Abstract
Objective:
The authors’ goal was to use a multicenter, observational cohort study to determine whether supramarginal resection (SMR) of FLAIR-hyperintense tumor beyond the contrast-enhanced (CE) area influences the overall survival (OS) of patients with isocitrate dehydrogenase–wild-type (IDH-wt) glioblastoma after gross-total resection (GTR).
Methods:
The medical records of 888 patients aged ≥ 18 years who underwent resection of GBM between January 2011 and December 2017 were reviewed. Volumetric measurements of the CE tumor and surrounding FLAIR-hyperintense tumor were performed, clinical variables were obtained, and associations with OS were analyzed.
Results:
In total, 101 patients with newly diagnosed IDH-wt GBM who underwent GTR of the CE tumor met the inclusion criteria. In multivariate analysis, age ≥ 65 years (HR 1.97; 95% CI 1.01–2.56; p < 0.001) and contact with the lateral ventricles (HR 1.59; 95% CI 1.13–1.78; p = 0.025) were associated with shorter OS, but preoperative Karnofsky Performance Status ≥ 70 (HR 0.47; 95% CI 0.27–0.89; p = 0.006), MGMT promotor methylation (HR 0.63; 95% CI 0.52–0.99; p = 0.044), and increased percentage of SMR (HR 0.99; 95% CI 0.98–0.99; p = 0.02) were associated with longer OS. Finally, 20% SMR was the minimum percentage associated with beneficial OS (HR 0.56; 95% CI 0.35–0.89; p = 0.01), but > 60% SMR had no significant influence (HR 0.74; 95% CI 0.45–1.21; p = 0.234).
Conclusion:
SMR is associated with improved OS in patients with IDH-wt GBM who undergo GTR of CE tumor. At least 20% SMR of the CE tumor was associated with beneficial OS, but greater than 60% SMR had no significant influence on OS.
Keywords: Contrast Enhancement, Extent of Resection, Glioblastoma, IDH wild-type, Supramarginal Resection, Supratotal Resection, Survival, FLAIR
INTRODUCTION
Survival outcomes of patients with glioblastoma (GBM) remain unsatisfactory despite current surgical and medical therapies.1,2 Multiple factors are known to influence overall survival (OS), including age, preoperative and postoperative Karnofsky Performance Status (KPS), extent of resection (EOR), administration of adjuvant therapy, presence of isocitrate dehydrogenase (IDH) mutation, MGMT promoter methylation status, and tumor proximity to the lateral ventricles (LVs).2–7 EOR has been established as the main metric for assessment of resection.8,9 Previous studies have reported a survival benefit when a minimum EOR of approximately 70% is achieved on T1-weighted imaging with contrast enhancement.8,10 Driven by the positive outcomes seen with increased EOR, new technologies and surgical tools have been developed to further maximize safe resection.8,11,12
Resection of GBM is usually limited to the contrast-enhanced (CE) tumor. Survival remains dismal, even if gross-total resection (GTR) of the CE portion is achieved.2,5 GBM cells are highly infiltrative and migrate beyond the CE component, with a considerable amount of tumor cells present within the FLAIR-hyperintense area surrounding the CE region.8
Recent studies have evaluated the impact of supramarginal resection (SMR)—defined as resection beyond the CE portion on T1-weighted postcontrast imaging but within the boundaries of the FLAIR-hyperintense signal—on survival outcomes, but variable results have been reported.9,13–17 Furthermore, the benefit of SMR for patients with IDH–wild-type (wt) tumor remains unclear,18 with a recent study reporting beneficial OS in patients younger than 65 years with IDH-wt GBM who underwent SMR.19 In the present multicenter, observational cohort study, we tested the hypothesis that SMR may have a beneficial influence on OS, regardless of other influencing variables, in a homogenous cohort of patients diagnosed with IDH-wt GBM who underwent GTR of the CE portion.
METHODS
Patient Selection
The electronic medical records of 888 adult patients with a presumed diagnosis of GBM who underwent treatment at our three main campuses between January 2011 and December 2017 were reviewed. Diagnosis of GBM was based on the updated WHO classification system20 and determined from the pathology report. Inclusion criteria were as follows: 1) confirmed histopathologic diagnosis of GBM; 2) first-time resection; 3) preoperative MRI scan obtained within 4 weeks before surgery and postoperative MRI scan obtained within 48 hours after surgery; 4) achievement of GTR (defined as complete resection of the CE region on postoperative T1-weighted MRI); 5) IDH-wt status confirmed by the absence of IDH1 or IDH2 gene mutation; 6) known MGMT promoter methylation status; 7) adjuvant radiation therapy and chemotherapy administered according to the Stupp protocol;21 and 8) presence of preoperative FLAIR hyperintensity beyond the CE tumor (Fig. 1).21 This study was approved by the Mayo Clinic Institutional Review Board.
Figure 1:
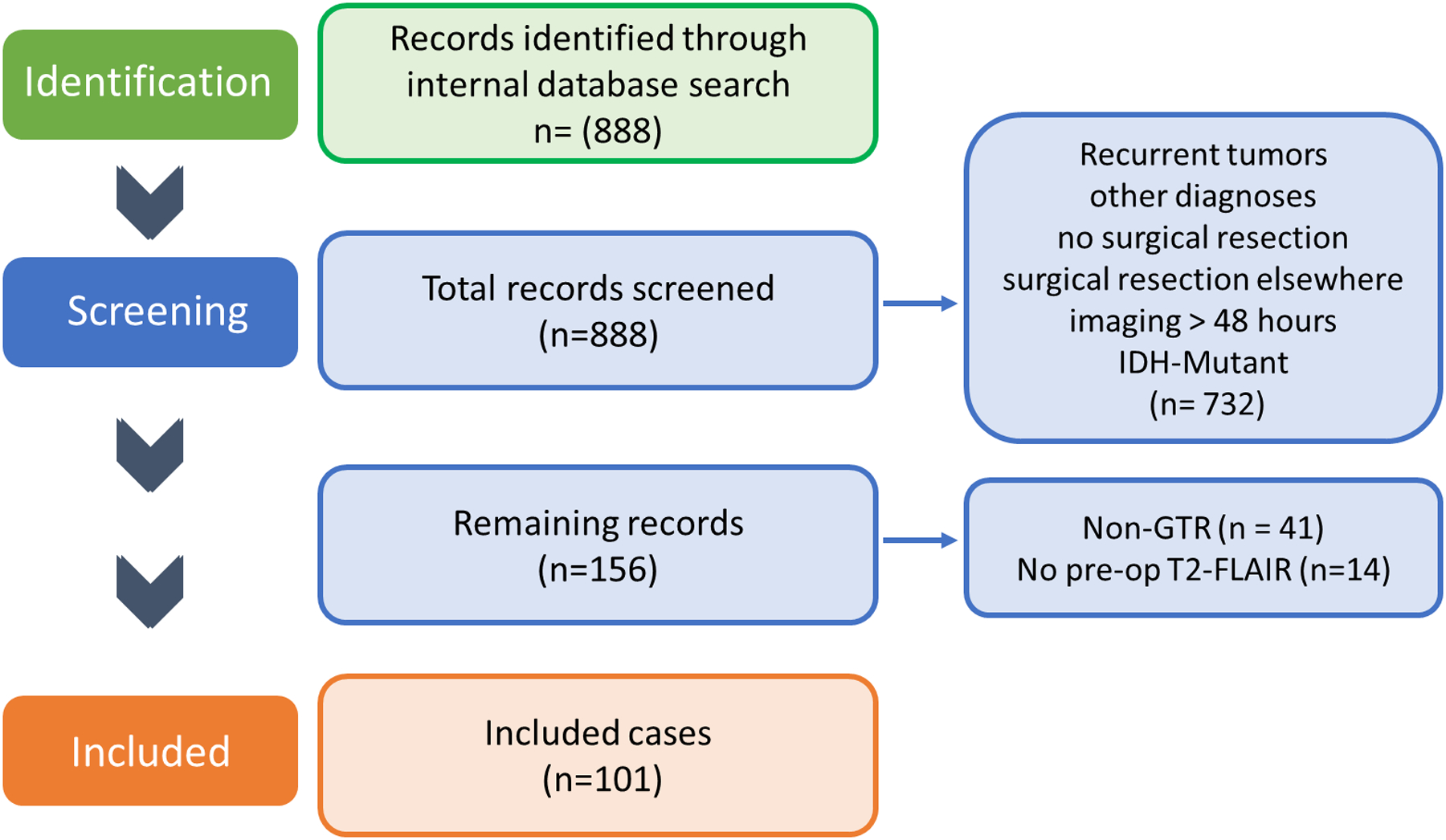
Flowchart illustrating the inclusion and exclusion criteria for this sudy.
