Abstract
BACKGROUND
Cerebral microbleeds (CMBs) may increase the risk of future intracerebral hemorrhage and ischemic stroke. However, It is unclear whether antiplatelet medication is associated with CMBs. This study aimed to investigate the association between antiplatelet medication and CMBs in a community-based stroke-free population.
METHODS
In this cross-sectional study, stroke-free participants aged 18–85 years were recruited from a community in Beijing, China. Demographic, clinical, and antiplatelet medication data were collected through a questionnaire, and all participants underwent blood tests and brain magnetic resonance imaging at 3.0T. The presence, count, and location of CMBs were evaluated using susceptibility-weighted imaging. The association between antiplatelet medication and the presence of CMBs was analyzed using multivariable logistic regression. The associations between antiplatelet medication and CMBs by location (lobar, deep brain or infratentorial, and mixed regions) were also analyzed using multinomial logistic regression. A linear regression analysis was conducted to determine the association between antiplatelet medication and the log-transformed number of CMBs.
RESULTS
Of the 544 participants (mean age: 58.65 ± 13.66 years, 217 males), 119 participants (21.88%) had CMBs, and 64 participants (11.76%) used antiplatelet medication. Antiplatelet medication was found to be associated with CMBs at any location [odds ratio (OR) = 2.39, 95% CI: 1.24–4.58] and lobar region (OR = 2.83, 95% CI: 1.36–5.86), but not with the number of CMBs (β = 0.14, 95% CI: -0.21–0.48). Among antiplatelet medications, aspirin use was found to be associated with any CMB (OR = 3.17, 95% CI: 1.49–6.72) and lobar CMBs (OR = 3.61, 95% CI: 1.57–8.26).
CONCLUSIONS
Antiplatelet medication was associated with CMBs in stroke-free participants, particularly lobar CMBs. Among antiplatelet medications, aspirin use was associated with any CMB and lobar CMBs. Our findings suggest that it might be essential to optimize the management of antiplatelet medication in the stroke-free population with a higher burden of vascular risk factors to reduce the potential risk of CMBs.
Cerebral microbleeds (CMBs) are hypointense lesions detected by susceptibility-weighted imaging (SWI). Pathologically, CMBs are small foci of blood cell leakage, representing perivascular hemosiderin deposits.[1] As a subclinical period of cerebrovascular disease, CMBs can predict the future risks of stroke, death, and dementia in the general population.[2–4] It is beneficial to identify the risk factors for CMBs to facilitate the primary prevention of CMBs and subsequent intracerebral hemorrhage.
Antiplatelet medication is crucial for managing vascular diseases, mainly to prevent ischemic stroke and myocardial infarction, but its use can increase the risk of intracerebral hemorrhage simultaneously. Although increasing evidence has shown that antiplatelet medication may play a role in the occurrence of CMBs,[5–8] studies from the Atherosclerosis Risk in Communities (ARIC) and Mayo Clinic Study of Aging (MCSA) showed that antiplatelet medication did not increase the risk of CMBs.[9,10] In addition, most previous studies were conducted in general populations, including stroke and stroke-free participants, which may have introduced potential confounding effects.[8–11] Up to now, few studies have focused on stroke-free populations.
This study aimed to investigate the association between antiplatelet medication and CMBs in the community-based participants without history of stroke or transient ischemic attack (TIA) and to explore whether the association varies in different CMB locations and specific agents.
METHODS
Study Population
Our study participants were enrolled from an ongoing community study of Cardio- and cerebrovascular Accident Monitoring, Epidemiology, and caRe quAlity system (CAMERA), which aimed to investigate the risk factor of cerebrovascular disease in a community-based population in Beijing, China.[12] In this cross-sectional study, we recruited participants aged 18–85 years who participated in the CAMERA study from January 2015 to September 2019. The exclusion criteria of the present study were as follows: (1) known malignant tumors; (2) severe clinical conditions (such as heart failure, hepatic failure, or renal failure); (3) stenting therapy history; (4) refusal or contraindications to magnetic resonance imaging (MRI); (5) pregnancy; (6) dementia; (7) no SWI or MRI with poor image quality; and (8) stroke or TIA history. In our study, we excluded the participants with a history of stroke or TIA to avoid the potential overestimation or underestimation of the association between antiplatelet medication and CMBs.[5] All participants underwent standardized questionnaire interviews, physical examinations, serological tests, and brain MRI.
The Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University, Beijing, China approved this study (KY2014-005-02), and all participants provided written informed consent before participating in the study.
Data Collection
Uniformly trained coordinators conducted anthropometric measurements and face-to-face interviews using a standard questionnaire to collect information on demographic characteristics (age and sex), behavioral lifestyle [history of smoke (self-reported, defined as ever versus never) and alcohol consumption], medical history [history of hypertension, dyslipidemia, diabetes mellitus, and atrial fibrillation (AF)], and medication history (use of antiplatelet medication, antihypertensive medication, lipid-lowering medication, and oral hypoglycemic agents or insulin). Hypertension was defined as self-reported physician diagnosis or treatment with antihypertensive medication in the previous two weeks. Diabetes mellitus was determined by self-reported physician diagnosis or treatment with either oral agents or insulin in the last two weeks or fasting blood glucose (FBG) ≥ 7.0 mmol/L, or hemoglobin A1c (HbA1c) level ≥ 6.5%.[13] Dyslipidemia was defined according to a self-reported disease history or if the participant took lipid-lowering medication treatment in the previous two weeks. A trained nurse performed a physical examination to measure height, weight, and blood pressure (BP). The body mass index was calculated as weight in kilograms divided by height in meters squared. The BP was an average of two right arm measurements, with a 5-min break in between.
Measurements of Biochemical Parameters
Fasting blood samples were collected in the morning, and FBG, HbA1c, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and high sensitivity C-reactive protein were tested in the central laboratory.
Imaging Data Collection and Analysis
All participants underwent brain MRI on a 3.0T MR scanner (Philips Achieva TX, Philips Healthcare, Best, the Netherlands) using a custom-designed 36-channel neurovascular coil. For CMB detection, we used the following imaging protocol and parameters: SWI, fast field echo sequence; repetition time, 24 ms; echo times, 5 ms, 10 ms, 15 ms, and 20 ms; flip angle, 17 °; field of view, 25.6 cm × 19.2 cm × 12.8 cm; slice thickness, 2 mm; in-plane resolution, 0.6 mm × 0.6 mm; and scan time, 4.03 min.
Two observers reviewed the SWI images independently using a Digital Imaging and Communications in Medicine (DICOM) viewer (RadiAnt DICOM Viewer, Medixant, Poznan, Poland) with consensus, who were experienced in neuroimaging and blinded to the clinical information. The presence, count, and location of CMBs were evaluated. CMBs were defined as very low signal intensity lesions on SWI, which were small (typically 2–5 mm in diameter, up to 10 mm), circular, and homogeneous. CMBs, as the paramagnetic substance, are similar to the venous signal on the SWI phase image and opposite the calcification (antimagnetic substances) signal.[14,15] The identified CMBs were classified into three types according to different locations: (1) lobar CMBs, located in the cortical gray or subcortical white matter; (2) deep brain or infratentorial CMBs, located in the basal ganglia, thalamus, white matter of the internal and external capsules, brainstem, or cerebellum; and (3) mixed CMBs, located in both lobar and deep brain or infratentorial regions.
Cohen’s kappa values of inter- and intra-observer reliability in identifying the presence of any CMB were 0.95 and 0.99, respectively. The intraclass correlation coefficients of inter- and intra-observer reliability in evaluating the count of CMBs were 0.96 and 0.98, respectively. Cohen’s kappa values of inter- and intra-observer reliability in assessing the location of CMBs were 0.95 and 0.99, respectively.
Statistical Analysis
Variables were presented as mean ± SD for normal distribution and median (interquartile range) for non-normal distribution of continuous variables, and counts (percentages) for categorical variables. The normal distribution of the data was determined using the Kolmogorov–Smirnov test. Continuous data were compared using the independent Student’s t-test (normal distribution) or Wilcoxon-Mann-Whitney test (non-normal distribution). Categorical data were compared using the Pearson’s chi-squared test or Fisher’s exact probability test (if ≤ 20% of the expected cell counts were < 5).
The association between antiplatelet medication and the presence of any CMB was analyzed using multivariable logistic regression, presenting as odds ratios (ORs) and 95% CIs. The associations between antiplatelet medication and CMBs by location were analyzed using multinomial logistic regression, with no CMB at any location as a reference for the response variable. A linear regression analysis was conducted to determine the association between antiplatelet medication and the log-transformed number of CMBs. Age, sex, history of smoke, hypertension, AF, antihypertensive medication use, lipid-lowering medication use, oral hypoglycemic agents or insulin, and the levels of HbA1c and LDL-C with P-value < 0.1 in the univariate analysis were adjusted as potential confounders.
SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used to analyze all data. A two-sided P-value < 0.05 was considered statistically significant.
RESULTS
General Characteristics of the Study Population
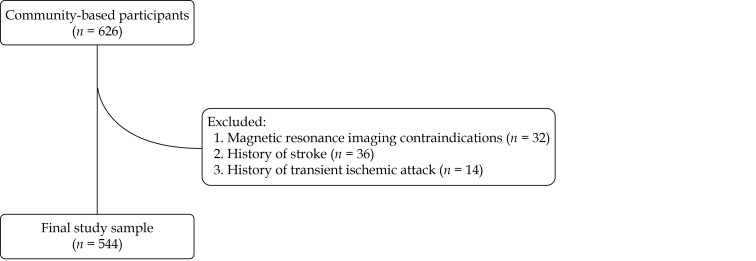
From January 2015 to September 2019, 626 participants from the CAMERA study were recruited. Of the 626 participants, 32 participants with MRI contraindications, 36 participants with a history of stroke, and 14 participants with a history of TIA were excluded, thus, a total of 544 stroke-free participants were included in the statistical analysis (Figure 1). Of the 544 participants, the mean age was 58.65 ± 13.66 years, and 217 participants (39.89%) are male, and the clinical characteristics of the study population are summarized in Table 1.
Figure 1.
Flow diagram of inclusion and exclusion of the study.
Table 1. Characteristics of participants stratified by CMB presence.
| Variables | Total (n = 544) | CMB present (n = 119) | CMB absent (n = 425) | P-value |
| Data are presented as means ± SD or n (%). *Presented as median (interquartile range). CMB: cerebral microbleed. | ||||
| Age, yrs | 58.65 ± 13.66 | 67.34 ± 10.83 | 56.21 ± 13.38 | 0.006 |
| Men | 217 (39.89%) | 65 (54.62%) | 152 (35.76%) | < 0.001 |
| Antiplatelet medication | 64 (11.76%) | 32 (26.89%) | 32 (7.53%) | < 0.001 |
| Aspirin alone | 44 (8.09%) | 26 (21.85%) | 18 (4.24%) | |
| Clopidogrel alone | 6 (1.10%) | 2 (1.68%) | 4 (0.94%) | |
| Cilostazol alone | 2 (0.37%) | 0 | 2 (0.47%) | |
| Dual antiplatelet therapy with aspirin and clopidogrel | 12 (2.21%) | 4 (3.36%) | 8 (1.88%) | |
| History of smoke | 76 (13.97%) | 25 (21.01%) | 51 (12.00%) | 0.012 |
| Alcohol consumption | 246 (45.22%) | 52 (43.70%) | 194 (45.65%) | 0.706 |
| Medical history | ||||
| Hypertension | 177 (32.54%) | 58 (48.74%) | 119 (28.00%) | < 0.001 |
| Dyslipidemia | 269 (49.45%) | 60 (50.42%) | 209 (49.18%) | 0.810 |
| Diabetes mellitus | 87 (15.99%) | 24 (20.17%) | 63 (14.82%) | 0.160 |
| Atrial fibrillation | 16 (2.94%) | 8 (6.72%) | 8 (1.88%) | 0.011 |
| History of medication | ||||
| Antihypertensive medication | 162 (29.78%) | 52 (43.70%) | 110 (25.88%) | < 0.001 |
| Lipid-lowering medication | 145 (26.65%) | 43 (36.13%) | 102 (24.00%) | 0.008 |
| Oral hypoglycemic agents or insulin | 58 (10.66%) | 18 (15.13%) | 40 (9.41%) | 0.074 |
| Physical examination | ||||
| Body mass index, kg/m2 | 24.30 ± 3.38 | 24.62 ± 3.59 | 24.21 ± 3.32 | 0.245 |
| Systolic blood pressure, mmHg | 126.50 ± 16.35 | 132.61 ± 17.46 | 124.81 ± 15.64 | < 0.001 |
| Diastolic blood pressure, mmHg | 75.27 ± 9.39 | 75.45 ± 9.45 | 75.22 ± 9.38 | 0.820 |
| Laboratory examination | ||||
| Fasting blood glucose, mmol/L | 5.00 ± 1.05 | 5.21 ± 1.39 | 4.94 ± 0.93 | 0.047 |
| Hemoglobin A1c, % | 5.79 ± 0.73 | 6.03 ± 0.93 | 5.73 ± 0.66 | 0.001 |
| Low-density lipoprotein cholesterol, mmol/L | 2.95 ± 0.84 | 2.81 ± 0.75 | 2.99 ± 0.86 | 0.038 |
| High-density lipoprotein cholesterol, mmol/L | 1.48 ± 0.37 | 1.46 ± 0.36 | 1.48 ± 0.38 | 0.527 |
| High-sensitivity C-reactive protein, mg/L | 0.90 (0.50–1.70)* | 0.90 (0.53–1.90)* | 0.8 (0.50–1.70)* | 0.346 |
Of the 544 participants, 119 participants (21.88%) had at least one CMB. The count of CMB lesions ranged from 1 to 40, 80 participants (14.71%) had one CMB lesion, 26 participants (4.78%) had two to four CMB lesions, and 13 participants (2.39%) had more than four CMB lesions. A total of 78 participants (14.34%) had CMBs in the lobar region, 23 participants (4.23%) had CMBs in the deep brain or infratentorial regions, and 18 participants (3.31%) had CMBs in both the lobar and deep brain or infratentorial regions (Table 2).
