Abstract
Background
Multiparametric risk assessment is used in pulmonary arterial hypertension (PAH) to target therapy. However, this strategy is imperfect because most patients remain at intermediate or high risk after initial treatment, with low risk being the goal. Metrics of right ventricular (RV) adaptation are promising tools that may help refine our therapeutic strategy.
Research Question
Does RV adaptation predict therapeutic response over time?
Study Design and Methods
We evaluated 52 incident treatment-naive patients with advanced PAH by catheterization and cardiac imaging longitudinally at baseline, follow-up 1 (∼3 months), and follow-up 2 (∼18 months). All patients received goal-directed therapy with parenteral treprostinil and/or combination therapy with treatment escalation if functional class I or II was not achieved. On the basis of their therapeutic response, patients were evaluated at follow-up 1 as nonresponders (died) or as responders, and again at follow-up 2 as super-responders (low risk) or partial responders (high/intermediate risk). Multiparametric risk was based on a simplified European Respiratory Society/European Society of Cardiology guideline score. RV adaptation was evaluated with the single-beat coupling ratio (Ees/Ea) and diastolic function with diastolic elastance (Eed). Data are expressed as mean ± SD or as OR (95% CI).
Results
Nine patients (17%) were nonresponders. PAH-directed therapy improved the European Respiratory Society low-risk score from 1 (2%) at baseline to 23 (55%) at follow-up 2. Ees/Ea at presentation was nonsignificantly higher in responders (0.9 ± 0.4) vs nonresponders (0.6 ± 0.4; P = .09) but could not be used to predict super-responder status at follow-up 2 (OR, 1.40 [95% CI, 0.28-7.0]; P = .84). Baseline RV ejection fraction and change in Eed were successfully used to predict super-responder status at follow-up 2 (OR, 1.15 [95% CI, 1.0-1.27]; P = .009 and OR, 0.29 [95% CI, 0.86-0.96]; P = .04, respectively).
Interpretation
In patients with advanced PAH, RV-pulmonary arterial coupling could not discriminate irreversible RV failure (nonresponders) at presentation but showed a late trend to improvement by follow-up 2. Early change in Eed and baseline RV ejection fraction were the best predictors of therapeutic response.
Key Words: hemodynamics, pulmonary hypertension, right ventricular function, treatment effect, ventricular/vascular coupling
Abbreviations: BNP, brain natriuretic peptide; Ea, pulmonary arterial elastance; EDP, end-diastolic pressure; EDV, end-diastolic volume; Eed, RV diastolic elastance; Ees, RV systolic elastance; ERS/ESC, European Respiratory Society/European Society of Cardiology; ESP, end-systolic pressure; ESV, end-systolic volume; FC, functional class; mPAP, mean pulmonary artery pressure; PA, pulmonary artery; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PH, pulmonary hypertension; Pmax, RV maximum isovolumic pressure; PVR, pulmonary vascular resistance; RV, right ventricle; REVEAL, US Registry to Evaluate Early and Long-Term PAH Disease Management; RVEF, RV ejection fraction; RV-PA, right ventricular-pulmonary arterial; sRVP, peak systolic right ventricular pressure; SV, stroke volume; WSPH, World Symposium on Pulmonary Hypertension
Pulmonary arterial hypertension (PAH) is a chronic progressive pulmonary vasculopathy resulting in increased right ventricular (RV) afterload and eventual RV failure. Although the prognosis for patients with PAH has improved since the introduction of PAH-directed therapies, the disease remains progressive in many patients. The World Symposium on Pulmonary Hypertension (WSPH) has adopted treatment recommendations based on serial assessment using a multiparametric risk score.1,2 However, therapeutic decisions are still marked by uncertainty, especially after the initial presentation: many patients remain at intermediate or high risk, despite the goal being to achieve low-risk status.3 In this setting, dynamic risk assessment based on RV adaptation to the disease may help us refine our treatment strategy.
Ideally, the therapeutic goal would be to predict those who be “super-responders”: that is, those who would experience sustained improvement, remaining at low risk for long periods.4 Further, early targeting of patients who progress despite therapy would offer additional opportunities regarding prognosis and potential treatments. Preliminary evidence suggests that aggressive treatment strategies are associated with both improvements in RV function and low-risk status.5 However, the most sensitive measure of RV function as a predictor of this response is unknown.
Increasingly, the consensus for RV-pulmonary arterial (RV-PA) adaptation is the coupling ratio (Ees/Ea). This ratio relates “load-independent” RV contractility (RV elastance, Ees) to afterload (PA elastance, Ea). In the adapted state, there is a commensurate increase in Ees to accommodate increasing Ea such that the ratio remains > 1.5 where RV work efficiency is optimized.6 In contrast to Ees/Ea, the right ventricular ejection fraction (RVEF) is often used as a metric of RV function and is a global measurement dependent on the composite of Ees, Ea, diastolic function, and preload conditions. The load independence of Ees makes Ees/Ea potentially more sensitive to adaptation and a more accurate metric of RV function than RVEF. Further, techniques7 have made Ees/Ea and RV diastolic function easier to quantify with standard catheterization, whereas RVEF by MRI is time- and cost-demanding. Therefore, Ees/Ea is an attractive measurement for real-time serial evaluation of RV function.
Although there is general interest in and acceptance of Ees/Ea as a potential reference standard for RV adaptation,8,9 little is known about how it can be used clinically in real time. Cross-sectional data on treated prevalent patients show that an RV coupling ratio ≤ 0.65 to 0.8, and increased diastolic stiffness (Eed), are related to reduced RVEF6,10 and clinical worsening.10, 11, 12 However, it is not clear from these studies how interpatient differences in coupling may be related to PAH-directed therapy. Short-term changes in coupling ratio are heterogeneous, and changes in RV Eed may prove a better indicator of therapeutic changes.13 Therefore, long-term serial assessment of RV coupling, Eed, and RVEF is needed to determine which of these metrics best predict therapeutic response.
In the present study, among advanced, treatment-naive subjects uniformly prescribed parenteral prostacyclin, we aimed (1) to assess whether longitudinal measurement of RVEF, Ees/Ea, and Eed can be used to predict therapeutic response defined by achievement of a low-risk multiparametric score3 and (2) to determine whether there is a coupling ratio that predicts therapeutic failure at diagnosis (irreversible RV failure). We hypothesized that RV-PA coupling and/or Eed may be an earlier and more predictive metric of super-responder status (European Respiratory Society [ERS] low-risk score) than RVEF.
