Abstract
Background
Diabetes remains poorly controlled in a high proportion of diabetes patients. This study examines the prevalence of poor glycaemic control and associated factors in type 2 diabetes patients in the Beni-Mellal Khenifra region in Morocco.
Methods
A cross-sectional survey was conducted in 2017 among 1456 diabetes patients attending primary health centres. Demographic and clinical data were collected through face-to-face interviews using structured and pre-tested questionnaires. Anthropometric measurements, including body weight, height and waist circumference were taken using standardized techniques and calibrated equipment. Glycaemic control was assessed in terms of the glycated haemoglobin (HbA1c) level and poor glycaemic control was defined as HbA1c ≥7% and a level <7% reflects good glycaemic control.
Results
Of the total participants, 66.3% had poor glycaemic control. Bivariate analysis showed that sex (p=0.010), education level (p=0.013), body mass index (p=0.048), duration of diabetes (p<0.0001) and type of therapeutic regimen (p<0.0001) were significantly associated with HbA1c level. However, multiple logistic regression analyses revealed that only a longer duration of diabetes (OR 1.525 [95% confidence interval {CI} 1.183–1.967], p=0.001) and receiving insulin therapy alone (OR 1.589 [95% CI 1.157–2.183], p=0.004) or a combination of oral antidiabetics with insulin (OR 2.554 [95% CI 1.786–3.653], p<0.001) were significantly associated with inadequate glycaemic control.
Conclusions
Despite the particularities of the region, the findings about glycaemic control and its cross-sectionally associated factors are in line with findings from other regions of Morocco. In this subgroup, the longer duration of diabetes and insulin treatment could constitute a cause leading to poor glycaemic control. However, inverse causality cannot be excluded.
Keywords: determinant factors, glycated haemoglobin, Morocco, poor glycaemic control, prevalence, type 2 diabetes patients
Introduction
Diabetes is a growing public health problem affecting people worldwide, with a rapidly increasing prevalence in both developing and developed countries.1 The number of diabetes patients 20–79 y of age is projected to rise from 425 million in 2017 to 629 million in 2045, which represents an increase of 48%.2 The Middle Eastern and North African regions are experiencing a significant increase in the prevalence of diabetes, recording the second highest rate of increase in diabetes patients, with >39 million diabetic patients 20–79 y of age in 2017; this number is expected to reach 82 million by 2045.2 Morocco is not an exception, as it is estimated that there are >1.641 million diabetic adults, accounting for >7.3% of their adult population.2
The burden of diabetes is higher in developing countries, where screening and access to care and treatment are not readily available.3
It has been shown that inadequate glycaemic control in type 2 diabetes mellitus (T2DM) patients contributes to increased rates of macrovascular and microvascular diabetic complications, which may increase the costs associated with healthcare.4 Despite this evidence, a high proportion of patients worldwide still have poorly controlled diabetes.5 Several studies have revealed that the rate of poor glycaemic control among diabetic patients in different parts of the world is high.6–8 Interestingly, equivalent high proportions of T2DM patients with poor glycaemic control have been reported in Morocco.9,10
Most of these previous studies have shown that achieving optimal glycaemic control is difficult, and the reasons for this poor control are complex. It has been demonstrated that the glycaemic level in patients with T2DM is affected by various factors, including age, sex, ethnicity, education, employment status, marital status, body mass index, smoking status, diabetes duration, presence of comorbidities, polypharmacy, diabetes-related knowledge, non-adherence to medication and type of medications used.11
Due to the scarcity of these kinds of studies in Morocco and because these investigations have concerned only particular regions in Morocco, and to extend our knowledge on the evolution of glycaemic control in Morocco, this study aims to examine the prevalence of poor glycaemic control and associated factors in T2DM patients in the Beni-Mellal Khenifra region of Morocco. This region has never been analysed before, despite its socio-economic and cultural characteristics. Indeed, according to the last General Census of Population and Housing in Morocco, the Beni-Mellal Khenifra region had 2 520 776 inhabitants and covered 4% of the area of the country, with an urbanization rate of 49.1% in 2014, which is lower than the national average (60.36%).12 The agricultural sector constitutes the essential part of the economic activity in the region, and the industry is essentially focused on the processing of agricultural products.12
Methods
Study participants and data collection
We conducted a cross-sectional survey in 2017 among 1456 diabetes patients attending primary health centres in the Beni-Mellal Khenifra region of Morocco. At the time of the survey and according to the Regional Observatory of Health in the Beni-Mellal Khenifra region, the primary health centres provide health services for 153 000 T2DM patients registered in five provinces (Beni-Mellal, 22 000; Azilal, 40 000; Fquih Ben Salah, 34 000; Khenifra, 21 000; Khouribga, 36 000), who receive regular medical follow-up and get their medications dispensed at the centres free of charge. For patient selection, a multilevel random-sampling method was used to recruit participants.
