Abstract
Glycogen storage disease type 1a (GSD Ia) is an inborn error of carbohydrate metabolism. Despite severe hyperlipidemia, GSD Ia patients show limited atherogenesis compared to age-and-gender matched controls. Employing a GSD Ia mouse model that resembles the severe hyperlipidemia in patients, we here found increased atherogenesis in GSD Ia. These data provide a rationale for investigating atherogenesis in GSD Ia in a larger patient cohort.
Keywords: Glycogen storage disease type 1a, Atherosclerosis, Hyperlipidemia
Abbrevations: GSD Ia, Glycogen storage disease type 1a; G6PC1, Glucose-6-phosphatase enzyme; G6P, Glucose-6-phosphate; HDL, High-density lipoprotein; Ldlr−/−, LDL receptor deficient; SFA, Saturated fatty acids; PUFA, Poly-unsaturated FA; TG, Triglycerides; VLDL, Very-low-density lipoprotein; WTD, Western-type diet
1. Introduction
Glycogen storage disease type 1a (GSD Ia) is an inborn error of carbohydrate metabolism, caused by mutations in the gene encoding the catalytic subunit of glucose-6-phosphatase enzyme (G6PC1) [1]. G6PC1 is expressed by liver, kidney, and intestine, and is essential for conversion of glucose-6-phosphate (G6P) into glucose [2]. GSD Ia patients show severe hypoglycemia upon fasting and show 10-fold higher plasma very-low-density lipoprotein (VLDL)-triglycerides (TG) and 2-fold higher VLDL- and LDL-cholesterol compared to age-and-gender matched controls [3,4].
Despite severe hyperlipidemia, GSD Ia patients show a ~ 10% reduction in carotid intima media thickness compared to age-and-gender matched controls [5], indicative of decreased atherosclerotic lesion size in the carotid arteries. This decrease has been attributed to LDL particles being less susceptible to oxidation [4], due to increased saturated fatty acids (SFA) in plasma VLDL/LDL [4]. However, diets rich in SFA are associated with increased atherosclerosis in humans [6], and replacement of SFA with polyunsaturated fatty acids decreases atherosclerosis in mice [7]. Therefore, the decrease in atherosclerosis in GSD Ia cannot be explained by increased plasma SFA levels. Further research into the mechanisms driving the decreased atherogenesis in GSD Ia patients is thus needed. We previously employed hepatocyte-specific G6pc deficient mice as a model of GSD Ia. Although these mice are slightly hyperlipidemic during fasting, their plasma cholesterol mainly circulates in high-density lipoproteins (HDL) and not in VLDL or LDL [8]. This is similar to wild-type mice. For this reason wild-type mice do not develop atherosclerosis [9]. For atherosclerosis studies, mice deficient in the LDL receptor (Ldlr−/− mice) have been generated [10]. When fed a cholesterol-rich Western-type diet (WTD), Ldlr−/− mice show high levels of VLDL/LDL-cholesterol, and develop advanced atherogenesis with plaques comparable to humans [9]. Therefore, to study atherogenesis under hyperlipidemic conditions similar to those in GSD Ia patients, we generated hepatocyte-specific G6pc deficient mice on the Ldlr−/− background and fed these mice WTD.
2. Methods
2.1. Animals
B6.G6pclox/lox and B6.G6pclox/lox.SACreERT2 mice were intercrossed with Ldlr−/− (stock 002207; Jackson Laboratories, Bar Harbor, ME, USA) mice to generate B6.G6pclox/loxLdlr−/− and B6.G6pclox/lox.SACreERT2 Ldlr−/− mice. At 8–12 weeks of age, mice received intraperitoneal tamoxifen injections (T5648; Sigma-Aldrich, St. Louis, MO, USA) (1 mg/day in 95% sunflower oil/5% ethanol) for five consecutive days to induce hepatocyte-specific G6pc deficiency, as described previously [11]. We refer to B6.G6pclox/lox.SACreERT2Ldlr−/− and littermate B6.G6pclox/loxLdlr−/− mice as L-G6pc−/−Ldlr−/− and Ldlr−/− mice. Mice were housed in a light (lights on at 7:00 AM, lights off at 7:00 PM) and temperature (21 °C)-controlled facility and had ad libitum access to water and food. After tamoxifen injections, male and female mice were fed a chow diet (RMH-B, AB diets, Woerden, The Netherlands) for 4 weeks (recovery period), followed by WTD (40% fat, 0.15% cholesterol; D12079B, Research Diets, New Brunswick, NJ, USA) for 8 or 15 weeks. Mice were randomly assigned to experimental groups. The number of mice used for each experiment and the period of WTD feeding are indicated in the figure legends. No inclusion or exclusion criteria were used. Experiments were performed at 8:00 AM in fed condition or at 2:00 PM after a 6 h fasting period during the inactive period. Mice were sacrificed after 8 (~20 weeks old) or 15 (~27 weeks old) weeks of WTD feeding. All animal studies were approved by the Institutional Animal Care and Use Committee from the University of Groningen under permit number AVD105002015244 and adhered to guidelines set out in the 2010/63/EU directive.