Study Design and Methods
Subjects
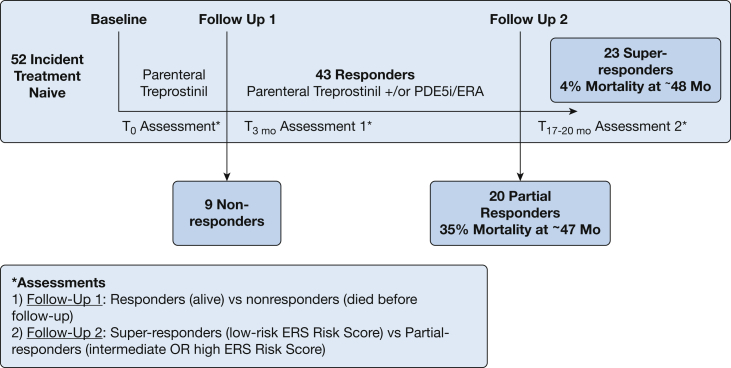
Fifty-two incident functional class (FC) III or IV patients with WSPH group 1 PAH gave informed consent to participate in the study, which was approved by the institutional review board at the University of Arizona (IRB #1100000621). This is part of a continuously enrolling study of all incident FC III or IV patients receiving sequential combination therapy with parenteral treprostinil and oral therapy according to guidelines at the time14 from December 2012 to November 2017. Short-term analysis of a cohort subset has been previously published.13 The diagnosis of PAH was established by dedicated PAH health care providers, with invasive confirmation based on updated guidelines,15 and all patients were naive for PAH-directed therapy at baseline (Fig 1).
Figure 1.
Schematic overview of the timeline to assessment and outcome of enrolled patients by therapeutic response. The first assessment was conducted at follow-up 1, during which patients were classified as responders or nonresponders (died before the assessment). Nonresponders experienced irreversible right ventricular failure. The second assessment was done at follow-up 2, during which patients were categorized as super-responders (low risk, European Respiratory Society/European Society of Cardiology [ERS/ESC] score) and partial responders (high or intermediate ERS/ESC risk score). Mortality at > 47 months was higher in partial responders than in super-responders (35% vs 4%). ERA = endothelin receptor antagonist; PDE5i = phosphodiesterase-5 inhibitor.
Study Protocol and Assessment of Therapeutic Response
After study inclusion, a pulmonary artery catheter was advanced via the antecubital vein or the internal jugular vein for measurements of pulmonary artery pressure (systolic, mean, and diastolic PAP), RV pressure, right atrial pressure, wedged PAP, and cardiac output (direct Fick or thermodilution). Baseline hemodynamics, echocardiography, cardiac MRI, and brain natriuretic peptide (BNP) levels were obtained within 48 h.
All patients underwent initiation of parenteral treprostinil monotherapy by aggressive inpatient, and subsequently outpatient, titration as previously described.13 A 6-min walk test was conducted before discharge. Patients were assessed at follow-up 1 (∼3 months) for therapeutic response to treprostinil monotherapy. Nonresponders died before follow-up 1. Patients received sequential combination therapy if they remained in FC III or IV at follow-up 1 per recommendations at the time.14 Responders continued to follow-up 2 (∼17-20 months), at which point the therapeutic response was again assessed (Fig 1). Patients underwent echocardiography, BNP determination, cardiac MRI, and hemodynamic assessment16 at follow-up 1 and follow-up 2.
Response to therapy was scored by the Swedish/COMPERA (Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension)3,17 approach, using the cutoff values suggested by the current European Respiratory Society/European Society of Cardiology (ERS/ESC) pulmonary hypertension (PH) guidelines,1,2 termed the “ERS risk score.” Patients were categorized as nonresponders if they died before follow-up 1. Patients were categorized as super-responders4 if they met criteria for low-risk PAH2 and as partial responders if they remained in the intermediate- or high-risk category (Fig 1). The US Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) score18 and REVEAL 2.019 score were also calculated and presented similarly.
Cardiac Imaging
Cardiac MRI was performed as described previously.13 Briefly, standard volumetric measurements were made from short-axis cine projections for cardiac MRI. Contour smoothing was done to include trabeculations in end-systole (ESV) and diastole (EDV). Stroke volume (SV) was calculated as EDV – ESV and RVEF as [(SV/EDV) × 100]. All echocardiographic data were obtained in accordance with the American Society of Echocardiography guidelines.20 Parameters that were included were right atrial area, RV fractional area change % [(RV end-diastolic area – RV end-systolic area)/RV end-diastolic area × 100], tricuspid annular plane systolic excursion, and presence of pericardial effusion.
RV Coupling and Diastolic Function
The single-beat method was used to quantify RV function.21 The maximum isovolumic pressure, Pmax, was determined from a sinusoidal curve fit of the RV pressure-time waveform during early isovolumetric contraction (from end-diastolic to maximal dP/dt) and late isovolumetric relaxation (minimal dP/dt to beginning of diastole).21 RV systolic elastance (Ees), a measure of contractility, was calculated as [Pmax – peak systolic RV pressure (sRVP)]/SV. An additional calculation based on estimated end-systolic pressure (ESP) from conductance catheter-derived regression, using mPAP, is also presented for comparison.22 Arterial elastance, Ea, was calculated as sRVP/SV. Estimates of Ees and Ea were done using thermodilution/Fick-derived stroke volumes (as opposed to MRI) as previously described.7 RV-PA coupling was calculated as Ees/Ea, which simplifies to (Pmax/sRVP – 1). Diastolic function was assessed by diastolic elastance (Eed), which is the slope of the end-diastolic pressure-volume relationship (end-diastolic pulmonary vascular resistance [PVR]) at end-diastolic volume.10 To examine the potential contribution of hypertrophy to Eed, we examined Eed relative to RV mass/volume (relative wall thickness), Eedcorrected.10 Ees and Eed were analyzed with a semiautomated MATLAB program (MathWorks).
Statistical Analysis
Continuous data are expressed as means ± SD. Categorical data are expressed as counts and percentages. Baseline two-group comparisons were done with unpaired, two-tailed t tests for continuous variables or the Pearson χ2 test for categorical variables. If the data were not normally distributed, then the Wilcoxon rank-sum test was used. Normality was assessed by visually examining Q-Q plots and by the Shapiro-Wilk test. Within- and between-group longitudinal differences were evaluated with a mixed-model, repeated-measures analysis of variance. For the repeated-measures analysis of variance, the assumption of sphericity was met.
