ABSTRACT
Aims/Introduction
To assess the association between vitamin D and diabetic foot (DF) in patients with type 2 diabetes mellitus (T2DM), in order to summarize clinical evidence in the prevention and treatment of DF.
Materials and methods
Between January 2012 and December 2019, a total of 1,721 hospitalized patients with type 2 diabetes mellitus were continuously enrolled in West China Hospital, Sichuan University, and divided into DF and non‐DF groups according to whether they had DF, and divided into four subgroups according to the admission season. The 25‐OH‐vitamin D levels were compared between groups and subgroups, and independent risk factors discussed for the occurrence of DF.
Results
The vitamin D insufficiency and deficiency rate were higher in the DF group (77.51%) than in the non‐DF group (59.2%). The 25‐OH‐vitamin D levels were lower in the DF group (35.80 nmol/L) than in the non‐DF group (45.48 nmol/L) (P < 0.001). Patients with poor glycemic control had lower 25‐OH‐vitamin D levels (P = 0.01). The levels of 25‐OH‐vitamin D were lower in winter and spring. In the same season, the levels of 25‐OH‐vitamin D in patients with DF were still lower (P < 0.001). The 25‐OH‐vitamin D levels of patients with Wagner grades 0 to 5 showed a downward trend (P = 0.114). The 25‐OH‐vitamin D level was independently associated with diabetic foot (P < 0.001, OR = 0.986).
Conclusions
The low serum vitamin D level was significantly associated with a higher prevalence of DF among Chinese patients with type 2 diabetes mellitus. Although vitamin D levels vary seasonally, patients with DF were always at higher risk of having vitamin D insufficiency and deficiency.
Keywords: Diabetic foot, Type 2 diabetes, Vitamin D deficiency
We conducted an observational study including 1,721 subjects to explore the prevalence of vitamin D deficiency, and to address the association between serum 25‐OH‐vitamin D levels and diabetic foot in a Chinese hospitalized type 2 diabetes mellitus population. It was found that the vitamin D insufficiency and deficiency rate were higher in the DF group (77.51%) than in the non‐DF group (59.2%) and the 25‐OH‐vitamin D was independently associated with diabetic foot (P < 0.001, OR = 0.986). It was indicated that the low serum vitamin D level was significantly associated with a higher prevalence of DF and patients with DF were always at higher risk of having vitamin D insufficiency and deficiency.
INTRODUCTION
Diabetic foot (DF) is one of the most severe and painful chronic complications of diabetes mellitus. Poor wound healing leads to high hospitalization, high rates of lower extremity amputation, and also increases the risk of disability and mortality in patients with diabetes 1 , 2 . It has been estimated that the annual incidence of DF is about 2.4–2.6% 3 . There is a high prevalence of 3‐year recurrence in patients with healed foot ulcers, which exceeds 50% 4 . Thus, diabetic foot has become a great burden on public health.
Vitamin D, a pleiotropic steroid hormone, is essential to the metabolism of calcium and phosphorus and the regulation of bone turnover. Moreover, it is known to participate in inflammatory response, immune function, the regulation of cell cycle, as well as multiple chronic diseases, including diabetes and its complications 5 , 6 In addition, vitamin D is correlated with HbA1c levels in diabetic patients 7 and low vitamin D levels also have been reported to be associated with low muscle strength 8 . About one billion people are facing vitamin D deficiency all over the world, mainly in the Middle East, China, Mongolia, and India 9 . It is worthy of note that the proportion is even higher in winter 10 .
In the past few years, an inverse association between vitamin D levels and the occurrence and development of type 2 diabetes mellitus has been demonstrated 11 , 12 , 13 . Pena et al. studied the micronutrient status in diabetic patients with foot ulcers, which have revealed that vitamin D deficiency was the most common situation in patients with diabetic foot 14 . A number of preclinical evidence, as well as observational studies have reported a positive contribution of vitamin D to wound healing. However, whether vitamin D is associated with the occurrence and development of diabetic foot (DF) remains controversial.
More importantly, large‐scale epidemiological studies on the association of vitamin D levels and diabetic foot among the Chinese population are scarce 11 , 15 . Thus, it is necessary to evaluate the association of vitamin D levels and diabetic foot among the large Chinese population. The primary aim of this study was to explore the prevalence of vitamin D deficiency, and to address the association between serum 25‐OH‐vitamin D levels and DF in a Chinese hospitalized type 2 diabetes mellitus population, in order to summarize clinical evidence in the prevention and treatment of DF.
METHODS
Patients recruitment, grouping situation and ethical consideration
We recruited 1,721 consecutive inpatients, including 547 patients with DF (DF group) and 1,174 patients without DF (non‐DF group), from January 2012 to December 2019 at the Department of Endocrinology and Metabolism, West China Hospital, Sichuan University. The Biomedical Research Ethics Committee of West China Hospital of Sichuan University approved the study protocol, and the application for exemption of informed consent was passed. The diagnostic criteria of type 2 diabetes mellitus and diabetic foot were based on the American Diabetes Association classification 16 and the World Health Organization 17 . Only patients aged ≥18 years were included in this study. The exclusion criteria were as follows: (1) with other types of diabetes; (2) pregnant or lactating females; (3) with acute complications of diabetes or other stress states, such as surgery and trauma; (4) with rheumatologic, serious hepatic, cardiac, renal failure, malignancy, and endocrine diseases that affect the metabolism of vitamin D. Admission from December to May of each year was regarded as admission in winter and spring, and admission from June to November each year was regarded as admission in summer and autumn. Based on the above, the subjects were divided into two groups and four subgroups. The group assignment of population is presented in Figure 1.
Figure 1.
