Abstract
Background
Burundi has one of the highest rates of malnutrition in the world, particularly chronic malnutrition, which affects 55% of all children <5 y of age. Although it rolled out a national treatment programme to combat all forms of malnutrition, enrolment of children remains difficult. In this study, we use observational data from two screening approaches to assess the effectiveness in detection and enrolment into treatment.
Methods
Individual data from each screening approach was classified as either acutely malnourished or normal and either chronically malnourished or normal using a cut-off z-score between −2 and 2.
Results
While the Global Acute Malnutrition rate for the community-based mass screening was 8.3% (95% CI 5.6 to 11), with 8% enrolled in treatment, that of clinic-based systematic screening was 14.1% (95% CI 12.2 to 16.1), 98% of which were enrolled in treatment. Clinic systematic screening was 1.82 times (OR, 95% CI 1.26 to 2.62, p<0.001) and 1.35 times (95% CI 1.09 to 1.68, p=0.06) more likely to detect acute and chronic malnutrition, respectively, than community-based mass screening.
Conclusions
Although different mechanisms are relevant to proactively detect cases, strengthening the health system to systematically screen children could yield the best results, as it remains the primary contact for the sicker population, who may be at risk of increased infection as a result of underlying malnutrition.
Keywords: Burundi, child malnutrition, health systems, proactive screening, public health, sub-Saharan Africa
Introduction
In Africa today, malnutrition remains one of the leading public health threats, especially among children <5 y of age. In 2016, an estimated 45% of mortality in children <5 y of age was attributed to malnutrition-related factors.1 This amounts to 3.1 million malnutrition-related deaths annually.1 Of the 155 million children stunted and 52 million wasted globally, Africa accounts for 38.1% and 26.9%, respectively and while eastern Africa has made important strides to combat acute malnutrition (wasting), it still has the highest rates of chronic malnutrition (stunting) in Africa.2
Burundi, a small and densely populated low-income country in East Africa, has one of the highest rates of all forms of malnutrition in the world. According to the 2016–2017 Burundi Demographic and Health Survey,3 55.9% of all children <5 y of age are chronically malnourished, the highest in the world, with 5% experiencing different forms of acute malnutrition. In spite of 46.7% of its land area classified as arable,4 Burundi ranked number one in the 2014 Global Hunger Index, with a place in the extremely alarming category.5 The 2018 Global Food Security Report ranks Burundi ninth among countries with food security crises in the world, placing 50% of the population in the chronically food insecure category. This implies that total food production in a year will only cover 55 d per person.6 With a gross domestic product (GDP) of just US$3.478 billion, treatment for malnutrition directly costs the Burundian economy US$9.5 per capita annually, making it one of the highest in the world.7
In response to the devastating impact of malnutrition, on 23 February 2013, the Government of Burundi joined the global Scaling Up Nutrition initiative, with the aim of combating acute and chronic malnutrition. In 2014, it institutionalised the multisectoral food and nutritional platform as a way of coordinating efforts and resources towards a common results framework.8 At the end of 2014, a technical nutrition strategic plan had been drafted, with most of its content prioritised under the 2016–2020 National Health Policy.
Given the finite resources vis-à-vis the burgeoning rates of malnutrition in all provinces, Burundi has to invest in new ways of proactively detecting and treating malnutrition cases with integrated and innovative interventions.7 Proactive malnutrition detection, a terminology defined in this study as routinely screening children to detect early possible malnourished cases along with appropriate treatment, alleviates the impact of complications from severe malnutrition. The Burundian National Health Policy supports proactive screening and recommends that all children <5 y of age should be screened systematically for malnutrition during visits to facilities.9 Inclusive in the Integrated Management of Childhood Illness guidelines are different approaches for proactive community and clinical systematic screening for all children.10 In spite of this recommendation, studies have increasingly established non-adherence of these protocols, both at the community level and in health facilities. For example, a recent study found that of all children <5 y of age who received care in Burundian health facilities, only 38.6% (95% CI 31.0 to 46.9) were weighed, only 26.2% (95% CI 19.6 to 34.1) had their heights checked and only 15.9% (95% CI 10.7 to 22.8) were properly classified for malnutrition according to the weight-for-height (WFH) parameters.11
Disregard for this protocol has serious implications for early detection of malnourished children and also missed opportunities for prompt treatment. For this reason, Village Health Works (VHW), a grassroots Burundian–American organisation working to provide quality, compassionate and dignified healthcare, has invested in different models of proactive case detection for malnutrition both in the community and in the clinic. In this study we compare detection rates of community-based mass screening and clinic-based systematic screening approaches at VHW and argue that incorporating different models of proactive screening, especially in health facilities, results in early case detection and improved treatment outcomes among identified cases. We also create a logistic regression model to assess the effectiveness of these proactive case detection models.
