Abstract
Background
Smoking at the time of surgical treatment for lung cancer increases the risk for perioperative morbidity and mortality. The prevalence of persistent smoking in the postoperative period and its association with long-term oncologic outcomes are poorly described.
Research Question
What is the relationship between persistent smoking and long-term outcomes in early-stage lung cancer after surgical treatment?
Study Design and Methods
We performed a retrospective cohort study using a uniquely compiled Veterans Health Administration dataset of patients with clinical stage I non-small cell lung cancer (NSCLC) undergoing surgical treatment between 2006 and 2016. We defined persistent smoking as individuals who continued smoking 1 year after surgery and characterized the relationship between persistent smoking and disease-free survival and overall survival.
Results
This study included 7,489 patients undergoing surgical treatment for clinical stage I NSCLC. Of 4,562 patients (60.9%) who were smoking at the time of surgery, 2,648 patients (58.0%) continued to smoke at 1 year after surgery. Among 2,927 patients (39.1%) who were not smoking at the time of surgical treatment, 573 (19.6%) relapsed and were smoking at 1 year after surgery. Persistent smoking at 1 year after surgery was associated with significantly shorter overall survival (adjusted hazard ration [aHR], 1.291; 95% CI, 1.197-1.392; P < .001). However, persistent smoking was not associated with inferior disease-free survival (aHR, 0.989; 95% CI, 0.884-1.106; P = .84).
Interpretation
Persistent smoking after surgery for stage I NSCLC is common and is associated with inferior overall survival. Providers should continue to assess smoking habits in the postoperative period given its disproportionate impact on long-term outcomes after potentially curative treatment for early-stage lung cancer.
Key Words: cigarettes, non-small cell lung cancer, smoking, thoracic surgery
Abbreviations: aOR, adjusted OR; CDW, Corporate Data Warehouse; HF, Health Factors; ICD, International Classification of Diseases; NSCLC, non-small cell lung cancer; VHA, Veterans Health Administration
FOR EDITORIAL COMMENT, SEE PAGE 1442
Take-home Points.
Study Question: What is the relationship between persistent smoking after surgery and long-term outcomes after non-small cell lung cancer (NSCLC) resection?
Results: Among 7,489 veterans undergoing surgical treatment for clinical stage I NSCLC, persistent smoking was observed in 3,221 patients (43.0%) at 1 year after surgery. Persistent smoking was associated with significantly shorter overall survival.
Interpretation: Persistent smoking is common among veterans with early-stage lung cancer after curative-intent surgery and is associated with inferior overall survival.
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related mortality in the United States, including among veterans.1,2 Approximately 90% of newly diagnosed cases of lung cancer are attributable to cigarette smoking.3,4 The gold standard treatment for early-stage NSCLC is surgery, with a subsequent 5-year survival ranging from 60% to 80%.5 Continued smoking at the time of surgery is a well-established risk factor for postoperative complications and mortality.6,7 Therefore, most professional guidelines recommend aggressive smoking cessation therapy for patients with newly diagnosed lung cancer who smoke.8, 9, 10 Perioperative smoking cessation interventions are especially important in the veteran population, which has a disproportionately higher prevalence of smoking.11
Despite the large body of evidence outlining the relationship between smoking and treatment-related adverse events in lung cancer, most cancer registries and surgical databases are static, assessing smoking status at a single preoperative time point, if at all.12, 13, 14 Consequently, smoking habits in the postoperative period and during extended follow-up are poorly understood. For example, a small study found that nearly half of patients with early-stage lung cancer who smoke at diagnosis continue to smoke within a year after surgery.15 Furthermore, because long-term smoking habits are not captured routinely in large cancer databases, the association between persistent postoperative smoking habits and oncologic outcomes like disease-free survival and overall survival is poorly understood and is limited to small studies.16
To address this gap in knowledge, we performed a retrospective cohort study using a cohort of patients from the Veterans Health Administration (VHA) with clinical stage I NSCLC. Our objectives were to describe the prevalence of persistent smoking 1 year after surgery using the granular electronic health records maintained by the VHA and to examine the association between persistent postoperative smoking and long-term oncologic outcomes. We hypothesized that persistent smoking after surgery would be associated with inferior overall and disease-free survival.
Study Design and Methods
Study Population
We performed this retrospective cohort study of adults with clinical stage I NSCLC undergoing surgical treatment in the VHA from 2006 through 2016. The VHA Informatics and Computing Infrastructure system, which contains clinical and administrative data from multiple platforms in the Corporate Data Warehouse (CDW), was used to generate our initial dataset. NSCLC cases were identified using International Classification of Diseases (ICD) for Oncology, Third Edition codes. Patients were verified as having undergone surgery using ICD, Ninth and Tenth Revisions, procedure codes and Current Procedural Terminology codes. To ensure greater accuracy and validity, we refined the study cohort via additional data abstraction performed by a dedicated team of researchers over a period of nearly 24 months. Exclusion criteria were patients younger than 18 years, patients receiving neoadjuvant chemotherapy or radiotherapy, patients undergoing surgery for recurrent disease, and patients with unavailable smoking data 1 year after surgery. We also excluded individuals who never smoked from the present analysis because this population of patients was exceedingly small in the cohort (< 5%), precluding clinically meaningful analysis. Additionally, given the nature of our analysis, we excluded patients who died within 30 days of surgery as well as patients whose operations led to a diagnosis of pathologic stage IV disease.
