Abstract
Aims/Introduction
Many East Asians with type 2 diabetes are elderly and have a low body mass index (BMI), especially in 'super‐aged' populations, such as Japan. This post‐hoc analysis assessed once‐weekly semaglutide efficacy and safety in Japanese individuals with type 2 diabetes across baseline age and BMI subgroups.
Materials and Methods
Data were derived from the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) Japan monotherapy and SUSTAIN Japan oral antidiabetes drug (OAD) combination trials comparing once‐weekly semaglutide with sitagliptin or OADs, respectively. Participants were grouped by baseline age (<65 and ≥65 years) and/or BMI (<25 and ≥25 kg/m2). Reductions from baseline in glycosylated hemoglobin and bodyweight (efficacy), and adverse events (safety) were assessed.
Results
In this analysis, participants from the SUSTAIN Japan monotherapy trial (n = 308; n per subgroup; range, 8–73) and SUSTAIN Japan OAD combination trial (n = 601; n per subgroup; range, 20–168) were included. Reductions in glycosylated hemoglobin and bodyweight were numerically greater with semaglutide versus comparators across all age and BMI subgroups. Reductions from baseline in glycosylated hemoglobin ranged from –1.7 to –2.1 with semaglutide 0.5 mg, –1.8 to –2.4 with semaglutide 1.0 mg and –0.6 to –1.0 with comparators. Corresponding ranges for bodyweight (kg) were –1.0 to –2.5, –2.4 to –4.3 and 1.0 to –1.0 kg, respectively. The safety profile of semaglutide was broadly similar across BMI and age subgroups.
Conclusions
In this post‐hoc analysis with modest subgroup numbers, once‐weekly semaglutide appeared consistently more efficacious versus comparators across age and BMI subgroups in Japanese patients, with a similar safety profile.
Keywords: Body mass index, Diabetes mellitus, type 2, Glucagon‐like peptide‐1
In this post‐hoc analysis of data from the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) Japan monotherapy and SUSTAIN Japan oral antidiabetes drug combination trials, which compared once‐weekly semaglutide with sitagliptin or oral antidiabetes drugs, respectively, individuals with type 2 diabetes were grouped by baseline age (<65 and ≥65 years) and/or body mass index (<25 and ≥25 kg/m2). In Japanese patients with type 2 diabetes, once‐weekly semaglutide appeared to be more efficacious versus comparators in terms of reductions in glycosylated hemoglobin and bodyweight across age, body mass index and age plus body mass index subgroups, with a similar safety profile. These exploratory results are consistent with those from other trials in the SUSTAIN clinical trial program.
![]()
INTRODUCTION
In East Asian people, the etiology of type 2 diabetes is characterized by β‐cell dysfunction 1 , 2 , 3 and relatively low insulin secretion capacity has been reported. 4 Furthermore, East Asian people with type 2 diabetes on average have a lower body mass index (BMI), compared with white and Western populations, 5 with dietary differences potentially influencing the effect of antihyperglycemic medications. 1 Japan is also a ‘super‐aged’ society with a large number of elderly people (aged ≥75 years) with late‐stage type 2 diabetes. In elderly populations with a low BMI, individualized treatment and considerations of frailty may be particularly important. 6
For approval in Japan, regulations require antihyperglycemic drugs to be evaluated in Japanese people with type 2 diabetes. Once‐weekly (OW) subcutaneous semaglutide, a glucagon‐like peptide‐1 (GLP‐1) analog approved for the treatment of type 2 diabetes in Japan, 7 was studied in the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) clinical trial program, which included two trials in Japanese individuals. These trials evaluated the safety and efficacy of OW semaglutide as monotherapy (SUSTAIN Japan monotherapy trial [NCT02254291] vs sitagliptin monotherapy) 8 and as monotherapy or in addition to one baseline oral antidiabetes drug (OAD; SUSTAIN Japan OAD combination trial [NCT02207374] vs additional OAD). 9
The aim of this post‐hoc exploratory analysis was to assess semaglutide efficacy and safety versus comparators in Japanese individuals with type 2 diabetes across subgroups defined by baseline age and BMI.
MATERIALS AND METHODS
The SUSTAIN Japan monotherapy trial (NCT02254291) compared OW semaglutide with sitagliptin, 8 the most widely used antihyperglycemic drug in Japan. The trial included 308 Japanese individuals with type 2 diabetes aged ≥20 years and treated with either diet and exercise alone or with OAD monotherapy who were randomized 1:1:1 to receive OW semaglutide 0.5 mg, OW semaglutide 1.0 mg or sitagliptin 100 mg for 30 weeks (after an 8‐week washout period, for those taking an OAD). This trial was carried out to meet the ‘Guideline for Clinical Evaluation of Oral Hypoglycemic Agents’ issued by the Japanese Ministry of Health, Labor and Welfare, which advises that new drugs are assessed as monotherapy to investigate their isolated effects. 10
The SUSTAIN Japan OAD Combination trial (NCT02207374) compared OW semaglutide with OAD therapy added to either diet/exercise or existing OAD monotherapy (sulfonylurea, glinide, α‐glucosidase inhibitor or thiazolidinedione). 9 In the trial, 601 Japanese individuals with type 2 diabetes aged ≥20 years were randomized 2:2:1 to OW semaglutide 0.5 mg, OW semaglutide 1.0 mg or one additional OAD with a different mode of action to background therapy, for 56 weeks. The SUSTAIN Japan OAD Combination trial was carried out to meet the “Guideline for Clinical Evaluation of Oral Hypoglycemic Agents” issued by the Japanese Ministry of Health, Labor and Welfare, which advises that new drugs are assessed against a marketed OAD to investigate their safety. 10
Both of the SUSTAIN Japan trials were carried out in compliance with the International Conference on Harmonization Good Clinical Practice guidelines 11 and the Declaration of Helsinki. 12 The protocols for the trials were reviewed and approved as required according to local regulations (a full list of the institutional review boards is provided in Table S1). Written informed consent was obtained from all participants.
The primary end‐point in the SUSTAIN Japan trials was safety, including the incidence of adverse events (AEs) in the monotherapy and OAD combination trials. 8 , 9
Post‐hoc analyses
Data from the SUSTAIN Japan monotherapy and the SUSTAIN Japan OAD Combination trials were evaluated separately according to baseline age subgroups and baseline BMI subgroups.
Baseline age subgroups were defined according to the traditional Japanese definition of ‘elderly’ (i.e. age <65 years or ≥65 years). 13 Baseline BMI subgroups were defined according to the Japanese definition for obesity (i.e. BMI <25 kg/m2 or ≥25 kg/m2). 14 Combined age and BMI subgroups were defined by age <65 years and BMI <25 kg/m2, age <65 years and BMI ≥25 kg/m2, age ≥65 years and BMI <25 kg/m2 or age ≥65 years and BMI ≥25 kg/m2.
Efficacy end‐points evaluated according to the baseline subgroups included: the changes in glycosylated hemoglobin (HbA1c) and bodyweight from baseline to end of trial, the proportion of participants achieving HbA1c <7.0% and ≤6.5% at end of trial, and the proportions of participants with ≥5% and ≥10% bodyweight loss from baseline to end of trial.
Safety end‐points evaluated according to the baseline subgroups included: the incidence of overall AEs, including data by severity; incidence of AEs leading to premature treatment discontinuation; incidence of gastrointestinal AEs, including nausea, vomiting, diarrhea, constipation and abdominal discomfort; and incidence of blood glucose (BG)‐confirmed symptomatic hypoglycemia, which was defined as an episode that was severe according to the American Diabetes Association classification 15 or confirmed by plasma glucose <3.1 mmol/L (56 mg/dL), with symptoms consistent with hypoglycemia.
