ABSTRACT
Aims/Introduction
To explore the predicting factors of exercise response (whether the participants converted to diabetes) in elderly patients with prediabetes.
Materials and Methods
This is a retrospective subgroup analysis of the registered clinical trial with previous publication of the same cohort. A total of 248 participants with prediabetes were randomized to the aerobic training (n = 83) group, resistance training (n = 82) group and control group (n = 83). The patients who finished the 2‐year exercise intervention were included in this analysis to explore the factors impacting exercise response.
Results
A total of 113 patients with prediabetes completed 2 years of exercise, with 56 participants in the aerobic exercise group and 57 in the resistance exercise group. Patients who reversed to normal glucose tolerance, remained in prediabetes and developed diabetes were 18 (15.90%), 70 (62.00%) and 25 (22.10%), respectively. Logistic regression showed that baseline, homeostatic model 2 assessment of β‐cell function (β = −0.143, P = 0.039), hemoglobin A1c (β = 3.301, P = 0.007) and body mass index (β = 0.402, P = 0.012) were related to exercise response, whereas the waist‐to‐hip ratio (β = −3.277, P = 0.693) and types of exercise (β = 1.192, P = 0.093) were not significantly related to exercise response.
Conclusions
Baseline homeostatic model 2 assessment of β‐cell function, hemoglobin A1c and body mass index were the predictors for the response to exercise in elderly patients with prediabetes.
Keywords: Elderly patients, Exercise response, Prediabetes
Baseline homeostatic model 2 assessment of β‐cell function and waist‐to‐hip ratio were the predictors for the response to exercise in elderly patients with prediabetes.

INTRODUCTION
Prediabetes, also known as impaired glucose regulation, is defined as a pre‐disease state between normal blood glucose and diabetes mellitus 1 . Approximately 5–10% of people with prediabetes eventually progress to type 2 diabetes annually 2 . Exercise has been widely recognized to ameliorate insulin resistance, and therefore, prevent or delay the onset of diabetes3, 4. However, despite the overall health benefits brought by regular exercise, not all patients showed the expected results after exercise. People whose responses do not improve or even have adverse reactions are often referred to as “non‐responders” or “exercise resistors” 5 . Therefore, it is important to evaluate exercise response predictors to enhance the effectiveness of exercise intervention.
After a 2‐year exercise intervention in elderly patients with prediabetes, our team found that aerobic training (AT) and resistance training (RT) improved β‐cell function, blood glucose and lipid profiles, as well as reduced insulin resistance and type 2 diabetes risk6, 7, 8. Despite the beneficial effects achieved in the study, there were still some patients with poor exercise response and converted to type 2 diabetes. To our knowledge, there is no study analyzing the predictors of exercise response as conversion to type 2 diabetes mellitus in elderly patients with prediabetes. Therefore, the purpose of the present study was to evaluate predictors of exercise response in our cohort of elderly patients with prediabetes who participated in a 2‐year exercise intervention.
MATERIALS AND METHODS
Study design and sample selection
This was a retrospective subgroup analysis of the registered clinical trial with previous publication of the same cohort6, 7, 8. An analysis was carried out in elderly prediabetes patients who completed regular exercise (AT and RT) for up to 2 years in several medicine centers, including the Affiliated Hospital of Integrative Medicine of Nanjing University of Traditional Chinese Medicine, Danyang People's Hospital and the First Affiliated Hospital of Guangxi Medical University, from January 2014 to December 2016. The participants who completed the 2‐year study with an attendance rate ≥70% in the exercise groups were considered to have finished the exercise training, and were included in the exercise response analysis.
The program (NCT02561377, registered at www.clinicaltrial.gov) was approved by the ethics committee of Jiangsu Provincial Hospital of Integrated Traditional Chinese and Western Medicine, and all participants signed an informed consent form, in accordance with the principles of the Declaration of Helsinki.
Study participants
Retired individuals (had time to go to the centers for exercise) aged <75 years were included in the present study if they had prediabetes, as defined by any of the following criteria: (i) fasting blood glucose between 5.6–6.9 mmol/L, indicating impaired fasting glucose, on two separate occasions; (ii) blood glucose concentration between 7.8–11.0 mmol/L, 2 h after ingestion of a 75‐g oral glucose load (indicating impaired glucose tolerance); and (iii) hemoglobin A1c (HbA1c) between 5.7% and 6.4% 9 on two occasions. Participants were excluded if they had been diagnosed with diabetes, cardiovascular or cerebrovascular disease, were pregnant or breastfeeding, had severe physical disability, or lacked the intellectual or emotional capability to adhere to the study protocol 6 .
Exercise programs
Patients participated in the AT sessions three times each week for 60 min per session under the supervision of a qualified research nurse (including 5 min of warm up, aerobic exercises (dancing with music) for 50 min and 5 min of stretching exercises). Participants carried out aerobic exercises at 60–70% of their maximal heart rate determined in the treadmill test. During training, heart rate was monitored using a heart rate watch (Polar A370; Polar, Oulu, Finland), which was connected to a computer. When heart rate exceeded 60–70% of maximal heart rate, we would let participants slow down the movement or reduce the exercise time, until the heart rate returned to 60–70% of the maximal heart rate, then continue to exercise.