To determine the relationship of RV coupling, Eed, or RVEF to therapeutic response at follow-up 2 (super-responders vs partial responders), univariate logistic regression model analysis was conducted using these variables as well as variables likely related to therapeutic response such as age,23 sex,24 race/ethnicity,25 and connective tissue disease.26 Variables subsumed within the multiparametric ERS risk score (ie, BNP, 6-min walk test distance) were not used in the model, given this would be self-referential. Therapeutic response at follow-up 2 was used as the dependent variable. A three-model multivariate analysis was conducted to examine for potential mediation/confounding (Table 2). RVEF and diastolic elastance were added to a multivariate logistic regression, model A based on P < .2 in the univariate analysis and their relevance to our primary hypothesis. Model B consisted of model A, substituting Eedcorrected for Eed. Because age was the only significant demographic/clinical variable on univariate analysis, it was added to model A as a model C analysis. Collinearity was assessed by linear regression for continuous variables and the Spearman rank correlation for categorical variables. All statistical analyses were performed with SPSS software (version 27.0; IBM). Statistical tests were two-sided, and a P value < .05 was considered statistically significant.
Table 2.
Univariate and Multivariate Predictors of Therapeutic Response at Follow-Up 2
| Characteristic | Super-Responders | Partial Responders | Univariate Analysis OR (95% CI) | Multivariate Analysisa |
||
|---|---|---|---|---|---|---|
| Model A OR (95% CI) |
Model B OR (95% CI) |
Model C OR (95% CI) |
||||
| Demographics and clinical | ||||||
| Age (mean ± SD), y | 48 ± 11 | 59 ± 12 | 0.92 (0.85-0.98)b | … | … | 0.9 (0.83-0.99)b |
| Sex (male), No. (%) | 18 (81) | 16 (76) | 0.63 (0.14-2.8) | … | … | … |
| Ethnicity (Hispanic), No. (%) | 5 (25) | 7 (30) | 1.31 (0.34-5.0) | … | … | … |
| CTD, No. (%) | 3 (13) | 6 (31) | 0.35 (0.08-1.6) | … | … | … |
| RV imaging, mean ± SD | ||||||
| Baseline RVEF | 23.8 ± 7.2 | 19.0 ± 8.06 | 1.15 (1.0-1.27)b | 1.31 (1.0-1.27)b | 1.17 (1.03-1.4)b | 1.14 (1.0-1.29)b |
| RVEF at FU1 | 32.4 ± 9.1 | 30.2 ± 11.0 | 1.0 (0.96-1.08) | … | … | … |
| ΔRVEFc | 9.2 ± 7.7 | 10.7 ± 7.0 | 0.99 (0.89-1.09) | … | … | … |
| Baseline SV/ESV | 0.43 ± 0.27 | 0.34 ± 0.17 | 6.9 (0.28-171.6) | … | … | … |
| SV/ESV at FU1 | 0.52 ± 0.23 | 0.47 ± 0.25 | 2.5 (0.17-36.1) | … | … | … |
| ΔSV/ESVc | 0.12 ± 0.27 | 0.15 ± 0.19 | 0.6 (0.04-8.6) | … | … | … |
| RV-PA coupling and diastolic function, mean ± SD | ||||||
| Baseline Ees/Ea | 0.9 ± 0.4 | 0.8 ± 0.4 | 1.40 (0.28-7.0) | … | … | … |
| Ees/Ea at FU1 | 1.1 ± 0.5 | 1.1 ± 0.5 | 1.15 (0.31-4.2) | … | … | … |
| ΔEes/Eac | 0.2 ± 0.6 | 0.2 ± 0.5 | 0.89 (0.27-2.87) | … | … | … |
| Baseline Eed | 1.4 ± 0.8 | 1.1 ± 0.6 | 2.03 (0.75-5.51) | … | … | … |
| Eed at FU1 | 0.7 ± 0.5 | 0.9 ± 0.6 | 0.67 (0.12-2.0) | … | … | … |
| ΔEedc | –0.7 ± 0.6 | –0.2 ± 0.8 | 0.24 (0.07-0.88)b | 0.27 (0.07-0.98)b | … | 0.31 (0.07-1.33) |
| ΔEedcorrectedc | –2.2 ± 1.8 | –0.8 ± 2.2 | 0.70 (0.48-0.95) | … | 0.82 (0.51-1.2) | … |
Data are expressed as No. (%) or mean ± SD. Logistic regression analysis was used to predict super-responders relative to partial responders (reference category). Multivariate models A and B include variables significant in the univariate analysis at P < .2 whereas model C also includes age. Δ = change; CTD = connective tissue disease; Eed = RV diastolic elastance; Eedcorrected = Eed corrected for RV mass/volume; Ees/Ea = RV-PA coupling ratio; FU = follow-up; PH = pulmonary hypertension; RVEF = right ventricular ejection fraction; SV/ESV = RV stroke volume/end-systolic volume.
Multivariate analysis models: Model A = baseline RVEF + ΔEed (change from baseline to follow-up 1); model B = baseline RVEF + ΔEedcorrected (Eed corrected for RV mass/volume); model C = model A + age.
P < .05.
Change from baseline to follow-up 1.
Results
Patient Characteristics and Therapeutic Response
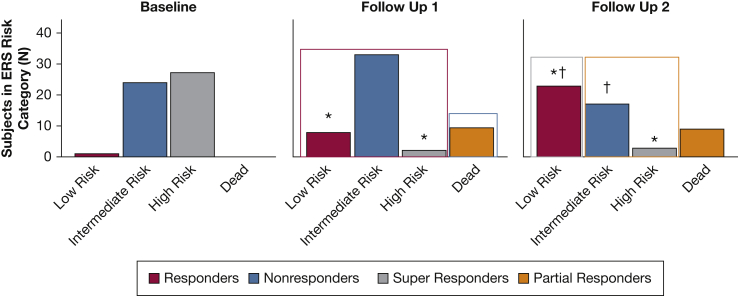
During the study period, 52 patients were enrolled. Subjects were predominantly female and 17% had mixed PH. At enrollment, the subjects had very advanced PAH with RV dilation, reduced RVEF, and significantly elevated afterload (Table 1). Baseline ERS risk scores and REVEAL scores demonstrated nearly all patients as high or intermediate risk. There was a significant increase in both low- and intermediate-risk ERS scores at follow-up 1 and a corresponding decrease in high risk on therapy (Fig 2). For follow-up 2, there was a corresponding increase in low risk and reduction in intermediate risk (Fig 2). REVEAL scores showed a similar trend (Table 1 and e-Fig 1). Therapeutic improvements in RVEF were accompanied by improvements in PVR, pulmonary compliance, cardiac output, and exercise capacity (Table 1).
Table 1.