Statistical analysis
All efficacy end‐points are summarized or analyzed using ‘on‐treatment without rescue medication’ data from participants in the full analysis set, whereas the safety end‐points are descriptively summarized using ‘on‐treatment’ data from participants in the safety analysis set.
Change from baseline at scheduled post‐baseline visits in HbA1c and bodyweight were analyzed separately using a mixed model for repeated measurements with treatment, subgroup at baseline, and interaction between treatment and subgroup as fixed factors, and baseline value as the covariate, all nested within visit. From the model, treatment means and treatment differences (semaglutide – comparator) for each subgroup were estimated. Mean estimates were adjusted according to the observed baseline distribution in each subgroup. For participants achieving HbA1c targets, or weight loss ≥5% or ≥10%, missing data at week 30 (SUSTAIN Japan monotherapy) or week 56 (SUSTAIN Japan OAD Combination) were imputed from predicted values from the mixed model for repeated measurements applied in the primary manuscripts and subsequently classified. 8 , 9 The proportions of participants achieving these targets were reported for the subgroups.
AE data were summarized descriptively as the number of participants, percentage of the safety analysis set, number of events and event rate per 100 years of treatment exposure.
RESULTS
Participants and subgroup stratification
In total, 308 and 601 participants were enrolled in the SUSTAIN Japan monotherapy and SUSTAIN Japan OAD combination trials, respectively. One participant in the OAD arm for the SUSTAIN Japan OAD combination trial was not exposed to an additional OAD, so was not included in the full analysis set. 9
Of the participants randomized to the semaglutide 0.5 mg (n = 103), semaglutide 1.0 mg (n = 102) and sitagliptin (n = 103) arms of the SUSTAIN Japan monotherapy trial, 66, 63 and 73, respectively, were aged <65 years; 37, 39 and 30, respectively, were aged ≥65 years; 57, 56 and 59, respectively, had a BMI <25 kg/m2; and 46, 46, and 44, respectively, had a BMI ≥25 kg/m2. After stratification by both age and BMI, the respective numbers of participants in each subgroup were: 32, 29 and 37 (age <65 years and BMI <25 kg/m2); 34, 34 and 36 (age <65 years and BMI ≥25 kg/m2); 25, 27 and 22 (age ≥65 years and BMI <25 kg/m2); and 12, 12 and 8 (age ≥65 years and BMI ≥25 kg/m2).
Of the participants randomized to semaglutide 0.5 mg (n = 239), semaglutide 1.0 mg (n = 241) and additional OAD (n = 120) arms of the SUSTAIN Japan OAD trial, 162, 168 and 77, respectively, were aged <65 years; 77, 73 and 43, respectively, were aged ≥65 years; 105, 102 and 49, respectively, had a BMI <25 kg/m2; and 134, 139 and 71, respectively, had a BMI ≥25 kg/m2. After stratification by both age and BMI, respective participant numbers were 57, 59 and 29 for age <65 years and BMI <25 kg/m2; 105, 109 and 48 for age <65 years and BMI ≥25 kg/m2; 48, 43 and 20 for age ≥65 years and BMI <25 kg/m2; and 29, 30 and 23 for age ≥65 years and BMI ≥25 kg/m2.
Baseline characteristics
The baseline characteristics of the participants, according to combined baseline age and BMI subgroups, are shown in Table 1; baseline characteristics according to individual baseline age or baseline BMI subgroups are shown in Table S2.
Table 1.
Participant disposition and baseline characteristics according to combined baseline age and body mass index subgroups in the SUSTAIN Japan monotherapy trial and the SUSTAIN Japan OAD Combination trial
| SUSTAIN Japan monotherapy trial | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OW semaglutide 0.5 mg | OW semaglutide 1.0 mg | Sitagliptin | ||||||||||
| Age (year) | <65 | ≥65 | <65 | ≥65 | <65 | ≥65 | ||||||
| BMI (kg/m2) | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 |
| Participant disposition, n (%) | ||||||||||||
| Randomized | 32 | 34 | 25 | 12 | 29 | 34 | 27 | 12 | 37 | 36 | 22 | 8 |
| Trial completers | 32 (100) | 34 (100) | 25 (100) | 12 (100) | 28 (96.6) | 33 (97.1) | 26 (96.3) | 12 (100) | 36 (97.3) | 36 (100) | 21 (95.5) | 8 (100) |
| Premature treatment discontinuation | 1 (3.1) | 0 | 1 (4.0) | 1 (8.3) | 5 (17.2) | 2 (5.9) | 6 (22.2) | 2 (16.7) | 2 (5.4) | 0 | 1 (4.5) | 0 |
| Participant characteristics | ||||||||||||
| Age (years) | 53.9 (7.9) | 51.9 (8.0) | 69.5 (3.6) | 68.9 (4.0) | 54.1 (7.3) | 48.1 (8.6) | 69.9 (3.9) | 69.7 (3.6) | 55.1 (6.2) | 50.9 (7.9) | 69.2 (4.1) | 71.5 (6.1) |
| Male, n (%) | 24 (75.0) | 25 (73.5) | 20 (80.0) | 10 (83.3) | 22 (75.9) | 24 (70.6) | 19 (70.4) | 10 (83.3) | 28 (75.7) | 28 (77.8) | 18 (81.8) | 7 (87.5) |
| HbA1c (%) | 8.3 (1.0) | 8.4 (1.2) | 7.8 (0.7) | 8.6 (0.8) | 8.3 (0.8) | 8.2 (1.0) | 7.7 (0.6) | 7.5 (0.4) | 8.2 (0.9) | 8.1 (0.9) | 8.3 (1.0) | 8.3 (0.7) |
| HbA1c (mmol/mol) | 67.3 (11.3) | 68.0 (12.8) | 61.5 (8.1) | 70.0 (8.9) | 67.1 (9.1) | 66.3 (11.0) | 60.5 (6.5) | 58.7 (4.8) | 66.4 (9.5) | 65.2 (9.5) | 66.7 (11.5) | 67.6 (8.1) |
| FPG (mg/dL) | 163.6 (36.6) | 176.2 (44.1) | 155.8 (29.1) | 165.3 (30.9) | 172.7 (37.3) | 171.7 (30.4) | 156.8 (30.1) | 145.5 (20.7) | 173.8 (31.1) | 165.2 (30.0) | 173.7 (49.6) | 183.8 (37.0) |
| FPG (mmol/L) | 9.1 (2.0) | 9.8 (2.5) | 8.6 (1.6) | 9.2 (1.7) | 9.6 (2.1) | 9.5 (1.7) | 8.7 (1.7) | 8.1 (1.1) | 9.6 (1.7) | 9.2 (1.7) | 9.6 (2.8) | 10.2 (2.1) |
| Diabetes duration (years) | 9.0 (5.4) | 5.2 (4.1) | 10.6 (4.4) | 7.9 (6.1) | 8.0 (4.8) | 4.6 (3.8) | 12.1 (9.4) | 7.0 (6.6) | 7.0 (4.4) | 4.8 (3.6) | 14.3 (8.7) | 11.0 (8.0) |
| Bodyweight (kg) | 62.3 (8.1) | 78.2 (10.9) | 60.0 (7.6) | 69.2 (7.0) | 63.2 (9.7) | 85.1 (17.0) | 58.8 (7.7) | 75.8 (7.0) | 65.3 (8.9) | 80.8 (10.1) | 57.2 (7.7) | 70.8 (8.9) |
| BMI (kg/m2) | 22.6 (1.9) | 28.7 (3.4) | 22.6 (1.9) | 26.7 (2.2) | 22.7 (2.2) | 31.2 (5.0) | 22.2 (1.9) | 28.2 (1.6) | 23.3 (1.4) | 28.9 (2.7) | 21.8 (1.9) | 26.1 (1.2) |
