Abstract
OBJECTIVE:
Recent studies have proposed resection of the T2 FLAIR hyperintensity beyond the T1 contrast enhancement (supramarginal resection [SMR]) for IDH–wild-type glioblastoma (GBM) to further improve patients’ overall survival (OS). GBMs have significant variability in tumor cell density, distribution, and infiltration. Advanced mathematical models based on patient-specific radiographic features have provided new insights into GBM growth kinetics on two important parameters of tumor aggressiveness: proliferation rate (ρ) and diffusion rate (D). The aim of this study was to investigate OS of patients with IDH–wild-type GBM who underwent SMR based on a mathematical model of cell distribution and infiltration profile (tumor invasiveness profile).
METHODS:
Volumetric measurements were obtained from the selected regions of interest from pre- and postoperative MRI studies of included patients. The tumor invasiveness profile (proliferation/diffusion [ρ/D] ratio) was calculated using the following formula: ρ/D ratio = (4π/3)2/3 × (6.106/[VT21/1 − VT11/1])2, where VT2and VT1 are the preoperative FLAIR and contrast-enhancing volumes, respectively. Patients were split into subgroups based on their tumor invasiveness profiles. In this analysis, tumors were classified as nodular, moderately diffuse, or highly diffuse.
RESULTS:
A total of 101 patients were included. Tumors were classified as nodular (n = 34), moderately diffuse (n = 34), and highly diffuse (n = 33). On multivariate analysis, increasing SMR had a significant positive correlation with OS for moderately and highly diffuse tumors (HR 0.99, 95% CI 0.98–0.99; p = 0.02; and HR 0.98, 95% CI 0.96–0.99; p = 0.04, respectively). On threshold analysis, OS benefit was seen with SMR from 10% to 29%, 10% to 59%, and 30% to 90%, for nodular, moderately diffuse, and highly diffuse, respectively.
CONCLUSIONS:
The impact of SMR on OS for patients with IDH–wild-type GBM is influenced by the degree of tumor invasiveness. The authors’ results show that increasing SMR is associated with increased OS in patients with moderate and highly diffuse IDH–wild-type GBMs. When grouping SMR into 10% intervals, this benefit was seen for all tumor subgroups, although for nodular tumors, the maximum beneficial SMR percentage was considerably lower than in moderate and highly diffuse tumors.
Keywords: Glioblastoma, IDH wild type, Mathematical Model, Supramarginal Resection, Supratotal Resection, Oncology
INTRODUCTION
Current standard of care for glioblastoma (GBM) patients consists of maximal safe resection of the contrast-enhancing portion of the tumor followed by radiation therapy with concomitant and adjuvant temozolomide.1–5 Multiple factors influence overall survival (OS). Among these are age, the Karnofsky Performance Status (KPS) score, extent of resection (EOR), adjuvant therapy, contact with the lateral ventricles, and IDH mutation and O6-methylguanine-DNA methyltransferase (MGMT) methylation status.6–13 Despite improvements in surgical and medical therapies, the median survival of patients with GBM remains at 15 months.14,15 Supramarginal resection (SMR)—defined as resection beyond the T1 contrast enhancement within the boundaries of the FLAIR hyperintense signal—has been shown to improve overall OS in GBM.16–22 However the benefit of SMR may not be uniform across all GBM patients, and intrinsic tumor characteristics such as tumor cell distribution and genetic profiles might impact the survival benefit conferred by SMR.19
New advanced mathematical models based on patient-specific radiographic characteristics have provided new insights into GBM behavior and might help elucidate SMR benefit on OS. Of the various mathematical models available, the proliferation-diffusion model is among the most studied.23–29 This model relies on two important parameters of tumor aggressiveness: proliferation rate (ρ) and diffusion rate (D) which are mainly estimated by T1 postgadolinium and T2/FLAIR imaging signal volumes. The ratio of ρ/D classifies the tumor invasiveness profile; tumors with high ρ/D ratio are considered nodular, while tumors with a low ρ/D ratio are considered diffuse.23–29 Gross-total resection (GTR) of the contrast-enhancing region for nodular tumors resects a larger percentage of tumor cell burden than for diffuse tumors. However, for more diffuse tumors, a greater tumor cell burden remains after GTR of the contrast-enhancing region.24,30
In this study, we hypothesized that the ρ/D ratio (tumor invasiveness profile) is key in determining which GBM patients will benefit from SMR. We present a multicenter, retrospective cohort study, reporting the correlation of the ρ/D ratio on survival benefit of SMR of newly diagnosed IDH–wild-type GBMs that underwent SMR.
METHODS
Patient Selection
We reviewed electronic records of all consecutive adult patients (aged ≥ 18 years) who underwent resection of newly diagnosed GBM at our three main sites (Rochester, Minnesota; Phoenix, Arizona; and Jacksonville, Florida) from January 2011 to December 2017. Inclusion criteria were 1) GBM diagnosis based on the WHO classification system as determined from the pathology report, 2) first-time resection, 3) GTR (complete resection of the contrast-enhancing region on postoperative T1-weighted MRI, 4) availability of preoperative and immediately postoperative MRI studies within 48 hours of surgery, 5) known MGMT methylation status, 6) adjuvant radiation and chemotherapy according to the Stupp protocol,1 and 7) absent IDH mutation (wild type). This study was approved by the Mayo Clinic institutional review board.