Demographics and Clinical Data, Multiparametric Risk Score, Resting Hemodynamics, Cardiac Imaging, and Right Ventricular-Pulmonary Arterial Coupling and Diastolic Function by Assessment Time
| Variable | Event |
||
|---|---|---|---|
| Baseline | Follow-Up 1 | Follow-Up 2 | |
| Age, y | 54 ± 14 | … | … |
| Sex (female), No. (%) | 41 (78.8) | … | … |
| Ethnicity (Hispanic), No. (%) | 12 (23.1) | … | … |
| BMI, kg/m2 | 30.8 ± 8.4 | … | … |
| WSPH category, No. (%) | |||
| CTD | 12 (23) | … | … |
| Drugs/toxins | 8 (15) | … | … |
| HIV | 1 (2) | … | … |
| iPAH | 25 (48) | … | … |
| Portal hypertension | 4 (8) | … | … |
| PVOD | 1 (2) | … | … |
| CHD | 1(2) | … | … |
| Diabetes (yes) | 9 (17) | … | … |
| BNP, pg/mL | 698 ± 646 | 251 ± 285a | 112 ± 134b |
| 6-Min walk distance, m | 288 ± 98 | 345 ± 123 | 380 ± 129b |
| Therapy | |||
| Combination therapy, No. (%) | … | … | 34 (81) |
| Monotherapy, No. (%) | … | 45 (100) | 8 (19) |
| Treprostinil dosage, ng/kg/min | 9.8 ± 2.8 | 44.5 ± 8.9a | 65 ± 19.1a,b |
| Risk scoresc | |||
| ERS low risk, No. (%) | 1 (1.9) | 8 (18.6)a | 23 (54.8)a,b |
| ERS intermediate risk, No. (%) | 24 (46.2) | 33 (76.7)a | 17 (40.5) |
| ERS high risk, No. (%) | 27 (51.9) | 2 (4.7)a | 2 (4.8)a,b |
| REVEAL risk score | 10.0 ± 1.6 | 7.5 ± 2.1a | 6.0 ± 2.4a,b |
| REVEAL 2.0 risk score | 10.0 ± 2.0 | 6.8 ± 2.7a | 5.1 ± 2.9a,b |
| Hemodynamics | |||
| Mean PAP, mm Hg | 55.9 ± 10.9 | 45.7 ± 9.8a | 39.0 ± 9.4a,b |
| Cardiac output, L/min | 4.0 ± 1.3 | 5.4 ± 1.5a | 5.3 ± 1.2a |
| PCWP, mm Hg | 9.0 ± 4.1 | 6.9 ± 3.7a | 7.0 ± 2.9a,b |
| Mean RAP, mm Hg | 12.0 ± 6.0 | 6.0 ± 5.0a | 5.0 ± 4.0a,b |
| PVR, Wood units | 12.8 ± 5.1 | 7.8 ± 3.0a | 6.4 ± 2.5a,b |
| Compliance, mL/mm Hg | 1.0 ± 0.4 | 1.6 ± 0.7a | 2.0 ± 0.9a,b |
| PAO2sat, % | 59.1 ± 9.4 | 64.9 ± 7.5a | 66.5 ± 7.3b |
| Cardiac imaging | |||
| Echo RA area, cm2 | 26.2 ± 5.7 | 24.4 ± 6.8 | 24.3 ± 8.6 |
| Echo TAPSE, cm | 1.4 ± 0.4 | 2.1 ± 2.6 | 2.0 ± 0.4 |
| Echo FAC, % | 19.4 ± 8.5 | 23.6 ± 9.4 | 24.1 ± 11.0 |
| MRI RVESVI, mL/m2 | 87.6 ± 43.6 | 80.5 ± 45.8 | 79.1 ± 54.2 |
| MRI RVEDVI, mL/m2 | 116.1 ± 45.0 | 113.8 ± 48.0 | 117.3 ± 57.4 |
| MRI RVEF, % | 20.2 ± 8.3 | 31.9 ± 10.0a | 35.1 ± 10.9a |
| MRI SV/ESV | 0.38 ± 0.23 | 0.50 ± 0.23a | 0.60 ± 0.24a |
| MRI RV mass, g/m2 | 45.2 ± 18.3 | 46.7 ± 20.4 | 46.5 ± 34.2 |
| RV mass/volume, g/mL | 0.42 ± 0.13 | 0.43 ± 0.14 | 0.40 ± 0.15 |
| RV-PA coupling and diastolic function | |||
| Ees, mm Hg/mL | 1.5 ± 0.8 | 1.3 ± 0.7 | 1.2 ± 0.8 |
| Ea, mm Hg/mL | 2.0 ± 1.0 | 1.2 ± 0.5a | 1.0 ± 0.4a,b |
| Pmax, mm Hg | 159.9 ± 33.4 | 147.2 ± 36.8 | 132.5 ± 39.7a |
| RV-PA coupling ratio, Ees/Ea | 0.8 ± 0.4 | 1.1 ± 0.5 | 1.2 ± 0.8a |
| Eed, mm Hg/mL | 1.2 ± 0.7 | 0.8 ± 0.6a | 0.8 ± 0.4a |
| Eedcorrected, mm Hg/mL | 3.3 ± 2.1 | 1.9 ± 1.4a | 2.3 ± 1.7 |
Between-group differences over time were evaluated by repeated-measures analysis of variance. Post hoc comparisons were evaluated by the Bonferroni method. Data are expressed as means ± SD unless indicated otherwise. BNP = brain natriuretic peptide; CHD = congenital heart disease; CTD = connective tissue disease; Ea = pulmonary arterial elastance; Echo = echocardiography; Eed = right ventricular diastolic elastance; Eedcorrected = right ventricular Eed corrected for mass/volume; Ees = right ventricular systolic elastance; ERS = European Respiratory Society; FAC = fractional area change; iPAH = idiopathic PAH; PA = pulmonary artery; PAH = pulmonary arterial hypertension; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; Pmax = maximal RV isovolumic pressure; PVOD = pulmonary veno-occusive disease; PVR = pulmonary vascular resistance; RA = right atrium; RAP = right atrial pressure; REVEAL = US Registry to Evaluate Early and Long-Term PAH Disease Management; RV = right ventricle; RVEDVI = right ventricular end-diastolic volume index; RVEF = right ventricular ejection fraction; RVESVI = right ventricular end-systolic volume index; PAO2sat = pulmonary arterial oxygen saturation; SV/ESV = stroke volume/end-systolic volume; TAPSE = tricuspid annular plane systolic excursion; WSPH = World Symposium on Pulmonary Hypertension.
P < .05 vs baseline.
P < .05 vs follow-up 1.
Figure 2.
Therapeutic changes in European Respiratory Society (ERS) risk score. There is an improvement from high and intermediate risk at baseline to low and intermediate risk at follow-up 1. ERS within-group differences were tested by Pearson χ2 test. ∗P < .05 vs baseline; †P < .05 vs follow-up 1.