Radiographic Characteristics and Volumetric Measurements
The MRI scans of all included patients were retrieved from our institutional archive of medical images. Every scan was obtained at our institution with a 1.5- or 3-T scanner according to a standardized internal protocol. For every patient, preoperative and postoperative MRI scans were evaluated. Preoperative scans were acquired within 4 weeks prior to surgery, and postoperative scans were acquired no later than 48 hours after the surgical procedure. Postcontrast FLAIR and T1-weighted images were evaluated. Tumors were subclassified according to their anatomical location of the involved lobe as frontal, parietal, temporal, or occipital; as cortical or deep-seated (defined as CE tumor not in contact with cortical gray matter); and as eloquent (defined as lesion located in the left hemisphere or right parietal/occipital lobes) or non-eloquent (defined as lesion located in the right frontal/temporal lobes).22 Additionally, the relationship with the LVs was classified on the basis of the location of the CE tumor as 1) CE tumor bordering the LVs or 2) CE tumor not bordering the LVs (Supplementary Fig. 1), as previously reported.4,23
Volumetric measurements were obtained by manually defining the region of interest on each scan of each slide in the axial plane, with subsequent automatic computerization of the volumetric data from the selected regions of interest with DICOM medical image viewer software OsiriX (Pixmeo SARL). Two authors (R.A.D. and G.D.B.), who were blinded to any clinical or demographic patient information except the images, double-coded 20 (20%) randomly chosen patients to ensure no significant differences between measurements; intraclass correlation was used to measure intergrader reliability. Intraclass correlation was calculated to be 0.85; therefore, the remaining volumes were equally distributed and measured.
A total of three volumes corresponding to the following characteristics were directly measured on each scan (Supplementary Fig. 2). 1) CE volume was defined as the CE area on postgadolinium T1-weighted images and re-reflected disrupted blood-brain barrier, including regions of central necrosis. 2) Cystic/necrotic volume was defined as a nonenhanced region within the CE portion of the tumor on postgadolinium T1-weighted images. The region was considered cystic if the borders of the cavity were regular, whereas irregular borders reflected central necrosis. 3) FLAIR volume was defined as a hyperintense area on FLAIR sequence and likely represented a combination of tumor and vasogenic edema.
SMR was defined as the percentage of the FLAIR volume beyond the CE area that was resected. Therefore, to calculate SMR, CE tumor volume was subtracted from preoperative FLAIR volume to determine the preoperative FLAIR volume beyond the CE area. A positive SMR value indicated a decrease in postoperative FLAIR hyperintensity, whereas a negative SMR value indicated an increase in postoperative FLAIR hyperintensity in comparison with the preoperative value; this was potentially related to increased vasogenic edema secondary to the surgical procedure.
Statistical Analysis
OS was defined as the period from surgery to death. The data of patients who were still alive at data compilation were censored at the date of the most recent clinical follow-up. OS was plotted using the Kaplan-Meier method, and log-rank analysis was used to analyze differences in Kaplan-Meier curves. Univariate and multivariate Cox proportional hazards models were used to investigate the associations between variables, percentage of SMR, and OS. A p value < 0.05 (2-sided) was considered statistically significant. RStudio version 3.6.0 was utilized to analyze patient data. Explorative multivariable Cox proportional hazards regression analysis was performed by binning SMR into 10% increments for inclusion in further analyses. Progression-free survival (PFS) was defined as the period from surgery to radiographic evidence of tumor recurrence and analyzed as a secondary outcome of interest.
RESULTS
We included 101 patients with a new diagnosis of GBM who underwent GTR at our institution and met the inclusion criteria. Demographic and clinical characteristics are summarized in Table 1. The mean (range) age was 59.8 (18–86) years, and 68 (67%) patients were male. The mean (range) KPS was 80 (30–100), and the mean (range) OS was 18.3 (2.7–50) months. The most common locations were the frontal (n = 47 [46.5%]) and temporal (n = 46 [45.5%]) lobes, followed by the parietal (n = 26 [25.7%]) and occipital (n = 14 [13.9%]) lobes. Fifty-one (50.5%) tumors were considered deep-seated because they were not in contact with cortical gray matter, and 52 (51%) were in contact with the LVs. The mean (range) preoperative CE tumor volume was 36.2 (1.03–124.15) cm3, and the mean (range) preoperative FLAIR-hyperintense volume was 42.2 (0.48– 182.74) cm3. After GTR, the mean (range) residual volume on FLAIR was 28.4 (0–120) cm3, and the mean percentage of SMR on FLAIR was 28.4% (Supplementary Fig. 3).
TABLE 1:
Characteristics of the study cohort (n = 101)
| Characteristics | Value |
|---|---|
| Age, yrs | 59.8 (18 to 86) |
| Male sex | 68 (67) |
| KPS | 80 (30 to 100) |
| Charlson Comorbidity Index | 4.4 (2 to 9) |
| MGMT Methylated | 32 (32) |
| Preop CE vol, cm3 | 36.2 (1.03 to 124.15) |
| Preop FLAIR vol, cm3 | 42.2 (0.48 to 182.74) |
| Preop Cystic vol cm3 | 16.1 (0 to 113.25) |
| Contact w/ LVs | 52 (51) |
| % SMR on T2-weighted FLAIR | 28.4 (−128.13 to 100) |
| Residual FLAIR vol, cm3 | 28.4 (0 to 120) |
| Location | |
| Frontal Lobe | 47 (46.5) |
| Parietal Lobe | 26 (25.7) |
| Temporal Lobe | 46 (45.5) |
| Occipital Lobe | 14 (13.9) |
| Deep Seated | 51 (50.5) |
Values are shown as mean (range) or number (%)
Patient-Specific and Tumor-Specific Variables
Univariable Cox proportional hazards regression analysis identified age ≥ 65 years (HR 1.99; 95% CI 1.31–3.02; p < 0.001), deep-seated tumor location (HR 1.54; 95% CI 1.01–2.42; p = 0.05), and contact with LVs (HR 1.61; 95% CI 1.06–2.39; p = 0.02) as associated with shorter OS (Table 2). Additionally, preoperative KPS ≥ 70 (HR 0.47; 95% CI 0.29–0.81; p = 0.01), postoperative KPS ≥ 70 (HR 0.57; 95% CI 0.35–0.94; p = 0.03), and MGMT promoter methylation (HR 0.61; 95% CI 0.40–0.99; p = 0.03) were associated with longer OS (Table 2 and Fig. 2). On multivariate analysis, age ≥ 65 years (HR 1.61; 95% CI 1.01–2.56; p = 0.04), contact with LVs (HR 1.26; 95% CI 1.13–1.78; p = 0.03), preoperative KPS ≥ 70 (HR 0.48; 95% CI 0.27–0.89; p = 0.02), and MGMT promoter methylation (HR 0.63; 95% CI 0.52–0.99; p = 0.04) were still found to have a significant influence on OS (Table 3). Finally, tumor location (frontal, parietal, temporal, or occipital lobe) and laterality (left vs right) had no significant influence on OS (Table 2).
