Abstract
We have developed a fast and accurate method that uses a small volume of sample to determine over 25 of the typically reported amino acids in human plasma. Samples were prepped with a single step using a spin filter to remove proteins, avoiding the decreased sensitivity from dilution in acid precipitation. Using a reverse phase (RP) High Performance Liquid Chromatography (HPLC) system with O-phthaldehyde (OPA) as the pre-column derivatization reagent, and UV detection at 338 nm, we did a direct comparison with the most common ion exchange/ninhydrin method used in clinical labs on the same plasma samples with 95% concurrence, analysis of amino acid standard solutions returned 99% concurrence. With a sample preparation time of 30 min, utilizing less than 25 μl of sample and with a chromatography run of 30 min, this method can substantially increase access to analysis in both clinical and research laboratories using instruments that are more widely available.
Synopsis
We describe a rapid and easily deployed method for sensitive amino measurement in biological samples.
Keywords: Amino acid, Inborn error of metabolism, IEM, HPLC, Clinical biochemistry
1. Introduction
The measurement of amino acids in fluids is a basic analytic tool for the field of inborn errors of metabolism. Amino acid concentration levels in plasma are in the micromolar (uM) range and can also be found in most body fluids and tissues [1]. Their role in measurement is primarily in diagnosing and monitoring inborn errors of metabolism like, maple syrup urine disease [2], phenylketonuria [3], organic acidemias [4], homocystinuria [5], tyrosinemia [6], and urea cycle disorders [7]. Their importance can be summarized by the many different methods developed over the years for measuring these biomarkers [[8], [9], [10], [11], [12]]. The timely measurement of amino acids is highly impactful on the care of these patients [13]. With the widespread growth of newborn screening internationally, a rapid, small-volume, and reliable method of amino acid measurement on widely available standard lab equipment can benefit the field.
Currently the most common method of AA measurement utilizes ion-exchange chromatography with UV-colorimetric detection based on the chromophore formed when a primary amino acid reacts with ninhydrin [14,15]. This reaction has been used for over 60 years with a high degree of confidence; however, a shortcoming of this method is the 2-to-3-h runtimes per sample [14]. In the last 20 years, other methods using HPLC, and LCMS have been published with varying degrees of adaptation but often do not cover the full range of clinically relevant amino acids described here [8,9,12,[14], [15], [16], [17]].
We examined each step in the process of AA measurement and sought to optimize it for sample use, time, reproducibility, and equipment use. In this work, we outline the specifics of the resulting method from this work, show results from biological samples, demonstrate the linearity of the method across a range of concentrations, measure the recovery using biological samples spiked with known amounts of several amino acids, and show the increase in sensitivity of a spin-filter preparation method over acid precipitation. In this method, all amino acid measurements were performed by reverse phase HPLC, using a two buffers gradient system (instead of the five buffer systems used commonly with most ion exchange systems) with a widely available C18 column [9].
Sample preparation was done through the use of an economical 3 K centrifuge filter instead of an acid or alcohol precipitation. This filtration step avoids the formation of precipitates in the sample which can clog the analytical column. The filter has an added advantage of not diluting the sample, which improves the detection limits of the assay and allows less sample to be used.
The authors hope that this method may increase access to amino acid measurement in laboratories where standard HPLC equipment is available. This method can also decrease the required sample volume which is important for newborns and patients already having large volume blood draws. The reduction in analytical time may also provide more timely information for clinical decision making.
2. Materials & methods
2.1. Chemicals and columns
The Infinity Lab Poroshell 120, 2.7 μm analytical column (C18) and the corresponding guard column, borate buffer and O-phthaldehyde (OPA) reagent were purchased from Agilent Technologies (Santa Clara, CA). HPLC grade Acetonitrile, methanol, and water were purchased from VWR International (Radnor, PA). Individual amino acids as well as an amino acid standard, and all other chemicals were purchased from Sigma Aldrich (St. Louis, MO). Argon gas was purchased from Roberts Oxygen Co (Rockville Md).