Nonresponders vs Responders (Therapeutic Response at Follow-Up 1)
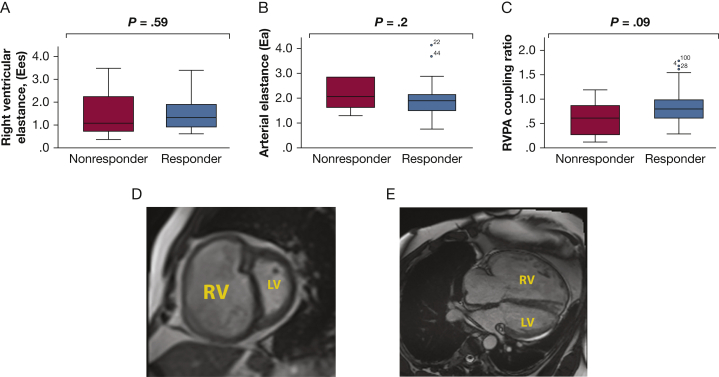
Nonresponders had elevated baseline BNP and lower 6-min walk test distance than responders (e-Table 1). Eight of nine nonresponders (89%) died at their presenting hospitalization, and one subject died right after discharge. Therefore, the treprostinil dosage was lower (15.6 ± 4.6 ng/kg/min in nonresponders vs 26.1 ± 19.1 ng/kg/min in responders). Follow-up 1 occurred for responders at 3 ± 1.5 months (range, 2-7 months). Figure 3 demonstrates that RV coupling was nonsignificantly higher in responders. Notably, 50% of responders had an RV coupling ratio < 0.8 at presentation.
Figure 3.
Right ventricular (RV) function at presentation in treatment nonresponders and responders. A-C, Baseline RV contractility (Ees) (A), afterload (Ea) (B), and RV-pulmonary arterial coupling ratio (RV-PA [Ees/Ea]) (C). Nonresponders experienced irreversible RV failure manifested by death at initial hospitalization or soon after. Although Ees/Ea was nonsignificantly higher in responders, there was no discrete cutoff defining irreversible RV failure. D and E, A representative example of baseline severe enlargement of the RV and underfilling of the left ventricle in the short axis (D) and long axis (E) in a responder. Cardiac imaging parameters were similar in responders and nonresponders.
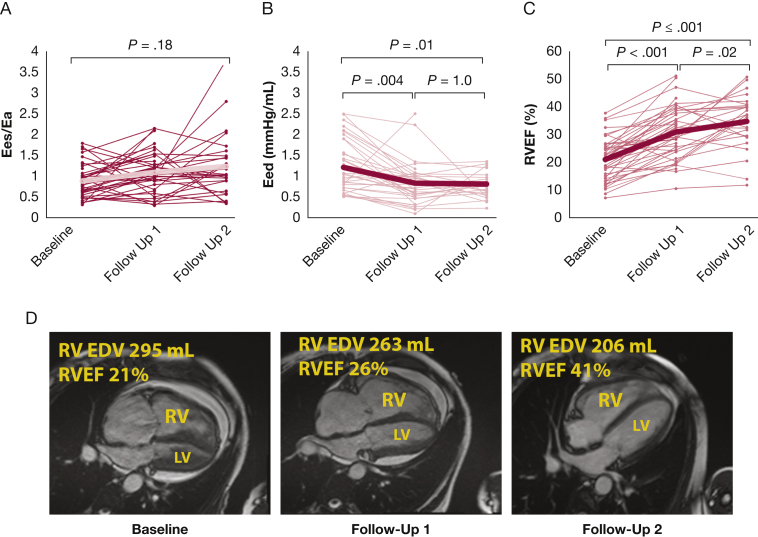
Among treatment responders, baseline RV Ees/Ea demonstrated substantial RV uncoupling and elevated diastolic stiffness (e-Table 1 and Figs 4A and 4B). RV coupling showed high interpatient variability and was not significantly changed over time in response to therapy (Fig 4A). However, RV Eed and Eedcorrected significantly improved over time in a more uniform fashion (Fig 4B and e-Fig 2). Early improvements in Eed were associated with improvements in RV size and ejection fraction (Figs 4C and 4D).
Figure 4.
Longitudinal assessment of right ventricular (RV) function among treatment responders. A and B, Spaghetti plots demonstrate changes in right ventricular-pulmonary arterial coupling ratio (Ees/Ea) (A) and diastolic elastance (Eed) (B) over time. C, Intersubject heterogeneity in Ees/Ea was high, but early improvements in Eed were associated with subsequent improvements in RV size and function. D, Cardiac MR long-axis images from a single patient who was classified as a super-responder at follow-up 2. Ees/Ea and Eed were tested by repeated-measures analysis of variance. In A-C, large brackets indicate overall model P value; smaller brackets indicate pairwise comparisons. Eed = right ventricular diastolic elastance; Ees/Ea = RV-pulmonary artery coupling ratio; LV = left ventricle; RV EDV = RV end-diastolic volume; RVEF = RV ejection fraction.
Among responders, estimating ESP from sRVP resulted in a consistently lower Ees/Ea than when using ESP estimated from mPAP (e-Fig 3; bias, –0.143). Limits of agreement were large, 0.18 to –0.49. Further, there was proportional bias at larger coupling ratios (β, –0.13; P < .0001). This proportional bias was consistent over time between baseline and follow-ups 1 and 2 (P = .12 within subjects’ difference by time).
Super-Responders vs Partial Responders (Therapeutic Response at Follow-Up 2)
Super-responders were younger and had lower REVEAL 2.0 scores and higher RVEF than partial responders at baseline (e-Table 1). Follow-up 2 occurred at 17 ± 13 months (range, 4-45 months) in super-responders. Some super-responders continued to receive treprostinil monotherapy (8 of 23; 35%) as they achieved FC I or II at follow-up 1, whereas the others were given combination therapy (15 of 23; 65%) (Fig 1). Follow-up 2 occurred at 20 ± 15 months (range, 6-49 months) on combination therapy (19 of 19; 100%) in partial responders (Fig 1). Both groups had similar treprostinil dosing and diuretic use at follow-ups 1 and 2 (e-Tables 2 and 3). Super-responders were monitored for 48 months (range, 10-86 months), and one of 23 (4%) died, whereas partial responders were monitored for 47 months (range, 21-85), during which seven of 20 (35%) died (Fig 1).