TABLE 2:
Univariate analysis of OS
| Variable | HR (95% CI) | p Value |
|---|---|---|
| Age ≥ 65 yrs | 1.99 (1.31 – 3.02) | <0.01 |
| Male sex | 0.92 (0.61 – 1.48) | 0.54 |
| KPS ≥ 70 | 0.47 (0.29 – 0.81) | 0.01 |
| Charlson Comorbidity Index | 1.21 (1.07 – 1.37) | 0.02 |
| MGMT promoter Methylation | 0.61 (0.40 – 0.99) | 0.03 |
| CE vol | 1.00 (0.99 – 1.01) | 0.18 |
| Preop Flair vol | 1.03 (0.99 – 1.04) | 0.28 |
| Preop cystic/necrotic vol | 1.00 (0.99 – 1.01) | 0.51 |
| Cystic/necrotic vol | 0.98 (0.60 – 1.52) | 0.89 |
| T1/T2-weighted FLAIR ratio | 0.99 (0.96 – 1.03) | 0.76 |
| % SMR on T2-weighted FLAIR | 0.99 (0.98 – 0.99) | <0.01 |
| Location | ||
| Contact w/ LVs | 1.61 (1.06 – 2.39) | 0.02 |
| Deep Seated | 1.54 (1.01 – 2.42) | 0.05 |
| Lt hemisphere | 0.80 (0.52 – 1.25) | 0.33 |
| Frontal | 1.22 (0.79 – 1.90) | 0.36 |
| Parietal | 0.63 (0.37 – 1.07) | 0.09 |
| Temporal | 1.11 (0.71 – 1.71) | 0.65 |
| Occipital | 1.28 (0.67 – 2.42) | 0.45 |
Boldface type indicates statistical significance (p < 0.05).
Figure 2:
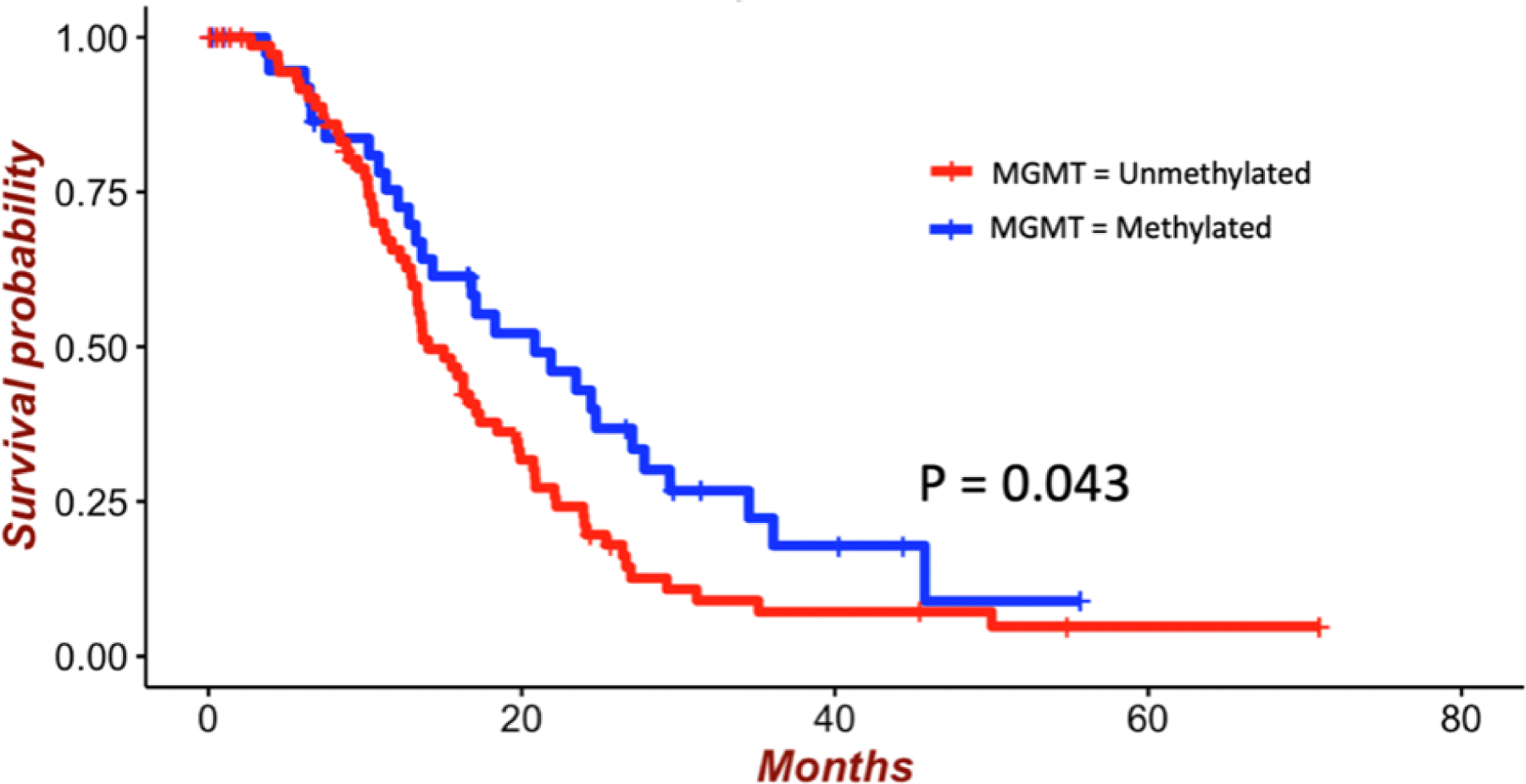
Survival of patients with GBM who underwent GTR of CE tumor according to MGMT promoter methylation status. The mean survival of patients with methylated MGMT promoter was 21.4 months compared with 18.6 months for patients with unmethylated MGMT promoter (p = 0.043).