Volumetric measurements and Calculation of Tumor Invasiveness
MRI scans of all included patients were retrieved from our institutional archive of medical images. Every scan was done at our institution on a 1.5T or 3T scanner following a standardized internal protocol. Image volumes were obtained by manually defining the region of interest for each measurement and subsequent automatic computerization of the volumetric data from the selected region of interest using a DICOM Medical Image Viewer software. Using methods previously described by Swanson et al., we calculated the tumor invasiveness profile or the proliferation/diffusion (ρ/D) ratio using the following formula: ρ/D ratio = (4π/3)2/3 × (6.106/[VT21/3 − VT11/3])2, where VT2 and VT1 are the preoperative T2/FLAIR volume and contrast-enhancing volume.23–29 Patients were divided into subgroups based on tumor invasiveness.23,24 In this analysis, highly diffuse tumors had a low ρ/D ratio (< 0.55 mm−2), moderately diffuse tumors had a moderate ρ/D ratio (0.55–1.80 mm−2), and nodular tumors had a high ρ/D ratio (> 1.80 mm−2). The cutoffs between highly diffuse, moderately diffuse, and nodular tumors have not been well established in the literature. Different studies have used varying cutoffs; the analysis in our current study was conducted using the different characterization cutoffs presented in Baldock et al.24 Additional calculations with different cutoffs from other studies were done for comparative purposes, with highly diffuse tumors having a low ρ/D ratio (< 0.38 mm−2), moderately diffuse tumors having a moderate ρ/D ratio (0.38–1.30 mm−2), and nodular tumors having a high ρ/D ratio (> 1.30 mm−2).24
Statistical Analysis
The association between different tumor invasiveness subgroups was analyzed using Fisher’s exact test and Wilcoxon rank-sum test where appropriate. OS was defined as the time from surgery to death. The OS was analyzed using a log-rank test and plotted using the Kaplan-Meier method. Threshold analysis was performed by grouping the extent of T2/FLAIR resection into 10% intervals to identify the maximal resection interval associated with greater significant OS. Univariate and multivariate Cox proportional hazards models were recruited to investigate the association between age, sex, KPS score, MGMT status, contact of the contrast-enhancing portion of the tumor with the lateral ventricles, contrast-enhancing preoperative volume, preoperative and postoperative FLAIR volumes, and FLAIR SMR and OS. Unfortunately, proof of dexamethasone use or administered dosages was not widely available in this data set and was insufficient to be included in the analysis. Significance was considered at α ≤ 0.05 (two-sided). R (version 3.6.0) was utilized to analyze patient data. Only variables with p < 0.05 at any of the ρ/D ratio groups were included in the multivariable analysis.
RESULTS
We screened 888 patients with newly diagnosed IDH–wild-type GBM at our institution between January 2011 and December 2017; 101 patients met all inclusion criteria for this study. The demographic and clinical characteristics are listed in Table 1. All patients were classified into different subgroups based on the tumor invasiveness ratio (ρ/D); highly diffuse tumors had a low ρ/D ratio (< 0.55 mm−2), moderately diffuse tumors had a moderate ρ/D ratio (0.55–1.80 mm−2), and nodular tumors had a high ρ/D ratio (> 1.80 mm−2) (Fig. 1).
Table 1:
Study Population (n = 101)
| Characteristics | Value |
|---|---|
| Mean age, yrs | 59.8 (18 to 86) |
| Male, n | 68 (67) |
| Mean KPS score | 80 (30 to 100) |
| Methylated MGMT | 32 (32) |
| Mean preop CE vol, cm3 | 36.2 (1.03 to 124.15) |
| Mean preop FLAIR vol, cm3 | 42.2 (0.48 to 182.74) |
| Mean preop cystic vol, cm3 | 16.1 (0 to 113.25) |
| Contact w/ ventricles | 52 (51) |
| Mean FLAIR SMR % | 28.4 (−128.13% to 100%) |
| Mean residual FLAIR vol, cm3 | 28.4 (0 to 120) |
| Mean OS (SD), mos | 16.1 (9.2) |
| ND | 15.2 (9.7) |
| MD | 15.7 (7.5) |
| HD | 17.1 (10.4) |
CE = contrast-enhancing; HD = highly diffuse; MD = moderately diffuse; ND = nodular.
Values are presented as the number of patients (%) or mean (range) unless stated otherwise.
Figure 1.
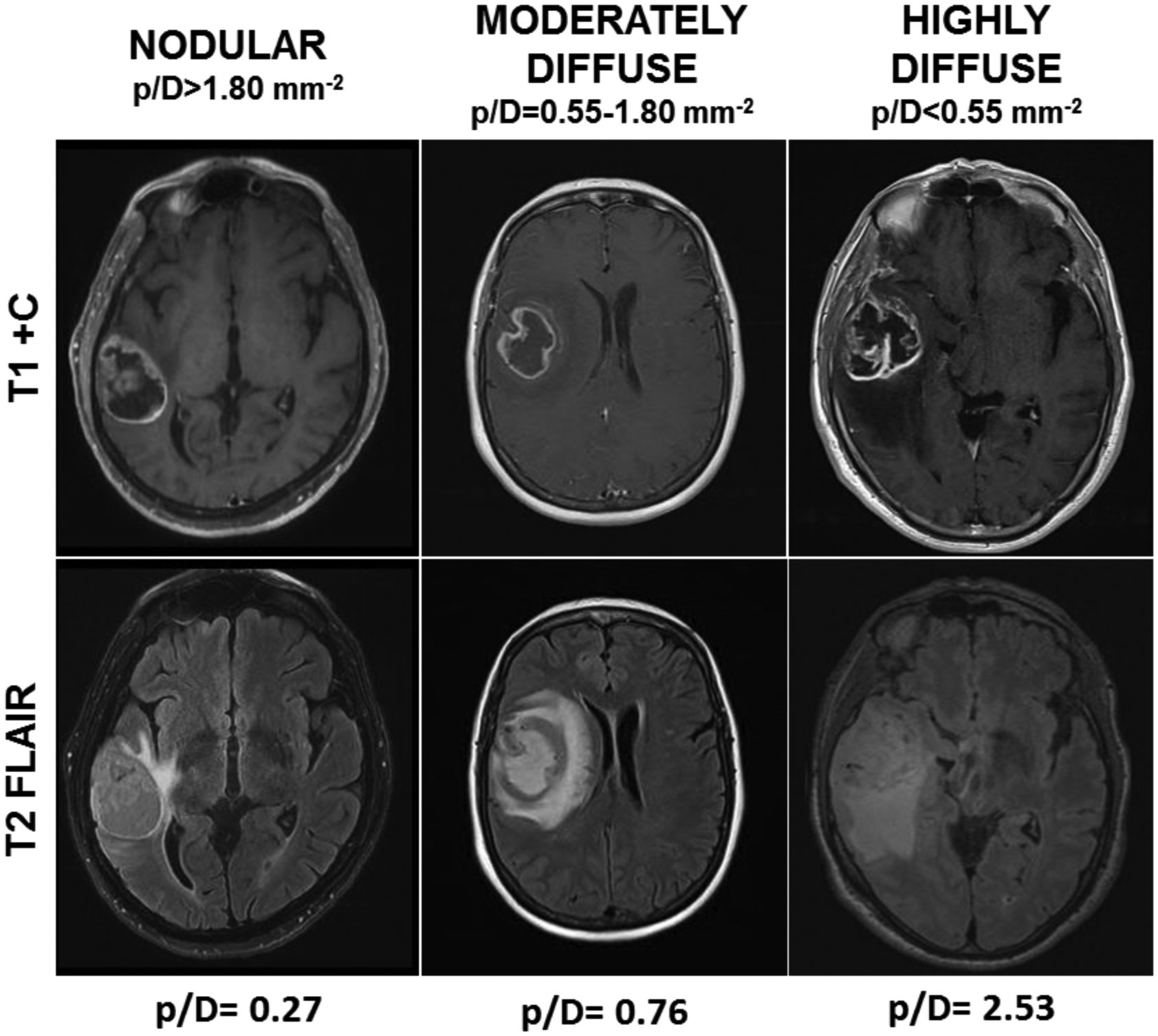
Axial MR images showing IDH–wild-type GBM classification according to the ρ/D ratio in a nodular tumor with a ρ/D ratio > 1.80 mm−2, moderately diffuse tumor with a ρ/D between 0.55 and 1.80 mm−2, and highly diffuse tumor with ρ/D ratio < 0.55 mm−2. The ρ/D ratios beneath the figure represent the individual ρ/D ratio’s resulting value for each representative case.