Participants were involved in RT sessions three times per week, supervised by research nurses, in the gardens of two hospitals (Nanjing and Danyang) and in community squares (Guilin). There were 13 exercises in the RT protocol: leg presses, leg extensions, chest presses, pull downs, rowing motions, calf raises, seated leg curls, shoulder presses, straight arm forwards, straight arm backwards, leg rotation left, leg rotation right and abdominal crunches. The protocol took approximately 50 min to complete (plus 5 min of warm up and 5 min of stretching exercises). The detailed methods of AT and RT were described in previous studies6, 7, 8. The present study assessed the response to exercise, so we mainly focused on the individuals in the exercise groups (AT and RT).
Data collection
Demographic data, such as age, sex, smoking, body mass index (BMI), waist‐to‐hip ratio and blood pressure, were collected. Bodyweight and height were measured for all participants wearing light clothing and standing barefoot. BMI was calculated from weight and height squared (kg/m2). Waist circumference was measured at the midpoint between the lower ribs and the iliac crest at the end of normal expiration.
The oral glucose tolerance test (OGTT) was carried out by administering 75 g glucose solution to participants after a 10‐h fast, with plasma glucose sampling before and 2 h after glucose administration (2hPG). Plasma glucose was analyzed using a YSI 2700 Select Biochemistry Analyzer (YSI, Yellow Springs, OH, USA). Fasting serum insulin was measured using a solid‐phase, enzyme‐labeled chemiluminescent immunometric assay (Immulite 2000; Diagnostic Products, Los Angeles, CA, USA) and homeostatic model 2 assessment of β‐cell function (HOMA2‐β) and insulin resistance (HOMA2‐IR) were calculated based on fasting serum insulin and fasting blood glucose. HbA1c was determined by HPLC (Bio‐Rad Diamat, Munich, Germany).
Blood lipid assays: Fasting serum concentrations of total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol and plasma triacylglycerol were tested by colorimetric methods using commercially available kits (Abbott, Abbott Park, IL, USA) with an Architect c8000 analyzer (Abbott) according to the manufacturer's instructions.
Data collection was carried out at baseline, 6 months, 12 months and after the completion of the 24‐month exercise.
Exercise response determination
After 2 years of exercise intervention (AT or RT), the participants who remained in prediabetes or reversed to normal glucose tolerance were defined as beneficial response to exercise, whereas the patients who converted to type 2 diabetes were defined as poor response. We repeated OGTT and HbA1c at 6 months, 12 months and 24 months. In both the AT and RT group, patients once diagnosed with diabetes at any of these time points were considered as having diabetes, regardless of their subsequent results of OGTT or HbA1C, and they were categorized to the poor exercise response groups. For example, if a patient was diagnosed as diabetes after 6 months, but their blood glucose and HbA1c were within the normal limits after 24 months of intervention, they were still considered to have diabetes.
Statistical analysis
We estimated that a sample size of 70 patients per group would provide 80% power to detect a mean (±standard deviation) clinically important difference between groups of 0.66 ± 1.2 in the change in HbA1c, at an alpha level of 0.05. SPSS 22.0 (IBM Corp., Armonk, NY, USA) was applied for statistical analysis. Baseline characteristics were compared with the use of analysis of variance or the Kruskal–Wallis test. Longitudinal changes between AT, RT and control groups were tested with the use of repeated measures analysis of variance, and intention‐to‐treat analyses were carried out. During 2 years of study, patients from the AT and RT groups who converted to diabetes were future categorized to the poor response group, whereas those who reversed to normal glucose tolerance or remained in prediabetes were categorized to the beneficial response group. In comparing the difference between the beneficial response group and poor response group, normally distributed data were shown as the mean ± standard deviation, and the independent samples t‐test was used for comparison between two groups, otherwise data were described by the median (interquartile range) and a non‐parametric rank sum test was used. The χ2‐test was used to compare the categorical variables between two different response groups. Binary logistic regression analysis was used to determine the factors impacting the response to exercise (0 = beneficial response, 1 = poor response) in elderly patients with prediabetes, and variables, such as sex, age, smoking status, types of exercise, research site, baseline HbA1c, BMI, waist‐to‐hip ratio, HOMA2‐IR and HOMA2‐β, were entered into the model simultaneously. Odds ratios (OR) and their respective 95% confidence intervals (CI) were calculated. P‐values <0.05 were considered to show statistical significance.
RESULTS
Adherence
248 elderly participants with pre‐diabetes were randomized to the AT (n = 83) group, RT (n = 82) group and control group (n = 83). A total of 172 patients completed the 2‐year study, with 58 participants in the AT group, 60 in the RT group and 55 in the control group. Two out of 58 in AT group and three out of 60 in the RT group had missing data, so 56 patients in the AT and 57 patients in the RT group were finally included in the exercise response analysis.
Baseline data and changes after 24 months in the AT, RT and control groups
Table 1 presents the characteristics and laboratory values of patients with prediabetes in the AT, RT and control groups at baseline and changes after 24 months of intervention. There were no significant differences between the groups at baseline (all P > 0.05).
Table 1.