Table 2. Descriptive statistics stratified by CMB location in the 544 participants.
| Variables | Number | Explanation |
| Data are presented as n (%). CMB: cerebral microbleed. | ||
| Any CMB | 119 (21.88%) | |
| Lobar CMBs | 78 (14.34%) | Cortical gray or subcortical white matter |
| Deep brain or infratentorial CMBs | 23 (4.23%) | Basal ganglia, thalamus, white matter of the internal and external capsules, brainstem or cerebellum |
| Mixed CMBs | 18 (3.31%) | Both lobar CMBs and deep brain or infratentorial CMBs |
Compared to the participants without CMB, those with CMBs were significantly older (67.34 ± 10.83 years vs. 56.21 ± 13.38 years, P = 0.006), predominantly male (54.62% vs. 35.76%, P < 0.001), more likely to use antiplatelet medication (26.89% vs. 7.53%, P < 0.001), had higher percentages of the history of smoke (21.01% vs. 12.00%, P = 0.012), hypertension (48.74% vs. 28.00%, P < 0.001), and AF (6.72% vs. 1.88%, P = 0.011), more likely to use antihypertensive medication (43.70% vs. 25.88%, P < 0.001), lipid-lowering medication (36.13% vs. 24.00%, P = 0.008), and had higher systolic BP (132.61 ± 17.46 mmHg vs. 124.81 ± 15.64 mmHg, P < 0.001), FBG (5.21 ± 1.39 mmol/L vs. 4.94 ± 0.93 mmol/L, P = 0.047) and HbA1c level (6.03% ± 0.93% vs. 5.73% ± 0.66%, P < 0.001).
Antiplatelet Medication Use
Of the 544 participants, 64 participants (11.76%) received antiplatelet therapy, of whom 44 participants (68.75%) used aspirin alone, six participants (9.38%) used clopidogrel alone, two participants (3.12%) used cilostazol alone, and 12 participants (18.75%) used dual antiplatelet therapy with aspirin and clopidogrel.
Association Between Antiplatelet Medication and CMBs
The multivariable logistic regression analysis revealed that, after adjusting for age and sex (Model 1, Table 3), antiplatelet medication was significantly associated with the presence of any CMB (OR = 2.38, 95% CI: 1.33–4.26, P = 0.003). After further adjustment for a history of smoke, hypertension, AF, and antihypertensive medication use, lipid-lowering medication use, oral hypoglycemic agents or insulin, HbA1c and LDL-C levels (Model 2), the association between antiplatelet medication and any CMB remained statistically significant (OR = 2.39, 95% CI: 1.24–4.58, P = 0.009).