The sample size was calculated based on the following parameters: prevalence of poor glycaemic control (50%) among T2DM patients, 4% margin of error (e=0.04) and 99% confidence level (z=2.57); thus, the minimum study sample size was 1032, which was rounded up to 1500 persons for more accuracy and in order to account for possible exclusions and the need to carry out subgroup analysis.
The minimum sample size (n) was calculated using the following equation, with a total target population of N=153 000:
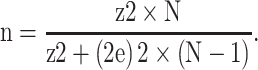 |
The sample surveyed in the five provinces of the Beni-Mellal Khenifra region was proportional to the total T2DM population in each province: 216 patients from Beni-Mellal, 392 from Azilal, 333 from Fquih Ben Salah, 206 from Khenifra and 353 from Khouribga. All primary health centres providing diabetes care in each province were counted and centres were randomly selected from these. The number of primary health centres was chosen based on proportions of diabetes patients recorded in each province. Thus 15 primary health centres were the setting for the survey.
Every workday, a list of expected participants was obtained from the healthcare centres. The value of K participants depended on the number of people attending the centre each day, which varies between centres. The first K participant to be recruited into the study and who met the inclusion criteria was randomly selected by the investigator and then every Kth patient was recruited into the study. If the Kth person declined, the next person was invited. The recruitment was continued until data were collected from 1500 patients.
After cleaning of the files, 44 questionnaires with missing data or unreadable handwriting were eliminated; the sample size remains 1456. During the study, 80 eligible participants (5.33%) declined to participate, mainly because of lack of time.
A face-to-face interview was carried out by trained interviewers to collect data, including sociodemographic and cultural information such as age, sex, place of residence, marital status, family size, level of education and occupational status. The participants’ education levels were classified into four categories: illiterate (unable to read and write and without formal education), primary (had 1–6 y of formal education), secondary (had 7–12 y of formal education) and university (had at least 13 y of formal education). The employment status was categorized as working or not currently employed.
In addition, we collected information about diabetes, such as the duration (years) of diabetes, family history of diabetes (defined as having a parent or sibling with diabetes), treatment type and complications linked to diabetes.
The inclusion criteria for this study were as follows: patients diagnosed with T2DM for ≥1 y, with an available medical file; age ≥18 y; had a haemoglobin A1c (HbA1c) test during the last 3 months; physically and mentally able to provide all data required for the study and willing to participate in the study.
Patients with type 1 diabetes, hospitalized patients and pregnant women with diabetes were excluded from this study. The participation was voluntary and anonymous. Participants were informed about the study objective and they also read carefully and signed a consent form. All data were confidential and protected at all stages of the study.
Anthropometric measurements and clinical parameters
Height and body weight were measured for all participants by trained research staff; body weight was measured to the nearest 0.1 kg using a digital scale (Seca 877, Seca, Hamburg, Germany) and height was recorded to the nearest 0.1 cm using a wall-mounted stadiometer (Seca 216). Measurements were taken for each participant with light clothing and without shoes, and body mass index (BMI) was calculated as weight in kilograms divided by height in metres squared and categorized as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obese (≥30 kg/m2).13 Waist circumference was measured to the nearest 0.5 cm and abdominal obesity was defined as a waist circumference ≥102 cm in men and ≥88 cm in women.13
For biological indicators, the most recent HbA1c measurements (if not exceeding 3 months previously) were extracted from the patients’ medical records. According to the American Diabetes Association, we defined glycaemic status as good glycaemic control if HbA1c was <7% and poor glycaemic control as HbA1c ≥7%.14
Data analysis
Statistical analysis was carried out using Statistical Package for Social Sciences, version 19.0 (SPSS, Chicago, IL, USA) software. Data are presented as the mean±standard deviation (SD) for continuous variables and proportions for categorical variables. The χ2 test was used to assess statistical significance between the dependent variable (poor glycaemic control) and potential explanatory variables. All significant variables in the χ2 test analysis (p<0.05) were considered in the multivariate logistic regression model to determine independent factors associated with poor glycaemic control.