Univariate Analysis for OS
For nodular tumors, univariable Cox proportional hazards regression analysis identified age ≥ 65 years (HR 1.83, 95% CI 1.10–3.97; p = 0.03) and contact of the contrast-enhancing portion of the tumor with the lateral ventricles (HR 1.19, 95% CI 1.01–2.41; p = 0.04) as being associated with shorter OS. Additionally, KPS score ≥ 70 (HR 0.27, 95% CI 0.10–0.70; p < 0.01) and MGMT promoter methylation (HR 0.43, 95% CI 0.21–0.91; p = 0.03) were associated with longer OS.
For moderately diffuse tumors, univariable Cox proportional hazards regression analysis identified age ≥ 65 years (HR 2.43, 95% CI 1.13–5.24; p = 0.02) and contact of the contrast-enhancing region with the lateral ventricles (HR 2.39, 95% CI 1.11–5.14; p < 0.01) as being associated with shorter OS. Additionally, KPS score ≥ 70 (HR 0.34, 95% CI 0.12–0.94; p = 0.04), MGMT promoter methylation (HR 0.48, 95% CI 0.25–0.93; p = 0.02), and increasing FLAIR SMR percentage (HR 0.98, 95% CI 0.98–0.99; p = 0.05) were associated with longer OS.
For highly diffuse tumors, univariable Cox proportional hazards regression analysis identified age ≥ 65 years (HR 1.14, 95% CI 1.01–2.87; p = 0.03) and contact of the contrast-enhancing region with the lateral ventricles (HR 2.43, 95% CI 1.13–5.24; p = 0.02) as being associated with shorter OS. Additionally, KPS score ≥ 70 (HR 0.66, 95% CI 0.25–0.98; p = 0.04) and increasing FLAIR SMR percentage (HR 0.98, 95% CI 0.97–0.99; p = 0.02) were associated with longer OS. Furthermore, for all 3 tumor invasiveness profiles (nodular, moderately diffuse, and highly diffuse), postoperative KPS score was not significantly associated with increasing SMR percentage (p = 0.56, p = 0.71, and p = 0.85, respectively).
Multivariate Analysis for OS
For nodular tumors, contact of the contrast-enhancing region with the lateral ventricles (HR 1.21, 95% CI 1.10–1.63; p = 0.02), KPS score ≥ 70 (HR 0.4, 95% CI 0.16–0.99; p = 0.05), and MGMT promoter methylation (HR 0.32, 95% CI 0.13–0.79; p = 0.01) remained significant in the multivariate analysis (Table 2). Next, for moderately diffuse tumors, contact of the contrast-enhancing region with the lateral ventricles (HR 1.26, 95% CI 1.13–1.78; p = 0.03), KPS score ≥ 70 (HR 0.34, 95% CI 0.12–0.94; p = 0.03), MGMT promoter methylation (HR 0.63, 95% CI 0.52–0.99; p = 0.04), and increasing FLAIR SMR percentage (HR 0.99, 95% CI 0.98–0.99; p = 0.02) remained significant in the multivariate analysis (Table 2). Finally, for highly diffuse tumors, contact of the contrast-enhancing region with the lateral ventricles (HR 1.56, 95% CI 1.34–1.85; p = 0.03), KPS score ≥ 70 (HR 0.27, 95% CI 0.08–0.95; p = 0.04), and increasing FLAIR SMR percentage (HR 0.98, 95% CI 0.96–0.99; p = 0.04) remained significant in the multivariate analysis (Table 2).