Methods
Study area
The study was conducted in the VHW catchment area in the southwestern part of Burundi. The activities of VHW extend over 18 collines in Bururi and Rumonge provinces, as shown in the area marked in pink in Figure 1. Seven collines are located in Vyanda commune (Kabwayi, Mirango, Migera, Mushishi, Kirungu, Kigutu and Karirimvya) and 11 in Rumonge commune (Nyakuguma, Mayengo, Kanenge, Gashasha, Cabara, Busebwa, Mugara, Gatete, Mutambara, Gitwe and Karagara).
Figure 1.
Map of Study Area (marked in pink) in Rumonge and Bururi provinces of Burundi
Approaches to proactive malnutrition screening
Between March and September 2018, VHW implemented two primary models of proactive case detection: clinic-based systematic screening and community-based mass screening during nutrition health promotion activities. These models are further described below.
Clinic-based systematic screening
In January 2018, a policy of systematic screening for all forms of malnutrition among children <5 y of age was initiated at the VHW clinic. According to the clinic screening policy, all patients have vitals checked on arrival at the clinic. For children <5 y of age, parameters for malnutrition screening are included in these vital signs assessments. Weight, age and height are measured and a corresponding z-score is determined immediately using the WHO growth standards.9 Children with a WFH z-Score<−2 (SD <−2) are supplemented and treated according to the national treatment standards; specifically, severely uncomplicated malnourished children are treated with a course of oral antibiotics and 5000 IU of vitamin A daily along with ready-to-use therapeutic food. Moderately malnourished children are given fortified blended foods containing cereals mixed with oil, sugar and quality protein additives.9 Those with complicated severe acute malnutrition and/or other nutrition-related complications are hospitalised for further treatment and monitoring. When a child recovers, they are linked to community health workers (CHWs), who conduct routine visits, monitor growth and make referrals when indicated.
Main factors that prevented the full realisation of this policy included emergency cases that demanded urgent care and failure to screen children with visibly good nutritional status. Nonetheless, between March and September, when this study was conducted, of all the children screened, 1370 were included in the study, representing 82% of all children <5 y of age who visited the health facility. Originally 89% of all children <5 y of age via the systematic strategy were screened; however, after applying data quality checks in the Emergency Nutrition Assessment (ENA), cases with wrong height, weight, age or z-score >−5 were excluded from the study.
Community-based mass screening
During the same period (March–September 2018), VHW conducted three mass health promotion exercises in communities. During these activities, 445 children were screened for malnutrition. The same methods for malnutrition detection in the clinic were employed in the community screening. Children whose WFH was <−2 (<−2 SD) were issued an appointment date and referred to the VHW clinic for further assessment and treatment. To ensure that they adhered to the appointment schedule, they were linked to CHWs, who facilitated follow-up on their appointment date. On arrival at the clinic, children were given the appropriate treatment and complicated severe acute cases or cases with nutrition-related medical complications were hospitalised, supplemented, treated and monitored according to global WHO standards, which specify a combination of antibiotics, vitamin A supplementation (5000 IU daily) and ready-to-use therapeutic food during the rehabilitation phase.10 Upon treatment completion, cases are relinked with CHWs, who conduct periodic visits and refer children with relapses and complications (Figure 2). In addition, CHWs ensure the children adhere to their appointment dates and treatment regimen. Uncomplicated severely acute malnourished children are treated as outpatients and also linked with CHWs, who then monitor treatment and report outcomes. Likewise, moderately acute malnourished cases are treated according to the guidelines described in the clinic-based approach.
Figure 2.