| eGFR (mL/min/1.73 m2) | 99.2 (19.4) | 96.6 (19.6) | 97.7 (22.0) | 88.3 (14.9) | 109.0 (20.8) | 109.8 (23.6) | 92.1 (17.6) | 95.3 (18.6) | 104.1 (17.4) | 99.7 (20.2) | 86.0 (12.6) | 94.4 (17.2) |
| SUSTAIN Japan OAD Combination trial | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OW semaglutide 0.5 mg | OW semaglutide 1.0 mg | Additional OAD | ||||||||||
| Age (years) | <65 | ≥65 | <65 | ≥65 | <65 | ≥65 | ||||||
| BMI (kg/m2) | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 |
| Participant disposition, n | ||||||||||||
| Randomized | 57 | 105 | 48 | 29 | 59 | 109 | 43 | 30 | 29 | 48 | 20 | 23 |
| Trial completers | 57 (100) | 100 (95.2) | 47 (97.9) | 29 (100) | 54 (91.5) | 105 (96.3) | 42 (97.7) | 30 (100) | 29 (100) | 45 (93.8) | 20 (100) | 21 (91.3) |
| Premature treatment discontinuation | 1 (1.8) | 6 (5.7) | 6 (12.5) | 2 (6.9) | 11 (18.6) | 8 (7.3) | 14 (32.6) | 1 (3.3) | 0 | 4 (8.3) | 1 (5.0) | 2 (8.7) |
| Participant characteristics | ||||||||||||
| Age (years) | 56.5 (5.4) | 50.2 (8.2) | 70.2 (4.3) | 69.1 (3.5) | 56.3 (6.6) | 52.6 (8.5) | 70.2 (3.8) | 69.4 (3.7) | 57.7 (5.2) | 51.2 (8.4) | 70.0 (4.1) | 68.4 (3.2) |
| Male, n (%) | 41 (71.9) | 75 (71.4) | 31 (64.6) | 19 (65.5) | 47 (79.7) | 83 (76.1) | 28 (65.1) | 16 (53.3) | 24 (82.8) | 34 (70.8) | 14 (70.0) | 17 (73.9) |
| HbA1c (%) | 7.9 (0.8) | 8.3 (1.0) | 7.9 (0.8) | 7.8 (0.7) | 7.9 (0.7) | 8.4 (1.1) | 7.9 (0.7) | 8.1 (1.1) | 7.8 (0.7) | 8.3 (0.9) | 8.1 (0.8) | 8.2 (1.1) |
| HbA1c (mmol/mol) | 62.6 (9.2) | 66.8 (10.4) | 62.9 (9.0) | 61.8 (8.0) | 63.1 (7.9) | 68.1 (11.9) | 62.7 (7.4) | 64.8 (11.6) | 61.6 (8.0) | 66.9 (9.7) | 64.9 (9.0) | 65.7 (11.9) |
| FPG (mg/dL) | 158.9 (32.6) | 170.4 (36.4) | 142.4 (29.4) | 151.7 (25.1) | 155.1 (31.3) | 167.9 (40.8) | 151.8 (38.6) | 155.9 (35.4) | 160.6 (34.6) | 168.8 (36.7) | 150.8 (25.8) | 157.6 (36.5) |
| FPG (mmol/L) | 8.8 (1.8) | 9.5 (2.0) | 7.9 (1.6) | 8.4 (1.4) | 8.6 (1.7) | 9.3 (2.3) | 8.4 (2.1) | 8.7 (2.0) | 8.9 (1.9) | 9.4 (2.0) | 8.4 (1.4) | 8.7 (2.0) |
| Diabetes duration (years) | 9.0 (6.9) | 5.7 (3.7) | 10.9 (6.8) | 10.5 (6.4) | 9.9 (7.0) | 7.8 (6.0) | 11.3 (7.1) | 11.3 (4.7) | 9.2 (6.7) | 6.7 (5.4) | 14.6 (8.8) | 10.0 (6.1) |
| Bodyweight (kg) | 62.2 (7.7) | 82.0 (14.6) | 57.4 (7.5) | 70.9 (9.2) | 62.1 (7.5) | 82.4 (15.7) | 58.3 (7.8) | 71.0 (9.3) | 62.5 (7.3) | 83.3 (12.9) | 57.2 (8.1) | 74.1 (10.8) |
| BMI (kg/m2) | 22.7 (1.6) | 29.6 (4.4) | 22.0 (2.1) | 28.1 (2.3) | 22.7 (1.5) | 29.5 (4.3) | 22.2 (2.0) | 28.5 (2.9) | 22.6 (1.8) | 30.4 (3.5) | 22.1 (1.7) | 28.3 (2.3) |
| eGFR (mL/min/1.73 m2) | 104.6 (20.5) | 108.2 (20.3) | 89.1 (19.4) | 90.4 (20.1) | 100.0 (18.7) | 108.5 (27.3) | 92.9 (19.4) | 91.9 (19.2) | 100.3 (17.7) | 110.0 (25.0) | 92.5 (19.4) | 95.7 (20.6) |
All data are the mean (standard deviation) unless otherwise stated, in the full analysis set. The estimated glomerular filtration rate (eGFR) was estimated from serum creatinine using the Modification of Diet in Renal Disease formula. BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; n, number of participants; OAD, oral antidiabetes drug; OW, once weekly.
HbA1c
Reductions in HbA1c were greater in participants receiving semaglutide (both doses) compared with those receiving sitagliptin monotherapy or additional OAD regardless of baseline age (Figure 1a) or baseline BMI (Figure 1b), and across all age and BMI subgroups (Figure 1c). In the SUSTAIN Japan monotherapy trial, reductions in HbA1c were greater in participants receiving semaglutide 0.5 mg (range across subgroups –1.7 to –2.1%‐points) or semaglutide 1.0 mg (range across subgroups –1.8 to –2.4%‐points) compared with sitagliptin monotherapy (range across subgroups –0.6 to –1.0%‐points), with little variation in the magnitude of the change across subgroups; a similar pattern was seen in the SUSTAIN Japan OAD combination trial (semaglutide 0.5 mg range across subgroups: –1.7 to –1.9% points; semaglutide 1.0 mg range across subgroups: –1.8 to –2.1% points; additional OAD range across subgroups: –0.6 to –0.8% points). HbA1c reductions in participants receiving semaglutide 1.0 mg were numerically greater than or similar to those observed in participants receiving semaglutide 0.5 mg across all subgroups.
Figure 1.

Change from baseline to trial end in glycosylated hemoglobin (HbA1c) according to (a) baseline age subgroup, (b) baseline body mass index (BMI) subgroup, and (c) baseline age and BMI subgroup. *Overall means are based on values from participants across all three treatment arms. Data from the ‘on‐treatment without rescue medication’ observation period from the full analysis set were included in the analysis. The mean change from baseline at the end of the treatment and mean treatment differences (semaglutide – comparator) were estimated using a mixed model for repeated measurements with treatment, subgroup at baseline, and interaction between treatment and subgroup as fixed factors, and the baseline value as covariate, all nested within the visit. CI, confidence interval; ETD, estimated treatment difference; mono, monotherapy; OAD, oral antidiabetes drug; OW, once weekly.
Bodyweight
Reductions in bodyweight were highest in participants receiving semaglutide 1.0 mg, and greater with semaglutide (both doses) than with sitagliptin monotherapy or an additional OAD across all age subgroups (Figure 2a), all BMI subgroups (Figure 2b), and all age and BMI subgroups (Figure 2c). In the SUSTAIN Japan monotherapy trial, reductions in bodyweight were greater in participants receiving semaglutide 0.5 mg (range across subgroups –2.0 to –2.5 kg) or semaglutide 1.0 mg (range across subgroups –2.9 to –4.3 kg) compared with sitagliptin monotherapy (range across subgroups: +0.4 to –1.0 kg). In the SUSTAIN Japan OAD combination trial, reductions were also greater in participants receiving semaglutide 0.5 mg (range across subgroups –1.0 to –2.4 kg) or semaglutide 1.0 mg (range across subgroups –2.4 to –4.0 kg) than in those receiving an additional OAD (range across subgroups –0.0 to 1.0 kg).