Table 2:
Multivariate Analysis of OS
| ND (n = 34) | MD (n = 34) | HD (n=33) | ||||
|---|---|---|---|---|---|---|
| Variable | HR (95% CI) | p Value | HR (95% CI) | P-Value | HR (95% CI) | p Value |
| Age | ||||||
| >=65 yrs | 1.02 (1.00 – 1.06) | 0.06 | 1.51 (0.52 – 4.43) | 0.45 | 1.71 (0.78 – 3.73) | 0.17 |
| KPS score | ||||||
| >=70 | 0.41 (0.16 – 0.99) | 0.05 | 0.34 (0.12 – 0.94) | 0.03 | 0.27 (0.08 – 0.95) | 0.04 |
| MGMT | ||||||
| Methylated | 0.32 (0.13 – 0.79) | 0.01 | 0.63 (0.52 – 0.99) | 0.04 | 0.75 (0.39 – 1.41) | 0.36 |
| Contact w/ Ventricles | 1.21 (1.10 – 1.63) | 0.02 | 1.26 (1.13 – 1.78) | 0.03 | 1.56 (1.34 – 1.85) | 0.03 |
| FLAIR SMR | 0.99 (0.98 – 1.00) | 0.53 | 0.99 (0.98 – 0.99) | 0.02 | 0.98 (0.96 – 0.99) | 0.04 |
| > 10% | 0.28 (0.09 – 0.93) | 0.03 | 0.35 (0.13 – 0.91) | 0.03 | 0.48 (0.16 – 1.44) | 0.19 |
| > 20% | 0.56 (0.35 – 0.89) | 0.01 | 0.27 (0.12 – 0.66) | <0.01 | 0.58 (0.24 – 1.38) | 0.22 |
| > 30% | 0.75 (0.51 – 3.49) | 0.55 | 0.26 (0.10 – 0.63) | <0.01 | 0.39 (0.17 – 0.92) | 0.03 |
| > 40% | 0.98 (0.35 – 2.91) | 0.97 | 0.27 (0.11 – 0.63) | <0.01 | 0.36 (0.16 – 0.83) | 0.02 |
| > 50% | 0.88 (0.29 – 2.28) | 0.78 | 0.36 (0.15 – 0.86) | 0.02 | 0.26 (0.10 – 0.63) | <0.01 |
| > 60% | 0.82 (0.37 – 1.81) | 0.62 | 0.68 (0.29 – 1.60) | 0.38 | 0.27 (0.11 – 0.63) | <0.01 |
| > 70% | 0.68 (0.29 – 1.60) | 0.38 | 0.77 (0.32 – 1.84) | 0.55 | 0.35 (0.13 – 0.91) | 0.03 |
| > 80% | 0.64 (0.24 – 1.70) | 0.37 | 0.68 (0.25 – 1.82) | 0.44 | 0.56 (0.35 – 0.89) | 0.01 |
| > 90% | 0.52 (0.18 – 1.51) | 0.23 | 0.82 (0.35 – 2.26) | 0.33 | 0.62 (0.39 – 0.99) | 0.04 |
Boldface type indicates statistical significance.
Effects of Tumor Invasiveness on Survival Benefit of Supramarginal Resection
As the optimal threshold of SMR correlating with maximal OS may vary with tumor invasiveness levels, explorative multivariable Cox proportional hazards regression analyses were performed to determine the minimum and maximum T2/FLAIR resection associated with survival benefit.
For highly diffuse tumors, resection of up to 90% of the T2/FLAIR volume was associated with survival benefit (Fig. 2). The mean survival for patients receiving < 90% SMR was 13.2 months compared with 22.1 months for patients receiving > 90% SMR (p = 0.047). Significant benefit in OS was seen with thresholds from 30% (HR 0.39, 95% CI 0.17–0.92; p = 0.03) to 90% (HR 0.62, 95% CI 0.39–0.99; p = 0.04) (Table 2). For moderately diffuse tumors, resection up to 50% of the T2/FLAIR volume was associated with survival benefit (Fig. 3). The mean survival for patients receiving < 50% SMR was 16.1 months compared with 23.4 months for those receiving > 50% SMR (p = 0.017). Significant benefit in OS was seen with thresholds from 10% (HR 0.35, 95% CI 0.13–0.91; p = 0.03) to 50% (HR 0.36, 95% CI 0.15–0.86; p = 0.02) (Table 2). For nodular tumors, resection up to 20% of the T2/FLAIR volume was associated with survival benefit (Fig. 4). The mean survival for patients receiving < 20% SMR was 11.2 months compared with 21.5 months for those receiving > 20% SMR (p = 0.027). Significant benefit in OS was seen with thresholds from 10% (HR 0.28, 95% CI 0.09–0.93; p = 0.03) to 20% (HR 0.56, 95% CI 0.35–0.89; p = 0.01) (Table 2).
Figure 2:

Survival of patients with highly diffuse tumors by SMR threshold of 90% among patients with GBM with GTR of the contrast-enhancing portion. The mean survival for patients receiving < 90% SMR was 13.2 months compared with 22.1 months for those receiving > 90% SMR (p = 0.047).
Figure 3:
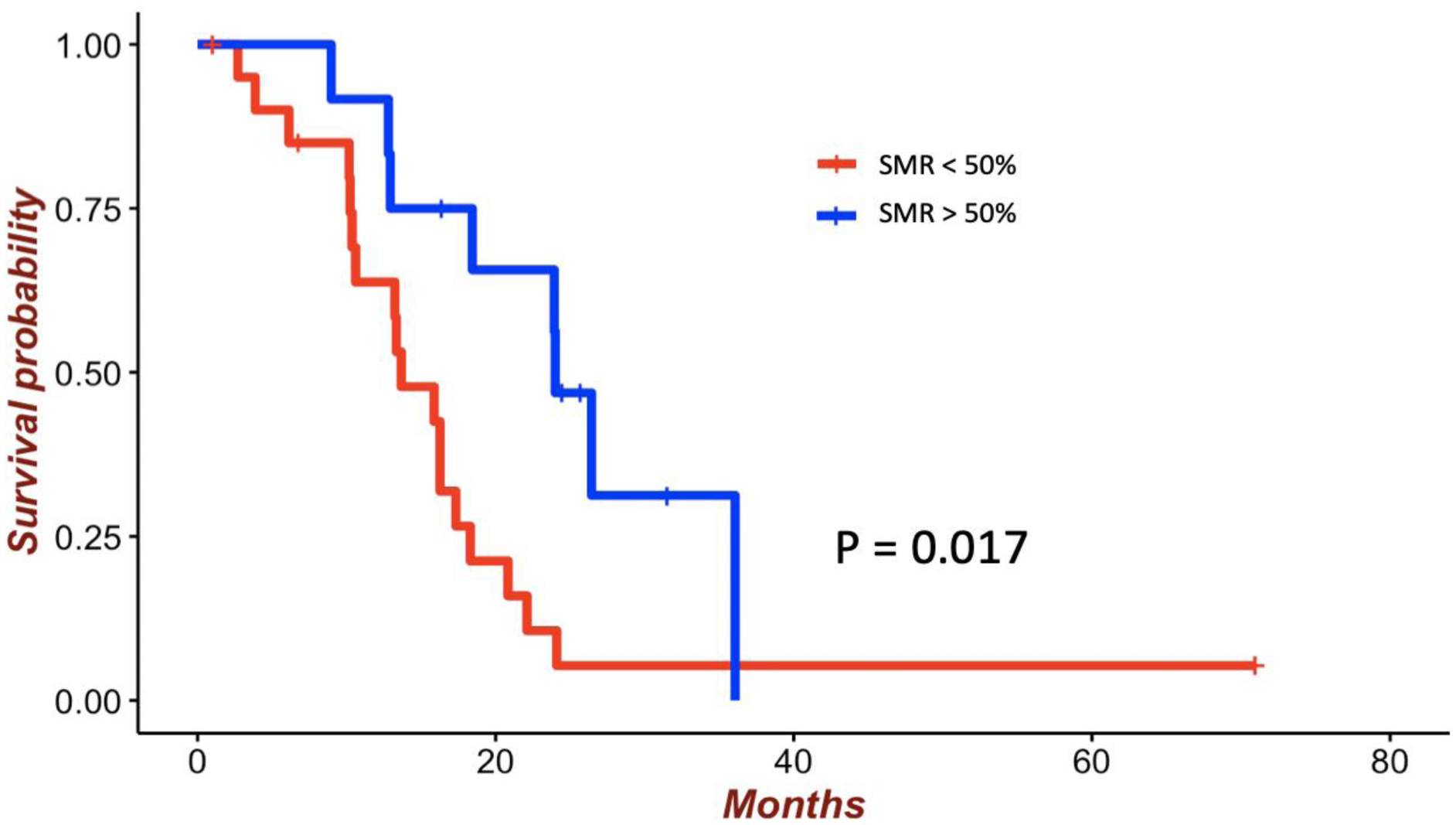
Survival of patients with moderately diffuse tumors by SMR threshold of 50% among patients with GBM with GTR of the contrast-enhancing portion. The mean survival for patients receiving < 50% SMR was 16.1 months compared with 23.4 months for those receiving > 50% SMR (p = 0.017). Figure is available in color online only.