Table 3. Logistic regression analyses for the association between antiplatelet medication and CMBs.
| Variables | Unadjusted | Model 1 | Model 2 | |||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| Model 1: adjusted for age and sex. Model 2: adjusted for variables in model 1 plus history of smoke, hypertension, atrial fibrillation, antihypertensive medication use, lipid-lowering medication use, oral hypoglycemic agents or insulin, hemoglobin A1c level, and low-density lipoprotein cholesterol level. CMB: cerebral microbleed; OR: odds ratio. | ||||||||
| Any CMB | 4.52 (2.63–7.77) | < 0.001 | 2.38 (1.33–4.26) | 0.005 | 2.39 (1.24–4.58) | 0.009 | ||
| Lobar CMBs | 5.46 (2.99–9.95) | < 0.001 | 2.90 (1.53–5.49) | 0.001 | 2.83 (1.36–5.86) | 0.005 | ||
| Any lobar CMBs (Lobar CMBs + Mixed CMBs) | 5.06 (2.86–8.93) | < 0.001 | 2.62 (1.43–4.82) | 0.002 | 2.53 (1.27–5.04) | 0.008 | ||
| Deep or infratentorial CMBs | 2.59 (0.83–8.06) | 0.102 | 1.48 (0.46–4.81) | 0.511 | 1.63 (0.44–6.02) | 0.461 | ||
| Any deep CMBs
(Deep or infratentorial CMBs + Mixed CMBs) |
2.98 (1.27–6.98) | 0.012 | 1.55 (0.64–3.78) | 0.332 | 1.63 (0.62–4.32) | 0.324 | ||
Considering the CMB location, the use of antiplatelet medication was significantly associated with lobar CMBs (OR = 2.83, 95% CI: 1.36–5.86, P = 0.005), whereas no significant association was found between antiplatelet medication and deep or infratentorial CMBs (OR = 1.63, 95% CI: 0.44–6.02, P = 0.461). Regarding the specific medications of antiplatelet therapy, the use of aspirin was significantly associated with CMBs at any location (OR = 3.17, 95% CI: 1.49–6.72, P = 0.003) and lobar CMBs (OR = 3.61, 95% CI: 1.57–8.26, P = 0.002; Table 4).
Table 4. Logistic regression analyses for the association between aspirin use and CMBs.
| Variables | Unadjusted | Model 1 | Model 2 | |||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| Model 1: adjusted for age and sex. Model 2: adjusted for variables in model 1 plus history of smoke, hypertension, atrial fibrillation, antihypertensive medication use, lipid-lowering medication use, oral hypoglycemic agents or insulin, hemoglobin A1c level, and low-density lipoprotein cholesterol level. CMB: cerebral microbleed; OR: odds ratio. | ||||||||
| Any CMB | 6.53 (3.43–12.43) | < 0.001 | 3.18 (1.61–6.31) | 0.001 | 3.17 (1.49–6.72) | 0.003 | ||
| Lobar CMBs | 7.68 (3.80–15.54) | < 0.001 | 3.78 (1.79–7.96) | 0.001 | 3.61 (1.57–8.26) | 0.002 | ||
| Any lobar CMBs (Lobar CMBs + Mixed CMBs) | 7.39 (3.79–14.41) | < 0.001 | 3.53 (1.74–7.18) | 0.001 | 3.40 (1.55–7.45) | 0.002 | ||
| Deep or infratentorial CMBs | 3.45 (0.93–12.73) | 0.063 | 1.85 (0.48–7.14) | 0.371 | 2.06 (0.47–9.00) | 0.336 | ||
| Any deep CMBs
(Deep or infratentorial CMBs + Mixed CMBs) |
4.63 (1.81–11.89) | 0.001 | 2.23 (0.83–5.98) | 0.110 | 2.41 (0.83–6.99) | 0.107 | ||
Considering the CMB number, after full adjustment, the linear regression analysis showed that neither antiplatelet medication (β = 0.14, 95% CI: -0.21–0.48, P = 0.444) nor aspirin medication (β = 0.10, 95% CI: -0.27–0.48, P = 0.585) was significantly associated with the number of CMBs.
Among the 64 participants with antiplatelet medication, 32 participants (50%) had CMBs, including 24 participants (37.5%) with lobar CMBs, four participants (6.25%) with deep or infratentorial CMBs, and four participants (6.25%) with mixed CMBs. Among the 64 antiplatelet medication users, participants with CMBs are predominantly males (71.88% vs. 37.50%, P = 0.006) and had lower HDL-C levels than those without CMB (1.37 ± 0.35 mmol/L vs. 1.56 ± 0.35 mmol/L, P = 0.035) (supplemental material, Table 1S).
DISCUSSION
In this cross-sectional, observational study, we investigated the association between antiplatelet medication and CMBs in community-based participants without stroke or TIA. We found that antiplatelet medication was independently associated with CMBs, particularly lobar CMBs. Moreover, we found that aspirin use may be associated with CMBs at any location and lobar CMBs. Our study suggests that optimizing antiplatelet treatment strategies in the stroke-free population might be helpful to decrease the risk of CMBs.