Figure 2.

Change from baseline to trial end in bodyweight according to (a) baseline age subgroup, (b) baseline body mass index (BMI) subgroup, and (c) baseline age and BMI subgroup. *Overall means are based on values from participants across all three treatment arms. Data from the ‘on‐treatment without rescue medication’ observation period in the full analysis set were included in the analysis. The mean change from baseline at the end of the treatment and mean treatment differences (semaglutide – comparator) were estimated using a mixed model for repeated measurements with treatment, subgroup at baseline, and interaction between treatment and subgroup as fixed factors, and the baseline value as covariate, all nested within the visit. BW, body weight; CI, confidence interval; ETD, estimated treatment difference; mono, monotherapy; OAD, oral antidiabetes drug; OW, once weekly.
Treatment targets
The proportion of participants achieving HbA1c <7.0% and HbA1c ≤6.5% was greater with semaglutide (both doses) than in those receiving sitagliptin monotherapy or additional OAD across all age, BMI, and age and BMI subgroups (Figure 3a,b; Figure S1a,b); these proportions were numerically greater with semaglutide 1.0 mg versus 0.5 mg, with the exception of the ≥65 years and ≥25 kg/m2 BMI subgroup. The proportion of participants in this subgroup achieving HbA1c <7% in the SUSTAIN Japan monotherapy trial was equal for both semaglutide doses, and the proportion of participants in this subgroup achieving HbA1c <7% and ≤6.5% in the SUSTAIN Japan OAD combination trial was equal or greater in those receiving semaglutide 0.5 mg versus 1.0 mg (Figure 3a,b). Across all subgroups in the SUSTAIN Japan monotherapy trial, the proportions of participants achieving HbA1c <7.0% ranged from 74 to 100% with semaglutide 0.5 mg, and 93 to 100% with semaglutide 1.0 mg, versus 28 to 50% with sitagliptin monotherapy; the proportions achieving HbA1c <6.5% ranged from 59 to 78% with semaglutide 0.5 mg. and 82 to 93% with semaglutide 1.0 mg, versus 13 to 23% with sitagliptin monotherapy. Across all subgroups in the SUSTAIN Japan OAD combination trial, the proportions of participants achieving HbA1c <7.0% ranged from 74 to 100% with semaglutide 0.5 mg, and 83 to 100% with semaglutide 1.0 mg, versus 31–50% for those receiving an additional OAD; the proportions achieving HbA1c <6.5% ranged from 59 to 100% with semaglutide 0.5 mg, and 71 to 91% with semaglutide 1.0 mg, versus 9 to 25% for those receiving an additional OAD.
Figure 3.
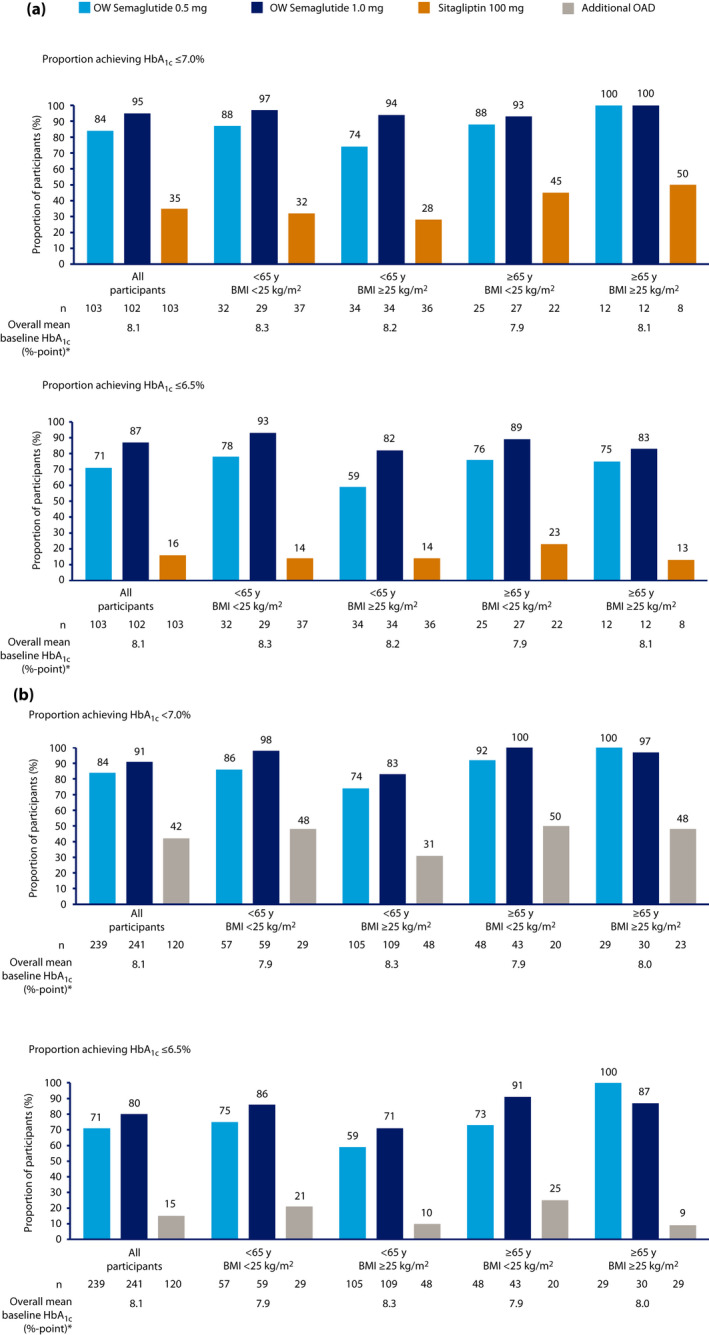
Proportion of participants achieving glycosylated hemoglobin (HbA1c) <7.0% and ≤6.5% by end of treatment, by baseline age and body mass index (BMI) subgroup, in (a) the SUSTAIN Japan monotherapy trial and (b) the SUSTAIN Japan OAD Combination trial. *Overall means are based on values from participants across all three treatment arms. On‐treatment without rescue medication data in the full analysis set. Numbers of participants refer to the numbers with available target data. Missing end‐of‐treatment data were imputed from a mixed model for repeated measurements with treatment and pre‐trial treatment at screening as fixed factors, and baseline value as the covariate, all nested within visit, and subsequently classified. mono, monotherapy; OAD, oral antidiabetes drug; OW, once weekly.
The proportion of participants with a weight loss from baseline of ≥5% was highest in those receiving semaglutide 1.0 mg, and greater with semaglutide (both doses) than in those receiving sitagliptin monotherapy or additional OAD across all age and BMI subgroups (Figure 4a,b). Across subgroups in the SUSTAIN Japan monotherapy trial, the proportions of participants with a ≥5% weight loss ranged from 24 to 38% with semaglutide 0.5 mg, and 41 to 78% semaglutide 1.0 mg, versus 0 to 11% for sitagliptin monotherapy. Across subgroups in the SUSTAIN Japan OAD combination trial, the proportion of participants with a ≥5% weight loss ranged from 24 to 31% with semaglutide 0.5 mg and 35–63% with semaglutide 1.0 mg, vs 3 to 10% of those receiving an additional OAD.
Figure 4.