2.2. Equipment
The 1260 Infinity II LC System was purchased from Agilent Technologies (Santa Clara, CA) It should be noted we have a 40 uL syringe and sample loop for our HPLC system. The Centrifuge 5417c was purchased from Eppendorf (Hamburg, Germany). The vortex mixer was purchased from BioExpress (Kaysville, UT). The 3 k centrifuge filters (VWR Spin filter 3 k, 82,031–346) were purchased from VWR international.
2.3. Chromatographic conditions
A binary mobile phase consisting of solution A, 20 mM sodium phosphate (dibasic), 20 mM sodium borate, and 5 mM sodium azide, adjusted pH to 7.2 and solution B, a mixture of 45% acetonitrile, 45% methanol, and 10% water were used. Programming for the chromatographic run starts with 100% of solvent A, 0% solvent B and over a 30-min time course reduces A to 0% and B to 100% as shown in Table 1. Then the column will equilibrate back to 100% solvent A over 10 min. The column temperature was held constant at 40 °C while the sample tray was maintained at 4 °C. UV detection was performed at 338 nm.
Table 1.
Chromatographic program.
| Time (min) | Solvent A (%) | Solvent B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 6 | 90 | 10 |
| 13.5 | 80 | 20 |
| 18 | 80 | 20 |
| 25 | 60 | 40 |
| 26 | 60 | 40 |
| 29 | 40 | 60 |
| 30 | 0 | 100 |
| 34 | 0 | 100 |
| 35 | 100 | 0 |
| 40 | 100 | 0 |
Mobile Phase Concentration Over Time (includes column equilibration min 30–40).
2.4. Derivatization and injection
OPA purchased from Agilent was used to derivatize the primary amino group. OPA has been tested before but not commonly used since its formed derivatives are only stable for 2–6 h [12]. We have taken advantage of the advent of programable injectors, and derivatize samples in the injector's needle immediately before column injection. This allows us the benefit of the stronger absorbance of the OPA reagent in the 30-min run time. The injector was programmed to draw 16 μl of borate buffer pH 10.4 then 4 μl of the sample and mix with 10 μl of air, then draw 3 μl from OPA reagent, mix with 10 μl of air, wait 2 min, and inject 23 μl. It is important to note if you store the OPA solution under Argon in a sample vial, it will be stable for up to 2 weeks. The program steps are outlined in Table 2.
Table 2.
Programming steps for RD HPLC run. Specific for an Agilent model 1260.
| Injector Program Primary AA on Agilent model 1260 or 1290 | |
|---|---|
| Draw | Draw 16 μl of Borate buffer with default speed using default offset. |
| Draw | Draw 4 μl from sample with default speed using default offset. |
| Mix | Mix 10 μl from air with default speed 5 times. |
| Wait | Wait 0.2 min. |
| Draw | Draw 3 μl of OPA with default speed using default offset. |
| Mix | Mix 10 μl from air with default speed 7 times. |
| Wait | Wait 2 min. |
| Inject | Inject. |
| Wait | Wait 0.5 min. |
| Valve | Switch valve to “bypass”. |
2.4.1. Biological sample:
As part of this quality improvement effort, human samples were collected in standard EDTA tubes for routine amino acid analysis and frozen residuals used for our analysis without identifiers. An aliquot of sample was centrifuged at 1200g for 10 min. Plasma and red blood cells were collected in 100 μl aliquots and stored in −80 °C. Personnel were blinded to the origin of the sample and comparison results were provided without patient ID or clinical information.
2.4.2. Biological sample preparation
Filter Sample Preparation:
A plasma (or other liquid sample) aliquot is transferred to a pre-wet 3 K centrifugal filter and centrifuged for 20 min at 9000g. The liquid is collected and transferred to vials for injection on the HPLC. The 3 K filter is a polypropylene 1.7 ml sample tube with a PES membrane insert which traps structures greater than 3000 MW to remove excess proteins which could clog HPLC column and filter. The filter has a 5 ul hold back volume which requires a pre-wet of the filter before use. We pass 50 ul of PBS and discard the eluent before adding any sample.
2.4.3. Acid precipitation sample preparation
The method used in most clinical labs uses acid precipitation. To a 50 μl aliquot of plasma add 100 μl of 0.15 M sulfosalicylic acid, and place on ice for 30 min to allow the proteins to precipitate before centrifuging for 10 min at 14000g. Supernatant is collected and transferred to vials for injection on HPLC.