TABLE 3:
Multivariate analysis of SMR and OS
| Variable | HR (95% CI) | p Value |
|---|---|---|
| % SMR on T2-weighted FLAIR | ||
| Increased Resection | 0.99 (0.98 – 0.99) | 0.02 |
| > 20% | 0.56 (0.35 – 0.89) | 0.01 |
| > 30% | 0.55 (0.35 – 0.87) | 0.01 |
| > 40% | 0.52 (0.33 – 0.81) | <0.01 |
| > 50% | 0.62 (0.39 – 0.99) | 0.04 |
| > 60% | 0.74 (0.45 – 1.21) | 0.34 |
| Age ≥ 65 yrs | 1.61 (1.01 – 2.56) | 0.04 |
| KPS ≥ 70 | 0.48 (0.27 – 0.89) | 0.02 |
| Charlson Comorbidity Index | 1.07 (1.01 – 1.16) | 0.03 |
| MGMT promoter methylation | 0.63 (0.52 – 0.99) | 0.04 |
| Contact w/ LVs | 1.26 (1.13 – 1.78) | 0.03 |
| T1/T2-weighted FLAIR ratio | 0.97 (0.94 – 1.02) | 0.38 |
| Deep-seated tumor | 1.21 (0.66 – 2.16) | 0.46 |
Boldface type indicates statistical significance (p < 0.05).
Association Between Percentage of SMR on FLAIR and Survival Outcomes
In univariate analysis, the percentage of SMR on FLAIR was associated with improved OS (HR 0.99; 95% CI 0.98–0.99; p < 0.01) (Table 2). Whether a tumor was deep-seated (24.8% of patients vs 31.9% of patients with non–deep-seated tumors, p = 0.50) (Supplementary Table 1) or located in a noneloquent area of the brain (30% of patients vs 26.4% of patients with tumors in an eloquent area, p = 0.73) (Supplementary Table 1) had no significant effect on percentage of SMR observed. In multivariate analysis, after we controlled for other variables (age, KPS, MGMT promoter methylation, contact with LVs, and contrast enhancement/FLAIR ratio), an increased SMR on FLAIR was still significantly associated with OS (HR 0.99; 95% CI 0.98–0.99; p = 0.02) (Table 3). Furthermore, explorative multivariable Cox proportional hazards regression analysis was used to determine whether significant cutoff values existed. Here, significant benefit in OS was seen in patients who underwent 20% (HR 0.56; 95% CI 0.35–0.89; p = 0.01) (Fig. 3) to 50% SMR (HR 0.62; 95% CI 0.39–0.99; p = 0.04) (Fig. 4), but no significant influence was seen in patients who underwent 60% SMR (HR 0.74; 95% CI 0.45–1.21; p = 0.34) and greater (Table 3). Additionally, postoperative KPS was not significantly different between the patients with ≤ 0% SMR and those with > 0% SMR at the last follow-up (80 vs 80, respectively; p = 0.15).
Figure 3:
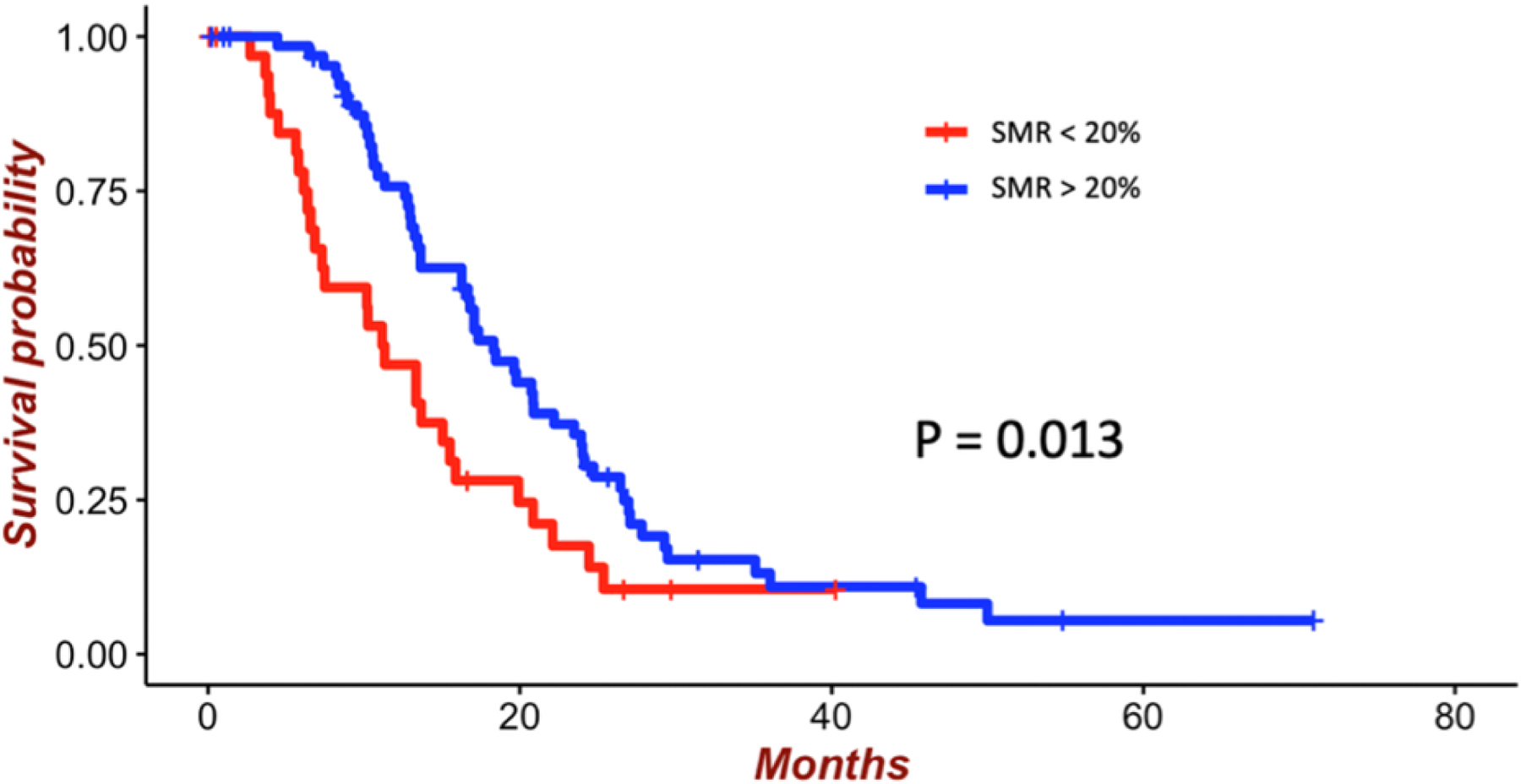
Survival of patients with GBM who underwent GTR of CE tumor according to a 20% SMR threshold. The mean survival of patients who underwent > 20% SMR was 19.1 months compared with 16.8 months for patients who underwent < 20% SMR (p = 0.013).