Figure 4:
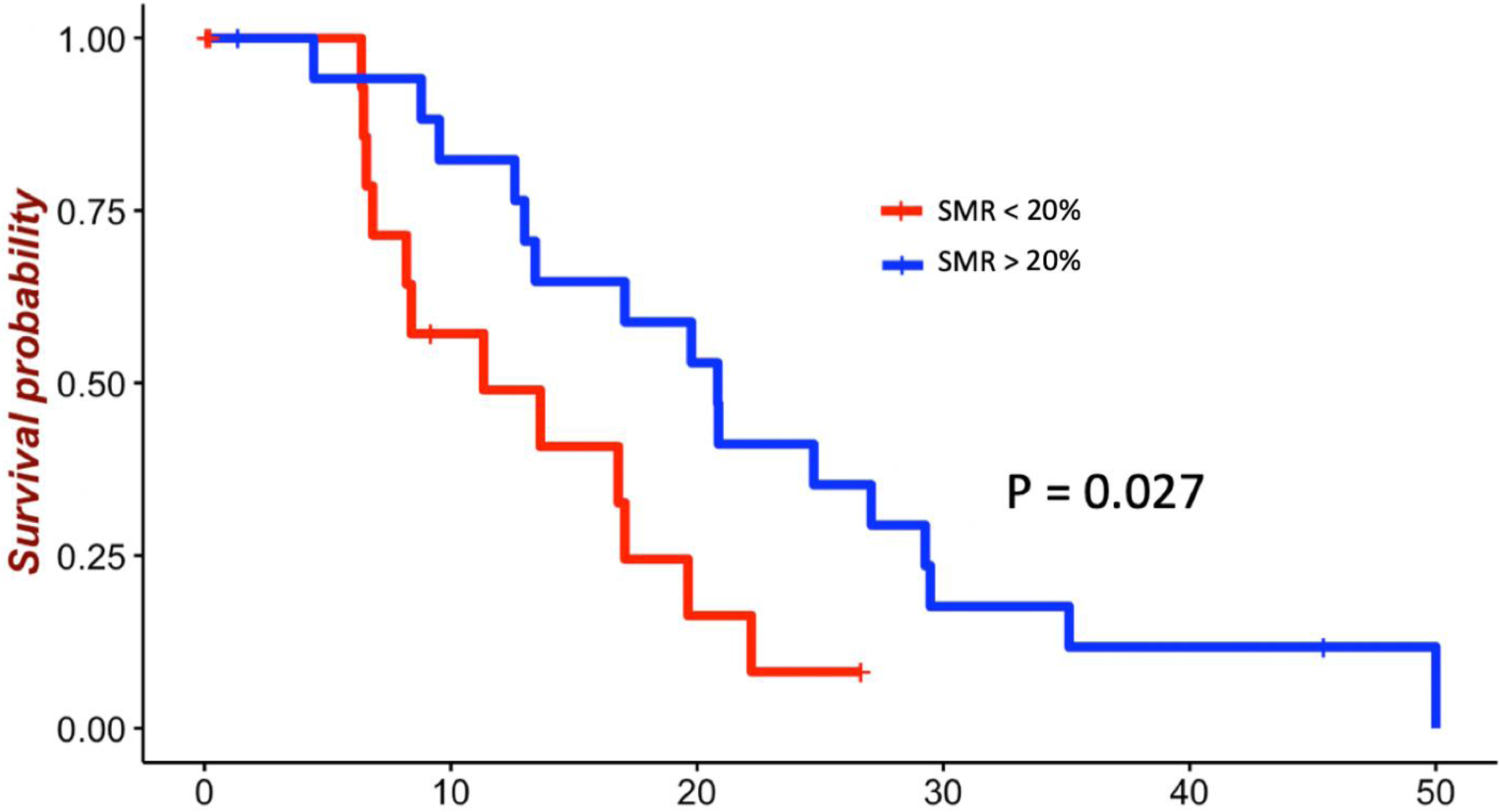
Survival of patients with nodular tumors by SMR threshold of 20% among patients with GBM with GTR of the contrast-enhancing portion. The mean survival for patients receiving < 20% SMR was 11.2 months compared with 21.5 months for those receiving > 20% SMR (p = 0.027).
In the repeat analysis with the different cutoffs (highly diffuse with a low ρ/D ratio (< 0.38 mm−2), moderately diffuse with a moderate ρ/D ratio (0.38–1.30 mm−2), and nodular with a high ρ/D ratio (> 1.30 mm−2), the thresholds for each subgroup in this analysis remained unchanged with 90% as the maximum significant SMR for highly diffuse, 50% SMR for moderately diffuse, and 20% SMR for nodular tumors; additionally minimum significant SMR was still 30% for highly diffuse, 10% SMR for moderately diffuse, and 10% SMR for nodular tumors.