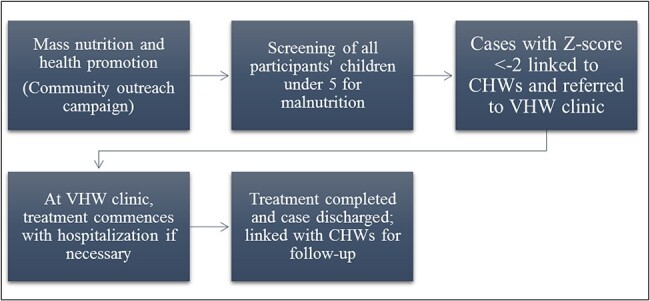
Process chart of the community-based proactive malnutrition case detection approach
Outcome variables under investigation
Two main outcome indicators were used for measuring acute and chronic malnutrition. Consistent with the WHO’s growth standards,12 acute malnutrition (wasting) was mainly classified under the indicator global acute malnutrition (GAM). The WHO defines GAM as children with a corresponding WFH z-score <−2 SD compared with the normal growth standards. The percentage of detected GAM enrolled in treatment was considered as the next-level outcome.
For chronic malnutrition, height-for-age (HFA; stunting) was used as a parameter to determine the stunting status of children <5 y of age. Global chronic malnutrition (GCM) was defined as children with a corresponding HFA z-score <−2 SD compared with the normal growth standards.
Data collection, processing and analysis
Community data were collected manually during mass screenings using a paper form. Data were then entered into Excel (Microsoft, Redmond, WA, USA) and imported into the WHO Anthro software13 to calculate the acute and chronic malnutrition rates. Routine clinic data were collected during vital signs measurements at triage. Health professionals at the triage area then entered data into an electronic database–OpenClinic.14 Data from March to September 2018 were queried and exported to Excel. Data were cleaned and duplicates were deleted. Final data were then transferred into the WHO Anthro software to determine the rates for acute and chronic malnutrition for both the community data and the routine clinical dataset.
To create a statistical model, the two datasets were imported into the ENA software15 to determine individual z-scores for all cases. For both acute and chronic malnutrition, cases were classified as ‘normal’ or ‘stunted’ (for chronic malnutrition) or ‘wasted’ (for acute malnutrition). These results were then exported into Stata version 13 (StataCorp, College Station, TX, USA). Binary and multinomial logistic regression were conducted to assess the crude odds and adjusted odds of detecting acute and chronic malnutrition via each of the two models of screenings. The significance of relationships was pegged at the 95% level and CIs were calculated for results of the outcome variables under consideration.
Results
Description of screening populations
In the clinic, as shown in Table 1, 1370 children <5 y of age were routinely screened between March and September. Among the age groups, the highest number of screenings was for those between 12 and 23 mo, while the lowest was for those between 48 and 60 mo. The mass screening recorded 445 children in attendance. Among the age groups, the highest number of screenings was for those between the ages of 48 and 60 mo for both boys (n=78) and girls (n=71).
Table 1.
Numbers of children <5 y of age screened at the clinic aggregated by sex and age
| Age group (mo) | Clinic screening, n | Mass screening, n | ||
|---|---|---|---|---|
| Boys | Girls | Boys | Girls | |
| 0–5 | 120 | 111 | 0 | 0 |
| 6–11 | 95 | 111 | 19 | 20 |
| 12–23 | 189 | 202 | 48 | 47 |
| 24–35 | 113 | 110 | 40 | 39 |
| 36–47 | 83 | 84 | 36 | 47 |
| 48–60 | 79 | 73 | 78 | 71 |
| Total | 679 | 691 | 221 | 224 |
Case detection for acute malnutrition via clinic-based systematic screening and community-based mass screening
Table 2 shows that overall, routine screening in the clinic detected a GAM rate of 14.1% (95% CI 12.2 to 16.1) compared with 8.3% (95% CI 5.6 to 11) for community-based mass screening. χ2 analysis showed a significant association between clinic-based systematic screening and higher GAM rates. Through the implementation of the clinical model at VHW, 98% of all cases detected were enrolled in treatment, with 87.7% successfully treated, reducing the GAM rate to just 1.6% among this cohort after 3 mo. This compared with only 8% of children detected in the community who actually visited the health facility and received treatment in an appropriate health facility.
Table 2.
Comparative GAM rate between clinic systematic screening and community-based mass screening
| Screening | GAM, % (95% CI) | p-Value |
|---|---|---|
| Clinic systematic | 14.1 (12.2 to 16.1) | 0.001 |
| Community-based mass | 8.3 (5.6 to 11) |
Case detection for chronic malnutrition via clinic-based systematic screening and community-based mass screening
Consistent with GAM rates, the clinic systematic screening detected more cases for enrolment into treatment (53.9% [95 CI 51.2 to 56.7]) chronically malnourished compared with community-based mass screening. Again, detection of GCM rates was significantly associated with clinic systematic screening (p=0.006) Table 3.