The study protocol was approved by the St. Louis VHA Research and Development Committee, and given the deidentified nature of the analysis, a waiver for consent was granted by the institutional review board. Data were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Smoking Exposure
In VHA datasets, smoking status typically is determined by using Health Factors (HFs) data, which are maintained within the CDW.17 These data are collected nationally and are validated against prospective data for determining veteran smoking status.17,18 HFs data are compiled from automated prompts, which VHA clinicians must complete on a regular basis, that include information on patient cigarette use.17 Therefore, these data can be used not only to determine smoking habits at a given time (ie, at the time of surgery), but also to assess changes in smoking status longitudinally (ie, smoking cessation).19 We assessed smoking status (current vs former) at two time points: the date of surgery and 1 year after the date of surgery. Because the content and frequency of smoking status in HFs can fluctuate,19,20 we queried the VHA Informatics and Computing Infrastructure Text Integration Utility data files—which contain free-text documents like progress and consultation notes—to augment the preoperative assessment of smoking status (ie, to assess better if individuals were still smoking on the date of surgery), as described previously by our group.21 For the postoperative assessment, HFs data were used without such augmentation because smoking status in the postoperative period is less prone to the temporal limitations of the preoperative assessment. For patients who died within 1 year of surgery, the last recorded smoking status in HFs was used for the analysis.
For subsequent analyses, we defined several terms as follows. Continued smoking describes patients who were smoking at the time of surgical treatment and who continued to smoke 1 year after surgery. Relapsed smoking describes patients who were not smoking at the time of surgical treatment, but who relapsed and began smoking again at 1 year after surgery. For the survival analyses, we analyzed individuals classified as having continued and relapsed smoking habits together (termed persistent smoking) to highlight best the effects of postoperative smoking on long-term outcomes.
We also collected several other variables related to smoking status. Prescriptions for smoking cessation pharmacotherapy (nicotine replacement therapy, varenicline, or bupropion) within 1 year after surgery were assessed using the CDW Pharmacy Outpatient file. We also assessed whether patients followed up with a cardiothoracic surgeon (more than one visit, because patients were assumed to have undergone at least one postoperative visit), oncologist, or primary care physician within 1 year after surgery.
Covariates
We extracted several additional covariates including age, sex, BMI, and comorbidities. Comorbidities were measured from 5 years before surgery to 1 month after surgery and were determined using ICD, Ninth and Tenth Revisions, codes to calculate a composite Charlson/Deyo score.22 Residential zip codes were used to calculate distance from the treating hospital (measured as the straight-line distance between the center of patient zip code and the facility address). Area deprivation index, a county-level measure of socioeconomic deprivation that incorporates several poverty, education, housing, and employment indicators from the US census, also was obtained.23,24
Several oncologic and treatment covariates also were extracted. These included tumor size, histologic findings (adenocarcinoma, squamous cell carcinoma, other), grade, year of operation, hospital caseload (lung cancer treatment volume in the year before surgery at that VHA facility), wait time to surgery (time between radiographic diagnosis and surgery, with delayed surgery defined as a wait time of > 12 weeks25), type of operation (lobectomy, segmentectomy, wedge resection, or pneumonectomy), surgical approach (video-assisted thoracoscopic surgery or thoracotomy), number of lymph nodes assessed (adequacy defined as ≥ 10 based on prior guidelines26,27), and final pathologic stage.
We also extracted several short-term outcomes to include as covariates in our disease free-survival and overall survival analyses. These included length of stay, major postoperative complications (pneumonia, empyema, myocardial infarction, respiratory failure, renal failure, or stroke),28,29 and 30-day readmission.
Outcomes
Our primary outcomes of interest were disease-free survival and overall survival. Overall survival was determined using the CDW Vital Status File. Patients were censored at the date of last follow-up (May 1, 2020). Disease-free survival (or time until cancer recurrence) was defined using the CDW Oncology database and was supplemented with additional diagnoses suggestive of recurrence using ICD, Ninth and Tenth Revisions, codes, as described previously in VHA literature and by our group.25,30 In particular, patients with any of the following occurrences based on ICD codes also were deemed to demonstrate cancer recurrence: additional chemotherapy or radiation therapy (in the absence of another cancer diagnosis), additional lung resection (in the absence of another cancer diagnosis), malignant pleural effusion, a secondary malignant neoplasm resulting from lung cancer, or lung biopsy (that was followed by additional chemotherapy or radiation therapy).