2.4.4. Amino acid standards solutions
5 mM stocks of the individual amino acids were prepared separately in 0.1 N hydrochloric acid and stored at 4 °C. To check linearity and reproducibility, concentrations of 5–200 μM were made by diluting stocks with PBS. Standard concentrations were prepared fresh on the day of analysis.
3. Results and discussion
3.1. Standard curves over a range of concentration
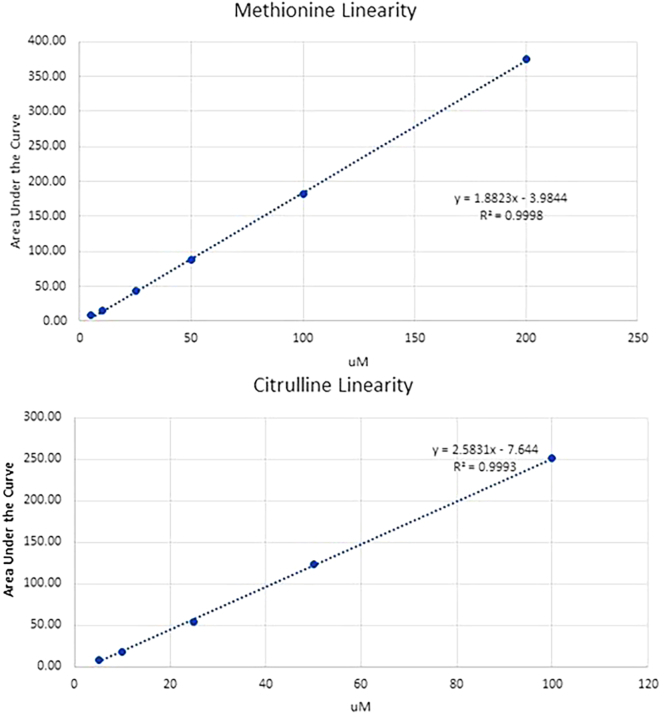
We show, as examples of linearity of measurement, two amino acids over a concentration range of 5 to 200 uM and 5 to 100 uM in Fig. 1A and b. These standards were done in triplicates for each concentration, and it should be noted the r2 values of 0.98 or higher are typical for all amino acids (data available).
Fig. 1.
A-B Methionine and citrulline concentration curve.
3.2. Amino acids chromatograph
Each amino acid was injected independently to resolve its retention time, and if needed the chromatic program was adjusted to avoid co-migrating of amino acids in order to achieve near baseline resolution. Fig. 2 shows a sample of our amino acid standard run under our method's conditions.
Fig. 2.
Sample chromatogram from our method. 100 μM concentration of all amino acid except cystine, beta-alanine, and homocystine which are 50 μM and our internal standard D-2-Aminobutyric acid (AABA) at 500 μM.
3.2.1. Comparing 3 K filter results against the acid precipitation method
In comparing the methods of using a 3 K centrifugal filter for protein removal to the more common method of acid precipitation used in plasma preparation we compared a split sample with 3 repeats for each method. The results are shown in Table 3. The ratio of the two signals confirms that using the filters increases the signal strength of most amino acids. This is probably the result of increased sensitivity by not diluting the sample and avoiding loss through precipitation or acid modification.
Table 3.
Comparison of detected concentrations between preparation with a 3 K filter and acid precipitation. Three repeats for each method were performed.
