To the Editor,
The prevalence of IgE-mediated food allergy (IgE-FA) has dramatically increased in the United States in recent decades.1–3 This rapid increase strongly suggests environmental contributions and recent attention has focused on the gut microbiome.1,3 Acid suppressive therapy (AST) has been found to impact the developing gut microbiome and is frequently prescribed in early childhood despite limited evidence supporting its use.2–5 AST is quite frequently prescribed in infants with food protein-induced allergic proctocolitis (FPIAP), given symptoms of reflux or fussiness, and FPIAP has been associated with later onset of IgE-FA.2 While recent studies have found associations between atopic disease and use of AST in infancy, none have accounted for FPIAP as a possible confounder.1,3 AST can disrupt oral immune tolerance in animal models by altering the gut microbiome, impairing protein digestion, disrupting inflammatory signalling, and regulating immune responses.3–6 More specifically, aluminium-containing antacids such as Maalox or Mylanta have been shown to serve as Th2 adjuvants and disrupt microbial composition through direct effects of aluminium.3,4 Proton pump inhibitors (PPI) have been shown to decrease the diversity of the gut microbiome, interrupt inflammatory pathways, inhibit the immune function of neutrophils, and facilitate systemic allergic reactions through impaired pepsin digestion.3,5 Histamine type-2 receptor antagonists (H2RA) have similarly been shown to alter the gut microbiome and modulate immune responses, although mechanisms are highly variable with histamine serving both anti- and pro-inflammatory roles.3,6 We sought to prospectively evaluate the association between AST in infancy and development of IgE-FA in our healthy infant cohort.
The Gastrointestinal Microbiome and Allergic Proctocolitis (GMAP) Study is a prospective observational cohort study of 1003 healthy infants studying the development of IgE- and non-IgE-FA.2 Infants were serially recruited at their first newborn visit and followed prospectively at all routine well-child visits (age 1 week, 2 weeks, and 1, 2, 4, 6, 9, 12, 15, 18, 24 and 36 months) and unscheduled sick visits.2 Parents completed a questionnaire at each visit assessing the use of acid suppressive therapy and development of allergic or gastrointestinal symptoms.2 From those who met inclusion criteria for the GMAP cohort, we excluded infants with inadequate questionnaire completion regarding acid suppression and 797 children were included in these exploratory analyses (Figure 1). Exposure to AST within the first 6 months of life was determined by prospective questionnaires. Incomplete data on length of therapy were clarified by chart review (and when remained unclear, estimated based on standard practice of a 14-day empiric trial). The diagnosis of IgE-FA was determined from birth through age 3 by independent agreement of two study staff allergists based on clinical history and sensitization data (positive skin prick test, serum-specific immunoglobulin E, or oral food challenge) as previously published.7 The diagnosis of FPIAP was made by the primary care provider based on clinical symptoms and documented blood in the stool (grossly bloody or guaiac positive) not attributable to an alternate aetiology as previously published.2 Univariable and multivariable logistic regression with significance set at α <0.05 were performed using R version 4.0.3.8 All covariates which were significantly associated with the IgE-FA outcome (p < .05) in the univariable screen were included in the multivariable regression (sex, atopic dermatitis, FPIAP, Maalox/Mylanta, H2RA). Since this multivariable regression model did not change any of the findings, and there was potential for collinearity among several variables, we show the univariable analysis results in Figure 2. The GMAP study was approved by the Massachusetts General Hospital Institutional Review Board.
FIGURE 1.