The prevalence of CMBs in our study was slightly higher than that reported in a study conducted by Li, et al.[16] (21.88% vs. 18.51%), but lower than that in the ARIC study (24%)[17] and two studies from the MCSA study (prevalence: 22.6%[18] and 26.3%[10]). Many factors are related to the prevalence of CMBs, such as detection technique, age of participants, and history of hypertension.[6,19,20] The use of SWI in the present study is more sensitive in detecting CMBs than the T2-weighted gradient-recalled echo sequence used by Cheng, et al.,[19] which may contribute to the contradiction. In addition, the older age (mean age: 76 ± 5 years for the ARIC study,[17] 74.1 ± 8.6 years[18] and 72.6 ± 8.4 years[10] for the two MCSA studies) and the higher prevalence of hypertension in the participants (75% for the ARIC study,[17] 65%[18] and 63%[10] for the two MCSA studies) may be responsible for the inconsistency.
In our study population, most CMBs were found in the lobar region (14.34%), followed by the deep or infratentorial regions (4.23%) and mixed regions (3.31%). The Framingham Heart Study (FHS) also demonstrated that the prevalence of CMBs in the lobar region was higher than that in the deep or infratentorial regions (5.55% vs. 1.93%).[6] In contrast, another study in a Japanese population reported that CMBs were more prevalent in the deep or infratentorial regions than in the lobar region (18.7% vs. 9.6%).[11] These contradictory findings may be due to the higher prevalence of hypertension in the study of the Japanese population (56% for the FHS,[6] 70.7% for the Japanese population[11]). Although the mechanism of CMBs is complex, it seems that CMBs in different locations correspond to different etiologies. Deep or infratentorial CMBs were more strongly associated with hypertension than lobar CMBs.[21–23]
It has been widely evidenced that antiplatelet medication use was a risk factor for CMBs in stroke or TIA patients,[24] but the association between antiplatelet medication use and CMBs in the stroke-free population is controversial.[5,6,25] In our study, we found that antiplatelet medication was independently associated with CMBs at any location in a community-based stroke-free population, which suggests that proper use of antiplatelet medication in primary prevention may need to be considered due to its potential benefits and risks. Similar to our findings, the Rotterdam Scan study in 2013 reported that CMBs were more prevalent among users of antiplatelet medication in stroke-free Dutch participants (OR = 1.55, 95% CI: 1.01–2.37).[5] On the contrary, Kim, et al.[25] reported that antiplatelet medication use was not associated with CMBs in Korean asymptomatic older adults without a history of stroke or TIA (OR = 1.10, 95% CI: 0.73–1.66). A possible reason for this inconsistency may be the low detection rate of CMB (9.5%) in the study by Kim, et al.[25] We found that the prevalence of CMB was higher in participants with antiplatelet medication than in those without, suggesting a higher burden of vascular risk factors in such a population. Participants with atherosclerotic cardiovascular disease may take antiplatelet medication to prevent cardiovascular events. The antiplatelet medication inhibits platelet adhesion, aggregation, and activation, and prevents platelet-vessel wall interaction, vascular inflammatory responses, and atherosclerotic plaque formation. Thus, long-term antiplatelet medication may damage the vessel walls, increase leakage of the arterial wall, result in subsequent hemorrhage. This finding may serve as a reminder to clinicians to carefully weigh the benefits and risks of antiplatelet medication.
