring thermal sensitivity, we found that after the ABA injection the paw withdrawal latencies in the pSNL + ABA group were significantly increased at 15 min in comparison with their own values before the injection (n = 6; p < 0.05), and at 15 min and 30 min in comparison with those in the pSNL + Vehicle group (n = 5; p < 0.05 to 0.001; Figure 3(b)). These effects dissipated 60 min after the injection. We did not observe significant changes in mechanical and thermal thresholds of hind paw withdrawal responses in sham-operated rats treated with vehicles or ABA (Figure 3(a) and (b)). These results indicate that enhancing activation of LANCL2 with ABA can reverse established neuropathic pain induced by nerve injury.
Figure 3.
ABA treatment ameliorates pre-existing mechanical allodynia and thermal hyperalgesia induced by nerve injury. (a) and (b): Intraperitoneal treatment. Line plots show the hind paw mechanical withdrawal threshold (±SE) and the withdrawal latency (mean ± SE) to heat stimuli collected at baseline, 10 DPS, and then at 15 min, 30 min, 60 min, 90 min, 120 min, 150 min, and 180 min after Intraperitoneal administration of the tested agent. (c) and (d): Intrathecal treatment. Line plots show the hind paw mechanical withdrawal threshold (±SE) and the withdrawal latency (mean ± SE) to heat stimuli collected at baseline, 10 DPS, and then at 15 min, 30 min, 60 min, 90 min, 120 min, 150 min, and 180 min after intrathecal injection of the tested agents. Comparisons between the pSNL + ABA group and the pSNL + Vehicle group are labeled with *. Comparisons between time points before and after ABA treatment in the pSNL + ABA group are labeled with +. One symbol: p < 0.05; Two symbols: p < 0.01; Three symbols: p < 0.001.
Intrathecal injection of ABA attenuates pre-existing mechanical allodynia and thermal hyperalgesia induced by nerve injury
To confirm that direct spinal action of ABA account for its effects on mechanical allodynia and thermal hyperalgesia, ABA was applied directly onto the spinal cord via an intrathecal catheter. Rats with pre-implanted intrathecal catheters were grouped into: Sham + Vehicle (saline) group, Sham + ABA (at a dose of 15 μg/rat) group, pSNL + Vehicle group, pSNL + ABA (15 μg/rat) group, and pSNL + ABA (1.5 μg/rat) group. Ten days after confirming the development of mechanical allodynia and thermal hyperalgesia, ABA or vehicle was intrathecally administered in the ABA treated groups or vehicle treated group respectively. We found that intrathecal administration of ABA (15 μg/rat) significantly (n = 8, p < 0.01) raised the mechanical thresholds of hind paw withdrawal responses from 15 min after the injection (Figure 3(c)) in comparison with their own baseline values before the injection. These effects reached its peak at 30 min and maintained the plateau for at least another 60 min before it waned at 120 min after the injection. In comparison with pSNL rats treated with vehicle (n = 6), pSNL rats treated with ABA at a dose of 15 μg/rat (n = 6) had significantly (p < 0.01) higher mechanical thresholds of withdrawal responses between the 30 min and 90 min time points. When ABA at a reduced dosage (1.5 μg/rat, n = 6) or vehicle (n = 6) was applied to rats receiving pSNL, mechanical thresholds of hind paw withdrawal responses were not significantly altered (Figure 3(c)). We did not observe a significant alteration in mechanical thresholds of hind paw withdrawal responses in sham-operated rats treated with vehicle or ABA (15 μg/rat) administered in the same fashion.
In thermal sensitivity measurements, we found that ABA (15 μg/rat, i.t.) significantly (p < 0.001) increased the latencies of hind paw withdrawal responses to heat stimuli from 7.26 ± 0.29 s (n = 7) before injection to 13.55 ± 0.32 s (n = 7) at 30 min after the injection. These analgesic effects lasted for at least another 60 min (Figure 3(d)). We did not observe significant alteration in latencies of hind paw withdrawal responses to heat stimuli in pSNL rats receiving ABA (n = 6) at a reduced dosage (1.5 μg/rat, i.t.) or vehicle (i.t., n = 5). Compared with pSNL rats treated with vehicle (n = 5), latencies of withdrawal responses to heat stimuli in pSNL rats treated with ABA at a dose of 15 μg/rat (n = 5) were also significantly (p < 0.001) increased between the 30 min and 90 min time after the ABA injection. Meanwhile, vehicle (i.t.) or ABA (15 μg/rat, i.t.) treatment did not significantly altered latencies of withdrawal responses to heat stimuli in sham-operated rats (Figure 3(d)). These data suggest that the decreased activation of the ABA/LANCL2 system in the spinal cord contributes to the maintenance of neuropathic pain, and activation of spinal LANCL2 with ABA can attenuate pre-existing mechanical allodynia and thermal hyperalgesia induced by nerve injury. The therapeutic effects of ABA in pSNL rats further suggest that even though LANCL2 expression is reduced in pSNL rats, these receptors are not saturated by the endogenous ABA. The lack of effects of ABA on sham-operated rats suggest that ABA treatment does not impact nociception under normal conditions.
ABA/LANCL2 is expressed in spinal microglia but not astrocytes or neurons
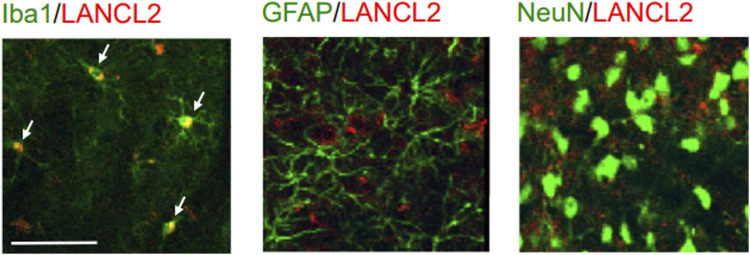
To understand molecular and cellular mechanisms underlying the role of the ABA/LANCL2 system in spinal nociceptive processing, we determined which cellular type expresses LANCL2 in the spinal dorsal horn. As LANCL2 expression is reduced in animals with nerve injury, spinal slices obtained from normal rats were used for our immunohistological experiments. The spinal slices were co-stained with LANCL2 and the markers for microglia (Iba1), astrocytes (GFAP), and neurons (NeuN). We found that LANCL2 staining was solely colocalized with the microglial marker (Iba1), but not with GFAP or NeuN (Figure 4). These data indicate that LANCL2 is expressed in microglia but not in astrocytes or neurons in the spinal dorsal horn of rats. Thus, the effects produced by the ABA/LANCL2 system must be through modulating microglial function.
Figure 4.
LANCL2 is expressed in microglia but not in neurons or astrocytes in the spinal cord. Samples of double labeling obtained from the spinal dorsal horn of naïve rats. Microglia, astrocytes, neurons in spinal slices were respectively labeled with Iba1, GFAP, NeuN antibodies (in green) while LANCL2 was stained in red. Colocalization between the Iba1 (in green) and LANCL2 (in red) is indicated with arrows. Scale bar: 50 μm.