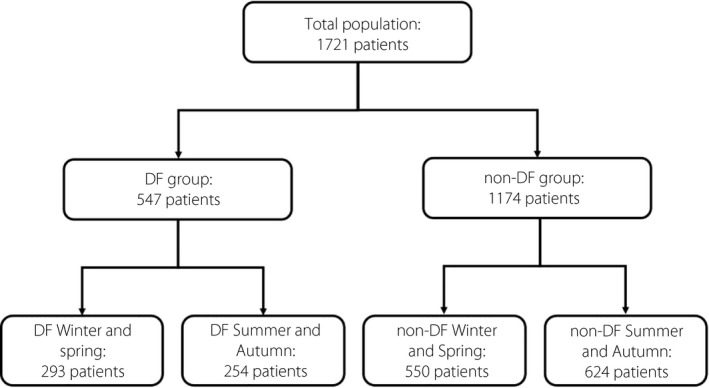
Flow chart showing the grouping situation for study subjects. In the diabetic foot group, 293 patients were admitted in winter and spring, and 254 patients were admitted in summer and autumn. In the non‐DF group, 550 patients were admitted in winter and spring, and 624 patients were admitted in summer and autumn.
Clinical and biochemical characteristics and vitamin D assessments
Demographics, comorbidities, and laboratory data were extracted from the electronic medical record system. The demographics included age, sex, body mass index (BMI), duration of diabetes, and smoking. The severity of diabetic foot was assessed by Wagner classification. The laboratory measurements collected included 25‐OH‐vitamin D, glycated hemoglobin (HbA1c), triglycerides (TG), total cholesterol (TC), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), albumin (ALB), creatinine (Cr), estimated glomerular filtration rate (eGFR), and serum uric acid (UA).
It was worth noting that the electrochemiluminescence immunoassay (Roche Cobas e601 analyzer) was used to determine the total serum 25‐OH‐vitamin D concentration and the functional sensitivity was 10.03 nmol/L. Combining the recommendations of the Institute of Medicine (IOM) 18 , the US Endocrine Society 19 and the latest evaluation results of vitamin D levels worldwide in 2020 20 , 21 , in this study, a serum level of 25‐OH‐vitamin D ≥ 50 nmol/L was defined as normal, 30 nmol/L ≤ 25‐OH‐vitamin D < 50 nmol/L as vitamin D insufficiency (VDI), and serum 25‐OH‐vitamin D < 30 nmol/L as vitamin D deficiency (VDD).
Statistical analysis
SPSS 18.0 software was used for statistical analyses. All continuous variables involved in this study did not conform to a normal distribution. Non‐normally distributed continuous variables were reported as median and interquartile range (IQR, 25–75%) and compared by the Mann‐Whitney test. The chi‐square test was used for categorical variables, which were summarized by frequency counts with percentages (n/%). The multivariate regression analysis was used to identify the association between the variables. A P value below 0.05 was considered statistically significant. Graphs were drawn using GraphPad Prism 7 software.
RESULTS
Characteristics of participants and vitamin D status
The demographic, clinical, and laboratory characteristics are shown in Tables 1 and 2. The median age was 66 (IQR 57, 74) years, and 49.2% of patients were men. In the total population, 48.5% of patients had a diabetes duration of more than 10 years, and 33.9% of patients had a history of smoking. The median BMI was 24.16 (IQR 21.97, 26.67) kg/m2, with a median glycated hemoglobin (HbA1c) level of 7.8 (IQR 6.7, 9.6) %. The vitamin D insufficiency and deficiency rate in the study population was 64.96%. The vitamin D insufficiency and deficiency rates in the DF group were higher than that in the non‐DF group (77.51% vs 59.2%, P < 0.001). The vitamin D status of patients is displayed in detail in Figure 2. The median level of 25‐OH‐vitamin D in the total population was 42.03 (IQR 30.79, 55.60) nmol/L. The level of 25‐OH‐vitamin D was lower in the DF group than that in the non‐DF group [35.80(IQR 26.19, 48.09) vs. 45.48(IQR 33.44, 59.25) nmol/L, P < 0.001].
Table 1.
Demographic and clinical characteristics in the two groups
| Total (n = 1721) | DF group (n = 547) | non‐DF group (n = 1174) | P | |
|---|---|---|---|---|
| Age | 66 (57, 74) | 67 (59, 75) | 66 (56, 74) | 0.001 |
| BMI | 24.16 (21.97, 26.67) | 23.31 (21.48, 25.39) | 24.52 (22.27, 27.10) | <0.001 |
| Gender | ||||
| Male | 847 (49.2%) | 346 (63.3%) | 501 (42.7%) | <0.001 |
| Female | 874 (50.8%) | 201 (36.7%) | 673 (57.3%) | |
| Duration of type 2 diabetes mellitus | ||||
| <5 years | 444 (25.8%) | 100 (18.3%) | 344 (29.3%) | |
| 5–10 years | 442 (25.7%) | 145 (26.5%) | 297 (25.3%) | <0.001 |
| >10 years | 835 (48.5%) | 302 (55.2%) | 533 (45.4%) | |
| Smoking history | ||||
| Smoking | 584 (33.9%) | 260 (47.5%) | 324 (27.6%) | <0.001 |
| Non‐smoking | 1137 (66.1%) | 287 (52.5%) | 850 (72.4%) | |
The two groups, DF group and non‐DF group; DF, diabetic foot; BMI, body mass index.
Table 2.