Figure 4:
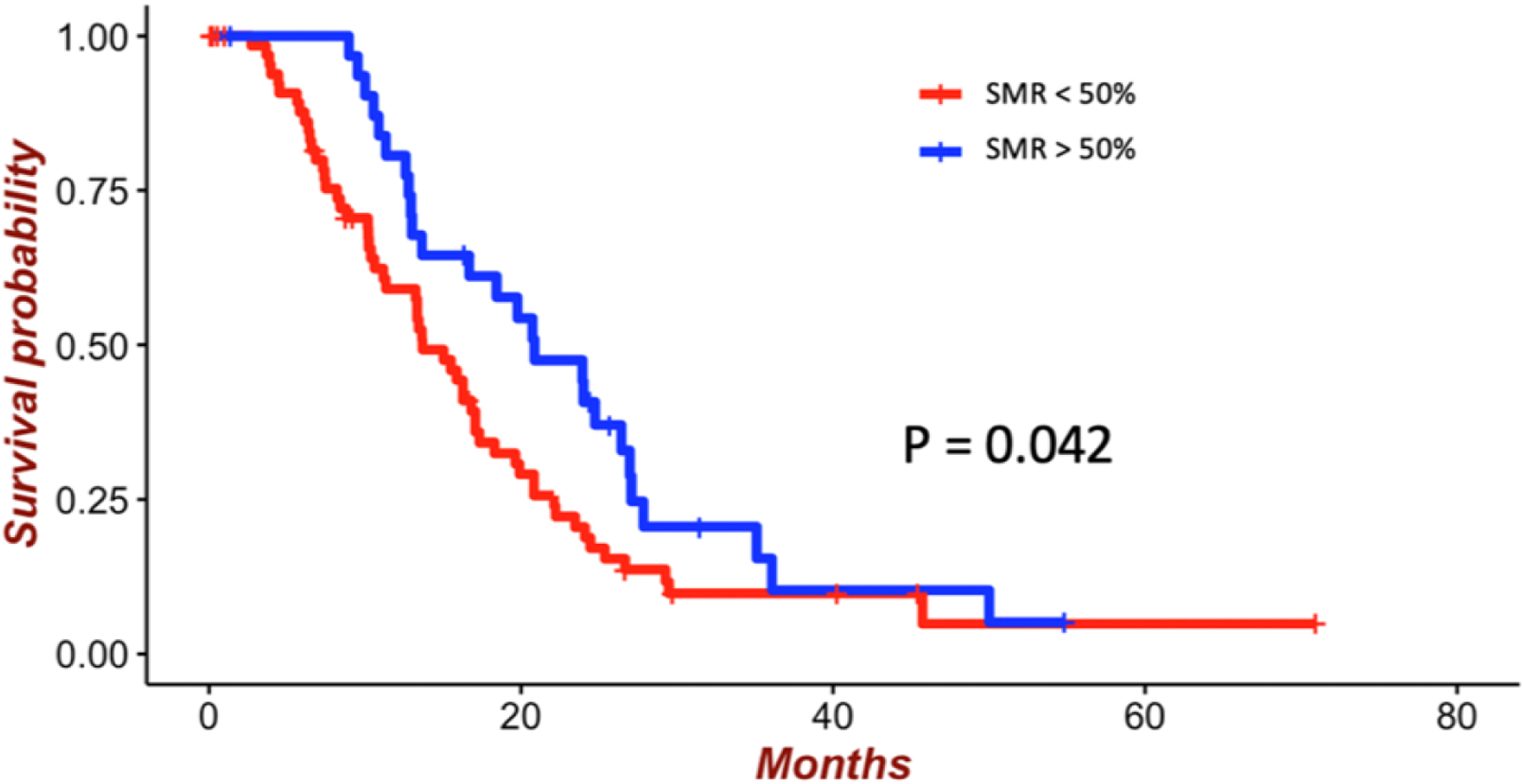
Survival of patients with GBM who underwent GTR of CE tumor according to a 50% SMR threshold. The mean survival of patients who underwent > 50% SMR was 20.1 months compared with 18.3 months for patients who underwent < 50% SMR (p = 0.042).
PFS was analyzed as a secondary outcome of interest. PFS was radiographically determined in 69 (68%) patients. Because the cohort size was small and this study was powered for analysis of the primary outcome of OS, no multivariate analysis of PFS was conducted. Univariable Cox proportional hazards regression analysis identified age ≥ 65 years (HR 2.34; 95% CI 1.31–4.19; p < 0.01) and increased Charlson Comorbidity Index (HR 1.20; 95% CI 1.01–1.43; p = 0.03) as associated with shorter PFS (Supplementary Table 2). Additionally, KPS ≥ 70 (HR 0.48; 95% CI 0.24–0.86; p = 0.03) and MGMT promoter methylation (HR 0.61; 95% CI 0.48–0.94; p = 0.04) were associated with longer OS (Supplementary Table 2). In univariate analysis, 10% increments in SMR on FLAIR were associated with improved PFS (HR 0.92; 95% CI 0.85–0.99; p = 0.04) (Supplementary Table 2). Furthermore, explorative Cox proportional hazards regression analysis was used to determine if significant cutoff values existed. Here, significant benefit in PFS was seen in patients who underwent 20% (HR 0.58; 95% CI 0.34–0.99; p = 0.05) (Supplementary Fig. 4) to 40% SMR (HR 0.52; 95% CI 0.31–0.86; p = 0.01) (Supplementary Fig. 5), but no significant influence was seen in patients who underwent 50% (HR 0.64; 95% CI 0.38–1.06; p = 0.08) or greater SMR (Supplementary Table 2).
DISCUSSION
The survival benefits of EOR of CE tumor in patients with GBM have been widely studied. Maximum safe resection and particularly GTR are associated with improved OS.8,24–26 Nonetheless, OS remains dismal, and the median OS is 15 months for patients who receive maximal therapy.2 Recent studies have evaluated the impact of SMR on OS, but variable results have been reported.1,9,13–15 The inclusion of patients with different molecular subtypes of GBM and intrinsic differences in prognosis and response to therapy,17 variability in EOR of CE tumor, and diversity of nonvolumetric measurements methods may have potentially influenced these findings.
For the purposes of this study, we aimed to evaluate the potential influence of SMR on OS in a homogeneous cohort of patients diagnosed with IDH-wt GBM. Thus, we minimized variability within our cohort while including patients with the most common subtype of GBM.27 Furthermore, to account for variability in EOR of CE tumor, only patients who underwent GTR of CE tumor were included. All included patients completed an adjuvant radio-chemotherapy regimen according to the Stupp protocol to account for variability in adjuvant treatment. This is the first study to report the benefit of SMR for OS in patients with IDH-wt GBM, regardless of other influencing variables.
Patient and Tumor-Specific Variables
The survival outcomes of patients with GBM are strongly linked to patient age and functional status at time of diagnosis.26 Our results are consistent with those in the literature, with age > 65 years and KPS < 70 negatively influencing OS. Tumor proximity to the LVs has also been associated with increased aggressiveness in tumor behavior. However, data supporting its influence on survival are inconsistent throughout the literature.4 A recent meta-analysis compared survival of patients with GBM in contact with the LVs versus that of patients with GBM without LV contact, and GBM without LV contact reportedly had a potentially negative influence on OS; however, no definitive conclusions were drawn.28 In our study, patients with tumors in contact with the LVs had a significantly lower mean OS (16.6 months) than patients with GBM without LV contact (20.2 months). The current literature suggests that the migration of GBM cells through the CSF and involvement with the neurogenic niche of the subventricular zone are potential mechanisms for increased tumor aggressiveness and decreased survival.4,28 Furthermore, no significant association with OS was observed when tumors were classified by location (deep-seated, cortical, and left/right hemispheres) or lobe (frontal, parietal, temporal, and occipital).
In recent years, molecular markers have played an important role in subclassification and characterization of gliomas and are considered to influence survival outcomes and response to treatment in patients with GBM.17,29 IDH and MGMT methylation status are most strongly associated with OS, and patients with IDH-wt tumors are believed to have worse response to adjuvant treatment and more aggressive tumor behavior.17,24 Our cohort included only patients with IDH-wt GBM, and our results were consistent with those in the available literature, showing that MGMT promoter methylation is associated with increased survival.