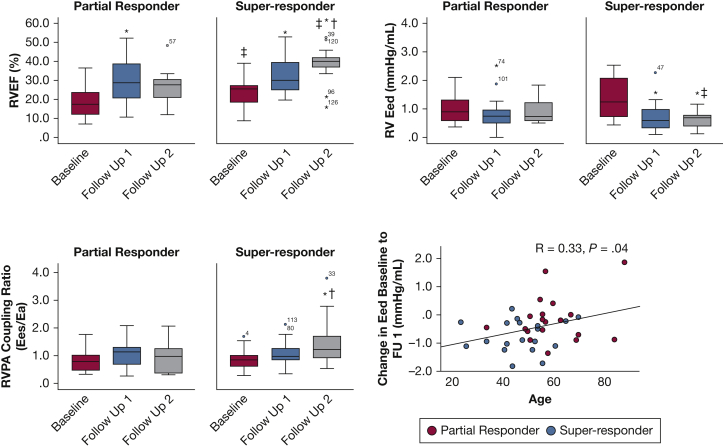
Both groups had improvements in resting hemodynamics, RV ESV, and RVEF by follow-up 1, but only super-responders had continued improvement in hemodynamics and ventricular volumes at follow-up 2 (Fig 5 and e-Tables 2 and 3). Only super-responders showed a significant improvement in Eed, which preceded improvements in coupling ratio (Figs 5B and 5C) and persisted after correction for RV mass/volume (e-Table 3). At follow-up 2, super-responders demonstrated a significant improvement in RV-PA coupling (Fig 5C) from baseline and a trend to higher Ees/Ea vs partial responders (P = .09).
Figure 5.
A-C, Right ventricular (RV) function, RV-pulmonary artery (PA) coupling, and diastolic function by partial or super-responder status at follow-up 2. Shown are RV ejection fraction (RVEF) (A), RV diastolic elastance (Eed) (B), and RV-PA coupling ratio (Ees/Ea) (C) as a function of time. Patients are categorized as partial responders (European Respiratory Society [ERS] risk score, intermediate or high) or super-responders (ERS risk score, low) at follow-up 2. D, There was a correlation between Eed and age by treatment response at follow-up 2. Repeated-measures analysis of variance was used to test intergroup differences (partial vs super-responders) and intragroup differences (ie, baseline vs follow-up). ∗P < .05 vs baseline; †P < .05 vs follow-up 1; ‡P < .05, super-responder vs partial responder. FU = follow-up.
Univariate logistic regression (using baseline, change from baseline to follow-up 1, and follow-up 1 variables) was done to predict therapeutic response at follow-up 2 (Table 2). Lower age, higher baseline RVEF, and decreasing Eed and Eed corrected for RV mass/volume were associated with super-responder status. On multivariate analysis, model A confirmed baseline RVEF and change in Eed as predictors. When model A was modified to substitute Eedcorrected for Eed (model B), only RVEF was still significant. Model C showed that lower age and higher baseline RVEF were associated with therapeutic response, but reduced the predictive power of Eed. This may indicate age as a potential modifier of the relationship of Eed to therapeutic response (Fig 5D).
Discussion
This study is the first to demonstrate in patients with advanced PAH, all receiving similar goal-directed therapy, that (1) early change in Eed is a predictor of long-term “super” therapeutic response, and (2) a coupling ratio that clearly defines RV failure unresponsive to therapy, irreversible RV failure, is difficult to define. We hypothesized that Ees/Ea or Eed could be used to predict eventual response and, thus, could be a target for future study by individualizing care for patients with RV dysfunction. Although there were clear improvements in coupling among super-responders, Ees/Ea demonstrated significant interpatient heterogeneity. Eed, however, demonstrated a uniform and early change allowing for prediction of response to therapy.
The WSPH recommends combination therapy based on dynamic risk assessment using serial measurements, with the goal to attain/sustain low-risk status.2 Although current guidelines recommend up-front combination therapy,2 sequential combination was the therapeutic standard at the time of this study.14 Further, we thought it more important to match the physiologic changes to sequential assessment under a uniform goal-directed therapy approach. In other words, our hypothesis would be best tested by seeing what changes in coupling occur with the addition of therapy to a set goal rather than waiting for clinical deterioration on up-front combination therapy.
RV diastolic stiffening (Eed) has gained attention in PAH because of its relationship to prognosis.10 Intuitively, this association would be tied to the improvement in cardiac output, stroke volume, and RVEF.6 The relationship of RV diastolic function to stroke volume is likely complex and dependent on loading conditions, relative wall thickness, myocardial fibrosis, pericardial conditions, and myocyte properties. We speculate that the effect of Eed on RVEF is related to changes in both intramyocyte conditions as well as wall thickness.27 Therapeutic changes in diastolic function are seen acutely (within 2 days) before changes in mass or fibrosis can occur.13,28 In addition, high cardiac myocyte passive stiffness has been seen in PAH.27,29,30 However, our study showed that Eed correction for mass/volume did alter the relationship to therapeutic response. Although speculative, it is possible that either direct or indirect (through lowering RV afterload) therapeutic effects may alter RV diastolic stiffness. This process may restore the myocyte length-systolic tension reserve (Starling reserve), termed “heterometric” autoregulation, and thereby improvements in stroke volume in a contractility (Ees) or “homeometric” autoregulation-independent manner.31 Thus, improvements in Eed may be linked with stroke volume and ejection fraction independent of RV-PA coupling.
Individual heterogeneity in Ees/Ea makes it difficult to apply to RV compensation. It is generally viewed that heterometric autoregulation is a secondary compensatory mechanism and would not begin until the coupling response is maximized.32 This is supported by cross-sectional studies including one demonstrating that treated patients exhibit RV dilation at an Ees/Ea cutoff of ≤ 0.8.6 However, other studies have demonstrated that RV dilation occurs even in early PH (mPAP, ∼22 mm Hg) possibly indicating early augmentation of heterometric autoregulation.33 Early use of heterometric autoregulation is seen in patients with scleroderma34 and may account for some differences in this study. It is unlikely that heterometric vs homeometric autoregulation is an “on-off” phenomenon, especially if the afterload is high. Therefore, it is quite possible that the predictive ability of Ees/Ea is greater after PVR drops to 50% to 60% of baseline, where investigators have shown dramatic drops in RV end-diastolic volume (reverse remodeling).35, 36, 37
As reviewed,9 there are few to no longitudinal studies on RV-PA coupling and/or diastolic function, with only one study10 examining, as a secondary end point, Eed changes at ∼12 months follow-up in patients receiving therapy. This study showed a nonsignificant decrease in Eed after therapy (P = .06). However, in these patients the disease was not as advanced, the study did not have a similar goal-oriented therapeutic approach, and both responders and nonresponders were analyzed similarly.