Laboratory characteristics in the two groups
| Variable | Total (n = 1721) | DF group (n = 547) | non‐DF group (n = 1174) | P |
|---|---|---|---|---|
| 25‐(OH)‐VD (nmol/L) | 42.03 (30.79, 55.60) | 35.8 (26.19, 48.09) | 45.48 (33.44, 59.25) | <0.001 |
| HbA1c (%) | 7.8 (6.7, 9.6) | 7.8 (6.8, 9.5) | 7.8 (6.6, 9.6) | 0.560 |
| TG (mmol/L) | 1.37 (1.01, 1.98) | 1.3 (0.98, 1.83) | 1.39 (1.02, 2.06) | 0.010 |
| TC (mmol/L) | 4.13 (3.40, 4.94) | 3.89 (3.19, 4.72) | 4.25 (3.55, 5.01) | <0.001 |
| HDL‐C (mmol/L) | 1.15 (0.92, 1.42) | 1.03 (0.85, 1.3) | 1.19 (0.97, 1.46) | <0.001 |
| LDL‐C (mmol/L) | 2.26 (1.69, 2.91) | 2.12 (1.58, 2.74) | 2.32 (1.75, 3) | <0.001 |
| ALB (g/L) | 41.4 (37.7, 44.4) | 38.4 (34.1, 41.6) | 42.6 (39.2, 45.3) | <0.001 |
| Cr (μmol/L) | 71 (58, 90.5) | 83 (65, 110.7) | 66 (56, 82) | <0.001 |
| eGFR (mL/min/1.73 m2) | 85.05 (63.79, 97.45) | 76.21 (54.41, 93.68) | 87.57 (69.03, 99.41) | <0.001 |
| UA (μmol/L) | 328 (269, 394) | 324 (252, 398) | 331 (275, 391) | 0.083 |
| Ca2+ (mmol/L) | 2.25 (2.16, 2.35) | 2.21 (2.09, 2.3) | 2.27 (2.18, 2.37) | <0.001 |
The two groups, DF group and non‐DF group; DF, diabetic foot; HbA1c, glycated hemoglobin; TG, triglycerides; TC, total cholesterol; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; ALB, albumin; Cr, creatinine; eGFR, estimated glomerular filtration rate; UA, serum uric acid.
Figure 2.
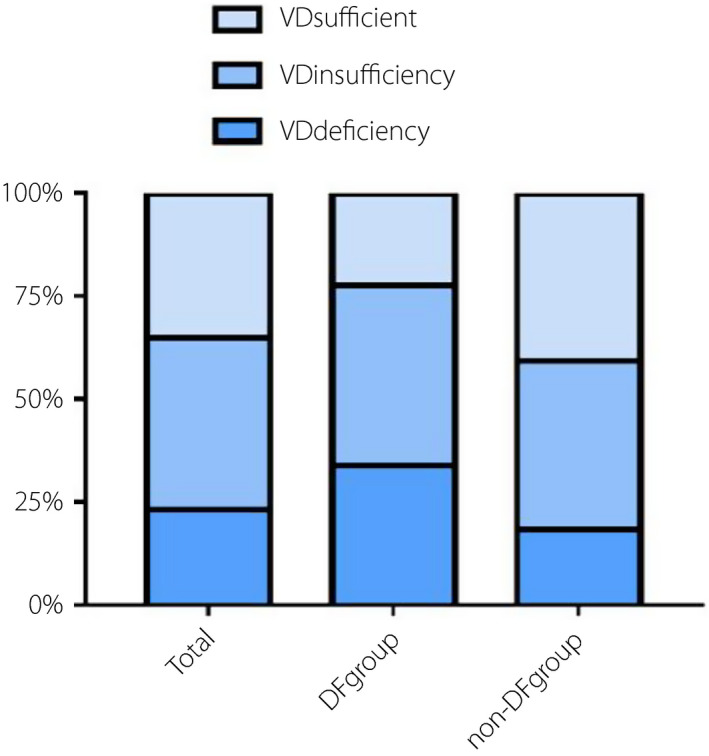
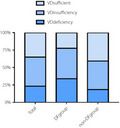
Bar graphs show the prevalence rates of vitamin D sufficiency, insufficiency, and deficiency among groups. The rates of vitamin D sufficiency, insufficiency, and deficiency in the total population were respectively 35.04, 41.72, and 23.24%. The rates of vitamin D sufficiency, insufficiency, and deficiency in diabetic foot group were respectively 22.49, 43.69, and 33.82%. The rates of vitamin D sufficiency, insufficiency, and deficiency in non‐DF group were respectively 40.80, 40.80, and 18.40%.
Vitamin D and glycemic control
Glycemic control was classified based on HbA1c levels as either good (<7%) or poor (≥7%) 22 . Compared with those with good glycemic control (n = 531), patients with poor glycemic control (n = 1,190) had lower 25‐OH‐vitamin D levels [40.98(IQR 30.17, 53.98) vs. 44.82 (IQR 32.30, 59.56) nmol/L, P = 0.01].
Seasonal fluctuation of vitamin D
As Figure 3 shows, there was a seasonal fluctuation with lower 25‐OH‐vitamin D levels in winter and spring than in summer and autumn. In the DF group, the vitamin D levels of those admitted in winter and spring were lower than those admitted in summer and autumn [33.05(IQR 23.86, 43.97) vs. 39.77(IQR 29.47, 50.70) nmol/L, P < 0.001]. The same was true in the non‐DF group [40.47(IQR 28.48, 54.10) vs. 49.15(IQR 38.37, 61.15) nmol/L, P < 0.001]. Among those admitted in winter and spring, the vitamin D levels of the DF group were lower than that of the non‐DF group [33.05(IQR 23.86, 43.97) vs. 40.47(IQR 28.48, 54.10) nmol/L, P < 0.001]. The same was true in those admitted in summer and autumn [39.77(IQR 29.47, 50.70) vs. 49.15(IQR 38.37, 61.15) nmol/L, P < 0.001].
Figure 3.