Results
Participants’ characteristics
Among the 1456 participants included in the present investigation, women represented 73.4% and men represented 26.6% (Table 1) of the participants. Participants ranged in age from 19 to 86 y, with a mean age of 56.16 y (SD 11.76).
Table 1.
The sociodemographic, clinical and anthropometric characteristics of participants (n=1456)
| Variable | Values | |
|---|---|---|
| Gender, n (%) | Male | 388 (26.6) |
| Female | 1068 (73.4) | |
| Age (years), n (%) | ≤40 | 125(8.8) |
| 41–50 | 296 (20.8) | |
| 51–60 | 501(35.3) | |
| ≥61 | 499 (35.1) | |
| Marital status, n (%) | Single | 91 (6.3) |
| Married | 994 (68.3) | |
| Divorced | 80 (5.5) | |
| Widow/widower | 290 (19.9) | |
| Education level, n (%) | Illiterate | 975 (67.0) |
| Primary | 229 (15.7) | |
| Secondary | 183 (12.6) | |
| University | 69 (4.7) | |
| Occupation, n (%) | Unemployed/housewife | 1141 (78.4) |
| Employed | 315 (21.6) | |
| Body mass index, n (%) | Underweight | 18 (1.4) |
| Normal | 382 (29.6) | |
| Overweight | 544 (42.1) | |
| Obese | 348 (26.9) | |
| Abdominal obesity, n (%) | Normal | 239 (26.8) |
| Obese | 654 (73.2) | |
| Duration of diabetes (years), n (%) | Mean duration of diabetes (years), mean±SD | 8.63±6.8 |
| ≤7 | 759 (53.0) | |
| >7 | 673 (47.0) | |
| Glycaemic control, n (%) | HbA1c (%), mean±SD | 8.4±1.98 |
| HbA1c ≤7% | 491 (33.7) | |
| HbA1c >7% | 965 (66.3) | |
| Management of diabetes, n (%) | OA alone | 773 (53.5) |
| Insulin alone | 326 (22.6) | |
| Combination of OA and insulin | 285 (19.7) | |
| Diet only | 60 (4.2) | |
OA, oral antidiabetic
In our sample we noted that the duration of diabetes ranged from 1 to 36 y, with a mean of 8.63 y (SD 6.8). The majority of participants were overweight (69.0%) and 26.9% were obese. Furthermore, HbA1c values ranged from 5.0% to 12.8% with a mean of 8.4% (SD 1.98), and the glycaemic control measured by HbA1c showed that 66.3% of the participants were classified as having poor glycaemic control. Finally, 53.5% of the participants used oral antidiabetic (OA) agents, 22.6% used insulin alone and 19.7% used a combination of OA agents and insulin (Table 1).
Poor glycaemic control and its determinant factors among T2DM participants
A total of 66.3% (n=965) of the participants investigated had poor glycaemic control. The details of the association between HbA1c and various demographic and clinical parameters using the χ2 test are outlined in Table 2.
Table 2.