DISCUSSION
GBM prognosis remains dismal despite millions spent developing aggressive multimodal treatment.1–5,31,32 While tumor molecular features such as the absence of an IDH mutation and MGMT promoter methylation strongly predict treatment response and OS in patients with GBM, there are several additional tumor- and patient-specific factors that also contribute to prognostication and to inform surgical planning.33–35 In recent years, the tumor invasiveness profile has emerged as a potential new marker predicting GBM response to resection.23–29 This approach groups tumors into 3 categories: 1) nodular, 2) moderately diffuse, and 3) highly diffuse. A study by Baldock et al. using this model showed that for nodular tumors, GTR is associated with survival benefit; however, a similar survival benefit was not observed for diffuse tumors.24 Specifically, they predicted that to resect 99% of tumor cells in a diffuse tumor (ρ/D ratio = 0.302 cm−2), an additional 237 cm3 of brain tissue would need to be removed beyond that of GTR of contrast-enhancing portion.24 On the other hand, a nodular tumor (ρ/D ratio = 367 cm−2) was predicted to require further resection of only 25 cm3 to obtain the same result.24 According to prior studies implementing this model, diffuse tumors have a greater tumor cell burden in the FLAIR-hyperintense region than do nodular tumors.24,27,29,36
The survival benefit associated with increased EOR has been widely studied in GBM patients. Despite improvements in treatment paradigms, the OS of GBM patients remains dismal, with a median survival of 15 months even after GTR. This has led to several studies proposing that SMR of the T2/FLAIR region beyond the contrast-enhancing portion might provide an additional benefit on a patient’s OS.16–22 However, there has been variability in these results, likely due to tumor subtypes and individual characteristics.33 In this current study, we implemented tumor invasiveness profiling to assess the influence of SMR on OS in patients with the most aggressive GBM subtype (IDH–wild type).
Tumor Invasiveness Profile Predicts SMR Efficacy
Our results show for the first time that SMR as a continuous variable is associated with a significant beneficial OS only for moderately and highly diffuse tumors (Table 2). Based on our results, the survival effects of SMR vary in accordance with tumor invasiveness profile subgroups. GTR of the contrast-enhancing portion in nodular tumors resects a larger percentage of tumor cell burden, resulting in a less significant role for SMR in terms of cellular load resection given the lower FLAIR cell invasiveness extension (Fig. 5). On the other hand, moderately diffuse and highly diffuse tumors have a greater tumor cell burden in the FLAIR region that remains after GTR of the contrast-enhancing portion.24,30 Therefore, for these tumors, resection of the surrounding FLAIR tissue (SMR) provides an additional survival benefit over GTR. These results highlight the limited influence of increasing SMR on nodular tumors, as there was no survival benefit when evaluated as a continuous variable. However, patients with moderately and highly diffuse tumors represent a population with a potentially greater likelihood of benefiting from SMR when possible and safe. This is especially important for IDH–wild-type tumors due to the key role of resection in the treatment of these tumors given their poor response to chemo-radiation.33–35
Figure 5:

Depiction of the cumulative percentage of tumor cells as a function of distance from tumor center for nodular (A) and diffuse (B) tumors. Tumor cells were assumed to be normally distributed around the center of the tumor. The blue area represents the percentage of cells present on T1-weighted imaging; the T1 cell threshold is larger for nodular tumor than for diffuse tumor. The area under the curve depicted between blue line and red line represents the percentage of cells present on T2-weighted FLAIR imaging beyond the contrast-enhancing tumor but not on T1-weighted imaging. For nodular tumors, this is a smaller percentage than for diffuse tumors. The white area above the red line represents tumor cells not present on any MRI. For the nodular tumor (A), the difference between 100% resection of T2 and 100% resection of T1 is only approximately 4%. For the diffuse tumor (B) the difference between 100% resection of T2 and 100% resection of T1 is 15%. In other words, for nodular tumors, increasing the resection to 100% of T2 only removes an additional 4% of tumor cell burden. In diffuse tumors, however, increasing the resection to 100% of T2 removes an additional 15% of tumor cell burden. This highlights why, in theory, SMR of diffuse tumors might show a survival benefit, whereas no such benefit is seen for nodular tumors.
SMR Percentages
Clinically significant survival benefit is seen when grouping SMR into 10% intervals for all tumor subgroups; however, different minimum and maximum SMR thresholds were found for each tumor invasiveness profile (Table 2). Based on this patient series, for highly diffuse tumors, resection of at least 30% of the FLAIR hyperintensity beyond the contrast enhancement showed a survival benefit, with no data supporting SMR greater than 99% of the FLAIR region to be beneficial. For moderately diffuse tumors, resection of at least 10% of the FLAIR hyperintensity beyond the contrast enhancement showed a survival benefit, with no data supporting that SMR greater than 60% provides further benefit. For nodular tumors, only resection of at least 10% of the FLAIR hyperintensity beyond the contrast enhancement showed a survival benefit, with no supported benefit above 29%.
SMR threshold percentages for each tumor invasiveness profile are summarized in Fig. 6, which outlines an ideal treatment paradigm based on our data. The results of this study should be further explored through prospective studies that include analysis of mutations and amplifications (i.e., EGFR, PTEN, and p53) and standardized preoperative tumor invasiveness profile characterization for surgical planning to evaluate the impact of safe SMR among the GBM population. Awake craniotomies with direct cortical and subcortical real-time mapping as well as in-depth neuropsychological testing during the procedure may help maximize resection within the illustrated beneficial SMR percentages for each tumor category and minimize potential neurological deficit.9,37–45
Figure 6:

SMR threshold percentages and the ideal treatment paradigm. Based on this patient series, for highly diffuse tumors, resection of at least 30% of the FLAIR hyperintensity beyond the contrast enhancement showed a survival benefit, with no data supporting SMR above 99% of the FLAIR region to be beneficial. For moderately diffuse tumors, resection of at least 10% of the FLAIR hyperintensity beyond the contrast enhancement showed a survival benefit, with no data supporting that SMR above 60% provides further benefit. For nodular tumors, only resection between 10% and 29% of the FLAIR hyperintensity beyond the contrast enhancement showed a survival benefit.