2.2. Plasma lipoprotein analysis
Blood samples were collected by tail bleeding into EDTA-coated tubes. Plasma was separated by centrifugation and plasma cholesterol and TG levels were measured using enzymatic kits (113,009,910,026 and 157,109,910,917, respectively; Diasys Diagnostic Systems, Holzheim, Germany) with Cholesterol FS or Precimat Glycerol standard (113,009,910,030; Diasys Diagnostic Systems and 10,166,588; Roche, Mannheim, Germany, respectively) for the calibration curve. Lipoprotein cholesterol and triglyceride distribution were measured by fast performance liquid chromatography (FPLC) using a system containing a PU-4180 pump with a linear degasser and UV-4075 UV/VIS detectors (Jasco, Tokyo, Japan). Pooled plasma samples were injected onto a Superose 6 Increase 10/300 GL column (GE Healthcare, Hoevelaken, The Netherlands) and eluted at a constant flow rate of 0.31 mL/min in PBS (pH 7.4). Cholesterol or triglycerides were measured in line by addition of cholesterol or triglyceride reagent at a constant flow rate of 0.1 mL/min using an additional PU-4080i infusion pump (Jasco, Tokyo, Japan). Data acquisition and analysis were performed using ChromNav software (version 1.0; Jasco, Tokyo, Japan).
2.3. Atherosclerotic lesion analysis
Female Ldlr−/− and L-G6pc−/−Ldlr−/− mice were fed a WTD for 8 weeks and males for 15 weeks. Mice were sacrificed, hearts were isolated and fixed in 4% phosphate buffered paraformaldehyde, embedded in paraffin, and 4 μm sections of the aortic root area were made and stained with hematoxylin-eosin (H&E). Atherosclerotic lesion area was quantified using Image J software (NIH) and the average of 5 sections with 40 μm distance between the sections was calculated for each mouse.
2.4. Plasma uric acid measurement
Blood samples were collected by tail bleeding into EDTA-coated tubes. Plasma was separated by centrifugation and uric acid levels were measured using a uric acid kit (KA1651, Abnova, Tapei, Taiwan) according to the manufacturer's instructions.
2.5. Statistical analysis
All data are presented as mean ± SEM. The unpaired t-test was used to compare two datasets. Group size and statistical test are reported in the figure legends. The criterion for significance was set at P < 0.05. Statistical analysis was performed using GraphPad Prism 5.