Table 3.
Comparative GCM rate between clinic systematic screening and community-based mass screening
| Screening | GCM, % (95% CI) | p-Value |
|---|---|---|
| Clinic systematic | 53.9 (51.2 to 56.7) | 0.006 |
| Community-based mass | 41.4 (36.6 to 46.2) |
Comparison of the two malnutrition screening models for acute malnutrition
Detection of acute malnutrition cases was higher via the clinic-based screening. While the GAM rate for the community-based mass screening was 8.3%, that of the clinic-based systematic screening was 14.1%. Logistic regression shows that the clinic-based systematic screening model was 1.82 times more likely to detect acutely malnourished cases than community-based mass screening (95% CI 1.26 to 2.62, p<0.001). After adjusting for the sex and age of the children in a multivariable logistic model, the odds of detecting acute malnutrition was significantly associated with clinic systematic screening (adjusted OR 1.9 [95% CI 1.30 to 2.80], p<0.001). Figure 3 confirms the higher deviation compared with the WHO growth standards in the cohort of children detected in the clinic vs those detected in community-based mass screening.
Figure 3.
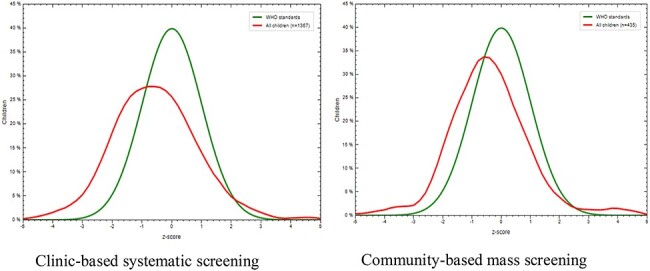
Graph showing distribution of GAM results in comparison to WHO growth standards
Comparison of the two malnutrition screening models for chronic malnutrition
Similarly, the rates of chronic malnutrition cases were higher in the clinic compared with the mass community screening. Compared with community-based screening, where the GCM rate was 41.4 (95% CI 36.6 to 46.2), the clinic recorded a GCM rate of 53.9 (95% CI 51.2 to 56.7).
As confirmed in the distribution graphs in Figure 4, the odds of detecting a chronically malnourished case in the clinic was 1.35 times greater than in the community mass screenings (95% CI 1.09 to 1.68, p=0.06). In a multivariable logistic regression after adjusting for sex and age, the adjusted odds of detecting chronic malnutrition were 2.2 times (95% CI 1.70 to 2.76, p<0.001) greater than in clinic systematic screening.
Figure 4.
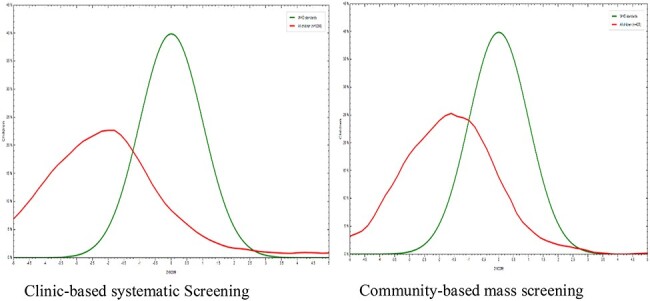
Graph showing distribution of GCM results in comparison to WHO growth standards
Discussion
Regarding the 2030 timeline for the achievement of the Sustainable Development Goals, this study assesses two models for proactively screening children <5 y of age for acute and chronic malnutrition. Through the implementation of these two models, we recorded a GAM rate of 12.7% (95% CI 11.2 to 14.3) in a total of 1815 children screened and treated. In both screening models we also found a total GCM rate of 46.5% (95% CI 44.2 to 48.8).
Proactively screening children for all forms of malnutrition has been recognised as an effective mechanism for early case detection and further treatment.16–23 In Cambodia, proactive screening by the UNICEF of all children in Phnom Penh’s urban poor communities showed a prevalence of 11.2% acute malnourished cases, who were expressly referred to secondary-level care.21 Similarly, in Malawi, as a key strategy to combat complications of malnutrition, UNICEF expanded its proactive screening programme to 90% of all health facilities, which identified >80% of all malnutrition cases detected nationally.20 The WHO endorses proactive screening of children for malnutrition and recommends different techniques to screen and treat malnourished children.10 At VHW, a ‘screen–detect–treat–monitor’ model uses screening and treatment methods in accordance with national standards, which are also consistent with the WHO guidelines.10 Malnourished cases were subsequently tracked via the CHW programme and received ongoing support along with preventive health promotion from nutrition promoters (community health extension workers with a sole focus on nutrition).