Statistical Analysis
Cohort characteristics are presented using means ± SDs for continuous variables and frequencies (proportions) for categorical variables. To compare between groups, two-tailed t tests were used for continuous variables and χ2 tests were used for categorical variables. Factors associated with continued smoking and smoking relapse at 1 year were assessed in multivariate logistic regression models adjusting for the variables specified in the corresponding tables. The association between persistent smoking and overall survival was assessed using Kaplan-Meier curves and a multivariate Cox proportional hazards model (for all-cause mortality), controlling for the aforementioned covariates (available in supplement). The association between persistent smoking and disease-free survival was assessed using a multivariate competing risk model (Fine and Gray subdistribution hazard function) with recurrence as the outcome and death as a competing event, controlling for the same aforementioned covariates (available in supplement). Because the occurrence of death precludes the possibility of recurrence in those who die, this time-to-event competing risk model was used for the recurrence analysis and was displayed using a cumulative incidence plot.31 Missing data were reported in the univariate analyses (available in supplement), and complete case analyses was used for the multivariate models (except where missing categories are specified). All analyses were performed using SAS version 9.3 software (SAS Institute).
Results
Study Cohort Characteristics
Among 7,489 patients who underwent surgical treatment for clinical stage I NSCLC, 7,189 patients (96.0%) were men, the mean ± SD age was 67.0 ± 7.3 years, and 4,562 patients (60.9%) were smoking at the time of surgical treatment (Fig 1). The median wait time between cancer diagnosis and surgery was 62 days (interquartile range, 40-94 days), with 2,282 patients (30.5%) experiencing a delay of longer than 12 weeks.25 Most veterans underwent lobectomy (5,295 patients [70.8%]) and 4,183 patients (55.9%) underwent a thoracotomy. Most patients had received a diagnosis of adenocarcinoma (3,935 patients [52.6%]) with tumor grades of more than I (grade II, 3,688 patients [52.9%]; grade III, 2,320 patients [33.3%]; and grade IV, 102 patients [1.5%]). The median length of stay was 7 days (interquartile range, 4-10 days). The most common complications were pneumonia (411 patients [5.5%]) and cardiorespiratory failure (300 patients [4.0%]). At 30 days after surgery, 567 patients (7.6%) had been readmitted. Additional patient demographics, treatment characteristics, and outcomes of the study cohort are presented in Table 1.
Figure 1.
Consolidated Standards of Reporting Trials diagram showing study progression.
Table 1.
Characteristics of Veterans With Clinical Stage I NSCLC Undergoing Surgical Treatment in the VHA
| Variable | Overall Cohort (N = 7,489) |
|---|---|
| Demographics | |
| Age, y | 66.95 ± 7.26 |
| Sex | |
| Male | 7,189 (95.99) |
| Female | 300 (4.01) |
| Race | |
| White | 6,168 (82.36) |
| Black | 1,154 (15.41) |
| Other | 102 (1.36) |
| Unknown | 65 (0.87) |
| Smoking status (at time of surgical treatment) | |
| Current | 4,562 (60.92) |
| Former | 2,927 (39.08) |
| BMI, kg/m2 | 27.00 (5.40) |
| Area deprivation index | 54.41 ± 14.96 |
| Charlson/Deyo score | 7 (5-8) |
| Smoking cessation pharmacotherapy prescribed after surgery (within 1 y) | 2,666 (35.6) |
| Postoperative appointments (in 1 y after surgery) | |
| Cardiothoracic surgeon (> 1 visit) | 4,609 (61.54) |
| Oncologist | 3,301 (44.08) |
| Primary care provider | 7,031 (93.88) |
| Treatment characteristics | |
| Wait time to surgery, d | |
| Median (IQR) | 62 (40-94) |
| > 12 wk | 2,282 (30.47) |
| Tumor size, Mm | 22.96 ± 10.58 |
| Resection | |
| Lobectomy | 5,295 (70.83) |
| Wedge | 1,637 (21.90) |
| Segment | 444 (5.94) |
| Pneumonectomy | 100 (1.34) |
| Surgical approach | |
| VATS | 2,988 (39.90) |
| Thoracotomy | 4,183 (55.86) |
| Histologic finding | |
| Adenocarcinoma | 3,935 (52.56) |
| Squamous cell | 2,589 (34.58) |
| Other | 962 (12.85) |
| Grade | |
| I | 859 (12.33) |
| II | 3,688 (52.92) |
| III | 2,320 (33.29) |
| IV | 102 (1.46) |
| Pathologic stage | |
| I | 6,373 (85.10) |
| II | 565 (7.54) |
| III | 291 (3.89) |
| Outcomes | |
| Length of stay, d | 7 (4-10) |
| Major complications | |
| Pneumonia | 411 (5.49) |
| MI | 67 (0.89) |
| Empyema | 62 (0.83) |
| Renal failure | 82 (1.09) |
| Respiratory/cardiac failure | 300 (4.01) |
| Stroke | 18 (0.24) |
| 30-d readmission | 567 (7.57) |
Data are presented as No. (%), mean ± SD, or median (IQR). IQR = interquartile range; MI = myocardial infarction; NSCLC = non-small cell lung cancer; VATS = video-assisted thoracoscopic surgery; VHA = Veterans Health Administration.