SMR and Survival Outcomes
GBM cells are highly invasive. By the time of diagnosis, cells are often present in locations distant from the CE foci and often extend into the contralateral hemisphere.30–34 This infiltrative behavior makes complete resection of GBM virtually impossible. For glioma surgery, the general consensus is that more resection is better, as long as it can be safely performed without compromising the neurological function of the patient.24,35 However, particularly for GBM, resection is usually limited to the CE tumor, with GTR of the CE portion considered the ideal outcome.5,8,11,14,15,26,36
Although GTR of the CE portion of the tumor is associated with survival benefits in patients with GBM, the percentage of SMR on FLAIR may not follow the same principle because this region comprises both edematous brain parenchyma and infiltrative tumor cells with a continuous reduction in tumor cell density toward the periphery.37 Furthermore, functional tissue is often present within this region, representing a challenge to the surgeon during the decision-making process to find a balance between functional tissue preservation and diffuse tumor resection. This may explain why, in our study, patients who underwent SMR greater than 60% did not have a statistically significant increase in OS. Our experience has been to perform more awake craniotomies with direct cortical and subcortical real-time mapping and neuropsychological testing at the time of surgery to maximize resection and minimize potential neurological deficit, and no significant difference in postoperative KPS has been observed between patients with ≤ 0% SMR and those with > 0% SMR.6,12,38–44 Additionally, no significant differences in percentages of SMR were noted between patients with deep-seated versus cortical tumor, or between those with tumors in eloquent versus noneloquent areas.
The survival benefit of SMR for patients with GBM has been assessed in prior studies that used various cohorts and methodologies, such as the inclusion of patients regardless of EOR of CE tumor, molecular status, presence of recurrent tumors, use of adjuvant therapy, and tumor measurement modalities, and contrasting results have been reported.9,14–16,22,24 A recent study reported significantly favorable OS for patients who underwent resection of CE and non-CE tumors, regardless of MGMT promoter methylation status, and for all patients with IDH-mutant tumors. For patients with IDH-wt tumor who underwent SMR, beneficial OS was only observed in those younger than 65 years.19 In the present study, we analyzed the impact of SMR on the survival outcomes of only patients with newly diagnosed IDH-wt GBM who had undergone first-time resection since 2011.
Our results show, for the first time, that increased SMR is associated with significantly improved OS in patients with IDH-wt GBM, regardless of age or other variables. Although statistically significant, the HR (0.99; 95% CI 0.98–0.99) of this finding is not as robust as the significance obtained for specific SMR percentages. In these analyses, 20% was the minimum percentage of SMR associated with beneficial OS, but > 60% SMR was not significantly associated with beneficial OS. However, as the percentage of SMR increased, we may not have had enough patients to identify statistical differences. Therefore, we conclude, on the basis of the current study, that resection of at least 20% of the FLAIR-hyperintense tumor beyond contrast enhancement should be attempted when safely possible. At this time, no data support improved OS with > 60% SMR (Fig. 5). PFS was analyzed as a secondary outcome; SMR was significantly associated with improved PFS only when pooled into 10% intervals. Threshold analysis demonstrated a significant benefit on PFS with SMR of at least 20%, while statistical significance was lost at SMR of 50%. Given the retrospective nature of the current study and its limited cohort size, the clinical impact of SMR for patients with GBM should be further explored by using prospective studies with standardized surgical strategies to maximize safe SMR.
Figure 5:
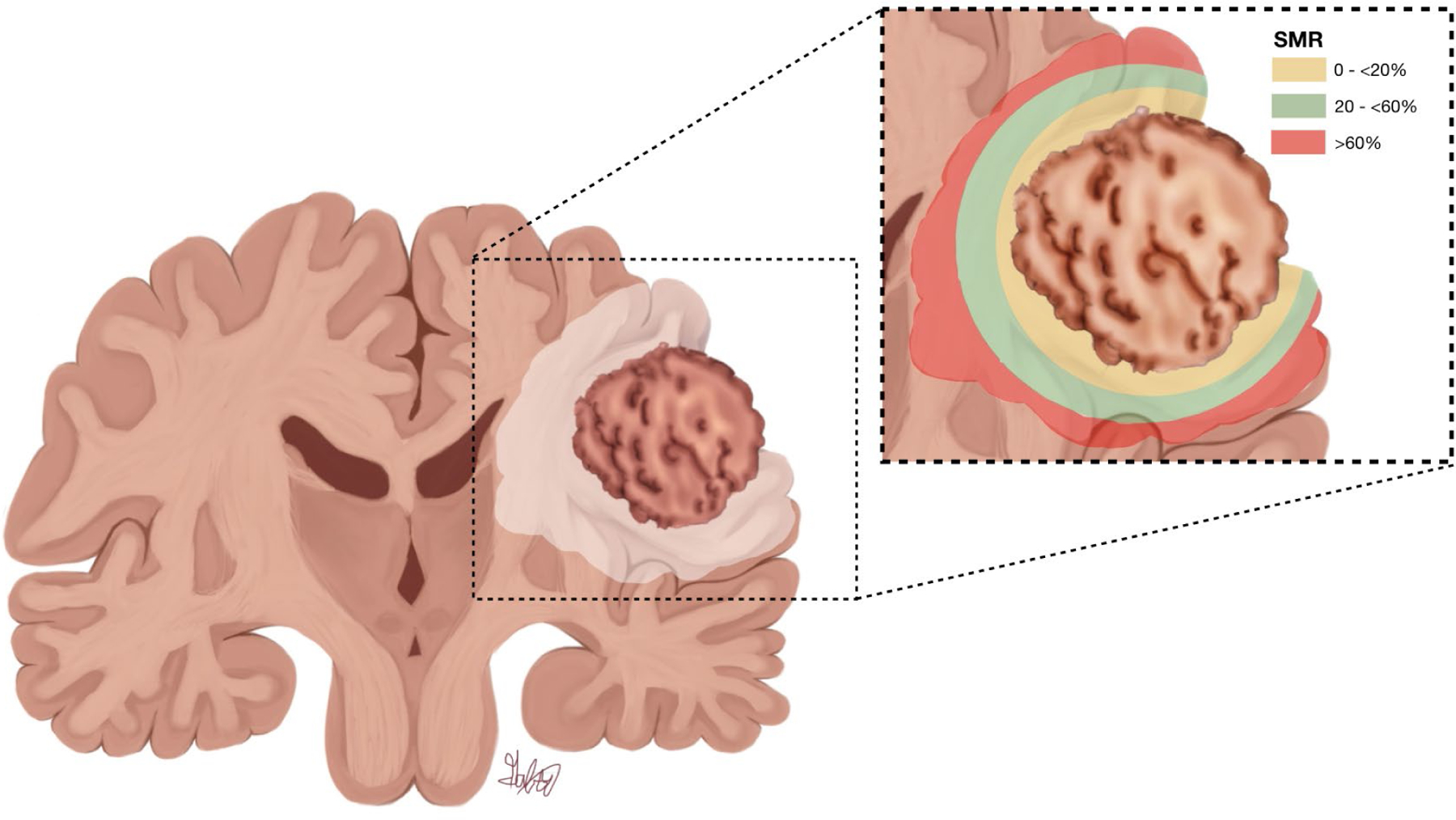
SMR percentages significantly associated with beneficial OS in patients with IDH-wt GBM who underwent GTR of CE tumor. A coronal section of the brain is shown, illustrating the CE portion of the tumor surrounded by FLAIR hyperintensity, as well as a color-coded magnified view of the tumor region (inset). Yellow represents proximal SMR percentages that did not show a significant benefit in OS. Green represents SMR percentages that were significantly associated with benefit in OS. Red represents the distal FLAIR-hyperintense area in which SMR was not significantly associated with OS. Copyright Alfredo Quinones-Hinojosa. Published with permission.