Generally, comparing the two models of proactively screening children, which is most effective in detecting and expressly treating identified cases? In this study we found that systematically screening children during clinic attendance was 1.9 times more effective in detecting and treating acutely malnourished children than mass community screening. Consistent with this finding, in a Canadian paediatric tertiary hospital, systematic screening of all children <5 y of age resulted in the detection of 6.9% (95% CI 3 to 13) with acute malnutrition and 13.4% (95% CI 10 to 18) with chronic malnutrition cases.24 Similarly, in rural Kenya, the implementation of systematic screening resulted in a GAM rate of 19.8% who received treatment.22
While community-based nutrition programmes are relevant in effectively combating all forms of malnutrition, attendance of sick children <5 y of age at health facilities presents a window of opportunity to systematically screen and treat malnutrition, which could be the main reason for recurrent health facility attendance. Malnutrition results in weakened immunity, which contributes to repeated infections and increased risk of child mortality.25–28 Therefore, incorporating rigorous systems to screen and treat malnutrition will be an effective strategy to mitigate the high infant and child mortality in Burundi and sub-Saharan Africa as a whole. Even though health facility-based systematic screening is generally effective, inaccuracies of screening tools may render this strategy less effective, leading to missed opportunities. Binagwaho et al.29 previously observed that in Rwanda, the use of inaccurate and outdated malnutrition screening tools resulted in a high number of undetected cases, potentially contributing to preventable morbidity and mortality among children <5 y of age. The authors further emphasise the need to couple accurate tools and protocols with robust systems, protocols and training.
While several studies support the effectiveness of mass screening,30,31 there are often barriers that prevent access to care for community-identified malnourished patients after they are referred to health facilities for treatment. This raises questions about the overall effectiveness of this strategy. Other studies have also questioned the effectiveness of community-based screening and treatment, attributing non-attendance at health facilities to transportation bottlenecks.32,33 The emergence of new approaches to community-based malnutrition management has seen some success in the management and treatment of acute malnutrition. However, lack of connections between these community-based initiatives and the formal health system may thwart the sustainability of these efforts.34 For example, in Bangladesh, the concentration of efforts on community management of acute malnutrition without concurrent health system strengthening, such as training health staff on acute malnutrition treatment and addressing the lack of referral systems from CHWs, culminated in poor management of severe acute malnutrition cases and also a high number of missed cases.35 Kouam et al.35 argue that since the WHO recommends that severely acute malnourished children with complications should be treated in health facilities, strengthening facilities to deliver these services results in important reductions in infant and child mortality rates. From our observations in health facilities, we realise that although the national policy supports systematic screening for all children, it was poorly conducted due to outmoded and inaccurate screening equipment, and children were only screened when the health worker suspected severe malnutrition. In fact, in most cases when malnutrition was detected among children (unless in severe cases, when devastating malnutrition symptoms were clinically visible), it was not considered as part of the diagnosis and therefore missed treatment.
Our study has limitations that must be acknowledged. First, the community-based mass screenings were delivered at a central point in a community during health promotion activities. This may have resulted in optimal attendance of people living close by, with a limited proportion of those living far from the central point. These farther areas could have experienced either better or worse nutritional outcomes, which could have underestimated or overestimated the community-based GAM and GCM rates. On the one hand, it may be that attendance signalled mobility and relative health, while on the other, it is conceivable that parents used the opportunity of a mass campaign to bring sicker children to be seen by health professionals. In addition, patients attending the hospital represent a generally sicker population and, as such, children could have been acutely malnourished from other causes instead of or in addition to primary nutritional deficiencies. This could have overestimated the GAM rate. To mitigate this effect, we also calculated the GCM, which is a more direct consequence of long-term protracted nutritional deficiencies.
Conclusions
Early detection and treatment of malnutrition among children is an important step in mitigating the consequences of malnutrition. The health system remains the primary contact to deliver interventions for children <5 y of age. This study demonstrates that systematic screening at health facilities is an effective approach to case identification. In the context of Burundi, given the high burden of all forms of malnutrition, strengthening the health system to deliver ‘detect-and-treat’ malnutrition interventions could be an important step in improving overall health outcomes among children <5 y of age.
