Abstract
Background
Anti-dipeptidyl-peptidase-like protein 6 (DPPX) encephalitis is a rare autoimmune encephalitis. The clinical symptoms of anti-DPPX encephalitis are often severe, manifested as diarrhea/weight loss, central nervous system hyperexcitability and cognitive dysfunction.
Case presentation
An 18-year-old boy was admitted for 1-week-long cerebellar symptoms including dizziness, unsteady gait and frequent vomiting. Magnetic resonance imaging (MRI) displayed no abnormal findings. However, autoimmune encephalitis panel revealed anti-DPPX antibody was positive in the serum. This patient completely recovered after immunoglobulin and corticoids therapy. In addition, repeat serum antibody test for DPPX was negative within one month.
Conclusion
In addition to the classic triad, anti-DPPX encephalitis may manifest as mild and rare symptoms due to lower antibody titers. Fast identification of rare symptoms can help to quickly diagnosis and effective treatment.
Keywords: Autoimmune encephalitis, Dipeptidyl-peptidase-like protein 6, Cerebellar ataxia, Antibody, Case report
Background
Autoimmune encephalitis is a debilitating neurological disease mediated by antibodies to neuronal surface receptors or ion channels on neurological tissue [1]. In the past ten years, with the reports of various antibodies against the surface antigens of central nervous system neurons, autoimmune encephalitis has gradually been recognized by clinicians [2]. Diverse antibodies may lead to a variety of clinical manifestations including behavioral and psychiatric symptoms, autonomic disturbances, movement disorders, and seizures [2]. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis, as the most frequently diagnosed encephalitis, often present with neuropsychiatric symptoms. However, morvan syndrome is the main feature of anti-contactin-associated protein 2 (CASPR2) encephalitis [2]. Anti-dipeptidyl-peptidase-like protein 6 (DPPX) encephalitis is a rare autoimmune encephalitis which was first described in 2013 [3]. DPPX is a regulatory subunit of Kv4.2 potassium channels and mainly expressed in the myenteric plexus, cerebellum, hippocampus and striatum [3]. Characteristically, most of these patients complain of diarrhea/weight loss, central nervous system hyperexcitability and cognitive dysfunction [3, 4]. Here, we describe a boy with only cerebellar symptoms and signs, a symptom rarely reported in anti-DPPX encephalitis. We think this case could enrich the symptom spectrum of this rare disease.
Case presentation
An 18-year-old boy presented with 1-week-long dizziness, unsteady gait and frequent vomiting. He had unexplained low-grade fever a few days but no diarrhea before symptom onset.
The patient presented drunken gait and could not ambulate independently. A neurological examination showed horizontal nystagmus although extraocular movements appeared full in all planes. In addition, coordination movement tests demonstrated cerebellar ataxia, mainly on finger-nose testing, heel-knee-tibia test was uncoordinated, and the Romberg sign positive with eyes closed and opened. However, mental status, limb muscle strength and sensory system examination were normal in this patient.
On hospitalization, there were no abnormal findings in laboratory analysis, brain magnetic resonance imaging (MRI), electroencephalogram and computed tomography imaging of the chest, abdomen. Cerebrospinal fluid (CSF) sample displayed normal white blood cells, protein and glucose levels. We sent an autoimmune encephalitis panel including serum and CSF samples, and anti-DPPX antibody was positive (1:10) in the serum through cell-based assay but CSF analysis revealed any abnormalities (Fig. 1 A-B). What’s more, other antibodies associated with tumors was negative.
Fig. 1.