Characteristics and laboratory values of the participants at baseline and 24 months
| Variables | Time | RT (n = 82) | AT (n = 83) | Control (n = 83) | Time × group | Main effects | P‐values | ||
|---|---|---|---|---|---|---|---|---|---|
| RT vs control | AT vs control | RT vs AT | |||||||
| Female (%) | Baseline | 52 (63.4%) | 59 (71.1%) | 50 (60.2%) | – | ||||
| Age (year) | Baseline | 59.91 ± 5.92 | 60.93 ± 5.71 | 60.73 ± 5.83 | – | ||||
| BMI | Baseline | 24.81 (24.11, 25.51) | 24.70 (24.09, 25.32) | 25.04 (24.42, 25.66) | |||||
| 24 months | 23.87 (22.34, 26.29) | 23.55 (21.80, 25.96) | 25.41 (23.52, 26.83) | 0.033* | 0.063 | 0.005** | 0.432 | ||
| FBG ‡ | Baseline | 2.44 (2.42, 2.47) | 2.42 (2.39, 2.44) | 2.41 (2.38, 2.44) | |||||
| 24 months | 2.38 (2.31, 2.50) | 2.36 (2.30, 2.42) | 2.52 (2.42, 2.60) | P < 0.001** | P < 0.001** | P < 0.001** | 0.204 | ||
| 2hPG ‡ | Baseline | 2.76 (2.70, 2.83) | 2.74 (2, 67, 2.80) | 2.82 (2.76, 2.89) | |||||
| 24 months | 2.62 (2.38, 2.98) | 2.60 (2.40, 2.95) | 2.78 (2.69, 3.01) | 0.047* | 0.020* | 0.025* | 0.704 | ||
| HbA1c (%) ‡ | Baseline | 2.43 (2.42, 2.45) | 2.44 (2.43, 2.46) | 2.44 (2.43, 2.45) | |||||
| 24 months | 2.40 (2.32, 2.49) | 2.38 (2.34, 2.44) | 2.49 (2.38, 2.56) | P < 0.001** | 0.001** | P < 0.001** | 0.306 | ||
| HOMA2‐IR | Baseline | 1.50 (1.37, 1.62) | 1.46 (1.35, 1.58) | 1.48 (1.37, 1.58) | |||||
| 24 months | 0.96 (0.82, 1.47) | 1.20 (0.99, 1.43) | 1.34. (1.09, 1.51) | 0.045* | 0.019* | 0.003* | 0.506 | ||
| HOMA2‐β ‡ | Baseline | 9.21 (8.89, 9.54) | 9.32 (9.00, 9.64) | 9.51 (9.11, 9.90) | |||||
| 24 months | 9.79 (7.76, 10.38) | 9.64 (8.79, 10.99) | 7.30 (6.14, 9.04) | P < 0.001** | P < 0.001** | P < 0.001** | 0.269 | ||
| TG † | Baseline | 0.21 (0.16, 0.26) | 0.23 (0.18, 0.27) | 0.21 (0.15, 0.26) | |||||
| 24 months | 0.21 (0.02, 0.34) | 0.22 (0.08, 0.31) | 0.25 (0.00, 0.39) | 0.387 | 0.585 | 0.125 | 0.089 | 0.951 | |
| HDL‐C | Baseline | 1.49 (1.26, 1.68) | 1.53 (1.25, 1.72) | 1.36 (1.16, 1.57) | |||||
| 24 months | 1.58 (1.33, 1.82) | 1.60 (1.27, 1.78) | 1.39 (1.20, 1.56) | 0.308 | 0.149 | 0.117 | 0.413 | 0.442 | |
| LDL‐C ‡ | Baseline | 1.74 (1.70, 1.78) | 1.78 (1.73, 1.820) | 1.70 (1.65, 1.75) | |||||
| 24 months | 1.75 (1.64, 1.89) | 1.73 (1.61, 2.00) | 1.79 (1.59, 1.94) | 0.046* | 0.017* | 0.034* | 0.938 | ||
| SBP ‡ | Baseline | 11.46 (11.32, 11.59) | 11.62 (11.46, 11.77) | 11.67 (11.53, 11.82) | |||||
| 24 months | 11.31 (10.95, 11.48) | 11.40 (11.09, 11.83) | 11.83 (11.44, 12.24) | P < 0.001** | 0.030* | 0.006** | 0.605 | ||
| DBP ‡ | Baseline | 8.81 (8.66, 8.95) | 8.96 (8.81, 9.10) | 9.01 (8.89, 9.13) | |||||
| 24 months | 8.83 (8.60, 9.11) | 8.71 (8.42, 9.27) | 9.11 (8.77, 9.38) | P < 0.001** | 0.004** | P < 0.001** | 0.046* | ||
At baseline, there were no significant differences between groups in all variables (all P > 0.05).
Intention‐to‐treat was used, so the data of all 248 participants were analyzed. Data were given as the mean (25%, 75% of confidence interval). HOMA2 Calculator Software (https://www.dtu.ox.ac.uk/homacalculator/) was used to calculate homeostatic model assessment of β‐cell function and insulin resistance (HOMA2‐β and HOMA2‐IR). 2hPG, 2‐h plasma blood glucose after 75 g glucose oral administration; BMI, body mass index; DBP, diastolic blood pressure; FINS, fasting insulin; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglyceride; WHR, waist‐to‐hip ratio.
Square root transformed.
Log10 transformed. P‐value significance in <0.05.
P < 0.05.
P < 0.01.