In the year after surgery, 2,666 patients (35.6%) were prescribed smoking cessation pharmacotherapy. In terms of postoperative follow-up in the year after surgery, 4,609 patients (61.5%) were seen by a cardiothoracic surgeon more than once, 3,301 patients (44.1%) were seen by an oncologist, and 7,031 patients (93.9%) were seen by a primary care physician.
Factors Associated With Continued Smoking
Among the 4,562 patients (60.9% of overall cohort) who were smoking at the time of surgical treatment, 2,648 patients (58.0%) continued to smoke at 1 year after surgery (e-Table 1). Factors associated with lower odds of continued smoking were increasing age (for every 1-unit increase; adjusted OR [aOR], 0.955; 95% CI, 0.944-0.966; P < .001) (Table 2), female sex (vs male; aOR, 0.648; 95% CI, 0.452-0.931; P = .02), Black race (vs White race; aOR, 0.796; 95% CI, 0.661-0.958; P = .02), and higher BMI (for every 1-unit increase; aOR, 0.943; 95% CI, 0.930-0.956; P < .001). Factors associated with higher odds of continued smoking were higher Charlson/Deyo score (for every 1-unit increase; aOR, 1.043; 95% CI, 1.005-1.083; P = .03), delayed surgery (> 12 weeks between diagnosis and surgery; aOR, 1.197; 95% CI, 1.027-1.395; P = .02), undergoing a wedge resection (vs lobectomy; aOR, 1.276; 95% CI, 1.064-1.531; P = .01), and receiving cessation pharmacotherapy after surgery (aOR, 1.817; 95% CI, 1.574-2.097; P < .001). Of note, continued smoking was not associated with tumor size, final pathologic stage, major complications after surgery, readmission after surgery, or the type of provider performing follow-up (surgeon, oncologist, or primary care provider).
Table 2.
Factors Independently Associated With Continued Smoking at 1 Y After Surgery (n = 4,562)
| Variable | OR | 95% CI (Wald) | P Value |
|---|---|---|---|
| Age | 0.955 | 0.944-0.966 | < .0001 |
| Sex (female vs male) | 0.648 | 0.452-0.931 | .0190 |
| Race (reference, White) | |||
| Black | 0.796 | 0.661-0.958 | .0158 |
| Other | 0.666 | 0.369-1.202 | .1773 |
| Unknown | 0.723 | 0.363-1.440 | .3561 |
| BMI | 0.943 | 0.930-0.956 | < .0001 |
| Charlson/Deyo score | 1.043 | 1.005-1.083 | .0250 |
| Area deprivation index | 1.004 | 1.000-1.009 | .0708 |
| Delayed surgery > 12 wk (yes vs no) | 1.197 | 1.027-1.395 | .0215 |
| Resection type (reference, lobectomy) | |||
| Pneumonectomy | 1.048 | 0.546-2.013 | .8875 |
| Segmentectomy | 1.088 | 0.801-1.478 | .5891 |
| Wedge | 1.276 | 1.064-1.531 | .0085 |
| Surgical approach (VATS vs thoracotomy) | 1.085 | 0.937-1.256 | .2768 |
| Tumor size (reference, ≤ 10 mm) | |||
| 11-20 | 0.829 | 0.644-1.067 | .1452 |
| 21-30 | 0.813 | 0.623-1.061 | .1276 |
| 31-40 | 0.822 | 0.612-1.105 | .1946 |
| 41-50 | 0.990 | 0.688-1.424 | .9563 |
| Unknown | 0.936 | 0.547-1.603 | .8104 |
| Pathologic stage (reference, I) | |||
| II | 0.803 | 0.621-1.040 | .0970 |
| III | 1.233 | 0.849-1.791 | .2717 |
| Major complication (yes vs no) | 0.848 | 0.676-1.065 | .1558 |
| Readmission (yes vs no) | 0.845 | 0.641-1.114 | .2330 |
| Smoking cessation pharmacotherapy prescribed after surgery (within 1 y) | 1.817 | 1.574-2.097 | < .0001 |
| Postoperative appointments (in 1 y after surgery) | |||
| Cardiothoracic surgeon (> 1 visit) | 1.029 | 0.889-1.191 | .7016 |
| Oncologist | 1.035 | 0.897-1.194 | .6400 |
| Primary care provider | 0.944 | 0.705-1.263 | .6959 |
Table displays the multivariate model controlling for all covariates. VATS = video-assisted thoracoscopic surgery.
Pack-year smoking history was available for 5,622 patients (75.1%). In a sensitivity analysis using a separate multivariate model, greater pack-year smoking history was associated with slightly higher odds of continued smoking 1 year after surgery (for every 1-unit increase; aOR, 1.003, 95% CI, 1.000-1.005; P = .03).