Abscisic acid attenuates microglial and astrocytic activation, ERK activity, and over-production of TNFa in the spinal dorsal horn following nerve injury
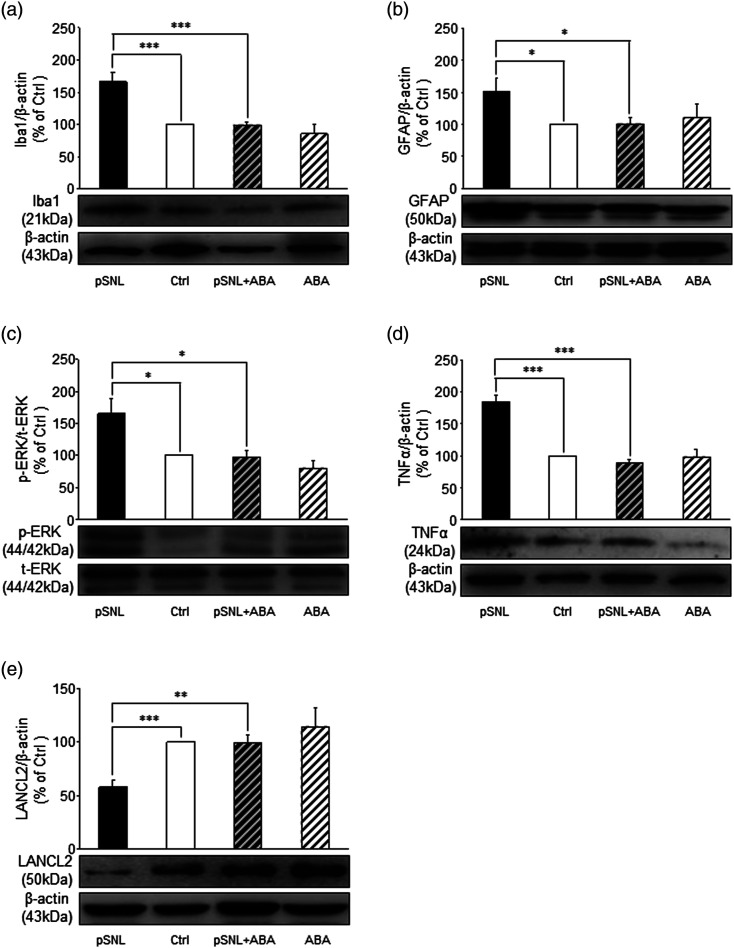
Given that LANCL2 is expressed in microglia, and glial activation is critically implicated in the genesis of neuropathic pain, we assumed that the status of microglia and astrocytes is controlled by the ABA/LANCL2 system. Rats receiving pre-emptive treatment of either ABA or vehicle that had completed the behavioral tests above (Figure 2(a) and (b)) were used for western blot experiments. We found that in comparison with the Sham + Vehicle group (n = 5), rats in the pSNL + Vehicle groups (n = 6) had significantly increased protein expression of Iba-1 (p < 0.001) and GFAP (p < 0.05) in the spinal dorsal horn (Figure 5(a) and (b)), indicating activation of microglia46,47 and astrocytes.48,49 Pre-emptive treatment of ABA (20 mg/kg, i.p. for 10 days) in the pSNL + ABA group (n = 8) significantly attenuated the increased expression of Iba-1 (p < 0.001) and GFAP (p < 0.05) in the spinal dorsal horn following nerve injury (Figure 5(a) and (b)), while ABA treatment in Sham operated animals had no effect on basal Iba1 or GFAP expression. The ERK signaling pathway is known for its regulation of activation of dorsal horn neurons, as well as activation of microglia and astrocytes in animals after nerve injury. 50 We measured ERK activity by measuring phosphorylated levels of ERK. Consistent with previous studies,4-6 ERK activity (phosphorylate ERK/total ERK) in the nerve injury group treated with saline (the pSNL + vehicle group) (n = 6) was significantly higher (p < 0.05) than that in the sham + vehicle group (n = 5). Daily pre-emptive treatment of ABA (20 mg/kg, i.p.) to rats with pSNL significantly (n = 8, p < 0.05) reduced the elevated phosphorylated level of ERK in the spinal dorsal horn (Figure 5(c)), while ABA treatment had no effect on basal phospho-ERK in Sham operated animals (n = 5). In neuropathic rats, glial activation leads to increased production of TNFα, which enhances neuronal activity. 51 We found that protein expression of TNFα in the pSNL + Vehicle group (n= 6) was significantly (p < 0.001) higher than that in Sham + Vehicle (n = 5). Daily treatment of ABA significantly (n = 8, p < 0.001) attenuated the TNFα protein level in rats with pSNL, but had no impact on basal TNFα levels in Sham operated animals (n = 5) (Figure 5(d)). These data indicate that increased activation of the ABA/LANCL2 system with ABA treatment attenuates the development of neuropathic pain via suppressing activation of microglia and astrocytes, ERK activity and production of TNFα in the spinal dorsal horn.
Figure 5.
Pre-emptive abscisic acid treatment suppresses the activation of microglia and astrocytes, and the increased ERK activity and TNFα protein production induced by pSNL, as well as blocks the suppression of LANCL2 expression induced by pSNL. Bar graphs show comparison of protein expression (mean + SE) ratios of Iba1, GFAP, TNFα, and LANCL2 to β-actin, and p-ERK to t-ERK in the pSNL + Vehicle (pSNL, n = 6), pSNL + ABA (n = 8), Sham + ABA (ABA, n = 5), and the Sham-Vehicle groups (Ctrl, n = 5). Samples of protein expression in each group are shown below. *: p < 0.05; **: p < 0.01; ***: p < 0.001.
Pre-emptive treatment of Abscisic acid blocks suppression of LANCL2 protein levels in the spinal dorsal horn following nerve injury
Since nerve injury reduces ABA/LANCL2 system function by lowering LANCL2 protein expression, we next asked whether such pathological change can be ameliorated by daily ABA treatment. As shown in Figure 5(e), systemic ABA treatment (20 mg/kg, i.p. for 10 days) significantly (p < 0.01) increased LANCL2 expression in the pSNL + ABA group (n = 8) compared to the pSNL + Vehicle group (n = 6). In contrast, the protein expression of LANCL2 in the Sham + Vehicle (n = 5) and Sham + ABA (n = 5) were similar. These results indicate that protein expression of LANCL2 under normal conditions is not controlled by exogenous ABA treatment, but under pathological conditions, exogenous ABA treatment can ameliorate the low protein expression of LANCL2 in the spinal dorsal horn.