The glycaemic control of participants according to demographic, anthropometric and clinical characteristics
| Variable | HbA1c, mean (SD) | Glycaemic control status, n (%) | p-Value (χ2 test) | |
|---|---|---|---|---|
| Good (HbA1c<7) | Poor (HbA1c ≥7) | |||
| Gender | 0.010* | |||
| Female | 8.28 (1.93) | 379 (35.5) | 689 (64.5) | |
| Male | 8.72 (2.11) | 112 (28.9) | 276 (79.1) | |
| Age (years) | NS | |||
| ≤40 | 8.92 (2.14) | 30 (24) | 95 (76) | |
| 41–50 | 8.44 (2.05) | 103(34.8) | 193(65.2) | |
| 51–60 | 8.30 (1.93) | 177 (35.3) | 374 (64.7) | |
| ≥61 | 8.40 (1.94) | 163 (32.7) | 336 (67.3) | |
| Education level | 0.013* | |||
| Illiterate | 8.30 (1.95) | 348 (35.7) | 627 (64.3) | |
| Literate | 8.60 (2.04) | 143 (29.7) | 338 (70.3) | |
| Body mass index | 0.048* | |||
| Underweight | 9.19 (1.86) | 2 (11.1) | 16 (88.9) | |
| Normal | 8.70 (2.16) | 120 (31.4) | 262 (68.6) | |
| Overweight | 8.25 (1.87) | 195 (35.8) | 349 (64.2) | |
| Obese | 8.10 (1.81) | 131 (37.6) | 217 (62.4) | |
| Abdominal obesity | NS | |||
| Normal | 8.20 (1.92) | 87 (36.4) | 152 (63.6) | |
| Obese | 8.15 (1.72) | 222 (33.9) | 432 (66.1) | |
| Duration of diabetes (years) | <0.001* | |||
| ≤7 | 8.07 (1.98) | 310 (40.8) | 449 (59.2) | |
| >7 | 8.75 (1.93) | 172 (25.6) | 501 (74.4) | |
| Management of diabetes | <0.001* | |||
| OA alone | 310 (40.1) | 463 (59.9) | ||
| Insulin alone | 81 (24.8) | 245 (75.2) | ||
| Combination of OA and insulin | 58 (20.4) | 227 (79.6) | ||
NS: not significant.
Statistically significant at p<0.05.
The results of the χ2 test analysis showed that male sex (p=0.010), low education level (p=0.013), underweight (p=0.048), longer duration of diabetes (p<0.001) and the use of a combination of OA drugs with insulin or insulin alone (p<0.001) were significantly associated with poor glycaemic control. In the multivariate analysis model, only increased duration of diabetes (OR 1.525 [95% CI 1.183–1.967], p=0.001) and the combined use of insulin and OA (OR 2.554 [95% CI 1.786–3.653], p<0.001) or insulin alone (OR 1.589 [95% CI 1.157–2.183], p=0.004) were significantly associated with increased odds of poor glycaemic control (Table 3).
Table 3.
Multivariate analysis of factors associated with poor glycaemic control among diabetic participants
| Variable | Poor glycaemic control (HbA1c ≥7), n (%) | Adjusted OR (95% CI) | p-Value |
|---|---|---|---|
| Gender | |||
| Female | 689 (64.5) | 1 | |
| Male | 276 (79.1) | 1.159 (0.853–1.575) | NS |
| Education level | |||
| Illiterate | 627 (64.3) | 1 | NS |
| Literate | 338 (70.3) | 1.238 (0.934–1.639) | |
| Duration of diabetes (years) | |||
| ≤7 | 449 (59.2) | 1 | |
| >7 | 501 (74.4) | 1.525 (1.183–1.967) | 0.001* |
| Body mass index | |||
| Underweight | 16 (88.9) | 4.209 (0.932–19.009) | NS |
| Normal | 262 (68.6) | 1.217 (0.869–1.705) | NS |
| Overweight | 349 (64.2) | 1.101 (0.813–1.492) | NS |
| Obese | 217 (62.4) | 1 | |
| Management of diabetes | |||
| OA alone | 463 (59.9) | 1 | |
| Insulin alone | 245 (75.2) | 1.589 (1.157–2.183) | 0.004* |
| Combination of OA and insulin | 227 (79.6) | 2.554 (1.786–3.653) | <0.001* |
Statistically significant at p<0.05.
The duration of diabetes was associated with poor glycaemic control. Participants who had been diagnosed with diabetes for >7 y were twice as likely to have poor glycaemic control compared with those who had diabetes for <7 y.
For the type of therapeutic regimen, compared with respondents who were using OA alone, participants who used insulin treatment alone or in combination with OA were two and three times, respectively, more likely to have poor glycaemic control.
Discussion
This study was carried out to assess the prevalence of poor glycaemic control and the factors that are significantly associated with increasing levels of HbA1c among a sample of Moroccan T2DM patients residing in the Beni-Mellal Khenifra region. The results show that the majority of studied patients had poor glycaemic control. Furthermore, a longer duration of diabetes and receiving insulin therapy alone or a combination of OAs with insulin was significantly associated with inadequate glycaemic control.