Factors Associated With Smoking Relapse
Among the 2,927 patients (39.1% of overall cohort) who were not smoking at the time of surgical treatment, 573 patients (19.6%) had relapsed and were smoking at 1 year after surgery (e-Table 1). Factors associated with higher odds of smoking relapse were individuals who quit more recently (ie, quitting between diagnosis and surgery vs quitting before diagnosis; aOR, 4.729; 95% CI, 3.711-6.025; P < .001) (e-Table 2), higher area deprivation index (for every 1-unit increase; aOR, 1.013; 95% CI, 1.005-1.021; P < .001), and higher pathologic stage (eg, II vs I; aOR, 1.793; 95% CI, 1.216-2.645; P = .003). Factors associated with lower odds of smoking relapse were increasing age (for every 1-unit increase; aOR, 0.951; 95% CI, 0.935-0.968; P < .001), Black race (vs White race; aOR, 0.660; 95% CI, 0.465-0.937; P = .02), higher BMI (for every 1-unit increase; aOR, 0.962; 95% CI, 0.941-0.982; P < .001), and delayed surgery (> 12 weeks between diagnosis and surgery; aOR, 0.776; 95% CI, 0.604-0.996; P = .05).
Persistent Smoking and Long-term Outcomes
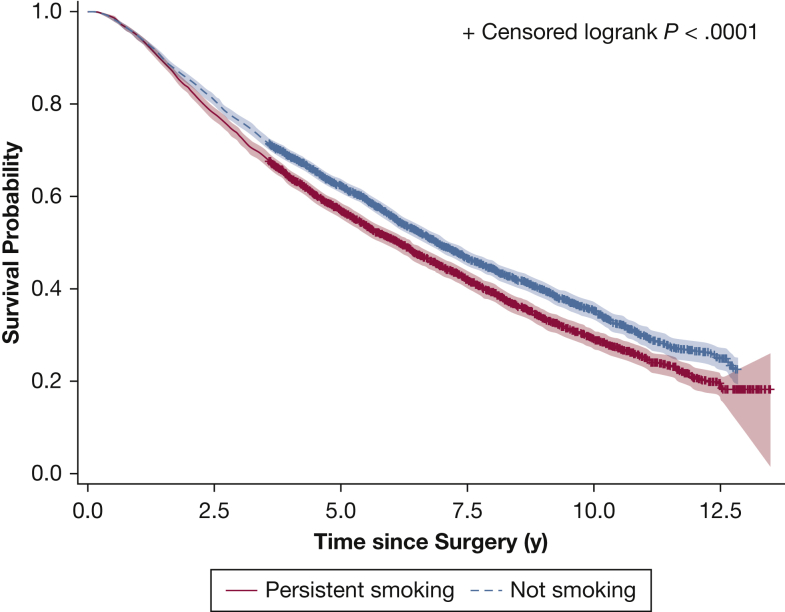
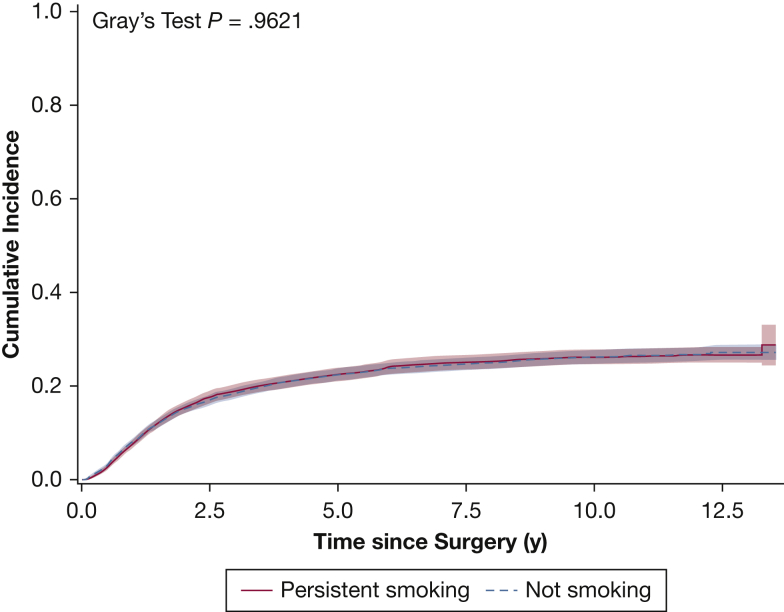
In the entire cohort, persistent smoking was observed in 3,221 patients (43.0%) at 1 year after surgery. Persistent smoking was associated with significantly shorter overall survival (adjusted hazard ratio of all-cause mortality, 1.291; 95% CI, 1.197-1.392; P < .001) (e-Table 3), with a median survival of 83.2 months in those who were not smoking compared with 73.7 months in individuals who persistently smoked (Fig 2). This survival difference was observed most prominently in individuals who continued smoking as opposed to those who relapsed (e-Figs 1, 2). With a median follow-up of 6.6 years, cancer recurrence was detected in 1,850 patients (24.7%). Persistent smoking was not associated with inferior disease-free survival (adjusted hazard ratio, 0.989; 95% CI, 0.884-1.106; P = .84) (Fig 3, e-Table 4).