Spinal knockdown of LANCL2 induces mechanical allodynia and thermal hyperalgesia
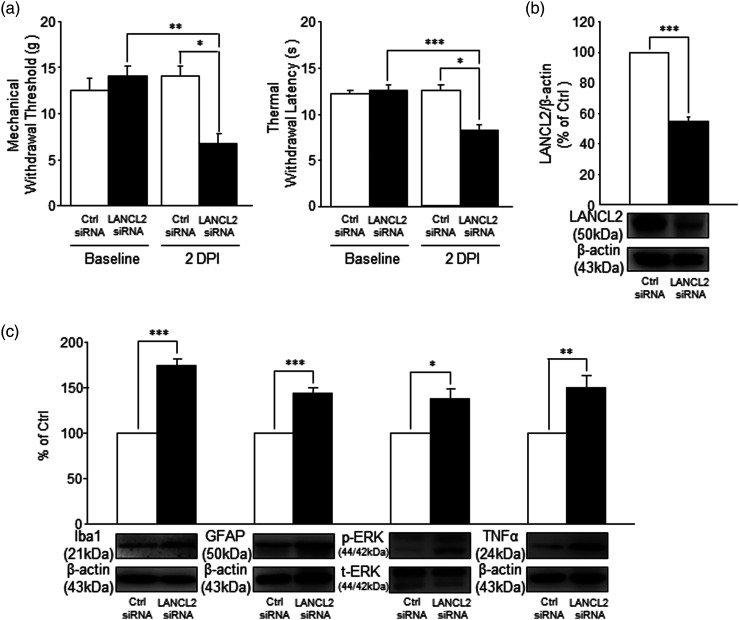
The data above suggest that the integrity of the spinal ABA/LANCL2 system is required to maintain normal nociception. To test this directly, we conducted experiments in which siRNA was used to genetically knockdown LANCL2 in the lumbar region of the spinal cord. Two groups of rats were used: LANCL2 siRNA group and Control siRNA group. After obtaining the baseline measurements for both mechanical thresholds and thermal latencies of hind paw withdrawal responses, rats were given lumbar injections of either a scrambled siRNA (Control siRNA) or LANCL2 siRNA at a dose of 2 μg/injection, twice/day for 2 days. The dosage and duration were known to be effective to suppress protein expression in the spinal dorsal horn. 38 Hind paw withdrawal responses to mechanical and thermal stimuli were examined 12 h after the final injection. As shown in Figure 6(a), the Control siRNA treated group did not show changes in the withdrawal responses to mechanical (n = 4) or thermal (n = 4) stimuli, respectively. In contrast, rats in the LANCL2 siRNA group developed a clear sign of mechanical allodynia, as demonstrated by a significantly decreased mechanical withdrawal threshold (n = 4, 7.0 ± 1.0 g) in comparison with the siRNA Control group (13.75 ± 1.25 g, n = 4, p < 0.05) and their own baseline measurements (13.75 ± 1.25 g, n = 4, p < 0.01) prior to the siRNA injection. At the same time, the thermal withdrawal latency in the LANCL2 siRNA group was significantly reduced to 8.24 ± 0.46 s (n = 4) in comparison with their own baseline readings (12.36 ± 0.42 s, n = 4, p < 0.001), and the rats receiving Control siRNA (p < 0.05). To verify whether the protein expression of LANCL2 in the spinal dorsal horn is knocked down by LANCL2 siRNA, the spinal dorsal L4 to L5 region was removed and the protein expression of LANCL2 in the spinal dorsal horn was analyzed following the completion of the behavioral tests. We found that the protein expression of LANCL2 in the LANCL2 siRNA group (n = 4) was significantly (p < 0.001) reduced in comparison with the control siRNA group (n = 4; Figure 6(b)). Notably, the degree of LANCL2 reduction observed in response to siRNA is similar to that observed following pSNL (about 50%), suggesting that the pain behavior observed in response to direct suppression of LANCL2 is likely contributing to the change in nociception following pSNL. Given that LANCL2 is only expressed in microglia and presence of ABA in the spinal dorsal horn, these results indicate that an intact functionality of the ABA/LANCL2 system in microglia is crucial for maintaining normal nociceptive processes in the animals.
Figure 6.
Spinal LANCL2 genetic knockdown recapitulates the pathological changes in nociceptive behaviors and molecular protein expression in the spinal cord induced by nerve injury. (a) Shows the mean (+SEM) of mechanical (n = 4) and thermal withdrawal thresholds (n = 4) before (baseline) and after 2-day treatment of LANCL2 siRNA (2 μg/injection, twice/day) and control siRNA (scrambled siRNA) (2 μg/injection, twice/day). (b): Bar graphs show the mean protein expression ratio (+SE) of LANCL2 to β-actin in the spinal dorsal horn in the LANCL2 siRNA group (n = 4) compared to that in the Control siRNA group (n = 4). (c): Bar graphs show protein expression (mean + SE) ratios of Iba1, GFAP, TNFα to β-actin, and p-ERK to t-ERK in the LANCL2 siRNA group (n = 4) compared to those in the control siRNA group (n = 4). Samples of each protein molecule expression in each group are shown below. *: p < 0.05; **: p < 0.01; ***: p < 0.001.
Knockdown of LANCL2 causes activation of microglia and astrocytes, and increases ERK activity and TNFα production
Next, we determined whether deficiency of the ABA/LANCL2 system induced by LANCL2 knockdown in the spinal dorsal horn can recapitulate the pathological changes in the status of microglia and astrocytes, and signaling molecules induced by nerve injury. Similar to rats with nerve injury, we found that rats with LANCL2 knockdown had increased activation of microglia and astrocytes in the spinal dorsal horn, as evident by significantly increased (p < 0.001) protein expressions of Iba-1 and GFAP (Figure 6(c)) in rats (n = 4) compared to rats without LANCL2 knockdown (n = 4). Furthermore, ERK activity (the ratio of phosphorylated ERK/total ERK) and TNFα protein expression (Figure 6(c)) in the spinal dorsal horn of rats with LANCL2 knockdown were significantly (n = 4, p < 0.05 to 0.01) higher than those in rats treated with Control siRNA (n = 4). These data confirm that normal nociceptive signaling process in the spinal dorsal horn is dependent on the intact function of the ABA/LANCL2 system.