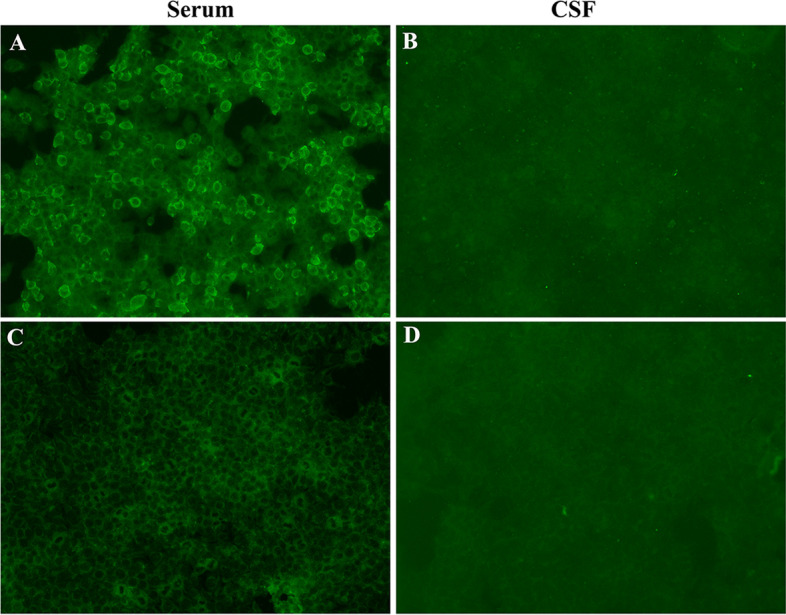
Positive reaction with transfected HEK293 cells expressing DPPX after incubation with the patient's serum (A) (titer 1:10) but negative with patient's CSF (B). The repeat (C) serum and (D) CSF antibody tests for DPPX were both negative after a 1-month follow-up visit
The patient was treated with intravenous 500 mg methylprednisolone per day for 5 days, 240 mg per day for 5 days, 120 mg per day for 5 days, and followed by a slow tapering dose of prednisone over half year. In addition, he also received intravenous immunoglobulin (0.4 g/kg) for 5 days. When the patient was discharged from the hospital, the symptoms of dizziness and vomiting were significantly improved, and he was able to ambulate independently. Within one month, repeat serum and CSF antibody tests for DPPX were both negative (Fig. 1C-D). At follow-up 10 months after symptom onset, the patient remained clinically stable and had no cerebellar symptoms.
Discussion and conclusions
Anti-DPPX encephalitis is a relatively rare autoimmune encephalitis. Up to now, no more than 50 cases have been reported [3–9]. The classic triad of clinical symptoms were diarrhea or weight loss, cognitive dysfunction and central nervous system hyperexcitability [4, 5], and 55% of patients had three classical symptoms in common (Table 1). Myoclonus, tremor, spasticity, rigidity, stiffness and hyperekplexia were the most common hyperexcitability in anti-DPPX encephalitis [4, 5]. Some rare symptoms have also been reported, including psychiatric symptoms (agitation, paranoia, hallucinations, anxiety, mutism, depression), brainstem disorders (eye movement disturbances, dysphagia, dysarthria, respiratory failure), dysautonomia (constipation, thermoregulation, urine retention, tachycardia), sleep disorder and seizures, occurring in 65%, 25%, 25%, 42.5%, and 12.5% of cases, respectively (Table 1). We presented a patient with pure cerebellar ataxia but no three classical symptoms. Although 57.5% of patients with anti-DPPX encephalitis displayed cerebellar ataxia, they all had other symptoms, and cerebellar ataxia was not the main clinical manifestation (Table 1). As far as we know, the case we presented was the first anti-DPPX encephalitis with cerebellar ataxia as the only symptom.
Table 1.