The mean HbA1c value recorded in the investigated population was 8.4±1.98%. This result is in accordance with previous Moroccan studies that reported a mean HbA1c of 8.3±1.9% and 8.0±1.9%.9,10 This result was also in accordance with other studies conducted outside Morocco, such as in the UK (8.16%) and Jordan (8.10±1.80%).15,16 However, our HbA1c levels were higher compared with the values reported in Canada (7.3%) and Germany (7.1%).17,18
Our study showed that 66.3% of diabetes patients had poor glycaemic control, which was slightly lower but in the range of previously reported proportions in other Moroccan regions. For example, a study conducted in 2009 among 356 T2DM patients in Marrakesh revealed that 68% of diabetic patients had poor glycaemic control.19 High proportions of patients with poor glycaemic control were also reported by the International Diabetes Management Practice Study (IDMPS) wave 2 conducted from 2006 to 20079 and IDMPS wave 5 in 2011,10 which reported that 69.1% and 73.2% of patients, respectively, had poor glycaemic control. These findings were generally in line with studies carried out in other Arabian countries reporting that a large proportion of studied patients exhibit poor glycaemic control, but the percentages recorded in Morocco are less than the majority of those reported for these countries.
In Tunisia, a North African country, a study of 404 patients with T2DM also revealed that >83% of the population studied had poor glycaemic control.8 Similarly, in Algeria, 81.3% of T2DM patients had HbA1c >7%.20 In Saudi Arabia, a study conducted at a national level, including 28 health centres, reported that 73% of the study patients did not achieve the HbA1c target of <7%.6 A high proportion of poor glycaemic control among patients with diabetes was also recorded in Jordan and Kuwait, with 65.1% and 66.7% of diabetic patients, respectively, having poorly controlled diabetes.7,21 Our results are in line with these findings.
In contrast, studies carried out in Germany22 and Japan23 showed that >45% and 65% of patients with T2DM, respectively, were able to achieve the target level of glycaemic control. The higher level of glycaemic control observed in Japan and Germany may be due to the high literacy rate in these developed countries and therefore probably better knowledge of the disease.22,23
The prevalence of poor glycaemic control in our study was comparable to previous studies in Morocco conducted in 2009 and 2011. This clearly revealed that, despite the great effort made by the Ministry of Health to improve the management of diabetic patients by providing free consultation and free medication in all Moroccan primary healthcare centres, poor glycaemic control was still present, which could constitute a real public health concern.
The results obtained from the multivariate logistic regression analysis indicated that poor glycaemic control was not associated with sex, age, education level or BMI. Indeed, although males, patients who could not read and patients with normal BMI and underweight showed slightly higher HbA1c values in the present study, these variables did not reach statistical significance.
We found that overweight and obesity were not associated with poor glycaemic control, in line with previous studies reporting the absence of a link between BMI and glycaemic control.24 In contrast, other research studies reported that overweight or obesity was associated with a significantly higher probability of having HbA1c ≥7%,25 a finding that may be explained by the fact that obese diabetic patients often reported irregular meal patterns, leading to poorer glycaemic control and reduced insulin sensitivity.26 On the other hand, other research has suggested that lower BMI is associated with poorer glycaemic control.27 The authors have shown that underweight patients are poorly controlled and have low C-peptide levels, reflecting inadequate β-cell reserves.27 This explanation may be somewhat misleading. Indeed, the reverse causation could not be excluded, as poor glycaemic control may constitute a cause of weight loss and therefore is more frequent in underweight patients.