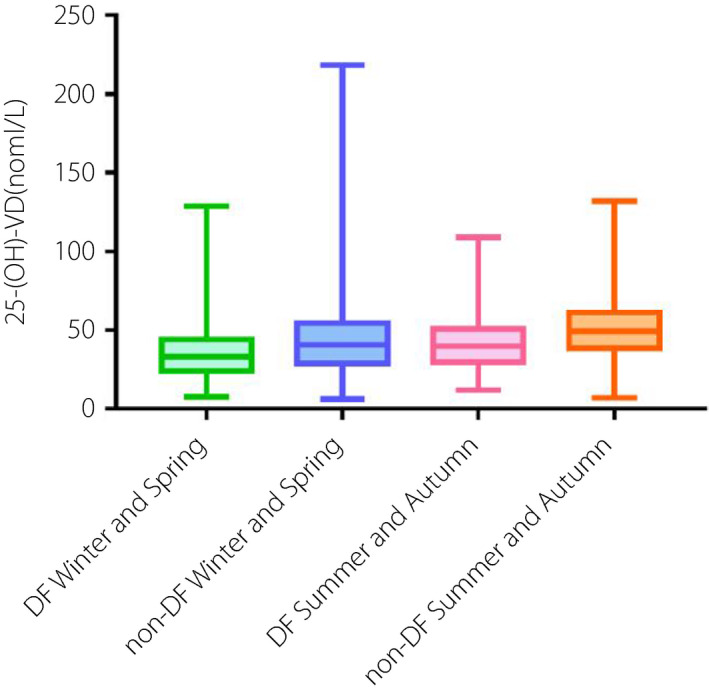
Bar graphs show the serum vitamin D levels in hospitalized patients with diabetic foot and non‐DF in different seasons.
Wagner classification and independent risk factor
As shown in Figure 4, in the DF group, the patients' vitamin D levels of Wagner grades 0–5 showed a downward trend, but the difference was not statistically significant(P = 0.114). In consideration of the effects of diabetic complications on diabetic foot and vitamin D levels, we evaluated diabetic complications in the study population, as shown in Table 3. These factors were taken into account in multivariate logistic regression analysis. Multivariate logistic regression analysis showed that 25‐OH‐vitamin D was independently related to diabetic foot, and it was a protective factor for DF (P < 0.001, OR = 0.986, 95% CI: 0.979–0.993), as shown in Table 4 and Figure 5. In addition, the multivariate logistic regression analysis was performed after grouping by vitamin D status (vitamin D deficiency vs. insufficiency vs. normal, and vitamin D deficiency was regarded as the control group). The results showed that patients with normal vitamin D were less likely to develop diabetic foot than those with vitamin D deficiency (P = 0.016, OR = 0.621, 95% CI: 0.421–0.915), as shown in Table 5.
Figure 4.
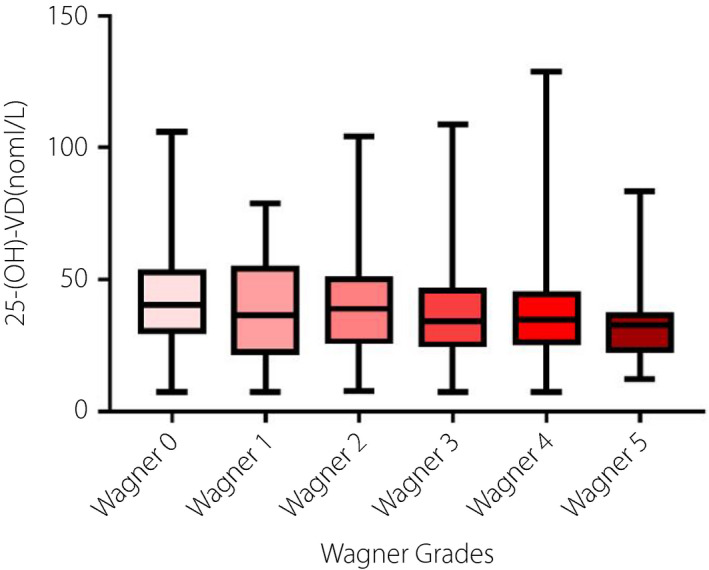
Bar graphs show the serum vitamin D levels among patients with diabetic foot with different Wagner grades.
Table 3.
The diabetic complications in the two groups
| Variable | Total (n = 1721) | DF group (n = 547) | non‐DF group (n = 1174) | P |
|---|---|---|---|---|
| DN, N (%) | 722 (42.0%) | 392 (71.7%) | 330 (28.1%) | <0.001 |
| DR, N (%) | 529 (30.7%) | 292 (53.4%) | 237 (20.2%) | <0.001 |
| DPN, N (%) | 1139 (66.2%) | 531 (97.1%) | 608 (51.8%) | <0.001 |
| PAD, N (%) | 459 (26.7%) | 283 (51.7%) | 176 (15.0%) | <0.001 |
| DAN, N (%) | 839 (48.8%) | 448 (81.9%) | 391 (33.3%) | <0.001 |
The two groups, DF group and non‐DF group; DF, diabetic foot; DN, diabetic nephropathy; DR, diabetic retinopathy; DPN, diabetic peripheral neuropathy; PAD, peripheral arterial disease; DAN, diabetic autonomic neuropathy.
Table 4.