ABA treatment ameliorates neuroinflammation induced by LPS
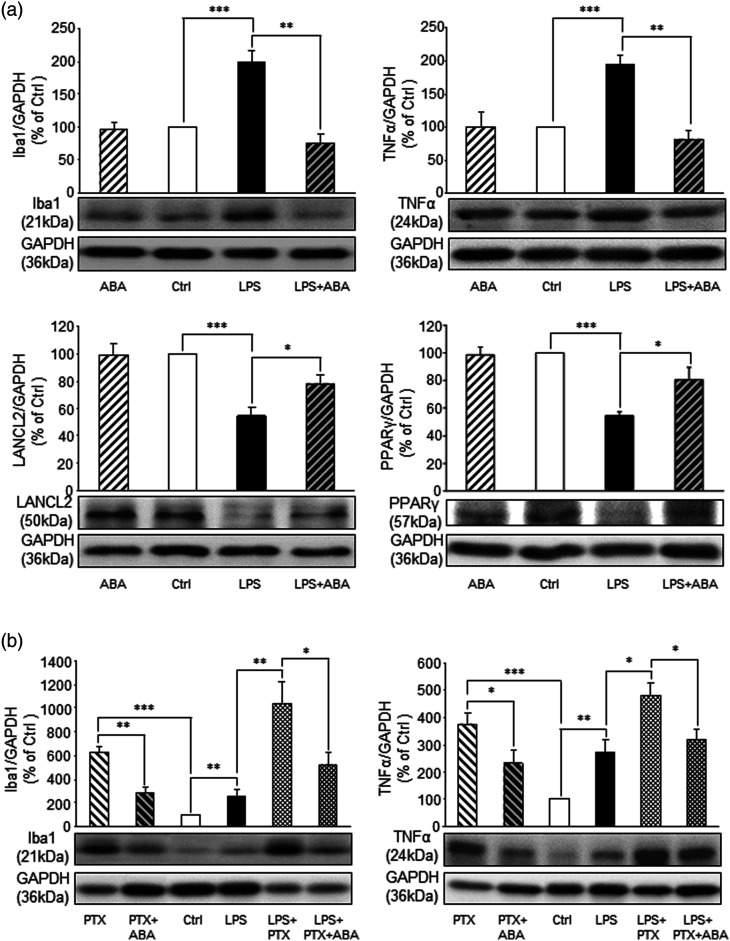
Toll like receptor 4 (TLR4) is present in the spinal microglia 26 and activation of spinal TLR4 plays a critical role in the genesis of neuropathic pain. 52 Thus, we investigated the interaction between TLR4 induced inflammatory responses and the ABA/LANCL2 system following in vivo drug incubation. 10 To activate spinal TLR4, a piece of cotton soaked with lipopolysaccharide (LPS, 0.1 μg/ml) in saline at 35oC was placed onto the dorsal surface of the L4-5 spinal segment for 2 h in rats anesthetized with urethane (1.3 g/kg, i.p.). Rats in the control group received saline treatment in the same fashion. We found that activation of TLR4 with LPS led to significantly (n = 4, p < 0.001) increased protein expression of Iba1 (sign of microglia activation) and TNFα (Figure 7(a)), indicating an inflammatory response induced by LPS. These were concurrently accompanied by a significant (n = 4, p < 0.001) reduction in protein abundance of LANCL2 in comparison with those receiving saline treatment (n = 4). The LPS-induced effects were attenuated when the spinal cord was incubated with ABA (20 μM) for 30 min earlier and then co-incubated with LPS (0.1 μg/ml; Figure 7(a)) for 2 h. Spinal cords receiving ABA treatment alone for 2.5 h did not significantly alter the protein expression of the same molecules (Figure 7(a)). These findings demonstrated that: 1. Spinal LANCL2 protein abundance is suppressed by activation of TLR4; 2. Activation of LANCL2 with ABA ameliorates the inflammatory responses and the reduced LANCL2 protein abundance induced by TLR4 activation. Previous studies demonstrated that anti-inflammatory effects induced by ABA treatment are mediated by peroxisome proliferator activated-receptor γ (PPARγ), 53 a nuclear receptor regulating transcription expression of anti-inflammatory cytokines. 54 We then determined protein expression levels of PPARγ in the same groups above. We found that protein expression of PPARγ was significantly (n = 4, p < 0.001) reduced in the spinal cord treated with LPS, and such change was suppressed by ABA treatment (Figure 7(a)). These findings suggest that increased PPARγ function may be involved in the anti-inflammatory signaling pathways activated by ABA in the spinal cord.
Figure 7.
ABA treatment blocks the induction of neuroinflammation markers induced by LPS independent of Gi protein activity. (a): Bar graphs show protein expression (mean + SE) ratios of Iba1, TNFα, LANCL2, and PPARγ to GAPDH in the ABA treated group (n = 4), saline treated (Ctrl) group (n = 4), LPS treated group (n = 4), and LPS + ABA treated group (n = 4). (b): Bar graphs show protein expression (mean + SE) ratios of Iba1, TNFα to GAPDH in the PTX treated group (n = 4), PTX + ABA group (n = 4), saline treated (Ctrl) group (n = 4), LPS treated group (n = 4), LPS + PTX treated group (n =4), and LPS + PTX + ABA treated group (n = 4). Samples of each protein molecule expression in each group are shown below. *: p < 0.05; **: p < 0.01; ***: p < 0.001.
Anti-inflammatory effects induced by ABA is independent of Gi protein
The effects of ABA on human granulocytes were reported to be abolished when granulocytes are preincubated with a Gi protein inhibitor (pertussis toxin, PTX). 20 Thus, we investigated whether Gi protein mediates the effects induced by ABA on inflammation induced by LPS. Rats were randomly assigned into six treatment groups (4 animals/group). In the PTX group, spinal cords were incubated with only PTX for 3 h. In the PTX + ABA group, spinal cords were pre-incubated with PTX for 30 min and then PTX plus ABA for 2.5 h. In the saline control group, spinal cords were treated with saline for 3 h. In the LPS group, spinal cords were incubated with LPS for 2 h. In PTX + PLS group, spinal cords were pre-incubated with PTX for 60 min and then PTX plus LPS for 2 h. In PTX + ABA + LPS group, spinal cords were pre-incubated with PTX for 30 min, and then PTX + ABA for another 30 min, and then PTX + ABA + PLS for two more hours. We found that in comparison with the saline group (n = 4), the PTX group had a significantly higher protein expression of Iba1 (n = 4, p < 0.001) and TNFα (n = 4, p < 0.001) (Figure 7(b)), indicating global inhibition of Gi protein induces inflammation in the spinal cord. These data are in consistent with a previous report that activation of Gi-designer receptors exclusively activated by designer drugs (DREADDs) in BV2 cells suppresses inflammation responses induced by LPS in BV2 cells. 55 Interestingly, the increased protein expression of Iba1 and TNFα induced by PTX were significantly attenuated in the PTX + ABA group (n = 4, p < 0.05 to 0.01). Spinal cords treated with PTX + LPS had significantly stronger inflammatory responses than those treated with LPS (n = 4) alone (Figure 7(b)) as demonstrated by significantly stronger Iba1 (p < 0.01) and TNFα protein expression (p < 0.05). Interestingly, such strong inflammatory responses were also significantly suppressed by ABA in the PTX + ABA + LPS group (p < 0.05). These data indicate that: 1. Global blocking Gi causes neuroinflammation in the spinal dorsal horn; 2. The anti-inflammatory effect induced by ABA is not dependent on the activity of Gi proteins.