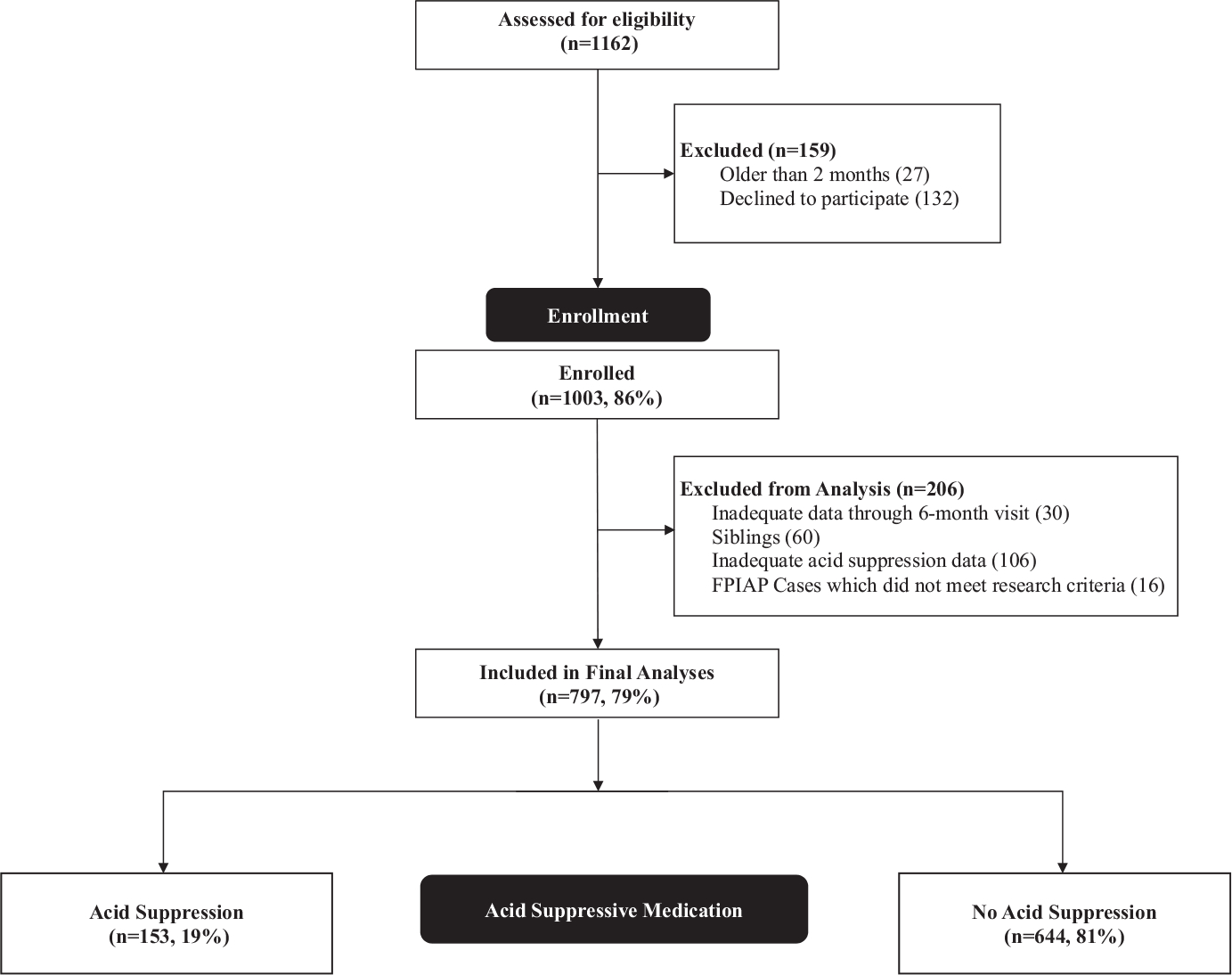
Study population
FIGURE 2.
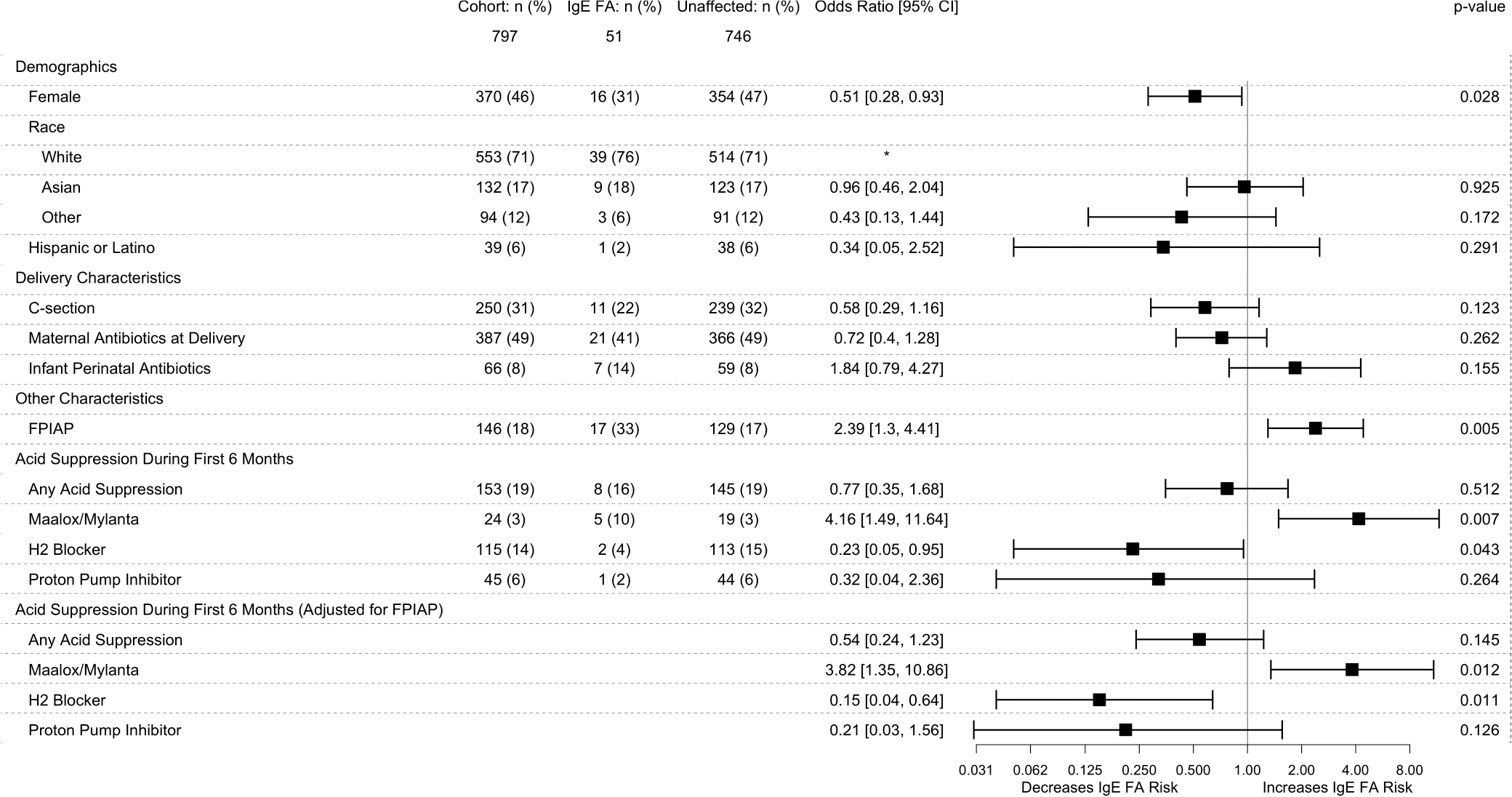
Risk Factors for development of IgE-FA. Results of univariable logistic regression of potential risk factors for IgE-FA. In the last section, FPIAP was added to the regression model and did not change the findings. A complete multivariable logistic regression including all covariates which were significantly associated with IgE-FA was also performed did not change any of these conclusions (not shown). Complete information on all demographics and risk factors for IgE-mediated food allergy can be found in our previously published manuscript2
Of the 797 children analysed, 46% were female, 93% were ever breastfed, 69% were born by vaginal delivery, 18% had FPIAP, and 46% had eczema. 153 (19%) infants were exposed to any AST, 45 (6%) were exposed to PPI, 115 (14%) were exposed to H2RA, 24 (3%) were exposed to Maalox/Mylanta in the first 6 months of life, and 13 (2%) were exposed to an unspecified AST. Of the 153 infants exposed to any AST, 112 were exposed to a single acid suppressive therapy: 77 infants were exposed to H2RA only, 14 were exposed to PPI only, 12 were exposed to Maalox/Mylanta only, and nine were exposed to an unspecified AST only. Alternatively, 41 infants were exposed to multiple ASTs: 27 were exposed to H2RA and PPI, seven were exposed to H2RA and Maalox/Mylanta, two were exposed to H2RA, PPI, and Maalox/Mylanta, two were exposed to Maalox/Mylanta and an unspecified AST, one was exposed to PPI and Maalox/Mylanta, one was exposed to H2RA and an unspecified AST and one was exposed to H2RA, PPI and an unspecified AST. Of those with complete data on age and length of therapy, the median age of initiation was 2 months [0.5, 6] and the median length of therapy was 2 months [0.5, 5.5]. Fifty-one (6%) of all children developed an IgE-FA: 26 to egg (3%), 25 to peanut (3%), 11 to tree-nuts (1%) and eight to milk (1%). There was no difference in the distribution of IgE-FA by food between those who received acid suppressive therapy and those who did not.