| Amino Acid | 3 K Filter Avg. μM | Acid Precipitation Avg. μM | Ratio Acid Precip. to 3 K Filter |
|---|---|---|---|
| Glutamic Acid | 87.05 ± 0.52 | 70.97 ± 3.04 | 1.23 |
| Asparagine | 54.13 ± 0.15 | 46.93 ± 1.04 | 1.15 |
| Serine | 97.05 ± 0.8 | 84.99 ± 4.32 | 1.14 |
| Glutamine | 601.06 ± 1.35 | 554.32 ± 12.83 | 1.08 |
| Histidine | 54.56 ± 0.15 | 48.11 ± 2.86 | 1.13 |
| Glycine | 233.06 ± 0.45 | 237.97 ± 8.58 | 0.98 |
| Threonine | 85.23 ± 0.75 | 76.69 ± 2.02 | 1.11 |
| Citrulline | 12.94 ± 0.34 | 11.68 ± 0.75 | 1.11 |
| Arginine | 74.89 ± 0.18 | 72.47 ± 1.7 | 1.03 |
| Alanine | 408.51 ± 40.97 | 334.81 ± 20.05 | 1.22 |
| Tyrosine | 59.23 ± 0.35 | 54.66 ± 1.73 | 1.08 |
| Valine | 218.43 ± 0.25 | 199.63 ± 4.49 | 1.09 |
| Methionine | 17.52 ± 0.14 | 16.38 ± 0.76 | 1.07 |
| Cystine | 144.29 ± 0.65 | 130.49 ± 3.35 | 1.11 |
| Beta alanine | 40.89 ± 0.36 | 37 ± 3.35 | 1.11 |
| Tryptophan | 6.34 ± 0.21 | 44.43 ± 1.24 | 0.14 |
| Isoleucine | 44.14 ± 0.49 | 44.7 ± 1.42 | 0.99 |
| Phenylalanine | 68.42 ± 0.38 | 62.53 ± 1.29 | 1.09 |
| Leucine | 123.74 ± 0.3 | 114.9 ± 2.97 | 1.08 |
| ornithine | 78.88 ± 0.72 | 72.85 ± 3.76 | 1.08 |
| Lysine | 173.1 ± 0.21 | 165.41 ± 5.92 | 1.05 |
| Ammonium | 11.98 ± 0.27 | 16.91 ± 3.03 | 0.71 |
Of note, the filter processed Tryptophan is much lower than the acid precipitation. The filter was tested for tryptophan retention and was negative. The lower levels are most likely a result of reported binding of tryptophan to albumin which would be released by acid precipitation [18]. Glutamate and alanine elevations in the non-acid precipitated sample suggesting an effect of the acid on the molecules.
3.3. Recovery study of spiked amino acids
A single plasma sample was divided into 10 equal aliquots. As described, the samples were spun using the 3 K filter. To five of these samples 50 μM each of arginine, tyrosine, methionine, and isoleucine was added and none to the other 5. Samples were derivatized and run as described. Table 4 compares the amino acids in the 5 plasma aliquots from each group. Good recovery of the 50uM was observed in the spiked aliquots (94–110%).
Table 4.
Recovery of Spiked 50 μM Amino Acids using one plasma sample split into 10 aliquots with amino acids added to each aliquot before processing.
| Amino Acid Added | Unspiked Plasma μM ± SD (n = 5) | Spiked Plasma μM ± SD (n = 5) | Average Difference μM ± SD (Recovery) | % Recovery Average |
|---|---|---|---|---|
| Arginine | 74 ± 2.7 | 123 ± 6.4 | 50 ± 3.9 | 100% |
| Tyrosine | 67 ± 2.7 | 118 ± 5.6 | 51 ± 3.7 | 102% |
| Methionine | 15 ± 1.3 | 61 ± 4.1 | 47 ± 3.2 | 94% |
| Isoleucine | 71 ± 5.2 | 126 ± 8.2 | 55 ± 5.5 | 110% |
3.3.1. Comparing clinical laboratory results to our protocol
Using ten de-identified 1-day old frozen discard samples from the clinical laboratory, we compared concentration measurements by our method and the normal clinical laboratory method. The clinical laboratory uses a standard ion-exchange chromatography method with ninhydrin derivatization (average run time 3 h). Table 5 shows the averages for the amino acid measures and the ratios between the methods (our method vs clinical lab). Agreement is close between the samples and except for tryptophan and glutamic acid which was expected from the filter vs acid precipitation measurements. Of note also is that glutamic acid had the highest patient to patient variability of the amino acids.
Table 5.