Discussion
In this study, we have characterized the ABA/LANCL2 system in the spinal cord for the first time. We found that deficiency of the ABA/LANCL2 system plays a critical role in the genesis of neuropathic pain. The deficient ABA/LANCL2 system and neuropathic pain can be remedied by exogenous ABA. We revealed signaling molecules used by the ABA/LANCL2 system to regulate the spinal nociceptive processing. We also identified that TLR4 signaling pathway regulates the protein abundance of LANCL2. Given that ABA is widely present in a normal diet (vegetables and fruits), our study provides a rationale to explore the nutraceutical application of ABA for the treatment of neuropathic pain.
Distribution and plasticity of the ABA/LANCL2 system in mammals
The discovery of ABA in mammals has triggered numerous studies in recent years. ABA has been shown to be present in many tissues and organs including brain, heart, lung, kidney, 19 and blood. 56 Endogenous ABA concentration in the brain is significantly higher than other tissues like the heart, lung, or kidney. 19 Human and murine pancreatic β-cells release ABA in response to glucose 57 and blood ABA levels in humans are increased by glucose intake. 56 Upon pro-inflammatory stimuli, ABA production and release are increased from human cultured granulocytes, monocytes, keratinocytes, and vascular smooth muscle cells.20,58,59 Currently, ABA analysis in the spinal cord has not been reported. In this study, we, for the first time, demonstrated the presence of ABA in the spinal dorsal horn. Interestingly, we found that ABA concentrations in the spinal dorsal horn tissue are not significantly altered by peripheral nerve injury despite concurrent neuroinflammation in the same area, which is evident by increased protein expression of Iba1 (a sign of microglia activation), GFAP (a sign of astrocytic activation), and pro-inflammatory cytokine TNFα. Thus, the regulation of ABA synthesis may be context and tissue/organ-dependent.
Previous studies have shown that the ABA receptor LANCL2 is widely expressed throughout the body, including heart, lung, and brain. 60 Immune cells like T cells, macrophages, endothelial and epithelial cells, and dendritic cells also express LANCL2. 61 Our study extends this observation to include LANCL2 protein expression in spinal microglia but not astrocytes or neurons, which is consistent with previous reports that cultured microglia respond to ABA treatment. 62 Interestingly, we found that the abundance of spinal LANCL2 was reduced in the spinal cord with neuroinflammation induced by nerve injury, or activation of TLR4 by LPS treatment. Furthermore, the reduction of LANCL2 protein expression is ameliorated by ABA treatment. These findings are consistent with previous reports where ABA treatment prevents the reduced LANCL2 protein expression in the cortex in an Alzheimer’s disease mouse model. 45 Given that activation of TLR4 is a well-known mechanism underlying the genesis of neuropathic pain, 52 it is conceivable that the reduction of LANCL2 protein expression in the spinal cord following nerve injury is ascribed to the activation of TLR4 signaling pathways.
Role of the ABA/LANCL2 system in the inflammatory processes
Mechanistic studies of the effects of the ABA/LANCL2 system on the regulation of mammalian inflammatory signaling pathways has yielded conflicting results. On the one hand, studies mainly based on cell culture experiments support that ABA produces pro-inflammatory effects. For example, human granulocytes treated with ABA have increased phagocytosis, and production of reactive oxygen species (ROS) and nitric oxide (NO). 20 ABA release from granulocytes and keratinocytes triggered by ultraviolet light enhances production of TNFα, NO, and ROS from the same cells. 58 On the other hand, ABA treatment reduces TNFα expression and macrophage infiltration in white adipose tissue in animals with inflammatory bowel disease. 63 Familial Alzheimer’s disease mice treated with ABA have less glial activation and production of TNFα and IL-1β in the brain and improved cognitive function. 45 ABA treatment reduces microglial activation and TNFα production in the hypothalamus induced by high fat diet in rats. 64 It was recently reported that brain intraventricular injection of ABA inhibits both the phase 1 and phase 2 responses induced by formalin injection. 65 Our present study demonstrated that mechanical allodynia and heat hyperalgesia in rats with nerve injury are ameliorated by systemic or intrathecal administration of ABA. Furthermore, pre-emptive treatment of ABA attenuates spinal neuroinflammation induced by nerve injury. The anti-inflammatory effects of ABA in the spinal cord were further confirmed in our in vivo incubation experiments, where increased protein expression of Iba1 and TNFα induced by LPS was reduced by ABA treatment. Given that ABA produces pro- and anti-inflammatory effects on different tissues/organs, it is conceivable that the role of ABA in regulating inflammatory processes is context and tissue/organ-specific. It was suggested that two different signaling pathways may be used for the opposite inflammatory responses induced by ABA treatment. 66 It was shown that pro-inflammatory responses induced by ABA in human granulocytes is mediated by pertussis toxin (PTX)-sensitive G protein. 20 On the other hand, anti-inflammatory effects induced by ABA treatment are proposedly linked to the LANCL2-PPARγ cascade. 66 Currently, it not known whether the anti-inflammatory effects induced by ABA are mediated by the PTX-sensitive G protein. Our current study demonstrated that in the presence of PTX, ABA treatment still attenuates microglial activation and TNFα production induced by LPS, suggesting that Gi protein is dispensable for ABA to exert its anti-inflammatory effects in the spinal cord.
Despite many reports of the anti-inflammatory effects by exogenous ABA treatment, the role of the endogenous ABA/LANCL2 system in the regulation of the inflammatory processes is unknown. In this study, we demonstrated that knockdown of LANCL2 gene with siRNA in the spinal dorsal horn recapitulates the spinal neuroinflammation and nociceptive behaviors induced by nerve injury. Furthermore, the reduction of neuroinflammation in the spinal cord induced by ABA treatment is associated with improvement in LANCL2 protein expression. These findings provide the first evidence that impairment of the endogenous spinal ABA/LANCL2 system contributes, at least in part, to the development of neuroinflammation at the spinal dorsal horn and the genesis of chronic pain induced by nerve injury.