Attention has been directed to the degree of phenotypic heterogeneity in PAH. Patients with group 1 PAH and who are older and have increased comorbidities have a worse prognosis23,38,39 and therapeutic response.23 These age-associated differences may be mediated in part by differences in RV function.40 This is the first study to demonstrate one potential mechanism of this age disparity as being mediated by RV diastolic function. Although RV diastolic function is altered by normal aging,41,42 previous evidence for an abnormal RV aging response in PAH comes mainly from experimental models.43,44
Our study has several limitations. It is possible that the heterogeneity in Ees/Ea is due to measurement error inherent in the single-beat method. However, recent work has validated the single-beat method to assess Ees relative to reference standard conductance catheterization, making this unlikely.11 In addition, the small number of patients limits our ability to examine the association of all factors related to a diastolic function-mediated therapeutic response, such as connective tissue disease and RV hypertrophy. The small sample size also limits our power to give a decisive Eed cutoff to apply clinically, and there is a strong potential of having excluded certain effects due to lack of power in the multivariate analysis. Our single-center study design limits the generalizability of our findings in addition to the sample size. Finally, it is possible that Ees/Ea may be more useful with patients with preserved or borderline RV function to detect occult RV dysfunction.12
Interpretation
Our data suggest that RV diastolic elastance (Eed) is an early marker of therapeutic response in patients with advanced PAH. RV-PA coupling shows a trend to improvement but with more intersubject variability. Changes in Eed appear to be, in part, age-dependent; however, it remains to be seen if an Eed-targeted approach can alter long-term outcome. Future research should examine the real-time use of the RV-PA coupling ratio as a marker of therapeutic response in less advanced forms of PAH.
Take-home Points.
Study Question: Does right ventricular (RV) adaptation characterized by right ventricular-pulmonary arterial (RV-PA) coupling or diastolic stiffness predict therapeutic response among patients with pulmonary arterial hypertension (PAH) over time?
Results: Our study evaluated an incident treatment-naive advanced PAH cohort for an average of 47 months. We longitudinally examined the RV-PA coupling ratio and diastolic stiffness as predictors of therapeutic response at follow-up 1 (∼3 months) and again at follow-up 2 (∼20 months). We found that there was no lower threshold of coupling ratio that predicted death at initial presentation. We also found that baseline RV ejection fraction and change in diastolic stiffness are the best early predictors of therapeutic response by follow-up 2.
Interpretation: RV diastolic stiffness is the best early predictor of therapeutic response in advanced PAH. The RV-PA coupling ratio may still hold promise as a predictor in less advanced disease.
Acknowledgments
Author contributions: F. P. R.: principal investigator, had access to all the study data and takes full responsibility for the integrity and accuracy of the data; F. P. R., R. R. V., and R. J. T.: study design; F. P. R. and M. I.: patient recruitment, care, and follow-up; R. R. V., F. P. R., E. J. B., M. I., and K. S. H.: data collection, maintenance, and analysis; F. P. R. and E. J. B.: statistical analysis; F. P. R., drafting of the original manuscript; R. R. V., M. I., R. J. T., K. S. H., E. J. B., and J. G. N. G.: critical revision of the manuscript for important intellectual content.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: F. P. R. has research funding and served an advisory function to the United Therapeutics Corporation, Actelion, Acceleron, Bayer, and Phase Bio. R. J. T. reports no conflicts related to this manuscript; other general disclosures include consulting relationships with Medtronic, Abbott, Aria CV Inc., Arena Pharmaceuticals, Acceleron, Eidos Therapeutics, Gradient, and United Therapeutics. R. J. T. is on a steering committee for Medtronic, Acceleron, and Abbott as well as a research advisory board for Abiomed. R. J. T. is a consultant for United Therapeutics, Arena Therapeutics, Gradient, Aria CV, Itamar, and Eidos Therapeutics. R. J. T. also does hemodynamic core laboratory work for Actelion and Merck. None declared (K. S. H., M. I., J. G. N. G., E. J. B.).
Other contributions: The authors acknowledge the gracious assistance of the UAHD Biorepository at the University of Arizona. The authors also acknowledge the many subjects with PH in the UA registry/biorepository and the coordinators who have dedicated significant effort in this academic pursuit to further our field.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: There are no direct funding sources for this study. U01 grant HL125208-01 from the National Heart, Lung, and Blood Institute of the NIH has provided indirect funding sources for related work in this field at the University of Arizona.
Supplementary Data
References
- 1.Galiè N., Humbert M., Vachiery J.-L., et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N., Channick R.N., Frantz R.P., et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoeper M.M., Kramer T., Pan Z., et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2):1700740. doi: 10.1183/13993003.00740-2017. [DOI] [PubMed] [Google Scholar]
- 4.Halliday S.J., Hemnes A.R. Identifying “super responders” in pulmonary arterial hypertension. Pulm Circ. 2017;7(2):300–311. doi: 10.1177/2045893217697708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badagliacca R., Raina A., Ghio S., et al. Influence of various therapeutic strategies on right ventricular morphology, function and hemodynamics in pulmonary arterial hypertension. J Heart Lung Transplant. 2018;37(3):365–375. doi: 10.1016/j.healun.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Tello K., Dalmer A., Axmann J., et al. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail. 2019;12(1) doi: 10.1161/CIRCHEARTFAILURE.118.005512. [DOI] [PubMed] [Google Scholar]
- 7.Vanderpool R.R., Puri R., Osorio A., et al. Surfing the right ventricular pressure waveform: methods to assess global, systolic and diastolic RV function from a clinical right heart catheterization. Pulm Circ. 2019;10(1) doi: 10.1177/2045894019850993. 2045894019850993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vonk Noordegraaf A., Chin K.M., Haddad F., et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. 2019;53(1):1801900. doi: 10.1183/13993003.01900-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahm T., Douglas I.S., Archer S.L., et al. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward [official American Thoracic Society research statement] Am J Respir Crit Care Med. 2018;198(4):e15–e43. doi: 10.1164/rccm.201806-1160ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trip P., Rain S., Handoko M.L., et al. Clinical relevance of right ventricular diastolic stiffness in pulmonary hypertension. Eur Respir J. 2015;45(6):1603–1612. doi: 10.1183/09031936.00156714. [DOI] [PubMed] [Google Scholar]
- 11.Richter M.J., Peters D., Ghofrani H.A., et al. Evaluation and prognostic relevance of right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med. 2020;201(1):116–119. doi: 10.1164/rccm.201906-1195LE. [DOI] [PubMed] [Google Scholar]