Figure 2.
Graph showing overall survival based on smoking status 1 year after surgical treatment.
Figure 3.
Graph showing disease-free survival based on smoking status 1 year after surgical treatment.
Discussion
This study explored the relationship between persistent postoperative smoking and long-term outcomes in veterans with clinical stage I NSCLC. We found that more than 60% of veterans with early-stage lung cancer smoke at the time of cancer diagnosis. At 1 year after surgery, both continued smoking and smoking relapse were common. Persistent smoking was associated with significantly shorter long-term survival, but did not influence the risk of cancer recurrence. In aggregate, these findings suggest that persistent smoking after potentially curative lung cancer surgery is an important modifiable factor for long-term survival and should be a key element of cancer survivorship plans.
Cancer survivorship—the experience of living for extended cancer-free periods after curative-intent treatment—is an increasingly common phenomenon in early-stage lung cancer. A major contributing factor to this is the implementation of lung cancer screening programs and the relative increase in patients with early-stage, potentially curative disease.32 However, a challenge of this survivorship period is the large number of patients who are lost in transition and do not receive appropriate long-term oncologic follow-up care.33 This has led several organizations, including the American Society for Clinical Oncology and the American College of Surgeons Commission on Cancer, to advocate for so-called survivorship care plans.34 These survivorship care plans are meant to be brief summaries of a patient’s treatments, their unique surveillance schedule, and other factors (like warning signs for late treatment effects and healthy lifestyle habits), all of which are designed to improve outcomes during this cancer survivorship period. Despite clear importance, the implementation of survivorship care plans has been poor for a variety of reasons.34 Nonetheless, our data support that smoking cessation should be an integral element of such care plans given the strong relationship between persistent smoking and worse survival.
Most large cancer databases assess preoperative smoking status only at a single time point, if at all, and lack longitudinal smoking data after treatment.13,14 Similarly, in administrative datasets where longitudinal data are more readily available, smoking status often is coded unreliably.35 Therefore, changes in preoperative and postoperative smoking status are poorly understood in the context of lung cancer surgery. A prior, single-institution study by Walker and colleagues15 examined 154 patients with early-stage lung cancer undergoing surgical treatment who smoked within 3 months of surgery; they found that at 12 months after surgery, 36.9% of these patients were still smoking and that this was associated with inferior outcomes. Our study demonstrated that an even higher proportion of veterans who smoked at the time of surgery continued to smoke 1 year after treatment (58.0%). Importantly, we further identified that persistent smoking significantly diminished long-term survival after accounting for tumor-related factors and other comorbidities.
Several factors likely contribute to diminished long-term survival in patients who persistently smoke after surgery. First, it is likely that persistent smoking contributes to accelerated cardiovascular and other smoking-related comorbidities. Although we did assess comorbidities that were present at the time of surgery, it is likely that these smoking-related comorbidities progress in the follow-up period, leading to higher rates of mortality in patients who continue to smoke. Second, persistent smoking likely is associated with higher risk for other smoking-related malignancies that may contribute to shorter survival in these patients.36 Third, prior studies have demonstrated that the benefits of smoking cessation, especially in reducing the risk of mortality, are realized relatively quickly.36,37 This finding should be emphasized to patients so that the treatment experience can be used effectively as a so-called teachable moment.38
A surprising finding was that persistent smoking was not associated with cancer recurrence. Several factors may contribute to this observation. For example, recurrence is relatively uncommon in patients with stage I NSCLC (20%-25%), limiting our study’s power. Similarly, surgery in the setting of early-stage disease is potentially curative, negating the risk of smoking-related recurrence. Despite this, it is established that persistent smoking results in higher rates of second primary lung cancers, at a rate of 1% to 2% per year.39 These second lung cancers in addition to other smoking-related malignancies, although not contributing to the incidence of recurrence, likely contribute to the inferior survival in individuals who persistently smoke.
We also assessed several patient- and treatment-related factors and their association with persistent smoking after surgery. Overall, we found through these analyses that it is challenging to identify individuals who are likely to continue smoking in the postoperative period. For example, postoperative smoking habits did not seem to differ based on cancer-related factors like tumor size or stage. Similarly, we did not observe differences in smoking habits in the setting of major complications or readmissions. Smoking habits also were similar among patients who followed up with a surgeon, oncologist, or primary care provider. These results indicate that careful attention must be paid to smoking status of all individuals with a smoking history during cancer follow-up.