Downstream signaling molecules used by the ABA/LANCL2 to regulate nociceptive behaviors
In this study, we found that in spinal cords treated with LPS, ABA suppresses microglial activation and production of TNFα, and concurrently, improves PPARγ protein expression in the spinal cord. These findings are in agreement with previous studies showing that ABA treatment in animals produces anti-inflammatory effects via PPARγ.53,66,67 For example, ABA treatment enhances expression of PPARγ, while inhibition of PPARγ abrogates the inhibitory effect of ABA on allergic airway inflammation.53,67 Pharmacological blockade of PPARγ abolishes the beneficial effects induced by ABA on inflammation and cell death induced by 6-hydroxydopamine in human dopaminergic neuroblastoma SH-SY5Y cell line. 68 Our findings on the correlation between PPARγ protein expression and neuroinflammation also are consistent with findings by others about the role of spinal PPARγ in the genesis of neuropathic pain. 54 It was reported that PPARγ protein expression in the spinal dorsal horn is reduced in animals with nerve injury. 69 Pharmacological activation of PPARγ produces analgesic effects in rats with neuropathic pain with concurrent suppression of microglial activation and expression of TNFα, IL-1β, and TLR4 in the spinal cord. 70 Numerous studies have shown the important role of TNFα in the regulation of spinal nociceptive processing. TNFα is produced in microglia, astrocytes, oligodendrocytes, 71 and neurons.61,62 In mice and rats, a single intrathecal injection of TNFα induces mechanical allodynia and heat hyperalgesia.72,73 Exogenous application of recombinant TNFα increases glutamate release, and AMPA and NMDA currents in the spinal dorsal horn neurons.73,74 TNFα has been suggested to induce a pro-inflammatory signaling cascade leading to recruitment and activation of inflammatory cells such as astrocytes and microglia.75,76 Moreover, in morphine tolerant rats, the increased gene expressions of TNFα, IL-1β, and IL-6 in the spinal dorsal horn are abolished upon intrathecal pretreatment with a TNFα antagonist. 77
Conclusions
In this study, we found that deficiency of the ABA/LANCL2 system plays a critical role in the genesis of neuropathic pain. The deficient ABA/LANCL2 system and neuropathic pain can be remedied by exogenous ABA. We revealed signaling molecules used by the ABA/LANCL2 system to regulate the spinal nociceptive processing and signaling molecules regulating the protein abundance of LANCL2. Our study provides a rationale to explore the use of ABA for the treatment of neuropathic pain.
Appendix.
Abbreviations:
- ABA
abscisic acid
- CCR2
C-C Motif Chemokine Receptor 2
- CX3CR1
C-X3-C Motif Chemokine Receptor 1
- CSF1
colony-stimulating factor 1 receptors
- ELISA
enzyme-linked immunosorbent assay
- ERK
Extracellular signal-regulated kinase
- GCP
G-protein coupled receptor
- GFAP
glial fibrillary acidic protein
- Iba1
ionized calcium binding adaptor molecule 1
- IL-1β
interleukin-1β
- i.p.
intraperitoneal injection
- LANCL2
lanthionine synthetase C-like protein 2
- L4-L5
Lumbar 4-Lumbar 5
- LPS
lipopolysaccharide
- MAP
mitogen-activated protein
- NFκB
nuclear factor-κB
- NO
nitric oxide
- pSNL
partial sciatic nerve ligation
- P2X4R
P2X Purinergic receptor 4
- P2X7R
P2X purinoceptor 7
- P2Y12
P2Y purinoceptor 12
- P2Y13
P2Y purinoceptor 13
- PPARγ
peroxisome proliferator activated-receptor γ
- PTX
pertussis toxin
- ROS
reactive oxygen species
- siRNA
small interfering ribonucleic acid
- TLR4
toll-like receptor 4
- TNFα
Tumor necrosis factor alpha
Footnotes
Authors’ contributions: D. W. Maixner, D. Christy, L. Kong, V. Viatchenko-Karpinski, H.-R. Weng performed the experiments and analyzed data. K. A. Horner and S. B. Hooks assisted with the manuscript. D.W. Maixner and H.-R. Weng conceived and designed the project. H.-R. Weng led the project and wrote the manuscript. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the NIH RO1 grants (NS064289, NS107569) to H.R.W.
ORCID iD
Han-Rong Weng https://orcid.org/0000-0003-2062-2782
References
- 1.van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014; 155(4): 654–662. [DOI] [PubMed] [Google Scholar]
- 2.Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang SH, Ding HK, Frankland PW, Salter MW. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med 2008; 14(12): 1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009; 32: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014; 13(7): 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol 2014; 14: 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med 2010; 16(11): 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nie H, Zhang H, Weng HR. Minocycline prevents impaired glial glutamate uptake in the spinal sensory synapses of neuropathic rats. Neuroscience 2010; 170(3): 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan X, Weng HR. Endogenous interleukin-1β in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic NMDA receptors. J Biol Chem 2013; 288(42): 30544–30557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao M, Yan X, Weng HR. Inhibition of glycogen synthase kinase 3beta activity with lithium prevents and attenuates paclitaxel-induced neuropathic pain. Neuroscience 2013; 254: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan X, Yadav R, Gao M, Weng HR. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia 2014; 62(7): 1093–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cata JP, Weng HR, Dougherty PM. The effects of thalidomide and minocycline on taxol-induced hyperalgesia in rats. Brain Res 2008; 1229: 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taves S, Berta T, Chen G, Ji RR. Microglia and spinal cord synaptic plasticity in persistent pain. Neural Plast 2013; 2013: 753656–753710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci 2008; 28(11): 2892–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. Microglial regulation of neuropathic pain. J Pharmacol Sci 2013; 121(2): 89–94. [DOI] [PubMed] [Google Scholar]
- 15.Yan X, Maixner DW, Li F, Weng HR. Chronic pain and impaired glial glutamate transporter function in lupus-prone mice are ameliorated by blocking macrophage colony-stimulating factor-1 receptors. J Neurochem 2017; 140: 963–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci 2016; 19(1): 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharath P, Gahir S, Raghavendra AS. Abscisic acid-induced stomatal closure: an important component of plant defense against abiotic and biotic stress. Front Plant Sci 2021; 12: 615114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotov AA, Kotova LM, Romanov GA. Signaling network regulating plant branching: Recent advances and new challenges. Plant Sci 2021; 307: 110880. [DOI] [PubMed] [Google Scholar]