As shown in Table 1, after 24 months of intervention, there were several significant differences in metabolic outcomes among the three groups. After training, there was no significant difference in the changes in HbA1c (square root mean, P = 0.306), fasting blood glucose (square root mean, P = 0.204) and 2hPG (square root mean, P = 0.704) between the AT and RT groups, but changes in HbA1c (group‐by‐time interaction P < 0.001), fasting plasma glucose (group‐by‐time interaction P < 0.001) and 2hPG (group‐by‐time interaction P = 0.047) in both exercise groups were significantly greater than in the control group. Participants in both the AT and RT groups showed significant improvement in HOMA2‐β (group‐by‐time interaction P < 0.001) and HOMA2‐IR (group‐by‐time interaction P < 0.045) compared with the control group. The improvement in HOMA2‐β was not significantly greater in the RT than the AT group (P = 0.269). BMI, systolic blood, diastolic blood pressure and low‐density lipoprotein cholesterol improved in both exercise groups compared with the control group (P < 0.05). Although the decreases in BMI (P = 0.432), HOMA2‐IR (P = 0.506), systolic blood pressure (P = 0.605) and low‐density lipoprotein cholesterol (P = 0.938) were greater in the AT than the RT group, the differences did not reach statistical significance.
Exercise response
Among 113 patients who completed the 2‐year exercise training and were included in the exercise response analysis, 88 patients had beneficial exercise response and 25 patients had poor response. Of the 18 patients who reversed to normal glucose tolerance, 10 were in the AT group and eight were in the RT group. Furthermore, there were 70 patients who remained prediabetes (AT n = 37 and RT n = 33). Together, this resulted in 47 (83.9%) AT patients and 41 (71.9%) RT patients categorized as good exercise responders. A total of 25 patients converted to type 2 diabetes, with nine (16.1%) patients in the AT group and 16 (28.1%) in the RT group (Figure 1). There was no significant difference between two groups with respect to exercise response results (P = 0.124; Figure 2).
Figure 1.
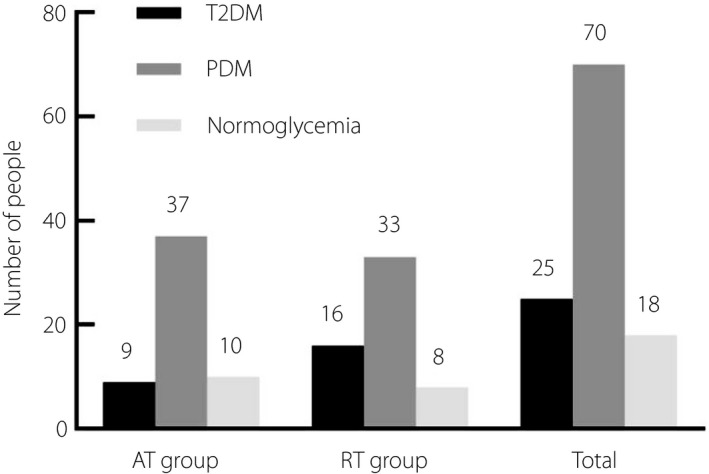
Exercise response of elderly prediabetes mellitus (PDM) patients with different types of exercise. AT, aerobic training; RT, resistance training; T2DM, type 2 diabetes mellitus.
Figure 2.
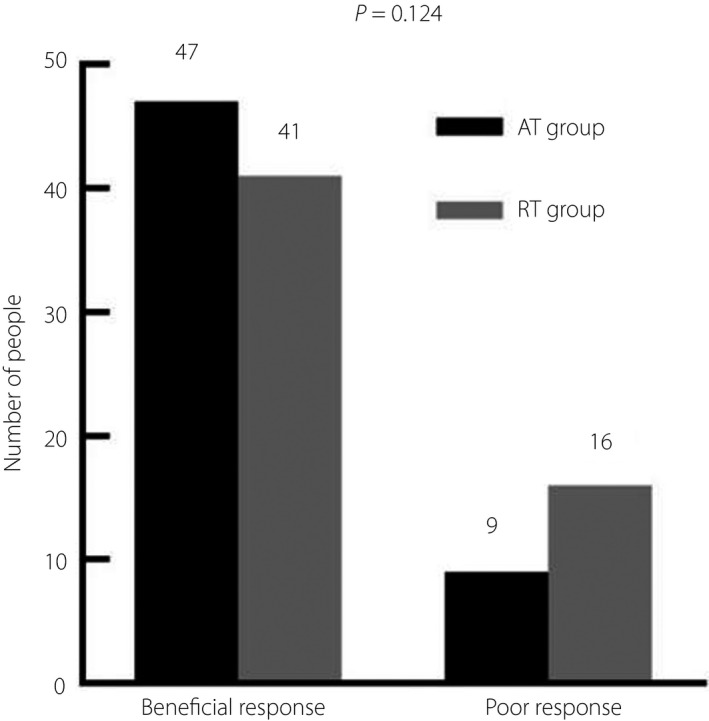
Comparison of exercise response in elderly prediabetes mellitus (PDM) patients with different types of exercise. AT, aerobic training; RT, resistance training.
Baseline information and metabolic outcome changes after 24 months of exercise training in the beneficial response group and poor response group
As presented in Table 2, 88 patients had beneficial exercise response and 25 patients had poor response. At baseline, the beneficial response group had lower BMI (P = 0.011), fasting plasma glucose (P = 0.002), 2hPG (P = 0.002), HOMA2‐IR (P = 0.015) and higher HOMA2‐β. After 24 months of training, the beneficial exercise response group had greater HOMA2‐β, and lower fasting plasma glucose, 2‐h post‐OGTT blood glucose, HbA1c, fasting insulin and HOMA2‐IR compared with the poor exercise response group (all P < 0.05).
Table 2.