This study has several strengths. Most notably, it harnesses well-maintained VHA electronic health records to assess smoking status longitudinally in a homogenous cohort of patients with stage I NSCLC undergoing definitive surgical treatment. Conversely, this study has some limitations. First, smoking status was not available for all patients at 1 year after surgery. This is related to the variability of the HFs prompts19,20 and to the subset of patients who may not receive follow-up care through the VHA. Second, smoking status in this study was determined through historical medical records, and therefore was not validated biochemically. Finally, the VHA serves a unique patient population that is largely male with significant comorbidities and histories of heavy smoking. However, with prior studies showing similar long-term outcomes in veteran and nonveteran populations undergoing lung cancer surgery,40 it is likely that our findings will be applicable generally to the broad population of patients with lung cancer.
Interpretation
In conclusion, persistent smoking after stage I NSCLC resection is common and dramatically worsens long-term survival. Providers—including surgeons—should counsel patients aggressively regarding smoking in the perioperative period and during cancer surveillance because successful cessation interventions can have a disproportionate impact on early-stage lung cancer outcomes.
Acknowledgments
Author contributions: B. T. H., D. B. E., and V. P. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. B. T. H., D. B. E., S.-H. C., Y. Y., M. W. S., L.-S. C., N. S., M. R. P., D. K., R. G. N., B. F. M., B. D. K., and V. P. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: None declared.
Roleofsponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figures and e-Tables are available online under “Supplementary Data.”
Footnotes
FUNDING/SUPPORT: This study was funded in part by the National Institutes of Health [Grants 5T32HL007776-25 (B. T. H.) and VA1 I01 HX002475-01A2 (V. P., S.-H. C., Y. Y., D. B. E.)].
Supplementary Data
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Zullig L.L., Sims K.J., McNeil R., et al. Cancer incidence among patients of the U.S. veterans affairs health care system: 2010 update. Mil Med. 2017;182(7):e1883–e1891. doi: 10.7205/MILMED-D-16-00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention What are the risk factors for lung cancer? Centers for Disease Control and Prevention website. https://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm
- 4.Cataldo J.K., Dubey S., Prochaska J.J. Smoking cessation: an integral part of lung cancer treatment. Oncology. 2010;78:289–301. doi: 10.1159/000319937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstraw P., Chansky K., Crowley J., et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Smoking greatly increases risk of complications after surgery. https://www.who.int/news/item/20-01-2020-smoking-greatly-increases-risk-of-complications-after-surgery World Health Organization website.
- 7.Grønkjær M., Eliasen M., Skov-Ettrup L.S., et al. Preoperative smoking status and postoperative complications: a systematic review and meta-analysis. Ann Surg. 2014;259(1):52–71. doi: 10.1097/SLA.0b013e3182911913. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network Non-small cell lung cancer, version 6.2020. NCCN Guidelines in Oncology. 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf National Comprehensive Cancer Network website.
- 9.Howington J.A., Blum M.G., Chang A.C., et al. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 10.Heiden B.T., Semenkovich T.R., Kozower B.D. Guide to enhanced recovery for cancer patients undergoing surgery. Ann Surg Oncol. 2021;28(12):6939–6942. doi: 10.1245/s10434-021-09882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odani S., Agaku I.T., Graffunder C.M., et al. Tobacco product use among military veterans—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7–12. doi: 10.15585/mmwr.mm6701a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian M.P., Hu Y., Puri V., et al. Administrative versus clinical databases. J Thorac Cardiovasc Surg. 2021;162(4):1173–1176. doi: 10.1016/j.jtcvs.2020.03.183. [DOI] [PubMed] [Google Scholar]
- 13.Merkow R.P., Rademaker A.W., Bilimoria K.Y. Practical guide to surgical data sets: National Cancer Database (NCDB) JAMA Surg. 2018;153(9):850–851. doi: 10.1001/jamasurg.2018.0492. [DOI] [PubMed] [Google Scholar]
- 14.Farjah F., Kaji A.H., Chu D. Practical guide to surgical data sets: Society of Thoracic Surgeons (STS) national database. JAMA Surg. 2018;153(10):955–956. doi: 10.1001/jamasurg.2018.0545. [DOI] [PubMed] [Google Scholar]
- 15.Walker M.S., Vidrine D.J., Gritz E.R., et al. Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2370–2377. doi: 10.1158/1055-9965.EPI-06-0509. [DOI] [PubMed] [Google Scholar]
- 16.Sheikh M., Mukeriya A., Shangina O., Brennan P., Zaridze D. Postdiagnosis smoking cessation and reduced risk for lung cancer progression and mortality: A prospective cohort study. Ann Intern Med. 2021;174(9):1232–1239. doi: 10.7326/M21-0252. [DOI] [PubMed] [Google Scholar]
- 17.McGinnis K.A., Brandt C.A., Skanderson M., et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13(12):1233–1239. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golden S.E., Hooker E.R., Shull S., et al. Validity of Veterans Health Administration structured data to determine accurate smoking status. Health Informatics J. 2020;26(3):1507–1515. doi: 10.1177/1460458219882259. [DOI] [PubMed] [Google Scholar]