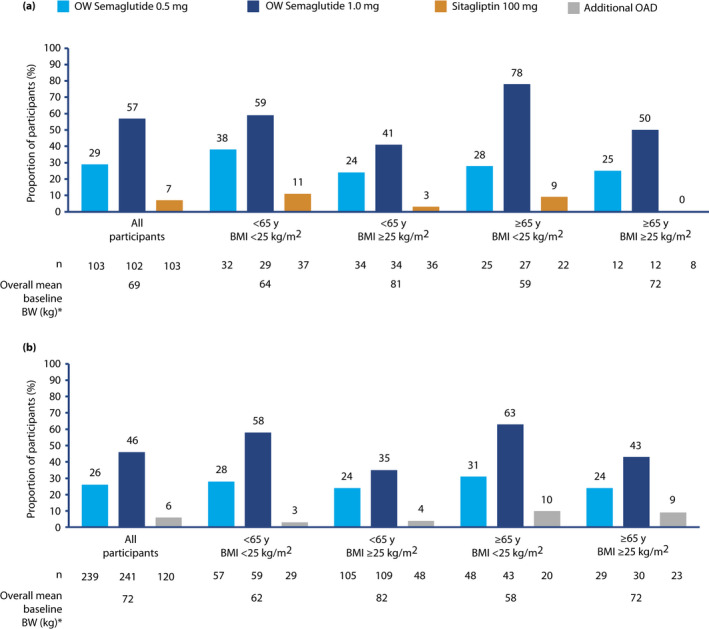
Proportion of participants achieving weight loss ≥5% by the end of treatment, by baseline age and body mass index (BMI) subgroup, in (a) the SUSTAIN Japan monotherapy trial and (b) the SUSTAIN Japan OAD Combination trial. *Overall means are based on values from participants across all three treatment arms. Numbers of participants refer to the numbers with available target data. On‐treatment without rescue medication data in the full analysis set. Missing end‐of‐treatment data imputed from a mixed model for repeated measurements with treatment and pre‐trial treatment at screening as fixed factors and baseline value as covariate, all nested within visit, and subsequently classified. BW, bodyweight; OAD, oral antidiabetes drug; OW, once weekly.
Safety
The proportions of participants with AEs, and the AE event rates, were broadly similar across treatment arms, regardless of baseline age and BMI (Table 2; Tables S2 and S3). Most AEs were mild or moderate regardless of age and BMI. Gastrointestinal AEs were more common with semaglutide (both doses) than with comparators in both studies and in all subgroups. There was no clear pattern across the treatment groups or subgroups. The proportions of participants prematurely discontinuing treatment due to AEs were highest in the semaglutide 1.0 mg group in both studies (range across subgroups 0–22.2% and 0–27.9% in the SUSTAIN Japan monotherapy and the SUSTAIN Japan OAD combination trial, respectively), and low in the groups receiving semaglutide 0.5 mg (range across subgroups 0–8.3% and 1.8–12.5% in the SUSTAIN Japan monotherapy and the SUSTAIN Japan OAD combination trial, respectively) or sitagliptin (range across subgroups 0–4.5% in the SUSTAIN Japan monotherapy trial). Premature treatment discontinuation due to AEs does not apply to the additional OAD group, because the additional OAD was not considered to be a trial product. The proportion of participants who discontinued study treatment was numerically higher in semaglutide‐treated participants aged ≥65 years than in semaglutide‐treated participants aged <65 years (Tables S3 and S4). In both trials, in the subgroup aged ≥65 years with BMI <25 kg/m2, the rate of premature study treatment discontinuation was higher with semaglutide 1.0 mg than with semaglutide 0.5 mg (Table 2).
Table 2.
Proportion of participants experiencing adverse events according to baseline age and body mass index subgroups in the SUSTAIN Japan monotherapy trial and the SUSTAIN Japan OAD Combination trial
| SUSTAIN Japan monotherapy trial | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OW semaglutide 0.5 mg | OW semaglutide 1.0 mg | Sitagliptin | ||||||||||
| Age (years) | <65 | ≥65 | <65 | ≥65 | <65 | ≥65 | ||||||
| BMI (kg/m2) | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 |
| N | 32 | 34 | 25 | 12 | 29 | 34 | 27 | 12 | 37 | 36 | 22 | 8 |
| Any AE, n (%) | 23 (71.9) | 25 (73.5) | 19 (76.0) | 10 (83.3) | 23 (79.3) | 21 (61.8) | 19 (70.4) | 10 (83.3) | 25 (67.6) | 22 (61.1) | 16 (72.7) | 5 (62.5) |
| E | 67 | 53 | 68 | 40 | 59 | 70 | 36 | 32 | 64 | 64 | 43 | 15 |
| R | 311.1 | 230.9 | 411.4 | 519.6 | 332.9 | 315.7 | 231.1 | 424.4 | 258.4 | 261.9 | 287.8 | 277.3 |
| Severity of AE, n (%) | ||||||||||||
| Mild AE | 21 (65.6) | 24 (70.6) | 18 (72.0) | 10 (83.3) | 22 (75.9) | 18 (52.9) | 19 (70.4) | 9 (75.0) | 25 (67.6) | 22 (61.1) | 15 (68.2) | 5 (62.5) |
| Moderate AE | 3 (9.4) | 4 (11.8) | 4 (16.0) | 2 (16.7) | 3 (10.3) | 5 (14.7) | 0 | 1 (8.3) | 6 (16.2) | 2 (5.6) | 2 (9.1) | 0 |
| Severe AE | 0 | 0 | 0 | 2 (16.7) | 0 | 1 (2.9) | 0 | 0 | 1 (2.7) | 0 | 1 (4.5) | 0 |
| Serious AEs, n (%) | 2 (6.3) | 0 | 1 (4.0) | 3 (25.0) | 1 (3.4) | 1 (2.9) | 0 | 0 | 1 (2.7) | 0 | 1 (4.5) | 0 |
| E | 2 | 0 | 2 | 3 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 0 |
| R | 9.3 | 0 | 12.1 | 39.0 | 5.6 | 4.5 | 0 | 0 | 8.1 | 0 | 6.7 | 0 |
| AE leading to premature treatment discontinuation, n (%) | 1 (3.1) | 0 | 1 (4.0) | 1 (8.3) | 4 (13.8) | 0 | 6 (22.2) | 1 (8.3) | 1 (2.7) | 0 | 1 (4.5) | 0 |
| E | 1 | 0 | 3 | 1 | 7 | 0 | 7 | 1 | 3 | 0 | 1 | 0 |
| R | 4.6 | 0 | 18.2 | 13.0 | 39.5 | 0 | 44.9 | 13.3 | 12.1 | 0 | 6.7 | 0 |
| GI AEs, n (%) | 10 (31.3) | 10 (29.4) | 12 (48.0) | 7 (58.3) | 12 (41.4) | 14 (41.2) | 11 (40.7) | 5 (41.7) | 6 (16.2) | 4 (11.1) | 4 (18.2) | 3 (37.5) |
| E | 19 | 14 | 20 | 10 | 17 | 38 | 14 | 13 | 10 | 7 | 4 | 7 |
| R | 88.2 | 61.0 | 121.0 | 129.9 | 95.9 | 171.4 | 89.9 | 172.4 | 40.4 | 28.6 | 26.8 | 129.4 |
| Nausea, n (%) | 4 (12.5) | 1 (2.9) | 3 (12.0) | 3 (25.0) | 4 (13.8) | 5 (14.7) | 4 (14.8) | 0 | 0 | 0 | 0 | 0 |
| Vomiting, n (%) | 0 | 2 (5.9) | 2 (8.0) | 0 | 0 | 2 (5.9) | 0 | 0 | 1 (2.7) | 0 | 0 | 0 |
| Diarrhea, n (%) | 0 | 2 (5.9) | 3 (12.0) | 2 (16.7) | 5 (17.2) | 2 (5.9) | 1 (3.7) | 1 (8.3) | 0 | 1 (2.8) | 1 (4.5) | 0 |