The spectrum of symptoms in anti-DPPX encephalitis
| Case | Age (years) | Sex | Symptoms: 1. Diarrhea or weight loss; 2. Hyperexcitability (myoclonus, tremor, spasticity, rigidity, stiffness, hyperekplexia; muscle cramps); 3. Cognitive dysfunction (memory loss, executive dysfunction); 4.Cerebellar ataxia (vertigo, ataxia, nystagmus, unsteady gait); 5. Psychiatric symptoms (agitation, paranoia, hallucinations, anxiety, mutism, depression); 6. Brainstem disorders (eye movement disturbances, dysphagia, dysarthria, respiratory failure); 7. Dysautonomia (constipation, thermoregulation, urine retention, tachycardia); 8. Sleep disorder; 9. Seizures; | Reference |
|---|---|---|---|---|
| 1 | 61 | Man | 1,2,3,5 | Boronat A et al. 2013 [3] |
| 2 | 45 | Female | 1,2,3,5,8,9 | Boronat A et al. 2013 [3] |
| 3 | 58 | Female | 2,4,5 | Boronat A et al. 2013 [3] |
| 5 | 15 | Man | 2,3,4 | Balint B et al. 2014 [6] |
| 6 | 27 | Man | 1,2,4,7 | Balint B et al. 2014 [6] |
| 7 | 26 | Man | 1,2,3,4,7 | Balint B et al. 2014 [6] |
| 8 | 18 | Man | 1,2,3,4,6,9 | Tobin WO et al [5]. 2014 |
| 9 | 57 | Man | 1,3,4,5,6,8 | Tobin WO et al [5]. 2014 |
| 10 | 37 | Female | 1,5,7,8 | Tobin WO et al [5]. 2014 |
| 11 | 36 | Female | 1,2,3,4,5,8 | Tobin WO et al. 2014 [5] |
| 12 | 51 | Female | 1,2,3,4,6,7 | Tobin WO et al. 2014 [5] |
| 13 | 75 | Man | 3,6,7 | Tobin WO et al. 2014 [5] |
| 14 | 61 | Man | 1,2,5,6 | Tobin WO et al. 2014 [5] |
| 15 | 70 | Man | 1,3,4,8 | Tobin WO et al. 2014 [5] |
| 16 | 63 | Man | 1,2,3,5,7,8 | Tobin WO et al. 2014 [5] |
| 17 | 39 | Female | 2,5 | Tobin WO et al. 2014 [5] |
| 18 | 52 | Female | 1,3 | Tobin WO et al. 2014 [5] |
| 19 | 66 | Man | 1,2,9 | Tobin WO et al. 2014 [5] |
| 20 | 24 | Man | 1,2,3,4,5,6,7,8 | Tobin WO et al. 2014 [5] |
| 21 | 13 | Female | 6,8 | Tobin WO et al. 2014 [5] |
| 22 | 49 | Man | 1,2,6,8 | Tobin WO et al. 2014 [5] |
| 23 | 53 | Female | 3,4,5 | Tobin WO et al. 2014 [5] |
| 24 | 55 | Man | 1,2,3,4,5,8 | Tobin WO et al. 2014 [5] |
| 25 | 46 | Man | 2,3,4,6 | Tobin WO et al. 2014 [5] |
| 24 | 40 | Female | 1,2,3,4 | Stoeck K et al. 2015 [7] |
| 26 | 52 | Man | 1,2,3,5 | Stokin GB et al. 2015 [7] |
| 27 | 36 | Female | 1,2,3,4,5,6 | Hara M et al. 2017 [4] |
| 28 | 52 | Man | 1,2,3,5,9 | Hara M et al. 2017 [4] |
| 29 | 68 | Man | 1,2,3,4,8 | Hara M et al. 2017 [4] |
| 30 | 67 | Man | 1,2,3,5,8,9 | Hara M et al [4]. 2017 |
| 31 | 49 | Man | 1,2,3,5 | Hara M et al [4]. 2017 |
| 32 | 57 | Man | 1,2,3,4,5 | Hara M et al. 2017 [4] |
| 33 | 45 | Female | 1,2,4,5,7 | Hara M et al. 2017 [4] |
| 34 | 57 | Man | 1,2,3,4,5,7 | Hara M et al. 2017 [4] |
| 35 | 69 | Man | 1,5 | Hara M et al. 2017 [4] |
| 36 | 72 | Man | 1,3,5,8 | Zhou Q et al. 2020 [10] |
| 37 | 77 | Man | 1,2,3,4,5,8 | Deuel LM et al. 2020 [9] |
| 38 | 53 | Man | 1,2,3,4,5,8 | Ye L et al. 2020 [11] |
| 39 | 44 | Female | 1,2,3,4,5,8 | Mbonde AA et al. 2021 [8] |
| 40 | 54 | Man | 1,2,3,4,5,7,8 | Mbonde AA et al. 2021 [8] |
In our patient, DPPX-IgG was detected positive in serum but not in CSF although we used the more sensitive cell-based assay rather than immunofluorescence. According to literature reports, in most patients with anti-DPPX encephalitis, both serum and CSF were positive for DPPX antibody [4, 5]. However, the serum DPPX antibody titers were significantly higher than the CSF DPPX antibody titer, which suggested that the positive rate of DPPX antibody in serum was higher than that in CSF [4, 5]. Combined with the clinical features, negative antibody profile of paraneoplastic syndromes, brain MRI, and cerebrospinal fluid characteristics, we