Comparison of 10 de-identified split patient samples between the clinical laboratory and our OPA method.⁎+
| Amino Acid |
|
|
Ratio A/B |
|---|---|---|---|
| Beta alanine | 0 | 0 | |
| Homocystine | 0 | 0 | |
| L-allo-isoleucine | 0 | 0 | |
| Aspartic Acid | 3.2 | 3.6 | 0.89 |
| Tryptophan | 41.8 | 4.5 | 9.29 |
| Methionine | 23.1 | 20.2 | 1.14 |
| Cystine | 35.3 | 32.1 | 1.10 |
| Phenylalanine | 46.6 | 42 | 1.11 |
| Citrulline | 58.1 | 51.9 | 1.12 |
| Asparagine | 54.2 | 54.3 | 1.00 |
| Isoleucine | 50.1 | 54.6 | 0.92 |
| Taurine | 57.2 | 58.6 | 0.98 |
| Glutamic Acid | 41.9 | 59.2 | 0.71 |
| Tyrosine | 71.1 | 63.6 | 1.12 |
| Arginine | 64.9 | 65.7 | 0.99 |
| Ornithine | 61.3 | 70 | 0.88 |
| Histidine | 73.8 | 70.1 | 1.05 |
| Threonine | 100.1 | 83.3 | 1.20 |
| Leucine | 95.5 | 90.4 | 1.06 |
| Lysine | 128.3 | 120.7 | 1.06 |
| Serine | 132.7 | 129.3 | 1.03 |
| Valine | 190.4 | 171.2 | 1.11 |
| Alanine | 320.8 | 319.8 | 1.00 |
| Glycine | 359.5 | 379.1 | 0.95 |
| Glutamine | 521.8 | 514.2 | 1.01 |
Note the expected differences of Tryptophan and Glutamate due to differences in preparation (see discussion). Samples are sorted by average concentration from lowest to highest.
4. Conclusion
We have shown that we can quantify amino acid levels in plasma via an RP HPLC method of 30 min run time followed by 10 min for wash and equilibration. This method uses 25 μl (or less) of sample with a preparation time of under 30 min. We are able to see the complete range of amino acid compounds detected by current clinical measurement. This method uses less volume and we have consistently achieved reproducible results with 10 μl of sample (data available). While the preparation time is comparable to current methods, the run time is only 15–20% of the current standard methods. The instrumentation and materials for this method are more cost-effective than traditional ion-exchange systems and widely available in most clinical labs. The use of filtration improves the sensitivity without the dilution from acid precipitation allowing more accuracy at lower concentrations of amino acids. The changes in sensitivity and the use of filtration would necessitate establishing local standards before use in clinical diagnostics. Furthermore, our data suggests that a number of amino acid concentrations are affected by acid precipitation such as the decreases in alanine and glutamate noted. It is well known that tryptophan binds to albumin and this difference is reflected in our results which measures only unbound or free tryptophan [18]. The consistent recovery of added amino acids shows the reliability and robustness of the assay.
As the number of patients diagnosed with inborn errors of amino acid metabolism has increased globally the access to rapid testing has become more important. Rapid turnaround of results can be critical to patient management and therapeutic decision making for patients in crisis and for routine care. The relative cost-effectiveness of this method can either increase the capacity of existing laboratories or create capacity in laboratories where it doesn't exist. In regions where there are resource limits, this can improve diagnostic access and outcome for patients and their families. In the research sphere, this lower cost method can increase throughput and provide broader sample analytics. The authors hope this method will expand access to amino acid measurements in clinical and research environments, leading to more rapid diagnosis of patients at a lower cost.
The following are the supplementary data related to this article.
Supplementary Fig.
Typical Plasma Amino Acid Profile Using our Method
Supplementary material
Acknowledgments
We want to thank Jessica Albert and Richard Scott of the clinical laboratory for their cooperation. We wish to thank the students and lab personnel: Clarissa Halpern, Fiona Lee, Angel Thompson, and Jenny Mak, who's hard work over the years helped so much with the development of this method.