Multivariate logistic regression analysis of risk factors for diabetic foot in patients with type 2 diabetes mellitus
| OR | 95% (CI) | P | ||
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| 25‐(OH)‐VD | 0.986 | 0.979 | 0.993 | <0.001 |
| TG | 0.745 | 0.652 | 0.852 | <0.001 |
| HDL‐C | 0.288 | 0.188 | 0.443 | <0.001 |
| DN | 2.297 | 1.706 | 3.093 | <0.001 |
| DR | 1.913 | 1.423 | 2.571 | <0.001 |
| DPN | 13.49 | 7.729 | 23.546 | <0.001 |
| PAD | 4.354 | 3.211 | 5.904 | <0.001 |
| DAN | 4.727 | 3.481 | 6.42 | <0.001 |
| Smoking history | 1.581 | 1.176 | 2.127 | 0.002 |
Did not enter the equation: age, sex, season of admission, BMI, LDL, CHOL; DF, diabetic foot; TG, triglycerides; HDL‐C, high‐density lipoprotein cholesterol; DN, diabetic nephropathy; DR, diabetic retinopathy; DPN, diabetic peripheral neuropathy; PAD, peripheral arterial disease; DAN, diabetic autonomic neuropathy.
Figure 5.
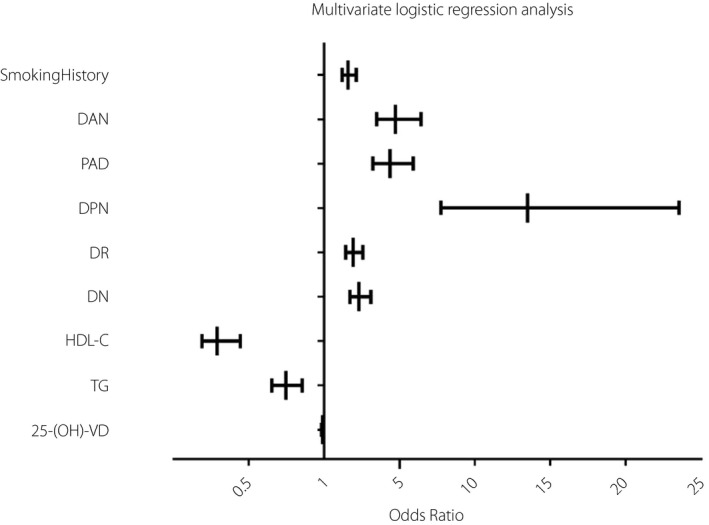
Forest map shows the results of the multivariate logistic regression analysis. The 25‐OH‐vitamin D was independently related to diabetic foot, and it was a protective factor for diabetic foot (P < 0.001, OR = 0.986, 95% CI: 0.979–0.993).
Table 5.
Multivariate logistic regression analysis of risk factors for diabetic foot in patients with type 2 diabetes mellitus
| OR | 95% (CI) | P | ||
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| VD grade | 0.019 | |||
| VD grade 2 | 0.961 | 0.676 | 1.366 | 0.823 |
| VD grade 3 | 0.621 | 0.421 | 0.915 | 0.016 |
| Admission time | 0.745 | 0.558 | 0.995 | 0.046 |
| TG | 0.751 | 0.657 | 0.858 | <0.001 |
| HDL‐C | 0.282 | 0.184 | 0.433 | <0.001 |
| DN | 2.376 | 1.762 | 3.204 | <0.001 |
| DR | 1.959 | 1.457 | 2.636 | <0.001 |
| DPN | 13.334 | 7.643 | 23.262 | <0.001 |
| PAD | 4.354 | 3.208 | 5.909 | <0.001 |
| DAN | 4.676 | 3.442 | 6.351 | <0.001 |
| Smoking history | 1.575 | 1.169 | 2.121 | 0.003 |
Did not enter the equation: age, sex, BMI, LDL, CHOL; DF, diabetic foot; VD grade: vitamin D deficiency and it was regarded as the control group; VD grade 2: vitamin D insufficiency; VD grade 3: vitamin D normal; admission time: season of admission; TG, triglycerides; HDL‐C, high‐density lipoprotein cholesterol; DN, diabetic nephropathy; DR, diabetic retinopathy; DPN, diabetic peripheral neuropathy; PAD, peripheral arterial disease; DAN, diabetic autonomic neuropathy.
DISCUSSION
This study found that the 25‐OH‐vitamin D levels in the DF group were significantly lower than that in the non‐DF group. Moreover, the proportion of vitamin D insufficiency and deficiency in the DF group were significantly higher than in the non‐DF group. In fact, Tiwari et al. firstly assessed the vitamin D status in patients with diabetic foot infection in India and concluded that vitamin D deficiency was more prevalent and severe in patients with diabetic foot infection than those without infection 23 . This conclusion was confirmed in their subsequent research 24 . Meanwhile, they reported that severe vitamin D deficiency was associated with elevated inflammatory cytokine concentrations and suggested a vitamin D concentration value of <25 nmol/L as the ‘cut‐off’ for unfavorable immunological alterations in diabetic patients. Since then, several observational studies have been reported in succession. However, the results of these studies have been inconsistent. Also in India, the difference in the serum level of vitamin D between diabetic patients with and without foot infections was not found to be statistically significant in a cross‐sectional study reported in 2019 25 . A similar conclusion has also been drawn by a recent study conducted in a Mediterranean country 26 . Nevertheless, Afarideh et al. 27 came to contrary conclusions in Iranian patients. Similar observational studies have been done in Europe 28 , 29 and the results show that patients with diabetic foot syndrome are at high risk of low vitamin D levels. At present, there have been only two research studies conducted in China 11 , 15 , and only one had a relatively large sample capacity 11 . It is worth mentioning that both of their findings are in accordance with ours. Moreover, our multivariate logistic regression analysis likewise revealed that vitamin D was an independent risk factor for diabetic foot and might have some level of protective effect on the occurrence of diabetic foot. However, the amplitude (OR = 0.986) was very small and false‐positive results cannot be ruled out, when we regarded vitamin D as a continuous variable. But when we grouped vitamin D status into vitamin D normal/insufficiency/deficiency groups, the results still showed that a normal vitamin D level was a protective factor for diabetic foot compared with vitamin D deficiency (P = 0.016, OR = 0.621, 95% CI: 0.421–0.915).