| Constipation, n (%) | 6 (18.8) | 2 (5.9) | 6 (24.0) | 1 (8.3) | 1 (3.4) | 6 (17.6) | 3 (11.1) | 2 (16.7) | 2 (5.4) | 1 (2.8) | 0 | 1 (12.5) |
| Abdominal discomfort, n (%) | 0 | 3 (8.8) | 1 (4.0) | 0 | 2 (6.9) | 3 (8.8) | 1 (3.7) | 1 (8.3) | 0 | 0 | 0 | 0 |
| Severe or BG‐confirmed symptomatic hypoglycemia † , n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.7) | 0 | 0 | 0 | 0 | 0 |
| SUSTAIN Japan OAD Combination trial | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OW semaglutide 0.5 mg | OW semaglutide 1.0 mg | Additional OAD | ||||||||||
| Age (years) | <65 | ≥65 | <65 | ≥65 | <65 | ≥65 | ||||||
| BMI (kg/m2) | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 | <25 | ≥25 |
| N | 57 | 105 | 48 | 29 | 59 | 109 | 43 | 30 | 29 | 48 | 20 | 23 |
| Any AE, n (%) | 52 (91.2) | 88 (83.8) | 39 (81.3) | 27 (93.1) | 51 (86.4) | 93 (85.3) | 41 (95.3) | 27 (90.0) | 18 (62.1) | 37 (77.1) | 11 (55.0) | 20 (87.0) |
| E | 220 | 360 | 193 | 136 | 203 | 456 | 183 | 112 | 50 | 126 | 35 | 58 |
| R | 333.7 | 300.8 | 371.0 | 408.5 | 331.4 | 373.9 | 467.2 | 325.7 | 146.4 | 235.3 | 154.6 | 226.5 |
| Severity of AE, n (%) | ||||||||||||
| Mild AE | 50 (87.7) | 86 (81.9) | 39 (81.3) | 27 (93.1) | 51 (86.4) | 92 (84.4) | 40 (93.0) | 26 (86.7) | 16 (55.2) | 36 (75.0) | 10 (50.0) | 18 (78.3) |
| Moderate AE | 4 (7.0) | 16 (15.2) | 6 (12.5) | 3 (10.3) | 3 (5.1) | 17 (15.6) | 5 (11.6) | 4 (13.3) | 3 (10.3) | 4 (8.3) | 2 (10.0) | 6 (26.1) |
| Severe AE | 2 (3.5) | 6 (5.7) | 1 (2.1) | 1 (3.4) | 1 (1.7) | 1 (0.9) | 1 (2.3) | 0 | 1 (3.4) | 1 (2.1) | 0 | 0 |
| Serious AEs, n (%) | 4 (7.0) | 9 (8.6) | 4 (8.3) | 2 (6.9) | 1 (1.7) | 5 (4.6) | 5 (11.6) | 1 (3.3) | 2 (6.9) | 2 (4.2) | 1 (5.0) | 3 (13.0) |
| E | 4 | 11 | 7 | 3 | 1 | 6 | 5 | 1 | 3 | 2 | 1 | 4 |
| R | 6.1 | 9.2 | 13.5 | 9.0 | 1.6 | 4.9 | 12.8 | 2.9 | 8.8 | 3.7 | 4.4 | 15.6 |
| AE leading to premature treatment discontinuation, n (%) | 1 (1.8) | 5 (4.8) | 6 (12.5) | 2 (6.9) | 8 (13.6) | 6 (5.5) | 12 (27.9) | 0 | N/A | N/A | N/A | N/A |
| E | 1 | 5 | 7 | 3 | 10 | 10 | 20 | 0 | N/A | N/A | N/A | N/A |
| R | 1.5 | 4.2 | 13.5 | 9.0 | 16.3 | 8.2 | 51.1 | 0 | N/A | N/A | N/A | N/A |
| GI AEs, n (%) | 30 (52.6) | 48 (45.7) | 32 (66.7) | 19 (65.5) | 29 (49.2) | 55 (50.5) | 33 (76.7) | 13 (43.3) | 2 (6.9) | 11 (22.9) | 2 (10.0) | 9 (39.1) |
| E | 66 | 81 | 70 | 45 | 65 | 124 | 73 | 38 | 2 | 21 | 2 | 15 |
| R | 100.1 | 67.7 | 134.6 | 135.2 | 106.1 | 101.7 | 186.4 | 110.5 | 5.9 | 39.2 | 8.8 | 58.6 |
| Nausea, n (%) | 6 (10.5) | 7 (6.7) | 10 (20.8) | 6 (20.7) | 8 (13.6) | 18 (16.5) | 16 (37.2) | 4 (13.3) | 0 | 0 | 0 | 1 (4.3) |
| Vomiting, n (%) | 3 (5.3) | 4 (3.8) | 4 (8.3) | 2 (6.9) | 4 (6.8) | 5 (4.6) | 3 (7.0) | 2 (6.7) | 0 | 0 | 0 | 2 (8.7) |
| Diarrhea, n (%) | 3 (5.3) | 10 (9.5) | 9 (18.8) | 2 (6.9) | 11 (18.6) | 17 (15.6) | 7 (16.3) | 3 (10.0) | 0 | 5 (10.4) | 0 | 3 (13.0) |
| Constipation, n (%) | 10 (17.5) | 16 (15.2) | 13 (27.1) | 6 (20.7) | 10 (16.9) | 12 (11.0) | 9 (20.9) | 5 (16.7) | 1 (3.4) | 3 (6.3) | 0 | 1 (4.3) |
| Abdominal discomfort, n (%) | 6 (10.5) | 6 (5.7) | 1 (2.1) | 2 (6.9) | 6 (10.2) | 3 (2.8) | 6 (14.0) | 0 | 0 | 0 | 0 | 0 |
| Severe or BG‐confirmed symptomatic hypoglycemia † , n (%) | 1 (1.8) | 1 (1.0) | 1 (2.1) | 0 | 2 (3.4) | 1 (0.9) | 2 (4.7) | 1 (3.3) | 1 (3.4) | 0 | 0 | 1 (4.3) |
Data from safety analysis set; percentages are based on the n for that subgroup; action taken to trial product was not collected for the additional oral antidiabetes drug (OAD) group, as the additional OAD was not considered as a trial product in this trial.
AE, adverse event; BMI, body mass index; GI, gastrointestinal; E, number of events; N, number of participants in specified subgroup in the safety analysis set; n, number of participants experiencing at least one event; N/A, not applicable; OW, once weekly; R, event rate per 100 years of treatment exposure.
An episode that is severe according to the American Diabetes Association (ADA) classification or blood glucose (BG)‐confirmed by a plasma glucose value <3.1 mmol/L (56 mg/dL), with symptoms consistent with hypoglycemia.
In the Japan monotherapy trial, no severe or BG‐confirmed symptomatic hypoglycemic episodes were reported with semaglutide 0.5 mg or comparator (Table 2). With semaglutide 1.0 mg, only one severe or BG‐confirmed symptomatic hypoglycemic episode was reported in a participant aged ≥65 years and BMI <25 kg/m2. In the Japan OAD Combination trial, severe or BG‐confirmed symptomatic hypoglycemic episodes were infrequent (three cases in the semaglutide 0.5 mg group, six in the semaglutide 1.0 mg group and two in the additional OAD group); 10 of the 11 of these cases of severe or BG‐confirmed hypoglycemic episodes occurred in combination with a sulfonylurea. No imbalance in hypoglycemia event numbers was observed in participants aged <65 versus ≥65 years or with BMI <25 kg/m2 vs ≥25 kg/m2.