Similarly, we reported for the first time in Moroccan diabetic patients that age had no significant association with glycaemic control. This finding was consistent with the findings of a similar study conducted in Iraq.28 A literature review of the factors associated with glycaemic control showed that there are conflicting relationships regarding age in relation to glycaemic control.11
The associated factors of glycaemic control in the present study were the duration of diabetes and type of treatment. Indeed, a longer duration of diabetes was significantly associated with poor glycaemic control. The worsening of glycaemic control over time has also been reported in other studies.7,29
A longer duration of diabetes is known to be a factor associated with poor glycaemic control, possibly because of the progressive impairment of insulin secretion with time due to β-cell failure, which makes a response to diet alone or to oral agents unlikely.30,31 This illustrates how difficult it is for physicians and patients to manage T2DM and achieve glycaemic control objectives when the disease progresses and multiple medications are subsequently needed.30
Our results revealed that patients using diet and OA alone were better controlled than those treated with insulin alone or with a combination of OA agents and insulin. Our finding was in line with a study carried out in Spain reporting that the worst levels of glycaemic control were observed in patients on insulin therapy.32 This result can be explained by the fact that many diabetic patients poorly comply with treatment that includes both OA and insulin.32 Another possible explanation for this might be that patients using diet modification and OA alone are newly diagnosed, as insulin is generally prescribed either as a single-agent therapy or in combination with OA therapy for T2DM patients not achieving glycaemic control with OA agents. Furthermore, patients with HbA1c >7% were more likely to be prescribed a combination of OA agents and insulin, which may indicate that physicians are attempting multitherapy to provide better disease control.
Although insulin and OA agents have been associated with poor glycaemic control, clinical trials have shown that insulin treatment significantly reduces HbA1c levels compared with diet or OA treatment. The UK Prospective Diabetes Study showed that the addition of insulin to sulphonamides taken at maximum doses significantly improved glycaemic control without an increase in hypoglycaemic events.25 In line with this, Weiss et al.33 showed that the addition of insulin to an OA was associated with a significant decrease in HbA1c levels in T2DM patients inadequately controlled by OA.
Notably, despite the free availability of drugs in Moroccan health centres, patients do not achieve the recommended glycaemic target. One possible explanation is that the available drugs (pre-mixed insulin and metformin) have poor efficacy in achieving good glycaemic control, an assertion that is supported by Giugliano et al.34 Indeed, they reported that patients treated with pre-mixed insulin had a higher likelihood of not achieving the target HbA1c level compared with patients treated with a basal plus prandial regimen.
Free healthcare in primary healthcare centres is available to all Moroccan patients, including free access to many drugs. Metformin, sulfonylureas and insulin are distributed in primary care units around the country. However, other medications used to treat diabetes are not supported by public health. Therefore, although the Moroccan public health system has made some advances in healthcare management, they are still insufficient to reach glycaemic control targets in diabetes care. This poor glycaemic control state we observed in the population could lead to the emergence of other health complications, notably retinopathy, which could lead to blindness; nephropathy, leading to renal failure or neuropathy, leading to impotence and diabetic foot disorders.
This was the first study conducted in the Beni-Mellal Khenifra region to determine factors associated with glycaemic control among T2DM patients; it investigated a relatively large sample but has some limitations. First, because this was a cross-sectional study, the causal relationship between significant factors and high HbA1c levels could not be well established, so a longitudinal study is needed to assess the relationship over time. Second, the possible better compliance of patients willing to participate can result in bias of the measures of outcome. Third, HbA1c was not measured at the time of the interview but was extracted from the medical files. This probably indicates that the patients who entered the analysis had a regular follow-up, once again opening up the possibility of a ‘best case’ (better monitored, more compliant patients, hence with better glycaemic control compared with patients with no HbA1c measurement in the previous 3 months). Finally, the patients’ compliance and adherence to medication was not assessed and may represent potential barriers to optimal glycaemic control.
Conclusions
Our study investigated the prevalence and factors associated with glycaemic control. It adds new evidence to the fact that glycaemic control in Moroccan diabetic patients is unsatisfactory. Despite the particularities of the region and the free availability of drugs in Moroccan health centres, glycaemic control remains poor and the study’s cross-sectionally associated factors are in line with findings from other regions of Morocco. In this subgroup, the longer duration of diabetes and insulin treatment could constitute a cause leading to poor glycaemic control. However, inverse causality cannot be excluded. Considering these conditions, this subgroup of patients may need additional therapies and targeted interventions, including counselling and behavioural skills training, in order to optimize the long-term self-management of patients with diabetes. Furthermore, studies on the impact of medication factors, including regimen complexity and medication adherence, are greatly needed.
Acknowledgements:
We would like to thank all health professional staff members for their support in the data collection and all the participants involved in this study.
Authors’ contributions:
AC, KK, FC and MN contributed to the conception and design of the study. AC wrote the first draft. AC, KK, KB and AE contributed to the data collection. AC, KK, KB, AE, FC and SE contributed to the analysis and interpretation of the data. AC, FC and MN critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. AC and MN are guarantors of the paper.