However, aside from the study conducted in Hunan and our study, the sample sizes of most existing studies are relatively small. Thus, the persuasiveness of these conclusions is open to question and the exact relationship between vitamin D and diabetic foot is still confusing. In spite of this contradiction, the abundance of preclinical data also tend to the favorable effects of vitamin D on diabetic foot until now, especially on wound healing 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 . Vitamin D may be involved in the wound healing by the following means: (1) regulates inflammation during wound healing through interacting with the TGF‐β signaling pathway to promote the normal inflammatory response 30 and suppresses NF‐κB‐mediated inflammatory gene expression to reduce the persistent inflammation 31 , just as observed in the study of Tiwari 24 ; (2) has an effect on vascular regeneration through promoting SDF1 expression by increasing hypoxia‐inducible factor signaling 32 and augmenting proangiogenic factors, such as VEGFA, HIF‐1α and angiogenin gene expression 33 ; (3) may be involved in the self‐renewal, migration, and differentiation of epidermal stem cells and progeny to promote wound re‐epithelialization 34 , 35 ; (4) induce the antimicrobial peptide gene expression 36 , 37 and suppress endoplasmic reticulum stress 38 and oxidative stress 39 ; (5) may play an indirect role during wound healing due to its effect on improving glycemic control 40 . We reported a lower level of 25‐OH‐vitamin D in patients with poor glycemic control when compared with good glycemic control patients, which was consistent with the results of Darraj et al 41 . That is to say, the positive contribution of vitamin D on glycemic control is also supported in our study. Therefore, from the pathophysiological mechanism, patients with type 2 diabetes with vitamin D deficiency are more likely to suffer from diabetic foot ulcer. In other words, we could regard vitamin D deficiency as the causative factor of the development of diabetic foot ulcer. On the other hand, patients who already have diabetic foot are associated with an increased risk of aggravating vitamin D deficiency due to long periods of being bedridden, immobilization of the affected limb, less physical exercise, decreased nutritional status, and other reasons. However, the causal relationship between vitamin D and diabetic foot is unclear. From current studies, we hold the opinion that diabetic foot and vitamin D level have an effect on each other.
As is well known, the vitamin D concentrations vary strongly by season 42 . To our knowledge, this is the first study to assess the seasonal fluctuation of vitamin D among patients with diabetic foot. We found a seasonal fluctuation of vitamin D with lower levels in winter and spring, particularly in patients with diabetic foot. Moreover, the vitamin D levels were also lower in the DF group during the same season when compared with patients without diabetic foot. Vitamin D is mainly derived from the synthesis in the skin under ultraviolet B exposure. Because of this, numerous factors could cause significant effects on vitamin D levels, such as season, time of day, latitude, altitude, air pollution, skin pigmentation, sunscreen use, and the rays passing through glass and plastic 43 . This may be part of the reason for the inconsistent results of current studies. Therefore, it is necessary to screen the vitamin D status of type 2 diabetes mellitus patients in winter and spring, especially those with diabetic foot. Besides, it could be of significance to advise timely vitamin D supplementation.
On the other hand, it was found that as the Wagner grade increases, the 25‐OH‐vitamin D levels of patients with diabetic foot showed a gradual, yet not significant, downward trend. It may be due to factors such as wound size, location, depth, infection, secretions, and treatment not being taken into account in the assessment of the severity of diabetic foot 44 .
Until now, only two randomized controlled trials have been done showing that supplementation of vitamin D could promote wound healing in DF patients 45 , 46 . Our study revealed that the vitamin D level in DF patients with type 2 diabetes mellitus was lower than those without diabetic foot. So, this gives us a hint that vitamin D supplementation may be a potential adjunctive therapeutic option for diabetic foot. In other words, preventing vitamin D deficiency and keeping appropriate levels of vitamin D could be helpful for the prevention and adjuvant treatment of diabetic foot. Based on the above, although there are contradictory results 25 , 26 , 27 , a large number of studies and meta‐analyses still show a clear connection between low vitamin D levels, vitamin D deficiency, and diabetic foot 11 , 15 , 23 , 24 , 28 , 29 , 47 , 48 . Although this connection does not mean necessarily correlation or causal connection, it also has great significance for the treatment and management of diabetic foot. Moreover, the screening of vitamin D levels in diabetic patients could assess the risk of diabetic foot to a certain extent.
There are a few limitations in this study. Firstly, we only included Chinese adults from West China Hospital, Sichuan University, who were Asian. This conclusion may not generalizable to other races or regions. Secondly, although the study population included were all inpatients, differences in outdoor activities and sun exposure between patients with and without diabetic foot were avoided to some extent. For all we know, patients with diabetic foot are bedridden for long periods as a result of the immobilization of the affected limb. Unfortunately, our results may not be applicable to the general household population. Thirdly, more than half of the diabetic patients were not included in the study because of the lack of data on vitamin D levels and incomplete data, which may lead to offset results. This means that more patients may have been facing vitamin D deficiency and the rate of vitamin D deficiency may be higher than reported in this article. Thus, the effects of vitamin D on diabetic foot might be underestimated. We believe that this condition could be avoided in the future with popularizing vitamin D screening. Moreover, this was a retrospective study and we did not check the level of vitamin D annually in the population, so that the temporality of the association between vitamin D and diabetic foot has not been assessed. Also this was a retrospective study and the assessment of lifestyles was very difficult, so lifestyle factors were not included. Last but not least, our study included the inherent limitations of a retrospective study. A RCT would be ideal, but a retrospective study with a relatively large sample size, well‐defined study population, and strong quality control could be acceptable if a sufficient number of RCTs is not conducted yet. Further well‐designed research should be done to verify whether there are associations between vitamin D and diabetic foot, and to assess the action of vitamin D in the prevention and treatment of diabetic foot.