Characteristics and laboratory values at baseline and changes after 24 months in the beneficial response group and poor response group
| Characteristics | Beneficial response (n = 88) | Poor response (n = 25) | T/x 2 | P‐value | |
|---|---|---|---|---|---|
| Female, n (%) | 53 (60.2%) | 12 (48.0%) | 2.090 | 0.054 | |
| Age (years) | 62.64 ± 5.89 | 64.92 ± 6.50 | 1.488 | 0.903 | |
| Smoker, n (%) | 14 (15.9%) | 7 (28.0%) | 1.213 | 0.234 | |
| AT group, n (%), | 47 (53.4%) | 9 (36%) | 2.360 | 0.124 | |
| BMI (kg/m2) | Baseline | 24.44 ± 2.72 | 26.26 ± 3.06 | 2.693 | 0.011* |
| Δ | −0.56 ± 2.17 | −1.50 ± 2.14 | −1.907 | 0.059 | |
| WHR | Baseline | 0.87 ± 0.51 | 0.90 ± 0.06 | 0.629 | 0.533 |
| Δ | 0.11 ± 0.07 | 0.13 ± 0.11 | 1.017 | 0.312 | |
| FPG (mmol/L) | Baseline | 5.84 ± 0.50 | 6.29 ± 0.58 | 3.447 | 0.002** |
| Δ | −0.34 ± 0.77 | 0.11 ± 1.24 | 2.221 | 0.028* | |
| 2hPG (mmol/L) | Baseline | 7.61 ± 1.23 | 8.41 ± 1.66 | 2.249 | 0.032* |
| Δ | −0.38 ± 1.98 | 1.85 ± 3.93 | 3.884 | 0.001* | |
| HbA1c (%) | Baseline | 5.90 (5.70, 6.10) | 6.40(5.85, 6.50) | 3.424 | 0.002** |
| Δ | −0.29 ± 0.46 | 0.58 ± 1.24 | 5.412 | 0.001** | |
| FINS (mmol/L) | Baseline | 11.51 (8.50, 13.47) | 10.61 (7.67, 12.80) | 1.67 | 0.664 |
| Δ | −1.11 ± 4.48 | 1.54 ± 5.79 | 2.444 | 0.016** | |
| TG (mmol/L) | Baseline | 1.88 ± 1.02 | 1.81 ± 0.82 | −0.388 | 0.700 |
| Δ | 0.03 ± 1.86 | −0.23 ± 0.58 | −1.117 | 0.267 | |
| HDL‐C (mmol/L) | Baseline | 1.48 ± 0.36 | 1.44 ± 0.35 | −0.554 | 0.583 |
| Δ | 0.12 ± 0.38 | 0.06 ± 0.19 | −0.768 | 0.444 | |
| LDL‐C (mmol/L) | Baseline | 3.10 ± 0.63 | 3.08 ± 0.73 | −0.164 | 0.871 |
| Δ | 0.03 ± 1.04 | 0.22 ± 1.08 | 0.814 | 0.417 | |
| SBP (mmHg) | Baseline | 132.50 (125, 140) | 136.00 (123, 145.5) | 0.986 | 0.332 |
| Δ | −2.93 ± 12.38 | −3.36 ± 16.50 | −1.141 | 0.888 | |
| DBP (mmHg) | Baseline | 80.00 (75.25, 86.00) | 84.00 (77.50, 87.50) | 0.884 | 0.382 |
| Δ | −4.20 ± 8.96 | −0.68 ± 8.86 | 1.739 | 0.085 | |
| HOMA2‐β | Baseline | 92.87 (71.63, 116.73) | 70.53 (45.57, 97.98) | −2.543 | 0.015* |
| Δ | 0.58 ± 1.23 | 0.37 ± 0.46 | −2.616 | 0.010* | |
| HOMA2‐IR | Baseline | 2.50 (1.95, 3.46) | 2.96 (1.81, 4.93) | −1.253 | 0.032* |
| Δ | −0.24 ± 1.34 | −0.13 ± 1.25 | 2.875 | 0.005* |
Data expressed as the mean ± standard deviation for continuous characteristics; n (%) for categorical characteristics; median (interquartile range) for variables with abnormal distributions. P‐value significance in <0.05. *P < 0.05, **P < 0.01. Δ, The biochemical measurements after 2 years of exercise minus the biochemical measurements at baseline; 2hPG, 2‐h plasma glucose after oral glucose tolerance test; AT, aerobic training; BMI, body mass index; DBP, diastolic blood pressure; FINS, fasting insulin; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HOMA2‐IR, homeostasis model assessment of insulin resistance; HOMA2‐β, homeostasis model assessment of β‐cell function; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglyceride; WHR, waist‐to‐hip ratio.
Factors at baseline related to exercise response in elderly patients with prediabetes
Logistic multiple regression was carried out to analyze the predictive factors at baseline to exercise response after 2 years of exercise training. The results are presented in Table 3. Baseline HbA1c (β = 3.301, P = 0.007), BMI (β = 0.402, P = 0.012) and HOMA2‐β (β = −0.143, P = 0.039) were significantly related to exercise response in elderly prediabetes patients. Baseline BMI and HbA1c were risk factors of poor response. As baseline HbA1c and BMI increased, the risk for prediabetes patients conversion to diabetes increased. Baseline HOMA2‐β was a protective factor for exercise response. Waist‐to‐hip ratio (β = −3.277, P = 0.693) and types of exercise (β = 1.192, P = 0.093) were not significantly related to exercise response (Table 3).
Table 3.