Funding:
This work was supported by the National Center for Scientific and Technical Research (Priority Research Projects type B [to MN]).
Competing interests:
None declared.
Ethical approval:
Ethical approval for this study was obtained from the Health Ministry of Morocco on 3 March 2016 (reference no. 6397-3/3/2016). For the questionnaire, informed written consent was obtained from all respondents after explaining the purpose of the study, the importance of their contribution and their right to refuse participation. The data are anonymized and free of personally identifiable information.
References
- 1. Sicree R, Shaw J, Zimmet P, Heart BI. The global burden. In: Diabetes atlas, 4th edn. Brussels: International Diabetes Federation, 2009; p. 22–38. [Google Scholar]
- 2. International Diabetes Federation . Diabetes atlas, 8th ed. Brussels: International Diabetes Federation; 2017, p. 150. Available from:https://diabetesatlas.org/IDF_Diabetes_Atlas_8e. [Google Scholar]
- 3. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. [DOI] [PubMed] [Google Scholar]
- 4. Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycaemic control from 1988 to 2000 among U.S. adults diagnosed with type 2 diabetes: a preliminary report. Diabetes Care. 2004;27(1):17–20. [DOI] [PubMed] [Google Scholar]
- 5. Bi Y, Zhu D, Cheng J, et al.. The status of glycaemic control: a cross-sectional study of outpatients with type 2 diabetes mellitus across primary, secondary, and tertiary hospitals in the Jiangsu province of China. Clin Ther. 2010;32(5):973–983. [DOI] [PubMed] [Google Scholar]
- 6. Al-Elq AH. Current practice in the management of patients with type 2 diabetes mellitus in Saudi Arabia. Saudi Med J. 2009;30(12):1551–1556. [PubMed] [Google Scholar]
- 7. Khattab M, Khader YS, Al-Khawaldeh A, Ajlouni K. Factors associated with poor glycaemic control among patients with type 2 diabetes. J Diabetes Complications. 2010;24(2):84–89. [DOI] [PubMed] [Google Scholar]
- 8. Ben Abdelaziz A, Soltane I, Gaha K, et al.. Predictive factors of glycaemic control in patients with type 2 diabetes mellitus in primary health care. Rev Epidemiol Sante Publique. 2006;54(5):443–452. [DOI] [PubMed] [Google Scholar]
- 9. Farouqui A, Harti M, Nejjari C. Management of diabetes in Morocco: results of the International Diabetes Management Practices Study (IDMPS) – wave 2. Méd Malad Métab. 2010;4(6):704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chadli A, El Aziz S, El Ansari N, et al.. Management of diabetes in Morocco: results of the International Diabetes Management Practices Study (IDMPS) – wave 5. Ther Adv Endocrinol Metab. 2016;7(3):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng LJ, Wang W, Lim ST, Wu VX. Factors associated with glycaemic control in patients with diabetes mellitus: a systematic literature review. J Clin Nurs. 2019;28(9–10):1433–1450. [DOI] [PubMed] [Google Scholar]
- 12. Haut Commissariat au Plan . Annuaire statistique de la Région Béni Mellal- Khénifra 2016. p. 279. Available from:https://www.hcp.ma/region-drta/Annuaire-statistique-de-la-Region-Beni-Mellal-Khenifra-2016_a79.html.