Limitations
The study has the inherent limitations of a retrospective analysis and is prone to errors due to inconsistent or inaccurate medical records and selection bias extending from the inclusion criteria of IDH–wild-type GBM, first time resection, and GTR. Other limitations include the limited cohort size and influence of human error on radiographic measurements. Furthermore, the decision process to perform greater SMR in each case is unknown. In regard to the assumptions made in the model, since the presence of nonenhancing gross infiltrative tumor (as assessed by T2-weighted imaging) was uncommon in this cohort, we did not discriminate between the nonspecific FLAIR hyperintensity and solid, nonenhancing tumor. Similarly, we assume that all FLAIR hyperintensities are similar between tumors and follow the same distribution of cells related to distance from center. Future studies of SMR will require further investigation into accuracy of predictions of tumor density. Other standard MRI sequences, such as T2-weighted imaging and DWI, have also been shown to correlate with tumor cell density at the group level and may provide additional accuracy in future models. Importantly, increasing complexity of the models does present both pros and cons, so our current approach establishes a proof of concept in a straightforward model.
Finally, in this study, we utilized a quantitative method to calculate the tumor invasiveness profile; however, some literature has called into question the ability of this model to get reliable results. For example, when Amelot et al. repeated the study conducted by Baldock et al., different results were reported.23,24 This may be potentially related to the fact that the cutoff values between nodular, moderately diffuse, and highly diffuse tumors have not yet been established. To better account for this, we conducted the threshold analysis twice, first by splitting the patients into 3 equal subgroups (the same protocol used by both Amelot et al. and Baldock et al.) and then using the cutoff presented in Baldock et al.23,24 We acknowledge the practical limitations of implementing this model on a population basis. This model requires determining preoperative volumetric measurements of contrast enhancement and T2/FLAIR signal, which can be time consuming, and then the tumor invasiveness profile needs to be calculated. Therefore, efforts to create an automated integrated system to calculate specific ρ/D ratios for each patient are being planned to facilitate and streamline data collection across multiple institutions.
CONCLUSIONS
The impact of SMR on OS in IDH–wild-type GBM is influenced by the degree of tumor invasiveness. Our results show that increasing SMR is associated with a significant beneficial OS in moderate and highly diffuse IDH–wild-type GBMs. When grouping SMR into 10% intervals, this benefit was seen for all tumor subgroups; however, for nodular tumors, the maximum beneficial SMR percentage was considerably lower than in moderately and highly diffuse tumors.
Disclosure of Funding:
AQH was supported by the Mayo Clinic Professorship and a Clinician Investigator award, and Florida State Department of Health Research Grant, and the Mayo Clinic Graduate School, as well as the NIH (R43CA221490, R01CA200399, R01CA195503, and R01CA216855).
ABBREVIATIONS
- CE
contrast enhancement
- CI
confidence interval
- D
diffusion
- EOR
extent of resection
- FLAIR
fluid-attenuated inversion recovery
- GBM
glioblastoma
- GTR
gross-total resection
- HR
hazard ratio
- IDH
isocitrate dehydrogenase
- KPS
Karnofsky performance status
- MGMT
O6-MethylGuanine-DNA Methyltransferase
- MRI
magnetic resonance imaging
- OS
overall survival
- ρPFS
progression-free survival
- SMR
supramarginal resection
- ρ
perfusion
Footnotes
Conflict of Interest: EHM is a consultant for Boston Scientific Corp.
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. Journal of neurosurgery. 2011;115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Guerrero-Cazares H, Ye X, et al. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. International journal of radiation oncology, biology, physics. 2013;86(4):616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I, et al. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg. 2014;82(1–2):e257–265. [DOI] [PubMed] [Google Scholar]
- 5.Almeida JP, Chaichana KL, Rincon-Torroella J, Quinones-Hinojosa A. The value of extent of resection of glioblastomas: clinical evidence and current approach. Curr Neurol Neurosci Rep. 2015;15(2):517. [DOI] [PubMed] [Google Scholar]
- 6.Miranda A, Blanco-Prieto M, Sousa J, Pais A, Vitorino C. Breaching barriers in glioblastoma. Part I: Molecular pathways and novel treatment approaches. Int J Pharm. 2017;531(1):372–388. [DOI] [PubMed] [Google Scholar]
- 7.Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89(2):219–224. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda M, Kohzuki H, Ishikawa E, et al. Prognostic analysis of patients who underwent gross total resection of newly diagnosed glioblastoma. J Clin Neurosci. 2018;50:172–176. [DOI] [PubMed] [Google Scholar]
- 9.Eseonu CI, Rincon-Torroella J, ReFaey K, et al. Awake Craniotomy vs Craniotomy Under General Anesthesia for Perirolandic Gliomas: Evaluating Perioperative Complications and Extent of Resection. Neurosurgery. 2017;81(3):481–489. [DOI] [PubMed] [Google Scholar]
- 10.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. The New England journal of medicine. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 11.Al-Kharboosh R, Lara-Velazquez M, Prieto L, Sarabia-Estrada R, Quiñones-Hinojosa A. The Study of Brain Tumor Stem Cell Invasion. Methods in molecular biology (Clifton, NJ). 2019;1869:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lara-Velazquez M, Al-Kharboosh R, Prieto L, Schiapparelli P, Quiñones-Hinojosa A. The Study of Brain Tumor Stem Cell Migration. Methods in molecular biology (Clifton, NJ). 2019;1869:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiapparelli P, Guerrero-Cazares H, Magaña-Maldonado R, et al. NKCC1 Regulates Migration Ability of Glioblastoma Cells by Modulation of Actin Dynamics and Interacting with Cofilin. EBioMedicine. 2017;21:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bush NA, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. 2017;40(1):1–14. [DOI] [PubMed] [Google Scholar]