The association between antiplatelet medication and the location of CMBs was controversial in previous studies, even in general populations.[5,6,8] In this study, we found that antiplatelet medication was associated with lobar CMBs but not with deep or infratentorial CMBs. Similarly, the risk of lobar CMBs was higher in antiplatelet medication users in the Rotterdam Scan study in 2009 (OR = 1.63, 95% CI: 1.08–2.48), which was conducted in the Dutch community, including cerebrovascular disease participants.[8] However, the Rotterdam Scan study in 2013 and the FHS, which was conducted in stroke-free participants, reported that antiplatelet medication had a stronger association with deep or infratentorial CMBs (OR = 1.90, 95% CI: 1.05–3.45 and OR = 2.14, 95% CI: 1.08–4.25; respectively), but no significant association between antiplatelet medication and lobar CMBs was found in either study (the Rotterdam Scan study in 2013: OR = 1.34, 95% CI: 0.81–2.21; the FHS: OR = 1.20, 95% CI: 0.79–1.83).[5,6] Inhomogeneous population selection may have contributed to the inconsistency. Lobar CMBs were considered to indicate cerebral amyloid angiopathy, whereas deep or infratentorial CMBs were predominantly caused by hypertensive microangiopathy.[22] Our findings suggest that antiplatelet-associated CMBs may be more likely associated with cerebral amyloid angiopathy than hypertension.[18,26] Furthermore, when considering the specific medications, we found that aspirin rather than clopidogrel or cilostazol was significantly associated with CMB at any location and lobar CMBs. In the Rotterdam Scan studies, aspirin use was associated with an increased risk of lobar CMBs,[8] and clopidogrel increased the prevalence of deep or infratentorial CMBs.[5] On the contrary, the FHS by Romero, et al.[6] and the study by Kim, et al.[25] suggested no significant association between aspirin use and CMBs in the stroke-free older-adult population. Nevertheless, the utilization rate of clopidogrel or cilostazol was low in our study. Future studies are warranted to illustrate the association between specific antiplatelet medications and CMB locations.
In the present study, we found that the presence of CMBs was higher in participants on lipid-lowering medications (36.13% vs. 24.00%), but after full adjustment, the association between lipid-lowering medications and CMBs presence was no longer significant, which is consistent with the previous meta-analysis.[27]
STRENGTHS AND LIMITATIONS
The major strengths of our study are as follows: (1) in order to investigate the association between antiplatelet medication and CMB in stroke-free adults, we excluded all patients with stroke or TIA to minimize the selection bias; and (2) the use of SWI guaranteed the high detection rate of CMBs.[15,19] However, several limitations of the present study should be noted. Firstly, the number of participants with deep or infratentorial CMBs in our study population was relatively small (n = 23). Secondly, residual confounding influences due to unmeasured confouders may have affected our results. Since our participants only contained one anticoagulant user, this potential confounding effect could not be adjusted in the analysis. Thirdly, the use of anticoagulant medication (n = 1), clopidogrel (n = 6), and cilostazol (n = 2) was relatively low in our study, limiting the investigation of the association between these medicines and CMBs. Fourthly, we found the difference in sex and HDL-C between presence and absence of CMB among antiplatelet medication users in the univariate analyses. However, we failed to further explore their association due to the limited sample size. Last but not least, this cross-sectional study can only investigate the association between antiplatelet medication and CMBs but not the causal effect. Therefore, our results need to be confirmed in multicenter, large-scale, prospective cohort studies in the future.
CONCLUSIONS
Antiplatelet medication was independently associated with CMBs in community-based adults without stroke or TIA, particularly lobar CMBs. Among antiplatelet medications, aspirin use was associated with CMB at any location and lobar CMBs. Our findings suggest that it might be essential to optimize the management of antiplatelet medication in the stroke-free population with a higher burden of vascular risk factors to reduce the potential risk of CMBs.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Program of the Ministry of Science and Technology of China (2017YFC1307702), and the Capital’s Funds for Health Improvement and Research (No.2020-1-2041). All authors had no conflicts of interest to disclose. The authors thank all the participants of the CAMERA (Cardio- and cerebrovascular Accident Monitoring, Epidemiology, and caRe quAlity system) study for their contribution.
Contributor Information
Xi-Hai ZHAO, Email: xihaizhao@tsinghua.edu.cn.
Gai-Fen LIU, Email: liugaifen@ncrcnd.org.cn.