Limitations
The study has the inherent limitations of a retrospective analysis and is prone to errors due to inconsistent or inaccurate medical records and potential selection bias. Specifically, given the restrictive inclusion criteria of IDH-wt status, first-time resection, and GTR, the results are subject to selection bias. Other limitations include a robust but limited cohort size given our inclusion/exclusion criteria, variability of the infiltrative patterns between tumors, and potential influence of human error on radiographic measurements. Because the cohort size was small and this study was powered for analysis of PFS was conducted. We are aware of the potential effect of increasing resection on functional status and the risk of new or worsened postoperative neurological deficits on GBM patients.45,46 Although this study showed no significant differences in overall preoperative and postoperative functional status in terms of KPS and Charlson comorbidity index, we emphasize the need for future prospective studies that include a detailed assessment of the neurological and cognitive function through tailored neuropsychological testing. Moreover, the possibility of hidden bias cannot be excluded because the decision-making process used to determine the extent of SMR for each patients cannot be defined owing to the retrospective nature of this study.
The percentage of SMR associated with maximal benefit in OS was not established owing to the limited number of patients included in the threshold subgroups. Because we exclusively evaluated patients with IDH-wt GBM, it is uncertain if these results are directly applicable to other infiltrative gliomas. In particular, because the presence of non-CE gross infiltrative tumor (as assessed with T2-weighted imaging) was uncommon in this cohort, we did not discriminate between nonspecific FLAIR hyperintensity and solid non-CE tumor. Such discrimination may be essential for evaluation of lower-grade gliomas. Likewise, it is uncertain if the benefit of SMR can be extended to patients with IDH-mutant gliomas owing to their increased responsiveness to radiation therapy and chemotherapy, and future studies are needed to directly assess SMR in this setting.
CONCLUSIONS
SMR is associated with beneficial OS in patients with IDH-wt GBM who undergo GTR of the CE portion. Resection of 20% of the FLAIR-hyperintense tumor beyond the CE portion was associated with beneficial OS, but no significant influence was seen in patients who underwent >60% SMR. Further studies are required to support these findings and determine the SMR percentage associated with maximal benefit in OS.
Supplementary Material
Disclosure of Funding:
Dr. Quinones-Hinojosa was supported by the Mayo Clinic Professorship and a Clinician Investigator Award, a Florida State Department of Health Research Grant, the Mayo Clinic Graduate School, and National Institutes of Health grants (nos. R43CA221490, R01CA200399, R01CA195503, and R01CA 216855).
ABBREVIATIONS
- CE
contrast enhancement
- CI
confidence interval
- EOR
extent of resection
- FLAIR
fluid-attenuated inversion recovery
- GBM
glioblastoma
- GTR
gross-total resection
- HR
hazard ratio
- ICC
intraclass correlation
- IDH
isocitrate dehydrogenase
- KPS
Karnofsky performance status
- LV
lateral ventricle
- MGMT
O6-MethylGuanine-DNA Methyltransferase
- MRI
Magnetic Resonance Imaging
- PFS
Progression-Free Survival
- SMR
supramarginal resection
Footnotes
Conflict of Interest: None
REFERENCES
- 1.Bush NA, Chang SM, Berger MS. Current and future strategies for treatment of glioma. Neurosurg Rev. 2016;40(1):1–14. doi: 10.1007/s10143-016-0709-8 [DOI] [PubMed] [Google Scholar]
- 2.Marenco-Hillembrand L, Wijesekera O, Suarez-Meade P, et al. Trends in glioblastoma: outcomes over time and type of intervention: a systematic evidence based analysis. J Neurooncol. 2020;147(2):297–307. doi: 10.1007/s11060-020-03451-6 [DOI] [PubMed] [Google Scholar]
- 3.Miranda A, Blanco-Prieto M, Sousa J, Pais A, Vitorino C. Breaching barriers in glioblastoma. Part I: Molecular pathways and novel treatment approaches. Int J Pharm. 2017;531(1):372–388. [DOI] [PubMed] [Google Scholar]
- 4.Chaichana KL MJM, F J F A, G-C H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89(2):219–24. doi: 10.1007/s11060-008-9609-2 [DOI] [PubMed] [Google Scholar]
- 5.Matsuda M, Kohzuki H, Ishikawa E, et al. Prognostic analysis of patients who underwent gross total resection of newly diagnosed glioblastoma. J Clin Neurosci. 2018;50:172–176. doi: 10.1016/j.jocn.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 6.Eseonu CI, Rincon-Torroella J, ReFaey K, et al. Awake Craniotomy vs Craniotomy Under General Anesthesia for Perirolandic Gliomas: Evaluating Perioperative Complications and Extent of Resection. Neurosurgery. Sep 1 2017;81(3):481–489. doi: 10.1093/neuros/nyx023 [DOI] [PubMed] [Google Scholar]
- 7.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. The New England journal of medicine. Mar 10 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331 [DOI] [PubMed] [Google Scholar]
- 8.Almeida JP, Chaichana KL, Rincon-Torroella J, Quinones-Hinojosa A. The value of extent of resection of glioblastomas: clinical evidence and current approach. Curr Neurol Neurosci Rep. 2014;15(2):517. doi:doi: 10.1007/s11910-014-0517-x [DOI] [PubMed] [Google Scholar]
- 9.Certo F, Stummer W, Farah JO, et al. Supramarginal resection of glioblastoma: 5-ALA fluorescence, combined intraoperative strategies and correlation with survival. J Neurosurg Sci. 2019;63(6):625–632. doi: 10.23736/S0390-5616.19.04787-8 [DOI] [PubMed] [Google Scholar]
- 10.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. Journal of neurosurgery. Jul 2011;115(1):3–8. doi: 10.3171/2011.2.jns10998 [DOI] [PubMed] [Google Scholar]
- 11.Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I, et al. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World neurosurgery. 2014;82(1–2):257–65. doi: 10.1016/j.wneu.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 12.Lara-Velazquez M, Al-Kharboosh R, Jeanneret S, et al. Advances in Brain Tumor Surgery for Glioblastoma in Adults. Brain sciences. Dec 20 2017;7(12)doi: 10.3390/brainsci7120166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Leeuw CN, Vogelbaum MA. Supratotal resection in glioma: a systematic review. Neuro Oncol. 2019;21(2):179–188. doi: 10.1093/neuonc/noy166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mampre D, Ehresman J, Pinilla-Monsalve G, et al. Extending the resection beyond the contrast-enhancement for glioblastoma: feasibility, efficacy, and outcomes. Br J Neurosurg. 2018;32(5):528–535. doi: 10.1080/02688697.2018.1498450 [DOI] [PubMed] [Google Scholar]
- 15.Altieri R, Melcarne A, Soffietti R, et al. Supratotal Resection of Glioblastoma: Is Less More? Surgical technology international. Nov 10 2019;35:432–440. [PubMed] [Google Scholar]