- 15.Lara-Velazquez M, Al-Kharboosh R, Jeanneret S, et al. Advances in Brain Tumor Surgery for Glioblastoma in Adults. Brain sciences. 2017;7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Certo F, Stummer W, Farah JO, et al. Supramarginal resection of glioblastoma: 5-ALA fluorescence, combined intraoperative strategies and correlation with survival. J Neurosurg Sci. 2019;63(6):625–632. [DOI] [PubMed] [Google Scholar]
- 17.de Leeuw CN, Vogelbaum MA. Supratotal resection in glioma: a systematic review. Neuro Oncol. 2019;21(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mampre D, Ehresman J, Pinilla-Monsalve G, et al. Extending the resection beyond the contrast-enhancement for glioblastoma: feasibility, efficacy, and outcomes. Br J Neurosurg. 2018;32(5):528–535. [DOI] [PubMed] [Google Scholar]
- 19.Molinaro AM, Hervey-Jumper S, Morshed RA, et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol. 2020;6(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah AH, Mahavadi A, Di L, et al. Survival benefit of lobectomy for glioblastoma: moving towards radical supramaximal resection. J Neurooncol. 2020;148(3):501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? Journal of neurosurgery. 2016;124(4):977–988. [DOI] [PubMed] [Google Scholar]
- 22.Vivas-Buitrago T, Domingo RA, Tripathi S, et al. Influence of supramarginal resection on survival outcomes after gross-total resection of IDH-wild-type glioblastoma. J Neurosurg. 2021:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amelot A, Deroulers C, Badoual M, et al. Surgical Decision Making From Image-Based Biophysical Modeling of Glioblastoma: Not Ready for Primetime. Neurosurgery. 2017;80(5):793–799. [DOI] [PubMed] [Google Scholar]
- 24.Baldock AL, Ahn S, Rockne R, et al. Patient-specific metrics of invasiveness reveal significant prognostic benefit of resection in a predictable subset of gliomas. PLoS One. 2014;9(10):e99057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldock AL, Yagle K, Born DE, et al. Invasion and proliferation kinetics in enhancing gliomas predict IDH1 mutation status. Neuro Oncol. 2014;16(6):779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang CH, Rockhill JK, Mrugala M, et al. Prognostic significance of growth kinetics in newly diagnosed glioblastomas revealed by combining serial imaging with a novel biomathematical model. Cancer Res. 2009;69(23):9133–9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson KR, Alvord EC Jr., Murray JD. Virtual brain tumours (gliomas) enhance the reality of medical imaging and highlight inadequacies of current therapy. Br J Cancer. 2002;86(1):14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swanson KR, Alvord EC Jr., Murray JD. A quantitative model for differential motility of gliomas in grey and white matter. Cell Prolif. 2000;33(5):317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swanson KR, Bridge C, Murray JD, Alvord EC Jr. Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J Neurol Sci. 2003;216(1):1–10. [DOI] [PubMed] [Google Scholar]
- 30.Chang P, Malone H, Bowden S, et al. A multiparametric model for mapping cellularity in glioblastoma using radiographically localized biopsies. American Journal of Neuroradiology. 2017;38(5):890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ReFaey K, Tripathi S, Grewal SS, et al. Cancer Mortality Rates Increasing vs Cardiovascular Disease Mortality Decreasing in the World: Future Implications. Mayo Clin Proc Inn Qual Out. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ReFaey K, Freeman WD, Tripathi S, et al. NIH Funding Trends for Neurosurgeon-Scientists from 1993–2017: Biomedical Workforce Implications for NeuroOncology. J Neurooncol. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Yu J, Tu L, Huang N, Li H, Luo Y. Isocitrate Dehydrogenase Mutations in Glioma: From Basic Discovery to Therapeutics Development. Front Oncol. 2019;9:506–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadjipanayis CG, Widhalm G, Stummer W. What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery. 2015;77(5):663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang K, Wang XQ, Zhou B, Zhang L. The prognostic value of MGMT promoter methylation in Glioblastoma multiforme: a meta-analysis. Fam Cancer. 2013;12(3):449–458. [DOI] [PubMed] [Google Scholar]
- 36.Swanson KR, Alvord EC Jr. A Biomathematical and Pathological Analysis of an Untreated Glioblastoma. Helsinki, Finland: 2002. [Google Scholar]
- 37.Domingo R, Vivas-Buitrago T, Sabsevitz DS, Middlebrooks EH, Quinones-Hinojosa A. Awake Craniotomy with Cortical and Subcortical Speech Mapping for Supramarginal Cavernoma Resection. World neurosurgery. 2020. [DOI] [PubMed] [Google Scholar]
- 38.Eseonu CI, Eguia F, Garcia O, Kaplan PW, Quiñones-Hinojosa A. Comparative analysis of monotherapy versus duotherapy antiseizure drug management for postoperative seizure control in patients undergoing an awake craniotomy. Journal of neurosurgery. 2018;128(6):1661–1667. [DOI] [PubMed] [Google Scholar]
- 39.Eseonu CI, Rincon-Torroella J, ReFaey K, et al. Awake Craniotomy vs Craniotomy Under General Anesthesia for Perirolandic Gliomas: Evaluating Perioperative Complications and Extent of Resection. Neurosurgery. 2017. [DOI] [PubMed] [Google Scholar]
- 40.ReFaey K, Tripathi S, Bhargav AG, et al. Potential differences between monolingual and bilingual patients in approach and outcome after awake brain surgery. J Neurooncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ReFaey K, Chaichana KL, Feyissa AM, et al. A 360° electronic device for recording high-resolution intraoperative electrocorticography of the brain during awake craniotomy. J Neurosurg. 2019:1–8. [DOI] [PubMed] [Google Scholar]
- 42.Eseonu CI, Rincon-Torroella J, Lee YM, ReFaey K, Tripathi P, Quinones-Hinojosa A. Intraoperative Seizures in Awake Craniotomy for Perirolandic Glioma Resections That Undergo Cortical Mapping. Journal of neurological surgery Part A, Central European neurosurgery. 2018;79(3):239–246. [DOI] [PubMed] [Google Scholar]
- 43.Eseonu CI, Rincon-Torroella J, ReFaey K, Quinones-Hinojosa A. The Cost of Brain Surgery: Awake vs Asleep Craniotomy for Perirolandic Region Tumors. Neurosurgery. 2017;81(2):307–314. [DOI] [PubMed] [Google Scholar]
- 44.Feyissa AM, Worrell GA, Tatum WO, et al. High-frequency oscillations in awake patients undergoing brain tumor-related epilepsy surgery. Neurology. 2018;90(13):e1119–e1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ReFaey K, Tripathi S, Grewal S, Chaichana KL, Quinones-Hinojosa A. Awake craniotomy operating room setup and surgical instruments. In: Awake craniotomy operating room setup and surgical instruments. Vol 1. United States: Thieme; 2019. [Google Scholar]