While it is still relevant to implement preventive multisectoral nutrition interventions, increased efforts should be directed towards proactively detecting, treating and monitoring ‘at-risk’ children. The health system presents a window of opportunity to deliver this cost-effective intervention. The global successes of the human immunodeficiency virus ‘test-and-treat’ initiative further justifies the approach of combining community initiatives with clinic-based case detection and treatment. The overall potential of the health system to deliver this service effectively will depend on training, the consistent availability of screening equipment and treatment commodities throughout all facilities in the country and the engagement of competent health workers who are able to administer effective and compassionate care. Government and donor investment in health system readiness for malnutrition initiatives could be that great leap in protecting malnourished children who are most often at the highest risk of mortality.
Acknowledgements
The authors are thankful to all the clinical and administrative staff of Village Health Works, Kigutu, Burundi who are involved in daily screening of children either in the health facility or communities. The anonymised primary dataset is available upon request from the corresponding author.
Authors’ contributions
ENO conceptualised and designed the study. ENO, GG, HM, SH and J-BM cleaned and analysed the data. ENO wrote the first draft of the manuscript and CC provided technical support and wrote sections of subsequent versions of the manuscript. CC, TM, GG, HM and SH provided around of reviews. TM supervised the entire study and provided technical guidance at every stage from conceptualisation to manuscript writing and wrote sections of the discussion. All authors read and approved the final version of this manuscript. ENO, CC and TM are the guarantors of this paper.
Funding
None.
Competing interests
None declared.
Ethical approval
Data were observational from programme activities of VHW and, as such, ethical clearance was granted internally by the organisation’s leadership. In addition, the study was approved by the Burundi National Ethics Committee, represented by the health representatives in the Bururi and Rumonge provinces (VHW catchment provinces). Careful steps were taken to secure the children’s identity during the analysis. The names of the children screened during the study period and included in the analysis were removed and replaced with unique codes before analysis.
References
- 1. Black R, Victora C, Walker S, et al. . Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Nutrition. Joint child malnutrition estimates 2018 edition. http://apps.who.int/gho/tableau-public/tpc-frame.jsp?id=402 (accessed 17 October 2018).
- 3. Ministère à la Présidence chargé de la Bonne Gouvernance et du Plan (MPBGP), Ministère de la Santé Publique et de la Lutte contre le Sida (MSPLS), Institut de Statistiques et d'Études Économiques du Burundi (ISTEEBU), ICF. Troisième Enquête Démographique et de Santé. Bujumbura: ISTEEBU, MSPLS and ICF; 2017. [Google Scholar]
- 4. Kaboneka, S. Soil fertility status and challenges in Burundi: an overview. In EGU General Assembly Conference Abstracts, vol. 17. 2015. [Google Scholar]
- 5. Grebmer K, Saltzman A, Birol E, et al. . Global hunger index: the challenge of hidden hunger. Washington, DC: International Food Policy Research Institute; 2014, p. 2014. [Google Scholar]
- 6. World Food Program . 2018. Global report on food. Available from:https://www.wfp.org/content/global-report-food-crises-2018 (accessed 23 October 2018).
- 7. United Nations Children’s Fund . A strike against chronic malnutrition in Burundi. Available from:https://www.unicef.org/nutrition/burundi_69651.html (accessed 18 October 2018).
- 8. Scaling Up Nutrition. Burundi . Available from:https://scalingupnutrition.org/sun-countries/burundi/ (accessed 23 October 2018).
- 9. MSPLS, 2010. Protocole national de prise en charge intégrée de la malnutrition aigìe globale.
- 10. World Health Organization . Integrated management of childhood illness. Geneva: World Health Organization; 1997. [Google Scholar]
- 11. Nimpagaritse M, Korachais C, Nsengiyumva G, Macq J, Meessen B. Addressing malnutrition among children in routine care: how is the integrated management of childhood illnesses strategy implemented at health centre level in Burundi? BMC Nutr. 2019;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO Multicentre Growth Reference Study Group, de Onis M.. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;95:76–85. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization . WHO Anthro for personal computers, version 3.2.2: software for assessing growth and development of the world’s children. Geneva: World Health Organization; 2010. [Google Scholar]
- 14. Cavelaars M, Rousseau J, Parlayan C, et al. . OpenClinica. J Clin Bioinforma. 2015;5(Suppl 1):S2. [Google Scholar]
- 15. Erhardt J, Seaman J, Bilukha O, and Golden M. Software for Emergency Nutrition Assessment (ENA SMART). 2011.