- 19.Le Page-Degivry MT, Bidard JN, Rouvier E, Bulard C, Lazdunski M. Presence of abscisic acid, a phytohormone, in the mammalian brain. Proc Natl Acad Sci U S A 1986; 83(4): 1155–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruzzone S, Moreschi I, Usai C, Guida L, Damonte G, Salis A, Scarfi S, Millo E, De Flora A, Zocchi E. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc Natl Acad Sci U S A 2007; 104(14): 5759–5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zocchi E, Hontecillas R, Leber A, Einerhand A, Carbo A, Bruzzone S, Tubau-Juni N, Philipson N, Zoccoli-Rodriguez V, Sturla L, Bassaganya-Riera J. Abscisic acid: a novel nutraceutical for glycemic control. Front Nutr 2017; 4: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li HH, Hao RL, Wu SS, Guo PC, Chen CJ, Pan LP, Ni H. Occurrence, function and potential medicinal applications of the phytohormone abscisic acid in animals and humans. Biochem Pharmacol 2011; 82(7): 701–712. [DOI] [PubMed] [Google Scholar]
- 23.Mayer H, Bauer H, Prohaska R. Organization and chromosomal localization of the human and mouse genes coding for LanC-like protein 1 (LANCL1). Cytogenet Cell Genet 2001; 93(1–2): 100–104. [DOI] [PubMed] [Google Scholar]
- 24.Magnone M, Sturla L, Guida L, Spinelli S, Begani G, Bruzzone S, Fresia C, Zocchi E. Abscisic acid: a conserved hormone in plants and humans and a promising aid to combat prediabetes and the metabolic syndrome. Nutrients 2020; 12(6): 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seltzer Ze, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 1990; 43(2): 205–218. [DOI] [PubMed] [Google Scholar]
- 26.Yan X, Li F, Maixner DW, Yadav R, Gao M, Ali MW, Hooks SB, Weng HR. Interleukin-1beta released by microglia initiates the enhanced glutamatergic activity in the spinal dorsal horn during paclitaxel-associated acute pain syndrome. Glia 2019; 67(3): 482–497. [DOI] [PubMed] [Google Scholar]
- 27.Sakai A, Saitow F, Maruyama M, Miyake N, Miyake K, Shimada T, Okada T, Suzuki H. MicroRNA cluster miR-17-92 regulates multiple functionally related voltage-gated potassium channels in chronic neuropathic pain. Nat Commun 2017; 8: 16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo X, Lu J, Yan M, Wang Y, Yang Y, Li H, Shen H, Diao S, Ni J, Lu H, Zhao H, Chen G. MicroRNA-133b-3p Targets Purinergic P2X4 Receptor to Regulate Central Poststroke Pain in Rats. Neuroscience 2021; 481: 60–72. [DOI] [PubMed] [Google Scholar]
- 29.Yan X, Weng H-R. Endogenous Interleukin-1β in Neuropathic Rats Enhances Glutamate Release from the Primary Afferents in the Spinal Dorsal Horn through Coupling with Presynaptic N-Methyl-d-aspartic Acid Receptors. J Biol Chem 2013; 288(42): 30544–30557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan X, Maixner DW, Yadav R, Gao M, Li P, Bartlett MG, Weng H-R. Paclitaxel induces acute pain via directly activating toll like receptor 4. Mol Pain 2015; 11(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32(1): 77–88. [DOI] [PubMed] [Google Scholar]
- 32.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science 1976; 192(4246): 1357–1358. [DOI] [PubMed] [Google Scholar]
- 33.Yadav R, Yan X, Maixner DW, Gao M, Weng HR. Blocking the GABA transporter GAT-1 ameliorates spinal GABAergic disinhibition and neuropathic pain induced by paclitaxel. J Neurochem 2015; 133(6): 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weng HR, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain 2003; 103(1–2): 131–138. [DOI] [PubMed] [Google Scholar]
- 35.Xu JJ, Walla BC, Diaz MF, Fuller GN, Gutstein HB. Intermittent lumbar puncture in rats: a novel method for the experimental study of opioid tolerance. Anesth Analgesia 2006; 103(3): 714–720. [DOI] [PubMed] [Google Scholar]
- 36.Luo M-C, Zhang D-Q, Ma S-W, Huang Y-Y, Shuster SJ, Porreca F, Lai J. An efficient intrathecal delivery of small interfering RNA to the spinal cord and peripheral neurons. Mol Pain 2005; 1(1): 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weng H-R, Gao M, Maixner DW. Glycogen synthase kinase 3 beta regulates glial glutamate transporter protein expression in the spinal dorsal horn in rats with neuropathic pain. Exp Neurol 2014; 252(0): 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maixner D, Yan X, Gao M, Yadav R, Weng H. Adenosine Monophosphate-activated Protein Kinase Regulates Interleukin-1β Expression and Glial Glutamate Transporter Function in Rodents with Neuropathic Pain. Anesthesiology 2015; 122: 1401–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva RV, Oliveira JT, Santos BLR, Dias FC, Martinez AMB, Lima CKF, Miranda ALP. Long-chain omega-3 fatty acids supplementation accelerates nerve regeneration and prevents neuropathic pain behavior in mice. Front Pharmacol 2017; 8: 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brenchat A, Nadal X, Romero L, Ovalle S, Muro A, Sanchez-Arroyos R, Portillo-Salido E, Pujol M, Montero A, Codony X, Burgueno J, Zamanillo D, Hamon M, Maldonado R, Vela JM. Pharmacological activation of 5-HT7 receptors reduces nerve injury-induced mechanical and thermal hypersensitivity. Pain 2010; 149(3): 483–494. [DOI] [PubMed] [Google Scholar]
- 41.Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 2009; 89(2): 707–758. [DOI] [PubMed] [Google Scholar]
- 42.Qi C-C, Zhang Z, Fang H, Liu J, Zhou N, Ge J-F, Chen F-H, Xiang C-B, Zhou J-N. Antidepressant effects of abscisic acid mediated by the downregulation of corticotrophin-releasing hormone gene expression in rats. Int J Neuropsychopharmacol 2015; 18(4): pyu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi C-C, Ge J-F, Zhou J-N. Preliminary evidence that abscisic acid improves spatial memory in rats. Physiol Behav 2015; 139: 231–239. [DOI] [PubMed] [Google Scholar]
- 44.Guri AJ, Hontecillas R, Si H, Liu D, Bassaganya-Riera J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr 2007; 26(1): 107–116. [DOI] [PubMed] [Google Scholar]
- 45.Jeon SH, Kim N, Ju YJ, Gee MS, Lee D, Lee JK. Phytohormone abscisic acid improves memory impairment and reduces neuroinflammation in 5xFAD mice by upregulation of LanC-like protein 2. Int J Mol Sci 2020; 21(22): 8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Periyasamy P, Thangaraj A, Guo ML, Hu G, Callen S, Buch S. Epigenetic promoter DNA methylation of miR-124 promotes HIV-1 Tat-mediated microglial activation via MECP2-STAT3 axis. J Neurosci 2018; 38(23): 5367–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ullah I, Choe YH, Khan M, Bharti D, Shivakumar SB, Lee HJ, Son YB, Shin Y, Lee SL, Park BW, Ock SA, Rho GJ. Dental pulp-derived stem cells can counterbalance peripheral nerve injury-induced oxidative stress and supraspinal neuro-inflammation in rat brain. Sci Rep 2018; 8(1): 15795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci 2007; 27(22): 6006–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang XS, Guan SY, Liu A, Yue J, Hu LN, Zhang K, Yang LK, Lu L, Tian Z, Zhao MG, Liu SB. Anxiolytic effects of Formononetin in an inflammatory pain mouse model. Mol Brain 2019; 12(1): 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain 2005; 114(1–2): 149–159. [DOI] [PubMed] [Google Scholar]