References
- 1.Fazekas F, Kleinert R, Roob G, et al Histopathologic analysis of foci of signal loss on gradient-echo T2-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999;20:637–642. doi: 10.1097/00002093-199904001-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akoudad S, Portegies ML, Koudstaal PJ, et al Cerebral microbleeds are associated with an increased risk of stroke: the Rotterdam Study. Circulation. 2015;132:509–516. doi: 10.1161/CIRCULATIONAHA.115.016261. [DOI] [PubMed] [Google Scholar]
- 3.Akoudad S, Ikram MA, Koudstaal PJ, et al Cerebral microbleeds and the risk of mortality in the general population. Eur J Epidemiol. 2013;28:815–821. doi: 10.1007/s10654-013-9854-3. [DOI] [PubMed] [Google Scholar]
- 4.Ding J, Sigurðsson S, Jónsson PV, et al Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology. 2017;88:2089–2097. doi: 10.1212/WNL.0000000000003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darweesh SK, Leening MJ, Akoudad S, et al Clopidogrel use is associated with an increased prevalence of cerebral microbleeds in a stroke-free population: the Rotterdam Study. J Am Heart Assoc. 2013;2:e000359. doi: 10.1161/JAHA.113.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero JR, Preis SR, Beiser A, et al Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke. 2014;45:1492–1494. doi: 10.1161/STROKEAHA.114.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu J, Ye H, Wang J, et al Antiplatelet therapy, cerebral microbleeds, and intracerebral hemorrhage: a meta-analysis. Stroke. 2018;49:1751–1754. doi: 10.1161/STROKEAHA.118.021789. [DOI] [PubMed] [Google Scholar]
- 8.Vernooij MW, Haag MD, van der Lugt A, et al Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2009;66:714–720. doi: 10.1001/archneurol.2009.42. [DOI] [PubMed] [Google Scholar]
- 9.Sharma R, Matsushita K, Wu A, et al Common medications and intracerebral hemorrhage: the ARIC study. J Am Heart Assoc. 2021;10:e014270. doi: 10.1161/JAHA.120.014270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graff-Radford J, Lesnick T, Rabinstein AA, et al Cerebral microbleeds: relationship to antithrombotic medications. Stroke. 2021;52:2347–2355. doi: 10.1161/STROKEAHA.120.031515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yubi T, Hata J, Ohara T, et al Prevalence of and risk factors for cerebral microbleeds in a general Japanese elderly community. Neurol Clin Pract. 2018;8:223–231. doi: 10.1212/CPJ.0000000000000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han H, Zhang R, Liu G, et al Reduction of cerebral blood flow in community-based adults with subclinical cerebrovascular atherosclerosis: a 3.0T magnetic resonance imaging study. Neuroimage. 2019;188:302–308. doi: 10.1016/j.neuroimage.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association Professional Practice Committee Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S17–S38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 14.Haacke EM, Xu Y, Cheng YC, et al Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52:612–618. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg SM, Vernooij MW, Cordonnier C, et al Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Fang F, Cui M, et al Incidental findings on brain MRI among Chinese at the age of 55–65 years: the Taizhou Imaging Study. Sci Rep. 2019;9:464. doi: 10.1038/s41598-018-36893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graff-Radford J, Simino J, Kantarci K, et al Neuroimaging correlates of cerebral microbleeds: the ARIC Study (Atherosclerosis Risk in Communities) Stroke. 2017;48:2964–2972. doi: 10.1161/STROKEAHA.117.018336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graff-Radford J, Botha H, Rabinstein AA, et al Cerebral microbleeds: prevalence and relationship to amyloid burden. Neurology. 2019;92:e253–e262. doi: 10.1212/WNL.0000000000006780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng AL, Batool S, McCreary CR, et al Susceptibility-weighted imaging is more reliable than T2-weighted gradient-recalled echo MRI for detecting microbleeds. Stroke. 2013;44:2782–2786. doi: 10.1161/STROKEAHA.113.002267. [DOI] [PubMed] [Google Scholar]
- 20.Han F, Zhai FF, Wang Q, et al Prevalence and risk factors of cerebral small vessel disease in a Chinese population-based sample. J Stroke. 2018;20:239–246. doi: 10.5853/jos.2017.02110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poels MM, Ikram MA, van der Lugt A, et al Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42:656–661. doi: 10.1161/STROKEAHA.110.607184. [DOI] [PubMed] [Google Scholar]
- 22.Vernooij MW, van der Lugt A, Ikram MA, et al Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 23.Jeerakathil T, Wolf PA, Beiser A, et al Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2004;35:1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- 24.Lovelock CE, Cordonnier C, Naka H, et al Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke. 2010;41:1222–1228. doi: 10.1161/STROKEAHA.109.572594. [DOI] [PubMed] [Google Scholar]
- 25.Kim CK, Kwon HT, Kwon HM No significant association of aspirin use with cerebral microbleeds in the asymptomatic elderly. J Neurol Sci. 2012;319:56–58. doi: 10.1016/j.jns.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Mesker DJ, Poels MM, Ikram MA, et al Lobar distribution of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2011;68:656–659. doi: 10.1001/archneurol.2011.93. [DOI] [PubMed] [Google Scholar]
- 27.Katsanos AH, Lioutas VA, Charidimou A, et al Statin treatment and cerebral microbleeds: a systematic review and meta-analysis. J Neurol Sci. 2021;420:117224. doi: 10.1016/j.jns.2020.117224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.