We first looked at the odds of IgE-FA in infants exposed to AST in the first 6 months of life (including infants exposed to single or multiple ASTs) compared to those never exposed. The odds of IgE-FA was significantly greater in infants exposed to Maalox/Mylanta (OR, 4.16; 95% CI, 1.49–11.64; p = .007), significantly lower in infants prescribed H2RA (OR, 0.23; 95% CI, 0.05–0.95; p = .043), and not significantly different in infants prescribed PPI (OR, 0.32; 95% CI, 0.04–2.36; p = .264) when compared with infants without any exposure to AST (Figure 2). Adjusting for infants with concomitant AST usage did not significantly change the above findings. There was additionally no difference in the odds of IgE-FA in infants exposed to a single versus multiple ASTs in the first 6 months of life. We next adjusted our model for FPIAP given that infants with FPIAP had an independently greater risk of IgE-FA (OR, 2.39; 95% CI, 1.3–4.41; p = .005) (Figure 2) and were more likely to receive AST in infancy (OR, 4.73; 95% CI, 3.18–7.03; p = 1.44e−14). Adjusting for FPIAP did not significantly change our findings (Figure 2).
In summary, in these exploratory analyses from a large healthy infant prospective cohort study, we found that infants exposed to Maalox/Mylanta were more likely to be diagnosed with IgE-FA, whereas infants prescribed H2RA were less likely. Exposure to PPI was not significantly associated, though we were underpowered for these AST subgroup analyses. Limitations of this study include the relatively small number of IgE-FA diagnoses limiting our power (due to the healthy cohort design), the exploratory nature of these analyses, and the lack of complete data on dose or duration of AST precluding dose or duration-based subgroup analyses. Consistent with our findings, aluminium-containing antacids have been shown to increase the risk of IgE-FA in animal models likely via Th2 adjuvant effect and microbiome modulation.3,4 While H2RA use in other cohorts was associated with an increased risk of IgE-FA, histamine is known to elicit a widely variable immune response and these studies did not address the role of reverse causality due to FPIAP, which we have independently shown is associated with increased risk of IgE-FA.2,3,6,9 AST is frequently prescribed in early infancy despite limited evidence supporting its use and growing evidence of lasting effects on the developing microbiome.2,4,5 Our findings suggest that H2RA may be a protective factor, whereas aluminium-containing antacids may be a risk factor in the development of IgE-FA. Validation of these exploratory findings in larger studies is needed, and further research into the opposing effects of different types of AST on the development of IgE-FA is necessary to elucidate mechanisms and better inform clinical practice.
Key Messages.
Acid suppressive therapy is frequently prescribed in infancy despite limited evidence supporting its use
Aluminium-containing antacids were associated with increased rates, whereas H2RA with decreased rates of IgE-FA
Diagnosis of FPIAP did not alter the association between AST and IgE-FA
ACKNOWLEDGEMENTS
Funding for this study was provided by the Gerber Foundation (1685–3680), the Demarest Lloyd Jr Foundation (230465), and the Food Allergy Science Initiative (229711). V.M.M. and Y.V.V. were supported by a grant from the National Institute of Allergy and Infectious Diseases of the US National Institutes of Health (1K23AI151555-01A1 and K23AI130408, respectfully).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest relevant to this article to disclose.
ETHICAL APPROVAL
The GMAP study was approved by the Massachusetts General Hospital Institutional Review Board protocol number 2013P002374 on 4/03/2014. Informed written consent was obtained from a parent of all enrolled infants.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Mitre E, Susi A, Kropp LE, Schwartz DJ, Gorman GH, Nylund CM. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr. 2018;172(6):e180315. doi: 10.1001/jamapediatrics.2018.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin VM, Virkud YV, Seay H, et al. Prospective assessment of pediatrician-diagnosed food protein-induced allergic proctocolitis by gross or occult blood. J Allergy Clin Immunol Pract. 2020;8(5):1692–1699.e1. doi: 10.1016/j.jaip.2019.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Untersmayr E Acid suppression therapy and allergic reactions. Allergo J Int. 2015;24(8):303–311. doi: 10.1007/s40629-015-0085-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assefa S, Köhler G. Intestinal microbiome and metal toxicity. Curr Opin Toxicol. 2020;19:21–27. doi: 10.1016/j.cotox.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson MA, Goodrich JK, Maxan M-E, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–756. doi: 10.1136/gutjnl-2015-310861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Mahony L, Akdis M, Akdis CA. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol. 2011;128(6):1153–1162. doi: 10.1016/j.jaci.2011.06.051 [DOI] [PubMed] [Google Scholar]
- 7.Martin VM, Virkud YV, Phadke NA, et al. Increased IgE-mediated food allergy with food protein-induced allergic proctocolitis. Pediatrics. 2020;146(3):e20200202. doi: 10.1542/peds.2020-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. https://www.R-project.org/ [Google Scholar]
- 9.Martin V, Keet C, Yuan Q. Antibiotics and acid-suppressing medications in early life and allergic disorders. JAMA Pediatr. 2018;172(10):989–990. doi: 10.1001/jamapediatrics.2018.2507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