- 12.Hsu S., Simpson C.E., Houston B.A., et al. Multi-beat right ventricular-arterial coupling predicts clinical worsening in pulmonary arterial hypertension. J Am Heart Assoc. 2020;9(10) doi: 10.1161/JAHA.119.016031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderpool R.R., Desai A.A., Knapp S.M., et al. How prostacyclin therapy improves right ventricular function in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700764. doi: 10.1183/13993003.00764-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galiè N., Corris P.A., Frost A., et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D60–D72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 15.Simonneau G., Montani D., Celermajer D.S., et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang W.H.W., Wilcox J.D., Jacob M.S., et al. Comprehensive diagnostic evaluation of cardiovascular physiology in patients with pulmonary vascular disease: insights from the PVDOMICS program. Circ Heart Fail. 2020;13(3) doi: 10.1161/CIRCHEARTFAILURE.119.006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kylhammar D., Kjellstrom B., Hjalmarsson C., et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018;39(47):4175–4181. doi: 10.1093/eurheartj/ehx257. [DOI] [PubMed] [Google Scholar]
- 18.Benza R.L., Miller D.P., Gomberg-Maitland M., et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 19.Benza R.L., Gomberg-Maitland M., Elliott C.G., et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156(2):323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Rudski L.G., Lai W.W., Afilalo J., et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Brimioulle S., Wauthy P., Ewalenko P., et al. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol. 2003;284(5):H1625–H1630. doi: 10.1152/ajpheart.01023.2002. [DOI] [PubMed] [Google Scholar]
- 22.Tello K., Richter M.J., Axmann J., et al. More on single-beat estimation of right ventriculoarterial coupling in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;198(6):816–818. doi: 10.1164/rccm.201802-0283LE. [DOI] [PubMed] [Google Scholar]
- 23.Hoeper M.M., Pausch C., Grünig E., et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant. 2020;39(12):1435–1444. doi: 10.1016/j.healun.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Tello K., Richter M.J., Yogeswaran A., et al. Sex differences in right ventricular-pulmonary arterial coupling in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2020;202(7):1042–1046. doi: 10.1164/rccm.202003-0807LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medrek S.K., Sahay S. Ethnicity in pulmonary arterial hypertension: possibilities for novel phenotypes in the age of personalized medicine. Chest. 2018;153(2):310–320. doi: 10.1016/j.chest.2017.08.1159. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee M., Mercurio V., Tedford R.J., et al. Right ventricular longitudinal strain is diminished in systemic sclerosis compared with idiopathic pulmonary arterial hypertension. Eur Respir J. 2017;50(5):1701436. doi: 10.1183/13993003.01436-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rain S., Handoko M.L., Trip P., et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation. 2013;128(18):2016–2025. doi: 10.1161/CIRCULATIONAHA.113.001873. [DOI] [PubMed] [Google Scholar]
- 28.Gan C.T., Holverda S., Marcus J.T., et al. Right ventricular diastolic dysfunction and the acute effects of sildenafil in pulmonary hypertension patients. Chest. 2007;132(1):11–17. doi: 10.1378/chest.06-1263. [DOI] [PubMed] [Google Scholar]
- 29.Hsu S., Kokkonen-Simon K.M., Kirk J.A., et al. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension. Circulation. 2018;137(22):2360–2370. doi: 10.1161/CIRCULATIONAHA.117.033147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rain S., Bos Dda S., Handoko M.L., et al. Protein changes contributing to right ventricular cardiomyocyte diastolic dysfunction in pulmonary arterial hypertension. J Am Heart Assoc. 2014;3(3) doi: 10.1161/JAHA.113.000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ait-Mou Y., Hsu K., Farman G.P., et al. Titin strain contributes to the Frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins. Proc Natl Acad Sci U S A. 2016;113(8):2306–2311. doi: 10.1073/pnas.1516732113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vonk-Noordegraaf A., Haddad F., Chin K.M., et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62(25 suppl):D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 33.Lamia B., Muir J.F., Molano L.C., et al. Altered synchrony of right ventricular contraction in borderline pulmonary hypertension. Int J Cardiovasc Imaging. 2017;33(9):1331–1339. doi: 10.1007/s10554-017-1110-6. [DOI] [PubMed] [Google Scholar]
- 34.Hsu S., Houston B.A., Tampakakis E., et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation. 2016;133(24):2413–2422. doi: 10.1161/CIRCULATIONAHA.116.022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badagliacca R., Poscia R., Pezzuto B., et al. Prognostic relevance of right heart reverse remodeling in idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2018;37(2):P195–P205. doi: 10.1016/j.healun.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 36.Badagliacca R., D’Alto M., Ghio S., et al. Risk reduction and hemodynamics with initial combination therapy in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2021;203(4):484–492. doi: 10.1164/rccm.202004-1006OC. [DOI] [PubMed] [Google Scholar]
- 37.van de Veerdonk M.C., Huis in ‘t Veld A.E., Marcus J.T., et al. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. Eur Respir J. 2017;49(6):1700007. doi: 10.1183/13993003.00007-2017. [DOI] [PubMed] [Google Scholar]
- 38.Ginoux M., Turquier S., Chebib N., et al. Impact of comorbidities and delay in diagnosis in elderly patients with pulmonary hypertension. ERJ Open Res. 2018;4(4):00100–02018. doi: 10.1183/23120541.00100-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoeper M.M., Huscher D., Ghofrani H.A., et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168(2):871–880. doi: 10.1016/j.ijcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 40.Badagliacca R., Rischard F., Papa S., et al. Clinical implications of idiopathic pulmonary arterial hypertension phenotypes defined by cluster analysis. J Heart Lung Transplant. 2020;39(4):310–320. doi: 10.1016/j.healun.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Fiechter M., Fuchs T.A., Gebhard C., et al. Age-related normal structural and functional ventricular values in cardiac function assessed by magnetic resonance. BMC Med Imaging. 2013;13(1):1–6. doi: 10.1186/1471-2342-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Andrea A., Vriz O., Carbone A., et al. The impact of age and gender on right ventricular diastolic function among healthy adults. J Cardiol. 2017;70(4):387–395. doi: 10.1016/j.jjcc.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Chouabe C., Ricci E., Amsellem J., et al. Effects of aging on the cardiac remodeling induced by chronic high-altitude hypoxia in rat. Am J Physiol Heart Circ Physiol. 2004;287(3):H1246–H1253. doi: 10.1152/ajpheart.00199.2004. [DOI] [PubMed] [Google Scholar]
- 44.Kuroha M., Isoyama S., Ito N., Takishima T. Effects of age on right ventricular hypertrophic response to pressure-overload in rats. J Mol Cell Cardiol. 1991;23(10):1177–1190. doi: 10.1016/0022-2828(91)90206-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.