Vitamin D deficiency is a common condition among Chinese patients with type 2 diabetes mellitus. The low serum vitamin D level was significantly associated with a higher prevalence of diabetic foot among Chinese patients with type 2 diabetes mellitus. We firstly assessed the seasonal fluctuation of vitamin D in patients with diabetic foot. Although vitamin D levels vary seasonally, patients with diabetic foot were still at higher risk of having vitamin D insufficiency and deficiency. Vitamin D screening or supplementation in Chinese patients with type 2 diabetes mellitus may prevent diabetic foot or improve the prognosis of diabetic foot, especially in winter and spring.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The study was approved by Biomedical Research Ethics Committee of West China Hospital of Sichuan University.
Informed consent: Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Approval date of registry and the registration no. of the study/trial: January 1, 2019 and No. ZYGD18025.
Animal studies: N/A.
ACKNOWLEDGMENTS
This study was partial supported by Science and Technology Bureau of Sichuan Province [Grant No. 2021JDKP004], 1.3.5 Project for disciplines of excellence, West China Hospital, Sichuan University [Grant No. ZYGD18025], West China Nursing Discipline Development Special Fund Project, Sichuan University [Grant No. HXHL20005], Health Medical Big Data Application and Innovation Project in Sichuan [Grant No. 2018gfgw001], Science and Technology Bureau of Chengdu city [Grant No. 2017‐CY02 to 00028‐GX].
J Diabetes Investig. 2022; 13: 1213–1221
REFERENCES
- 1. Kateel R, Augustine AJ, Prabhu S, et al. Clinical and microbiological profile of diabetic foot ulcer patients in a tertiary care hospital. Diabetes Metab Syndr 2018; 12: 27–30. [DOI] [PubMed] [Google Scholar]
- 2. Hobizal KB, Wukich DK. Diabetic foot infections: current concept review. Diabet Foot Ankle 2012; 3: 1840. 10.3402/dfa.v3i0.18409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lozano‐Platonoff A, Florida Mejía‐Mendoza MD, Ibáñez‐Doria M, et al. Estándar de oro en el manejo del pie diabético: yeso de contacto total [The gold standard in diabetic foot treatment: total contact cast]. Gac Med Mex 2014; 150: 58–64. [PubMed] [Google Scholar]
- 4. Dubský M, Jirkovská A, Bem R, et al. Risk factors for recurrence of diabetic foot ulcers: prospective follow‐up analysis in the Eurodiale subgroup. Int Wound J 2013; 10: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord 2017; 18: 153–165. [DOI] [PubMed] [Google Scholar]
- 6. Erkus E, Aktas G, Atak BM, et al. Haemogram parameters in vitamin D deficiency. J Coll Physicians Surg Pak 2018; 28: 779–782. [PubMed] [Google Scholar]
- 7. Erkus E, Aktas G, Kocak MZ, et al. Diabetic regulation of subjects with type 2 diabetes mellitus is associated with serum vitamin D levels. Rev Assoc Med Bras 2019; 65: 51–55. [DOI] [PubMed] [Google Scholar]
- 8. Kocak MZ, Aktas G, Atak B, et al. The association between vitamin D levels and handgrip strength in elderly men. Acta Endocrinol (Buchar) 2020; 16: 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sahota O. Understanding vitamin D deficiency. Age Ageing 2014; 43: 589–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Schoor N, Lips P. Global overview of vitamin D status. Endocrinol Metab Clin North Am 2017; 46: 845–870. [DOI] [PubMed] [Google Scholar]
- 11. Xiao Y, Wei L, Xiong X, et al. Association between vitamin D status and diabetic complications in patients with type 2 diabetes mellitus: a cross‐sectional study in Hunan China. Front Endocrinol (Lausanne) 2020; 11: 564738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mariam W, Garg S, Singh MM, et al. Vitamin D status, determinants and relationship with biochemical profile in women with type 2 diabetes mellitus in Delhi, India. Diabetes Metab Syndr 2019; 13: 1517–1521. [DOI] [PubMed] [Google Scholar]
- 13. Heath AK, Williamson EJ, Hodge AM, et al. Vitamin D status and the risk of type 2 diabetes: the Melbourne Collaborative Cohort Study. Diabetes Res Clin Pract 2019; 149: 179–187. [DOI] [PubMed] [Google Scholar]
- 14. Pena G, Kuang B, Cowled P, et al. Micronutrient status in diabetic patients with foot ulcers. Adv Wound Care 2020; 9: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dai J, Yu M, Chen H, et al. Association between serum 25‐OH‐vitamin D and diabetic foot ulcer in patients with type 2 diabetes. Front Nutr 2020; 7: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2013; 36: S67–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jeffcoate WJ, Macfarlane RM, Fletcher EM. The description and classification of diabetic foot lesions. Diabet Med 1993; 10: 676–679. [DOI] [PubMed] [Google Scholar]
- 18. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011; 96: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holick MF, Binkley NC, Bischoff‐Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline [published correction appears in J Clin Endocrinol Metab. 2011 Dec;96(12):3908]. J Clin Endocrinol Metab 2011; 96: 1911–1930. [DOI] [PubMed] [Google Scholar]
- 20. Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr 2020; 74: 1498–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cashman KD, Vitamin D. Deficiency: defining, prevalence, causes, and strategies of addressing. Calcif Tissue Int 2020; 106: 14–29. [DOI] [PubMed] [Google Scholar]
- 22. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018; 168: 569–576. [DOI] [PubMed] [Google Scholar]