References
- 1.Akram M., Asif H.M., Akhtar Naveed, Madni Asadullah, Shah S.M. Ali, ul Hasan Zahoor, Ullah Asmat. Amino acids: a review article. J. Med. Plants Res. 9 September 2011;5(17):3997–4000. [Google Scholar]
- 2.Blackburn Patrick R., Gass Jennifer M., e Vairo Filippo Pinto, Farnham Kristen M., Atwa Herjot K., Sarah Macklin, Klee Eric W., Atwal Paldeep S. Maple syrup urine disease: mechanisms and management. Appl. Clin. Genet. 2017;10:57–66. doi: 10.2147/TACG.S125962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John Christodoulou, Naz Al Hafid. Phenylketonuria: a review of current and future treatments. Translat. Pediat. October 2015;4(4):314–317. doi: 10.3978/j.issn.2224-4336.2015.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannan Vaidyanathan, Narayanan M.P., Vasudevan D.M. Organic acidurias: an updated review. Ind. J. Clin. Biochem. October. 2011;26(4):319–325. doi: 10.1007/s12291-011-0134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry I.J. Homocysteine, hypertension, and stroke. J. Hum. Hypertens. 1999;13:289–293. doi: 10.1038/sj.jhh.1000803. [DOI] [PubMed] [Google Scholar]
- 6.Chinsky Jeffrey M., Rani Singh, Can Ficicioglu, Clara van Karnebeek, Markus Gormpe, Grant Mitchell, Susan Waisbren, Muge Gucsavas-Calikoglu, Melissa Wasserstein, Katie Coakley. Vol. 3. August 2017. Scott Ronald. Diagnosis and treatment of tyrosinemia type I: a US and Canadian consensus group review and recommendations. American College of Medical Genetics and Genomics. Advance online publication. PP1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall Summar. 2018. Urea Cycle Disorders (UCD). NORD Physician Guide to the Urea Cycle Disorders (UCD) [Google Scholar]
- 8.Walker V., Mills G. Quantitative methods for amino acid analysis in biological fluids. Ann. Clin. Biochem. 1995;32:28–57. doi: 10.1177/000456329503200103. [DOI] [PubMed] [Google Scholar]
- 9.Ibolya Molnar-Perl. Advancement in the derivatizations of the amino groups with the o-phthaldehyde-thiol and with the 9-fluorenylmethyloxycarbonyl chloride reagents. J. Chromatogr. B. 2011;879:1241–1269. doi: 10.1016/j.jchromb.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Csapo J., Cs Albert, Loki K., Zs Csapo-Kiss. Separation and determination of the amino acids by ion exchange column chromatography applying postcolumn derivatization. Acta Univ. Sapientiae, Alimentaria. 2008;1 5{29. [Google Scholar]
- 11.Kaspar H., Dettmar K., Gronwald W., Oefner P. Automated GC–MS analysis of free amino acids in biological fluids. J. Chromatogr. B. 2008;870:222–232. doi: 10.1016/j.jchromb.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong M., Jonscher K., Reisdorph N. Analysis of 25 underivatized amino acids in human plasma using ion-pairing reversed-phase liquid chromatography/time-of-flight mass spectrometry. Rapid Commun. Mass Spectrum. 2007;21:2717–2726. doi: 10.1002/rcm.3124. [DOI] [PubMed] [Google Scholar]
- 13.Barbara Burton. Inborn errors of metabolism in infancy, a guide to diagnosis. Pediatrics. Dec 1998;102(6) doi: 10.1542/peds.102.6.e69. [DOI] [PubMed] [Google Scholar]
- 14.Hyman Rosen. A modified Ninhydrin colorimetric analysis for amino acids. Arch. Biochem. Biophys. March 1957;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- 15.Yemm E.W., Cocking C., Ricketts R.E. The determination of amino-acids with Ninhydrin. Analyst. 1955;80(948):209. [Google Scholar]
- 16.Durk Fekkes. State-of-the-art of high-performance liquid chromatographic analysis of amino acids in physiological samples. J. Chromatogr. B. 1996;682:3 22. doi: 10.1016/0378-4347(96)00057-6. [DOI] [PubMed] [Google Scholar]
- 17.Lookhart G., Jones B. High performance liquid chromatography analysis of amino acids at the picomole level. Cereal Chem. 2021;62(2):97–102. [Google Scholar]
- 18.Rapier McMenamy, Oncely J.L. The specific binding of L-tryptophan to serum albumin. J. Biochem. 1958;233:1436–1447. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material