3. Results
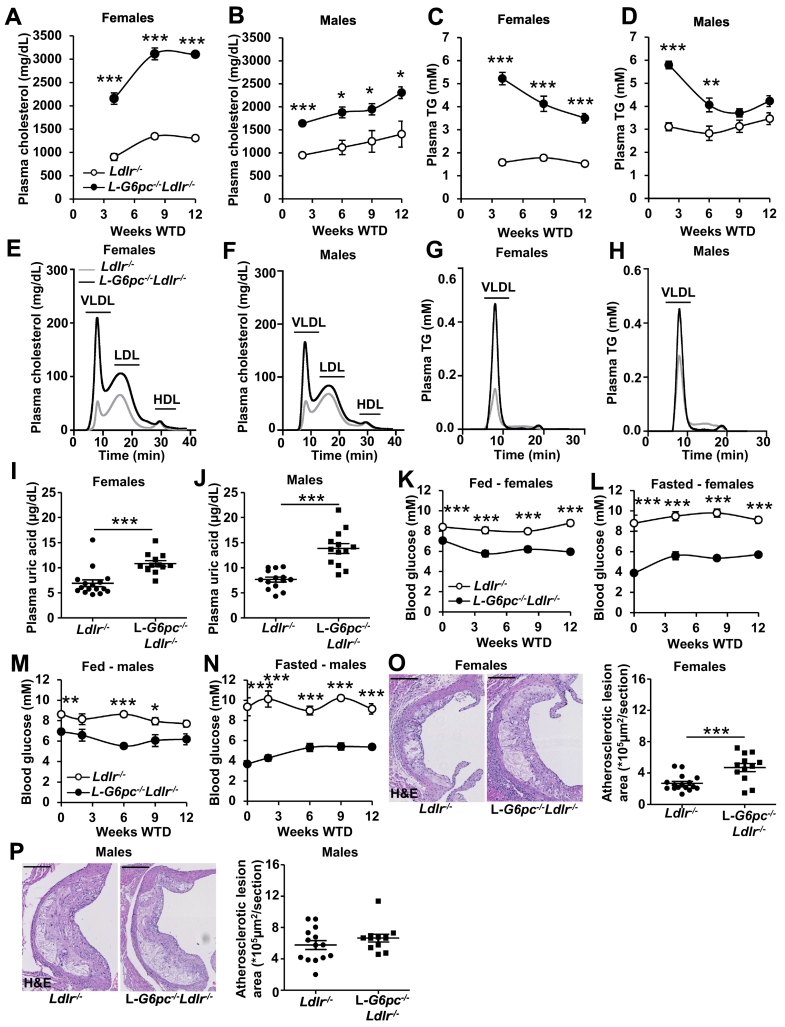
To investigate the role of hepatocyte-specific G6pc in atherogenesis, we generated L-G6pc−/−Ldlr−/− mice and Ldlr−/− littermate controls. On the Ldlr−/− background, hepatocyte-specific G6pc deficiency increased plasma cholesterol levels during WTD feeding by ~2.3-fold in female and by ~1.7-fold in male Ldlr−/− mice (Fig. 1A, B). Plasma cholesterol levels increased further over the course of WTD feeding, while the difference between the genotypes remained similar (Fig. 1A, B). Hepatocyte-specific G6pc deficiency increased plasma TG by ~2.3-fold in females and by ~1.9-fold in males at 2 weeks of WTD (Fig. 1C, D). These increases gradually declined during the study and were no longer different for male mice at 9 weeks of WTD (Fig. 1C, D). Sharing similarities with findings in GSD Ia patients, the increases in plasma lipids were reflected by an increase in VLDL- and LDL-cholesterol, as well as VLDL-TG (Fig. 1E-H). The decreased susceptibility of LDL to oxidation in GSD Ia patients has been attributed to elevated plasma concentrations of uric acid [12]. Consistently, L-G6pc−/−Ldlr−/− mice showed increased plasma uric acid levels compared to Ldlr−/− female and male mice fed WTD (Fig. 1I, J). We then measured blood glucose levels in mice fed chow or WTD. Similar to previous data [11,13], hepatocyte-specific G6pc1 deficiency induced hypoglycemia (blood glucose ≤4.0 mM) upon a 6 h fasting period (Fig. 1K-N). However, after 2 weeks of WTD, L-G6pc−/−Ldlr−/− mice showed blood glucose levels of ~5.5 mM in both fed and fasted conditions (Fig. 1K-N). This is likely due to the WTD being rich in sucrose (35% sucrose). Sucrose rapidly increases blood glucose levels to promote insulin release [14]. When fed chronically, sucrose lowers blood glucose levels in L-G6pc−/− mice as also shown upon high fat/high sucrose (HF/HS) diet feeding in a previous study [15].
Fig. 1.