- 23. Tiwari S, Pratyush DD, Gupta B, et al. Prevalence and severity of vitamin D deficiency in patients with diabetic foot infection. Br J Nutr 2013; 109: 99–102. [DOI] [PubMed] [Google Scholar]
- 24. Tiwari S, Pratyush DD, Gupta SK, et al. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br J Nutr 2014; 112: 1938–1943. [DOI] [PubMed] [Google Scholar]
- 25. Danny Darlington CJ, Suresh Kumar S, Jagdish S, et al. Evaluation of serum vitamin D levels in diabetic foot infections: a cross‐sectional study in a tertiary care center in South India. Iran J Med Sci 2019; 44: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsitsou S, Dimosthenopoulos C, Eleftheriadou I, et al. Evaluation of vitamin D levels in patients with diabetic foot ulcers [published online ahead of print, 2021 Jan 3]. Int J Low Extrem Wounds 2021: 1534734620984584. [DOI] [PubMed] [Google Scholar]
- 27. Afarideh M, Ghanbari P, Noshad S, et al. Raised serum 25‐hydroxyvitamin D levels in patients with active diabetic foot ulcers. Br J Nutr 2016; 115: 1938–1946. [DOI] [PubMed] [Google Scholar]
- 28. Feldkamp J, Jungheim K, Schott M, et al. Severe vitamin D3 deficiency in the majority of patients with diabetic foot ulcers. Horm Metab Res 2018; 50: 615–619. [DOI] [PubMed] [Google Scholar]
- 29. Todorova AS, Jude EB, Dimova RB, et al. Vitamin D status in a Bulgarian population with type 2 diabetes and diabetic foot ulcers [published online ahead of print, 2020 Oct 23]. Int J Low Extrem Wounds 2020: 1534734620965820. [DOI] [PubMed] [Google Scholar]
- 30. Luderer HF, Nazarian RM, Zhu ED, et al. Ligand‐dependent actions of the vitamin D receptor are required for activation of TGF‐β signaling during the inflammatory response to cutaneous injury. Endocrinology 2013; 154: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yuan Y, Das SK, Li M. Vitamin D ameliorates impaired wound healing in streptozotocin‐induced diabetic mice by suppressing NF‐κB‐mediated inflammatory genes. Biosci Rep 2018; 38: BSR20171294. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Wong MSK, Leisegang MS, Kruse C, et al. Vitamin D promotes vascular regeneration. Circulation 2014; 130: 976–986. [DOI] [PubMed] [Google Scholar]
- 33. Trujillo V, Marín‐Luevano P, González‐Curiel I, et al. Calcitriol promotes proangiogenic molecules in keratinocytes in a diabetic foot ulcer model. J Steroid Biochem Mol Biol 2017; 174: 303–311. [DOI] [PubMed] [Google Scholar]
- 34. Oda Y, Tu CL, Menendez A, et al. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol 2016; 164: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oda Y, Hu L, Nguyen T, et al. Vitamin D receptor is required for proliferation, migration, and differentiation of epidermal stem cells and progeny during cutaneous wound repair. J Invest Dermatol 2018; 138: 2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25‐dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression [published correction appears in J Immunol. 2004 Nov 15;173(10):following 6489. Hanrahan, JH [corrected to Hanrahan, JW]. J Immunol 2004; 173: 2909–2912. [DOI] [PubMed] [Google Scholar]
- 37. Liu PT, Stenger S, Li H, et al. Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science 2006; 311: 1770–1773. [DOI] [PubMed] [Google Scholar]
- 38. Yuan YF, Das SK, Li MQ. Vitamin D ameliorates impaired wound healing in streptozotocin‐induced diabetic mice by suppressing endoplasmic reticulum stress. J Diabetes Res 2018; 2018: 757925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wenclewska S, Szymczak‐Pajor I, Drzewoski J, et al. Vitamin D supplementation reduces both oxidative DNA damage and insulin resistance in the elderly with metabolic disorders. Int J Mol Sci 2019; 20: 2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu C, Qiu S, Zhu X, et al. Vitamin D supplementation and glycemic control in type 2 diabetes patients: a systematic review and meta‐analysis. Metabolism 2017; 73: 67–76. [DOI] [PubMed] [Google Scholar]
- 41. Darraj H, Badedi M, Poore KR, et al. Vitamin D deficiency and glycemic control among patients with type 2 diabetes mellitus in Jazan City, Saudi Arabia. Diabetes Metab Syndr Obes 2019; 12: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schottker B, Jorde R, Peasey A, et al. Vitamin D and mortality: meta‐analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ 2014; 348: g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wacker M, Holick MF. Sunlight and vitamin D: a global perspective for health. Dermatoendocrinol 2013; 5: 51–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Monteiro‐Soares M, Boyko EJ, Jeffcoate W, et al. Diabetic foot ulcer classifications: a critical review. Diabetes Metab Res Rev 2020; 36: e3272. [DOI] [PubMed] [Google Scholar]
- 45. Razzaghi R, Pourbagheri H, Momen‐Heravi M, et al. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double‐blind, placebo‐controlled trial. J Diabetes Complications 2017; 31: 766–772. [DOI] [PubMed] [Google Scholar]
- 46. Halschou‐Jensen PM, Sauer J, Bouchelouche P, et al. Improved healing of diabetic foot ulcers after high‐dose vitamin D: a randomized double‐blinded clinical trial [published online ahead of print, 2021 Jul 2]. Int J Low Extrem Wounds 2021: 15347346211020268. [DOI] [PubMed] [Google Scholar]
- 47. Yammine K, Hayek F, Assi C. Is there an association between vitamin D and diabetic foot disease? A meta‐analysis. Wound Repair Regen 2020; 28: 90–96. [DOI] [PubMed] [Google Scholar]
- 48. Dai J, Jiang C, Chen H, et al. Vitamin D and diabetic foot ulcer: a systematic review and meta‐analysis. Nutr Diabetes 2019; 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]