- 13. World Health Organization . Obesity: preventing and managing the global epidemic. Report No: 894. Geneva: World Health Organization; 2000, p. 268. Available from:http://www.who.int/entity/nutrition/publications/obesity/WHO_TRS_894/en/index.html. [PubMed] [Google Scholar]
- 14. American Diabetes Association . Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S48–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calvert MJ, McManus RJ, Freemantle N. Management of type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract. 2007;57(539):455–460. [PMC free article] [PubMed] [Google Scholar]
- 16. Adham M, Froelicher ES, Batieha A, Ajlouni K. Glycaemic control and its associated factors in type 2 diabetes patients in Amman, Jordan. East Mediterr Health J. 2010;16(7):732–739. [PubMed] [Google Scholar]
- 17. Harris SB, Ekoé J-M, Zdanowicz Y, Webster-Bogaert S. Glycaemic control and morbidity in the Canadian primary care setting (results of the diabetes in Canada evaluation study). Diabetes Res Clin Pract. 2005;70(1):90–97. [DOI] [PubMed] [Google Scholar]
- 18. Rothenbacher D, Rüter G, Saam S, Brenner H. Younger patients with type 2 diabetes need better glycaemic control: results of a community-based study describing factors associated with a high HbA1c value. Br J Gen Pract. 2003;53(490):389–391. [PMC free article] [PubMed] [Google Scholar]
- 19. Errajraji A, El-Anssari N, Ouhdouch F. Use of medicinal plants for type 2 diabetes treatment, in Morocco (Marrakech). Méd Malad Métab. 2010;4(3):301–304. [Google Scholar]
- 20. Belhadj M, Malek R, Boudiba A, et al.. DiabCare Algérie: epidemiology, costs and organization of care. Méd Malad Métab. 2012;5(4S1):88–92. [Google Scholar]
- 21. Al-Sultan F, Al-Zanki N. Clinical epidemiology of type 2 diabetes mellitus in Kuwait. Kuwait Med J. 2005;37(2):98–104. [Google Scholar]
- 22. Reisig V, Reitmeir P, Döring A, et al.. Social inequalities and outcomes in type 2 diabetes in the German region of Augsburg. A cross-sectional survey. Int J Public Health. 2007;52(3):158–165. [DOI] [PubMed] [Google Scholar]
- 23. Arai K, Hirao K, Matsuba I, et al.. The status of glycaemic control by general practitioners and specialists for diabetes in Japan: a cross-sectional survey of 15,652 patients with diabetes mellitus. Diabetes Res Clin Pract. 2009;83(3):397–401. [DOI] [PubMed] [Google Scholar]
- 24. Anari R, Amani R, Veissi M. Obesity and poor glycaemic control in patients with type 2 diabetes. Int J Res Med Sci. 2016;4(2):584–588. [Google Scholar]
- 25. Bae JP, Lage MJ, Mo D, Nelson DR, Hoogwerf BJ. Obesity and glycaemic control in patients with diabetes mellitus: analysis of physician electronic health records in the US from 2009–2011. J Diabetes Complicat. 2016;30(2):212–220. [DOI] [PubMed] [Google Scholar]
- 26. Ercan A, Kiziltan G. Obesity-related abnormal eating behaviors in type 2 diabetic patients. Pak J Med Sci. 2013;29(6):1323–1328. [PMC free article] [PubMed] [Google Scholar]
- 27. Chan WB, Tong PCY, Chow CC, et al.. The associations of body mass index, C-peptide and metabolic status in Chinese type 2 diabetic patients. Diabet Med. 2004;21(4):349–353. [DOI] [PubMed] [Google Scholar]
- 28. Mansour AA, Al-Maliky A, Kasem B. Determinants of loss of glycaemic control in patients with type 1 diabetes mellitus. Prospective cohort study from Iraq. J Diabetes Res Clin Metab. 2013;2:21. [Google Scholar]
- 29. Verma M, Paneri S, Badi P, Raman PG. Effect of increasing duration of diabetes mellitus type 2 on glycated haemoglobin and insulin sensitivity. Indian J Clin Biochem. 2006;21(1):142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) . UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 31. Leibowitz G, Kaiser N, Cerasi E. β-Cell failure in type 2 diabetes. J Diabetes Investig. 2011;(2, 2):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yurgin NR, Boye KS, Dilla T, Suriñach NL, Llach XB. Physician and patient management of type 2 diabetes and factors related to glycaemic control in Spain. Patient Prefer Adherence. 2008;2:87–95. [PMC free article] [PubMed] [Google Scholar]
- 33. Weiss SR, Cheng S-L, Kourides IA et al.. Inhaled insulin provides improved glycaemic control in patients with type 2 diabetes mellitus inadequately controlled with oral agents: a randomized controlled trial. Arch Intern Med. 2003;163(19):2277–2282. [DOI] [PubMed] [Google Scholar]
- 34. Giugliano D, Maiorino MI, Bellastella G, et al.. Efficacy of insulin analogs in achieving the haemoglobin A1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Care. 2011;34(2):510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]