Hepatocyte-specific G6pc1 deficiency increases plasma lipids and uric acid levels in female and male Ldlr−/−mice fed Western-type diet, and increases atherosclerotic lesion area in females. Hepatocyte-specific G6pc1 deficiency was induced by tamoxifen injections. After a recovery period of 4 weeks on chow diet, female and male L-G6pc−/−Ldlr−/− and Ldlr−/− mice were fed Western-type diet (WTD). Blood was collected after a 6 h fast (8:00 AM -2:00 PM) at the indicated time points on WTD and plasma cholesterol and triglyceride (TG) levels were determined. Lipoprotein fractions from pooled plasma samples (n = 8–15 per pool) were separated using fast performance liquid chromatography. (A, B) Plasma cholesterol levels in female (A) and male (B) L-G6pc−/−Ldlr−/− and Ldlr−/− mice. (C, D) Plasma TG levels in female (C) and male (D) L-G6pc−/−Ldlr−/− and Ldlr−/− mice. Data are shown as mean ± SEM. (E, F) Lipoprotein cholesterol distribution (8 weeks WTD) in female (E) and male (F) L-G6pc−/−Ldlr−/− and Ldlr−/− mice. (G, H) Lipoprotein TG distribution (8 weeks WTD) in female (G) and male (H) L-G6pc−/−Ldlr−/− and Ldlr−/− mice. (I, J) Plasma uric acid levels in female L-G6pc−/−Ldlr−/− and Ldlr−/− mice at 8 weeks WTD (I) and male L-G6pc−/−Ldlr−/− and Ldlr−/− mice at 15 weeks WTD (J). (K–N) At the indicated time points on WTD blood glucose levels were measured in the fed (8:00 AM) (K, M) and fasted (6 h fast from 8:00 AM - 2:00 PM) (L,N) condition in female (K,L) and male (M, N) L-G6pc−/−Ldlr−/− and Ldlr−/− mice. (n = 5–6 for males, n = 11–12 for females). (O, P) L-G6pc−/−Ldlr−/− and Ldlr−/− female mice were sacrificed after 8 weeks of WTD (O) and L-G6pc−/−Ldlr−/− and Ldlr−/− male mice after 15 weeks WTD (P). Hearts were isolated, sections were made of the aortic root, stained with hematoxylin-eosin (H&E) and atherosclerotic lesion area was quantified. Representative examples are shown. Scale bar represents 200 μm. (I, J, O, P) Each data point represents an individual mouse. (n = 13–14 for males, n = 12–16 for females). (I–P) Data are shown as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, by t-test.
We subsequently assessed atherosclerotic lesion area in these mice. In line with guidelines for atherosclerosis studies [9], we evaluated lesion area in mice of both genders. Given that atherosclerosis develops faster in female Ldlr−/− mice than in males [9], we fed female L-G6pc−/−Ldlr−/− and Ldlr−/− mice WTD for 8 weeks, and used 15 weeks of WTD for males. In contrast to findings on carotid intima media thickness in humans [5], but in line with the increases in plasma lipid levels, hepatocyte-specific G6pc deficiency increased atherosclerotic lesion area by ~1.5-fold in female Ldlr−/− mice after 8 weeks of WTD (Fig. 1O). Male Ldlr−/− mice fed WTD for 15 weeks showed larger atherosclerotic plaques than expected based on earlier studies [16]. Hepatocyte-specific G6pc deficiency tended to increase atherosclerotic lesion area in Ldlr−/− males fed WTD (Fig. 1P). As a consequence of the longer WTD feeding in males and the lesions in Ldlr−/− mice being larger than usual, we may have missed detecting a significant increase of hepatocyte-specific G6pc deficiency on atherosclerotic plaques, though we observed a tendency that did not reach statistical significance (p = 0.10). Together, hepatocyte-specific G6pc deficiency increases plasma lipid levels, which increases atherogenesis, at least in female WTD-fed Ldlr−/− mice, and shows a tendency to an increase in lesion size in males.
4. Discussion
GSD Ia patients show a ~ 10% decrease in carotid intima media thickness compared to age-and-gender matched controls [5], despite hyperlipidemia reflected by 10-fold higher plasma TG and 2-fold higher cholesterol levels [4,17]. Even though the patient cohort was small (n = 9 per group), these findings suggest that GSD Ia patients are less susceptible to atherogenesis [5]. To investigate mechanisms underlying this observation, we bred L-G6pc−/− mice on the Ldlr−/− background and fed them a cholesterol-rich WTD to induce hyperlipidemia and atherosclerosis. Similar to GSD Ia patients, hepatocyte-specific G6pc deficiency increased plasma lipids in mice of both genders, and increased plasma uric acid levels. However, unlike in GSD Ia patients, hepatocyte-specific G6pc deficiency increased atherosclerotic lesion size in female WTD-fed Ldlr−/− mice, while showing a tendency towards an increase in males.