- 16.Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? Journal of neurosurgery. Apr 2016;124(4):977–88. doi: 10.3171/2015.5.Jns142087 [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Yu J, Tu L, Huang N, Li H, Luo Y. Isocitrate Dehydrogenase Mutations in Glioma: From Basic Discovery to Therapeutics Development. Front Oncol. 2019;9:506–506. doi: 10.3389/fonc.2019.00506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81–91. doi: 10.1093/neuonc/not159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molinaro AM, Hervey-Jumper S, Morshed RA, et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol. 2020;6(4):495–503. doi: 10.1001/jamaoncol.2019.6143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis D, Ohgaki H, Wiestler O, Cavenee W. World Health Organization Histological Classification of Tumours of the Central Nervous System. International Agency for Research on Cancer. 2016; [Google Scholar]
- 21.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 22.Shah AH, Mahavadi A, Di L, et al. Survival benefit of lobectomy for glioblastoma: moving towards radical supramaximal resection. J Neurooncol. 2020;148(3):501–508. doi: 10.1007/s11060-020-03541-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Guerrero-Cazares H, Ye X, et al. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. International journal of radiation oncology, biology, physics. Jul 15 2013;86(4):616–22. doi: 10.1016/j.ijrobp.2013.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hadjipanayis CG, Widhalm G, Stummer W. What is the Surgical Benefit of Utilizing 5-Aminolevulinic Acid for Fluorescence-Guided Surgery of Malignant Gliomas? Neurosurgery. 2015;77(5):663–673. doi: 10.1227/NEU.0000000000000929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pino MA, Imperato A, Musca I, et al. New Hope in Brain Glioma Surgery: The Role of Intraoperative Ultrasound. A Review. Brain sciences. 2018;8(11):202. doi: 10.3390/brainsci8110202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haj A, Doenitz C, Schebesch K-M, et al. Extent of Resection in Newly Diagnosed Glioblastoma: Impact of a Specialized Neuro-Oncology Care Center. Brain sciences. 2017;8(1):5. doi: 10.3390/brainsci8010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15(7):405–417. doi: 10.1038/s41582-019-0220-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mistry AM, Hale AT, Chambless LB, Weaver KD, Thompson RC, Ihrie RA. Influence of glioblastoma contact with the lateral ventricle on survival: a meta-analysis. J Neurooncol. 2017;131(1):125–133. doi: 10.1007/s11060-016-2278-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang K, Wang XQ, Zhou B, Zhang L. The prognostic value of MGMT promoter methylation in Glioblastoma multiforme: a meta-analysis. Fam Cancer. 2013;12(3):449–58. doi: 10.1007/s10689-013-9607-1 [DOI] [PubMed] [Google Scholar]
- 30.Kim Y Regulation of Cell Proliferation and Migration in Glioblastoma: New Therapeutic Approach. 10.3389/fonc.2013.00053. Front Oncol. 2013;3:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbadi S, Rodarte JJ, Abutaleb A, et al. Glucose-6-phosphatase is a key metabolic regulator of glioblastoma invasion. Molecular cancer research : MCR. Nov 2014;12(11):1547–59. doi: 10.1158/1541-7786.Mcr-14-0106-t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiapparelli P, Guerrero-Cazares H, Magaña-Maldonado R, et al. NKCC1 Regulates Migration Ability of Glioblastoma Cells by Modulation of Actin Dynamics and Interacting with Cofilin. EBioMedicine. Jul 2017;21:94–103. doi: 10.1016/j.ebiom.2017.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lara-Velazquez M, Al-Kharboosh R, Prieto L, Schiapparelli P, Quiñones-Hinojosa A. The Study of Brain Tumor Stem Cell Migration. Methods in molecular biology (Clifton, NJ). 2019;1869:93–104. doi: 10.1007/978-1-4939-8805-1_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Kharboosh R, Lara-Velazquez M, Prieto L, Sarabia-Estrada R, Quiñones-Hinojosa A. The Study of Brain Tumor Stem Cell Invasion. Methods in molecular biology (Clifton, NJ). 2019;1869:105–116. doi: 10.1007/978-1-4939-8805-1_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duffau H Long-term outcomes after supratotal resection of diffuse low-grade gliomas: a consecutive series with 11-year follow-up. Acta neurochirurgica. 2016;158(1):51–8. doi: 10.1007/s00701-015-2621-3 [DOI] [PubMed] [Google Scholar]
- 36.Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16(1):113–122. doi: 10.1093/neuonc/not137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang P, Malone H, Bowden S, et al. A multiparametric model for mapping cellularity in glioblastoma using radiographically localized biopsies. American Journal of Neuroradiology. 2017;38(5):890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Domingo R, Vivas-Buitrago T, Sabsevitz DS, Middlebrooks EH, Quinones-Hinojosa A. Awake Craniotomy with Cortical and Subcortical Speech Mapping for Supramarginal Cavernoma Resection. World neurosurgery. Jun 22 2020;doi: 10.1016/j.wneu.2020.06.094 [DOI] [PubMed] [Google Scholar]
- 39.Eseonu CI, Eguia F, Garcia O, Kaplan PW, Quiñones-Hinojosa A. Comparative analysis of monotherapy versus duotherapy antiseizure drug management for postoperative seizure control in patients undergoing an awake craniotomy. Journal of neurosurgery. Jun 2018;128(6):1661–1667. doi: 10.3171/2017.1.Jns162913 [DOI] [PubMed] [Google Scholar]
- 40.Eseonu CI, Rincon-Torroella J, ReFaey K, Quinones-Hinojosa A. The Cost of Brain Surgery: Awake vs Asleep Craniotomy for Perirolandic Region Tumors. Neurosurgery. Aug 01 2017;81(2):307–314. doi: 10.1093/neuros/nyx022 [DOI] [PubMed] [Google Scholar]
- 41.Feyissa AM, Worrell GA, Tatum WO, et al. High-frequency oscillations in awake patients undergoing brain tumor-related epilepsy surgery. Neurology. Mar 27 2018;90(13):e1119–e1125. doi: 10.1212/wnl.0000000000005216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ReFaey K, Chaichana KL, Feyissa AM, et al. A 360° electronic device for recording high-resolution intraoperative electrocorticography of the brain during awake craniotomy. Journal of neurosurgery. Jul 2019:1–8. doi: 10.3171/2019.4.JNS19261 [DOI] [PubMed] [Google Scholar]
- 43.ReFaey K, Tripathi S, Bhargav AG, et al. Potential differences between monolingual and bilingual patients in approach and outcome after awake brain surgery. J Neurooncol. Jun 10 2020;doi: 10.1007/s11060-020-03554-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suarez-Meade P, Marenco-Hillembrand L, Prevatt C, et al. Awake vs. asleep motor mapping for glioma resection: a systematic review and meta-analysis. Acta neurochirurgica. Jul 2020;162(7):1709–1720. doi: 10.1007/s00701-020-04357-y [DOI] [PubMed] [Google Scholar]
- 45.McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. Sep 2009;65(3):463–9; discussion 469–70. doi: 10.1227/01.Neu.0000349763.42238.E9 [DOI] [PubMed] [Google Scholar]
- 46.Rahman M, Abbatematteo J, De Leo EK, et al. The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. Journal of neurosurgery. Jul 2017;127(1):123–131. doi: 10.3171/2016.7.Jns16396 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