excluded other diseases such as tumor, paraneoplastic syndrome and metabolic disease in this patient. Moreover, for acute or subacute cerebellar ataxia, we need to consider post-infectious cerebellar ataxia. Post-infectious cerebellar ataxia is an exclusive diagnosis and affects mainly younger children. Nussinovitch M et al. and Connolly AM et al. described in detail the clinical features of 39 and 73 children patients, respectively, and the mean age at presentation for these children was 4.8 ± 3.8 years and 7.4 ± 6.0 years [12, 13]. In addition, a prodromal varicella or mumps was noted in 31% or 20% children post-infectious cerebellar ataxia patients [12]. Of course, post-infectious cerebellar ataxia also occurs rarely in adults. Klockgether T et al. examined 11 adult patients (mean age, 40.7 ± 15.2 years) and 73% of the patients showed cerebellar oculomotor disturbances [14]. Our patient was 18 years old and had no prodromal varicella, mumps or cerebellar oculomotor disturbances so we didn’t consider this patient as post-infectious cerebellar ataxia. Furthermore, autoimmune encephalitis panel demonstrated positive DPPX antibody in serum, and the symptoms in this patient marked improved and DPPX antibody became negative after immunotherapy. Taken together, this patient should be considered as anti-DPPX antibody encephalitis. The antibody titer (1:10) in our patient was lower than other reported cases, which may explain the patient only has cerebellar symptoms.
The identified autoantibodies which present pure or primarily cerebellar ataxia can be divided into two categories: 1) specific autoantibodies, including anti-gliadin, TG2, and TG6 in gluten ataxia, and anti-Yo, Hu, CV2, Ri, Ma2 and Tr for paraneoplastic cerebellar degeneration. 2) nonspecific autoantibodies found in various neurological conditions, which assumed to have pathogenic roles in the cerebellar ataxia and include anti-VGCC, DPPX, LGI1, CASPR2, mGluR1, GAD65 and MAG [15, 16]. In order to reveal the cause of our patient, we sent autoimmune encephalitis antibody panel and paraneoplastic syndrome antibody panel which included the autoantibodies above. Finally, we found that only the DPPX antibody was positive, and the rest for pure or primarily cerebellar ataxia were negative.
In conclusion, it is important to recognize the uncommon symptoms of anti-DPPX encephalitis including cerebellar ataxia, psychiatric symptoms, dysautonomia, sleep disorder, and seizures in addition to the classic triad (diarrhea/weight loss, hyperexcitability and cognitive impairment). Fast identification of rare symptoms can lead to quickly diagnosis and effective treatment.
Acknowledgements
Not applicable.
Abbreviations
- NMDAR
N-methyl-D-aspartate receptor
- CASPR2
Contactin-associated protein 2
- DPPX
Dipeptidyl-peptidase-like protein 6
- MRI
Magnetic resonance imaging
- CSF
Cerebrospinal fluid
- TG
Transglutaminase
- VGCC
Voltage gated calcium channel
- LGI1
Leucine-rich glioma-inactivated 1
- mGluR1
Metabotropic glutamate receptor
- GAD65
Glutamic acid decarboxylase 65
- MAG
Myelin-associated glycoprotein
Authors’ contributions
J.L: patient management, literature review, initial draft manuscript preparation. M.Z: literature review, analysis of the radiologic data. X.C.M: perform autoimmune encephalitis panel. Z.Q.J: perform other antibodies panel associated with tumors. M.H.Z: patient management, literature review. D.J.H: concept and design of the study, revise the manuscript, final approval of the version to be published. All authors have read and approved the final manuscript.