Our data suggest, in contrast to findings in GSD Ia patients [5], that hyperlipidemia in a mouse model of GSD Ia does accelerate atherosclerosis. We also found that hepatocyte-specific G6pc deficiency increased plasma uric levels. While this increase is in line with findings in GSD Ia patients, it has been suggested that elevated plasma uric acids in GSD Ia decreases LDL oxidation, and may thus be athero-protective [12]. In contrast, several epidemiological studies and studies in animal models have shown that uric acid levels are associated with an increase in atherosclerosis [18]. These studies [18] thus suggest that the increase in plasma uric acid in mice with hepatocyte-specific G6pc deficiency enhances atherosclerosis.
Further, the discrepancy between our observations and the limited atherogenesis in GSD Ia patients may be due to the model that we used. Feeding Ldlr−/− mice WTD is one of the most frequently used models to induce atherogenesis [9]. WTD contains a high percentage of sucrose (35%), and sucrose rapidly increases blood glucose levels, leading to insulin release and hypoglycemia [14]. GSD Ia patients adhere to a strict diet to prevent fasting hypoglycemia [19], including avoiding sucrose intake [20]. The recommended diet for GSD Ia patients consists of 60–70% carbohydrates with a low glycemic index, 10–15% protein, and 15–30% fat [[20], [21], [22]]. We cannot exclude that the continuous low level of blood glucose in our model may have affected cells locally in the vessel wall, and therefore, increased atherogenesis, similar to findings in humans with hypoglycemia [23]. However, elevated plasma TG levels, as observed in GSD Ia patients [3,8,17,24], have, except for one study employing Apolipoprotein C3 (APOC3) overexpression in mice [25], a clear pro-atherogenic role in mice and in humans [[26], [27], [28], [29], [30], [31], [32]]. Since only 9 patients were included in the study on atherogenesis in GSD Ia [5], and in view of the pro-atherogenic role of triglycerides and plasma uric acid, our findings do provide a rationale to investigate atherogenesis in a larger GSD Ia patient cohort.
Source of funding
M.H. Oosterveer and M. Westerterp are supported by VIDI grants (917.17.373 and 917.15.350, respectively) from the Netherlands Organization of Scientific Research (NWO), and Rosalind Franklin Fellowships from the University of Groningen.
CRediT authorship contribution statement
Anouk M. La Rose: Conceptualization, Methodology, Investigation, Writing – original draft. Anouk G. Groenen: Resources, Writing – review & editing. Benedek Halmos: Resources, Writing – review & editing. Venetia Bazioti: Resources, Writing – review & editing. Martijn G.S. Rutten: Resources, Writing – review & editing. Kishore A. Krishnamurthy: Resources, Writing – review & editing. Mirjam H. Koster: Resources, Writing – review & editing. Niels J. Kloosterhuis: Resources, Writing – review & editing. Marieke Smit: Resources, Writing – review & editing. Rick Havinga: Resources, Writing – review & editing. Gilles Mithieux: Resources, Writing – review & editing. Fabienne Rajas: Resources, Writing – review & editing. Folkert Kuipers: Conceptualization, Writing – review & editing. Maaike H. Oosterveer: Conceptualization, Writing – review & editing. Marit Westerterp: Conceptualization, Methodology, Investigation, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
None.