- 16. World Health Organization . Malnutrition. Available from: https://www.who.int/maternal_child_adolescent/topics/child/malnutrition/en/(accessed 15 November 2018).
- 17. Durá-Travé T, San Martin-García I, Gallinas-Victoriano F, Vaquero Iñigo I, González-Benavides A. Prevalence of malnutrition in hospitalised children: retrospective study in a Spanish tertiary-level hospital. JRSM Open. 2016;7(9):205427041664388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Touzet S, Duclos A, Denis A, et al. . Multifaceted intervention to enhance the screening and care of hospitalised malnourished children: study protocol for the PREDIRE cluster randomized controlled trial. BMC Health Serv Res. 2013;13:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. United Nations Children’s Fund . UNICEF and WFP launch mass nutrition screening as hunger threatens lives of children in South Sudan. Available from:https://www.unicef.org/media/media_85970.html (accessed 15 November 2018).
- 20. United Nations Children’s Fund . UNICEF fears hunger in Malawi, embarks on mass screening for malnutrition across country. Available from:https://www.unicef.org/media/media_86549.html (accessed 15 November 2018).
- 21. United Nations Children’s Fund . Screening to treat child malnutrition in Cambodia’s urban poor communities. Available from: http://unicefcambodia.blogspot.com/2015/01/screening-to-treat-child-malnutrition.html (accessed 15 November 2018).
- 22. Bejon P, Mohammed S, Mwangi I, et al. . Fraction of all hospital admissions and deaths attributable to malnutrition among children in rural Kenya. Am J Clin Nutr. 2008;88(6):1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barker L, Gout B, Crowe T. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8(2):514–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baxter JAB, Al-Madhaki FI, Zlotkin SH. Prevalence of malnutrition at the time of admission among patients admitted to a Canadian tertiary-care paediatric hospital. Paediatr Child Health. 2014;19(8):413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beser O, Cokugras F, Erkan T, et al. . Evaluation of malnutrition development risk in hospitalized children. Nutrition. 2018;48:40–47. [DOI] [PubMed] [Google Scholar]
- 26. Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. Monogr Ser World Health Org. 1968;57:3–329. [PubMed] [Google Scholar]
- 27. Rytter M, Kolte L, Briend A, Friis H, Christensen V. The immune system in children with malnutrition–a systematic review. PLoS One. 2014;9(8):e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bourke C, Berkley J, Prendergast A. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016;37(6):386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Binagwaho A, Agbonyitor M, Rukundo A, et al. . Underdiagnosis of malnutrition in infants and young children in Rwanda: implications for attainment of the Millennium Development Goal to end poverty and hunger. Int J Equity Health. 2011;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Puett C, Sadler K, Alderman H, Coates J, Fiedler J, Myatt M. Cost-effectiveness of the community-based management of severe acute malnutrition by community health workers in southern Bangladesh. Health Policy Plan. 2012;28(4):386–399. [DOI] [PubMed] [Google Scholar]
- 31. Wilford R, Golden K, Walker DG. Cost-effectiveness of community-based management of acute malnutrition in Malawi. Health Policy Plan. 2011;27(2):127–137. [DOI] [PubMed] [Google Scholar]
- 32. Ndlovu G, Sokhela D, Sibiya M. Experiences of community caregivers in the assessment of malnutrition using mid-upper arm circumference measurement in children under 5 years old. Afr J Prim Health Care Fam Med. 2018;10(1):a1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park S, Kim S, Ouma C, Loha M, Wierzba T, Beck N. Community management of acute malnutrition in the developing world. Pediatr Gastroenterol Hepatol Nutr. 2012;15(4):210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ireen S, Raihan M, Choudhury N, et al. . Challenges and opportunities of integration of community based management of acute malnutrition into the government health system in Bangladesh: a qualitative study. BMC Health Serv Res. 2018;18(1):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kouam CE, Delisle H, Ebbing HJ, et al. . Perspectives for integration into the local health system of community-based management of acute malnutrition in children under 5 years: a qualitative study in Bangladesh. Nutr J. 2014;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]