- 51.Jara JH, Singh BB, Floden AM, Combs CK. Tumor necrosis factor alpha stimulates NMDA receptor activity in mouse cortical neurons resulting in ERK‐dependent death. J Neurochemistry 2007; 100(5): 1407–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018; 100(6): 1292–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hontecillas R, Roberts PC, Carbo A, Vives C, Horne WT, Genis S, Velayudhan B, Bassaganya-Riera J. Dietary abscisic acid ameliorates influenza-virus-associated disease and pulmonary immunopathology through a PPARgamma-dependent mechanism. J Nutr Biochem 2013; 24(6): 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okine BN, Gaspar JC, Finn DP. PPARs and pain. Br J Pharmacol 2019; 176(10): 1421–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grace PM, Wang X, Strand KA, Baratta MV, Zhang Y, Galer EL, Yin H, Maier SF, Watkins LR. DREADDed microglia in pain: Implications for spinal inflammatory signaling in male rats. Exp Neurol 2018; 304: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruzzone S, Magnone M, Mannino E, Sociali G, Sturla L, Fresia C, Booz V, Emionite L, De Flora A, Zocchi E. Abscisic acid stimulates glucagon-like peptide-1 secretion from L-cells and its oral administration increases plasma glucagon-like peptide-1 levels in rats. PLoS One 2015; 10(10): e0140588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruzzone S, Bodrato N, Usai C, Guida L, Moreschi I, Nano R, Antonioli B, Fruscione F, Magnone M, Scarfi S, De Flora A, Zocchi E. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic ADP ribose as second messenger. J Biol Chem 2008; 283(47): 32188–32197. [DOI] [PubMed] [Google Scholar]
- 58.Bruzzone S, Basile G, Mannino E, Sturla L, Magnone M, Grozio A, Salis A, Fresia C, Vigliarolo T, Guida L, De Flora A, Tossi V, Cassia R, Lamattina L, Zocchi E. Autocrine abscisic acid mediates the UV-B-induced inflammatory response in human granulocytes and keratinocytes. J Cell Physiol 2012; 227(6): 2502–2510. [DOI] [PubMed] [Google Scholar]
- 59.Magnone M, Bruzzone S, Guida L, Damonte G, Millo E, Scarfi S, Usai C, Sturla L, Palombo D, De Flora A, Zocchi E. Abscisic acid released by human monocytes activates monocytes and vascular smooth muscle cell responses involved in atherogenesis. J Biol Chem 2009; 284(26): 17808–17818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayer H, Pongratz M, Prohaska R. Molecular cloning, characterization, and tissue-specific expression of human LANCL2, a novel member of the LanC-like protein family. DNA Seq 2001; 12(3): 161–166. [DOI] [PubMed] [Google Scholar]
- 61.Lu P, Hontecillas R, Philipson CW, Bassaganya-Riera J. Lanthionine synthetase component C-like protein 2: a new drug target for inflammatory diseases and diabetes. Curr Drug Target 2014; 15(6): 565–572. [DOI] [PubMed] [Google Scholar]
- 62.Bodrato N, Franco L, Fresia C, Guida L, Usai C, Salis A, Moreschi I, Ferraris C, Verderio C, Basile G, Bruzzone S, Scarfi S, De Flora A, Zocchi E. Abscisic acid activates the murine microglial cell line N9 through the second messenger cyclic ADP-ribose. J Biol Chem 2009; 284(22): 14777–14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guri AJ, Hontecillas R, Si H, Liu D, Bassaganya-Riera J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr 2007; 26(1): 107–116. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez-Sarasua S, Moustafa S, Garcia-Aviles A, Lopez-Climent MF, Gomez-Cadenas A, Olucha-Bordonau FE, Sanchez-Perez AM. The effect of abscisic acid chronic treatment on neuroinflammatory markers and memory in a rat model of high-fat diet induced neuroinflammation. Nutr Metab 2016; 13: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mollashahi M, Abbasnejad M, Esmaeili-Mahani S. Phytohormone abscisic acid elicits antinociceptive effects in rats through the activation of opioid and peroxisome proliferator-activated receptors beta/delta. Eur J Pharmacol 2018; 832: 75–80. [DOI] [PubMed] [Google Scholar]
- 66.Balino P, Gomez-Cadenas A, Lopez-Malo D, Romero FJ, Muriach M. Is there a role for abscisic acid, a proven anti-inflammatory agent, in the treatment of ischemic retinopathies? Antioxidants 2019; 8(4): 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao CC, Xu J, Xie QM, Zhang HY, Fei GH, Wu HM. Abscisic acid suppresses the activation of NLRP3 inflammasome and oxidative stress in murine allergic airway inflammation. Phytother Res 2021; 35(6): 3298–3309. [DOI] [PubMed] [Google Scholar]
- 68.Rafiepour K, Esmaeili-Mahani S, Salehzadeh A, Sheibani V. Phytohormone abscisic acid protects human neuroblastoma SH-SY5Y cells against 6-hydroxydopamine-induced neurotoxicity through its antioxidant and antiapoptotic properties. Rejuvenation Res 2019; 22(2): 99–108. [DOI] [PubMed] [Google Scholar]
- 69.Jiang P, Jiang Q, Yan Y, Hou Z, Luo D. Propofol ameliorates neuropathic pain and neuroinflammation through PPAR gamma up-regulation to block Wnt/beta-catenin pathway. Neurol Res 2021; 43(1): 71–77. [DOI] [PubMed] [Google Scholar]
- 70.Jia H, Xu S, Liu Q, Liu J, Xu J, Li W, Jin Y, Ji Q. Effect of pioglitazone on neuropathic pain and spinal expression of TLR-4 and cytokines. Exp Ther Med 2016; 12(4): 2644–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan P, Li Q, Kim G-M, Xu J, Hsu CY, Xu XM. Cellular localization of tumor necrosis factor-α following acute spinal cord injury in adult rats. J Neurotrauma 2001; 18(5): 563–568. [DOI] [PubMed] [Google Scholar]
- 72.Gao Y-J, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu Z-Z, Park J-Y, Lind A-L, Ma Q, Ji R-R. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. The J Neuroscience 2009; 29(13): 4096–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Youn D-h, Wang H, Jeong S-J. Exogenous tumor necrosis factor-α rapidly alters synaptic and sensory transmission in the adult rat spinal cord dorsal horn. J Neurosci Res 2008; 86(13): 2867–2875. [DOI] [PubMed] [Google Scholar]
- 74.Kawasaki Y, Zhang L, Cheng J-K, Ji R-R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. The J Neurosci 2008; 28(20): 5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verri WA, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Therapeutics 2006; 112(1): 116–138. [DOI] [PubMed] [Google Scholar]
- 76.Tzeng S-F, Kahn M, Liva S, De Vellis J. Tumor necrosis factor-α regulation of the Id gene family in astrocytes and microglia during CNS inflammatory injury. Glia 1999; 26(2): 139–152. [DOI] [PubMed] [Google Scholar]
- 77.Shen C-H, Tsai R-Y, Shih M-S, Lin S-L, Tai Y-H, Chien C-C, Wong C-S. Etanercept restores the antinociceptive effect of morphine and suppresses spinal neuroinflammation in morphine-tolerant rats. Anesth Analgesia 2011; 112(2): 454–459. [DOI] [PubMed] [Google Scholar]