References
- 1.Lei K.J., Chen Y.T., Chen H., Wong L.J.C., Liu J.L., McConkie-Rosell A., et al. Genetic basis of glycogen storage disease type 1a: prevalent mutations at the glucose-6-phosphatase locus. Am. J. Hum. Genet. 1995;57:766–771. [PMC free article] [PubMed] [Google Scholar]
- 2.Chou J.Y., Jun H.S., Mansfield B.C. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase/glucose-6-phosphate transporter complexes. J. Inherit. Metab. Dis. 2015;38:511–519. doi: 10.1007/s10545-014-9772-x. [DOI] [PubMed] [Google Scholar]
- 3.Bandsma R.H.J., Prinsen B.H., Van Der Velden M.D.S., Rake J.P., Boer T., Smit G.P.A., et al. Increased de novo lipogenesis and delayed conversion of large VLDL into intermediate density lipoprotein particles contribute to hyperlipidemia in glycogen storage disease type 1a. Pediatr. Res. 2008;63:702–707. doi: 10.1203/PDR.0b013e31816c9013. [DOI] [PubMed] [Google Scholar]
- 4.Bandsma R.H.J., Rake J.P., Visser G., Neese R.A., Hellerstein M.K., Van Duyvenvoorde W., et al. Increased lipogenesis and resistance of lipoproteins to oxidative modification in two patients with glycogen storage disease type 1a. J. Pediatr. 2002;140:256–260. doi: 10.1067/mpd.2002.121382. [DOI] [PubMed] [Google Scholar]
- 5.Ubels F., Rake J., Smit P., Smit A., Slaets J. Is glycogen storage disease 1a associated with atherosclerosis? Eur. J. Pediatr. 2002;161:S62–S64. doi: 10.1007/s00431-002-1006-9. [DOI] [PubMed] [Google Scholar]
- 6.Sacks F.M., Lichtenstein A.H., Wu J.H.Y., Appel L.J., Creager M.A., Kris-Etherton P.M., et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136:e1–23. doi: 10.1161/CIR.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 7.Lian Z., Perrard X.Y.D., Peng X., Raya J.L., Hernandez A.A., Johnson C.G., et al. Replacing saturated fat with unsaturated fat in western diet reduces foamy monocytes and atherosclerosis in male Ldlr−/− mice. Arterioscler. Thromb. Vasc. Biol. 2020;40:72–85. doi: 10.1161/ATVBAHA.119.313078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoogerland J.A., Peeks F., Hijmans B.S., Wolters J.C., Kooijman S., Bos T., et al. Impaired VLDL catabolism links hypoglycemia to hypertriglyceridemia in GSD Ia. J. Inherit. Metab. Dis. 2021;44:879–892. doi: 10.1002/jimd.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daugherty A., Tall A.R., Daemen M.J.A.P., Falk E., Fisher E.A., García-Cardeña G., et al. Recommendation on design, execution, and reporting of animal atherosclerosis studies: a scientific statement from the American Heart Association. Circ. Res. 2017;121:e53–e79. doi: 10.1161/RES.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 10.Getz G.S., Reardon C.A. Diet and murine atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006;26:242–249. doi: 10.1161/01.ATV.0000201071.49029.17. [DOI] [PubMed] [Google Scholar]
- 11.Mutel E., Abdul-Wahed A., Ramamonjisoa N., Stefanutti A., Houberdon I., Cavassila S., et al. Targeted deletion of liver glucose-6 phosphatase mimics glycogen storage disease type 1a including development of multiple adenomas. J. Hepatol. 2011;54:529–537. doi: 10.1016/j.jhep.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Wittenstein B., Klein M., Finckh B., Ullrich K., Kohlschütter A. Radical trapping in glycogen storage disease 1a. Eur. J. Pediatr. 2002;161:S70–S74. doi: 10.1007/s00431-002-1008-7. [DOI] [PubMed] [Google Scholar]
- 13.La Rose A.M., Bazioti V., Hoogerland J.A., Svendsen A.F., Groenen A.G., van Faassen M., et al. Hepatocyte-specific glucose-6-phosphatase deficiency disturbs platelet aggregation and decreases blood monocytes upon fasting-induced hypoglycemia. Mol. Metab. 2021;53 doi: 10.1016/j.molmet.2021.101265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laube H., Schatz H., Nierle C., Fussgänger R., Pfeiffer E.F. Insulin secretion and biosynthesis in sucrose fed rats. Diabetologia. 1976;12:441–446. doi: 10.1007/BF01219507. [DOI] [PubMed] [Google Scholar]
- 15.Gjorgjieva M., Calderaro J., Monteillet L., Silva M., Raffin M., Brevet M., et al. Dietary exacerbation of metabolic stress leads to accelerated hepatic carcinogenesis in glycogen storage disease type Ia. J. Hepatol. 2018;69:1074–1087. doi: 10.1016/j.jhep.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Frodermann V., van Duijn J., van Pel M., van Santbrink P.J., Bot I., Kuiper J., et al. Mesenchymal stem cells reduce murine atherosclerosis development. Sci. Rep. 2015;5:15559. doi: 10.1038/srep15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandsma R., Smit P., Kuipers F. Disturbed lipid metabolism in glycogen storage disease type 1. Eur. J. Pediatr. 2002;161:S65–S69. doi: 10.1007/s00431-002-1007-8. [DOI] [PubMed] [Google Scholar]