DISCUSSION
In the present post‐hoc exploratory analysis, the impact of age and BMI on the efficacy and safety profile of OW semaglutide versus comparators in the two SUSTAIN Japan trials was assessed. Reductions in HbA1c observed in the Japanese population were consistent with those observed in the global SUSTAIN clinical trial program, with numerically greater reductions in HbA1c observed in OW semaglutide‐treated participants compared with those receiving a comparator. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 In our post‐hoc analysis, changes in both HbA1c and weight from baseline were consistently larger with OW semaglutide (both doses) than with comparators across all baseline age subgroups, baseline BMI subgroups, and combined baseline age and BMI subgroups. The reduction in bodyweight was greater with semaglutide 1.0 mg than with semaglutide 0.5 mg, and reductions in bodyweight for each dose were similar across all baseline age, BMI, and combined age and BMI subgroups. There were no clear differences in the overall incidence of AEs observed between the BMI and age subgroups in any of the treatment groups. In particular, the present analysis adds to the available data regarding the effects of GLP‐1RAs in elderly Japanese patients with a low BMI, which are currently limited; the data from this analysis might assist evidence‐based clinical decision‐making.
In a previous cross‐sectional study of Japanese individuals with type 1 and type 2 diabetes, sarcopenia (age‐related muscle loss and weakness) and dynapenia (impaired muscle strength, but without muscle mass reduction) were more frequent in individuals with diabetes (type 1 and type 2) compared with those without diabetes. Furthermore, old age (≥65 years) and underweight status (BMI <18 kg/m2) were associated with a higher likelihood of developing sarcopenia. 26 The Japanese clinical practice guidelines recommend favorable and appropriate glycemic control for elderly individuals with diabetes who might be at risk of issues including sarcopenia and frailty, as well as well‐balanced intake of energy, adjusted according to changes in factors including weight and BMI. 6 Thus, in Japanese people, who typically have a lower BMI and adiposity than those in Western populations, 1 , 5 the effect of OW semaglutide in elderly (age ≥65 years) individuals with a low BMI (<25 kg/m2) is of particular interest and relevance to Japanese clinicians.
In the present analysis, both 0.5 mg and 1.0 mg doses of OW semaglutide appeared to be effective in elderly participants with a low BMI, although reductions in HbA1c and bodyweight were greater with the 1.0 mg dose than the 0.5 mg dose. A large weight reduction might not be considered desirable in people with an existing low BMI, particularly elderly Japanese individuals at risk of sarcopenia. In line with general geriatric principles, 27 it might be advisable to begin treatment with a low, on‐label dose of semaglutide in such individuals, monitor responses closely, and intensify dosage and treatment based on individual needs and characteristics.
The finding of a greater proportion of participants aged ≥65 years with a BMI ≥25 kg/m2 receiving semaglutide 0.5 mg versus 1.0 mg achieving HbA1c <7% and ≤6.5% in the SUSTAIN Japan OAD combination trial is unexpected. This result contrasts with those for the proportion of participants achieving HbA1c <7% and ≤6.5% in the other subgroups in the SUSTAIN Japan OAD combination trial and all subgroups in the SUSTAIN Japan monotherapy trial. The result also contrasts with the other efficacy data across all subgroups in both trials. The patient numbers in the ≥65 years and ≥25 kg/m2 BMI subgroup in the SUSTAIN Japan OAD combination trial were smaller than those in all other subgroups, and it is possible that the paradoxical finding in this subgroup is an anomaly. In addition, the baseline mean HbA1c was higher in the patients receiving 1.0 mg versus 0.5 mg semaglutide in this subgroup (8.1 vs 7.8%, respectively). With specific regard to the HbA1c ≤7.0% target, the percentage of responders in the subgroup aged ≥65 years with a BMI ≥25 kg/m2 was 100% (29/29) among those receiving 0.5 mg semaglutide, and 97% (29/30) semaglutide for 1.0 mg. The numerical difference on this end‐point is, therefore, based on only one non‐responder in those receiving the higher dose.
This post‐hoc analysis shows the likely consistency of OW semaglutide efficacy and safety in Japanese individuals with type 2 diabetes over a range of baseline ages and BMIs. The findings provide preliminary evidence to support Japanese clinicians in delivering individualized therapy for patients according to their specific characteristics, including age and BMI. This is of relevance, because use of a GLP‐1 analog, such as OW semaglutide, which increases insulin secretion in a glucose‐dependent manner, might be preferable for Japanese people with type 2 diabetes owing to the increased prevalence of reduced insulin secretion in this population compared with reduced insulin sensitivity, as observed in white populations. 3 Furthermore, in a systematic review and meta‐analysis of 15 randomized controlled trials, GLP‐1 analogs lowered HbA1c more in studies where there was a higher proportion (≥50%) of Asian participants versus studies where <50% of participants were of Asian ethnicity. 28
There are several limitations of this post‐hoc analysis. The numbers of participants were low after stratification to subgroups, especially in the SUSTAIN Japan monotherapy trial after stratification by both baseline age and baseline BMI (ranging from 8 in the ≥25 kg/m2 and ≥65 years subgroup to 37 in the <65 years and <25 kg/m2 subgroup). This low number of participants limits the robustness of the subgroup analyses. In addition, the subgroup cut‐off for age was 65 years (rather than 75 years, which typically represents the ‘super‐elderly’) to allow larger sample sizes for analyses. It is unknown whether the findings from the ≥65 years subgroup would be applicable to the ‘super‐elderly’ aged ≥75 years. Finally, the influence of confounding factors, such as comorbidity, cannot be ruled out.
The efficacy and safety of OW semaglutide appeared to be largely consistent across baseline age and BMI subgroups in Japanese individuals; these exploratory results were similar to those from other SUSTAIN trials, and no new safety issues were observed.
ETHICAL STATEMENT
The SUSTAIN Japan monotherapy trial was carried out to meet the “Guideline for Clinical Evaluation of Oral Hypoglycemic Agents” from the Japanese Ministry of Health, Labor and Welfare, which advises that new drugs are assessed as monotherapy to investigate their isolated effects. 10 The SUSTAIN Japan OAD Combination trial was carried out to meet the “Guideline for Clinical Evaluation of Oral Hypoglycemic Agents” from the Japanese Ministry of Health, Labor and Welfare, which advises that new drugs are assessed against a marketed OAD to investigate their safety. 10 Both SUSTAIN Japan trials were carried out in compliance with the International Conference on Harmonization Good Clinical Practice guidelines 11 and Declaration of Helsinki. 12
DISCLOSURES
D Yabe reports consulting/speaker fees from Astellas Pharma Inc., Dainippon Sumitomo Pharma, Eli Lilly Japan, MSD K.K., Novo Nordisk, Nippon Boehringer Ingelheim, Ono Pharmaceutical Co. Ltd., Taisho Pharmaceutical and Takeda, and has also received clinically commissioned/joint research grants from Taisho Pharmaceutical, Ono Pharmaceutical Co. Ltd., Novo Nordisk, Arklay and Nippon Boehringer Ingelheim. Y Yamada reports personal fees from MSD K.K., personal fees from Novo Nordisk, grants and personal fees from Ono Pharmaceutical Co., Ltd., personal fees from Sumitomo Dainippon Pharma Co., Ltd., grants and personal fees from Mitsubishi Tanabe Pharma Corp., grants and personal fees from Takeda, personal fees from Sanofi, grants and personal fees from Daiichi Sankyo, and grants from Sanwa Kagaku Kenkyusho, outside the submitted work. K Kaku is an advisor to, received honoraria for lectures from and received scholarship grants from Astellas Pharma, AstraZeneca, Boehringer Ingelheim Japan, Eli Lilly Japan, Sumitomo Dainippon Pharma, Kowa, MSD, Ono Pharmaceutical, Takeda, Mitsubishi Tanabe Pharma, Taisho Toyama Pharmaceutical, Sankyo Daiichi and Sanwa Kagaku Kenkyusho. T Nishida and T Sato are employees of Novo Nordisk, and own stock in the company. Y Seino received consulting or speaker fees from Eli Lilly Japan, Sanofi, Novo Nordisk, GlaxoSmithKline, Taisho Pharmaceutical, Taisho Pharmaceutical, Astellas Pharma, BD, Nippon Boehringer Ingelheim, Johnson & Johnson and Takeda Pharmaceutical. Y Seino also received clinically commissioned/joint research grants from Nippon Boehringer Ingelheim, Eli Lilly, Taisho Pharmaceutical, MSD, Ono Pharmaceutical, Novo Nordisk Pharma, Arklay and Terumo.