- 19.Melzer A.C., Pinsker E.A., Clothier B., et al. Validating the use of veterans affairs tobacco health factors for assessing change in smoking status: accuracy, availability, and approach. BMC Med Res Methodol. 2018;18(1):39. doi: 10.1186/s12874-018-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava A.B., Ramsey A.T., McIntosh L.D., et al. Tobacco use prevalence and smoking cessation pharmacotherapy prescription patterns among hospitalized patients by medical specialty. Nicotine Tob Res. 2019;21(5):631–637. doi: 10.1093/ntr/nty031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heiden BT, Eaton DB Jr, Chang S-H, et al. Assessment of duration of smoking cessation prior to surgical treatment of non-small cell lung cancer [published online ahead of print November 18, 2021]. Ann Surg. 10.1097/SLA.0000000000005312. [DOI] [PMC free article] [PubMed]
- 22.Quan H., Sundararajan V., Halfon P., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 23.Fairfield K.M., Black A.W., Ziller E.C., et al. Area Deprivation Index and rurality in relation to lung cancer prevalence and mortality in a rural state [published online ahead of print March 2, 2020]. JNCI Cancer Spectr. https://doi.org/10.1093/jncics/pkaa011 [DOI] [PMC free article] [PubMed]
- 24.Kind A.J.H., Jencks S., Brock J., et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765–774. doi: 10.7326/M13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heiden B.T., Eaton D.B., Jr., Engelhardt K.E., et al. Analysis of delayed surgical treatment and oncologic outcomes in clinical stage I non-small cell lung cancer. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samson P., Crabtree T., Broderick S., et al. Quality measures in clinical stage I non-small cell lung cancer: improved performance is associated with improved survival. Ann Thorac Surg. 2017;103(1):303–311. doi: 10.1016/j.athoracsur.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American College of Surgeons CoC quality of care measures 2020 surveys. https://www.facs.org/Quality-Programs/Cancer/NCDB/qualitymeasurescocweb American College of Surgeons website.
- 28.Broderick S.R., Patel A.P., Crabtree T.D., et al. Pneumonectomy for clinical stage IIIA non-small cell lung cancer: the effect of neoadjuvant therapy. Ann Thorac Surg. 2016;101(2):451–457. doi: 10.1016/j.athoracsur.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozower B.D., O’Brien S.M., Kosinski A.S., et al. The Society of Thoracic Surgeons composite score for rating program performance for lobectomy for lung cancer presented at the fifty-first annual meeting of the Society of Thoracic Surgeons, San Diego, CA, Jan 24-28, 2015. Ann Thorac Surg. 2016;101(4):1379–1387. doi: 10.1016/j.athoracsur.2015.10.081. [DOI] [PubMed] [Google Scholar]
- 30.Tarlov E., Lee T.A., Weichle T.W., et al. Reduced overall and event-free survival among colon cancer patients using dual system care. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2231–2241. doi: 10.1158/1055-9965.EPI-12-0548. [DOI] [PubMed] [Google Scholar]
- 31.Pintilie M. Analysing and interpreting competing risk data. Stat Med. 2007;26(6):1360–1367. doi: 10.1002/sim.2655. [DOI] [PubMed] [Google Scholar]
- 32.Krist A.H., Davidson K.W., Mangione C.M., et al. Screening for lung cancer. JAMA. 2021;325(10):962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 33.Committee on Cancer Survivorship: Improving Care and Quality of Life, National Cancer Policy Board, Hewitt M., Freenfield S., Stovall E., editors. From Cancer Patient to Cancer Survivor: Lost in Transition. National Academies Press; 2005. [Google Scholar]
- 34.Jacobsen P.B., DeRosa A.P., Henderson T.O., et al. Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery. J Clin Oncol. 2018;36(20):2088–2100. doi: 10.1200/JCO.2018.77.7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huo J., Yang M., Tina Shih Y.C. Sensitivity of claims-based algorithms to ascertain smoking status more than doubled with meaningful use. Value Heal. 2018;21(3):334–340. doi: 10.1016/j.jval.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 36.United States Public Health Service Office of the Surgeon General, National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health . United States Department of Health and Human Services; 2020. Smoking Cessation: A Report of the Surgeon General. [Google Scholar]
- 37.Doll R., Peto R., Boreham J., et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519–1528. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meltzer L.R., Unrod M., Simmons V.N., et al. Capitalizing on a teachable moment: development of a targeted self-help smoking cessation intervention for patients receiving lung cancer screening. Lung Cancer. 2019;130:121–127. doi: 10.1016/j.lungcan.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heiden B.T., Subramanian M.P., Puri V., Kozower B.D. Striking a balance: surveillance of non-small cell lung cancer after resection. J Thorac Cardiovasc Surg. 2021;162(3):680–684. doi: 10.1016/j.jtcvs.2020.10.166. [DOI] [PubMed] [Google Scholar]
- 40.Heiden B.T., Eaton D.B.J., Chang S.-H., et al. Comparison between veteran and non-veteran populations with clinical stage I non-small cell lung cancer undergoing surgery [published online ahead of print May 11, 2021]. Ann Surg. https://doi.org/10.1097/SLA.0000000000004928 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.