Logistic regression of factors (baseline) associated with exercise response in elderly patients with prediabetes
| Variable | β | SE | Wald | P‐value | OR | 95% Confidence interval | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | 1.426 | 0.744 | 3.670 | 0.055 | 0.240 | 0.056 | 1.033 |
| Sex | 1.172 | 0.756 | 2.402 | 0.121 | 3.228 | 0.733 | 14.212 |
| WHR | −3.277 | 8.308 | 0.156 | 0.693 | 0.038 | 0.000 | 6.425 |
| HbA1c | 3.301 | 1.226 | 7.249 | 0.007 ** | 27.127 | 2.454 | 299.821 |
| BMI | 0.402 | 0.161 | 6.283 | 0.012 * | 1.495 | 1.092 | 2.048 |
| Types of exercise | 1.192 | 0.710 | 2.820 | 0.093 | 3.294 | 0.819 | 13.243 |
| TG | −0.425 | 0.422 | 1.015 | 0.314 | 0.654 | 0.286 | 1.495 |
| HDL‐C | 0.552 | 1.129 | 0.239 | 0.625 | 1.737 | 0.190 | 15.874 |
| LDL‐C | −0.278 | 0.603 | 0.212 | 0.645 | 0.758 | 0.232 | 2.468 |
| Smoking | −0.047 | 0.849 | 0.003 | 0.956 | 1.048 | 0.198 | 5.540 |
| SBP | 0.008 | 0.031 | 0.068 | 0.794 | 1.008 | 0.949 | 1.070 |
| DBP | 0.070 | 0.056 | 1.528 | 0.216 | 1.072 | 0.960 | 1.198 |
| HOMA2‐β | −0.143 | 0.069 | 4.260 | 0.039 * | 0.867 | 0.757 | 0.993 |
| HOMA2‐IR | 2.742 | 5.175 | 0.281 | 0.596 | 15.517 | 0.001 | 394.725 |
| Research Site | 0.442 | 0.419 | 1.114 | 0.291 | 1.556 | 0.685 | 3.538 |
P‐value significance in <0.05.
2hPG, 2‐h plasma glucose after oral glucose tolerance test; AT, aerobic training; Β, standardized regression coefficient, indicates that one standard deviation change in the independent variable predicts how many standard deviations will change in the dependent variable; BMI, body mass index; DBP, diastolic blood pressure; FINS, fasting insulin; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; HOMA2‐IR, homeostasis model assessment of insulin resistance; HOMA2‐β, homeostasis model assessment of β‐cell function; LDL‐C, low‐density lipoprotein cholesterol; OR, odds ratio; RT, resistance training; SBP, systolic blood pressure; SE, standard error; TG, triglyceride; Wald, χ2 value of Wald test, = (β/SE)2; WHR, waist‐to‐hip ratio.
P < 0.05.
P < 0.01.
DISCUSSION
The present study found that after 2 years of exercise training, 88 of 113 patients (77.9%) had a benefical exercise response, and 25 (22.1%) had a poor response and converted to type 2 diabetes mellitus. Baseline HOMA2‐β, HbA1c and BMI were predictors to exercise response in elderly patients with prediabetes. Among the three predictors, as shown by their β values, the magnitude of effect of HbA1c on the odds of being a responder was greater than BMI and HOMA2‐β, and HOMA2‐β had the lowest magnitude of effect.
Despite the 2‐year exercise intervention, 22.1% of prediabetes patients still converted to diabetes, which was very high compared with the Insulin Resistance Atherosclerosis Study (IRAS), Daqing and Diabetes Prevention Program (DPP) studies. The participants’ average age in the present study (61.14 ± 6.06 years) was much higher than the IRAS (54.4 ± 0.32) 10 , Daqing (44.7 ± 0.4) 11 and DPP studies (50.6 ± 10.7) 12 . The effects of increasing insulin resistance and impaired pancreatic islet function with aging might contribute to the consequent risk of diabetes13, 14. Furthermore, in the IRAS study, together with impaired glucose tolerance patients, people with normal glucose tolerance were also included in the study, which would obviously dilute its incidence of diabetes. These might explain the higher conversion ratio in the present study compared with the IRAS, Daqing and DPP studies.
The present study found that baseline HbA1c was the strongest predictor of exercise response for patients with prediabetes, which was supported by evidence that chronic hyperglycemia was associated with smaller exercise‐induced improvements in peripheral insulin sensitivity, which in turn contributed to blunted exercise‐induced glucose response 15 . A randomized, cross‐over study found that exercise‐induced improvements in glucose response were impaired by pre‐exercise hyperglycemia 16 , which was in agreement with the present findings. Furthermore, in in vivo and in vitro studies, exposure to hyperglycemia impaired skeletal muscle cell insulin sensitivity17, 18, insulin secretion capacity19, 20 and hepatic insulin sensitivity 21 . Such hyperglycemia‐induced impairments could also explain the exercise response blunted by higher baseline HbA1c. This suggests intervening with exercise earlier (when the HbA1c levels of prediabetes patients are relatively low), rather than later. For those poor exercise responders, pharmacological or dietary intervention before introducing an exercise regimen might be an effective strategy to enhance their chances of success 22 .
The present study showed that baseline BMI was a predictor of poor exercise response. The prediabetes patients with higher baseline BMI had poorer response to exercise, which was supported by evidence that insulin resistance is one of the main pathophysiological mechanisms of prediabetes in the elderly, which is closely related to visceral fat deposition23, 24. Pancreatic islet function has declined in elderly prediabetes patients 25 . Previous studies showed that as BMI increased, the visceral fat continuously deposited, which contributes to inflammatory factors accumulating to produce oxidative stress, posing a high impact on the pancreas, leading islet function impairment, thus, exercise response is blunted26, 27. Therefore, for those who with high BMI, before exercise training, reducing BMI by diet or medication might improve their response to exercise.