Approval of the research protocol: The protocols for both trials were reviewed and approved as required according to local regulations (see Table S1 for a full list of the institutional review boards).
Informed consent: Written informed consent was obtained from all participants.
Approval date of registry and the registration no. of the study/trial: SUSTAIN Japan monotherapy trial (NCT02254291); SUSTAIN Japan OAD Combination trial (NCT02207374). Approval date of registry: N/A.
Animal studies: N/A.
Supporting information
Figure S1 | Proportion of participants achieving glycosylated hemoglobin <7.0% and ≤6.5% by the end of treatment, by baseline age or body mass index subgroup, in (a) the SUSTAIN Japan monotherapy trial and (b) the SUSTAIN Japan OAD Combination trial.
Table S1 | Institutional review boards for the (a) SUSTAIN Japan monotherapy trial and (b) SUSTAIN Japan monotherapy trial.
Table S2 | Participant disposition and baseline characteristics according to individual baseline age or body mass index subgroups in (a) SUSTAIN Japan monotherapy trial and (b) SUSTAIN Japan OAD Combination trial.
Table S3 | Summary of adverse events according to baseline age subgroups in (a) the SUSTAIN Japan monotherapy trial and (b) the SUSTAIN Japan OAD Combination trial.
Table S4 | Summary of adverse events according to baseline BMI subgroups in (a) the SUSTAIN Japan monotherapy trial and (b) the SUSTAIN Japan OAD Combination trial.
ACKNOWLEDGMENTS
The authors are grateful to the people who participated in the SUSTAIN Japan monotherapy and SUSTAIN Japan OAD Combination trials, and to Catherine Starling, AXON Communications (supported by Novo Nordisk), for writing assistance. This post‐hoc analysis was sponsored by Novo Nordisk.
J Diabetes Investig. 2022; 13: 1161–1174
REFERENCES
- 1. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig 2016; 7: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yabe D, Seino Y. Type 2 diabetes via β‐cell dysfunction in east Asian people. Lancet Diabetes Endocrinol 2016; 4: 2–3. [DOI] [PubMed] [Google Scholar]
- 3. Yabe D, Seino Y, Fukushima M, et al. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep 2015; 15: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013; 1281: 64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kusunoki‐Tsuji C, Araki S‐I, Kume S, et al. Impact of obesity on annual medical expenditures and diabetes care in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2018; 9: 776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020; 11: 165–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. PMDA New Drugs Approved in FY 2017. 2017. Available from: https://www.PMDA.go.jp/files/000232769.pdf Accessed September 16, 20021.
- 8. Seino Y, Terauchi Y, Osonoi T, et al. Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes Metab 2018; 20: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaku K, Yamada Y, Watada H, et al. Safety and efficacy of once‐weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: a randomized trial. Diabetes Obes Metab 2018; 20: 1202–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ministry of Health, Labour and Welfare . Guideline for Clinical Evaluation of Oral Hypoglycaemic Agents. Tokyo, Japan: Pharmaceuticals and Medical Devices Agency, 2010. Available from: https://www.pmda.go.jp/files/000208194.pdf Accessed September 16, 20021. [Google Scholar]
- 11. International Conference on Harmonisation Working Group, editor ICH harmonised tripartite guideline: guideline for good clinical practice E6 (R1) . International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Washington, DC, 1996.
- 12. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 13. Ouchi Y, Rakugi H, Arai H, et al. Redefining the elderly as aged 75 years and older: Proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr Gerontol Int 2017; 17: 1045–1047. [DOI] [PubMed] [Google Scholar]
- 14. Weir CB, Jan A. BMI Classification Percentile and Cut Off Points. StatPearls. Treasure Island, FL: StatPearls Publishing, 2021. [PubMed] [Google Scholar]
- 15. American Diabetes Association . 6. Glycemic targets: standards of medical care in diabetes—2020. Diabetes Care 2020; 43: S66–S76. [DOI] [PubMed] [Google Scholar]
- 16. Sorli C, Harashima S‐I, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 251–260. [DOI] [PubMed] [Google Scholar]
- 17. Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as an add‐on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56‐week, double‐blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol 2017; 5: 341–354. [DOI] [PubMed] [Google Scholar]
- 18. Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56‐week, open‐label, randomized clinical trial. Diabetes Care 2018; 41: 258–266. [DOI] [PubMed] [Google Scholar]
- 19. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once‐weekly semaglutide versus once‐daily insulin glargine as add‐on to metformin (with or without sulfonylureas) in insulin‐naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open‐label, parallel‐group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 2017; 5: 355–366. [DOI] [PubMed] [Google Scholar]
- 20. Rodbard HW, Lingvay I, Reed J, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab 2018; 103: 2291–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 22. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol 2018; 6: 275–286. [DOI] [PubMed] [Google Scholar]
- 23. Lingvay I, Catarig A‐M, Frias JP, et al. Efficacy and safety of once‐weekly semaglutide versus daily canagliflozin as add‐on to metformin in patients with type 2 diabetes (SUSTAIN 8): a double‐blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol 2019; 71: 834–844. [DOI] [PubMed] [Google Scholar]
- 24. Zinman B, Bhosekar V, Busch R, et al. Semaglutide once weekly as add‐on to SGLT‐2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo‐controlled trial. Lancet Diabetes Endocrinol 2019; 7: 356–367. [DOI] [PubMed] [Google Scholar]
- 25. Capehorn MS, Catarig A‐M, Furberg JK, et al. Efficacy and safety of once‐weekly semaglutide 1.0 mg vs once‐daily liraglutide 1.2 mg as add‐on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab 2020; 46: 100–109. [DOI] [PubMed] [Google Scholar]
- 26. Mori H, Kuroda A, Yoshida S, et al. High prevalence and clinical impact of dynapenia and sarcopenia in Japanese patients with type 1 and type 2 diabetes: findings from the Impact of Diabetes Mellitus on Dynapenia study. J Diabetes Investig 2020; 12: 1050–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valencia WM, Botros D, Vera‐Nunez M, et al. Diabetes treatment in the elderly: incorporating geriatrics, technology, and functional medicine. Curr Diab Rep 2018; 18: 95. [DOI] [PubMed] [Google Scholar]
- 28. Kim YG, Hahn S, Oh TJ, et al. Differences in the HbA1c‐lowering efficacy of glucagon‐like peptide‐1 analogues between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetes Obes Metab 2014; 16: 900–909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Proportion of participants achieving glycosylated hemoglobin <7.0% and ≤6.5% by the end of treatment, by baseline age or body mass index subgroup, in (a) the SUSTAIN Japan monotherapy trial and (b) the SUSTAIN Japan OAD Combination trial.
Table S1 | Institutional review boards for the (a) SUSTAIN Japan monotherapy trial and (b) SUSTAIN Japan monotherapy trial.
Table S2 | Participant disposition and baseline characteristics according to individual baseline age or body mass index subgroups in (a) SUSTAIN Japan monotherapy trial and (b) SUSTAIN Japan OAD Combination trial.
Table S3 | Summary of adverse events according to baseline age subgroups in (a) the SUSTAIN Japan monotherapy trial and (b) the SUSTAIN Japan OAD Combination trial.
Table S4 | Summary of adverse events according to baseline BMI subgroups in (a) the SUSTAIN Japan monotherapy trial and (b) the SUSTAIN Japan OAD Combination trial.