- 18.Kimura Y., Tsukui D., Kono H. Uric acid in inflammation and the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222212394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishnani P.S., Austin S.L., Abdenur J.E., Arn P., Bali D.S., Boney A., et al. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American college of medical genetics and genomics. Genet. Med. 2014;16:1–29. doi: 10.1038/gim.2014.128. [DOI] [PubMed] [Google Scholar]
- 20.Rake J.P., Visser G., Labrune P., Leonard J.V., Ullrich K., Smit G.P.A., et al. Guidelines for management of glycogen storage disease type I - European study on glycogen storage disease type I (ESGSD I) Eur. J. Pediatr. 2002;161:S112–S119. doi: 10.1007/s00431-002-1016-7. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg T., Slonim A.E. Nutrition therapy for hepatic glycogen storage diseases. J. Am. Diet. Assoc. 1993;93:1423–1430. doi: 10.1016/0002-8223(93)92246-t. [DOI] [PubMed] [Google Scholar]
- 22.Wolfsdorf J.I., Weinstein D.A. Glycogen storage diseases. Rev. Endocr. Metab. Disord. 2003;4:95–102. doi: 10.1023/a:1021831621210. [DOI] [PubMed] [Google Scholar]
- 23.Desouza C.V., Bolli G.B., Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389–1394. doi: 10.2337/dc09-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rake J.P., Visser G., Labrune P., Leonard J.V., Ullrich K., Smit G.P. Glycogen storage disease type I : diagnosis, management, clinical course and outcome. Results of the European study on glycogen storage disease type I (ESGSD I) Eur. J. Pediatr. 2002;161:S20–S34. doi: 10.1007/s00431-002-0999-4. [DOI] [PubMed] [Google Scholar]
- 25.Ebara T., Ramakrishnan R., Steiner G., Shachter N.S. Chylomicronemia due to apolipoprotein CIII overexpression in apolipoprotein E-null mice apolipoprotein CIII – induced hypertriglyceridemia is not mediated by effects on apolipoprotein E. J. Clin. Invest. 1997;99:2672–2681. doi: 10.1172/JCI119456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masucci-magoulas A.L., Goldberg I.J., Bisgaier C.L., Serajuddin H., Francone O.L., Breslow J.L., et al. A mouse model with features of familial combined hyperlipidemia. Science (80-) 1997;275:391–394. doi: 10.1126/science.275.5298.391. [DOI] [PubMed] [Google Scholar]
- 27.Li H., Han Y., Qi R., Wang Y., Zhang X., Yu M., et al. Aggravated restenosis and atherogenesis in ApoCIII transgenic mice but lack of protection in ApoCIII knockouts : the effect of authentic triglyceride-rich lipoproteins with and without ApoCIII. Cardiovasc. Res. 2015;107:579–589. doi: 10.1093/cvr/cvv192. [DOI] [PubMed] [Google Scholar]
- 28.Arca M., Veronesi C., D’erasmo L., Borghi C., Colivicchi F., De Ferrari G.M., et al. Association of hypertriglyceridemia with all-cause mortality and atherosclerotic cardiovascular events in a low-risk italian population: the tg-real retrospective cohort analysis. J. Am. Heart Assoc. 2020;9:1–9. doi: 10.1161/JAHA.119.015801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jørgensen A.B., Frikke-Schmidt R., West A.S., Grande P., Nordestgaard B.G., Tybjærg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 2013;34:1826–1833. doi: 10.1093/eurheartj/ehs431. [DOI] [PubMed] [Google Scholar]
- 30.Katzmann J.L., Werner C.M., Stojakovic T., März W., Scharnagl H., Laufs U. Apolipoprotein CIII predicts cardiovascular events in patients with coronary artery disease: a prospective observational study. Lipids Health Dis. 2020;19:1–10. doi: 10.1186/s12944-020-01293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Capelleveen J.C., Moens S.J.B., Yang X., Kastelein J.J.P., Wareham N.J., Zwinderman A.H., et al. Apolipoprotein C-III levels and incident coronary artery disease risk: the EPIC-Norfolk prospective population study. Arterioscler. Thromb. Vasc. Biol. 2017;37:1206–1212. doi: 10.1161/ATVBAHA.117.309007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarwar N., Danesh J., Eiriksdottir G., Sigurdsson G., Wareham N., Bingham S., et al. Triglycerides and the risk of coronary heart disease: 10 158 incident cases among 262 525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]