However, there is epidemiological evidence that the impact of BMI is different in terms of mortality and morbidity in the elderly, as compared with other ages. The BMI associated with the lowest mortality risk was higher in older individuals than in younger individuals 28 , and a higher BMI was associated with increased survival in older adults 29 . In clinical decision‐making on weight loss for elderly prediabetes patients, healthcare professionals have to weigh the pros and cons, and future studies aimed at finding an optimal BMI range for elderly prediabetes patients are warranted.
Interestingly, even though there was no statistical deference, the decrease of BMI was greater in the poor exercise response group (−1.50 ± 2.14) than that of beneficial the exercise response group (−0.56 ± 2.17, P = 0.059). At the baseline, the average BMI of the poor response group (26.26 ± 3.06) was much higher than that of the beneficial group (24.44 ± 2.72); therefore, under the same intensity and time of exercise, the patients in the poor response group had more room for reduction. This might partially explain the fact that the magnitude of BMI reduction was greater in the poor response group than that of the beneficial response group. This might also indicate that mild change in BMI did not have a strong impact on exercise response for prediabetes patients with BMI >25.
In the present study, we found that β‐cell function, not insulin resistance at baseline, was a predictive factor for beneficial exercise response in elderly patients with prediabetes. Kahn et al 30 reported that in patients with prediabetes, their insulin sensitivity and β‐cell function decreased. Whereas it has been widely confirmed that exercise improves insulin sensitivity in prediabetes patients3, 4, 31. However, improving β‐cell function through exercise was not that easy for prediabetes patients3, 4. Thus, it is not difficult to speculate that based on the improvement of insulin sensitivity, the exercise response would largely depended on baseline β‐cell function, which suggests that, when making a treatment plan (does the patient need antiglycemic medication?) for prediabetes patients, their baseline β‐cell function should be considered.
Finally, the present study also found that type of exercise is not a factor affecting exercise response in elderly prediabetes patients. The effects of aerobic exercise on blood sugar control in elderly prediabetes patients have been confirmed by a large number of studies32, 33. In the previous study, our team found that resistance exercise is as effective as aerobic exercise in reducing insulin resistance; improving β‐cell function, blood glucose and lipid metabolism; and reducing the risk of type 2 diabetes and cardiovascular disease6, 7, 8. This also shows that the different exercise types of elderly prediabetes patients will not affect their own exercise response. Different methods of long‐term regular exercise can effectively reduce the risk of diabetes and achieve the best exercise response.
The strengths of the present study include the multicenter, randomized controlled trial design and the up to 2 years long intervention, with the exercise training supervised by the researchers. The findings from our study might have pragmatic implications to help clinicians make individualized treatment plans for prediabetes patients. However, there were still some limitations in the present study. The adherence rate was relatively low; therefore, the number of patients included in the exercise response analysis was rather small. Studies with a large sample should be carried out to confirm our findings.
In conclusion, baseline HbA1c, BMI and β‐cell function were predictive factors for exercise response in elderly patients with prediabetes. This might indicate that in treating prediabetes patients with higher HbA1c, BMI and lower β‐cell function, on the basis of diet and exercise intervention, early medications therapy might be advised.
DISCLOSURE
The authors declare no conflict of interest.
Ethics committee: The Ethics Committee of Jiangsu Provincial Hospital of Integrated Traditional Chinese and Western Medicine.
The approval number: 2013LW017.
The date on which the approval was granted: 8 August 2013.
Informed consent: All participants signed an informed consent form.
Animal studies: N/A.
ACKNOWLEDGEMENT
The authors thank all the investigators and their staff for their help with this study. This study was funded by the National Natural Science Foundation of China (grant number 81370923) and the State Administration of Traditional Chinese Medicine of the People's Republic of China (grant number JDZX2015132).