Funding
Dr. Lin is supported by YFYPY202012, Young Talent Research and Cultivation Fund of the First Affiliated Hospital of Nanchang University. Dr. Hong is supported by JXSQ2019101021, Double Thousand Talents Program of Jiangxi Province. The design and data collection of this study were supported by YFYPY202012 and JXSQ2019101021. The funding body was not involved in the data interpretation and writing.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The study is approved by ethics committee of the first affiliated hospital of Nanchang University.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lancaster E, Dalmau J. Neuronal autoantigens–pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–390. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Rosenfeld MR. Autoimmune encephalitis update. Neuro Oncol. 2014;16:771–778. doi: 10.1093/neuonc/nou030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boronat A, Gelfand JM, Gresa-Arribas N, Jeong H-Y, Walsh M, Roberts K, et al. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of kv4.2 potassium channels. Ann Neurol. 2013;73:120–128. doi: 10.1002/ana.23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hara M, Ariño H, Petit-Pedrol M, Sabater L, Titulaer MJ, Martinez-Hernandez E, et al. Dppx antibody-associated encephalitis: Main syndrome and antibody effects. Neurology. 2017;88:1340–1348. doi: 10.1212/WNL.0000000000003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobin WO, Lennon VA, Komorowski L, Probst C, Clardy SL, Aksamit AJ, et al. Dppx potassium channel antibody: frequency, clinical accompaniments, and outcomes in 20 patients. Neurology. 2014;83:1797–1803. doi: 10.1212/WNL.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balint B, Jarius S, Nagel S, Haberkorn U, Probst C, Blöcker IM, et al. Progressive encephalomyelitis with rigidity and myoclonus: a new variant with dppx antibodies. Neurology. 2014;82:1521–1528. doi: 10.1212/WNL.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 7.Stoeck K, Carstens P-O, Jarius S, Raddatz D, Stöcker W, Wildemann B, et al. Prednisolone and azathioprine are effective in dppx antibody-positive autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 2015;2:e86. doi: 10.1212/NXI.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mbonde AA, Gritsch D, Arca K, Blech B, McKeon A, Carter J. Dppx autoimmune encephalitis: a short report on two patients presenting with classic features of this rare but treatable disease. Mult Scler Relat Disord. 2021;52:102934. doi: 10.1016/j.msard.2021.102934. [DOI] [PubMed] [Google Scholar]
- 9.Deuel LM, Yu CH, Vaughan CL, Piquet AL. Oro-bucco-lingual dyskinesia, weight loss, and cognitive decline in anti-dppx antibody-mediated encephalitis. Mov Disord Clin Pract. 2020;7:S80–S82. doi: 10.1002/mdc3.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, Zhu X, Meng H, Zhang M, Chen S. Anti-dipeptidyl-peptidase-like protein 6 encephalitis, a rare cause of reversible rapid progressive dementia and insomnia. J Neuroimmunol. 2020;339:577114. doi: 10.1016/j.jneuroim.2019.577114. [DOI] [PubMed] [Google Scholar]
- 11.Ye L, Schnegelsberg M, Obermann M. Dipeptidyl-peptidase-like protein 6 encephalitis treated with immunotherapy. Proc (Bayl Univ Med Cent) 2020;34:114–115. doi: 10.1080/08998280.2020.1822132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nussinovitch M, Prais D, Volovitz B, Shapiro R, Amir J. Post-infectious acute cerebellar ataxia in children. Clin Pediatr (Phila) 2003;42:581–584. doi: 10.1177/000992280304200702. [DOI] [PubMed] [Google Scholar]
- 13.Connolly AM, Dodson WE, Prensky AL, Rust RS. Course and outcome of acute cerebellar ataxia. Ann Neurol. 1994;35:673–679. doi: 10.1002/ana.410350607. [DOI] [PubMed] [Google Scholar]
- 14.Klockgether T, Döller G, Wüllner U, Petersen D, Dichgans J. Cerebellar encephalitis in adults. J Neurol. 1993;240:17–20. doi: 10.1007/BF00838440. [DOI] [PubMed] [Google Scholar]
- 15.Mitoma H, Manto M, Hampe CS. Immune-mediated cerebellar ataxias: practical guidelines and therapeutic challenges. Curr Neuropharmacol. 2019;17:33–58. doi: 10.2174/1570159X16666180917105033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitoma H, Manto M, Hadjivassiliou M. Immune-mediated cerebellar ataxias: clinical diagnosis and treatment based on immunological and physiological mechanisms. J Mov Disord. 2021;14:10–28. doi: 10.14802/jmd.20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