J Diabetes Investig. 2022; 13: 1253–1261
Clinical Trial Registry www.clinicaltrial.govNCT02561377
REFERENCES
- 1. American Diabetes Association . Summary of revisions: standards of medical care in diabetes—2021. Diabetes Care 2021; 44: S4–S6. [DOI] [PubMed] [Google Scholar]
- 2. Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all‐cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23‐year follow‐up study. Lancet Diabetes Endocrinol 2014; 2: 474–480. [DOI] [PubMed] [Google Scholar]
- 3. Naufahu J, Elliott B, Markiv A, et al. High‐intensity exercise decreases IP6K1 muscle content and improves insulin sensitivity (SI2*) in glucose‐intolerant individuals. J Clin Endocrinol Metab 2018; 103: 1479–1490. [DOI] [PubMed] [Google Scholar]
- 4. Ross LM, Slentz CA, Zidek AM, et al. Effects of amount, intensity, and mode of exercise training on insulin resistance and type 2 diabetes risk in the STRRIDE randomized trials. Front Physiol 2021; 12: 626142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaitan JM, Weltman A, Malin SK. Enhancing exercise responsiveness across prediabetes phenotypes by targeting insulin sensitivity with nutrition. J Diabetes Res 2017; 2017: 8314852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuan X, Dai X, Liu L, et al. Comparing the effects of 6 months aerobic exercise and resistance training on metabolic control and β‐cell function in Chinese patients with prediabetes: a multicenter randomized controlled trial. J Diabetes 2020; 12: 25–37. [DOI] [PubMed] [Google Scholar]
- 7. Dai X, Zhai LU, Chen Q, et al. Two‐year‐supervised resistance training prevented diabetes incidence in people with prediabetes: a randomised control trial. Diabetes Metab Res Rev 2019; 35: e3143. [DOI] [PubMed] [Google Scholar]
- 8. Yan J, Dai X, Feng J, et al. Effect of 12‐month resistance training on changes in abdominal adipose tissue and metabolic variables in patients with prediabetes: a randomized controlled trial. J Diabetes Res 2019; 2019: 8469739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Diabetes Association . Standards of medical care in diabetes‐2017. Diabetes Care 2017; 40: 1–142. [Google Scholar]
- 10. D’Agostino RB, Hamman RF, Karter AJ, et al. Insulin Resistance Atherosclerosis Study Investigators. Cardiovascular disease risk factors predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care 2004; 27: 2234–2240. [DOI] [PubMed] [Google Scholar]
- 11. Li G, Zhang P, Wang J, et al. The long‐term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20‐year follow‐up study. Lancet 2008; 371: 1783–1789. [DOI] [PubMed] [Google Scholar]
- 12. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012; 35: 2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu M, Liu X, Liu W, et al. β cell aging and age‐related diabetes. Aging 2021; 13: 7691–7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malin SK, Haus JM, Solomon TPJ, et al. Insulin sensitivity and metabolic flexibility following exercise training among different obese insulin‐resistant phenotypes. Am J Physiol Endocrinol Metab 2013; 305: E1292–E1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carter S, Solomon TPJ. Exercise‐induced improvements in postprandial glucose response are blunted by pre‐exercise hyperglycemia: a randomized crossover trial in healthy individuals. Front Endocrinol 2020; 15: 566548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aas V, Hessvik NP, Wettergreen M, et al. Chronic hyperglycemia reduces substrate oxidation and impairs metabolic switching of human myotubes. Biochim Biophys Acta 2011; 1812: 94–105. [DOI] [PubMed] [Google Scholar]
- 18. Shannon C, Merovci A, Xiong J, et al. Effect of chronic hyperglycemia on glucose metabolism in subjects with normal glucose tolerance. Diabetes 2018; 67: 2507–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vasu S, McClenaghan NH, McCluskey JT, et al. Cellular responses of novel human pancreatic β‐cell line, 1.1B4 to hyperglycemia. Islets. 2013; 5: 170–177. [DOI] [PubMed] [Google Scholar]
- 20. Solomon TP, Knudsen SH, Karstoft K, et al. Examining the effects of hyperglycemia on pancreatic endocrine function in humans: evidence for in vivo glucotoxicity. J Clin Endocrinol Metab 2012; 97: 4682–4691. [DOI] [PubMed] [Google Scholar]
- 21. Tripathy D, Merovci A, Basu R, et al. Mild physiologic hyperglycemia induces hepatic insulin resistance in healthy normal glucose‐tolerant participants. J Clin Endocrinol Metab 2019; 104: 2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sparks LM. Exercise training response heterogeneity: physiological and molecular insights. Diabetologia 2017; 60: 2329–2336. [DOI] [PubMed] [Google Scholar]
- 23. Aschner P. The importance of estimating abdominal obesity. Acta Méd Colomb 2013; 38: 112–113. [Google Scholar]
- 24. Thota P, Perez‐Lopez FR, Benites‐Zapata VA, et al. Obesity‐related insulin resistance in adolescents: a systematic review and meta‐analysis of observational studies. Gynecol Endocrinol 2017; 33: 179–184. [DOI] [PubMed] [Google Scholar]
- 25. Cohrs CM, Panzer JK, Drotar DM, et al. Dysfunction of persisting β cells is a key feature of early type 2 diabetes pathogenesis. Cell Rep 2020; 31: 107469. [DOI] [PubMed] [Google Scholar]
- 26. Sirbu AE, Buburuzan L, Kevorkian S, et al. Adiponectin expression in visceral adiposity is an important determinant of insulin resistance in morbid obesity. Endokrynol Pol 2018; 69: 252–258. [DOI] [PubMed] [Google Scholar]
- 27. Boersma G, Johansson E, Pereira M, et al. Altered glucose uptake in muscle, visceral adipose tissue, and brain predict whole‐body insulin resistance and may contribute to the development of type 2 diabetes: a combined PET/MR study. Horm Metab Res 2018; 50: 627–639. [DOI] [PubMed] [Google Scholar]
- 28. Bhaskaran K, dos‐Santos‐Silva I, Leon DA, et al. Association of BMI with overall and cause‐specific mortality: a population‐based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol 2018; 6: 944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reis JP, Macera CA, Araneta MR, et al. Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity 2009; 17: 1232–1239. [DOI] [PubMed] [Google Scholar]
- 30. Kahn SE, Porte D Jr. Pathophysiology of type II diabetes mellitus. In: Porte D Jr, Sherwin RS (eds). Diabetes mellitus. Stamford: Appleton and Lange, 1996; 487–512. [Google Scholar]
- 31. Malin SK, Gerber R, Chipkin SR, et al. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care 2012; 35: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pan X‐R, Li G‐W, Hu Y‐H, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997; 20: 537–544. [DOI] [PubMed] [Google Scholar]
- 33. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344: 1343–1350. [DOI] [PubMed] [Google Scholar]


